Abstract
Laboratory-markers of the systemic inflammatory-response, such as neutrophil/lymphocyte-ratio (NLR) have been studied as prognostic factors in several tumors but in OC-patients their role is still controversial and no data about the possible correlation with the BRCA-status has been ever reported. We consecutively enrolled a series of 397 newly diagnosed high-grade serous-advanced OC-patients. All patients were tested for BRCA-mutational-status and blood-parameters have been collected 48 h before staging-surgery. A significant correlation of NLR with disease distribution (p < 0.005) was found and patients with NLR < 4 underwent primary-debulking-surgery more frequently (p-value 0.001), with a lower surgical-complexity-score (p-value 0.002). Regarding survival-data, patients with NLR < 4 had a significant 7-month increase in mPFS (26 vs 19 months, p = 0.009); focusing on the BRCA-status, among both BRCA-mutated and BRCA-wild type patients, those with lower NLR had a significantly prolonged mPFS compared to patients with NLR > 4 (BRCA-mutated: 35 vs 23 months, p = 0.03; BRCA-wt: 19 vs 16 months, p = 0.05). At multivariate-analysis, independent factors of prolonged PFS were BRCA mutational status, having received complete cytoreduction and NLR < 4. Also, the strongest predictors of longer OS were BRCA-mutational status, having received complete cytoreductive surgery, NLR < 4 and age. NLR is confirmed to be a prognostic marker in OC-patients and it seems unrelated with BRCA-mutational status.
Subject terms: Gynaecological cancer, Ovarian cancer
Introduction
Ovarian cancer remains the most lethal gynecological malignancy in developed countries1.
Nearly 75% of OC-affected women present with advanced disease (stage III or IV) and most of these will die from their disease, with 5-year overall survival rates around 30%2.
Several prognostic factors have been identified to predict the outcome and guide personalized treatment of patients with advanced OC, including histological type, FIGO-stage, residual tumor after surgery, response to chemotherapy, and BRCA1/2-mutation status3. In addition to clinical and molecular features, recent data suggest the host-driven inflammatory response's influence on tumors' behavior and treatment outcome4,5. In fact, tumor growth and metastatic spread result from several interactions between tumoral and stromal factors, including blood vessels, inflammatory cells and the immune system, leading to a chronic inflammation status6,7.
Laboratory markers of the systemic inflammatory response, such as white blood cell count, have been studied as prognostic and predictive factors in several tumors8,9. High NLR, defined as the absolute neutrophils count divided by the lymphocytes count9–11, has shown a negative prognostic impact in the stomach, colorectal cancer and other cancers12–14. The mechanism underlying the association between high NLR and the worse outcome has not been clarified yet. Probably, neutrophilia can inhibit the immune system by blocking the cytolytic activity of immune cells15,16, and promotes tumor growth by producing vascular endothelial growth factor17,18. On the other hand, lymphocytopenia is frequently found in patients with advanced disease, indicating a lower immune activity against tumor antigens released by cancer cells19–21. Moreover, lymphocytes T seem keener to apoptosis22,23.
In OC, inflammation markers have shown interesting but controversial results24. In particular, recent trials have suggested that a high NLR is correlated with an immunosuppressive profile25 and with poorer overall survival and could be a predictive marker for treatment efficacy26–28. Rising literature has also demonstrated the correlation between NLR-ratio and more advanced ovarian cancer disease, with some series suggesting that preoperative higher NLR ratio is also associated with a greater risk of 30-day postoperative morbidity29–31. However, there is currently no established threshold value for the neutrophil-to-lymphocyte ratio.
Albeit OCs harboring a BRCA mutation are considered more immunoreactive and with higher mutational and neoantigen loads than BRCA wild-type tumors, no study has investigated the possible correlation between NLR values and BRCA mutational status. In light of these aspects, we investigated the relationship between BRCA status and systemic inflammatory factors in a large high-grade serous OC population.
Results
Patients’ characteristics
Overall, 397 patients fulfilled the inclusion criteria (primary diagnosis of high-grade serous ovarian cancer (HGSOC), had received 3 weekly carboplatin-paclitaxel as first line treatment, known BRCA mutational status, see “Materials and methods” section) and were evaluable for the biomarkers of interest.
