Abstract
How morphology informs function is a fundamental biological question. Here, we review the morphological features of the adrenal zona glomerulosa (zG), highlighting recent cellular and molecular discoveries that govern its formation. The zG consists of glomeruli enwrapped in a Laminin-β1-enriched basement membrane (BM). Within each glomerulus, zG cells are organized as rosettes, a multicellular structure widely used throughout development to mediate epithelial remodeling, but not often found in healthy adult tissues. Rosettes arise by constriction at a common cellular contact point mediated/facilitated by adherens junctions (AJs). In mice, small, dispersed AJs first appear postnatally and along the entire cell-cell contact around 10 days after birth. Subsequently, these AJ-rich contacts contract, allowing rosettes to form. Concurrently, flat sheet-like domains in the nascent zG, undergo invagination and folding, gradually giving rise to the compact round glomeruli that comprise the adult zG. How these structures impact adrenal function is discussed.
Introduction
Proper development and function of the adrenal gland is essential for survival [1]. The adrenal cortex is a continuously self-renewing organ, which is organized in distinct concentric zones [2]. Morphologically, the outermost zona glomerulosa (zG) consists of small compact aldosterone-producing cell clusters, whereas the innermost zona fasciculata (zF) consists of larger cells arranged in parallel cords that produce glucocorticoids. The cells of the zG migrate centripetally giving rise to zF cells, through a process of zonal transdifferentiation (Figure 1), which underlies both tissue maintenance as well as regeneration [3]. In the mouse adrenal, the zG arises within the first few weeks of postnatal life, coincident with the onset of aldosterone secretion [1]. The cellular and molecular mechanisms that govern zG formation, however, have been less well understood. It had previously been postulated that “rosette-like” structures exist in the adult zG [4], but the precise nature of these structures, how and when they arise, and their role in proper zG function has only recently been investigated. Here, we discuss the morphological features that comprise the zG and review recently discovered cellular and molecular mechanisms governing its formation.
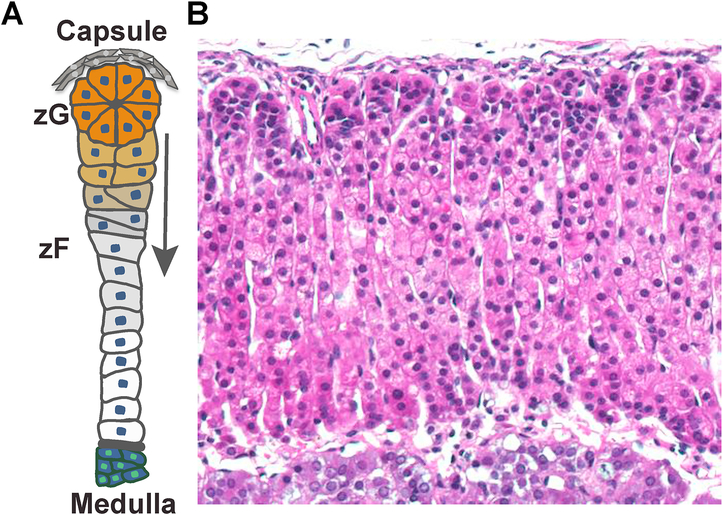
Figure 1. Adrenal cortex structure and histology.
A. Schematic of a single adrenal cortex structural unit. Beneath the capsule, zG cells are organized into rosettes and are in direct contact with zF cells organized in cords. zG cells migrate centripetally and transdifferentiate into zF cells (red arrow) during homeostatic maintenance. B. H&E staining of an adult mouse adrenal cortex cross section. zG cells are small and densely packed. zF cells appear columnar and have a larger cytoplasmic volume.
Rosette Formation, Adherens Junctions and Epithelial Remodeling
The question of how morphological features influence diverse cellular functions has long fascinated biologists. Rosettes are multicellular structures that are widely used to mediate epithelial remodeling during embryonic development and organogenesis, such as elongation of the kidney tubule, branching of pancreatic duct and closure of the neural tube [5–7]. In these contexts, they are short-lived. Rosette formation occurs when at least five neighboring epithelial cells self-organize into a flower-like structure (Figure 2A), whereas rosette resolution occurs upon the subsequent dissolution of the structure [8]. Rosette formation and resolution is observed throughout evolution, having been described in fruit flies, frogs, zebrafish, chicks, mice and humans [9, 10]. At the molecular level, rosette formation results from the specific molecular rearrangement of cell-cell junctional proteins that comprise the adherens junction (AJ) complex [9, 11].
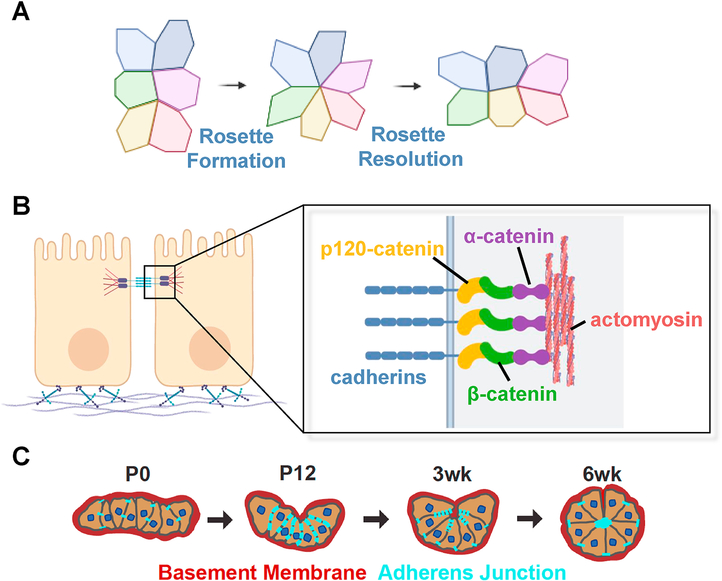
Figure 2. Mechanism of rosette formation and zG morphogenesis.
