Sir
Hanrath and colleagues, reported in this Journal that the prior SARS-CoV-2 infection was associated with protection against symptomatic reinfection.1 Authors suggested further studies to know the durability of protection. SARS-CoV-2 elicits rapid immune response, with seroconversion occurring in majority cases by 10 days post-symptom onset.2, 3, 4, 5 Information about longevity of antibody mediated immune response in convalescent COVID-19 patients is vital in understanding the duration of immunity. Several published studies have reported rapid waning of antibodies within 3–4 months.5 Others have reported presence of IgG antibodies up to 3 and 8-months post infection.6, 7, 8 Few studies are available about persistence of humoral immune response from low and middle-income countries. We estimated prevalence of SARS-CoV-2 specific antibody response among COVID-19 patients at multiple time points over a 7-month period post RT-PCR confirmation.
We conducted a cross-sectional study among recovered COVID-19 patients between November 10 and December 15, 2020 across all age groups in Chennai, India. We obtained the line-list of recovered COVID-19 patients from the local civic body and grouped these patients into seven time-points (i.e. 15–30, 31–60, 61–90, 91–120, 121–150, 151–180 and 181–232 days) based on days since their RT-PCR confirmation. We enrolled a minimum of 100 consenting individuals from each of the seven-time groups and interviewed them to collect information on basic demographic details, clinical history, comorbidity and current health status and collected 3–5 ml of venous blood.
The sera were tested for the presence of IgG antibodies against nucleocapsid (NC) (Abbott Park, IL, USA, Sensitivity: 100%, Specificity: 99.6%9) and spike (S1-RBD) (Siemens Healthineers India, Mumbai, Sensitivity: 100%, Specificity: 99.9%) proteins using chemiluminescent immunoassays, and neutralizing antibodies (Nabs) using surrogate virus neutralization test (sVNT) (GenScript, Piscataway, USA) (Supplementary material.8) The data were analyzed to estimate the proportion IgG positivity during different time-windows (Supplementary material). Institutional ethics committee of ICMR-National Institute of Epidemiology and ICMR-National Institute for Research in Tuberculosis, Chennai approved the study protocol.
We enrolled 755 individuals in the study (minimum 100 participants in each time-group). The mean age of the study participants was 41.8 (SD: 12.5) years, and 58.3% (n = 440) were males. 81 (10.7%) individuals reported that they were asymptomatic, 44 (5.8%) had severe illness (admitted in ICU or required supplemental oxygen while hospitalization) and 630 (83.4%) were classified into mild to moderate illness category. Majority were either isolated in COVID care centres (33.1%) or in their homes (37%) and 194 (25.7%) were directly admitted to a hospital or medical institution. 280 (37.1%) reported a chronic co-morbidity; the most common being diabetes mellitus (n = 176, 23.3%) and hypertension (n = 155, 20.5%) (Table-1).
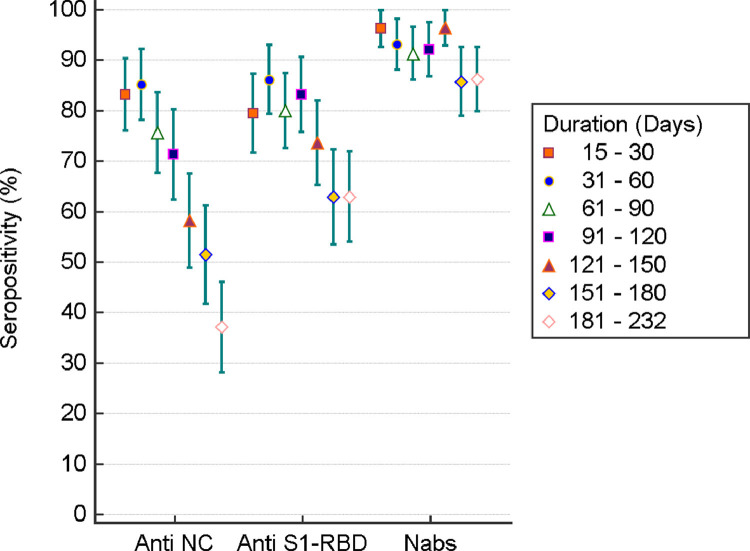
IgG seropositivity against NC protein 15–30, 31–60, 61–90, 91–120, 121–150, 151–180 and 181–232 days after RT-PCR diagnosis was 83.2% (95%CI: 76.1% - 90.3%), 85.1% (95%CI: 78.2% - 92.1%), 75.7% (95%CI: 67.8% - 83.5%), 71.3% (95%CI: 62.5% - 80.1%), 58.2% (95%CI: 49.0% - 67.4%), 51.4% (95%CI: 41.9% - 61.0%), and 37.1% (95%CI: 28.3% - 45.9%) respectively (Fig-1, Supplementary Table-1). Sero-positivity to S1-RBD was higher compared to that of NC protein at all time-windows except during the first-time-window of 15–30 days. The proportion of COVID-19 patients sero-positive to NC or S1-RBD declined over time, with respectively 43 (37.1%) and 73 (62.9%) of the 116 patients having antibodies against NC and S1-RBD 180 days , after RT-PCR diagnosis (Supplementary Table-1, Fig-1). More than 90% (range: 91.3%- 96.4%) of the recovered COVID-19 patients had NAbs till 121–150 days. NAbs during the time-window of 151–180 and 181–232 days after RT-PCR diagnosis was 85.7% (95%CI: 79.0% - 92.4%) and 86.2% (95%CI: 79.9% - 92.5%) respectively.
