Abstract
Objectives
To identify available literature on prevalence, severity and contributing factors of scan-associated anxiety (‘scanxiety’) and interventions to reduce it.
Design
Systematic scoping review.
Data sources
Ovid MEDLINE, Ovid EMBASE, Ovid PsycINFO, Ovid Cochrane Central Register of Controlled Trials, Scopus, EBSCO CINAHL and PubMed up to July 2020.
Study selection
Eligible studies recruited people having cancer-related non-invasive scans (including screening) and contained a quantitative assessment of scanxiety.
Data extraction
Demographics and scanxiety outcomes were recorded, and data were summarised by descriptive statistics.
Results
Of 26 693 citations, 57 studies were included across a range of scan types (mammogram: 26/57, 46%; positron-emission tomography: 14/57, 25%; CT: 14/57, 25%) and designs (observation: 47/57, 82%; intervention: 10/57, 18%). Eighty-one measurement tools were used to quantify prevalence and/or severity of scanxiety, including purpose-designed Likert scales (17/81, 21%); the State Trait Anxiety Inventory (14/81, 17%) and the Hospital Anxiety and Depression Scale (9/81, 11%). Scanxiety prevalence ranged from 0% to 64% (above prespecified thresholds) or from 13% to 83% (‘any’ anxiety, if no threshold). Mean severity scores appeared low in almost all measures that quantitatively measured scanxiety (54/62, 87%), regardless of whether anxiety thresholds were prespecified. Moderate to severe scanxiety occurred in 4%–28% of people in studies using descriptive measures. Nine of 20 studies assessing scanxiety prescan and postscan reported significant postscan reduction in scanxiety. Lower education, smoking, higher levels of pain, higher perceived risk of cancer and diagnostic scans (vs screening scans) consistently correlated with higher scanxiety severity but not age, gender, ethnicity or marital status. Interventions included relaxation, distraction, education and psychological support. Six of 10 interventions showed a reduction in scanxiety.
Conclusions
Prevalence and severity of scanxiety varied widely likely due to heterogeneous methods of measurement. A uniform approach to evaluating scanxiety will improve understanding of the phenomenon and help guide interventions.
Keywords: adult oncology, diagnostic radiology, anxiety disorders
Strengths and limitations of this study.
This is the first scoping review on scanxiety.
A comprehensive search strategy and broad inclusion criteria have resulted in an extensive summary of all available literature.
Summary statistics for prevalence and severity of scanxiety were not possible due to heterogeneity in the type and timing of measurement tools between the studies.
Introduction
Anxiety may increase when people have scans to screen for, diagnose, or stage cancer, or to monitor cancer for recurrence or progression. Scan-associated anxiety, or the distress before, during or after a scan, was first dubbed ‘scanxiety’ by a patient writing for the Time Magazine in 2011.1
Qualitative research on the experience of having a scan has shown some people experience dread in the weeks before a scan,2 perceive scans as dehumanising, unpleasant or causing claustrophobia,2–5 and find scans trigger fear of the unknown and fear of cancer recurrence.2 3 6 Scanxiety is recognised as a common clinical concern on social media and public forums, and is acknowledged by international cancer institutions7 8 and cancer-specific support networks.9–11 Despite this, scanxiety is not uniformly recognised or measured in published studies. We conducted a systematic scoping review to identify the available literature on scanxiety in people having cancer-related scans.
Methods
We conducted a systematic scoping review based on the six-step methodological framework developed by Arskey and O’Malley12 and modified by Levac et al,13 and guided by the Preferred Reporting Items for Systematic review and Meta-Analysis protocols extension for Scoping Reviews (PRISMA-ScR) checklist.14 The study protocol and amendments are available (online supplemental files 1 and 2).
bmjopen-2020-043215supp001.pdf (113.8KB, pdf)
bmjopen-2020-043215supp002.pdf (67.9KB, pdf)
Step 1: research question
Our aim was to increase the understanding of scanxiety by: determining the prevalence and severity of scanxiety; identifying contributing factors to scanxiety; identifying interventions to reduce scanxiety in people having cancer-related scans; and, exploring patient experiences with scanxiety.
Step 2: search strategy
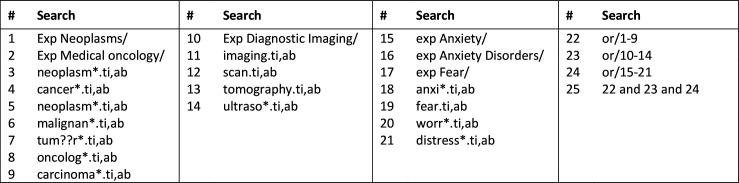
Published studies were identified from seven electronic databases: Ovid MEDLINE (1946 onwards), Ovid EMBASE (1947 onwards), Ovid PsycINFO (1806 onwards), Ovid Cochrane Central Register of Controlled Trials (1991 onwards), Scopus (any year), EBSCO CINAHL (any year) and PubMed (any year). The search strategy combined the subject headings and keywords of cancer, imaging and anxiety. An example is provided in figure 1. Reference lists of included articles were hand-searched for additional studies. All references were imported into Endnote V.9.
Figure 1.
Search strategy used for Ovid MEDLINE (1946 onwards).
The initial search was conducted on 11 April 2019 and updated on 3 July 2020.
Step 3: study selection
Inclusion criteria were full-text original research studies that recruited adults (≥18 years old) who had a non-invasive scan for a cancer-related reason, and which quantitatively assessed the prevalence or severity of scanxiety, reported a statistical comparison between prescan and postscan scanxiety, reported a statistical comparison between scanxiety and possible contributing factors, or evaluated the impact of an intervention on scanxiety.
Cancer-related reasons included screening (detection of cancer in asymptomatic person), diagnosis (detection of cancer in symptomatic person), staging (determining extent of cancer in person with confirmed or suspected cancer), surveillance (detection of recurrence in person with cancer treated with curative intent) or monitoring (detection of progression in person with cancer treated with non-curative intent).
The measurement of scanxiety was defined as any measure of anxiety, distress or worry occurring around the time of a scan. This included any period before, during or after a scan where the scan was used as a reference point for the measurement of scanxiety. All non-invasive imaging modalities were accepted. No date restrictions were applied. Foreign language material was included if an English translation was available.
After initial review of citations and based on increasing familiarity with the literature, and in line with recommendations on scoping review methodology,12 exclusion criteria were developed post hoc. Exclusion criteria were: studies involving invasive scans (eg, transvaginal ultrasound, ultrasound with fine needle aspirate or endoscopic ultrasound) due to differences in scan preparation and risk of adverse events and studies of scans performed to investigate a positive initial screening result because the psychological experiences of asymptomatic persons facing a potential new cancer diagnosis may lead to higher anxiety than is attributable to scanxiety. Due to feasibility of conducting quantitative and qualitative analysis with the volume of literature identified, studies reporting only a qualitative assessment of scanxiety were also excluded, and the objective to explore patient experiences was abandoned.
After removal of duplicate citations, two authors (KTB and RL) independently reviewed and screened publication titles and abstracts based on the eligibility criteria. Of the studies deemed potentially eligible, full texts were evaluated for final inclusion. Discrepancies were resolved by discussion between the two authors (KTB and RL) and were escalated to all authors if a consensus could not be reached.
Step 4: charting the data
Relevant data were independently extracted by two authors (KTB and RL) into an electronic data extraction form in Microsoft Excel, which included study demographics and methodology, scanxiety measurement tools, and the outcome measures of prevalence and severity of scanxiety, contributing factors to scanxiety, and interventions to reduce scanxiety.
Step 5: collating, summarising and reporting the results
Study data were tabulated to assist with a descriptive numerical summary of the range of cancer types, imaging modalities, study methodology and scanxiety measurement tools. Associations between scanxiety and potential contributing factors were tabulated if three or more studies reported a statistical comparison.
The prevalence of scanxiety was identified in two ways:
The percentage of people who scored above the prespecified clinically important anxiety threshold, if reported.
The percentage of people who scored any degree of anxiety, if no prespecified threshold was reported.
Severity of scanxiety was defined in three ways:
Any mean score of the anxiety measure above the prespecified clinically important anxiety threshold, if reported.
Any mean score of the anxiety measure that was at least half the total score, if an anxiety threshold was not reported.
At least ‘moderate’ anxiety (or its equivalent) on a descriptive range.
The definitions of prevalence and severity were purposed-designed to allow descriptive comparisons between the studies as we anticipated heterogeneity in scanxiety measurement would preclude meaningful summary statistics.
The components of intervention studies and their effect on scanxiety were summarised and reported descriptively.
Step 6: consultation
Medical oncologists (PB and BEK), a behavioural scientist (HD) and a statistician (CB) were consulted for content expertise to develop the study objectives and to improve clarity on clinically relevant interpretations of the data.
Patient and public involvement
This research did not directly involve patients and public. Our research was initiated by repeated observations of scanxiety in oncology patients.
Results
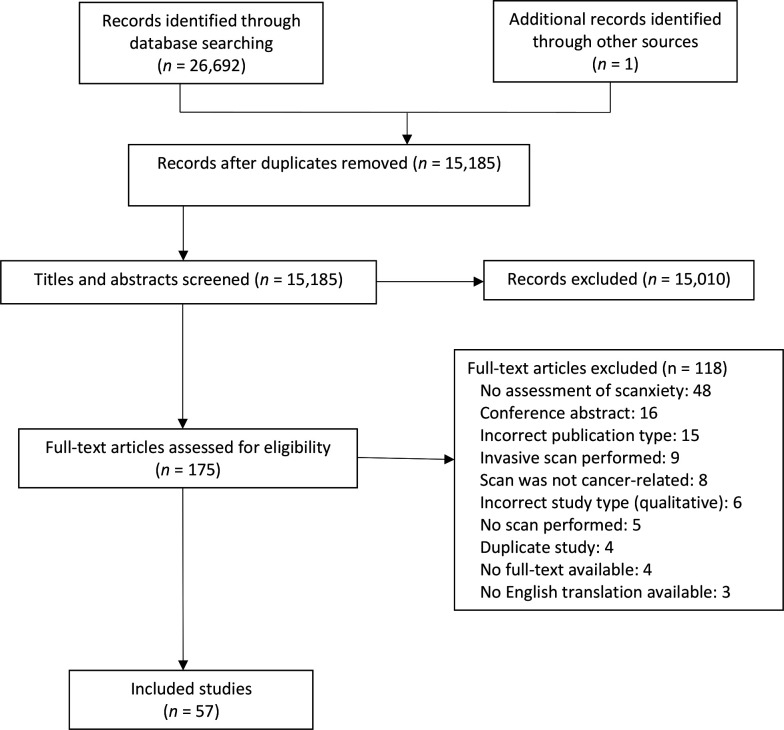
The study search identified 26 693 citations. The selection process is outlined in figure 2. After removal of duplicates, abstract and title screening, and full-text review, 57 eligible studies involving 21 352 people were included.
Figure 2.
Study search and selection flow diagram.
Demographics and study details
Observational studies
There were 47 observational studies (table 1) involving 19 498 people.15–61 Participants most commonly had scans for breast cancer (22 studies, n=14 338 women16 18–27 29 31 36 38 40 42 43 45 48 56 58), the most common scans were mammograms (21 studies16 18–27 29 31 36 38 40 42 43 45 48 56), and most studies used self-report surveys to assess scanxiety (40 studies15 16 18–36 38 40–54 56 58 59).