A. Classic mechanism of rosette formation and resolution in an epithelium. Polygons represent single epithelial cells viewed from the top. B. Schematic of the adherens junction (AJ) complex. Typical epithelial cells are polarized with a basal connection to the basement membrane consisting of laminin and collagen. AJ play a critical role in cell-cell contact and mechanotransduction. Cadherins provide extracellular cell-cell adhesion and serve as an intracellular scaffold for catenin proteins, which in turn bind to the actomyosin cytoskeleton network. C. Schematic of zG morphogenesis. Neonatal zG cells are loosely packed into sheet-like domains surrounded by the basement membrane. AJs are sparse along cell-cell contact. Gradually, AJs become enriched and zG undergoes extensive invagination and folding. In the adult, zG cells form rosettes and adopt the mature glomerular morphology.
AJ complex proteins play a critical role in development and tissue maintenance [11] mediating cell-cell contact and linking the cell membrane to the actin cytoskeleton (Figure 2B). AJs are comprised of core structural components, including β-catenin, α-catenin, 120-catenin and cadherins. Cadherins are membrane spanning adhesion proteins that form the backbone of AJs. Their extracellular domains mediate Ca2+-dependent connections between cells whereas their intracellular domains provide a scaffold for recruiting other adhesion and signaling molecules that link to and organize the cytoskeleton. Within the AJ complex, α-catenin is essential for junctional strength and AJ stability, which has important downstream modulatory effects on membrane stress and ion channel mechanosensitivity [9].
During subsequent steps of epithelial remodeling, rosettes typically undergo a process of convergent extension or mediolateral intercalation in the direction of tissue extension, resulting in resolution of the rosette (Figure 1, arrow) [9]. While rosette formation and resolution has been widely studied during embryonic development and has been described in the adult neural stem cell niche [9], the formation of rosettes during postnatal zG development and their presence in the mature zG of both mice and humans suggests an important role for these structures in the adrenal [10].
zG cells are organized as rosettes
The glomerular nature of the adult zG initially raised the possibility that multicellular rosettes play a key role in this tissue. Analysis of AJ complex proteins within adrenals would provide additional insight into whether rosette structures exist within the zG. Despite a previous report indicating that AJs were absent from the adrenal [12], we and others have demonstrated AJ proteins, such as β-catenin and N-cadherin at the plasma membrane of zG cells from adult mice [10, 13–15]. Actomyosin is also a key component of AJs and is essential for tissue morphogenesis [11]. Analysis of filamentous actin (F-actin), using phalloidin staining, revealed a punctate appearance of F-actin along zG cell-cell contacts, as well as large F-actin aggregates at the interface of multiple cells, representing rosette centers where as many as 10–15 cells come together [10]. As epithelial structures, zG rosettes are enwrapped by a basement membrane, enriched in laminin subunit β1 (Lamb1) and type IV collagen that outlines each glomerular unit within the adult zG layer [10]. Together, these observations establish that rosettes are present in the zG and raise intriguing possibilities about their role in adrenal development, function and disease.
Rosette formation during zG morphogenesis
Understanding how and when rosettes form is important to better understand postnatal zG development and morphogenesis. Using Lamb1 as a marker, adrenals from newborn (P0) mice demonstrate a nascent zG epithelium organized as flat sheet-like domains that lack bona fide rosette structures. In contrast, adrenals at the time of weaning (P21) demonstrate Lamb1-enwrapped domains that appear to be undergoing extensive folding and invagination. By five to six weeks of age, these structures assume the appearance of mature adult glomerular and rosette structures [10] (Figure 2C). Further analysis of F-actin localization during zG morphogenesis has shown it to be discretely distributed along cell-cell contacts as punctae at the time of birth, that later form lines and larger aggregates, before ultimately coalescing into distinct central clusters that demarcate mature rosette centers in the adult. These observations indicate that rosette formation occurs during postnatal life and may serve as a driving force underlying zG morphogenesis.
Importantly, the above observations do not prove causality between rosette formation and zG morphogenesis. In fact, it remains theoretically possible that although both occur concurrently, they are independent events influenced by distinct signals. To formally prove causation, one could use chemical inhibitors of actomyosin activity, such as blebbistatin [5], to disrupt rosette formation and follow glomerular morphogenesis. Such an approach, however, presents formidable technical challenges. First, rosette formation and glomerular morphogenesis take place during the first 3–5 weeks of postnatal life, and hence present a challenge to completely disrupt actomyosin activity in vivo for such an extensive period. This pharmacological approach may have toxic effects on the whole animal and introduce extra-adrenal complications, making interpretation of results difficult. Alternatively, ex vivo systems may allow for ease of manipulation, but the timeline of rosette formation will need to be reexamined. Furthermore, since the methodology for maintenance of differentiation in long-term adrenal slice cultures has not been established, it may prove to be technically challenging. In recent years, organotypic culture systems have become a powerful tool to model adult organ dynamics in vitro. Therefore, establishment of adrenal organoids will be an invaluable tool to study mechanisms of morphogenesis in the future.
Role of β-catenin in Rosette Formation
A growing body of genetic studies has contributed to a basic understanding of how the WNT/β-catenin signaling pathway regulates adrenocortical zonation and maintenance. WNT/β-catenin signaling specifies the identity of the zG layer, possibly by directly or indirectly inducing a zG-specific gene expression program [13, 16, 17]. WNT/β-catenin is also required for long-term cortical renewal, yet the mechanism(s) supporting this remain unclear [18]. As indicated above, β-catenin also plays a central structural role in AJ function. The impact of manipulating β-catenin on rosette biology within the zG has been studied in mice using zG-targeted β-catenin loss-of-function (βCat-LOF) [10]. βCat-LOF adrenals from both male and female adult mice showed a loss of AJ components, such as F-actin punctae and membrane-associated N-cadherin and K-cadherin, resulting in a reduction in rosette number. βCat-LOF also led glomeruli to be significantly smaller and less round, despite no change in the overall size of the zG layer. Hormonal analysis of βCat-LOF mice demonstrated no change in plasma aldosterone levels from controls in either male or female mice, though plasma renin activity (PRA) levels remain to be determined (Figure 3). Together, these data indicate that β66-catenin is necessary for generating intact rosette structures and appropriate glomerular morphology, but cannot distinguish between its structural role within the AJ and its role as a transcription factor within the WNT/β-catenin signaling pathway. In contrast, α-catenin functions as an essential structural molecule within the AJ complex, but does not function as a direct signaling molecule. Future studies exploring the role of β-catenin, given its more restricted role in AJ biology, will be required to discriminate between these possibilities.