Characteristics of evaluated patients are described in Table 1. The mean age at diagnosis was 60.2 years (range 27–89); 271 (68.3%) were BRCA wild-type (BRCA-WT), with 17 (4.3%) having a BRCA variant of uncertain significance (BRCA-VUS), and 127 (31.7%) had a BRCA 1/2 pathogenetic variants (BRCA-PVs). Among the latter, 79 (19.9%) patients presented with a BRCA1 mutation, and 47 (11.8%) presented with BRCA2. At diagnosis, the median value of NLR across the overall population was 4.02 (range 0.95–33). The association between the preoperative NLR score and clinicopathologic characteristics of EOC patients is shown in Table 1.
Table 1.
Characteristics of HGSOC patients by NLR.
| Total | Group 1 (NLR ≤ 4) | Group 2 (NLR > 4) | P-value | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| All cases | 397 | 196 (49.4) | 201 (50.6) | |
| Mean age at diagnosis (range, years) | 60.2 (27–89) | 60.8 (27–89) | 59.8 (30–85) | 0.837 |
| Type of BRCA mutation | 0.97 | |||
| No mutation | 271 (68.3) | 135 (68.3) | 136 (68.3) | |
| BRCA1 | 79 (19.9) | 39 (20.1) | 40 (20.1) | |
| BRCA2 | 47 (11.8) | 24 (12.1) | 23 (11.6) | |
| CA125, mean (SD), UI/mL | 2632 (4265) | 2085 (3152) | 1973 (3190) | 0.81 |
| FIGO stage at diagnosisa | ||||
| III | 282 (71.8) | 144 (73.1) | 138 (70.4) | 0.31 |
| IV | 111 (28.2) | 53 (26.9) | 58 (29.6) | |
| LPS-PIVb | ||||
| < 8 | 173 (43.9) | 108 (55.4) | 65 (32.7) | 0.0001 |
| ≥ 8 | 221 (56.1) | 87 (44.6) | 134 (67.3) | |
| Primary treatment strategy | ||||
| PDS | 186 (46.9) | 110 (55.6) | 76 (38.2) | 0.001 |
| NACT | 211 (53.1) | 88 (44.4) | 123 (61.8) | |
| Surgical complexity scorec,* | ||||
| 1–2 | 82 (44.6) | 59 (54.1) | 23 (30.7) | 0.002 |
| 3 | 102 (55.4) | 50 (45.9) | 52 (69.3) | |
| RT at primary surgery (PDS) | ||||
| 0 | 158 (84.9) | 92 (83.6) | 66 (86.8) | 0.75 |
| 1–10 mm | 18 (9.7) | 11 (10) | 7 (9.1) | |
| > 1 cm | 10 (5.4) | 7 (6.4) | 3 (3.9) | |
WT: wild type; VUS: variants of uncertain significance; PVs: pathogenetic variants; FIGO: International Federation of Gynecology and Obstetrics; LPS-PIV: laparoscopic predictive index value; PDS: primary debulking surgery; NACT: neoadjuvant chemotherapy; RT residual tumor; NLR: neutrophile/lymphocyte ratio.
aData calculated on 393 patients due to lack of data of 4 patients.
bData calculated on 394 patients due to lack of data of 3 patients.
cData calculated on 395 patients due to lack of data of 2 patients.
* Calculated only in women treated with PDS.
Regarding the BRCA status, no significant differences were found regarding NLR values (p-value: 0.97). The majority of patients presented as stage III of disease (282, 71.8%), without differences related to NLR value. We found a significant correlation of NLR with disease distribution, with more patients with low tumor load in Group 1 (NLR < 4) versus Group 2 (NLR > 4) (44.6% vs 67.3%) (p < 0.0001).
Moreover, patients in group 1 (110, 55.6%) underwent PDS more frequently than patients in group 2 (76, 38.2%) (p-value 0.001), with no statistically significant difference of complete/optimal cytoreduction in the overall population. Among patients undergoing PDS, those with lower NLR also had a lower surgical complexity score (59, 54.1%) compared with those in group 2 (23, 30.7%) (p-value 0.002).
Impact of NLR on survival
The median follow-up was 24 months (range 4–47). At the time of final analysis, 210 (52.9%) of patients have recurred in the overall population, with more recurrences among group 2 (57.8%) versus group 1 (48%), respectively (p = 0.03). Similarly, 105 (24.2%) patients were dead, 64 (32.2%) in group 2 and 41 (20.7%) in group 1 (p = 0.007).
