Abstract
Non-invasive vagus nerve stimulation (nVNS) is an established neurostimulation therapy used in the treatment of epilepsy, migraine and cluster headache. In this randomized, double-blind, sham-controlled crossover trial we explored the role of nVNS in the treatment of gait and other motor symptoms in Parkinson’s disease (PD) patients. In a subgroup of patients, we measured selected neurotrophin levels and markers of inflammation and oxidative stress in serum, before and after the experimental intervention. Thirty-three PD patients with associated freezing of gait were randomised to either nVNS or sham. After baseline assessments, patients were instructed to deliver 6 two-minute stimulations (total 12 min/day) of the nVNS/sham device (electroCore, Inc. USA) for one month at home. Patients were then re-assessed. After a washout period of one month, the same patients were allocated to the alternate treatment arm and the same process was followed. Significant improvements in key gait parameters were observed with nVNS, including walking speed, stance time and step length, compared to sham. Similarly, overall motor function (MDS-UPDRS III) also improved significantly following nVNS stimulation. Serum Tumor Necrosis Factor (TNF)-α and glutathione levels decreased and brain-derived neurotrophic factor (BDNF) levels increased significantly (p < 0.05) after treatment with nVNS. Here we present the first double-blind sham-controlled trial evidence of the efficacy and safety of nVNS in the treatment of gait and motor function in patients with PD.
Subject terms: Parkinson's disease, Predictive markers
Introduction
Vagus nerve stimulation (VNS), delivered via a surgically implanted device, has been approved as an adjunctive neuromodulation therapy for epilepsy for more than two decades1. It has also demonstrated efficacy in the treatment of migraine, cluster headache and depression2. Although the specific mechanisms of VNS are largely unknown, it is thought to affect various brain regions through direct effects on nucleus tractus solitarius and locus coeruleus 3,4. More recently, this mode of treatment has been simplified by the introduction of handheld non-invasive VNS (nVNS) devices5. This approach has several advantages, not least of which is the ability to trial the intervention in various disorders without the hazard of surgical and post-operative complications6. In addition to its effects on central neural networks, a number of studies have suggested that VNS might have anti-inflammatory properties7,8. Consequently, potential applications have been posited across a range of inflammatory disorders9, including rheumatoid arthritis, sepsis, diabetes, and cardiovascular diseases. Interestingly, neuroinflammation has been implicated in the pathogenesis of PD and a number of other neurodegenerative conditions10.
Parkinson’s Disease (PD) is the second most prevalent neurodegenerative disorder and most common movement disorder, defined by bradykinesia, resting tremor, rigidity and postural instability11. PD patients face difficulty in walking with a normal pace and rhythm12. In advanced stages of disease, patients freeze while walking, describing a sense of being ‘glued to the ground’ for seconds or minutes13. These symptoms are disabling and gradually worsen with the progressive degeneration of the nigrostriatal system14. Various pathophysiological mechanisms of neurodegeneration have been proposed in PD, including neuroinflammation, oxidative stress and impaired cellular metabolism etc.15; of these, inflammation is arguably one of the important players. Our group and others have demonstrated that neuroinflammatory mediators are upregulated in patients with PD16. As expected, inflammatory modulators have been extensively explored with a view to modifying progression of the disease. However, results to date have been inconclusive17.
Recently, VNS has been found to be beneficial in improving locomotion in a rat model of PD18. Moreover, two independent preliminary studies found improvement in gait in patients with PD after a single application of cervical nVNS12,19. There is growing evidence that VNS can reduce oxidative stress, downregulate inflammatory cytokines and enhance anti-oxidative mechanisms20, suggesting that VNS might be a potential treatment in a variety of inflammation-associated diseases21. Whilst, the mechanisms by which VNS might exert its effects in PD are largely unknown22, anti-inflammatory effects are unlikely to explain the immediate therapeutic response to nVNS23, given that expression of inflammatory proteins generally requires hours. The immediate improvement observed following single application of nVNS in pilot studies is more likely to result from indirect activation of central neural circuitry, including noradrenergic projections from locus coeruleus 21, a region of the brain implicated in the aetiopathogenesis of freezing of gait24, rather than by modulating neuroinflammation25. In spite of the promising results from preliminary studies of nVNS in PD, it is unclear whether sustained benefits will result from chronic stimulation and precisely what those benefits might be26.
In this randomized double-blind sham-controlled crossover trial, we examined the efficacy of cervical nVNS (gammaCore, electroCore, Inc., NJ, USA) as an adjunct to standard treatment for a month in treating PD patients with freezing of gait. Additionally, we measured serum levels of selected markers of inflammation and oxidative stress, and brain derived neurotrophic factor (BDNF) in a subgroup of patients to assess the effects of chronic stimulation with nVNS on neuro-inflammation and neuroplasticity in PD patients. Our findings indicate that the use of nVNS three times a day for one month improved gait and motor function in PD patients and reduced serum inflammatory markers significantly.
Results
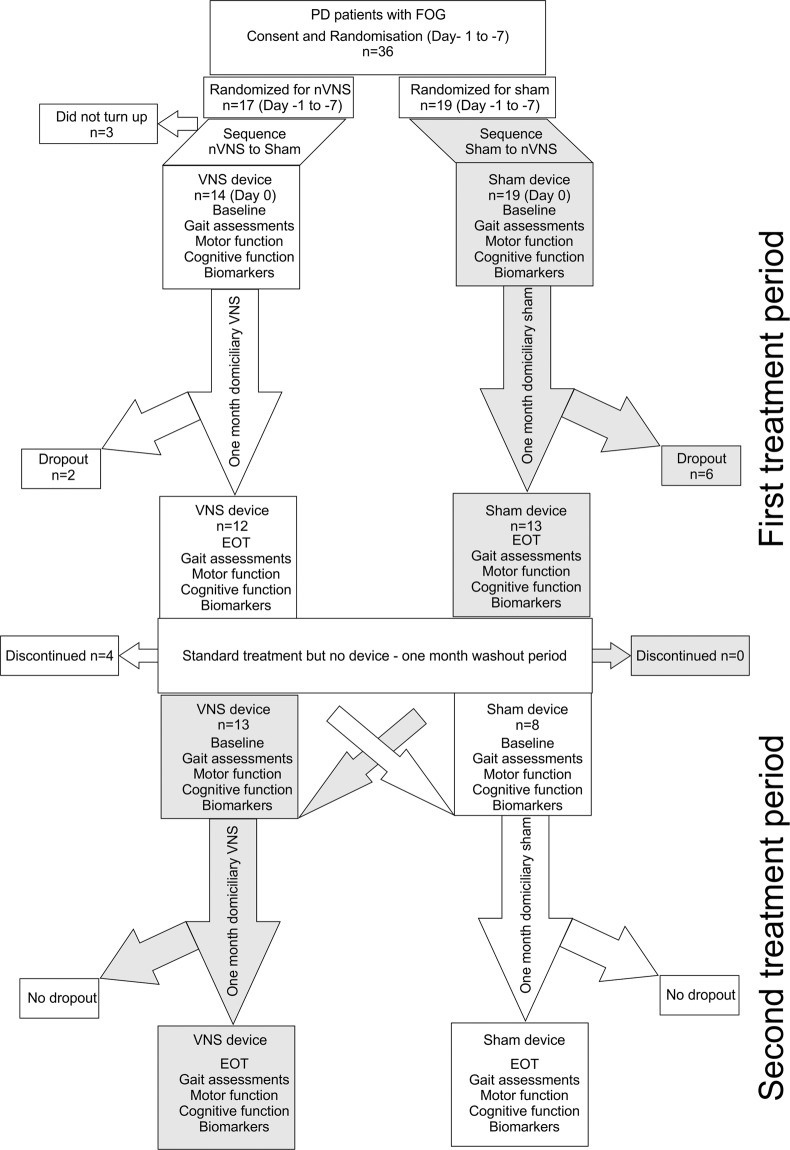
Thirty-six patients were recruited for this cross-over study, 17 were randomized initially to nVNS and 19 to sham stimulation. After the initial screening and randomization process 3 patients withdrew from the study. At the close of the study, 21 patients had completed both arms of the cross-over study and had thus received both nVNS and sham (Fig. 1 consort diagram). The pre-post analysis included all participants who completed one or both period(s). Twenty-five pairs of pre-post data for sham and twenty-one pairs of pre-post data for nVNS were available at the end of the study. Additionally, inter-group comparison of primary outcome measures was also performed in the 21 patients who completed both arms of the cross-over study.