Table 1.
Demographics and study details for the 47 observational studies
| First author | Year | N | Country of study | Cancer type | Age (years) (mean*) |
Female (%) |
Married or de facto (%) | At least secondary education (%) | First scan (%) |
Scan type | Reason for scan | Methods |
| Andolf15 | 1990 | 275 | Sweden | Ovarian | NR | 100 | NR | NR | NR | Abdominal ultrasound | Screening | Cross-sectional survey |
| Bull16†‡ | 1991 | 541 | UK | Breast | 50–54: 23% 55–59 s 29% 60–64: 34% 65–70: 7% Unknown: 7% |
100 | NR | NR | NR | Mammogram | Screening | Longitudinal surveys |
| Peteet17 | 1992 | 79 | USA | Any | NR | NR | NR | NR | 4 | CT | Any (except screening) | Cross-sectional interview |
| Cockburn18‡ | 1994 | 200 | Australia | Breast | NR | 100 | NR | NR | NR | Mammogram | Screening | Longitudinal surveys |
| Ellman19‡ | 1995 | 331 | UK | Breast | 50–64: 52% 65–78: 48% |
100 | NR | NR | NR | Mammogram | Screening or surveillance | Cross-sectional survey |
| Sutton20ठ| 1995 | 306 | UK | Breast | 58 | 100 | 76 | 50 | NR | Mammogram | Screening | Longitudinal surveys |
| Bakker21 | 1998 | 315 | Canada | Breast | 61 | 100 | 71 | 76 | 50 | Mammogram | Screening | Longitudinal surveys |
| Gupta22 | 1999 | 167 | Kuwait | Breast | Range 14–63 | 100 | NR | 82 | NR | Mammogram±ultrasound | Screening or diagnosis | Cross-sectional survey |
| Hafslund23 | 2000 | 170 | Norway | Breast | NR | 100 | NR | NR | NR | Mammogram | Diagnosis | Longitudinal surveys |
| Meystre-Agustoni24 | 2001 | 887 | Switzerland | Breast | 50–54: 36% 55–59: 22% 60–64: 20% 65–69: 22% |
100 | 77 | 62 | 27 | Mammogram | Screening | Longitudinal surveys |
| Drossaert25 | 2002 | 2657 | The Netherlands | Breast | 58 | 100 | 78 | 32 | NR | Mammogram | Screening | Longitudinal surveys |
| Sandin26ठ| 2002 | 598 | Spain | Breast | 51 | 100 | 77 | 41 | NR | Mammogram | Screening | Longitudinal surveys |
| Brunton27 | 2005 | 584 | New Zealand | Breast | 50–54: 38% 55–59: 35% 60–64: 27% |
100 | NR | 74 | <20% | Mammogram | Screening | Cross-sectional survey |
| Geurts28 | 2006 | 106 | The Netherlands | Head and neck | 56 | 36 | NR | 29 | NR | Chest X-ray | Surveillance | Cross-sectional survey |
| Tyndel29‡ | 2007 | 1174 | UK | Breast | 43 | 100 | 83 | 33 | 87 | Mammogram | Screening | Longitudinal surveys |
| Bunge30† | 2008 | 324 | The Netherlands, Belgium | Lung | 60 | 49 | NR | NR | NR | CT | Screening | Longitudinal surveys |
| Brown Sofair31† | 2008 | 47 | USA | Breast | 50 | 100 | 34 | 80 | NR | Mammogram | Screening | Longitudinal surveys |
| van den Bergh32† | 2008 | 324 | The Netherlands, Belgium | Lung | 60 | 49 | 64 | 82 | 66 | CT | Screening | Longitudinal surveys |
| Westerterp33† | 2008 | 82 | The Netherlands | Oesophageal | 64 | 18 | NR | NR | NR | CT+PET | Diagnosis and staging | Cross-sectional survey |
| Bastiaannet34 | 2009 | 59 | The Netherlands | Melanoma | Median: 59 | 44 | 69 | 66 | NR | CT, PET±chest X-ray | Staging | Cross-sectional survey |
| Vierikko35† | 2009 | 601 | Finland | Lung | 65 | 0 | 36 | NR | NR | CT | Screening | Longitudinal surveys |
| Bölükbaş36 | 2010 | 93 | Turkey | Breast | 48 | 100 | 97 | 10 | 45 | Mammogram | Screening or diagnosis | Cross-sectional survey |
| Thompson37 | 2010 | 70 | USA | Lymphoma | Median: 47 | 64 | 53 | 97 | NR | CT | Surveillance | Cross-sectional interview |
| Hutton38† | 2011 | 527 | UK | Breast | Median: 40 | 100 | 79 | NR | 75 | Mammogram±MRI | Screening | Longitudinal surveys |
| Pifarré39 | 2011 | 200 | Spain | Any | 52 | 51 | NR | NR | 67 | PET/CT | Any (except screening) | Cross-sectional interview |
| Steinemann40 | 2011 | 227 | USA | Breast | NR | 100 | NR | NR | NR | Mammogram | Screening or diagnosis | Cross-sectional survey |
| Yu41 | 2011 | 398 | Brazil | Any | 54 | 79 | 56 | 57 | 27 | Any | Any (except screening) | Cross-sectional survey |
| Brédart42† | 2012 | 637 | France | Breast | 50 | 100 | NR | 87 | NR | Mammogram± ultrasound±MRI |
Screening or surveillance | Longitudinal surveys |
| Hafslund43‡ | 2012 | 4249 | Norway | Breast | 58 | 100 | NR | 52 | NR | Mammogram | Screening | Cross-sectional survey |
| Adams(44¶ | 2014 | 36 | The Netherlands | Lymphoma | 50 | 42 | NR | NR | NR | CT and MRI | Staging | Cross-sectional survey |
| Baena-Cañada45 | 2014 | 434 | Spain | Breast | 54 | 100 | 72 | 43 | 18 | Mammogram | Screening | Cross-sectional survey |
| Andersson46 | 2015 | 169 | Sweden | Any | 64 | 47 | 62 | 62 | 100 | PET/CT | Any (except screening) | Cross-sectional survey |
| Elboga47 | 2015 | 144 | Turkey | Any | 63 | 46 | 83 | 52 | NR | PET/CT | Any (except screening) | Cross-sectional survey |
| Hobbs48 | 2015 | 49 | Australia | Breast | 55 | 100 | 79 | NR | 75 | Mammogram±MRI | Diagnosis | Longitudinal surveys |
| Bauml49 | 2016 | 103 | USA | Lung | Median: 67 | 61 | 73 | 53 | NR | CT, PET±MRI | Monitoring | Cross-sectional survey |
| Abreu50 | 2017 | 232 | Portugal | Any | 61 | 51 | NR | 73 | 71 | PET/CT | Any (except screening) | Longitudinal surveys |
| Grilo51 | 2017 | 81 | Spain and Portugal | Any | 55 | 53 | NR | 41 | 47 | PET/CT | Any (except screening) | Longitudinal surveys |
| Evans52 | 2018 | 115 | UK | Colorectal or lung | 66 | 33 | NR | NR | NR | Whole body MRI, PET+CT | Staging | Longitudinal surveys |
| Goense53 | 2018 | 27 | The Netherlands | Oesophageal | 64 | 15 | NR | NR | NR | MRI+PET/CT | Staging and monitoring | Cross-sectional survey |
| Hall54 | 2018 | 169 | USA | Lung | 64 | 51 | 58 | 96 | NR | Low-dose CT | Screening | Cross-sectional survey |
| Derry55 | 2019 | 94 | USA | Any | 61 | 72 | NR | 69 | 0 | Any | Monitoring | Longitudinal interview |
| Soriano56 | 2019 | 57 | USA | Breast | 58 | 100 | 93 | NR | 0 | Mammogram | Surveillance | Longitudinal survey |
| Taghizadeh57 | 2019 | 1237 | Canada | Lung | 63 | 56 | NR | 85 | NR | CT | Screening | Longitudinal interview |
| Bancroft58 | 2020 | 88 | UK and Ireland | Breast | 38 | 61 | 50 | 83 | NR | MRI | Screening | Longitudinal survey |
| Grilo59 | 2020 | 94 | Portugal | Any | 61 | 54 | NR | 99 | 77 | PET+bone scan | Staging, monitoring and surveillance | Longitudinal survey |
| Morreale60 | 2020 | 87 | USA | Gastrointestinal and lung | 62 | 55 | NR | 92 | NR | CT or MRI | Monitoring | Longitudinal interview |
| Paiella61 | 2020 | 54 | Italy | Pancreatic | 50 | 61 | NR | NR | NR | MRI – MRCP | Screening | Cross-sectional interview |
All percentages were rounded to the nearest whole number.
*Unless otherwise stated.
†Demographic data are based on participants who completed the first survey.
‡These studies collected data from other groups who were not included in this review as they did not meet eligibility criteria. This included people having invasive procedures such as fine-needle aspirate or open surgical biopsy,16 33 people with abnormal screening results18 26 29 and people who did not have a scan.18–20 43
§Demographics based on the entire population even if not all participants were eligible for this review.
¶Four paediatric participants were included in this study.
MRCP, magnetic resonance cholangiopancreatography; NR, not reported; PET, positron emission tomography.
Twenty-one studies were conducted in people having scans for screening.15 16 18 20 21 24–27 29–32 35 38 43 45 54 57 58 61 In the remaining studies, reasons for scanning included diagnosis,23 48 staging,34 44 52 monitoring,49 55 60 surveillance to detect recurrence28 37 56 or a combination of reasons in people with known or suspected cancers (17 studies17 39 41 46 47 50 51 53 59). Five studies permitted scans for both screening and non-screening reasons (namely, diagnosis22 36 40 or surveillance19 42).
The mean age of participants, reported by 33 studies, was 56.9 years (range 38–66 years).20 21 25 26 28–33 35 36 39 41–48 50–61 The majority of participants were women (87%).15 16 18–61 When studies involving scans for breast cancer were excluded, there were similar proportions of men and women (women 49% and men 51%).15 27 28 30 32–35 37 39 41 44 46 47 49–55 57 59–61 There was variation in the reporting and proportion of participants who were married (22 studies, range 34%–97%20 21 24–26 29 31 32 34–38 41 45–49 54 56 58), who received at least secondary education (29 studies, range 10%–99%20–22 24–29 31 32 34 36 37 41–43 45–47 49–51 54 55 57–60) and who were attending their first scan (18 studies, range 0%–100%17 21 24 27 29 32 36 38 39 41 45 46 48 50 51 55 56 59).
Intervention studies
There were 10 intervention studies (table 2) involving 1854 people.62–71 This included people having scans for breast cancer (six studies, n=1449 people62–65 69 70) and lung cancer (one study, n=16 people68). Scans included mammogram (five studies62–64 69 70), positron emission tomography (PET) with CT (three studies66 67 71), MRI,65 CT68 and ultrasound70 (one study each). Four studies involved scans for screening,63 64 68 69 one for diagnosis,65 three for any reason in people with known or suspected cancers66 67 71 and two where scans for screening, surveillance and/or diagnosis were permitted.62 70
Table 2.
