Abstract
Objective
The aim of this study was to investigate the cost effectiveness of nivolumab versus docetaxel in previously treated, advanced non–small-cell lung cancer (NSCLC) in England and assess how conditional reimbursement within the Cancer Drugs Fund (CDF) can be used to ensure timely patient access to effective treatments.
Methods
Cost-effectiveness models developed for the National Institute for Health and Care Excellence (NICE) TA483 (squamous) and TA484 (non-squamous) technology appraisals were supplemented with updated overall survival (OS), progression-free survival (PFS), and time-to-treatment discontinuation data collected as part of the CDF data collection agreement. Both models were developed by using a partitioned-survival approach based on PFS and OS predictions from CheckMate 017 and CheckMate 057 to estimate the projected proportion of patients in each health state (progression free, progression, death) throughout the model’s time horizon. The primary outcomes were estimated costs, quality-adjusted life-years (QALYs), and the resulting incremental cost-effectiveness ratio (ICER) expressed as cost/QALY gained.
Results
Base-case ICERs for treating patients with nivolumab versus docetaxel were £35,657/QALY and £38,703/QALY for squamous and non-squamous NSCLC patients, respectively, which are substantially lower than those obtained from what were deemed to be the most appropriate analyses for decision making in the original submissions when run with the same patient access scheme discount: £68,576/QALY and £73,189/QALY gained for squamous and non-squamous NSCLC, respectively.
Conclusions
Nivolumab versus docetaxel is cost effective for treating locally advanced/metastatic NSCLC after prior chemotherapy in adults, regardless of tumour histology or programmed death-ligand 1 expression status.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41669-020-00245-4.
Introduction
Lung cancer is the third most common cancer in the United Kingdom (UK), and non–small-cell lung cancer (NSCLC) accounts for approximately 87% of lung cancers [1]. Chemotherapy has long been the mainstay treatment for NSCLC, with docetaxel being standard-of-care treatment for locally advanced or metastatic NSCLC after prior chemotherapy in adults in the UK until immune checkpoint inhibitors, such as pembrolizumab, atezolizumab and nivolumab, were introduced to market. As NSCLC patients are often diagnosed at a late stage of disease, conventional therapies such as surgery, chemotherapy or radiation may not be feasible or may offer limited benefit. Recent developments in targeted immuno-oncology treatments have significantly improved prognosis in terms of overall survival for patients with NSCLC [2, 3]. Nivolumab received European marketing authorisation in 2015 for patients with squamous NSCLC and in 2016 for patients with non-squamous NSCLC as a monotherapy for the treatment of locally advanced or metastatic NSCLC after prior chemotherapy in adults, regardless of programmed death-ligand 1 (PD-L1) expression status [4]. This approval was based on the randomised CheckMate 017 and CheckMate 057 phase III clinical studies in squamous and non-squamous NSCLC, respectively [5, 6]. In these studies, it was shown that nivolumab, at a dose of 3 mg/kg of body weight every 2 weeks, resulted in superior overall survival compared with docetaxel, at a dose of 75 mg/m2 of body surface area every 3 weeks (minimum follow-up of 11 months for CheckMate 017 and 17 months for CheckMate 057).
To determine whether nivolumab would be recommended as standard of care for the treatment of locally advanced or metastatic NSCLC after prior chemotherapy in adults in England, Bristol-Myers Squibb (the manufacturer of nivolumab) was invited in 2015 to submit evidence on the clinical and cost effectiveness for National Institute for Health and Care Excellence (NICE) technology appraisals (TAs). Following NICE TAs 483 (squamous) and 484 (non-squamous), nivolumab, with an incremental cost-effectiveness ratio (ICER) < £50,000 [7], was deemed a plausibly cost-effective option for treating locally advanced or metastatic NSCLC in adults after chemotherapy, only if nivolumab is stopped after 2 years of uninterrupted treatment (or earlier in the event of disease progression) and conditions in a managed access agreement were followed [8, 9]. In the recommendation for non-squamous NSCLC, nivolumab was limited to patients whose tumours express PD-L1. However, the NICE committee felt that the cost effectiveness was uncertain given limited trial follow-up data available at the time of submission. Therefore, nivolumab was recommended for use within the then newly relaunched Cancer Drugs Fund (CDF) to allow for further evidence to be collected on overall survival and duration of therapy.
The CDF is a source of funding for cancer drugs in England, and it aims to provide access to promising new treatments through managed access agreements while additional evidence is collected to address existing uncertainty [10]. The data collection agreements for these appraisals stated that further data regarding long-term overall survival (OS), progression-free survival (PFS), duration of therapy and the clinical effectiveness of nivolumab across PD-L1 expression levels should be collected to reduce these key areas of uncertainty [11, 12]. Data with a minimal follow-up of 60 months from the CheckMate 017 and CheckMate 057 clinical trials as well as long-term data from CheckMate 003 are now available, and reappraisals were published for both indications in early 2020 [13, 14]. The 5-year data from both CheckMate 017 and CheckMate 057 show that long-term treatment effects of nivolumab observed in the earlier data cuts have been maintained. In both trials, 5-year overall survival estimates have outperformed the predictions from the original TAs.