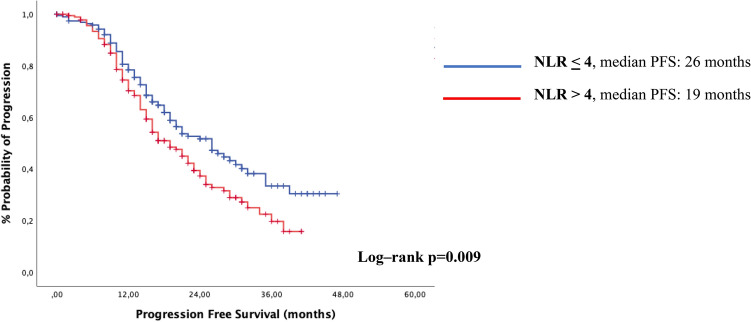
In the overall population, median progression-free survival (mPFS) was 21 months; those with NLR < 4 had a significant 7-month increase in mPFS, compared with patients with NLR > 4 (26 months vs. 19 months, p = 0.009, Fig. 1).
Figure 1.
Kaplan–Meyer plots for progression free survival (PFS) according to NLR-value, overall population.
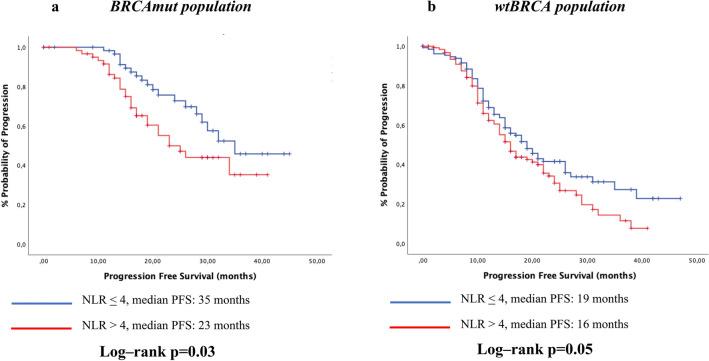
Focusing on the BRCA status, among BRCAmut patients, those with lower NLR had a significantly prolonged mPFS (35 months vs. 23 months, p = 0.03, Fig. 2a); similarly, among BRCA wild-type patients, those with lower NLR had a slightly significant 3-month increase in mPFS, compared with patients in group 2 (19 months vs. 16 months, p = 0.05, Fig. 2b). At multivariate analysis for PFS, independent factors of prolonged PFS were BRCA mutational status (HR 0.50, CI 95% 0.35–0.71), having received complete cytoreduction (HR 0.51, CI 95% 0.35–0.75) and NLR < 4 (HR 0.69, CI 95% 0.51–0.95) (Table 2).
Figure 2.
Kaplan–Meyer plots for progression free survival (PFS) according to NLR-value and BRCA status, subgroup analysis.
Table 2.
Cox univariate and multivariate analysis for progression-free survival (PFS).
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | 1.01 (1.00–1.03) | 0.008 | 1.00 (0.09–1.02) | 0.34 |
| LPS-PIV < 8/ ≥ 8 | 0.61 (0.46–0.81) | 0.001 | 0.98 (0.64–1.52) | 0.96 |
| BRCA status mut/wt | 0.43 (0.31–0.60) | 0.0001 | 0.50 (0.35–0.71) | 0.0001 |
| PDS/NACT | 0.61 (0.46–0.81) | 0.001 | 0.79 (0.52–1.20) | 0.27 |
| RT 0/ > 0 | 0.49 (0.33–0.71) | 0.0001 | 0.51 (0.35–0.75) | 0.001 |
| NLR ≤ 4/ > 4 | 0.69 (0.52–0.91) | 0.01 | 0.69 (0.51–0.95) | 0.023 |
LPS-PIV: laparoscopic predictive index value; mut: mutated; WT: wild type; PDS: primary debulking surgery; NACT: neoadjuvant chemotherapy; RT: residual tumor; NLR: neutrophil/lymphocyte ratio.