Demographics and study details for the 10 intervention studies to reduce scanxiety
| First author | Year | N | Country of study | Cancer type | Age (years) (mean*) |
Female (%) |
Married or de facto (%) | At least secondary education (%) | First scan (%) |
Scan type | Reason for scan | Allocation | Intervention and control groups |
| Mainiero62 | 2001 | 613 | USA | Breast | <40: 8% 50–50: 39% 50–60: 28% >70: 9% |
100 | NR | 95 | 7 | Mammogram | Screening or surveillance | Consecutive† | Educational or entertaining video in waiting room |
| Domar63 | 2005 | 143 | USA | Breast | 52 | 100 | NR | 81 | 8 | Mammogram | Screening | Randomised | Relaxation, music or blank audiotape in waiting room and during scan |
| Fernández-Feito64 | 2005 | 436 | Spain | Breast | 50–54: 24% 55–59: 30% 60–64: 23% 65–69: 22% |
100 | 73 | 28 | 4 | Mammogram | Screening | Randomised | Prescan nursing intervention or usual care |
| Caruso65 | 2006 | 44 | Italy | Breast | 47 | 100 | 75 | 89 | NR | MRI | Diagnosis | Randomised | Prescan informative-emotive psychological support or routine information |
| Vogel66 | 2012 | 101 | The Netherlands | Any | Median: 58 | 51 | NR | NR | 41 | PET/CT | Any (except screening) | Randomised | Audiovisual installation or usual care during FDG uptake |
| Acuff67 | 2014 | 180 | USA | Any | NR | NR | NR | NR | NR | PET/CT | Any (except screening) | Unclear | Handheld communication device or usual care during scan |
| Raz68 | 2014 | 16 | USA | Lung | 65 | 75 | NR | 100 | NR | CT | Screening | Sequential‡ | Prescan multimedia education or usual care |
| Zavotsky69 | 2014 | 100 | USA | Breast | 54 | 100 | NR | 98 | NR | Mammogram | Screening | Non-randomised§ | Music or no music during scan |
| Ashton70 | 2019 | 113 | USA | Breast | 18–39: 3.6% 40–59: 51.8% 60–79: 39.3% >80: 5.4% |
100 | NR | NR | NR | Mammogram±ultrasound | Screening, surveillance or diagnosis | NA¶ | Shoulder and neck massage±hand massage |
| Lorca71 | 2019 | 108 | Spain | Any | 59 | 57 | NR | NR | 54 | PET/CT | Any (except screening) | Randomised | Mindfulness meditation or usual care during FDG uptake |
All percentages were rounded to the nearest whole number.
*Unless otherwise stated.
†Each intervention was administered during one half of the study period.
‡Participants were enrolled into the control arm first, followed by the intervention arm.
§Participants attending on Mondays, Wednesdays and Fridays were allocated to the intervention arm, and participants attending on Tuesdays and Thursdays were allocated to the control arm.
¶All participants received the intervention.
FDG, fluorodeoxyglucose; NR, not reported; PET, positron emission tomography.
The mean age of participants was reported by five studies and ranged from 47 to 65 years.63 65 68 69 71 The majority were women (94%62–66 68–71). There was variation in the reporting and proportion of participants who were married (two studies, 73% and 75%64 65), received at least secondary education (six studies, range 28%–100%62–65 68 69) and participants attending their first scan (five studies, range 4%–54%62–64 66 71).
Eight studies allocated participants to an intervention or control group,63–69 71 one study compared two interventions62 and one study delivered the intervention to all participants.70 Two interventions were multifaceted.64 65 Types of interventions included: relaxation, distraction and/or meditation (six studies62 63 66 69–71); education (four studies62 64 65 68); emotional or psychosocial support (two studies64 65); or adjustments to routine logistics of the scan (one study67).
Scanxiety measurement
Anxiety measurements varied across the studies, with different measurement tools, variants of the same tool, and different range and thresholds applied to tools.
Observational studies
The 47 observational studies (table 3) used a total of 81 measures of anxiety, with 30 studies using one measure only,15–19 21 22 25–28 30 33 34 36 39 40 43 44 46 48–51 53 55–57 59 61 and 17 studies using at least two measures.20 23 24 29 31 32 35 37 38 41 42 45 47 52 54 58 60
Table 3.
Prevalence and severity of scanxiety
| First author | Year | Measurement of scanxiety | Results of scanxiety measurement | ||||
| Name of tool | Range of tool (anxiety threshold*) | Timing of assessment | Prevalence (%) | Severity (mean±SD†) | Prescan and postscan comparison | ||
| Andolf15 | 1990 | Visual analogue scale | 0–100 (NA) | Postscan: 1–3 years | 81 | Median 3.5 (range 0–100) | NA |
| Bull16 | 1991 | HADS: anxiety subscale | 0–21 (≥11)‡ | Prescan: specific timing NR | 4.9 | 4.97 (range 0–20) | Less severe postscan scanxiety, p<0.001 |
| Postscan: postresult, specific timing NR | 4 | 4.43 (range 0–17) | |||||
| Peteet17 | 1992 | 10-point Likert scale | 1–10 (NA) | Postscan: specific timing NR | NR | First scan 5.5, recent scan 3.5 | NA |
| Cockburn18 | 1994 | PCQ: emotional subscale | 0–15 (NA) | Prescan: day of scan | NR | <2 | No difference |
| Postscan: preresults, 1 week postresult and at 8 months | NR | <2 | |||||
| Ellman19 | 1995 | HADS: anxiety subscale | 0–21 (≥11) | Prescan: day of scan | 6 | NR | NA |
| Sutton20 | 1995 | STAI: state anxiety subscale | 1–4 (NA) | Prescan: at invitation to screening, specific timing NR | NR | Between 1.65 and 1.95 | No significant differences scanxiety at any time point |
| Periscan: day of scan | NR | ||||||
| Postscan: 9 months | NR | ||||||
| STAI: trait anxiety subscale | 1–4 (NA) | Prescan: at invitation to screening, specific timing NR | NR | Between 1.65 and 1.95 | No significant differences in scanxiety at any time point | ||
| Periscan: day of scan | NR | ||||||
| Postscan: 9 months | NR | ||||||
| GHQ: anxiety subscale | 0–3 (NA) | Prescan: at invitation to screening, specific timing NR | NR | <1 | Less severe postscan scanxiety, p<0.001 | ||
| Postscan: 9 months | NR | <1 | |||||
| 3-point Likert scale | 1–3 (NA) | Prescan: at invitation to screening, specific timing NR | NR | <2 | Less severe postscan scanxiety, p<0.001 | ||
| Postscan: 9 months | NR | <2 | |||||
| Bakker21 | 1998 | 5-point Likert scale | Descriptive range (NA) | Postscan: immediate and at 3 weeks | 39–40 | Somewhat, very or extremely: 9%–15% | NA |
| Gupta22 | 1999 | HSCL-25 | 0–3 (NA) | Postscan: specific timing NR | 40 | Moderate to severe: 25% | NA |
| Hafslund23 | 2000 | STAI: state anxiety subscale | 20–80 (NA) | Prescan: day of scan | NR | 35.5±11.0 | No statistical comparison reported |
| Postscan: day of scan | NR | 32.1±10.9 | |||||
| STAI: trait anxiety subscale | 20–80 (NA) | Prescan: day of scan | NR | 35.9±9.1 | No statistical comparison reported | ||
| Postscan: day of scan | NR | NR | |||||
| Meystre-Agustoni24 | 2001 | PCQ: negative consequences subscale | 0–36 (NA) | Prescan: day of scan | NR | <1 | No statistical comparison reported |
| Postscan: preresult, 2 weeks postresult and 8 weeks postresult | NR | <2 | |||||
| 6-point Likert scale | 0–5 (NA) | Prescan: immediate | 26 | <1 | |||
| Postscan: preresult, 2 weeks postresult and 8 weeks postresult | NR | <1 | |||||
| Drossaert25 | 2002 | Composite seven-item score of 4-point Likert scales | 1–4 (NA) | Baseline: 8 weeks post-first scan | NR | 1.6 | No statistical comparison reported |
| Prescan: 6 weeks (second and third scans) | NR | 1.6 to 1.7 | |||||
| Postscan: 6 weeks (second and third scans) | NR | 1.5 | |||||
| Descriptive range (NA) | Baseline: 8 weeks post-first scan | NR | Moderate to severe: 10% | NA | |||
| Sandin26 | 2002 | HSCL-90-R: anxiety subscale | 0–4 (NA) | Pr-scan: day of scan | NR | 0.41±0.33 | No statistical comparison reported |
| Postscan: 2 weeks | NR | 0.28±0.30 | |||||
| Brunton27 | 2005 | 4-point Likert scale, three items | Descriptive range (NA) | Postscan: within 4 years | 56–77 | Quite or very: 11%–28% | NA |
| Geurts28 | 2006 | 4-point Likert scale | 1–4 (NA) | Periscan: specific timing NR | 61 | Moderate to severe: 21% | NA |
| Tyndel29 | 2007 | PCQ: negative consequences subscale | 0–36 (NA) | Prescan: 1 month | NR | 5.1±6.7 | Less severe postscan scanxiety, p=0.000 |
| Postscan: 1 month post result and 6 months postresult | NR | 3.8±6.0 to 4.2±6.2 | |||||
| Cancer Worry Scale – Revised | 6–24 (NA) | Prescan: 1 month | NR | 11.0±2.9 | Less severe postscan scanxiety, p=0.000 | ||
| Postscan: 1 month post result and 6 months postresult | NR | 10.1±2.5 to 10.6±2.6 | |||||
| Bunge30 | 2008 | IES in low affective risk people | 0–75 (NA) | Prescan: 1 day | NR | 5.6±7.9 | Less severe postscan scanxiety in both low and high affective risk groups, p<0.05 |
| Postscan: 6 months | NR | 4.3±7.2 | |||||
| IES in high affective risk people | 0–75 (NA) | Prescan: 1 day | NR | 14.7±14.4 | |||
| Postscan: 6 months | NR | 10.3±11.0 | |||||
| Brown Sofair31 | 2008 | Penn State Worry Questionnaire | 16–80 (60) | Prescan: within 1 month | NR | 50.18 (range 40–60) | No statistical comparison reported |
| Postscan: day of scan (postresult) | NR | NR | |||||
| SCL-90-R: anxiety subscale | NR (NA) | Prescan: within 1 month | NR | 48.75 | No difference | ||
| Postscan: day of scan (postresult) | NR | 42.07 | |||||