The study objective was to investigate the cost effectiveness of nivolumab compared with docetaxel in patients with previously treated advanced NSCLC, from the perspective of the National Health Service (NHS) in England. The survival data collected during the CDF were compared with the analysis based on the data cut at CDF entry, serving as a case study of how managed access agreements within the CDF can be used effectively to inform reimbursement decisions.
Methods
The analyses were based on the cost-effectiveness models developed for the NICE TA483 (squamous) and TA484 (non-squamous) [15, 16], supplemented with the updated OS, PFS and time-to-treatment discontinuation (TTD) data collected as part of the CDF data collection agreement. Data other than OS, PFS and TTD (e.g. costs, utilities and resource utilization) were kept in accordance with the original models to enable assessment of data collected through the CDF without other changes. The primary outcomes of the analysis were estimated costs, quality-adjusted life-years (QALYs), and the resulting ICER expressed as cost per QALY gained. The time horizon of the analysis was 20 years and both costs and outcomes were discounted at 3.5% annually, in accordance with NICE guidelines.
Patient Population
The patient population, adults aged ≥ 18 years with advanced or metastatic squamous or non-squamous NSCLC after failure of prior platinum doublet-based chemotherapy [5, 6], is aligned with that of CheckMate 017 and CheckMate 057.
Intervention
The intervention considered in the economic models was nivolumab, which was compared with docetaxel. In the clinical trials, nivolumab was administered at 3 mg/kg every 2 weeks (Q2W) and docetaxel was administered at 75 mg/m2 every 3 weeks (Q3W) [5, 6]. However, dosing of nivolumab was updated to a flat dosing schedule of 240 mg Q2W in 2019, which is deemed to be clinically equivalent and is the recommended dose for NSCLC in the summary of product characteristics [4], subsequent to the trials being conducted. Therefore, to reflect the current clinical use of nivolumab in the economic models and facilitate comparison, we used 240 mg Q2W dosing as the base case in both the original and the updated models.
Model Structure
Both models were developed by using a partitioned-survival approach based on PFS and OS predictions from CheckMate 017 and CheckMate 057 to estimate the projected proportion of patients in each health state (progression free, progression, and death) throughout the model’s time horizon. Costs and health outcomes of patients with squamous and non-squamous NSCLC were assigned to the cohort based on the projected proportion of patients in each health state over time. Drug, administration and monitoring costs of nivolumab and docetaxel were based on the TTD observed in CheckMate 017 and CheckMate 057. Key elements of the model structure and input parameters are detailed in Table 1.
Table 1.
Model inputs: base-case parameters
| Item | Squamous | Non-squamous |
|---|---|---|
| Population | Previously treated locally advanced or metastatic non-small cell lung cancer after prior chemotherapy | |
| Comparator | Docetaxel | |
| Proportional hazards on OS | Yes | No |
| Proportional hazards on PFS | No | No |
| Extrapolation of OS: nivolumab arm | Spline 2-knot hazard (generalised gamma) | Log-normal (hybrid-exponential) |
| Extrapolation of OS: docetaxel arm | Spline 2-knot hazard (generalised gamma) | Log-normal (hybrid exponential) |
| Extrapolation of PFS: nivolumab arm | Spline 1-knot hazard (hybrid exponential) | Spline 2-knot odds (hybrid exponential) |
| Extrapolation of PFS: docetaxel arm | Spline 1-knot hazard (hybrid exponential) | Spline 2-knot odds (hybrid exponential) |
| TTD nivolumab arm | KM | KM |
| TTD docetaxel arm | KM | KM |
| PF utility | 0.693 | 0.713 |
| PD utility | 0.509 | 0.688 |
| Clinical stopping rule | 2-year stopping rule | |
| Continued treatment effect | Nivolumab’s treatment effect could last up to 20 years | |
KM Kaplan-Meier, OS overall survival, PD progressed disease, PF progression free, PFS progression-free survival, TTD time-to-treatment discontinuation
Selection of Survival Models
OS and PFS were based on parametric survival models fitted to the 60-month data cut of CheckMate 017 and CheckMate 057 clinical trial data. All analyses followed the decision support unit guidelines by fitting both standard parametric functions and spline models to the available data [17]. For the spline models, up to three knots were fitted; however, in line with the analyses for the original TA, knots were limited to two for selection of base-case distributions in order to avoid overfitting the data. For instances where a good visual fit to the Kaplan–Meier data could not be achieved with curves other than three-knot splines due to more complex shapes of the survival pattern, scenario analyses were included. Whether proportional hazard could be assumed was explored based on log-cumulative hazards and log-cumulative odds plots to determine if parallel lines were evident. In instances where proportional hazards could not be ruled out, survival models with treatment as a covariate were fitted. Models were fitted to each arm separately where proportional hazards assumption did not hold. Selection of the best fitting distribution for each outcome was informed by the Akaike information criterion (AIC), the Bayesian information criterion (BIC) and the visual fit to the data. For AIC, distributions with a difference of < 4 to the distribution with the lowest AIC were considered appropriate based on the Burnham and Anderson rule of thumb [18]. Similarly, based on Raftery’s rule of thumb, it was considered that a difference in BIC > 10 to the distribution with the lowest BIC was inappropriate [19] (see Online Resource 1, Table A-1, in the electronic supplementary material [ESM] for AIC and BIC values). Furthermore, if statistical and visual fit for both arms could be achieved by using the same distribution, use of a common distribution was preferred over different distributions between arms.