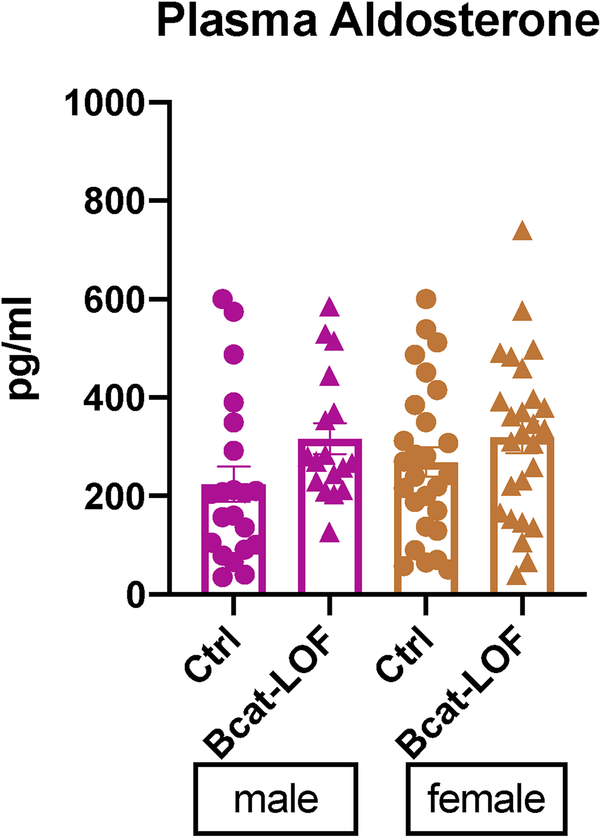
Figure 3. Plasma aldosterone level in βCat-Loss-of-Function mice.
Plasma aldosterone levels from zG-specific beta-catenin loss-of-function male and female adult mice were measured by RIA assay. ns, not significant, t-test. N = 21, 17 for male, N = 27, 26 for female.
The impact of stabilizing β-catenin on rosette number, AJ dynamics and glomerular morphology within the zG has also been studied in mice using zG-targeted β-catenin gain-of-function (βCat-GOF). Remarkably, the adrenals from βCat-GOF mice showed a progressive expansion of the zG that results from a block in zonal (zG→zF) transdifferentiation [15]. Whether the block in zonal transdifferentiation results from enhanced rosette formation, impaired rosette resolution, or a combination of both remains unknown. βCat-GOF mice also demonstrate mild hyperaldosteronism without suppression of their PRA [15]. Analysis of the expanded zG from adult βCat-GOF female mice revealed an increase in the number of rosettes along with glomeruli that were larger and more elongated than controls [10]. In contrast, male βCat-GOF mice showed a more modest zG expansion, with a more limited increase in rosette number compared to females, as well as glomeruli that were similar in size and shape to controls. Given no sexual dimorphism has been observed during initial rosette formation and zG morphogenesis, the extent to which this sexually dimorphic result reflects differences in adult zG turnover rates between male and female [19] remains to be established. Finally, analysis of AJ components (N-cadherin and K-cadherin) revealed similar increases in both male and female βCat-GOF adrenals. These results indicate that β-catenin stabilization within zG cells is sufficient for expansion of intact rosette structures and glomerular morphology, but, as in the βCat-LOF model, whether β-catenin is functioning within the AJ primarily in a structural or signaling role cannot be distinguished and remains unresolved. Understanding how zG rosette biology influences transdifferentiation and, conversely, how blocking transdifferentiation influences rosette morphology and function remain important
Rosette resolution and cellular signaling during zonal transdifferentiation
The above findings further strengthen the popular model that WNT/β-catenin dictates zG identity, implying that WNT/β-catenin activity may need to be down regulated for rosette resolution and/or zonal transdifferentiation to occur during normal homeostatic turnover within the adrenal cortex. The mechanisms underlying such downregulation, however, remain to be determined. Recent studies focusing on the ACTH/cAMP/PKA pathway in the zF show that PKA signaling inhibits β-catenin activation [20], suggest upregulation of PKA activity may be critical to allow rosette resolution and/or zonal transdifferentiation to occur. Interestingly, several cAMP/PKA inhibitors, including Pde2a, a cyclic nucleotide phosphodiesterase, are upregulated in β-catenin GOF adrenals, implying overt β-catenin activity may cause PKA inhibition. Intriguing questions such as how a cell changes its identity from a zG to a zF cell in response to this “tug of war” of WNT and PKA antagonism, and what role rosette resolution might play in mediating the migration of cells across this “war zone” require further studies.
βCat-GOF is associated with an increase in FGFR2
To explore potential mechanisms by which βCat-GOF may influence rosette formation, whole transcriptome analysis comparing adrenals from βCat-GOF and control mice was performed [10]. Consistent with the above findings, analysis of the top differentially expressed transcripts enriched in βCat-GOF mice revealed biological processes such as epithelial morphogenesis, regulation of cell adhesion and AJ assembly. Among the upregulated factors associated with changes in epithelial morphogenesis were known regulators of rosette formation, such as Fgfr2, a component of the FGF signaling pathway, and Shroom3. Both the FGF pathway and Shroom3 play a critical role in rosette formation during development. Shroom3 is a downstream target of FGFR signaling and mediates AJ constriction via actin-myosin phosphorylation at cell membranes [21]. Further analysis of βCat-GOF mice revealed an increase in FGFR2 protein levels as well as Fgfr2 and Shroom3 transcripts. Single molecule RNA in situ assays revealed that Shroom3 expression is highly specific to the zG while Fgfr2 was expressed in both zG and zF, albeit slightly more enriched in the zG. Both transcripts are markedly enriched in the expanded zG in βCat-GOF Taken together, changes in zG gene expression following βCat-GOF revealed well-known pathways with critical roles in mediating epithelial morphogenesis, cell-cell contact and AJ dynamics, suggesting a role for these pathways in zG rosette formation and morphogenesis.