| Individualised Questionnaire: anxiety response | 1–3 (2) | Prescan: within 1 month | 35 | NR | No statistical comparison reported | ||
| Postscan: day of scan (postresult) | 24 | NR | |||||
| van den Bergh32 | 2008 | STAI-6 | 20–80 (NA) | Prescan: 1 day | NR | 34.1±7.7 | Less severe postscan scanxiety, p<0.01 |
| Postscan: within 1 week and at 6 months | NR | 32.7±8.4 to 34.3±9.1 | |||||
| IES | 0–75 (NA) | Prescan: 1 day | NR | 6.9±9.6 | Less severe postscan scanxiety, p<0.01 | ||
| Postscan: within 1 week and at 6 months | NR | 5.1±8.0 to 5.6±8.8 | |||||
| EuroQol questionnaire: anxiety subscale | 1–3 (NA) | Prescan: 1 day | 23 | NR | No statistical comparison reported | ||
| Postscan: 6 months | NR | NR | |||||
| Westerterp33 | 2008 | 5-point Likert scale | 1–5 (NA) | Postscan (after both scans): 2 weeks | NR | CT 1.2±0.6, PET 1.4±1.0 | NA |
| Descriptive range (NA) | Postscan (after both scans): 2 weeks | CT 13, PET 23 | Moderate to severe: CT 4%, PET 10% | NA | |||
| Bastiaannet34 | 2009 | 5-point Likert scale | 1–5 (NA) | Postscan: 2–6 weeks after lymph node dissection | Chest x-ray 20, CT 31, PET 36 | Moderate to severe: chest X-ray 13%, CT 5%, PET: 9% | NA |
| Vierikko35 | 2009 | Health anxiety inventory | 0–24 (NA) | Prescan: specific timing NR | NR | 6.7±4.7 | Less severe postscan scanxiety, p<0.001 |
| Postscan: 1 year | NR | 5.8±4.6 | |||||
| Worry about lung cancer | 0–8 (NA) | Prescan: specific timing NR | NR | 3.0±2.4 | No difference | ||
| Postscan: 1 year | NR | 3.1±2.3 | |||||
| Bölükbaş36 | 2010 | STAI: state anxiety subscale | 0-NR (20–39 mild, 40–59 moderate, 60–79 severe,≥80 help needed) | Periscan: specific timing NR | NR | 46.2±4.9 | NA |
| Thompson37 | 2010 | STAI | 40–160 (NA) | Postscan: specific timing NR | 37 | 65.8±21.0 | NA |
| STAI: state anxiety subscale | 20–80 (≥40) | Postscan: specific timing NR | NR | 30.4±10.9 | NA | ||
| STAI: trait anxiety subscale | 20–80 (≥40) | Postscan: specific timing NR | NR | 35.4±11.3 | NA | ||
| Hutton38 | 2011 | HADS: anxiety subscale | 0–14 (≥11) | Baseline: 4 weeks pre-first scan | 20 | 6.9±4.2 | No difference |
| Prescan: day of each scan (for five scans) | MRI 17, mammogram 20 | MRI 5.2±4.0 to 6.5±4.2, mammogram 5.0±3.9 to 6.5±4.1 |
|||||
| Postscan: 6 weeks (for five scans) | ten to 13 | 5.1±4.2 to 5.9±4.1 | |||||
| STAI-6 | 20–80 (NA) | Prescan: day of scan (for five scans) | NR | MRI 10.8±3.8 to 12.1±4.0, mammogram 10.1±3.9 to 11.3±4.1 |
Less severe postscan scanxiety for MRI (p<0.0005) and mammogram (p=0.002) | ||
| Postscan: day of scan (for five scans) | NR | MRI 9.6±3.2 to 10.7±3.8, mammogram 9.7±3.1 to 10.5±3.9 |
|||||
| IES | 0–75 (NA) | Postscan: 6 weeks (for five scans) | NR | MRI 17.8±5.8 to 19.3±7.0, mammogram 17.2±4.4 to 18.6±5.2 |
NA | ||
| Pifarré39 | 2011 | STAI | 0–60 for each subscale (state more than 10 than trait) | Prescan: day of scan | 68 | NR | NA |
| Steinemann40 | 2011 | 7-point Likert scale | 1–7 (NA) | Prescan: day of scan | NR | 4.1 | NA |
| Yu41 | 2011 | HADS: anxiety subscale | 0–21 (≥8) | Prescan: day of scan | 38 | NR | NA |
| STAI: state anxiety subscale | NR-80 (≥40) | Prescan: day of scan | 46 | 39.4±12.2 | NA | ||
| STAI: trait anxiety subscale | NR-80 (≥40) | Prescan: day of scan | 46 | 39.9±12.2 | NA | ||
| Dichotomous reporting§ | Yes/No (NA) | Prescan: day of scan | 41 | NR | NA | ||
| Brédart42 | 2012 | STAI: state anxiety subscale | 20–80 (≥46) | Prescan: 1 week | NR | MRI 42.1, mammogram 41.1 |
No statistical comparison reported |
| Postscan: day of scan and between 15 days to 3 months | NR | MRI 34.9, 40.8, mammogram 34.3, 38.8 |
|||||
| IES: intrusion subscale | 0–35 (≥20) | Prescan: 1 week | NR | MRI 8.9, mammogram 8.4 |
No statistical comparison reported | ||
| Postscan: day of scan and between 15 days to 3 months | NR | MRI 8.5, mammogram 7.7 |
|||||
| IES: avoidance subscale | 0–40 (≥21) | Prescan: 1 week | NR | MRI 12.1, mammogram 9.8 |
No statistical comparison reported | ||
| Postscan: day of scan nd between 15 days to 3 months | NR | MRI 11.8, mammogram 8.9 |
|||||
| Hafslund43 | 2012 | HADS: anxiety subscale | 0–21 (≥8) | Prescan: within 2 weeks | 15 | 4.1±3.3 | NA |
| Adams44 | 2014 | 4-point Likert scale | 1–4 (NA) | Postscan: day of scan (after each scan) | NR | MRI 1.5±0.7, CT 1.8±0.8 | NA |
| Baena-Cañada45 | 2014 | HADS: anxiety subscale | 0–21 (≥11) | Postscan: specific timing NR | 4 | 1.86±3.26 | NA |
| Cancer Worry Scale | 6–24 (NA) | Postscan: specific timing NR | NR | 9.4±3.0 | NA | ||
| Andersson46 | 2015 | Sum of three items on 5-point Likert scale | 0–12 (NA) | Postscan: within 4 weeks | NR | 4 (range 0–10) | NA |
| Elboga47 | 2015 | HADS: anxiety subscale | 0–21 (≥10) | Prescan: day of scan | NR | 9.2±3.8 | NA |
| STAI: state anxiety subscale | NR (NA) | Prescan: day of scan | NR | 40.4±8.5 | NA | ||
| STAI: trait anxiety subscale | NR (NA) | Prescan: day of scan | NR | 46.6±7.8 | NA | ||
| Hobbs48 | 2015 | 5-point Likert scale | 1–5 (NA) | Postscan (after both scans), specific timing NR | Mammogram 17, MRI 44 | NR | NA |
| Bauml49 | 2016 | IES-6 | 0–24 (NA) | Postscan: specific timing NR | 83 | 6.4±5.3 | NA |
| Abreu50 | 2017 | 10-point Likert scale | 1–10 (NA) | Prescan: day of scan | NR | 6.4±2.7 | Less severe postscan scanxiety, p=0.000 |
| Postscan: day of scan | NR | 5.7±2.6 | |||||
| Grilo51 | 2017 | STAI: state anxiety subscale | 0–60 (NA) | Prescan: day of scan | NR | 31.1±5.2 | More severe postscan scanxiety, p=0.000 |
| Postscan: day of scan | NR | 33.9±4 | |||||
| Evans52 | 2018 | GHQ-12 | 0–12 (≥4) | Periscans: specific timing NR | 42 | NR | NA |
| 7-point Likert scale | 1–7 (NA) | Postscan: 1 month | NR | MRI 2.5±1.3, CT or PET/CT 2.2±1.2 | NA | ||
| Goense53 | 2018 | 5-point Likert scale | 1–5 (NA) | Postscan (after both scans): day of scan | NR | MRI 1.0±0.2, PET 1.0±0.2 | NA |
| Hall54 | 2018 | Generalised Anxiety Disorder two-item | 0–6 (≥3) | Periscan: specific timing NR | 26 | 1.62±1.78 | NA |
| Perceived Stress Scale 4 | 0–16 (NA) | Periscan: specific timing NR | NR | 5.14±3.35 | NA | ||
| Derry55 | 2019 | 4-point Likert scale | Descriptive range (NA) | Periscan: preresult | NR | ‘A great deal’ or ‘completely’: 23% | NA |
| Soriano56 | 2019 | PROMIS Anxiety Short Form | 1–5 (NA) | Prescan: 2 weeks | NR | 1.55±0.64 | NA |
| Taghizadeh57 | 2019 | STAI: state anxiety subscale | NR (39) | Baseline | NR | 30.9 | More severe postscan scanxiety, p<0.001 |
| Postscan: 1 month postresult and at 12 months | NR | 33.1, 31.7 | |||||
| Bancroft58 | 2020 | HADS: anxiety subscale | 0–21 (11) | Baseline | Carriers¶: 14 Controls: 7 |
Carriers: 6.2±3.9 Controls: 4.9±3.3 |
No difference in prevalence Less severe postscan in carriers (p=0.04) |
| Postscan: preresults, at 12 weeks, 26 weeks and 52 weeks | Carriers: 5 to 14 Controls: 2 to 7 |
Carriers: 5.3±3.9 to 5.9±4.1 Controls: 4.1±3.1 to 4.6±3.3 |
|||||
| Cancer Worry Scale – Revised | 8–32 (NA) | Baseline | NR | Carriers: 14.4±3.6 Controls: 12.2±1.7 |
No difference | ||
| Postscan: at 12 weeks, 26 weeks and 52 weeks | NR | Carriers: 13.6±4.4 to 14.7±4.2 Controls: 11.9±1.4 to 12.1±1.9 |
|||||
| IES-cancer: intrusion subscale | 0–35 (8.5) | Postscan: preresults, at 12 weeks, 26 weeks and 52 weeks | Carriers: 35 to 58 Controls: 5 to 13 |
Carriers: 8.3±9.1 to 11.4±9.1 Controls: 1.7±3.5 to 3.0±4.9 |
NA | ||
| IES-cancer: avoidance subscale | 0–40 (8.5) | Postscan: preresults, at 12 weeks, 26 weeks and 52 weeks | Carriers: 55 to 64 Controls: 12 to 37 |
Carriers: 9.9±9.0 to 13.3±10.5 Controls: 2.6±4.6 to 7.0±8.2 |
NA | ||
| IES-MRI: intrusion subscale | 0–35 (8.5) | Postscan: at 12 weeks, 26 weeks and 52 weeks | Carriers: 4 to 7 Controls: 0 to 3 |
Carriers: 1.2±3.2 to 3.1±8.8 Controls: 0.1±0.3 to 0.5±1.8 |
NA | ||
| IES-MRI: avoidance subscale | 0–40 (8.5) | Postscan: at 12 weeks, 26 weeks and 52 weeks | Carriers: 14 Controls: 8 |
Carriers: 1.8±3.4 to 4.1±9.3 Controls: 0.8±1.4 to 2.8±1.8 |
NA | ||
| STAI-6 | 6–24 (NA) | Prescan: day of scan | NR | Carriers: 7.2±3.3 Controls: 7.3±3.2 |
NA | ||
| Health Questionnaire | 0–14 (NA) | Baseline | NR | Carriers: 7.0±2.6 Controls: 6.8±2.2 |
No difference | ||
| Postscan: preresults, at 12 weeks, 26 weeks and 52 weeks | NR | Carriers: 7.1±2.5 to 8.1±2.8 Controls: 6.9±2.2 to 7.7±2.1 |
|||||
| Grilo59 | 2020 | STAI: state anxiety subscale | 20–80 (NA) | Prescan: day of scan | NR | Bone scan: 51.75±3.77 PET/CT: 44.76±10.0 |
Less severe postscan scanxiety for both: bone scan. p=0.02 PET/CT, p<0.001 |
| Postscan: day of scan | NR | Bone scan: 36.70±12.12 PET/CT: 38.82±11.33 |
|||||
| Morreale60 | 2020 | Distress thermometer | 0–10 (4) | Periscan: day of scan | NR | 3.73±2.60 | No statistical comparison |
| Postscan: 1 week postresult | NR | 3.91±2.69 | |||||
| HADS: anxiety subscale | 0–21 (0–7 none, 8–10 mild, 11–14 moderate, 15–21 high) | Periscan: day of scan | NR | 6.12±3.98 | No statistical comparison | ||
| Postscan: 1 week postresult | NR | 5.32±4.31 | |||||
| Paiella61 | 2020 | Perceived Stress Scale | 0–40 (15–18 moderate, ≥19 high) | Postscan: preresult | NR | 14.8 | NA |
All percentages were rounded to the nearest whole number.