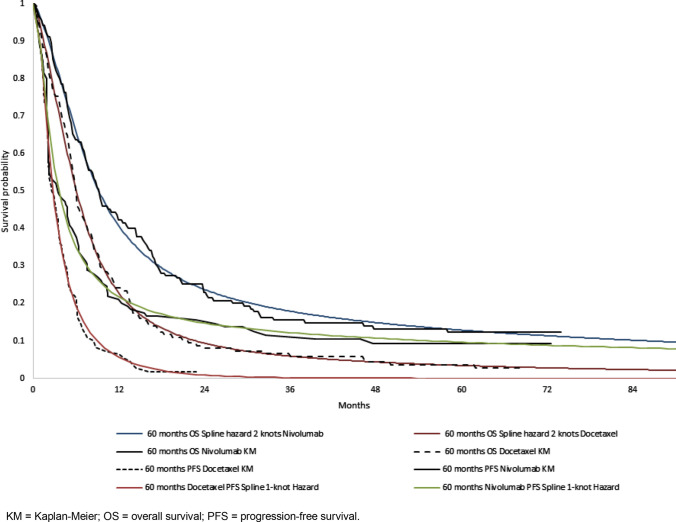
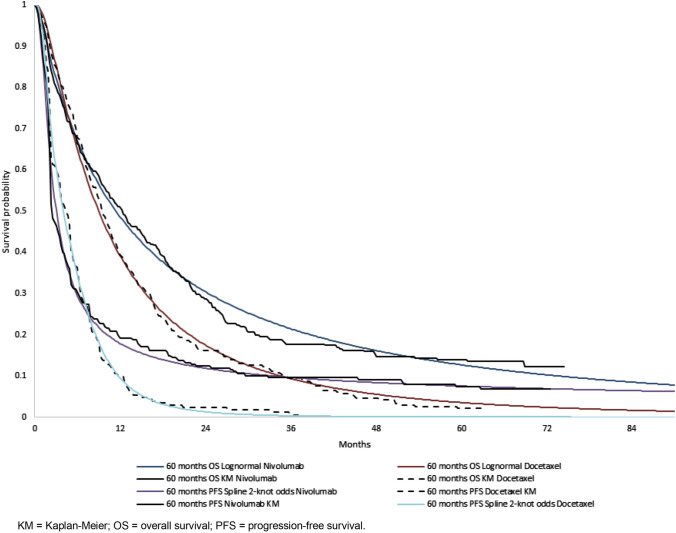
The distributions selected for the base-case analyses of each model are presented in Table 1, and Figs 1 and 2 present the predicted survival overlaid with the CheckMate 017 and CheckMate 057 clinical trial data. Distributions for OS and PFS preferred by the committee in the original submission are shown in parentheses in Table 1. All distributions considered for the updated analyses are presented in Online Resource 1, Figs A-1 to A-4 (see ESM). Long-term extrapolations of the selected distributions are presented in Online Resource 1, Figs A-5 to A-6 (see ESM). Duration of treatment data were available beyond the 2-year stopping rule agreed on in the original TA; therefore, it was modelled using the Kaplan-Meier curves for TTD, without need for extrapolation.
Fig. 1.
Kaplan-Meier and fitted overall survival and progression-free survival curves for squamous non-small-cell lung cancer. KM Kaplan-Meier, OS overall survival, PFS progression-free survival
Fig. 2.
Kaplan-Meier and fitted overall survival and progression-free survival curves for non-squamous non-small-cell lung cancer. KM Kaplan-Meier, OS overall survival, PFS progression-free survival
Health-State Utility
For the NICE appraisals, health-state utility values were taken from the pre-progression and post-progression utility values from the EQ-5D preference-based health-state utility questionnaire (EQ-5D utility index) and visual analogue scale for overall health status data collected in CheckMate 017 and CheckMate 057. The EQ-5D data from the trials were converted to health-state utilities using the published UK value set [20] resulting in utility values of 0.750 and 0.739 for the progression-free health state and 0.592 and 0.688 for the progressed-disease health state in squamous and non-squamous NSCLC, respectively [21, 22]. These values were deemed too high by the evidence review group (ERG) on the basis that the trial population was not representative of NSCLC patients in the UK and values from the published literature were argued to be more appropriate. For the squamous appraisal, the ERG suggested a utility value of 0.65 for the progression-free health state and 0.43 for the progressed-disease health state from Nafees et al. [23]. For the non-squamous appraisal and in order to account for low completion rates of EQ-5D questionnaires, the ERG used a subset of early EQ-5D responses from the CheckMate 057 trial to estimate a progression-free health-state value of 0.713. Further, the ERG suggested using a utility value of 0.545 from van den Hout et al. [24] and applied a terminal care disutility, which resulted in a final utility value of 0.476 for the progressed-disease health state. The committee felt that the health-state utility values proposed by the ERG were too low, those proposed by the company were too high, and the true value would be somewhere in between the two. The base-case utility values in the squamous model reflect the committee’s preferred values, in between the trial utility values and those provided by the ERG from the literature. The base-case utility values in the non-squamous model reflect the committee-preferred assumption for the progression-free state and the trial value in the progressed-disease health state (Table 1). Utility values based fully on the trial were included in a scenario analysis.