Fig. 1. Consort diagram for the randomised cross-over controlled trial comparing active non-invasive VNS (nVNS) and sham nVNS.
PD = Parkinson’s disease; FOG = freezing of gait; n=number/sample size; VNS = Vagus nerve stimulation; EOT = End of treatment visit.
The mean UPDRS III score was not significantly different at baseline between sham and nVNS (40.3 vs 38.5; p = 0.328). These values indicate mild to moderate disease, which is supported by the H&Y scores of around 2.4. The baseline summary scores comparing both groups are presented in Table 1. Table 1 also shows the demographics, gait parameters, clinical characteristics and levels of serum markers, none of which were significantly different between groups at baseline. Table 2 shows the difference in outcome measures (gait parameters and clinical characteristics) before and after intervention (nVNS and sham) in the two groups separately.
Table 1.
Comparing the baseline characteristics of demographics, clinical characteristics and serum biomarkers between active and sham nVNS groups.
| Both groups Mean (SD) | Baseline – Sham Group Mean (SD) | Baseline – Active Group Mean (SD) | Group Comparisons (P value) | |
|---|---|---|---|---|
| Demography | ||||
| Age (years) | 62.5 ± 10.3 | 60.8 ± 14.4 | 62.26 ± 10.5 | 1.0 |
| Sex (n) (female) | 3 (10.2%) | 3 (11.5%) | 2 (8.7%) | 1.0 |
| Gait | ||||
| Velocity (cm/s) | 64.5 ± 20.6 | 66.9 ± 19.4 | 61.9 ± 20.3 | 0.13 |
| Average Step Length (cm) | 25 ± 20.5 | 36.8 ± 10.4 | 36.2 ± 10.3 | 0.3 |
| Average Stance time (s) | 0.8 ± 0.2 | 0.780 ± 0.17 | 0.825 ± 0.17 | 0.03 |
| Clinical scores | ||||
| MDS-UPDRS I | 15.9 ± 7.3 | 15.6 ± 6.8 | 16.3 ± 8.1 | 0.67 |
| MDS-UPDRS II | 21.4 ± 5.5 | 20.8 ± 5.8 | 22.1 ± 5.1 | 0.14 |
| MDS-UPDRS III | 39.5 ± 11.6 | 40.3 ± 12.7 | 38.5 ± 10.4 | 0.33 |
| H & Y | 2.4 ± 0.6 | 2.4 ± 0.5 | 2.3 ± .7 | 0.26 |
| TUG (s) | 42 ± 55.2 | 39.2 ± 77.5 | 45.4 ± 67.5 | 0.71 |
| FES | 55.2 ± 10.6 | 54.2 ± 12.8 | 56.4 ± 7.3 | 0.25 |
| MMSE | 26.4 ± 3.9 | 25.9 ± 3.8 | 26.5 ± 3.8 | 0.92 |
| RBDSQ | 4.7 ± 2.9 | 4.9 ± 2.9 | 5.2 ± 2.9 | 0.27 |
| FOGQ1 | 2.9 ± 0.5 | 2.9 ± 0.53 | 2.9 ± 0.54 | 1.00 |
| FOGQ2 | 2.7 ± 0.5 | 2.6 ± 0.5 | 2.8 ± 0.5 | 0.16 |
| FOGQ3 | 3.2 ± 0.7 | 3.1 ± 0.6 | 2.5 ± 0.7 | 0.26 |
| FOGQ4 | 2.5 ± 0.9 | 2.4 ± 0.8 | 2.6 ± 0.9 | 0.61 |
| FOGQ5 | 2.3 ± 0.9 | 2.3 ± 0.8 | 2.4 ± 1.1 | 0.88 |
| FOGQ6 | 2.4 ± 0.9 | 2.3 ± 0.8 | 2.5 ± 0.9 | 0.45 |
| Total FOG-Q score | 14.7 ± 5.4 | 15.5 ± 3.1 | 13.9 ± 6.9 | 0.38 |
| Biomarkers | ||||
| Serum TNF-α (pg/ml) | 25.6 ± 4.1 | 23.2 ± 2.2 | 28.1 ± 4.1 | 0.1 |
| Serum reduced glutathione (pg/ml) | 6.4 ± 0.7 | 6.7 ± 0.7 | 6.0 ± 0.6 | 0.3 |
| Serum BDNF (pg/ml) | 1945.2 ± 256.6 | 1943.7 ± 348.1 | 1943.7 ± 146.4 | 0.3 |
The differences were assessed by Wilcoxon Sign Rank Test for numerical variables and Fisher’s exact test for categorical variables (e.g. sex); P < 0.05 (*) was considered significant. [SD = Standard Deviation; MDS-UPDRS = MDS-Unified Parkinson’s disease Rating Scale; H&Y = Hoehn and Yahr scale; TUG = Timed Up and Go test; FES = Falls Efficacy Scale; MMSE = Mini Mental State Examination; RBD-Q = REM Sleep Behaviour Disorder Questionnaire; FOG-Q = Freezing of Gait Questionnaire; TNF-α = Tumour Necrosis Factor-α; BDNF = Brain Derived Neurotrophic Factor].
Table 2.
Pre-post differences in clinical profile and gait characteristics for active nVNS and sham nVNS groups.