*NA is listed as the anxiety threshold when the study did not state a prespecified threshold. In these cases, the definition of scanxiety prevalence was the percentage of people who reported any degree of anxiety.
†Mean listed unless otherwise described; SD listed only when available.
‡This study did not specify an anxiety threshold; however, the Anxiety subscale of the Hospital Anxiety and Depression Scale has validated thresholds. These thresholds were included in this table
§Dichotomous reporting assumed given description of question (self-perception of anxiety) and results ‘40.5% of the patients considered themselves to be anxious’.41
¶This study included participants who were TP53 mutation carriers and population controls.
GHQ, General Health Questionnaire; HADS, Hospital Anxiety and Depression Scale; HSCL, Hopkins Symptom Checklist; HSCL-90-R, Hopkins Symptom Checklist 90-Revised; IES, Impact of Event Scale; NA, not applicable; NR, not reported; PCQ, Psychological Consequences Questionnaire; PET, positron emission tomography; PROMIS, Patient-Reported Outcomes Measurement Information System; SCL-90-R, Symptom Checklist-90-Revised; STAI, State-Trait Anxiety Inventory.
The most common measures used were: purpose-designed Likert scales (17 studies); the State-Trait Anxiety Inventory (STAI) (14 studies); the anxiety subscale of the Hospital Anxiety and Depression Scale (HADS) (nine studies); the Impact of Event Scale (IES) (six studies); the Psychological Consequences Questionnaire (PCQ) (three studies), the Cancer Worry Scale (three studies); and the Perceived Stress Scale (two studies). There were 17 measures used by one study only.15 20 22 26 31 32 35 52 54 56 58 60
Likert scales were varied, with a numerical lower range limit of 0 or 1, and an upper range limit between 3 and 12.17 20 24 25 33 40 44 46 48 50 52 53 Seven studies used a descriptive range.21 25 27 28 33 34 55 Two studies used both a numerical and a descriptive range.25 33
The STAI compromises state and trait anxiety subscales with a possible subscale range of 20– 80. It has no validated anxiety threshold and is usually calculated as a sum of four-point response options.72 Included studies used and reported the STAI as a total score,37 39 using one or both subscales,20 23 36 37 41 42 47 51 57 59 or as a variant (eg, STAI-632 38 58). There were different ranges: none reported47 57; no reported lower limit41; no reported upper limit36; 0– 60;39 51 or based on a mean of individual item scores.20 Some studies prespecified an anxiety threshold of 39,57 40 and37 41 46,42 calculated based on the relationship between the anxiety and trait subscales,39 or based on investigator-determined categories.36 One study used a different method to calculate scores (ie, subtracting the points of reversed statements from direct statements, which were valued at 1, 2, 3 and 20, and then added to a constant of 5036).
The HADS anxiety subscale has a range of 0–21 and a validated anxiety threshold of 11.73 One study reported a range of 0–14,38 one study reported anxiety categories rather than a threshold,60 two studies reported an anxiety threshold of 841 43 and one study reported an anxiety threshold of 10 (though there was overlap the ‘tendency to anxiety’ and ‘anxiety’ categories, classified as scores of 8–10 and 10 or more, respectively).47
The IES was used in its original form30 32 38 42 58 or as a variant (IES-649) and was reported as a total score30 32 38 49 or as intrusion and avoidance subscale scores.42 58 The two studies using subscale scores reported threshold levels of 20 or 2142 and 8.5.58 When using the PCQ, researchers used either the emotional subscale18 or the negative consequences subscale.24 29 The Cancer Worry Scale and the Perceived Stress Scale were used in original45 61 or variant29 54 58 forms. The Symptom Checklist-90-Revised score could not be interpreted because the authors did not report a range,31 and a raw score or a transformed score could have been used.74
Intervention studies
The 10 intervention studies (table 4) used 19 measures of anxiety, with five studies using one measure only,62 66 67 69 70 and five studies at least two.63–65 68 71 The measures included subscales of the STAI (seven studies), Likert scales (five studies), a variant of the Psychological Consequences Questionnaire (one study68) and the Crown Crisp Experimental Index (one study65).
Table 4.
Effect of interventions to reduce scanxiety
| First author | Year | Intervention | Measurement of scanxiety | Impact of intervention on scanxiety | |||
| Name of tool | Range of tool (anxiety threshold) | Timing of assessment | Description of results | P value | |||
| Mainiero62 | 2001 | Arm A: an educational video about breast cancer and mammography Arm B: an entertaining movie (from the 1940s to 1960s) |
6-point Likert score | 0–5 (NA) | Prescan: immediate Postscan: immediate |
No difference | NR |
| Domar63 | 2005 | Arm A: relaxation audiotape or Arm B: music audiotape or Arm C: control (blank audiotape) |
STAI: state anxiety subscale | NR (NA) | Prescan: immediate | No difference Arm A versus arm B versus arm C: 34.8 versus 33.6 versus 33.2 |
0.18 |
| Postscan: immediate | No difference Arm A versus arm B versus arm C: 30.4 versus 30.9 versus 33.2 |
0.78 | |||||
| STAI: trait anxiety subscale | NR (NA) | Prescan: immediate | No difference Arm A versus arm B versu arm C: 32.6 versus 32.7 versus 32.5 |
0.99 | |||
| 11-point Likert scale | 1–10 (NA) | Postscan | No difference Arm A versus arm B versus arm C: 2.6 versus 3.2 versus 2.8 |
0.43 | |||
| Postscan: immediate | NR | NR | |||||
| Fernández-Feito64 | 2005 | Arm A: a protocolised nursing intervention (information and emotional support) and usual care or arm B: usual care alone | STAI: state anxiety subscale | 0–60 (NA) | Prescan: immediate (postintervention) | Less severe | <0.001 |
| Less severe if fear of cancer present | 0.002 | ||||||
| Less severe if no fear of cancer present | 0.003 | ||||||
| No difference if fear of cancer outcome present | 0.09 | ||||||
| Less severe if no fear of scan outcome | <0.001 | ||||||
| STAI: trait anxiety subscale | 0–60 (NA) | Prescan: immediate (postintervention) | No difference | 0.34 | |||
| Caruso65 | 2006 | Arm A: routine information and 45 min of informative-emotive psychological support with a psychologist or arm B: routine information | Crown Crisp Experimental Index | NR (0–96) | Prescan: immediate (postintervention) | Less severe Arm A versus arm B: 39.4 versus 42.3 |
0.03 |
| STAI: state anxiety subscale | NR (NA) | Prescan: immediate (postintervention) | No difference Arm A versus arm B: 57.7 versus 58.6 |
0.77 | |||
| Postscan: immediate | Less severe | 0.048 | |||||
| STAI: trait anxiety subscale | NR (NA) | Prescan: immediate (postintervention) | NR | NR | |||
| Vogel66 | 2012 | Arm A: uptake room with an audio-visual installation involving a video of nature scenes on a 119 cm television, dynamic lighting and ambient electronic music Arm B: uptake room without the audio-visual installation |
8-item STAI | 18–32 (≥16) | Prescan: immediately before and immediately after fluorodeoxyglucose uptake period | Less severe Arm A versus arm B: reduction by 2.39 versus 1.02 |
0.04 |
| Acuff67 | 2014 | Arm A: receive a handheld device to contact imaging staff during the scan Arm B: no device |
STAI: state anxiety subscale | 20–80 (NA) | During scan: immediately before completion of the scan | Less severe Arm A versus arm B: 22.87 versus 26.45 |
0.014 |
| Less severe if previous PET/CT Arm A versus arm B: 20.78 versus 24.64 |
0.023 | ||||||
| No difference if first time PET/CT Arm A versus arm B: 23.09 versus 27.25, p=0.249 |
0.249 | ||||||
| Raz68 | 2014 | Arm A: multimedia education session and usual care or arm B: usual care |
STAI: state anxiety subscale | 20–80 (≥40) | Prescan: within 2 weeks Postscan: immediate, at 1 week and 3–7 months postscan |
No difference at any time point | NR |
| STAI: Trait Anxiety subscale | 20–80 (≥40) | No difference at any time point | NR | ||||
| PCQ: lung cancer adaptation, anxiety subscale | 0–18 (NR) | No difference at any time point | 0.11 to 0.76 | ||||
| Zavotsky69 | 2014 | Arm A: music of their choice played via dock during the scan Arm B: no music |
11-point Likert scale | 0–10 (NA) | Postscan: immediate | No difference Arm A versus arm B: 2.36 versus 2.98 |
0.21 |
| Ashton70 | 2019 | All participants: 10 min shoulder and neck massage and/or hand massage before, during or after imaging, or between two imaging tests | 11-pointLikert scale | 0–10 (NA) | Postintervention (prescan or postscan) | 81% had a reduction in anxiety following massage* | <0.01 |
| Lorca71 | 2019 | Arm A: mindfulness meditation Arm B: routine care |
STAI: State Anxiety subscale | NR (NA) | Postscan: immediate | Less severe Arm A versus arm B: 10.47 versus 29.07 |
0.000 |
| STAI: Trait Anxiety subscale | NR (NA) | No difference | NS | ||||
| 11-item Likert scale | 0–10 (NA) | Less severe Arm A versus arm B, 1.07 versus 5.70 |
0.000 | ||||
*Mean scores for overall study population not provided.
NA, not applicable; NR, not reported; PCQ, Psychological Consequences Questionnaire; STAI, State-Trait Anxiety Inventory.
Likert scales were varied, with a lower range limit of 0 or 1, and an upper range limit between 5 and 10.62 63 69–71 The STAI was used and reported using one or both subscales,63–65 67 68 71 or as a variant (eight-item STAI66). There was variation from the usual STAI parameters, with studies using a different range (ie, not reported,63 65 0–60,64 or 18–3266) or prespecified anxiety thresholds of 4068 or 16.66
Scanxiety outcomes
Prevalence and severity of scanxiety for each study are provided in table 3. Summary statistics for prevalence and severity were not calculated due to heterogeneity in the type and timing of measurement between the studies.
Prevalence of scanxiety
Twenty-four of the 47 studies reported the prevalence of scanxiety. The prevalence of scanxiety above prespecified anxiety thresholds ranged between 0% and 64% across the 16 measures,16 19 31 38 41 43 45 52 54 58 though eight of these measures came from only two studies.41 58 In the 14 measures without a prespecified anxiety threshold, the prevalence of any degree of scanxiety ranged between 13% and 83%.15 21 22 24 27 28 32–34 37 39 41 48 49
There were insufficient numbers to compare the prevalence of scanxiety using measures with prespecified anxiety thresholds of people having scans for screening (11 measures16 31 38 43 45 54 58), reasons other than screening (four measures41 52) and for screening or non-screening reasons (one measure19). When no threshold was reported, the prevalence of scanxiety had a similar range (screening 23%–81%, five measures15 21 24 27 32; reasons other than screening 14% to 83%, eight measures28 33 34 37 39 41 48 49; either screening or reasons other than screening 40%, one measure22).