Adverse Events
Data on the safety outcomes for each arm were captured in the CheckMate 017 and CheckMate 057 clinical trials. Grade 3–4 adverse events that were reported in ≥ 5% of patients and ≥ 2% of patients in CheckMate 017 and CheckMate 057, respectively, were included in the analyses. Adverse events were accounted for in the model by applying a unit cost and a disutility value for each event, as shown in Online Resource 1, Tables A-2 and A-3 (see ESM).
Cost and Resource Use
Drug acquisition unit costs are shown in Table 2. The list price for nivolumab is shown; however, a confidential patient access scheme (PAS) was applied in the model and is reflected in the results presented. As described earlier, the drug, administration and monitoring costs of both nivolumab and docetaxel were based on the TTD from the CheckMate 017 and CheckMate 057 clinical trials. This was done to adequately reflect that some patients could be treated beyond progression or discontinue treatment prior to progression. Further, a 2-year stopping rule was applied to the nivolumab drug costs in both models to reflect NICE recommendations [8, 9]. In the base case, treatment effect of nivolumab following the 2-year stopping rule was assumed to be durable over the lifetime of the patients. A continued treatment effect of nivolumab for 3 years following treatment discontinuation was explored in scenario analyses. This scenario is applied such that after 3 years following discontinuation, patients in the nivolumab arm switch to the docetaxel hazard.
Table 2.
Model inputs: drug costs
| Drug | Pack/vial size (mg) | Cost per vial/pack (£) | Source |
|---|---|---|---|
| Nivolumab | 100 | 1097.00 | List price |
| Nivolumab | 40 | 439.00 | List price |
| Docetaxel | 20 | 138.33 | [34] |
| Docetaxel | 140 | 900.00 | [34] |
| Subsequent treatment | |||
| Cisplatin | 100 | 50.22 | [34] |
| Carboplatin | 50 | 20.00 | [34] |
| Carboplatin | 450 | 160.00 | [34] |
| Gemcitabine | 1000 | 154.62 | [34] |
| Gemcitabine | 200 | 29.80 | [34] |
| Vinorelbine | 50 | 139.00 | [34] |
Healthcare resource use was taken from previous NICE appraisals in NSCLC and were validated with a UK clinician [25, 26]. For each health state, the number of each element of resource use required per 4-week period was estimated, and unit costs were applied to these values. Unit costs for each element of resource use were estimated based on UK sources and are shown in Online Resource 1, Tables A-2 and A-3 (see ESM).
The cost of subsequent treatments was included in the analyses. Drug acquisition unit costs of subsequent therapies are included in Table 2. The proportion of patients in each arm receiving subsequent therapy and the type of subsequent therapy that they received were estimated from the CheckMate 017 and CheckMate 057 clinical trials.
Finally, a terminal cost reflecting end-of-life care was included in the models. So that the impact of only the newly collected data could be assessed, costs were set to be consistent with the original TA submissions. Therefore, the cost-year was 2015, and costs quoted for other cost-years were inflated to 2015 using inflation indices from the Personal Social Services Research Unit [27].
Comparison with Original Cost-Effectiveness Analyses
The analyses that were originally submitted during NICE TA483 (squamous) and TA484 (non-squamous) [15, 16] were conducted with a confidential NHS England (NHSE) PAS discount specific to the CDF, as well as the original dosing of nivolumab, 3 mg/kg every 2 weeks. Therefore, the cost-effectiveness analysis deemed plausible under the appraisal committee’s preferred assumptions cannot be easily compared with the updated analyses.
To isolate the impact of the additional data and to facilitate comparison, the original analyses were conducted with the current confidential NHSE PAS discount and with the nivolumab dosing of 240 mg every 2 weeks. Thus, the change in ICER resulting from additional information and reduction of uncertainty provided during the CDF data collection period can then be assessed.
Scenario Analyses
Beyond the base-case analysis, several scenarios were explored to investigate areas of particular uncertainty. The scenarios explored included the following:
- Selection of survival models: Scenario analyses were undertaken to assess other plausible distributions for the extrapolation of survival data beyond the trial.