Role of FGFR2 in Rosette Formation and Adrenal Development
In addition to its role in rosette biology, FGF signaling plays an important role during fetal adrenal development [22–24]. Its role in the adult cortex and interplay with the WNT/β-catenin pathway, however, has not been thoroughly investigated. FGF signaling regulates proliferation, survival, and differentiation during tissue development and maintenance [25]. Moreover, FGF signaling has a well-described role in rosette-based morphogenesis [21]. Four distinct FGF receptors (FGFR1–4) activate diverse intracellular signaling cascades [25]. In the developing adrenal, FGFR2 promotes the expansion of the adrenal primordium [23]. Consistent with this, deletion of Fgfr2 during embryonic development results in severe adrenal hypoplasia [22–24]. While FGFR2 is expressed in the adult zG [10], its role during postnatal development and tissue maintenance is less clear.
The impact of manipulating FGFR2 on rosette biology within the zG has been studied using zG-targeted Fgfr2 loss-of-function (Fgfr2-LOF) mice [10]. Analysis of the adrenals from both male and female adult mice revealed zG morphology to be significantly disrupted, without an obvious change in the overall thickness of the zG. Fgfr2-LOF led to a failure of F-actin punctae to aggregate into rosette centers and a decrease in AJ components, such as membrane-associated β-catenin, consistent with a defect in AJ integrity. In addition, Fgfr2-LOF was associated with a marked reduction in rosette number. Further analysis of cross-sectional area revealed a clear reduction in the size of zG glomeruli. Despite the changes observed in β-catenin, and the possibility that Fgfr2-LOF could directly impact canonical WNT/β-catenin signaling, transcriptional targets of β-catenin were not directly impacted by Fgfr2 deletion. Finally, the functional impact of Fgfr2-LOF on the Renin-Angiotensin-Aldosterone-System (RAAS) was assessed, which revealed upregulation of PRA but no change in aldosterone levels, suggesting a state of compensated hypoaldosteronism [10]. Together, these results indicate that FGFR2 is necessary for normal rosette formation, AJ integrity, glomerular morphology and aldosterone secretion.
While the regulatory effects of β-catenin and FGFR2 on zG morphology may be directly linked, the available data suggest that they more likely alter zG morphology in distinct ways. Whereas β-catenin is an integral component of the AJ, and its deletion leads to an expected loss of AJs and subsequent loss of rosettes, β-catenin may also directly or indirectly regulate transcripts that are potentially important mediators of rosette formation. On the other hand, loss of Fgfr2 results in small, dispersed F-actin punctae, reminiscent of the AJs in nascent zG. This effect may be mediated by Shroom3, which is required for positioning ROCK at the junctional complex to activate actomyosin in many morphogenetic contexts [21, 26, 27]. Notably, glomeruli in βCat-LOF and FGFR2-LOF adrenals do not fully resemble zG structures in wild type newborn (P0) animals, as one would expect if rosette formation were simply blocked in these mutants. As discussed below, it is possible this finding could be explained by gradual onset of ASCre/+-mediated recombination during zG morphogenesis with wild type zG cells in these LOF mutants driving a partially normal morphogenetic program.
Rosettes are organizing centers for coordinated Ca2+ signaling in the zG
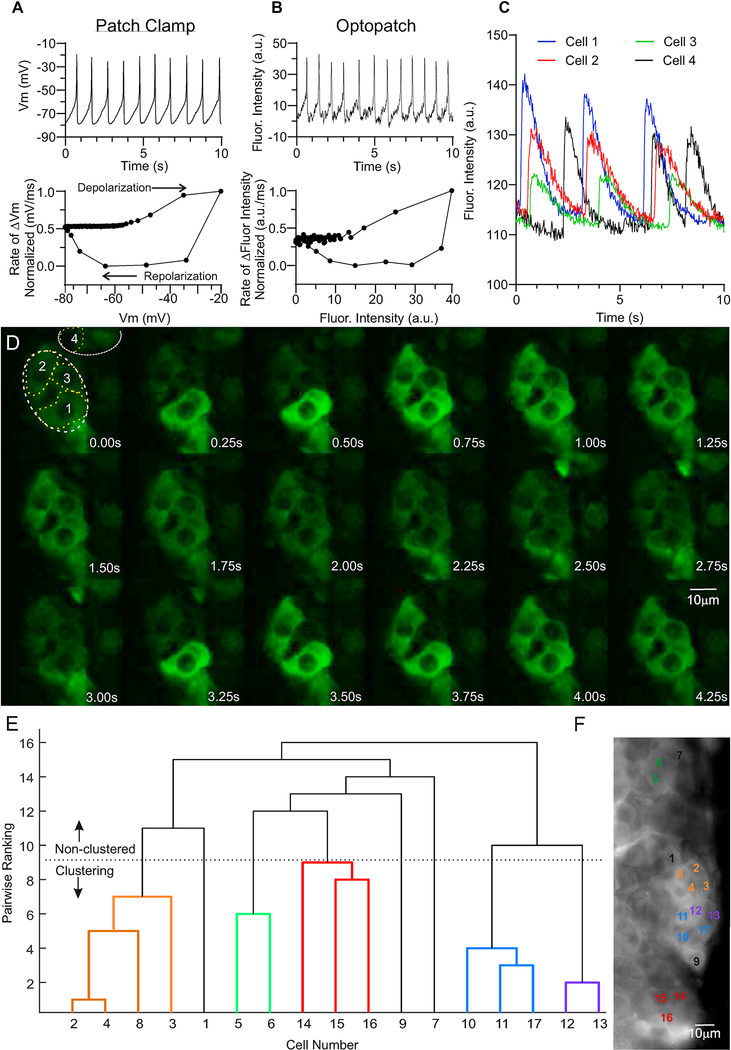
The first evidence of rosette importance to zG function was obtained using whole-cell patch-clamp electrophysiology [28]. These studies showed that zG cells in a tissue slice stimulated by Angiotensin II (Ang II) responded as electrical oscillators, demonstrating rhythmic changes in membrane voltage; a behavior not observed in dispersed zG cells (Figure 4A). Based on these findings in rosettes, zG Ca2+ wouldbe expected tobedynamic,encodedincytosolic Ca2+ oscillations driven primarily by fluctuations in membrane voltage. However, it is also formally possible that the observed electrical excitability may have been triggered by membrane disruption, cytosolic dialysis, and/or membrane suction imposed by the patch-clamp technique itself. To confirm if electrical excitability is actually an innate property of stimulated zG cells in native rosette structures, adrenal slices expressing a zG-targeted near-infrared archaerhodopsin-based voltage indicator, Optopatch, were studied [29]. In agreement with patch-clamp data, Ang II elicited rhythmic changes in fluorescent intensity in