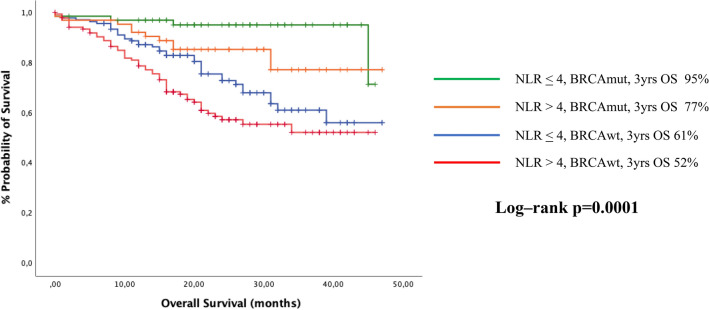
Median overall survival (mOS) at 60 months was still not reached and 3 years OS was 72% in group 1 and 60% in group 2 (p = 0.007). Statistically significant differences related to NLR were found in the BRCAmut (mOS not reached in both groups, p = 0.05), and BRCA wild-type (mOS not reached in both groups, p = 0.027) populations (Fig. 3). In the multivariate analysis, the strongest predictors of longer OS were BRCA mutational status (HR 0.47, CI 95% 0.26–0.85) having received complete cytoreduction (HR 0.42, CI 95% 0.25–0.72), NLR < 4 (HR 0.58, CI 95% 0.36–0.95) and younger age (HR 1.03, CI 95% 1.01–1.06) (Table 3).
Figure 3.
Kaplan–Meyer plots for overall survival (OS) according to NLR-value and BRCA status, overall population.
Table 3.
Cox univariate and multivariate analysis for overall-survival (OS).
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | 1.05 (1.03–1.07) | 0.0001 | 1.03 (1.01–1.06) | 0.001 |
| LPS-PIV < 8/ ≥ 8 | 0.46 (0.30–0.71) | 0.0001 | 0.81 (0.39–1.65) | 0.563 |
| BRCA status mut/wt | 0.29 (0.16–0.50) | 0.0001 | 0.47 (0.26–0.85) | 0.013 |
| PDS/NACT | 0.43 (0.28–0.65) | 0.0001 | 0.79 (0.39–1.57) | 0.497 |
| RT 0/ > 0 | 0.40 (0.24–0.68) | 0.001 | 0.42 (0.25–0.72) | 0.001 |
| NLR ≤ 4/ > 4 | 0.59 (0.39–9.87) | 0.008 | 0.58 (0.36–0.95) | 0.032 |
LPS-PIV: laparoscopic predictive index value; mut: mutated; WT: wild type; PDS: primary debulking surgery; NACT: neoadjuvant chemotherapy; RT: residual tumor; NLR: neutrophil/lymphocyte ratio.
Discussion
In this study, we found for the first time that high NLR (> 4) has a negative prognostic role in patients with primary advanced OC, in terms of both PFS and OS, regardless of BRCA status.
Several evidences suggest that BRCA-mutated OC disease exhibits statistically significantly higher mutational and neoantigen loads and might be more immunologically "hot"/T-cell inflamed than BRCAwt and HR proficient ovarian cancers. Therefore, we wanted to explore whether or not a simple blood biomarker could predict this. Nevertheless, we weren't able to find such a correlation, as NLR values seem unrelated to the presence/absence of the BRCA mutation. In other words, if we assume that NLR is correlated, at least indirectly, with the immune status, based on our results, individual immunoreactivity to cancer is independent of BRCA status. We shouldn't be surprised that BRCA, as well as HRD status, do not linearly predict response to immune checkpoint inhibitors (ICIs)32,33 and we should consider NLR as a more reliable predictor of immunotherapy response even in OC, as it has been recently demonstrated in other cancers34–36.
More importantly, we came up with the evidence that OC BRCA-mutated disease is not "one disease" with peculiar and good survival outcomes. Even in the presence of a BRCA mutation, the prognosis can be determined by other factors, of which NLR is one easily identifiable. Indeed, among BRCAmut patients, those with low NLR had seven months advantage of mPFS with respect to women with high NLR.
Moreover, it is now recognized that PARPi, which are known to achieve their most remarkable efficacy in BRCA mutated and HRD cancer cells, can also get a response in HR proficient cancer37. This can be explained by both a cytotoxic activity (depending on HR deficiency) and antitumor immune activity, which might be more relevant on HR proficient cells38–41. This hypothesis should be further investigated in the HR proficient population to provide NLR as a marker able to identify those patients who can rely on their reactive antitumor-immune response to benefit from PARPi.