- Spline-normal two-knots distribution for OS was tested in the squamous analysis representing the second-best fit based on AIC and BIC.
A spline hazards three-knots distribution for nivolumab and spline-normal one knot for docetaxel OS were tested in the squamous analysis, representing the overall best fit based on AIC and BIC and the best visual fit to the Kaplan Maier data, as well as relaxing the restriction on using three-knot spline models.
Using trial-based health-state utility values.
Application of a cap on treatment effect of nivolumab after 3 years following treatment discontinuation.
Sensitivity Analyses
To fully characterise uncertainty around the results of both analyses, probabilistic and one-way sensitivity analyses were carried out. Tested ranges in the one-way sensitivity analysis were based on 20% increase or decrease on the mean value. The probabilistic sensitivity analyses were conducted by using second-order Monte Carlo simulation for 1000 iterations, sufficient to reach a stable probabilistic result. Model validation was performed in alignment with best practices [28] and included face validity and internal validity (verification of input data and coding). The model also was externally reviewed by the ERG, per NICE’s TA process [21, 22].
Results
Compared with docetaxel, nivolumab was associated with improved survival and QALYs in both squamous and non-squamous NSCLC populations, with survival improving by 1.49 and 1.23 life-years (LYs) and QALYs by 0.88 and 0.73, respectively. After the confidential discount provided for nivolumab was taken into account, nivolumab was associated with an increase in cost of £31,281 and £28,360 for squamous and non-squamous NSCLC populations, respectively.
The resulting base-case ICERs for treating patients with nivolumab compared with docetaxel were £35,657 and £38,703 per QALY for squamous and non-squamous NSCLC patients (Table 3). Assuming 35.65% of patients with second-line NSCLC are squamous and 64.35% are non-squamous [29, 30], the resulting ICER for the entire population, regardless of histology, was £37,422.
Table 3.
Deterministic results
| Costs (£) | Total LYG | QALY | Incremental cost (£) | Incremental QALY | Incremental cost per QALY gained (£/QALY) | |
|---|---|---|---|---|---|---|
| Squamous | ||||||
| Nivolumab | 46,711 | 2.55 | 1.39 | |||
| Docetaxel | 15,430 | 1.06 | 0.51 | 31,281 | 0.88 | 35,657 |
| Non-squamous | ||||||
| Nivolumab | 47,569 | 2.53 | 1.54 | |||
| Docetaxel | 19,209 | 1.30 | 0.81 | 28,360 | 0.73 | 38,703 |
LYG life-year gained, QALY quality-adjusted life-year
The ICERs above are substantially lower than those obtained from what was deemed to be the most appropriate analyses for decision making in the original submissions when run at the PAS discount used for this analysis, which were £68,576 and £73,189 per QALY gained for squamous and non-squamous NSCLC, respectively.
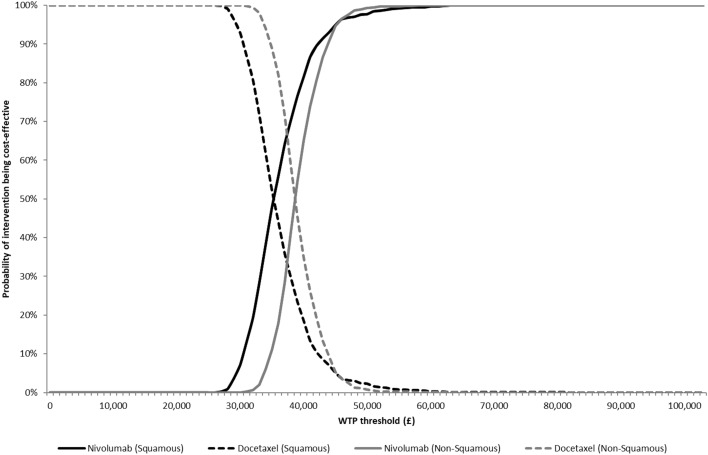
The probabilistic sensitivity analysis showed that nivolumab had a probability of being cost effective at a willingness-to-pay threshold of £50,000 per QALY of 97.7% for squamous NSCLC and 99.3% for non-squamous (Fig. 3 and Online Resource 1, Figs A-7 and A-8, see ESM). The one-way sensitivity analysis showed that the parameters having the greatest impact on the model results were the cost value for the progression-free health state and the administration cost of nivolumab (Online Resource 1, Figs A-9 and A-10, see ESM). The scenario analyses (Table 4) showed that the results were robust to changes in all scenarios. For scenario analyses investigating the most plausible alternative distributions to model OS, in the squamous model, the spline-normal two-knot model resulted in an ICER of £38,888; in the non-squamous model, the spline hazards three-knot distribution for nivolumab and spline-normal one-knot distribution for docetaxel OS resulted in an ICER of £33,832, as the observed plateau of the nivolumab arm was better reflected when a three-knot spline model was used.
Fig. 3.
Cost-effectiveness acceptability curves. WTP willingness to pay
Table 4.