Optopatch-expressing zG cells (Figure 4B). Congruence between electrical and optical measurements of membrane voltage can be evaluated by constructing a phase-plane plot of an averaged oscillation quantifying the rate of change of each signal with voltage [d(signal-intensity)/dt) vs signal intensity] which is a sensitive index of response shape. Normalization of this rate of change (maximum value=1, minimum value=0) allows comparison of the recorded voltage trajectories (direct reporting-patch clamp; indirect reporting archaerhodopsin fluoresence-optopatch). The close correspondence of these voltage slopes indicates that voltage oscillations of zG cells are not artificial but rather are an intrinsic property of stimulated zG cells in rosettes. (Figure 4A-B).
Figure 4. Intrinsic activities of zG cells in rosettes.
A-B. Representative electrophysiological (A) and optical (B) recordings of membrane voltage oscillations evoked by 1μM Ang II from zG cells residing in rosettes. A. Upper: Whole-cell current-clamp recording; data acquisition was collected at 2.5KHz, but subsequently reduced to 100Hz to match the Archaerhodopsin fluorescence acquisition rate using the Decimate data reduction method with Clampfit v10.4 software. Lower: Normalized phase-plane plot of averaged voltage oscillation, showing arc of voltage change. B. Upper: Archaerhodopsin fluorescent trace. Lower: Phase-plane plot of voltage oscillation recorded optically showing similar voltage trajectory, verifying voltage oscillations in zG cells with preserved intracellular milieu. To compare fluorescent intensity (a.u.) and voltage (mV) data, values were scaled between 0 (Xmin) and 1 (Xmin), and calculated as: . C. The time-dependent changes in fluorescent intensity [arbitrary units (a.u.)] within regions of cells 1 (blue), 2 (red), 3 (green), and 4 (black) from A are plotted over 10 seconds, illustrating coordinated activity among zG cells within a rosette (1–3), but often asynchronous activity among cells within different rosettes (e.g., Cells 1–3 vs 4). D. Time lapse (0.25 sec intervals) of Ca2+-evoked fluorescent activity of zG cells in an adrenal slice elicited by 3nM Ang II as reported by genetically encoded GCaMP3. Images were acquired at 40Hz at 63x using a custom spinning disc confocal microscope and Slidebook 6.0.18 software [Intelligent Imaging Innovations, Inc. (3i)]. Output levels were adjusted to optimize clarity but include all information-containing pixels. Images were cropped to highlight a rosette (white dotted lines, cells 1–3), as well as a few extra-rosette cells (cell 4). Scale bar represents 10μm. E. Dendrogram illustrating Ca2+ activity coordination among zG cells expressing GCaMP3 in an adrenal slice elicited by 3nM Ang II, as determined by a functional clustering algorithm. Clearly defined cells given arbitrary number designations (X-axis) were grouped pair-wise by the degree of temporal synchrony among Ca2+ oscillatory events, with lower values indicating a greater degree of synchrony. zG cell clusters paired below the dotted line (color-coded) represent coordinated activity groups that statistically differ from pairwise comparison of 10,000 semi-randomly generated surrogate data sets per cell. Non-clustering cells (black) do not have significant coordinated activity with other cells/clusters analyzed. F. Image of averaged pixel intensity (acquired on Zeiss Examiner. Z1 at 20Hz, Slidebook 6.0.18 software, 1,000 frames averaged using ImageJ software), showing the anatomical location of cells in (E, color-matched). zG cells within a given rosette are more likely to have coordinated Ca2+ activity, whereas those outside of that rosette are rarely assigned to the same functional cluster. Scale bar represents 10μm.
A rise in intracellular Ca2+ is a critical signal driving aldosterone production [30], but whether the rosette further shapes and times the Ca2+ signal, remains incompletely understood (34). To investigate the relationship between rosette structure and Ca2+ signaling directly, live adrenal slices from mice expressing a zG-targeted Ca2+ indicator, GCaMP3 were studied [30]. Oscillatory Ca2+ signals with a slow periodicity were elicited by Ang II over a large dose range (50pM-1μM) that overlapped with zG voltage oscillations ranging from 0.5–2.0Hz (Figure 4A-B). Furthermore, pharmacological blockade of the voltage-gated T-type Ca2+ channel (Cav3.2) halted both Ca2+ and voltage oscillations. Together, these findings suggest that in the electrically excitable zG cell, in situ, oscillatory changes in zG cell cytosolic Ca2+ are driven by membrane voltage.