Severity of scanxiety
Severity of scanxiety was reported in 44 of 47 observational studies. Mean severity scores appeared low in almost all measures, which quantitatively measured scanxiety (54/62, 87%).
The mean severity scores were below prespecified anxiety thresholds on 17 of the 19 measures where a threshold was reported.16 31 37 38 41–43 45 47 54 57 58 The two exceptions were observed in a study comparing people with TP53 mutations (‘carriers’) to controls, with all participants undergoing screening scans. In carriers, mean scores were maximally 11.4 (IES intrusion subscale, threshold 8.5) and 13.3 (IES avoidance subscale, threshold 8.5). Mean severity scores for controls were below the thresholds.58
Of the 43 measures without a prespecified threshold, the majority had mean scores that were less than half the total scores.15 18 20 23–26 29 30 32 33 35 37 38 44–46 49 52–54 56 58 60 61 There were six exceptions, which reported maximal mean severity scores of: 5.5 out of 10 (Likert scale)17; 6.4 out of 10 (Likert scale)50; 4.1 out of 7 (Likert scale),40 33 out of 60 (STAI state anxiety subscale),51 8.1 out of 14 (Health Questionnaire)58; and 51.75 out of 80 (STAI).59 Four of these scores occurred in studies where scans were performed for reasons other than screening,17 50 51 59 one allowed scans for diagnosis or screening40 and one allowed scans for screening only.58
Eight measures used a descriptive range of severity, with more severe levels of scanxiety in 4%–28% of participants.21 22 25 27 28 33 34 55
Four measures could not be interpreted because they failed to report a range and anxiety threshold.31 36 47
Scanxiety before and after a scan
Of the 20 studies that reported a prescan and postscan scanxiety measurement, 14 studies reported a statistical comparison16 18 20 29–32 35 38 50 51 57–59 and six did not23–26 42 60 (table 3). There was variation in the timing of scanxiety measurement before a scan from 4 weeks before the scan until immediately before the scan, and after a scan from immediately after the scan until 1 year after the scan. Five studies reported a postscan reduction in scanxiety severity compared with prescan levels.16 29 30 32 50 59 Two studies reported an increase in postscan scanxiety severity51 57 and two studies no difference in prescan and postscan scanxiety severity.18 31
Four studies reported mixed findings on the change in scanxiety severity across different measures (table 5).
Table 5.
Studies with discrepant results on prescan and postscan scanxiety severity using different measures
| First author | Measurement tool | |
| Postscan reduction in scanxiety | No difference in prescan or postscan scanxiety | |
| Sutton20 | General Health Questionnaire: anxiety subscale | STAI: state anxiety subscale |
| 3-point Likert scale | STAI: Trait Anxiety subscale | |
| Vierikko35 | Health Anxiety Inventory | Worry about lung cancer |
| Hutton38 | 6-item STAI | HADS: anxiety subscale |
| Bancroft58 | HADS: anxiety subscale | Cancer Worry Scale – Revised |
| Health Questionnaire | ||
HADS, Hospital Anxiety and Depression Scale; STAI, State Trait Anxiety Inventory.
Although Bancroft et al58 reported a reduction in scanxiety severity using HADS (anxiety subscale), there was no difference in scanxiety prevalence.
Contributing factors to scanxiety
Multiple comparisons were made between scanxiety and possible contributing factors across the included studies (table 6).
Table 6.
Contributing factors to scanxiety
| Variable | Comparison | Effect on scanxiety | Studies | N | P value* |
| Age | Younger versus older | More prevalent | 1 | 398 | 0.00841 |
| No difference in prevalence | 2 | 338 | NS28 50 | ||
| More severe | 5 | 1883 | 0.005,45 <0.01,20 <0.01 (for screening),70 0.01,24 NR63 | ||
| No difference in severity | 11 | 6804 | NS,22 27 36 37 42 43 49 51 59 62 NS (for surveillance)70 | ||
| Gender | Men versus women | More prevalent | 1 | 200 | <0.00139 |
| Less prevalent | 1 | 298 | 0.02141 | ||
| No difference in prevalence | 1 | 106 | NS28 | ||
| More severe | 1 | 232 | 0.033 (postscan)50 | ||
| Less severe | 2 | 1381 | 0.000,47 <0.0557 | ||
| No difference in severity | 5 | 580 | NS37 49 51 59, NS (prescan)50 | ||
| Ethnicity | White versus other races | More severe | 1 | 143 | NR63 |
| Maori and Pacific Islanders versus New Zealand European or Asian | More severe | 1 | 584 | <0.00127 | |
| Any | No difference in severity | 5 | 1454 | NS22 24 37 40 49 | |
| Education | Lower versus higher | More prevalent | 1 | 398 | <0.00141 |
| No difference in prevalence | 2 | 338 | NS28 50 | ||
| More severe | 8 | 7400 | 0.003,62 0.007,36 <0.01,22 ≤0.01,42 0.012,24 0.018,27 0.04,43 <0.0523 | ||
| No difference in severity | 6 | 591 | NS37 49 51 59 63 69 | ||
| Employment | Unemployed versus employed | More prevalent | 1 | 398 | 0.04641 |
| More severe | 3 | 5056 | 0.01,43 0.05,23 ≤0.0542 | ||
| No difference in severity | 2 | 654 | NS27 37 | ||
| Income | Higher versus lower | No difference in severity | 3 | 757 | NS27 37 49 |
| Marital status | Married or de facto versus single | More severe | 1 | 637 | ≤0.01 (using IES – intrusion subscale)42 |
| No difference in severity | 5 | 1790 | NS24 36 37 49, NS (using STAI – state anxiety subscale)42 | ||
| Children | Children versus no children | No difference in severity | 3 | 5206 | NS24 37 43 |
| Smoking status | Current versus non-smoking† | More severe | 3 | 4562 | <0.001,43 54 0.03147 |
| No difference in severity | 2 | 330 | NS40 49 | ||
| Reason for scan | Diagnostic versus screening | More severe | 3 | 1104 | 0.007,41 0.047,36 NR62 |
| Staging or surveillance versus monitoring | More severe | 1 | 200 | <0.00139 | |
| Lower versus higher referral clarity | More severe | 1 | 169 | 0.04854 | |
| Type of scan | MRI versus mammogram | More severe | 1 | 49 | 0.00948 |
| Less severe | 1 | 637 | NR42 | ||
| CT versus MRI | More severe | 1 | 36 | 0.00744 | |
| Less severe | 1 | 115 | NR52 | ||
| PET versus CT | More severe | 1 | 82 | 0.0133 | |
| Nuclear medicine scan versus non-nuclear medicine scan | More severe | 1 | 398 | 0.00441 | |
| MRI versus PET/CT | No difference in severity | 2 | 142 | NS52 53 | |
| CT versus PET versus chest X-ray | No difference in severity | 1 | 59 | NS34 | |
| Bone scan versus PET scan | More severe | 1 | 94 | <0.001 (postscan)59 | |
| No difference in severity | 1 | 94 | NS (prescan)59 | ||
| Scan-naïve | First versus subsequent scans | More prevalent | 1 | 398 | 0.00141 |
| No difference in prevalence | 1 | 200 | NS39 | ||
| More severe | 5 | 3796 | <0.0005,38 <0.01,25 <0.02,19 <0.05,67 NR66 | ||
| Less severe | 1 | 93 | 0.03836 | ||
| No difference in severity | 6 | 2491 | NS24 27 50 51 59 62 | ||
| Pain | Pain versus no pain during scan | More severe | 6 | 4291 | <0.0001,25 <0.001,27 0.001,62 <0.01,23 69 <0.0522 |
| Risk of cancer | Past history versus no history of cancer | More severe | 2 | 864 | ≤0.001,42 <0.0540 |
| Less severe | 1 | 434 | 0.01345 | ||
| No difference in severity | 3 | 1206 | NS15 24 58 | ||
| Family history versus no family history of cancer | More severe | 1 | 584 | 0.00227 | |
| No difference in severity | 3 | 1255 | NS15 24 36 | ||
| Mutation carrier versus not a carrier | More severe | 1 | 88 | <0.05 (three comparisons, using IES cancer – Intrusion and Avoidance subscales, and postscan Health Questionnaire)58 | |
| No difference | 1 | 88 | NS (five comparisons, using HADS- Anxiety subscale, Cancer Worry Scale – Revised, IES MRI – Intrusion and Avoidance subscales, and prescan Health Questionnaire)58 | ||
| Higher, not otherwise specified versus lower | More severe | 1 | 70 | <0.0537 | |
| Perceived risk of cancer | Higher versus lower | More severe | 3 | 1545 | <0.001,27 ≤0.00142 <0.0130 |
*The p values listed in this table were reported by individual studies based on their own datasets. This scoping review has not performed additional analysis or attempted quantitative comparisons between studies.
†One study compared current smokers versus former smokers,54 and one study compared current and former smokers versus never smokers.49
HADS, Hospital Anxiety and Depression Scale; IES, Impact of Event Scale; NR, not reported; NS, not significant; STAI, State Trait Anxiety Inventory.
In summary, higher scanxiety severity was associated with people with:
Lower education (compared with higher education, eight of 14 studies22–24 27 36 37 42 43 49 51 59 62 63 69).
A history of smoking (compared with non-smoking, three of five studies40 43 47 49 54).
Higher pain levels during the scan (compared with no pain, all six studies22 23 25 27 62 69).
Higher perceived risk of cancer (compared with lower perceived risk of cancer, all three studies27 30 42).
Diagnostic scans (compared with screening scans, all three studies36 41 62).
The prevalence or severity of scanxiety was not consistently affected by age (13 of 19 comparisons20 22 24 27 28 36 37 41–43 45 49–51 59 62 63 70), gender (6 of 11 comparisons28 37 39 41 47 49–51 57 59), ethnicity (five of seven comparisons22 24 27 37 40 49 63), income (all three comparisons27 37 49), marital status (five of six comparisons24 36 37 42 49) or having children (all three comparisons24 37 43).
Inconclusive results occurred in the following comparisons:
Employment (unemployed compared with employed, four of six comparisons23 27 37 41–43).
Scan-naivety (first scan compared with subsequent scans, six of 13 comparisons19 24 25 27 36 38 39 41 50 51 62 66 67).
Risk of cancer (higher compared with lower risk of cancer, 7 of 19 comparisons15 24 27 36 37 40 42 45 58).
Although nine studies reported differences in scanxiety between different imaging modalities, the number of comparisons between specific scans were insufficient to draw conclusions.33 34 41 42 44 48 52 53 59
Interventions that reduce scanxiety
Five of the 10 intervention studies showed a reduction in scanxiety compared with controls.64–67 71 Four studies reported no difference in scanxiety between the intervention arms.62 63 68 69 The study where all participants received the same intervention showed a reduction in anxiety.70 Details of these results are listed in table 4.