Scenario results
| Scenario | Incremental cost-effectiveness ratio |
|---|---|
| Squamous scenario analyses | |
| Overall survival extrapolation: spline normal 2-knot distribution | £38,888 |
| Treatment effect of nivolumab continues for 3 years following treatment discontinuation | £36,249 |
| Trial-based utility values | £33,134 |
| Non-squamous scenario analyses | |
| Overall survival extrapolation: spline hazard 3-knot distribution | £33,832 |
| Treatment effect of nivolumab continues for 3 years following treatment discontinuation | £39,848 |
| Trial-based utility values | £37,582 |
Discussion
The results of the current cost-effectiveness analyses suggest that nivolumab is cost effective in treating locally advanced or metastatic NSCLC after prior chemotherapy in adults, regardless of histology. This is based on a £50,000 willingness-to-pay threshold being used for treatments meeting the end-of-life criteria and is in line with the previous assessment of nivolumab by NICE [8, 9].
To the authors’ knowledge, this is the first cost-effectiveness analysis in which updated cost-effectiveness estimates have been presented following data collection through the CDF implemented in 2016. Given that long-term survival data was the key uncertainty preventing the NICE committee from approving nivolumab through routine commissioning, the 60-month-minimum follow-up data now incorporated into the analysis provide significant reduction of uncertainty around the estimated long-term outcomes of nivolumab treatment.
In the analyses underpinning the CDF approval, it was predicted that nivolumab treatment would result in 0.80 and 0.44 LYs gained and 0.46 and 0.32 QALYs gained for patients with squamous and non-squamous NSCLC, respectively [8, 9]. Using mature survival data, the updated analysis shows a substantial increase in the expected QALYs gained in the nivolumab arm versus the docetaxel arm. The incremental QALY gain observed in the squamous model almost doubles from 0.46 to 0.88. In the non-squamous model, the incremental QALY gain more than doubles from 0.32 to 0.73. Thus, the results of the current analysis show that the predictions made both by the ERG and the company during the NICE appraisal underestimated the LYs and QALYs gained from treatment with nivolumab. The updated analysis with reduced uncertainty around long-term survival results in a marked decrease in the predicted ICER for both squamous and non-squamous patients and allows us to quantify the value of the additional data collection period in this case.
A key result of this analysis is the reduced uncertainty around the long-term extrapolation of OS. In the original TAs, alternative assumptions regarding the most appropriate extrapolation to be used for OS resulted in large differences in ICERs. With the more mature data now available, the choice of distribution for the extrapolations has a limited impact on the ICER, as confirmed by the ERG in the CDF exit appraisal [31, 32]. Thus, the data collection through CDF fulfilled its purpose by granting patients access to a new effective treatment while collecting additional data to reduce the clinical uncertainty.
Only data that were specified in the data collection period could be collected and updated in order to reduce uncertainty. Therefore, uncertainty remains around other inputs that were not included in the data collection agreement. Thus, uncertainty around the correct choice of utility values and the correct approach to modelling treatment effect waning remains in the updated analysis. However, as can be seen in Table 4, scenarios investigating alternative options for utility values and waning scenarios did not alter the overall conclusion. In fact, the choice of utility values had a smaller impact on the results in the current updated analysis compared with the original analysis. Thus, the overall uncertainty in the analyses informing NICE’s decision has further decreased compared with the original appraisal.
The key strength of this case study was that the OS, PFS and TTD data were relatively mature and that the statistical models used to extrapolate survival beyond the case study’s time horizon provided good visual fit to the within-trial data. Further, the study tested a wide range of parametric and spline-based survival models to achieve best fit and tested various scenarios in the sensitivity analysis. The framework for selection of most appropriate survival curves remained consistent between the original and updated analyses, though mature data allowed a much better understanding of the long-term survival profile of nivolumab in this indication.
There are also some limitations of the analyses that should be taken into consideration when interpreting the results of the models. Only standard parametric and spline-based survival models were tested when data were extrapolated beyond the study’s time horizon. Recent work by Bullement et al. [30] suggests that standard parametric or spline-based models may underestimate long-term survival benefits that have been observed for immuno-oncology products. This observation was also clear for non-squamous NSCLC, where three-knot spline models were needed to provide better fit to the tail of the nivolumab Kaplan–Meier curve, as other distributions did not seem to fully capture the plateau developing toward the end of the current follow-up. This is further supported by external data published by Antonia et al. [33], in which 6-year survival rates of patients with advanced NSCLC treated with nivolumab in CheckMate 003 continued to follow a similar plateau.
Further limitations include that, to remain consistent with the original submissions to NICE, the cost data that were used in the analyses were not updated using the most recently published sources. Similarly, differing thresholds for inclusion of adverse events are the result of the original submissions being developed separately, and these thresholds have been retained to remain consistent with the modelling approach at CDF entry.