We also found that high NLR in patients with primary advanced OC is predictive of larger tumor burden (expressed as LPS-PIV score) and those with higher NLR have higher chances of receiving NACT instead of PDS, compared with those with lower NLR. These observations are in line with the negative prognostic value of high NLR reported in other retrospective series of different OC settings30,42. Finally, our data confirm that NLR at baseline has an independent prognostic impact for both PFS and OS.
Our cohort's strength relies on collecting data from a large single-center population consecutively enrolled in a prospective study for tissue-BRCA status investigation. Furthermore, no data about NLR according to BRCA status in OC have been published before. However, it should be recognized that neutrophils and lymphocytes counts are non-specific parameters because they could be influenced by concurrent conditions, such as infections or inflammation. The cut-off value to discriminate between the high or low group using NLR is not clearly established. We decided to use the cut-off closer to the median value, which was 4 in our population, as it has been previously proposed43 and considering that the more often used values are 3 or 519,44. In particular, in a previous meta-analysis of 12 studies including patients with ovarian cancer, an elevated preoperative NLR was associated with more advanced stage and worse OS and PFS30; interestingly, all the included studies used different neutrophil-to-lymphocyte ratio cut-offs (ranging from 2.1 to 4), and finally, the Authors concluded that a neutrophil-to-lymphocyte ratio ≥ 3 is associated with poorer survival.
Of course, the lack of a definitive cut-off might be a critical limitation in our assessment's general application, though it would reasonably not change the final findings of our analysis.
In conclusion, NLR is confirmed to be a prognostic marker in OC patients. The information obtained from our study has revealed a potential new biologic subtype of BRCA patients, correlated with inflammation status and easily detectable. Next research should be focused on the role of NLR with regard to PARPi and ICIs response, regardless of BRCA/HRD status, underlining others less common but not less effective mechanisms of action of these drugs and allowing further personalization of treatment.
Materials and methods
Patients
Between January 2017 and December 2019, newly diagnosed high grade serous ovarian cancer (HGSOC) patients with FIGO Stage IIIC-IV, admitted at the Gynecologic Oncology Unit, Fondazione Policlinico A. Gemelli IRCCS in Rome were consecutively tested for the tissue/blood BRCA mutation within a prospective study45.
All women received gynecologic oncologist counseling before BRCA testing and a signed written informed consent. BRCA-mutations were classified according to the ENIGMA BRCA1/2 Gene Variant Classification Criteria (http://www.enigmaconsortium.org/) and women with variants of uncertain significance (VUS) were considered wild-type. Tissue samples for somatic testing were obtained during surgery. Patients were included if they had a primary diagnosis of high-grade serous ovarian cancer (HGSOC), if they had received 3 weekly carboplatin-paclitaxel as first line treatment, with or without maintenance therapy, if their BRCA mutational status was available. Their blood parameters should have been collected in the local laboratory at Fondazione Policlinico A. Gemelli IRCCS 48 h before staging laparoscopy/laparotomy.
All women gave written informed consent for their data to be collected and analyzed for scientific purposes. The Institutional Review Board of the Catholic University of the Sacred Hear approved the study (CICOG-01-07-19/35).
Clinical data and follow-up
According to our Institutional model, patients were initially submitted to clinical evaluation, CT-scan and staging laparoscopy (S-LPS)46 to be triaged to primary debulking surgery (PDS) or neoadjuvant chemotherapy (NACT). Intraperitoneal tumor burden was evaluated at diagnosis using a laparoscopic predictive-index value (LPS-PIV)46, classifying women as having: low tumor load in the presence of LPS-PIV < 8, and high tumor load when an LPS-PIV ≥ 8 was observed. A maximal surgical effort was attempted in all patients selected for PDS, and the residual tumor was recorded. The Complexity of surgical procedures in patients receiving PDS was graded according to the surgical complexity score (SCS) by Aletti et al.47. Regardless of upfront treatment strategy, all women received six cycles of carboplatin (area under the curve [AUC] 5 or 6) plus paclitaxel (175 mg/mq) every 21 days (Q3W); maintenance therapy was also administrated as indicated according to internal protocol and included Bevacizumab (15 mg/kg) in combination with chemotherapy and maintenance Q3W for 22 cycles or Olaparib 300 mg tablets orally two times per day until disease progression or toxicity for a maximum of 2 years, in BRCA mutated patients (since April 2019).