| Clinical outcome variables | Baseline (Pre for nVNS) Mean (SD) | Post-intervention nVNS Mean (SD) | P value pre-post nVNS | Baseline (Pre for sham) Mean (SD) | Post-intervention sham Mean (SD) | P value pre-post sham |
|---|---|---|---|---|---|---|
| Gait outcome variables | ||||||
| Velocity | 61.9 ± 20.3 | 72 ± 19.1 | 0.003* | 66.6 ± 20.3 | 68.31 ± 18.2 | 0.689 |
| Step length | 36.2 ± 10.3 | 40.3 ± 10.15 | 0.007* | 36.8 ± 10.4 | 37.2 ± 10 | 0.797 |
| Swing time variability | 0.04 ± 0.02 | 0.03 ± 0.02 | 0.085 | 0.04 ± 0.03 | 0.04 ± 0.03 | 0.432 |
| Step time | 0.6 ± 0.10 | 0.57 ± 0.08 | 0.003* | 0.57 ± 0.099 | 0.55 ± 0.08 | 0.059 |
| Swing time | 0.37 ± 0.06 | 0.38 ± 0.07 | 0.970 | 0.36 ± 0.07 | 0.35 ± 0.07 | 0.338 |
| Stance time | 0.83 ± 0.17 | 0.75 ± 0.12 | 0.001* | 0.77 ± 0.16 | 0.74 ± 0.12 | 0.304 |
| Stride velocity variability | 6.4 ± 3.2 | 6.9 ± 3.4 | 0.846 | 6.9 ± 2.55 | 6.9 ± 2.43 | 0.841 |
| Step length variability | 3.9 ± 1.5 | 4.0 ± 2.3 | 0.440 | 4.1 ± 1.2 | 4.3 ± 1.3 | 0.543 |
| Step time variability | 0.05 ± 0.03 | 0.04 ± 0.02 | 0.114 | 0.05 ± 0.03 | 0.05 ± 0.035 | 0.920 |
| Step time asymmetry | 0.04 ± 0.04 | 0.02 ± 0.02 | 0.056 | 0.03 ± 0.03 | 0.03 ± 0.04 | 0.819 |
| Step length asymmetry | 3.1 ± 2.4 | 2.5 ± 2.2 | 0.149 | 2.7 ± 2.4 | 2.7 ± 2.1 | 0.808 |
| Step width | 11 ± 2.9 | 10.7 ± 2.9 | 0.357 | 10.8 ± 2 | 10.7 ± 3.7 | 0.424 |
| Clinical characteristics | ||||||
| MDS-UPDRS I | 16 ± 7 | 13 ± 8 | 0.004* | 16 ± 7 | 13 ± 8 | 0.030 |
| MDS-UPDRS II | 21 ± 6 | 17 ± 7 | 0.001* | 22 ± 5 | 18 ± 5 | 0.009* |
| MDS-UPDRS III | 40 ± 1 | 33 ± 1 | 0.002* | 39 ± 10 | 32 ± 12 | 0.002* |
| H & Y | 2 ± 0.5 | 2 ± 0.5 | 0.083 | 2 ± 0.7 | 2 ± 0.7 | 0.655 |
| TUG (s) | 39 ± 77 | 42 ± 101 | 0.033 | 45 ± 67 | 35 ± 47 | 0.098 |
| FES | 54 ± 13 | 48 ± 13 | 0.001* | 56 ± 7 | 50 ± 8 | 0.003* |
| MMSE | 26 ± 4 | 25 ± 6 | 0.195 | 26 ± 4 | 27 ± 3 | 0.905 |
| RBDSQ | 5.2 ± 2.9 | 4.1 ± 3 | 0.036 | 4 ± 2.8 | 3.6 ± 2.9 | 0.177 |
| Total FOG-Q score | 16.5 ± 3.5 | 13.2 ± 3.9 | 0.001* | 15.5 ± 3 | 11.9 ± 4.3 | 0.001* |
| DRS Total | 124.8 ± 14.8 | 120.6 ± 28.9 | 0.727 | 120 ± 18.4 | 114 ± 31.6 | 0.819 |
The differences were assessed by Wilcoxon Sign Rank Test; P < 0.05 (*) was considered significant after correction for multiple comparisons. [SD = Standard Deviation; MDS-UPDRS = MDS-Unified Parkinson’s disease Rating Scale; H&Y = Hoehn and Yahr scale; TUG = Timed Up and Go test; FES = Falls Efficacy Scale; MMSE = Mini Mental State Examination; RBDSQ = REM Sleep Behaviour Disorder Screening Questionnaire; FOG-Q = Freezing of Gait Questionnaire].
On pairwise pre-post analysis, we observed that the velocity increased by 16% (p = 0.018), step length increased by 11% (p = 0.021) and step time reduced by 16% (p = 0.003) in the active nVNS group, whereas the changes in velocity (2.3%; p = 1.0), step length (1%; p = 1.0) and step time (1.7%; p = 0.708) were not significant with sham. Velocity (p = 0.018), step time (p = 0.012) and step length (p = 0.021) showed significant improvement with nVNS but not with sham. The effect size for the nVNS group was 0.45 and for sham it was 0.06; nVNS therefore has a moderate effect on gait velocity in contrast to sham (no effect demonstrated with sham nVNS).
When we compared the change in clinical scores before and after treatment in the two groups separately, we found clinical outcome measures improved significantly in both groups. UPDRS II, III, falls efficacy scale score and freezing of gait questionnaire score all improved significantly in both groups.
A small subset (less than one third) of patients with freezing of gait experienced freezing episodes whilst gait assessments were being performed (and captured simultaneously on video). The average duration of freezing episodes whilst walking around the laboratory gait assessment circuit (see Supplementary Fig.1A) reduced from 21 ± 47 to 15 s ± 37 (s) in the nVNS group (p = 0.042), whereas the duration of freezing episodes did not change significantly after treatment with sham (27 ± 67 to 72 ± 268 (s); p = 0.575). However, the average difference in freezing duration did not constitute a clinically meaningful change in either group. The total time taken to complete the laboratory gait assessment circuit in the sham group did not change significantly (128 ± 130 to 159 ± 299 (s); p = 0.968), whereas this measure was reduced significantly with nVNS (116 ± 55 to 94 ± 32 (s); p = 0.007). There was no significant difference in the average time taken to complete the laboratory gait assessment circuit at baseline for both the nVNS and sham groups (130 and 116 (s); p = 0.897).
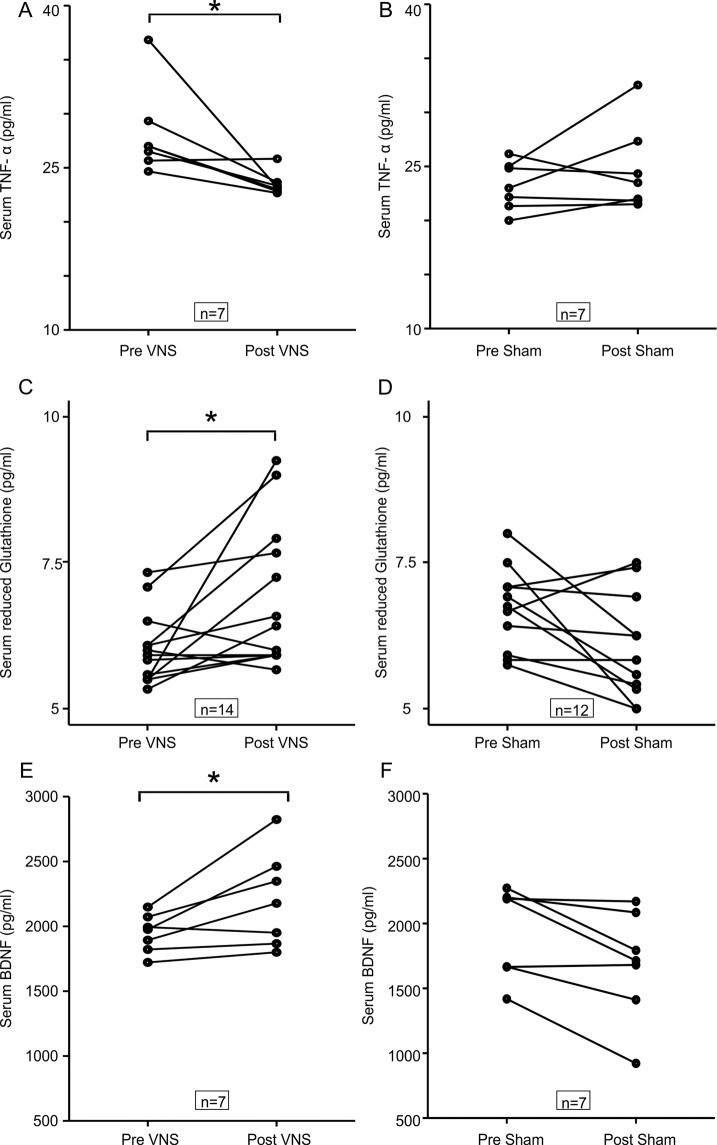
Of all the biochemical parameters measured, TNF-α levels were significantly reduced from baseline in patients receiving nVNS (28.1 to 23.5 pg/ml; p = 0.028) but not in patients receiving sham (23.2 to 24.7 pg/ml; p = 0.499; Fig. 2A). From Fig. 2B it can be seen that the reduced glutathione concentration increased after nVNS (6.1 to 6.8 pg/ml; p = 0.02) but did not change significantly after sham stimulation (6.7 to 6.1 pg/ml; p = 0.05). BDNF levels significantly increased with the nVNS intervention (1946.7 to 2204.1 pg/ml; p = 0.028) but decreased with sham stimulation (1943.7 to 1682.7 pg/ml; p = 0.028). We did not find any significant differences in the levels of IL-6 (p = 0.128), IL-10 (p = 0.108) and the specific activity of superoxide dismutase between groups (p = 0.058).