Both multifaceted interventions studies incorporating education and emotional or psychological support showed a reduction in scanxiety.64 65
Of the six studies with relaxation, distraction and/or meditation components, three studies showed a reduction in scanxiety,66 70 71 while three studies did not.62 63 69
Interventions with only educational components did not show a reduction in scanxiety.62 68
A reduction in scanxiety severity was also observed when a handheld device was available to communicate with radiology staff. This reduction was observed in the subgroup of participants who had had a previous scan but not in participants having their first scan.67
Discussion
This is the first systematic scoping review aimed at quantifying the phenomenon of scanxiety in people having cancer-related scans. Scanxiety is a common and important clinical problem, as supported by the large number of studies identified by our search. There is a wide range of reported scanxiety prevalence (0%–83%), and scanxiety is generally not severe. Severity of scanxiety may be lower after a scan and is higher in people who have a lower education, currently smoke, experience pain during a scan, have higher perceived risk of cancer and who are having diagnostic (rather than screening) scans. Interventions may be more likely to reduce scanxiety if they involve active participation (eg, psychological and emotional support, meditation or a handheld communication device) rather than passive participation (listening to music or education only).
Firm conclusions about prevalence and severity could not be drawn due to considerable methodological heterogeneity of the included studies, especially in relation to scanxiety measurement tools. None were designed and validated for scanxiety, and some tools and their thresholds were not designed and/or validated for anxiety. This review did use purpose-designed definitions of prevalence and severity to allow some comparison between studies; however, the lack of a universal definition or specific measurement tool for scanxiety limits confidence in the interpretation of the results and interstudy comparisons. This highlights the need for a universally accepted measure to quantify scanxiety and evaluate scanxiety interventions in the future. A recent literature review by Al-Dibouni75 provided a narrative overview of scanxiety in people having scans for any reason and also recognised the lack of a specific measurement tool for scanxiety and variable scanxiety prevalence among studies.75
Given the STAI and Likert scales were the most common tools used, we propose that future studies use the state anxiety subscale of the STAI, with a range of 20–80 and no specific anxiety threshold72 (or variants, such as the STAI-676), and/or the distress thermometer, with a range of 0–10 and a clinically significant threshold of ≥4,77 to measure scanxiety. These tools can be combined with other validated anxiety measures, such as the HADS, to further refine the relationship between tools. Using existing measures rather than developing a scanxiety specific tool allows scanxiety assessment to occur immediately and broadly in clinical research.
Strengths of this scoping review include the rigorous methodology using a published framework,12 13 two independent researchers for study selection and data extraction and the implementation of a comprehensive search strategy and broad inclusion criteria to achieve an exhaustive review of the available literature. Limitations include the use of purpose-designed definitions of prevalence and severity and the limited generalisability of the results due to heterogeneity in cancer type, reason for scan, imaging modality and timing of scanxiety measurement between the studies and because the search strategy was restricted to English language databases. Finally, scanxiety in people who were recalled after an abnormal screening result were excluded from this review due to confounding and feasibility. These populations may be at higher risk of scanxiety, and further research may provide further insight about the scanxiety experience in this population.
Additional research implications of our review include the need for research into high-risk populations for scanxiety, including people with advanced cancer. This population was included in only three studies49 55 60; however, people with cancer have higher rates of anxiety compared with the general population.78 As they may be more likely to develop scanxiety, experience more severe scanxiety, or have higher postscan scanxiety while waiting for scan results, longitudinal assessment of scanxiety is required. Further research into effective and feasible interventions is also required, though these will face implementation challenges due to variations in health systems and available resources.
Conclusions
Prevalence and severity of scanxiety varied widely, although heterogeneity in scanxiety measurement interpretation. A uniform approach to evaluating scanxiety will improve understanding of the phenomenon and help guide the development of interventions to high-risk populations.
Supplementary Material
Footnotes
Twitter: @ktambui
Contributors: KTB, PB, BEK, HD and CB contributed to the concept and design of this review. KTB developed and implemented the search strategy. KTB and RL independently screened and reviewed titles, abstracts and full-text articles for inclusion. KTB and RL independently extracted data from the included studies. PB, BEK, HD and CB contributed content expertise to ensure clinically relevant interpretation of the data. KTB drafted the initial manuscript, and RL, PB, BEK, HD and CB reviewed and approved the manuscript prior to submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as supplemental information. The additional data are the data extraction forms for each study.
References
- 1.Feiler B, Scanxiety FB. Scanxiety. fear of a postcancer ritual. Time 2011;177:56. [PubMed] [Google Scholar]
- 2.Brandzel S, Rosenberg DE, Johnson D, et al. Women's experiences and preferences regarding breast imaging after completing breast cancer treatment. Patient Prefer Adherence 2017;11:199–204. 10.2147/PPA.S122244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans R, Taylor S, Janes S, et al. Patient experience and perceived acceptability of whole-body magnetic resonance imaging for staging colorectal and lung cancer compared with current staging scans: a qualitative study. BMJ Open 2017;7:e016391. 10.1136/bmjopen-2017-016391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathers SA, McKenzie GA, Robertson EM. A necessary evil: the experiences of men with prostate cancer undergoing imaging procedures. Radiography 2011;17:284–91. 10.1016/j.radi.2011.06.005 [DOI] [Google Scholar]
- 5.Strand T, Törnqvist E, Rask M, et al. The experience of patients with neoplasm metastasis in the spine during a magnetic resonance imaging examination. J Radiol Nurs 2014;33:191–8. 10.1016/j.jradnu.2014.09.001 [DOI] [Google Scholar]
- 6.Truesdale-Kennedy M, Taggart L, McIlfatrick S. Breast cancer knowledge among women with intellectual disabilities and their experiences of receiving breast mammography. J Adv Nurs 2011;67:1294–304. 10.1111/j.1365-2648.2010.05595.x [DOI] [PubMed] [Google Scholar]
- 7.Memorial Sloan Kettering . Coping with “Scanxiety” during and after Cancer Treatment, 2013. Available: https://www.mskcc.org/blog/coping-scanxiety-during-and-after-treatment/ [Accessed May 2020].
- 8.Dana-Farber Cancer Institute . Scan Anxiety (or ‘Scanxiety’): 5 Approaches to Coping, 2019. Available: https://blog.dana-farber.org/insight/2019/02/5-tips-for-reducing-scanxiety/ [Accessed May 2020].
- 9.Barter K. Scans + anxiety = Scanxiety, 2019. Available: https://pinkhope.org.au/scans-anxiety-scanxiety/ [Accessed May 2020].
- 10.brainstrust . Scanxiety. Available: https://brainstrust.org.uk/brain-tumour-support/quality-of-life/living-well-with-a-brain-tumour/scanxiety/ [Accessed May 2020].
- 11.Fight Colorectal Cancer . Waiting for test results: 5 tips to minimize Scanxiety, 2019. Available: https://fightcolorectalcancer.org/blog/scanxiety/ [Accessed May 2020].
- 12.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 13.Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5:69. 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 15.Andolf E, Jørgensen C, Uddenberg N, et al. Psychological effects of ultrasound screening for ovarian carcinoma. J Psychosom Obstet Gynaecol 1990;11:155–62. 10.3109/01674829009084412 [DOI] [Google Scholar]
- 16.Bull AR, Campbell MJ. Assessment of the psychological impact of a breast screening programme. Br J Radiol 1991;64:510–5. 10.1259/0007-1285-64-762-510 [DOI] [PubMed] [Google Scholar]
- 17.Peteet JR, Stomper PC, Ross DM, et al. Emotional support for patients with cancer who are undergoing CT: semistructured interviews of patients at a cancer Institute. Radiology 1992;182:99–102. 10.1148/radiology.182.1.1727318 [DOI] [PubMed] [Google Scholar]
- 18.Cockburn J, Staples M, Hurley SF, et al. Psychological consequences of screening mammography. J Med Screen 1994;1:7–12. 10.1177/096914139400100104 [DOI] [PubMed] [Google Scholar]
- 19.Ellman R, Thomas BA. Is psychological wellbeing impaired in long-term survivors of breast cancer? J Med Screen 1995;2:5–9. 10.1177/096914139500200103 [DOI] [PubMed] [Google Scholar]
- 20.Sutton S, Saidi G, Bickler G, et al. Does routine screening for breast cancer raise anxiety? results from a three wave prospective study in England. J Epidemiol Community Health 1995;49:413–8. 10.1136/jech.49.4.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakker DA, Lightfoot NE, Steggles S, et al. The experience and satisfaction of women attending breast cancer screening. Oncol Nurs Forum 1998;25:115–21. [PubMed] [Google Scholar]
- 22.Gupta R, Nayak MB, Khoursheed M, et al. Emotional distress in women presenting for breast imaging. Ann Saudi Med 1999;19:511–4. 10.5144/0256-4947.1999.511 [DOI] [PubMed] [Google Scholar]
- 23.Hafslund B. Mammography and the experience of pain and anxiety. Radiography 2000;6:269–72. 10.1053/radi.2000.0281 [DOI] [Google Scholar]
- 24.Meystre-Agustoni G, Paccaud F, Jeannin A, et al. Anxiety in a cohort of Swiss women participating in a mammographic screening programme. J Med Screen 2001;8:213–9. 10.1136/jms.8.4.213 [DOI] [PubMed] [Google Scholar]
- 25.Drossaert CHC, Boer H, Seydel ER. Monitoring women's experiences during three rounds of breast cancer screening: results from a longitudinal study. J Med Screen 2002;9:168–75. 10.1136/jms.9.4.168 [DOI] [PubMed] [Google Scholar]
- 26.Sandin B, Chorot P, Valiente RM, et al. Adverse psychological effects in women attending a second-stage breast cancer screening. J Psychosom Res 2002;52:303–9. 10.1016/s0022-3999(01)00227-6 [DOI] [PubMed] [Google Scholar]
- 27.Brunton M, Jordan C, Campbell I. Anxiety before, during, and after participation in a population-based screening mammography programme in Waikato Province, New Zealand. N Z Med J 2005;118:U1299. [PubMed] [Google Scholar]
- 28.Geurts TW, Ackerstaff AH, Van Zandwijk N, et al. The psychological impact of annual chest X-ray follow-up in head and neck cancer. Acta Otolaryngol 2006;126:1315–20. 10.1080/00016480600868414 [DOI] [PubMed] [Google Scholar]
- 29.Tyndel S, Austoker J, Henderson BJ, et al. What is the psychological impact of mammographic screening on younger women with a family history of breast cancer? findings from a prospective cohort study by the PIMMS management group. J Clin Oncol 2007;25:3823–30. 10.1200/JCO.2007.11.0437 [DOI] [PubMed] [Google Scholar]