After treatment administration, patients were entered into a routine follow-up program including gynecological examination, CA125 assessment and CT-scan every 6 months.
Data from medical records were consecutively collected including medical history, surgery results, treatment approach, and genetic counseling.
Endpoints and statistical analysis
NLR was defined as the absolute neutrophils count divided by the absolute lymphocytes count. Neutrophils and lymphocytes count collected within 48 h before staging laparoscopy or laparotomy were taken into consideration. A cut-off value of 4 was adopted to discriminate patients with low (NLR < 4) (Group 1) versus high (NLR > 4) (Group 2) as primary analysis, according to previously published data and median value in the present series27,48–52.
The primary endpoint of the study was to investigate if exists a possible correlation between the BRCA status and NLR values in a large high-grade serous OC population; also, we evaluated the correlation between NLR values and clinical parameters of patients with advanced high-grade serous OC, as well as survival outcomes (progression-free survival and overall survival).
Chi-square or Fisher's exact tests were used for comparison of categorical variables.
Regarding survival analysis, PFS was defined as the time elapsed between the date of diagnosis (staging laparoscopy/laparotomy) and recurrence; patients without evidence of progressive disease at the time of the analysis were censored on the date of their last tumor evaluation. Overall survival (OS) was defined as the time interval between the diagnosis and death of any cause. Patients who were no longer alive at the time of the analysis or had been lost to follow-up were censored on the date of their last follow-up visit. PFS and OS were estimated by the Kaplan–Meier method, and curves were compared by the log-rank or Breslow (Generalized Wilcoxon) tests (at a significance level of 5%), as appropriate. Estimated hazard ratios (HRs) and their two-sided 95% confidence intervals (95% CIs) were calculated using the Cox proportional-hazard model. All statistical calculations were carried out using SPSS 26.0 for Mac (SPSS Inc., Chicago, IL, USA).
Ethical approval
All procedures performed in studies involving human participants were under the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author contributions
C.M. planned the study, conducted statistical analysis and drafted the manuscript. M.D. drafted the manuscript, advised on results interpretation. C.B. collected data and drafted the manuscript. C.D.I. and S.D.B. collected data. B.C., L.V. and A.M. revised the manuscript. G.S. planned the study, advised on results interpretation and revised the manuscript. A.F. planned the study, advised on results interpretation, drafted and revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cancer Statistics Center. American Cancer Society Web site. Updated 2020. https://cancerstatisticscenter.cancer.org/.
- 2.Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374:1371–1382. doi: 10.1016/S0140-6736(09)61338-6. [DOI] [PubMed] [Google Scholar]
- 3.Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240–1253. doi: 10.1016/S0140-6736(18)32552-2. [DOI] [PubMed] [Google Scholar]
- 4.Bot A. Editorial: In this issue. Int. Rev. Immunol. 2010;29(5):459–460. doi: 10.3109/08830185.2010.510390. [DOI] [PubMed] [Google Scholar]
- 5.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkwill F, Mantovani A. Cancer and inflammation: Implications for pharmacology and therapeutics. Clin. Pharmacol. Ther. 2010;87(4):401–406. doi: 10.1038/clpt.2009.312. [DOI] [PubMed] [Google Scholar]
- 7.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 9.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 10.Leitch EF, Chakrabarti M, Crozier JEM. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br. J. Cancer. 2007;97(9):1266–1270. doi: 10.1038/sj.bjc.6604027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J. Surg. Oncol. 2005;91(3):181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13(1):350. doi: 10.1186/1471-2407-13-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng H, Long F, Jaiswar M, et al. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: A meta-analysis. Sci. Rep. 2015;5(1):11026. doi: 10.1038/srep11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao W-K, Chen D, Li S-Q, et al. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: A meta-analysis. BMC Cancer. 2014;14(1):117. doi: 10.1186/1471-2407-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J. Immunol. 1985;134(1):230–234. [PubMed] [Google Scholar]