Fig. 2. Comparing levels of serum biomarkers before and after intervention in the active and sham nVNS groups.
A, C, E The change in serum TNF-α, reduced glutathione and BDNF concentration after active nVNS compared to baseline. B, D, F The change in serum TNF-α, reduced glutathione and BDNF concentration after sham nVNS compared to baseline. Statistical differences were assessed using the Wilcoxon Sign Rank Test, where p < 0.05 (*) was considered significant after correction for multiple comparisons with the Benjamini–Hochberg procedure.
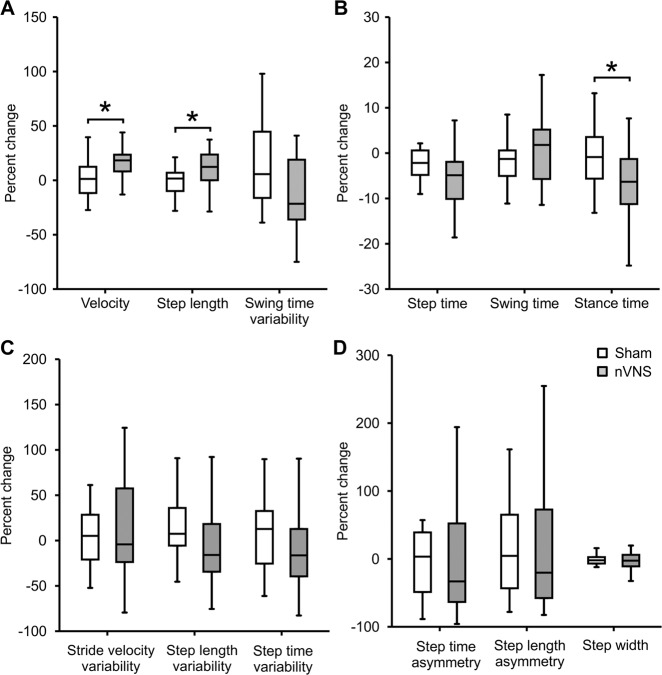
Percentage changes in gait parameters with respect to baseline are presented in Fig. 3. We found significant differences in percentage change of velocity (p = 0.014), step length (p = 0.017) and stance time (p = 0.006) between the active and sham nVNS groups.
Fig. 3. Comparing the percentage change (from baseline) in gait parameters between active and sham nVNS groups.
Representative gait parameters are presented. A Percentage change (from baseline) in gait parameters from the ‘pace’ domain between the active nVNS and sham nVNS groups. B Percentage change (from baseline) in gait parameters from the ‘rhythm’ domain for active and sham nVNS groups. C Percentage change (from baseline) in gait parameters from the ‘variability’ domain. D Percentage change (from baseline) in gait parameters from the ‘asymmetry’ and ‘postural control’ domains. Differences were assessed statistically using the Wilcoxon Sign Rank Test, where p < 0.05 (*) was considered significant without correction for multiple comparison.
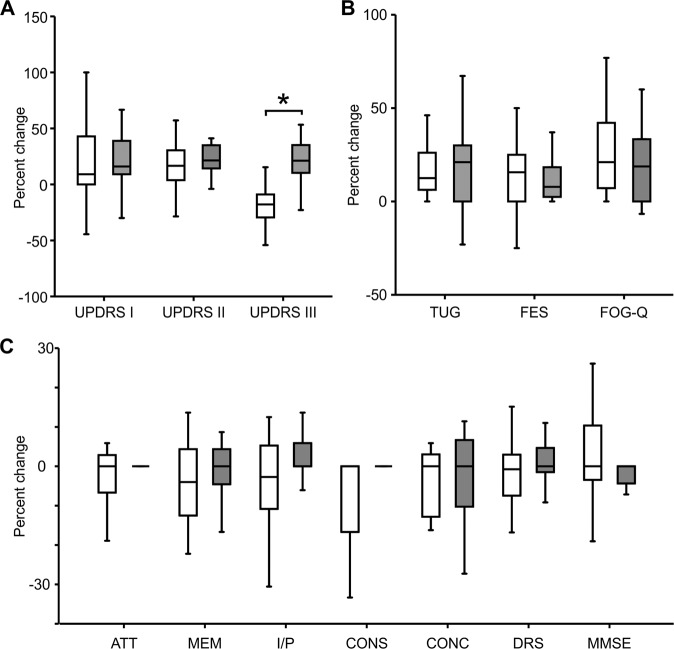
In Fig. 4 we present the comparative analysis of the percentage change in clinical scores between nVNS and sham treatment. We observed significant differences in the change in UPDRS III score between the nVNS and sham groups, with an increase in the nVNS group but not the sham group. There were no significant differences between the groups in the percentage change in scores of the RBD screening questionnaire, falls efficacy scale and freezing of gait questionnaire.
Fig. 4. Comparing the percentage change (from baseline) in clinical characteristics between active and sham nVNS groups.
A percentage change (from baseline) in MDS - Unified Parkinson’s disease Rating Scale (UPDRS Part I, II, III) between active and sham nVNS groups. B percentage change (from baseline in time taken for Timed Up and Go Test (TUG, Falls Efficacy Scale (FES score, and Freezing of Gait Questionnaire (FOG-Q) score between active and sham nVNS groups. C percentage change (from baseline) in total Dementia Rating Scale (DRS) score and scores in specific domains (ATT, MEM, I/P, CONS, CONC) and Mini Mental State Examination (MMSE) score between active and sham nVNS groups. [AAT = Attention; MEM = Memory; I/P = Initiation and Perseveration; CONS = Construction; CONC = Conceptualisation]. Statistical differences were assessed using the Wilcoxon Sign Rank Test, where p < 0.05 (*) was considered significant after correction for multiple comparison.
The perception of patients regarding their experience of freezing and their fear of falling, assessed through the freezing of gait questionnaire and falls efficacy scales respectively, showed unexpected findings. The six items of the freezing of gait questionnaire and the mean score reduced significantly in both groups. Total freezing of gait questionnaire scores were reduced by 26.3% (p = 0.001) and 21% (p = 0.001) in the sham and nVNS groups respectively. Similarly, mean falls efficacy scale scores were also reduced by 10.7% (p = 0.001) and 12% (p = 0.003) after nVNS and sham respectively.
The percentage change in cognitive scores between the two groups was comparable (Fig. 4C). The change in raw scores before and after treatment, calculated separately for each group, was not statistically significant.
There was no carry over effect with either intervention (Supplementary Table 1 and Table 2).