Seropositivity for IgG against NC, S1-RBD and NAbs observed during 15–30-day time period was higher among individuals with severe illness compared to those with a mild/moderate or asymptomatic illness. This pattern was observed during each of the time-window. In particular, IgG seropositivity against NC and S1-RBD protein during the time-window of 151–232 days was 37.5% and 50.0% among individuals with asymptomatic COVID-19 and 43.9% and 63.6% respectively among mild/moderate patients. However, individuals who had a severe illness had higher levels of IgG NC (60%) and S1-RBD (80%) during the same time-window. Similarly, percentage of NAbs among individuals with severe illness (90.0%) was higher compared to who had a mild/moderate (81.3%) or asymptomatic (70.8%). (Supplementary Table-2).
Seropositivity for IgG against NC, S1-RBD and NAbs was not different among males and females during all time-windows (Supplementary table-3). Seropositivity for NAbs was also not different among those with and without comorbidity during all time-windows. Although individuals with comorbidity had higher seropositivity for IgG against NC and S1-RBD during each time-windows, proportion seropositives for these antibodies were not significantly different among those with and without comorbidity (Supplementary table-4).
The decline of anti-NC and anti S1-RBD has an implication on the serosurveys conducted to estimate the proportion of population previously infected with SARS-CoV-2. Most serosurveys use NC or spike assays to estimate seropositivity.2 Since the pandemic is continuing for more than a year, serosurveys using only one assay would grossly underestimate the seroprevalence. Hence a standard algorithm to use laboratory assays for serosurvey needs to be developed to account for the waning of antibodies.
The IgG anti-NC, anti S1-RBD and neutralizing antibody waned faster among the individuals with no to mild/moderate symptoms than individuals who had severe illness. Antibody response was more pronounced and long-lasting in individual who had severe disease as documented in other studies.6, 10, 11 Lower antibody response and relatively faster waning among asymptomatic and individuals with mild/moderate symptoms might be because of strong innate immunity and T cell response in these individuals 2.
Our study has certain limitations. We used cross-sectional design to measure humoral response to SARS-CoV-2 over time whereas cohort design involving longitudinal measurement may be ideal for this purpose. Nevertheless, our cross-sectional estimates provide quick snapshot of durability of immune response among COVID-19 patients. We could not compare antibody response over time by age groups (Supplementary table-5) since majority of our study participants were in working age group than children and older adults. As a secondary objective, we examined host immune response by clinical severity based on self-reported symptoms, this could have led to misclassification specifically between those reporting asymptomatic status versus mild/moderate symptoms. However, we could validate the severity status from hospitalization records for those categorized as having severe illness.
In conclusion, findings of our study indicated that IgG antibodies against NC and S1-RBD waned over time but the neutralization function of the antibody remained stable in majority of the COVID-19 infected patients till 7 months of post-infection. These findings suggest a lower possibility of reinfection by the same viral strain among infected individuals during this time period.
Fig. 1.
SARS-CoV-2 specific IgG seropositivity (%) with 95% CIs among recovered COVID-19 patients by duration since infection (N = 755).
Table 1.