- 30.Bunge EM, van den Bergh KAM, Essink-Bot M-L, et al. High affective risk perception is associated with more lung cancer-specific distress in CT screening for lung cancer. Lung Cancer 2008;62:385–90. 10.1016/j.lungcan.2008.03.029 [DOI] [PubMed] [Google Scholar]
- 31.Brown Sofair J, Lehlbach M. The role of anxiety in a mammography screening program. Psychosomatics 2008;49:49–55. 10.1176/appi.psy.49.1.49 [DOI] [PubMed] [Google Scholar]
- 32.van den Bergh KAM, Essink-Bot M-L, Bunge EM, et al. Impact of computed tomography screening for lung cancer on participants in a randomized controlled trial (Nelson trial). Cancer 2008;113:396–404. 10.1002/cncr.23590 [DOI] [PubMed] [Google Scholar]
- 33.Westerterp M, van Westreenen HL, Deutekom M, et al. Patients' perception of diagnostic tests in the preoperative assessment of esophageal cancer. Patient Prefer Adherence 2008;2:157–62. [PMC free article] [PubMed] [Google Scholar]
- 34.Bastiaannet E, Hoekstra-Weebers JE, Francken AB, et al. Perception of burden experienced during diagnostic tests by melanoma patients with lymph node metastases. Melanoma Res 2009;19:36–41. 10.1097/CMR.0b013e32831993b7 [DOI] [PubMed] [Google Scholar]
- 35.Vierikko T, Kivistö S, Järvenpää R, et al. Psychological impact of computed tomography screening for lung cancer and occupational pulmonary disease among asbestos-exposed workers. Eur J Cancer Prev 2009;18:203–6. 10.1097/cej.0b013e328329d800 [DOI] [PubMed] [Google Scholar]
- 36.Bölükbaş N, Erbil N, Kahraman AN. Determination of the anxiety level of women who present for mammography. Asian Pac J Cancer Prev 2010;11:495–8. [PubMed] [Google Scholar]
- 37.Thompson CA, Charlson ME, Schenkein E, et al. Surveillance CT scans are a source of anxiety and fear of recurrence in long-term lymphoma survivors. Ann Oncol 2010;21:2262–6. 10.1093/annonc/mdq215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hutton J, Walker LG, Gilbert FJ, et al. Psychological impact and acceptability of magnetic resonance imaging and X-ray mammography: the MARIBS study. Br J Cancer 2011;104:578–86. 10.1038/bjc.2011.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pifarré P, Simó M, Gispert JD, et al. [Diagnostic imaging studies: do they create anxiety?]. Rev Esp Med Nucl 2011;30:346–50. 10.1016/j.remngl.2011.03.013 [DOI] [PubMed] [Google Scholar]
- 40.Steinemann SK, Chun MBJ, Huynh DH, et al. Breast cancer worry among women awaiting mammography: is it unfounded? does prior counseling help? Hawaii Med J 2011;70:149–50. [PMC free article] [PubMed] [Google Scholar]
- 41.Yu LS, Chojniak R, Borba MA, et al. Prevalence of anxiety in patients awaiting diagnostic procedures in an oncology center in Brazil. Psychooncology 2011;20:1242–5. 10.1002/pon.1842 [DOI] [PubMed] [Google Scholar]
- 42.Brédart A, Kop J-L, Fall M, et al. Anxiety and specific distress in women at intermediate and high risk of breast cancer before and after surveillance by magnetic resonance imaging and mammography versus standard mammography. Psychooncology 2012;21:1185–94. 10.1002/pon.2025 [DOI] [PubMed] [Google Scholar]
- 43.Hafslund B, Espehaug B, Nortvedt MW. Health-Related quality of life, anxiety and depression related to mammography screening in Norway. J Clin Nurs 2012;21:3223–34. 10.1111/j.1365-2702.2012.04244.x [DOI] [PubMed] [Google Scholar]
- 44.Adams HJA, Kwee TC, Vermoolen MA, et al. Whole-Body MRI vs. CT for staging lymphoma: patient experience. Eur J Radiol 2014;83:163–6. 10.1016/j.ejrad.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 45.Baena-Cañada JM, Rosado-Varela P, Expósito-Álvarez I, et al. Women's perceptions of breast cancer screening. Spanish screening programme survey. Breast 2014;23:883–8. 10.1016/j.breast.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 46.Andersson C, Johansson B, Wassberg C, et al. Patient experience of an 18F-FDG-PET/CT examination: need for improvements in patient care. J Radiol Nurs 2015;34:100–8. 10.1016/j.jradnu.2014.11.008 [DOI] [Google Scholar]
- 47.Elboga U, Elboga G, Can C. Assessment of procedure related anxiety and depression in oncologic patients before F-18 FDG PET-CT imaging. J Psychiatry 2015;18. [Google Scholar]
- 48.Hobbs MM, Taylor DB, Buzynski S, et al. Contrast-Enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): patient preferences and tolerance. J Med Imaging Radiat Oncol 2015;59:300–5. 10.1111/1754-9485.12296 [DOI] [PubMed] [Google Scholar]
- 49.Bauml JM, Troxel A, Epperson CN, et al. Scan-associated distress in lung cancer: Quantifying the impact of "scanxiety". Lung Cancer 2016;100:110–3. 10.1016/j.lungcan.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abreu C, Grilo A, Lucena F, et al. Oncological patient anxiety in imaging studies: the PET/CT example. J Cancer Educ 2017;32:820–6. 10.1007/s13187-016-1069-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grilo A, Vieira L, Carolino E, et al. Anxiety in Cancer Patients during 18F-FDG PET/CT Low Dose: A Comparison of Anxiety Levels before and after Imaging Studies. Nurs Res Pract 2017;2017:3057495. 10.1155/2017/3057495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evans RE, Taylor SA, Beare S, et al. Perceived patient burden and acceptability of whole body MRI for staging lung and colorectal cancer; comparison with standard staging investigations. Br J Radiol 2018;91:20170731. 10.1259/bjr.20170731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goense L, Borggreve AS, Heethuis SE, et al. Patient perspectives on repeated MRI and PET/CT examinations during neoadjuvant treatment of esophageal cancer. Br J Radiol 2018;91:20170710. 10.1259/bjr.20170710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall DL, Lennes IT, Carr A, et al. Lung cancer screening uncertainty among patients undergoing LDCT. Am J Health Behav 2018;42:69–76. 10.5993/AJHB.42.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Derry HM, Maciejewski PK, Epstein AS, et al. Associations between anxiety, poor prognosis, and accurate understanding of scan results among advanced cancer patients. J Palliat Med 2019;22:961–5. 10.1089/jpm.2018.0624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soriano EC, Perndorfer C, Siegel SD, et al. Threat sensitivity and fear of cancer recurrence: a daily diary study of reactivity and recovery as patients and spouses face the first mammogram post-diagnosis. J Psychosoc Oncol 2019;37:131–44. 10.1080/07347332.2018.1535532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taghizadeh N, Tremblay A, Cressman S, et al. Health-Related quality of life and anxiety in the PAN-CAN lung cancer screening cohort. BMJ Open 2019;9:e024719. 10.1136/bmjopen-2018-024719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bancroft EK, Saya S, Brown E, et al. Psychosocial effects of whole-body MRI screening in adult high-risk pathogenic TP53 mutation carriers: a case-controlled study (SIGNIFY). J Med Genet 2020;57:226–36. 10.1136/jmedgenet-2019-106407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grilo AM, Vieira L, Carolino E, et al. Cancer Patient Experience in a Nuclear Medicine Department: Comparison Between Bone Scintigraphy and 18F-FDG PET/CT. J Nucl Med Technol 2020;48:254-262. 10.2967/jnmt.119.239285 [DOI] [PubMed] [Google Scholar]
- 60.Morreale MK, Moore TF, Kim S, et al. Preferences for notification of imaging results in patients with metastatic cancer. Patient Educ Couns 2020;103:392–7. 10.1016/j.pec.2019.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paiella S, Marinelli V, Secchettin E, et al. The emotional impact of surveillance programs for pancreatic cancer on high-risk individuals: a prospective analysis. Psychooncology 2020;29:1004–11. 10.1002/pon.5370 [DOI] [PubMed] [Google Scholar]
- 62.Mainiero MB, Schepps B, Clements NC, et al. Mammography-related anxiety: effect of preprocedural patient education. Womens Health Issues 2001;11:110–5. 10.1016/s1049-3867(00)00071-2 [DOI] [PubMed] [Google Scholar]
- 63.Domar AD, Eyvazzadeh A, Allen S, et al. Relaxation techniques for reducing pain and anxiety during screening mammography. AJR Am J Roentgenol 2005;184:445–7. 10.2214/ajr.184.2.01840445 [DOI] [PubMed] [Google Scholar]
- 64.Fernández-Feito A, Lana A, Baldonedo-Cernuda R, et al. A brief nursing intervention reduces anxiety before breast cancer screening mammography. Psicothema 2015;27:128–33. 10.7334/psicothema2014.203 [DOI] [PubMed] [Google Scholar]
- 65.Caruso A, Bongiorno L, Vallini L, et al. Breast cancer and distress resulting from magnetic resonance imaging (MRI): the impact of a psychological intervention of emotional and informative support. J Exp Clin Cancer Res 2006;25:499–505. [PubMed] [Google Scholar]
- 66.Vogel WV, Valdés Olmos RA, Tijs TJW, et al. Intervention to lower anxiety of 18F-FDG PET/CT patients by use of audiovisual imagery during the uptake phase before imaging. J Nucl Med Technol 2012;40:92–8. 10.2967/jnmt.111.097964 [DOI] [PubMed] [Google Scholar]
- 67.Acuff SN, Bradley YC, Barlow P, et al. Reduction of patient anxiety in PET/CT imaging by improving communication between patient and technologist. J Nucl Med Technol 2014;42:211–7. 10.2967/jnmt.114.139915 [DOI] [PubMed] [Google Scholar]
- 68.Raz DJ, Nelson RA, Kim JY, et al. Pilot study of a video intervention to reduce anxiety and promote preparedness for lung cancer screening. Cancer Treat Res Commun 2018;16:1–8. 10.1016/j.ctarc.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zavotsky KE, Banavage A, James P, et al. The effects of music on pain and anxiety during screening mammography. Clin J Oncol Nurs 2014;18:E45–9. 10.1188/14.CJON.E45-E49 [DOI] [PubMed] [Google Scholar]
- 70.Ashton JC, Bousquet D, Fevrier E, et al. Massage therapy in the breast imaging department: repurposing an ancient anxiety reducing method. Clin Imaging 2020;67:49–54. 10.1016/j.clinimag.2020.05.029 [DOI] [PubMed] [Google Scholar]
- 71.Lorca AM, Lorca MM, Criado JJ, et al. Using mindfulness to reduce anxiety during PET/CT studies. Mindfulness 2019;10:1163–8. 10.1007/s12671-018-1065-2 [DOI] [Google Scholar]
- 72.Spielberger CD, Gorsuch RL, Lushene R. Manual for the State-Trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press, 1983. [Google Scholar]
- 73.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 74.Rush Jr AJ, First MB, Blacker D. Handbook of psychiatric measures: symptom Checklist-90-Revised (SCL-90-R); brief symptom inventory (BSI). 2nd edn. Arlington, VA: American Psychiatric Publishing, Inc, 2008. [Google Scholar]
- 75.Al-Dibouni Z. Scanxiety. Improving the patient's experience. Part 1. Imaging & Therapy Practice 2019:21–5. [Google Scholar]
- 76.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait anxiety inventory (STAI). Br J Clin Psychol 1992;31:301–6. 10.1111/j.2044-8260.1992.tb00997.x [DOI] [PubMed] [Google Scholar]
- 77.Ownby KK. Use of the distress thermometer in clinical practice. J Adv Pract Oncol 2019;10:175-179. [PMC free article] [PubMed] [Google Scholar]
- 78.Pitman A, Suleman S, Hyde N, et al. Depression and anxiety in patients with cancer. BMJ 2018;361:k1415. 10.1136/bmj.k1415 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-043215supp001.pdf (113.8KB, pdf)
bmjopen-2020-043215supp002.pdf (67.9KB, pdf)
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as supplemental information. The additional data are the data extraction forms for each study.