Discussion
This is the first randomized, double-blind sham-controlled trial to confirm the efficacy of cervical nVNS as an adjunctive therapy in PD. Improvements in motor function and gait after one month of treatment with nVNS were significant. While nVNS undoubtedly has immediate modulatory effects on the central neural circuitry controlling gait (Mondal et al, 2019) (see Fig. 5A), the mechanisms by which long term effects are mediated is less clear. The ability of nVNS to significantly reduce pro-inflammatory cytokines such as TNF-α point to an anti-inflammatory role, whereas the increase in serum BDNF would appear to implicate neuroplasticity. The effects observed on anti-oxidant levels might also point to disease-modifying actions.
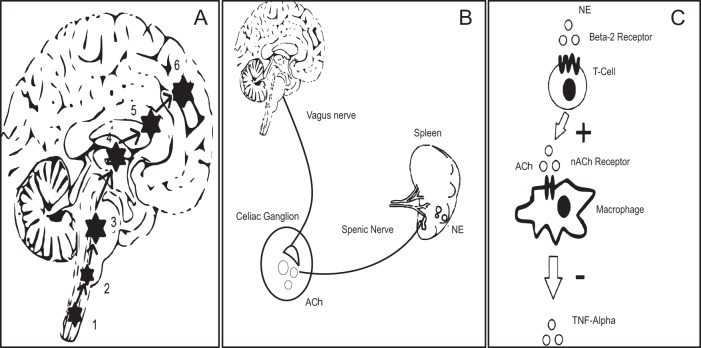
Fig. 5. Putative mechanism of nVNS action at circuit level and cellular level.
The pathway of direct stimulation of brain regions. 1&2, Dorsal motor nucleus of the vagus and nucleus tractus solitarius; 3, Locus coeruleus; 4&5, Basal ganglia and thalamus; 6, forebrain cholinergic nucleus (including nucleus basalis of Meynert). B inflammatory reflex through vagus nerve showing the efferent limb. Vagus nerve stimulation leads to secretion of ACh in the celiac ganglion. ACh in turn stimulates the splenic nerve, which provides direct adrenergic innervation to the spleen. [Ach = Acetyl Choline; NE = Norepinephrine/Noradrenaline]. C The cellular and molecular environment inside the spleen. NE secreted by splenic nerve stimulates T cells (cholinesterase positive to secrete Ach). The secreted neurotransmitter binds with the 7-α subunit of nicotinic ACh receptors on the surface of macrophages and inhibits secretion of TNF-α.
Previous animal studies have shown that VNS exerts its effects primarily via afferent inputs to nucleus tractus solitarius and thence through sequential activation of the locus coeruleus as illustrated in Fig. 527. Locus coeruleus is a noradrenergic nucleus projecting widely to cortical and subcortical locations28. Improvement in postural instability and gait in PD is expected if there is direct cortical activation through excitatory neurotransmitters such as noradrenaline29. The forebrain cholinergic nucleus basalis of Meynert, which provides extensive cholinergic projections to cerebral cortex, is also in receipt of afferent input from locus coeruleus; cortical cholinergic tone is thus also likely to be enhanced by nVNS27. Interestingly, reduced cortical cholinergic tone has been associated with slow gait speed in PD patients30. In this study, gait was assessed using a walkway with integrated pressure-sensors that provide precise measurements of two-dimensional gait parameters. In general, gait parameters are classified into five domains (pace, rhythm, asymmetry, variability, and postural control) based on principle component analysis of gait data from PD patients31. We observed significant increases in velocity and step length (pace domain), and a reduction in stance time (rhythm domain) with nVNS therapy, indicating that PD patients were walking not only with a faster pace but also with improved rhythm. Other gait parameters also showed significant improvement from baseline, specifically after nVNS treatment, in all five domains, suggesting therefore that nVNS results in a global improvement in the quality of gait in PD patients. Along similar lines, we also found significant improvement in the timed up and go test, which is another quantitative surrogate measure of gait speed.
One of the major outcome measures—video-based assessment of freezing of gait—provided mixed results. Although, the average duration of freezing episodes whilst walking around the laboratory gait assessment circuit reduced significantly only in the nVNS group, the perception of patients regarding disability caused by freezing of gait and fear of falling (as quantified through questionnaires) was reduced significantly from baseline in both groups. The difference identified in freezing duration is therefore of uncertain clinical significance. This clinically marginal result is not entirely unexpected given the methodological limitations of video-based assessment of freezing of gait. Freezing of gait is not only a highly variable phenomenon (less than one-third of our patient cohort demonstrated freezing episodes during video-recording) but the severity of freezing can change during a single clinic visit32. Moreover, we did not adopt procedures that might provoke freezing of gait while acquiring video. Ideally freezing of gait should be quantified over a longer period of assessment, preferably with covert video recording. This might be achieved either through a wearable monitoring device or through analysis of prolonged domiciliary video recordings. Such approaches might be incorporated into future, larger interventional trials of nVNS. Besides gait parameters, we also observed a significant difference in the change in overall motor function (UPDRS III) between the two groups. The change in UPDRS III was greater in the nVNS group.
Among non-motor features, we assessed two important aspects, cognition and sleep (in particular RBD), both of which are impaired in PD patients as disease progresses. Basal forebrain cholinergic neurons also play an important role in modulating attention, essential to healthy cognition33. Moreover, drugs that enhance cholinergic transmission are widely used in the treatment of cognitive impairment34. Given the potential cholinergic effects of nVNS via nucleus basalis of Meynert21, one might have expected an improvement in cognitive function in the nVNS group. Whilst reports on the effects of VNS upon cognition have been mixed35, most have failed to demonstrate significant improvements in cognition in patients receiving VNS as an adjunctive therapy for epilepsy36. The primary limitation of such studies is related to the short duration of follow-up; it is difficult to detect significant cognitive improvement (or a reduced rate of deterioration/progression) with less than one year of continuous treatment. The lack of improvement in cognitive tests in our cohort of patients is therefore not entirely unexpected given the relatively short duration of treatment with nVNS. RBD might also be expected to improve with nVNS35, particularly given evidence implicating the locus coeruleus as an important anatomical substrate of RBD37. Whilst we found no effect of nVNS in our study, the effects of nVNS on RBD should perhaps be revisited in future studies with polysomnography.
Evidence would also suggest that VNS can suppress inflammation through a reflex (Fig. 5B, C)38, whereby stimulation of vagal afferents activates vagal efferent fibres, which in turn stimulate splenic T-cells to secrete acetylcholine. This in turn binds to nicotinic receptors (7-α subunit) on the surface of macrophages in and around the spleen, reducing the secretion of pro-inflammatory cytokines. In this crossover study we therefore also tested a number of molecular biomarkers of inflammation and redox dysregulation, which, as established by others39, are upregulated in serum and cerebrospinal fluid of PD patients and in some studies appear to correlate with the severity of motor dysfunction and the extent of neurodegeneration in PD38, leading some to speculate that PD might be an inflammatory disorder40. Whilst we did not monitor the effect of nVNS on circulating T-cell subsets, encouragingly, we showed that nVNS significantly reduced TNF-α levels and increased concentrations of reduced glutathione. We did not observe significant changes in IL-6 and IL-10 levels or superoxide dismutase activity. However, this could be related to stimulation parameters41, which might be further optimised to obtain a more favourable anti-inflammatory effect.
BDNF has been studied widely as a peripheral biomarker of neuroplasticity in various neurodegenerative disorders including PD. Serum levels of BDNF are significantly reduced in PD patients compared to age-matched controls and the concentration was found previously to be negatively associated with disease severity42. Interestingly, BDNF is also strongly associated with inflammation and might thus be a bridge between neuroplasticity and inflammation43. In various neurostimulation studies, peripheral BDNF concentration has been used as a surrogate marker for the effects of intervention on neuroplasticity. BDNF expression was enhanced in rat brain following VNS44, suggesting a potential neuro-modulatory/neuro-protective effect. To translate this finding to humans we measured peripheral BDNF in selected patients from our cohort and found that BDNF concentration significantly increased after nVNS.