Conclusions
In the base-case analysis for nivolumab versus docetaxel in squamous NSCLC, the incremental cost per QALY was £35,657. In the base-case analysis for nivolumab versus docetaxel in non-squamous NSCLC, the incremental cost per QALY was £38,703. The results presented suggest that, based on NICE’s end-of-life criteria, nivolumab is a cost-effective strategy for the treatment of locally advanced or metastatic NSCLC after prior chemotherapy in adults, regardless of tumour histology or PD-L1 expression status when compared with docetaxel.
Early health technology assessment of oncology treatments often is accompanied by high levels of uncertainty around the long-term survival benefit of the intervention and, therefore, a high level of uncertainty around the ICER used in decision making. The appraisals discussed in this paper are an example of this persistent issue. These appraisals also show that longer follow-up data following conditional reimbursement through the CDF can be valuable in reducing uncertainty around long-term survival benefit. In this case, both the ERG and company appear to have been too pessimistic in their original estimates of long-term survival.
Ultimately, for this particular TA, the initial pessimism from both NICE and the ERG in terms of the survival extrapolations and the need for several assessment iterations before CDF entry contributed to delayed patient access. Because of this delay, patients did not receive treatment that could have extended their lives and improved their quality of life. Given the growing body of evidence regarding the long-term survival benefit of immuno-oncology treatments, NICE and ERGs should consider that long-term survival rates with these treatments are likely to be greater than those obtained with conventional oncology treatments. It may be appropriate to consider adapting their approach so that survival is more accurately extrapolated beyond the trial period in such cases.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank Caitlin Smare and team for their work on the survival analyses that were used in the models. The authors also wish to thank John Penrod and Mohammad Ashraf Chaudhary for their support throughout and valuable review of the manuscript.
Declarations
Funding
This study was funded by Bristol-Myers Squibb.
Conflict of interest
Thor-Henrik Brodtkorb, Caroline Ling and Ben Rothwell are full-time employees of RTI Health Solutions, which received funding from Bristol-Myers Squibb to conduct this study. The contract between RTI Health Solutions and the sponsor includes independent publication rights. RTI Health Solutions conducts work for government, public, and private organisations, including pharmaceutical companies. Christopher Kiff is an employee and stockholder of Bristol-Myers Squibb.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data from Phase III CheckMate-017 and CheckMate-057 trials are used in these analyses to inform efficacy, safety and health-related quality-of-life inputs. The patient-level data are not publicly available but have been presented in several publications. The trial results supporting these analyses are presented within the article and in the electronic supplementary material.
Code availability
The model was developed in Excel and is not publicly available.
Authors’ contributions
All authors were responsible for conception and design of the research. Economic modelling was carried out by BR and THB; BR and THB were responsible for development of the draft manuscript; all authors were responsible for critical revision of the manuscript for important intellectual content.
References
- 1.Cancer Research UK. Lung cancer statistics: Incidence. 2019. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer#heading-Zero. Accessed 11 Dec 2019.
- 2.Rolfo C, Caglevic C, Santarpia M, Araujo A, Giovannetti E, Gallardo CD, et al. Immunotherapy in NSCLC: a promising and revolutionary weapon. Adv Exp Med Biol. 2017;995:97–125. doi: 10.1007/978-3-319-53156-4_5. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Research Institute. How is immunotherapy for lung cancer changing the outlook for patients? 2019. https://www.cancerresearch.org/immunotherapy/cancer-types/lung-cancer. Accessed 17 Dec 2019.
- 4.European Medicines Agency. Opdivo: summary of product characteristics. 4 June 2019. https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-product-information_en.pdf. Accessed 18 June 2019.
- 5.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NICE. Consultation paper: Value based assessment of health technologies. 2014. https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisals/VBA-TA-Methods-Guide-for-Consultation.pdf. Accessed 12 Feb 2020.
- 8.NICE. Final appraisal determination: nivolumab for previously treated squamous non-small-cell lung cancer. Technology appraisal guidance [TA483]. 2017. https://www.nice.org.uk/guidance/ta483/documents/final-appraisal-determination-document. Accessed 24 July 2019.
- 9.NICE. Final appraisal determination: nivolumab for previously treated locally advanced or metastatic non-squamous non-small-cell lung cancer. Technology appraisal guidance [TA484]. 2017. https://www.nice.org.uk/guidance/ta484/documents/final-appraisal-determination-document. Accessed 26 July 2019.
- 10.NHS England. Cancer Drugs Fund. 2019. https://www.england.nhs.uk/cancer/cdf/. Accessed 11 Dec 2019.
- 11.NICE. Cancer drugs fund managed access agreement: Nivolumab for previously treated squamous non-small-cell lung cancer. 2017. https://www.nice.org.uk/guidance/ta483/resources/managed-access-agreement-november-2017-pdf-4659350653. Accessed 2 Aug 2019.
- 12.NICE. Cancer drugs fund managed access agreement: nivolumab for previously treated non-squamous non-small-cell lung cancer. 2017. https://www.nice.org.uk/guidance/ta484/resources/managed-access-agreement-november-2017-pdf-4659629293. Accessed 2 Aug 2019.