Notably, the Ca2+ oscillatory events elicited by Ang II were evoked in stereotypic bursts whose mean duration and number of events did not vary with dose. Ang II increased the activity of zG cells by increasing the number of bursts in a dose-dependent manner, a consequence of a decrease in the duration of inter-burst intervals [30]. Thus surprisingly, zG cells within the rosette structure do not respond to Ang II stimulation as frequency oscillators. In sum, Ang II intensifies Ca2+ signaling by generating more bursts of oscillatory activity, rather than by changing oscillatory frequency.
To determine whether the rosette acts as a functional unit to modulate Ca2+ signaling within the zG-layer, a functional clustering algorithm (FCA) was applied to test for correlated activity among the zG cell population (35). Following Ang II stimulation, oscillatory Ca2+ events of individual zG cells were observed to group into clusters that displayed synchronous Ca2+ activity (Figure 4C, E). These functional groups defined by the FCA tended to map to visually-identified individual rosette units (Figure 4F). Similarity in rosette assignment among cluster members was further verified by quantifying smaller proximate distances between clustered cell-pairs than between cell-pairs that did not cluster functionally. The importance of the rosette as a coordinator of activity was corroborated further using phase-analysis to test for fixed activity relationships among zG cells within a slice. This metric encompasses activity synchrony but expands the analysis to include cell-pairs that have correlated-activities that maintain a fixed relationship with time which are out of phase (>0º up to 359º). Again, fixed correlated-activity relationships were observed among cell-pairs within the same rosette but were seldom observed among cell-pairs that were located in different rosettes. Together, these results suggest that the rosette supports coordinated activity among zG cells and is the functional unit of the zG layer.
Finally, to assess the functional role of the AJ in the regulation of calcium activity within the zG-layer, a calcium switch assay was employed to disrupt junctions by combining calcium chelation with antibodies to the extracellular domain of cadherin proteins. Consistent with an important role for AJs in regulating Ca2+ oscillatory behavior, incubation with antibodies to the major zG cadherins following calcium chelation significantly reduced both the number and duration of zG cell Ca2+ bursts [30]. Taken together, these findings support the hypothesis that the rosette structure acts as a coordinating center for Ca2+ signaling and subsequent aldosterone production. Future studies will evaluate Ca2+ burst activity in transgenic mice, to better access how alterations in rosette morphology impact zG function.
The ASCre/+ mouse model
The heterozygous Cyp11b2(AS)Cre/+ mouse line (ASCre/+) represents a specific tool to dissect the genetic mechanisms underlying postnatal zG morphogenesis. This line was generated using homologous recombination to replace the coding region of Cyp11b2 with Cre recombinase. ASCre/+ activity begins around the time of birth [3], allowing investigators to bypass earlier stages of fetal adrenal development and evaluate the effects of genetic mutants on rosette formation and zG morphogenesis postnatally. One caveat of this model, however, is that ASCre/+-mediated recombination does not target every single zG cell simultaneously, suggesting heterogeneity among zG cells. Importantly, the AS locus is regulated by the RAAS and thus its level of activation serves as a read out for the animal’s set point/need for aldosterone at any given point in time. Under baseline conditions, when combined with conditional lineage reporters, ASCre/+-mediated recombination collectively marks nearly all zG cells by six weeks of age [31]. In contrast, the rate of recombination can appear to be much less efficient in mouse mutants where the morphology or function of the zG is impaired, as in βCat-LOF an dFGFR2-LOF mice. While an overall decrease in lineage marking may lead one to assume that recombination efficiency is reduced, such a conclusion is unlikely since these phenotypes are typically associated with decreased aldosterone production and therefore activation of the RAAS, which would favor an increase in recombination efficiency. Rather, RAAS activation likely leads to a compensatory drive to replace compromised zG cells (in an effort to restore homeostatic zG function) from undifferentiated progenitor/stem cells [31]. In this case, the continuous renewal of zG cells from undifferentiated progenitor/stem cells, that have not yet undergone ASCre lineage-marking, results in a heterogeneous zG containing both lineage-marked and -unmarked cells. Despite this, a dramatic reduction in rosette frequency and a disruption of glomerular morphology are readily observed in adrenals from βCat-LOF and FGFR2-LOF adult mice (>10 weeks of age), when homeostatic balance has presumably achieved a steady state. One approach to circumvent this potential issue involves use of the homozygous ASCre/Cre mouse model of aldosterone deficiency, where Cre activity (and recombination efficiency) is markedly enhanced (~5-fold) early in life due to enhanced activation of the RAAS [3]. It should be noted, however, that the absence of aldosterone in these mice could result in unexpected confounding effects.
What mechanism(s) underlie Rosette Resolution?
One outstanding question raised by the above findings is the mechanism(s) by which rosettes in the zG undergo resolution and whether or not this is coupled to transdifferentiation and centripetal migration. Because the zF mainly consists of single columns of cells, it is intriguing to consider how zG cells exit the rosette and reorganize into a single column of cells. One possible mechanism is through convergent extension, a developmental process whereby several columns of cells intercalate into a single column perpendicular to the axis of migration [32]. Such a mechanism would theoretically first require AJs to disaggregate from rosette centers followed by cells being guided to establish a circumferential contact with the basement membrane. A recent study in the developing intestine shows mid-gut tube elongation utilizes a process of filopodial pathfinding and cell intercalation mediated by Wnt5a/Ror2 signaling [33]. It will be of interest to study the effects of Ror2 and Wnt5a deletion on rosette resolution within the zG.
Do rosettes represent long-term structures?