Overall, our results provide the first evidence that nVNS downregulates major pro-inflammatory cytokines, upregulates BDNF and increases levels of the antioxidant (reduced glutathione) in PD patients, and that nVNS might have disease-modifying effects in PD. Moreover, BDNF, TNF-α and reduced glutathione might serve as biomarkers, alongside improvement in motor symptoms in PD patients, for optimizing therapeutic nVNS protocols for PD.
The primary objective of this trial was a need to translate a potentially effective technology to the clinic and to treat those symptoms of PD that are currently very difficult to manage. Importantly, such treatment should be easy to apply and safe. We, therefore, assessed the safety of nVNS through adverse event monitoring. Reassuringly, neither interventional group reported clinically meaningful adverse effects related to study devices. Blood pressure and pulse were checked in every subject at all visits and we found no significant deviation of these vital parameters from baseline. Although we advised patients to stimulate the left vagus nerve at the cervical region to avoid the theoretical risk of cardiac adverse effects, previous studies showed that the effects of stimulating the right vagus nerve on heart rate are minimal and did not present an additional risk of adverse cardiac effects45. Current literature suggests that treatment can be delivered safely on either side46. In general, patients were satisfied with the treatment and the majority were able to self-administer nVNS regularly, excepting 2 patients, who required assistance from their care giver to administer nVNS. Three patients could not tolerate nVNS and two patients were unable to tolerate sham stimulation, complaining that even at the lowest intensity of stimulation there was significant discomfort, and withdrew from the study. Grounds for withdrawal for other participants were unrelated to the study devices or adverse effects.
Whilst the results of this trial are very promising, there are nevertheless limitations, not least of which is that we found no significant difference between groups after correction for multiple comparisons. This is not unexpected; the trial was planned as a preliminary study from which the results could inform power calculations for a future trial. A relatively high number of patients dropped out from the initial arm of the study. This observation is potentially explained by the requirement for patients to commit to commuting between home and hospital for several study visits over a short time period. Moreover, because the patients were recruited from a relatively frail population, in whom a significant and progressive gait disorder was one of the inclusion criteria, commuting long distances and transferring between different transportation modalities may have become a particular issue for some. However, the frequencies of dropouts were equivalent in both groups and none were related to the safety of the study or difficulties with the stimulation procedure.
In such future trials, other limitations will also need to be addressed. Molecular biomarkers, including other emerging candidate molecules, should ideally be measured in all trial participants. The limitations of video-based estimation of freezing of gait (discussed above) might be addressed by the use of ambulatory monitoring devices. Finally, whether or not a care-giver is needed (see above), practical issues related to the delivery of nVNS in older populations may have to be addressed in future iterations of the device.
This study has provided preliminary evidence supporting the efficacy and safety of nVNS in treating motor and non-motor symptoms of PD. Future studies of nVNS in PD should not only confirm repeatability but should also focus on optimising treatment parameters, by first establishing how long treatment effects (and potential neuroprotective effects) of nVNS persist before significant motor symptoms develop. Larger, multi-centre trials of nVNS in PD are now warranted.
Methods
In this crossover study, thirty-three PD patients of both sexes with freezing of gait, aged 30–80 years, were recruited consecutively from the movement disorders outpatient clinic at a tertiary care hospital in Eastern India. Only patients who could walk independently and continuously for at least 30 metres without support, and with the ability to turn 180° on the spot, were included in the study. Patients were diagnosed according to UK Brain Bank Criteria47 and those with baseline scores ≥2 on both items 2.13 and 3.11 (specific to freezing of gait on the MDS-UPDRS rating scale) were included. We were careful to exclude patients with early signs of atypical Parkinsonism e.g. supranuclear gaze palsy.
Patients with significant visual impairment or coexisting local or systemic diseases (e.g. osteoarthritis or other neurological conditions) likely to affect gait were excluded from our study. Patients who underwent deep brain stimulation surgery or those with an implanted cardiac pacemaker were also excluded to ensure the safe use of nVNS, as were patients with metallic implants near the stimulation site (e.g. fusion of cervical vertebrae). Finally, patients with known or suspected cardiovascular disease, uncontrolled hypertension or recent myocardial infarction were also excluded from the study.
Ethics approval & trial registration
Written informed consent was obtained from each participant prior to the study in accordance with the Declaration of Helsinki. Protocols and procedures were approved by the Institutional Institute of Neurosciences Kolkata Ethics Committee (reference number I-NK/EComm/44/2016), dated 2nd April 2016 and the trial was registered with ISRCTN (International Standard Registered Clinical/soCial sTudy Number), registration number ISRCTN14797144.
Study procedure
Every patient was assessed four times over a minimum study period of 12 weeks (consort diagram; Fig. 1). At the screening visit, medical history and medications were reviewed, and patients screened for eligibility according to criteria, before randomization. Patients were requested to come for baseline assessments within seven days of the consent process, at which point devices were dispensed. A general physical examination including a detailed neurological examination was performed. PD-related motor and non-motor symptoms were assessed according to clinical scales (see section below). Assessments of gait and cognition were also performed on the same day. All assessments were performed in the OFF state after an overnight L-dopa free period. The patients were randomised to one of two sequences (nVNS first or sham first). Patients and carers were trained to administer nVNS or sham and instructed to use the treatment at home for one month. To confirm that the patients and/or carers remained proficient at delivering nVNS throughout the study, the patients and/or carers were instructed to apply the stimulation during each study-visit in the presence of the study team member who had been delegated to train the patients.
The patients returned after four weeks (first treatment period) for their first follow-up visit. After a minimum washout period of 4 weeks, patients from the same cohort returned for a second follow-up visit and were allocated to the alternate group for the second interventional phase of the study (second treatment period). The same set of evaluations was performed at all four visits. Patients maintained a stable dose of L-dopa and other anti-Parkinsonian medication throughout the study.
Only a subset of patients participated in the biomarker study. Serum samples from 14 patients in the nVNS and 12 patients in the sham arms of the study were suitable for the redox marker study. Paired samples for estimation of inflammatory biomarkers were available from seven patients. The blood samples were collected four times from six participants (at baseline and at the end of each treatment period). The remaining participants only provided samples relating to one treatment period (i.e. unpaired samples). Eight patients were from the sham followed by active VNS group and five patients were from the active followed by sham VNS group. One patient from each group dropped out after the first intervention.
Process of randomisation
The devices were dispensed in a randomised and blinded manner. Simple randomisation was performed using a computer-generated list of random numbers to assign either nVNS or sham devices in addition to standard treatment in a 1:1 ratio (Random Allocation version 2.0). Other than the device serial number, nVNS and sham devices were indistinguishable. A complete list of serial numbers and the stimulation mode of each device (sham or active and its serial number) was provided to the unblinded trial oversight committee (not involved in patient recruitment or assessment) by the commercial sponsor (electroCore, Inc.). Investigators, site coordinators and participants were blind to the allocation of devices until the trial had been completed.