- 16.el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J. Immunol. 1987;139(7):2406–2413. [PubMed] [Google Scholar]
- 17.Carlo E, Forni G, Musiani P. Neutrophils in the antitumoral immune response. Chem. Immunol. Allergy. 2003;83:182–203. doi: 10.1159/000071561. [DOI] [PubMed] [Google Scholar]
- 18.Jabłonska E, Kiluk M, Markiewicz W, et al. TNF-alpha, IL-6 and their soluble receptor serum levels and secretion by neutrophils in cancer patients. Arch. Immunol. Ther. Exp. 2001;49(1):63–69. [PubMed] [Google Scholar]
- 19.Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br. J. Cancer. 2011;104(8):1288–1295. doi: 10.1038/bjc.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maltoni M, Pirovano M, Nanni O, et al. Biological indices predictive of survival in 519 Italian terminally ill cancer patients. Italian Multicenter Study Group on Palliative Care. J. Pain Symptom Manag. 1997;13(1):1–9. doi: 10.1016/S0885-3924(96)00265-5. [DOI] [PubMed] [Google Scholar]
- 21.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 22.Saito T, Kuss I, Dworacki G, et al. Spontaneous ex vivo apoptosis of peripheral blood mononuclear cells in patients with head and neck cancer. Clin. Cancer Res. 1999;5(6):1263–1273. [PubMed] [Google Scholar]
- 23.Dworacki G, Meidenbauer N, Kuss I, et al. Decreased zeta chain expression and apoptosis in CD3þ peripheral blood T lymphocytes of patients with melanoma. Clin. Cancer Res. 2001;7(3 Suppl):947s–957s. [PubMed] [Google Scholar]
- 24.Ethier JL, Desautels DN, Templeton AJ, Oza A, Amir E, Lheureux S. Is the neutrophil-to-lymphocyte ratio prognostic of survival outcomes in gynecologic cancers? A systematic review and meta-analysis. Gynecol. Oncol. 2017;145(03):584–594. doi: 10.1016/j.ygyno.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Baert T, et al. Influence of CA125, platelet count and neutrophil to lymphocyte ratio on the immune system of ovarian cancer patients. Gynecol. Oncol. 2018;150:31–37. doi: 10.1016/j.ygyno.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Pinto MP, et al. Patient inflammatory status and CD4+/CD8+ intraepithelial tumor lymphocyte infiltration are predictors of outcomes in high-grade serous ovarian cancer. Gynecol. Oncol. 2018;151:10–17. doi: 10.1016/j.ygyno.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Farolfi A, et al. Inflammatory indexes as prognostic and predictive factors in ovarian cancer treated with chemotherapy alone or together with bevacizumab. A multicenter, retrospective analysis by the MITO group (MITO 24) Target. Oncol. 2018;13:469–479. doi: 10.1007/s11523-018-0574-1. [DOI] [PubMed] [Google Scholar]
- 28.Battaglia A, Buzzonetti A, Fossati M, et al. Translational immune correlates of indirect antibody immunization in a randomized phase II study using scheduled combination therapy with carboplatin/paclitaxel plus oregovomab in ovarian cancer patients [published correction appears in Cancer Immunol Immunother. 2020 Jul;69(7):1389] Cancer Immunol. Immunother. 2020;69(3):383–397. doi: 10.1007/s00262-019-02456-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng Z, Wen H, Bi R, et al. Preoperative neutrophil-to-lymphocyte ratio as a predictive and prognostic factor for high-grade serous ovarian cancer. PLoS ONE. 2016;11:e0156101. doi: 10.1371/journal.pone.0156101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Q-T, Zhou L, Zeng W-J, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in ovarian cancer: A systematic review and meta-analysis of observational studies. Cell Physiol. Biochem. 2017;41:2411–2418. doi: 10.1159/000475911. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen JMV, Ferguson SE, Bernardini MQ, May T, Laframboise S, Hogen L, Bouchard-Fortier G. Preoperative neutrophil-to-lymphocyte ratio predicts 30 day postoperative morbidity and survival after primary surgery for ovarian cancer. Int. J. Gynecol. Cancer. 2020;30(9):1378–1383. doi: 10.1136/ijgc-2020-001378. [DOI] [PubMed] [Google Scholar]