It has now been established that rosette formation facilitates postnatal zG morphogenesis. It remains to be determined, however, whether de novo formation of new rosettes, underlies zG homeostasis throughout adult life. The answer to this question will inform our understanding of whether and how the zG is maintained and renewed in response to ongoing physiological demands. One possibility is that zG cells (or a subpopulation of them) are maintained long-term and undergo continuous self-renewal. In this case, it is possible that zG cells reside in rosette structures that are initially established during the early postnatal period. Another possibility is that facultative progenitor/stem cells, potentially residing within the adrenal capsule or subcapsular space, give rise to new rosette structures and zG cells. In either case, newborn zG cells must either form new rosettes or integrate into existing rosettes. These possibilities are not necessarily mutually exclusive and could depend on the degree of cellular plasticity the adrenal (and zG cells in particular) possess. Whatever the underlying mechanism(s), it is likely that regulation of the AJ will play a central role. Live imaging of adrenal slice cultures and following cellular dynamics in the adult may provide further insights. Alternatively, one could potentially perform pulse-chase lineage-marking experiments using a zG-specific inducible mouse model, such as ASCreER, to elucidate adult zG turnover
Concluding Remarks
The unique morphological features of the zG and zF have long been recognized, yet the functional significance of these features has largely remained elusive. Here, we review the cellular and molecular details of zG morphology, providing a foundation for further studies to systematically address the question of how zG morphology informs function. Glomeruli of the zG consist of rosettes, a multicellular structure widely used in epithelial remodeling throughout development. Rosettes form postnatally and underlie a dynamic process of glomerular morphogenesis, likely mediated by AJ formation and constriction. Here, we focused on two important molecular regulators of glomerular morphology, β-catenin and FGFR2, and review the evidence that modulating these two factors has a significant impact on zG function. For example, stabilization of β-catenin, an integral component of the AJ, increases rosettes and glomeruli as well as FGFR2 levels whereas βCat-LOF and Fgfr2-LOF decreases rosettes and glomeruli. These findings cannot, however, establish whether the observed changes in zG function are strictly due to altered morphology or due to direct effects that arise from manipulating these factors. Finally, rosettes serve as an organizing center for coordinated Ca2+ signaling in response to angiotensin II-mediated zG depolarization. Together, these recent developments provide a new conceptual framework to better dissect the relationship between zG morphology and function in future studies while exploring the implications of these findings for disease pathogenesis.
Materials and Methods
Mice
All animal procedures were approved by the Boston Children’s Hospital’s Institutional Animal Care and Use Committee. Mouse strains used in this study were as follows. ASCre (Cyp11b2tm1.1(cre)Brlt) bred with Ctnnb1fl (Ctnnb1tm2Kem) (purchased from Jackson Laboratory, stock 004152) led to the generation of mice with zG specific beta-catenin deletion, as previously described [10]. ASCre (Cyp11b2tm1.1(cre)Brlt) bred with B6;129S-Gt(ROSA)26Sortm38(CAG-GCaMP3)Hze/J (purchased from Jackson Laboratory, stock 014538) led to the generation of mice with zG specific GCaMP3 expression (zG-GCaMP3 mice), as previously described [30].
Hematoxylin and Eosin Imaging
After dissection, adrenals were trimmed of surrounding fat and rinsed in phosphate-buffered saline (PBS) cut into halves with a surgical blade and fixed in 4% paraformaldehyde (PFA) at 4°C overnight. After fixation, adrenals were dehydrated in ethanol, xylene, and embedded in paraffin blocks. Paraffin sections were cut at 5 μm thickness. Sections were rinsed in xylene, an ethanol gradient and then PBS. Slides then underwent standard hematoxylin and eosin staining and imaged using a 90i microscope (Nikon).
Hormone analysis
Plasma was collected by rapid retro-orbital blood collection with hematocrit tubes. Plasma aldosterone levels were determined using an aldosterone I125 radioimmunoassay (Tecan, Morrisville, NC, MG13051), as previously described [10].
Calcium/Voltage Imaging
Imaging experiments were conducted and analyzed as previously described [30]. Briefly, freshly prepared adrenal sections from heterozygous Cyp11b2(AS)Cre/+ male mice expressing either a zG-targeted Ca2+ indicator (GCaMP3) or archaerhodopsin-based voltage indicator (Optopatch) were perfused in a PIPES buffer (in mM: 20 PIPES, 122 NaCl, 3 KCl, 1.25 CaCl2, 1 MgCl, 25 D-Glucose, 0.1% BSA, pH 7.3) containing Ang II: 3nM (GCaMP3) or 1μM (Optopatch), and imaged using a Zeiss Axio-Examiner spinning disk confocal (GCaMP3, 488nm laser) or widefield (Optopatch, 637nm laser) microscope (Intelligent Imaging Innovations, Inc. [3i]). Images were acquired at 63x using Slidebook 6 (3i) software at 40Hz (GCaMP3, ~10 minutes) or 100Hz (Optopatch, ~2 minutes) with a sCMOS camera (Hamamatsu Orca-Flash 4.0). Image series were exported as multipage TIFF files, and fluorescent intensity/time extracted from regions of interested delineating in-focus zG cells using Caltracer software (Yuste lab, Columbia U., Matlab).
Patch Clamp Electrophysiology
Whole-cell current-clamp recordings were conducted as previously described (2). Adrenal slices were perfused with an external recording solution containing (in mM): 140 NaCl, 3 KCl, 10 HEPES, 2 MgCl2, 2 CaCl2, and 10 D-Glucose (pH 7.3). The internal recording solution (patch pipette) contained (in mM): 135 KMeSO3, 4 NaCl, 10 HEPES, 1 MgCl2, 0.5 EGTA, 3 Mg-ATP, and 0.3 Tris-GTP (pH 7.2). Patch-clamp voltage recordings from zG cells were acquired at 2.5KHz, but reduced to 100Hz to match the Archaerhodopsin fluorescence acquisition rate by applying the signal processing decimate data reduction method within Clampfit v10.4 software.
Functional Clustering Algorithm
Adrenal slices from zG-specific GCaMP3 expressing mice were imaged at 20Hz for 10 minutes in PIPES buffer containing 3nM Ang II on a Zeiss Axio-Examiner widefield microscope with a 63x objective. A functional clustering algorithm (1,3) was used to analyze Ca2+ event patterns among zG cells, their degree of similarity and assign functional clusters. Cluster memberships are depicted in a dendrogram. Statistical significance of detected clusters is determined by comparisons to surrogate data sets, and pairings considered significant if P < 0.001 (35).