Demographic and clinical characteristics of the study participants.
| Characteristics | Number of study Participants (% of the total) N = 755 |
|---|---|
| Age (in years) | |
| 6 - 18 | 17 (2.3) |
| 19 - 45 | 413 (54.7) |
| 46 - 60 | 293 (38.8) |
| 61 - 82 | 32 (4.2) |
| Mean (SD) | 41.8 (12.5) |
| Gender | |
| Male | 440 (58.3) |
| Female | 314 (41.6) |
| Transgender | 1 (0.1) |
| Severity of illness | |
| Severe | 44 (5.8) |
| Mild/Moderate | 630 (83.4) |
| Asymptomatic during the entire course of illness | 81 (10.7) |
| Admission status | |
| Home isolation throughout the entire course of illness | 279 (37.0) |
| Initially was in home isolation, but later hospitalised | 14 (1.9) |
| COVID Care center throughout the entire course of illness | 250 (33.1) |
| Initially was in COVID care center, but later hospitalised | 18 (2.4) |
| Directly admitted to a hospital/medical institution | 194 (25.7) |
| Duration since RT-PCR confirmation | |
| 15–30 days | 107 (14.2) |
| 31–60 days | 101 (13.4) |
| 61–90 days | 115 (15.2) |
| 91–120 days | 101 (13.4) |
| 121–150 days | 110 (14.6) |
| 151–180 days | 105 (13.9) |
| 181–232 days* | 116 (15.4) |
| Symptoms (n = 674) | |
| Fever | 504 (74.8) |
| Muscle aches/Body pain | 460 (68.2) |
| Loss of smell | 335 (49.7) |
| Loss of taste | 334 (49.6) |
| Cough | 313 (46.4) |
| Headache | 303 (45.0) |
| Joint pain | 300 (44.5) |
| Sore throat | 272 (40.4) |
| Fatigue | 272 (40.4) |
| Shortness of Breath | 146 (21.7) |
| Running nose | 97 (14.4) |
| Diarrhea | 92 (13.6) |
| Chills | 85 (12.6) |
| Vomiting | 75 (11.1) |
| Abdominal pain | 51 (7.6) |
| Conjunctivitis | 33 (4.9) |
| Confusion | 18 (2.7) |
| Seizures | 1 (0.1) |
| Presence of Comorbidity (n = 280) | |
| Hypertension | 155 (20.5) |
| Diabetes mellitus | 176 (23.3) |
| Heart diseases | 14 (1.9) |
| Asthma | 19 (2.5) |
| Other diseases (CKD, Liver diseases, malignancies, neurological disorders, rheumatic disorders, etc.) | 28 (3.7) |
(*includes 16 patients between 210 and 232 days after RT-PCR infection).
Declaration of Competing Interest
NIL
Acknowledgements
This study was funded by Greater Chennai Corporation, public health department for conducting this survey. We acknowledge the support from Greater Chennai Corporation health officials in field operations.
Footnotes
Supplementary material associated with this article can be found, in the online version, at 10.1016/j.jinf.2021.05.026.
Appendix. Supplementary materials
References
- 1.Hanrath A.T., Payne B.A.I., Duncan C.J.A. Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. J Infect. 2021;82(4):e29–e30. doi: 10.1016/j.jinf.2020.12.023. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021 Feb 18;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020 Dec 4;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long Q.-.X., Liu B.-.Z., Deng H.-.J., Wu G.-.C., Deng K., Chen Y.-.K., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. Jun. [DOI] [PubMed] [Google Scholar]
- 5.Ripperger T.J., Uhrlaub J.L., Watanabe M., Wong R., Castaneda Y., Pizzato H.A., et al. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity. 2020;53(5):925–933. doi: 10.1016/j.immuni.2020.10.004. Nov 17e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529) doi: 10.1126/science.abf4063. Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5(52) doi: 10.1126/sciimmunol.abe0367. Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41(5):355–359. doi: 10.1016/j.it.2020.03.007. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Food & Drug Administration. EUA authorized serology test performance 2020. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance (accessed May 03, 2021).
- 10.Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442. doi: 10.1038/s41586-020-2456-9. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccoli L., Park Y.-.J., Tortorici M.A., Czudnochowski N., Walls A.C., Beltramello M., et al. Mapping neutralizing and immuno-dominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020 Nov 12;183(4):1024–1042. doi: 10.1016/j.cell.2020.09.037. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.