Intervention
The nVNS device (provided without restriction by electroCore, Inc.) generated a proprietary frequency-modulated electrical stimulus at low voltage (maximum 24 V) with a maximum current output of 60 mA. The signal consisted of 1 ms bursts of 5 kHz sine waves repeated at 25 Hz. Two stainless steel contact surfaces coated with conductive gel delivered the stimulus to the neck in the vicinity of the vagus nerve. The sham device (also provided by electroCore, Inc.) was identical in appearance, weight and user interface, and whilst it delivered perceptible electrical stimulation to the skin (maximum output of 14 V and 24 mA), the proprietary electrical sham stimulus was designed not to activate the vagus nerve by delivering a low-frequency signal (0.1 Hz biphasic DC). The treatment consisted of two, 2-min stimulation intervals delivered 5–10 min apart to the left vagus nerve to minimize potential cardiac side effects (cardiac vagal efferents generally travel in the right vagus nerve), using the medial borders of the sternocleidomastoid muscle and the carotid pulse as anatomical landmarks. Stimulus intensity was set to the maximum tolerated by the participant. The same stimulus strength was used throughout the study for each individual. All subjects were questioned regarding adverse events during or following nVNS. The intervention was delivered at 3 pre-specified times every day: (1) within 1 h of awakening; (2) 6–8 h after the first treatment; and (3) 6–8 h after the second treatment.
Assessment
All patients were assessed for PD-related motor and non-motor symptoms through a set of validated clinical rating scales and gait assessments, at each visit.
Motor function was assessed by gait assessments, MDS-UPDRS scale, freezing of gait questionnaire and falls efficacy scale. Gait was assessed using an instrumented walkway (GAITRite, USA) and the Timed Up and Go test. In addition to the freezing of gait questionnaire, post hoc video gait assessments were completed to estimate the severity of freezing of gait. The non-motor functional assessments included cognition, namely the Mini Mental State Examination and Mattis Dementia Rating Scale, and sleep, specifically the Rapid Eye Movement sleep behaviour disorder (RBD) screening questionnaire. Serum biomarkers were measured in a sub-group of patients (see above). Details of the assessment protocols are described in the supplementary material.
Inflammatory markers (TNF-α, IL-6, IL-10 and BDNF) were measured in serum using commercially available ELISA kits (Abcam, USA). Serum markers of oxidative stress, namely glutathione and superoxide dismutase, were analysed by standard spectrophotometric methods using an iMark Microplate Reader (BIORAD, USA). Detailed methods are described in the supplementary material.
Sample size calculation
A formal sample size calculation was not performed because we had no prior information regarding the expected treatment effect (and variability) of one month of treatment with nVNS. We recruited patients over a 36-month period.
Safety and compliance
Patient safety was assessed through adverse event reporting followed by causality assessments using standard WHO-UMC criteria. Sitting blood pressure and pulse were monitored at all visits. Patients/care givers were advised to complete a paper diary to record adverse events and compliance. All patients had a compliance of >95%.
Statistical analysis
Clinical and demographic variables were presented using mean (and standard deviation) for parametric data and median (and interquartile range) for nonparametric data. Data were tested using the Shapiro-Wilk test (and distribution histograms) for normality. Categorical data were presented as percentages. If no difference in left and right gait variables was identified these were pooled and averaged. Differential carryover effects were tested between the 2 sequences using the Wilcoxon Sign Rank test. Period effects were not anticipated due to the short duration of the study48. Irrespective of the sequence of device allocation the percentage change of outcome variables from each period was combined. Changes in absolute values of outcome measures (e.g., biomarkers, clinical rating scores) after the administration of nVNS or sham were compared using Wilcoxon signed rank test. The percentage change from baseline in outcomes was also compared between nVNS and sham groups using Wilcoxon signed rank test for paired samples. Categorical variables were compared using Fisher’s Exact Test. A p-value of <0.05 was considered statistically significant. Correction for multiple comparison employed the Benjamini Hochberg procedure49. Effect size was calculated to evaluate the effect of nVNS and sham on gait velocity using the formula: r = z/ √ (2n), where z is the z-score and n is the sample size. A large effect is defined as r > 0.6 and a moderate effect 0.6 > r > 0.4. The effect size for the nVNS group was 0.45, whereas for sham it was 0.06. Statistical analysis was performed using SPSS version 20 (IBM, USA).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We are grateful to electroCore, Inc. (USA) for providing active and sham nVNS devices without restriction. The authors would like to thank Prof. R P Sengupta MD, FRCS, OBE, for his kind support throughout the study and Mr Aditya Deorah for his unrestricted grant for the development of the research center, Institute of Neurosciences Kolkata.
Author contributions
1. Research project: A. Conception, B. Organization, C. Execution. 2. Statistical analysis: A. Design, B. Execution, C. Review and Critique. 3. Manuscript: A. Writing the first draft, B. Review and Critique. Banashree Mondal: 1A,1C, 2A, 2B, 3A. Supriyo Choudhury: 1A, 2A, 2B, 3A. Rebecca Banerjee: 1C, 3B. Akash Roy: 1C, 2B, 3B. Koustav Chatterjee: 2B, 3B. Purba Basu: 1C, 3B. Ravi Singh: 1C, 3B. Saptak Halder: 1C, 3B. Shantanu Shubham: 1A, 3B. Stuart N Baker: 2A, 2C, 3B. Mark R Baker: 1A, 2C, 3B. Hrishikesh Kumar: 1A, 1B, 2C, 3B
Data availability
The experimental data are available from the corresponding author, given reasonable requests.
Code availability
No software code has been used for this study.
Competing interests
The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41531-022-00424-6
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/3/2022
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41531-022-00424-6
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-021-00190-x.
References
- 1.Terry Jr, R. S. Vagus nerve stimulation therapy for epilepsy. Epilepsy Topics: InTech, 139–160 (2014).