Acnowledgements
We thank members of the Breault and Barrett laboratories for helpful discussions. This work was supported by DK-123694 and AHA 18TPA34230077 awarded to D.T.B. and HL-36977, HL-138241 awarded to P.Q.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared that no conflict of interest exist.
Literature Cited
- 1.Yates R, et al. , Adrenocortical development, maintenance, and disease. Curr Top Dev Biol, 2013. 106: p. 239–312. [DOI] [PubMed] [Google Scholar]
- 2.Pihlajoki M, et al. , Adrenocortical zonation, renewal, and remodeling. Front Endocrinol (Lausanne), 2015. 6: p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman BD, et al. , Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev Cell, 2013. 26(6): p. 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walczak EM and Hammer GD, Regulation of the adrenocortical stem cell niche: implications for disease. Nat Rev Endocrinol, 2015. 11(1): p. 14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lienkamp SS, et al. , Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension. Nat Genet, 2012. 44(12): p. 1382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villasenor A, et al. , Epithelial dynamics of pancreatic branching morphogenesis. Development, 2010. 137(24): p. 4295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura T,Honda H, and Takeichi M, Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell, 2012. 149(5): p. 1084–97. [DOI] [PubMed] [Google Scholar]
- 8.Blankenship JT, et al. , Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell, 2006. 11(4): p. 459–70. [DOI] [PubMed] [Google Scholar]
- 9.Harding MJ, Nechiporuk AHF, The roles and regulation of multicellular rosette structures during morphogenesis. Development, 2014. 141(13): p. 2549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leng S, et al. , beta-Catenin and FGFR2 regulate postnatal rosette-based adrenocortical morphogenesis. Nat Commun, 2020. 11(1): p. 1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeichi M, Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol, 2014. 15(6): p. 397–410. [DOI] [PubMed] [Google Scholar]
- 12.Black VH, et al. , A correlated thin-section and freeze-fracture analysis of guinea pig adrenocortical cells. Am J Anat, 1979. 156(4): p. 453–503. [DOI] [PubMed] [Google Scholar]
- 13.Berthon A, et al. , Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet, 2010. 19(8): p. 1561–76. [DOI] [PubMed] [Google Scholar]
- 14.Basham KJ, et al. , A ZNRF3-dependent Wnt/beta-catenin signaling gradient is required for adrenal homeostasis. Genes Dev, 2019. 33(3–4): p. 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pignatti E, et al. , Beta-Catenin Causes Adrenal Hyperplasia by Blocking Zonal Transdifferentiation. Cell Rep, 2020. 31(3): p. 107524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berthon A, et al. , WNT/beta-catenin signalling is activated in aldosterone-producing adenomas and controls aldosterone production. Hum Mol Genet, 2014. 23(4): p. 889–905. [DOI] [PubMed] [Google Scholar]
- 17.Vidal V, et al. , The adrenal capsule is a signaling center controlling cell renewal and zonation through Rspo3. Genes Dev, 2016. 30(12): p. 1389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim AC, et al. , Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development, 2008. 135(15): p. 2593–602. [DOI] [PubMed] [Google Scholar]
- 19.Grabek A, et al. , The Adult Adrenal Cortex Undergoes Rapid Tissue Renewal in a Sex-Specific Manner. Cell Stem Cell, 2019. 25(2): p. 290–296 e2. [DOI] [PubMed] [Google Scholar]
- 20.Drelon C, et al. , PKA inhibits WNT signalling in adrenal cortex zonation and prevents malignant tumour development. Nat Commun, 2016. 7: p. 12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst S, et al. , Shroom3 is required downstream of FGF signalling to mediate proneuromast assembly in zebrafish. Development, 2012. 139(24): p. 4571–81. [DOI] [PubMed] [Google Scholar]
- 22.Guasti L, et al. , FGF signalling through Fgfr2 isoform IIIb regulates adrenal cortex development. Mol Cell Endocrinol, 2013. 371(1–2): p. 182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hafner R, et al. , Fgfr2 is required for the expansion of the early adrenocortical primordium. Mol Cell Endocrinol, 2015. 413: p. 168–77. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y, et al. , Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc Natl Acad Sci U S A, 2007. 104(42): p. 16558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ornitz DM and Itoh N, The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol 2015. 4(3):p. 215–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura T and Takeichi M, Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development, 2008. 135(8): p. 1493–502. [DOI] [PubMed] [Google Scholar]
- 27.Mohan S, et al. , Structure of Shroom domain 2 reveals a three-segmented coiled-coil required for dimerization, Rock binding, and apical constriction. Mol Biol Cell, 2012. 23(11): p. 2131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu C, et al. , Zona glomerulosa cells of the mouse adrenal cortex are intrinsic electrical oscillators. J Clin Invest, 2012. 122(6): p. 2046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lou S, et al. , Genetically Targeted All-Optical Electrophysiology with a Transgenic Cre-Dependent Optopatch Mouse. J Neurosci, 2016. 36(43): p. 11059–11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guagliardo NA, et al. , Angiotensin II induces coordinated calcium bursts in aldosterone-producing adrenal rosettes. Nat Commun, 2020. 11(1): p. 1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pignatti E, et al. , Regulation of zonation and homeostasis in the adrenal cortex. Mol Cell Endocrinol, 2017. 441: p. 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rauzi M, Cell intercalation in a simple epithelium. Philos Trans R Soc Lond B Biol Sci, 2020. 375(1809): p. 20190552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, et al. , Radial WNT5A-Guided Post-mitotic Filopodial Pathfinding Is Critical for Midgut Tube Elongation. Dev Cell, 2018. 46(2): p. 173–188 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett PQ, et al. , Ion Channel Function and Electrical Excitability in the Zona Glomerulosa: A Network Perspective on Aldosterone Regulation. Annu. Rev. Physiol, 2021. 83:451–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldt S et al. , Functional clustering algorithm for the analysis of dynamic network data. Phys. Rev. E. Stat. Nonlin. Soft Matter Phys, 2009, 79 (5 Pt 2): 056104. [DOI] [PMC free article] [PubMed] [Google Scholar]