- 32.Matulonis UA. Ovarian cancer. Hematol Oncol Clin. N. Am. 2018;32(6):xiii–xiv. doi: 10.1016/j.hoc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Disis ML, Taylor MH, Kelly K, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: Phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019;5(3):393–401. doi: 10.1001/jamaoncol.2018.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, Kosteva JA, Ciunci CA, Gabriel PE, Thompson JC, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. doi: 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L, Audigier-Valette C, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–357. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorich MJ, Rowland A, Karapetis CS, Hopkins AM. Evaluation of the lung immune prognostic index for prediction of survival and response in patients treated with atezolizumab for NSCLC: Pooled analysis of clinical trials. J. Thorac. Oncol. 2019;14:1440–1446. doi: 10.1016/j.jtho.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 37.González-Martín A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2019;381(25):2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 38.Alvarado-Cruz I, Mahmoud M, Khan M, et al. Differential immunomodulatory effect of PARP inhibition in BRCA1 deficient and competent tumor cells. Biochem. Pharmacol. 2020;184:114359. doi: 10.1016/j.bcp.2020.114359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chabanon RM, Soria JC, Lord CJ, et al. Beyond DNA repair: The novel immunological potential of PARP inhibitors. Mol. Cell Oncol. 2019;6(2):1585170. doi: 10.1080/23723556.2019.1585170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee EK, Konstantinopoulos PA. Combined PARP and immune checkpoint inhibition in ovarian cancer. Trends Cancer. 2019;5(9):524–528. doi: 10.1016/j.trecan.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Lee EK, Konstantinopoulos PA. PARP inhibition and immune modulation: Scientific rationale and perspectives for the treatment of gynecologic cancers. Ther. Adv. Med. Oncol. 2020;24(12):1758835920944116. doi: 10.1177/1758835920944116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henriksen JR, Nederby L, Donskov F, et al. Prognostic significance of baseline T cells, B cells and neutrophil-lymphocyte ratio (NLR) in recurrent ovarian cancer treated with chemotherapy. J. Ovarian Res. 2020;13(1):59. doi: 10.1186/s13048-020-00661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dell'Aquila E, Cremolini C, Zeppola T, et al. Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer: A retrospective analysis of the TRIBE study by GONO. Ann. Oncol. 2018;29(4):924–930. doi: 10.1093/annonc/mdy004. [DOI] [PubMed] [Google Scholar]
- 44.Kaneko M, Nozawa H, Sasaki K, et al. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in advanced colorectal cancer patients receiving oxaliplatin-based chemotherapy. Oncology. 2012;82(5):261–268. doi: 10.1159/000337228. [DOI] [PubMed] [Google Scholar]
- 45.Marchetti C, Minucci A, D'Indinosante M, et al. Feasibility of tumor testing for BRCA status in high-grade serous ovarian cancer using fresh-frozen tissue based approach. Gynecol. Oncol. 2020;158(3):740–746. doi: 10.1016/j.ygyno.2020.06.479. [DOI] [PubMed] [Google Scholar]
- 46.Petrillo M, Vizzielli G, Fanfani F, et al. Definition of a dynamic laparoscopic model for the prediction of incomplete cytoreduction in advanced epithelial ovarian cancer: Proof of a concept. Gynecol. Oncol. 2015;139(1):5–9. doi: 10.1016/j.ygyno.2015.07.095. [DOI] [PubMed] [Google Scholar]
- 47.Aletti GD, Eisenhauer EL, Santillan A, Axtell A, Aletti G, Holschneider C, et al. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol. Oncol. 2011;120:23–28. doi: 10.1016/j.ygyno.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014;20:6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 49.Passardi A, Scarpi E, Cavanna L, Dall'Agata M, Tassinari D, Leo S, et al. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget. 2016;7:33210–33219. doi: 10.18632/oncotarget.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kubo T, Ono S, Ueno H, et al. Impact of the perioperative neutrophil-to-lymphocyte ratio on the long-term survival following an elective resection of colorectal carcinoma. Int. J. Colorectal Dis. 2014;29:1091–1099. doi: 10.1007/s00384-014-1964-1. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Q, Hong L, Zuo M-Z, et al. Prognostic significance of neutrophil to lymphocyte ratio in ovarian cancer: Evidence from 4,910 patients. Oncotarget. 2017;8:68938–68949. doi: 10.18632/oncotarget.20196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Z, Zhao X, Lu J, et al. Prognostic roles of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in ovarian cancer: A meta-analysis of retrospective studies. Arch. Gynecol. Obstet. 2018;297:849–857. doi: 10.1007/s00404-018-4678-8. [DOI] [PubMed] [Google Scholar]