- 13.NICE. Nivolumab for previously treated locally advanced or metastatic non-squamous non-small-cell lung cancer (CDF review TA484) [ID1572]. In development [GID-TA10513]. Expected publication date: TBC. 2019. https://www.nice.org.uk/guidance/indevelopment/gid-ta10513. Accessed 11 Dec 2019.
- 14.NICE. Nivolumab for previously treated squamous non-small-cell lung cancer (CDF Review TA483) [ID1559]. In development [GID-TA10516]. Expected publication date: TBC. 2019. https://www.nice.org.uk/guidance/indevelopment/gid-ta10516. Accessed 11 Dec 2019.
- 15.NICE. Nivolumab for previously treated squamous non-small-cell lung cancer. Technology appraisal guidance [TA483]. 1 November 2017. https://www.nice.org.uk/guidance/ta483. Accessed 5 July 2019.
- 16.NICE. Nivolumab for previously treated non-squamous non-small-cell lung cancer. Technology appraisal guidance [TA484]. 1 November 2017. https://www.nice.org.uk/guidance/ta484. Accessed 5 July 2019.
- 17.Latimer NR. NICE DSU technical support document 14: Survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data. 2013. http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf. Accessed 13 Dec 2019. [PubMed]
- 18.Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33(2):43. doi: 10.1177/0049124104268644. [DOI] [Google Scholar]
- 19.Raftery A. Bayesian model selection in social research. Sociol Methodol. 1995;25:111–163. doi: 10.2307/271063. [DOI] [Google Scholar]
- 20.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 21.NICE. Committee papers 1: nivolumab for previously treated squamous non-small-cell lung cancer. Technology appraisal guidance [TA483]. 2015. https://www.nice.org.uk/guidance/ta483/documents/committee-papers. Accessed 24 July 2019.
- 22.NICE. Committee papers 1: nivolumab for previously treated locally advanced or metastatic non-squamous non-small-cell lung cancer. Technology appraisal guidance [TA484]. 2016. https://www.nice.org.uk/guidance/ta484/documents/committee-papers Accessed 26 July 2019.
- 23.Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;21(6):84. doi: 10.1186/1477-7525-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Hout WB, Kramer GW, Noordijk EM, Leer JW. Cost-utility analysis of short- versus long-course palliative radiotherapy in patients with non-small-cell lung cancer. J Natl Cancer Inst. 2006;98(24):1786–1794. doi: 10.1093/jnci/djj496. [DOI] [PubMed] [Google Scholar]
- 25.NICE. Lung cancer (non-small cell, second line)—erlotinib and gefitinib (revision of TA162 and TA175) (ID620) Erlotinib and gefitinib for treating non-small-cell lung cancer that has progressed following prior chemotherapy (Review of TA162 and TA175): appraisal consultation document. 2015. http://www.nice.org.uk/guidance/gid-tag347/documents/erlotinib-and-gefitinib-for-treating-nonsmallcell-lung-cancer-that-has-progressed-following-prior-chemotherapy-review-of-ta162-and-ta175-appraisal-consultation-document. Accessed 15 May 2015.
- 26.NICE. Nintedanib for previously treated locally advanced, metastatic, or locally recurrent non‑small‑cell lung cancer. (ID438). 2015. [DOI] [PubMed]
- 27.Curtis L, Burns A. Unit Costs of Health and Social Care 2015, Personal Social Services Research Unit, University of Kent, Canterbury; 2015.
- 28.Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Mak. 2012;32(5):733–743. doi: 10.1177/0272989X12454579. [DOI] [PubMed] [Google Scholar]
- 29.Powell HA, Tata LJ, Baldwin DR, Stanley RA, Khakwani A, Hubbard RB. Early mortality after surgical resection for lung cancer: an analysis of the English National Lung cancer audit. Thorax. 2013;68(9):826–834. doi: 10.1136/thoraxjnl-2012-203123. [DOI] [PubMed] [Google Scholar]
- 30.Bullement A, Latimer NR, Bell GH. Survival extrapolation in cancer immunotherapy: a validation-based case study. Value Health. 2019;22(3):276–283. doi: 10.1016/j.jval.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 31.NICE. Final appraisal document. Nivolumab for advanced non-squamous non-small-cell lung cancer after chemotherapy. 2020. https://www.nice.org.uk/guidance/gid-ta10513/documents/final-appraisal-determination-document. Accessed 15 Oct 2020.
- 32.NICE. Final appraisal document. Nivolumab for advanced squamous non-small-cell lung cancer after chemotherapy. 2020. https://www.nice.org.uk/guidance/gid-ta10516/documents/final-appraisal-determination-document. Accessed 15 Oct 2020.
- 33.Antonia SJ, Borghaei H, Ramalingam SS, Horn L, De Castro CJ, Pluzanski A, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol. 2019;20(10):1395–1408. doi: 10.1016/S1470-2045(19)30407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Royal Pharmaceutical Society. British National Formulary. 2015. https://about.medicinescomplete.com/#/. Accessed 9 Oct 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.