- 2.Mauskop A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia. 2005;25:82–86. doi: 10.1111/j.1468-2982.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- 3.Kraus T, et al. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J. neural Transm. 2007;114:1485–1493. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- 4.Oshinsky ML, Murphy AL, Hekierski H, Jr, Cooper M, Simon BJ. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. PAIN®. 2014;155:1037–1042. doi: 10.1016/j.pain.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: part II. Headache.: J. Head. Face Pain. 2016;56:259–266. doi: 10.1111/head.12650. [DOI] [PubMed] [Google Scholar]
- 6.Ben‐Menachem E, Revesz D, Simon B, Silberstein S. Surgically implanted and non‐invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur. J. Neurol. 2015;22:1260–1268. doi: 10.1111/ene.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corcoran C, Connor TJ, O’Keane V, Garland MR. The effects of vagus nerve stimulation on pro-and anti-inflammatory cytokines in humans: a preliminary report. Neuroimmunomodulation. 2005;12:307–309. doi: 10.1159/000087109. [DOI] [PubMed] [Google Scholar]
- 8.Majoie H, et al. Vagus nerve stimulation in refractory epilepsy: effects on pro-and anti-inflammatory cytokines in peripheral blood. Neuroimmunomodulation. 2011;18:52–56. doi: 10.1159/000315530. [DOI] [PubMed] [Google Scholar]
- 9.Bonaz B, Sinniger V, Pellissier S. Anti‐inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J. Physiol. 2016;594:5781–5790. doi: 10.1113/JP271539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akiyama H, et al. Inflammation and Alzheimer’s disease. Neurobiol. aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sveinbjornsdottir S. The clinical symptoms of Parkinson’s disease. J. neurochemistry. 2016;139:318–324. doi: 10.1111/jnc.13691. [DOI] [PubMed] [Google Scholar]
- 12.Mondal B, et al. Analysis of gait in Parkinson’s disease reflecting the effect of l-DOPA. Ann. Mov. Disord. 2019;2:21. [Google Scholar]
- 13.Giladi N, et al. Freezing of gait in patients with advanced Parkinson’s disease. J. neural Transm. 2001;108:53–61. doi: 10.1007/s007020170096. [DOI] [PubMed] [Google Scholar]
- 14.Riederer P. Time course of nigrostriatal degeneration in Parkinson’s disease. J. neural Transm. 1976;38:277–301. doi: 10.1007/BF01249445. [DOI] [PubMed] [Google Scholar]
- 15.Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann.-NY Acad. Sci. 2003;991:120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee K, et al. Inflammasome and α-synuclein in Parkinson’s disease: a cross-sectional study. J. Neuroimmunol. 2020;338:577089. doi: 10.1016/j.jneuroim.2019.577089. [DOI] [PubMed] [Google Scholar]
- 17.Klegeris A, McGeer EG, McGeer PL. Therapeutic approaches to inflammation in neurodegenerative disease. Curr. Opin. Neurol. 2007;20:351–357. doi: 10.1097/WCO.0b013e3280adc943. [DOI] [PubMed] [Google Scholar]
- 18.Farrand AQ, et al. Vagus nerve stimulation improves locomotion and neuronal populations in a model of Parkinson’s disease. Brain stimulation. 2017;10:1045–1054. doi: 10.1016/j.brs.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris, R. et al. Noninvasive vagus nerve stimulation to target gait impairment in Parkinson’s disease. Mov. Disord.34, 918-919 (2019). [DOI] [PubMed]
- 20.Chen M, et al. Low‐level vagus nerve stimulation attenuates myocardial ischemic reperfusion injury by antioxidative stress and antiapoptosis reactions in Canines. J. cardiovascular Electrophysiol. 2016;27:224–231. doi: 10.1111/jce.12850. [DOI] [PubMed] [Google Scholar]
- 21.Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 2018;11:203. doi: 10.2147/JIR.S163248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun, P. et al. Involvement of MAPK/NF-κB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS ONE8, e69424 (2013). [DOI] [PMC free article] [PubMed]
- 23.Mondal B, Choudhury S, Simon B, Baker MR, Kumar H. Noninvasive vagus nerve stimulation improves gait and reduces freezing of gait in Parkinson’s disease. Mov. Disord. 2019;34:917–918. doi: 10.1002/mds.27662. [DOI] [PubMed] [Google Scholar]
- 24.Ono, S. A., Sato, T. & Muramatsu, S.-I. Freezing of gait in Parkinson’s disease is associated with reduced 6-[18F] Fluoro-lm-tyrosine uptake in the locus coeruleus. Parkinson’s Disease2016, 5430920 (2016). [DOI] [PMC free article] [PubMed]
- 25.Lewine JD, Paulson K, Bangera N, Simon BJ. Exploration of the impact of brief noninvasive vagal nerve stimulation on EEG and event‐related potentials. Neuromodulation: Technol. Neural Interface. 2019;22:564–572. doi: 10.1111/ner.12864. [DOI] [PubMed] [Google Scholar]
- 26.Hays, S. A., Rennaker, R. L. & Kilgard, M. P. in Prog. Brain Res. Vol. 207 275–299 (Elsevier, 2013). [DOI] [PMC free article] [PubMed]
- 27.Engineer ND, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frangos E, Komisaruk BR. Access to vagal projections via cutaneous electrical stimulation of the neck: fMRI evidence in healthy humans. Brain stimulation. 2017;10:19–27. doi: 10.1016/j.brs.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Grimbergen YA, Langston JW, Roos RA, Bloem BR. Postural instability in Parkinson’s disease: the adrenergic hypothesis and the locus coeruleus. Exp. Rev. Neurotherap. 2009;9:279–290. doi: 10.1586/14737175.9.2.279. [DOI] [PubMed] [Google Scholar]
- 30.Rochester L, et al. Cholinergic dysfunction contributes to gait disturbance in early Parkinson’s disease. Brain. 2012;135:2779–2788. doi: 10.1093/brain/aws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lord S, et al. Cognition and gait show a selective pattern of association dominated by phenotype in incident Parkinson’s disease. Front. aging Neurosci. 2014;6:249. doi: 10.3389/fnagi.2014.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nieuwboer A, Giladi N. The challenge of evaluating freezing of gait in patients with Parkinson’s disease. Br. J. Neurosurg. 2008;22:S16–S18. doi: 10.1080/02688690802448376. [DOI] [PubMed] [Google Scholar]
- 33.Sarter M, Bruno JP. Developmental origins of the age-related decline in cortical cholinergic function and associated cognitive abilities. Neurobiol. aging. 2004;25:1127–1139. doi: 10.1016/j.neurobiolaging.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Ellis JM. Cholinesterase inhibitors in the treatment of dementia. J. Am. Osteopath. Assoc. 2005;105:145. [PubMed] [Google Scholar]
- 35.Rizzo P, et al. Chronic vagus nerve stimulation improves alertness and reduces rapid eye movement sleep in patients affected by refractory epilepsy. Sleep. 2003;26:607–611. doi: 10.1093/sleep/26.5.607. [DOI] [PubMed] [Google Scholar]
- 36.Dodrill CB, Morris GL. Effects of vagal nerve stimulation on cognition and quality of life in epilepsy. Epilepsy Behav. 2001;2:46–53. doi: 10.1006/ebeh.2000.0148. [DOI] [PubMed] [Google Scholar]
- 37.García-Lorenzo D, et al. The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson’s disease. Brain. 2013;136:2120–2129. doi: 10.1093/brain/awt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tracey KJ. Reflex control of immunity. Nat. Rev. Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müller T, Blum‐Degen D, Przuntek H, Kuhn W. Short communication Interleukin‐6 levels in cerebrospinal fluid inversely correlate to severity of Parkinson’s disease. Acta Neurologica Scandinavica. 1998;98:142–144. doi: 10.1111/j.1600-0404.1998.tb01736.x. [DOI] [PubMed] [Google Scholar]
- 40.Pretorius E, et al. Parkinson’s disease: a systemic inflammatory disease accompanied by bacterial inflammagens. Front. Aging Neurosci. 2019;11:210. doi: 10.3389/fnagi.2019.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsaava T, et al. Specific vagus nerve stimulation parameters alter serum cytokine levels in the absence of inflammation. Bioelectron. Med. 2020;6:1–10. doi: 10.1186/s42234-020-00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scalzo P, Kümmer A, Bretas TL, Cardoso F, Teixeira AL. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J. Neurol. 2010;257:540–545. doi: 10.1007/s00415-009-5357-2. [DOI] [PubMed] [Google Scholar]
- 43.Calabrese F, et al. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front. Cell. Neurosci. 2014;8:430. doi: 10.3389/fncel.2014.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Follesa P, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28–34. doi: 10.1016/j.brainres.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 45.Yamakawa K, et al. Electrophysiological effects of right and left vagal nerve stimulation on the ventricular myocardium. Am. J. Physiol.-Heart Circulatory Physiol. 2014;307:H722–H731. doi: 10.1152/ajpheart.00279.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spuck S, Nowak G, Renneberg A, Tronnier V, Sperner J. Right-sided vagus nerve stimulation in humans: an effective therapy? Epilepsy Res. 2008;82:232–234. doi: 10.1016/j.eplepsyres.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Meara J, Bhowmick BK, Hobson P. Accuracy of diagnosis in patients with presumed Parkinson’s disease. Age Ageing. 1999;28:99–102. doi: 10.1093/ageing/28.2.99. [DOI] [PubMed] [Google Scholar]
- 48.Karl, J. A., Ouyang, B., Goetz, S. & Metman, L. V. A Novel DBS paradigm for axial features in Parkinson’s disease: a randomized crossover study. Mov. Disord.35, 1369–1378(2020). [DOI] [PubMed]
- 49.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The experimental data are available from the corresponding author, given reasonable requests.
No software code has been used for this study.