Abstract
Purpose
To evaluate diagnostic performance and safety of ultrasound-guided needle biopsy in the diagnosis of peripheral pulmonary nodules (PPLs) ≤ 2 cm, and the influence factors of sample adequacy and safety.
Materials and Methods
194 patients (99 men, 95 women; mean age, 56.2 ± 13.7 years) who received biopsy for PPLs ≤ 2 cm between January 2014 to January 2019 were included. Variables including patient demographics, lesion location, lesion size, presence of lesion necrosis, presence of emphysema on CT, patient position, biopsy needle size and number of needle passes were recorded. Univariate analysis and multivariate logistic regression analysis were performed to explore the influence factor of sample adequacy and safety.
Results
Biopsy specimens were adequate for diagnosis in 161/194 (83%) cases; the diagnostic accuracy was 81.4% (158/194). The overall complication rate was 8.8% (17/194), including pneumothorax, hemoptysis and pleural effusion, which occurred in 2.1% (4/194), 5.2% (10/194), and 1.5% (3/194) of patients, respectively. The incidence of pneumothorax in the 16-gauge-needle group were significantly higher than that of the 18-gauge-needle group (5.6% vs 0%, P=0.018). Adequate sampling of 16-gauge and 18-gauge needles were achieved in 90.3%(65/72) and 78.7%(96/122) cases, respectively. Multivariate logistic regression analysis revealed needle size (16-gauge vs 18-gauge) was an independent influence factors of sample adequacy (P=0.015, odds ratio=3.419). A receiver operating characteristic curve was plotted and the area under the curve was 0.774.
Conclusion
US-guided percutaneous needle biopsy is a feasible and safe technique for small PPLs ≤ 2 cm. Needle size is an independent influence factor of sample adequacy and post-procedure pneumothorax. Sixteen-gauge needle has the advantage of achieving adequate sample for pathological analysis, though the risk of pneumothorax should be alerted.
Keywords: peripheral pulmonary lesions, core needle biopsy, ultrasound-guided, diagnostic performance, safety
Introduction
With the application of computed tomography (CT) and advent of lung cancer screening in recent years, peripheral pulmonary lesions (PPLs) has been more frequently detected (1). PPLs are defined as lesions directly in contact with the chest wall without an intervening aerated lung (2). PPLs have an echogenic texture and a sharply defined border due to a strong reflective interface between the aerated lung and the lesion on ultrasound (US) (3). Percutaneous core needle biopsy plays an important role in diagnosing PPLs, as a precise diagnostic procedure is necessary for use in determining appropriate management (4). Compared with CT-guided procedures, US-guided percutaneous needle biopsy has some advantages such as free of radiation exposure, real-time monitoring, convenience and avoiding vessels with Color Doppler imaging (5). Therefore, ultrasound has the strength to be a feasible and reliable guiding tool as an alternative to CT.
In China, US-guided biopsy is usually preferred for PPLs, while CT-guided procedures are mainly recommended for lesions that cannot be displayed by ultrasound (6). Although CT-guided procedures have been well established in a previous study with a similar diagnostic rate compared with US-guided procedures for PPLs, a higher incidence of postprocedural pneumothorax were observed (7). Currently, percutaneous US–guided core needle biopsy plays an increasingly important role in the management of PPLs (8). Some published studies had shown the feasibility and efficacy of US-guided percutaneous biopsy for PPLs (2, 5, 6, 9–11). It was reported that US-guided procedures could achieve a high success rate of 84%~96% (2), a pneumothorax rate of 1%~6%, and a hemorrhage rate of 3.3%~5.1% (2, 6, 12).
However, the increased number of small PPLs detected at CT screening has prompted a new challenge in management (8). Although peripheral non-small cell lung cancer (NSCLC) ≤2 cm without nodal and distant metastasis is classified as T1 stage, tumor size >2 cm is considered a significant indicator of visceral pleural invasion (13). As a result, early diagnosis is crucial for improving prognosis. Regular and repeated CT examinations to evaluate the change of volume of the PPLs is one of the remedies for management (14). However, when PPLs were recognized with a size increase follow-up CT examination, a pathologic diagnosis was required. Adequate tissue acquisition for histologic and molecular characterization of PPL is paramount. Nevertheless, small PPLs was reported to be associated with lower diagnostic accuracy in biopsy (5). To the best of our knowledge, data about diagnostic performance and safety of US-guided biopsy for small PPLs are still deficient. Most previous studies included cases with medium or large PPLs and did not systematically provide detailed analysis on small PPLs due to limited sample size, nor the procedures were performed under CT-guidance (2, 5, 6, 9). To find out the influence factors of efficacy and safety would provide critical information to improve the diagnostic yield and lower the complication rate in biopsy for small PPLs. Therefore, the purpose of this study was to evaluate diagnostic performance and safety of ultrasound-guided needle biopsy in the diagnosis of PPLs ≤ 2 cm, and the influence factors of sample adequacy and safety.
Materials and Methods
Patients
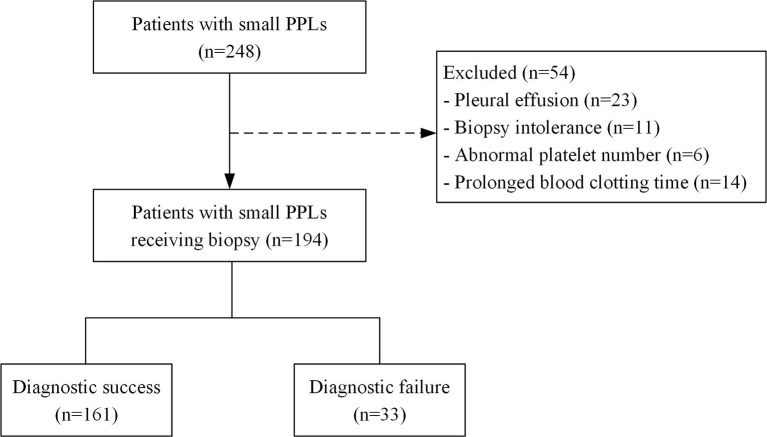
From January 2014 and January 2019, a cohort of 194 consecutive patients who received US-guided percutaneous biopsy procedures for PPLs ≤ 2 cm at our hospital were included in the study. Exclusion criteria for biopsy were as follows: patients with pleural effusion, biopsy intolerance resulted from severe cough or cardiopulmonary dysfunction, and abnormal platelet number or prolonged blood clotting time. The Flow chart of patient inclusion was shown in Figure 1 .
Figure 1.
Flow chart of patient inclusion. PPLs, peripheral pulmonary lesions.
Data Collection
The institutional review board of our hospital waived the requirement to obtain informed consent and approved the protocol for this retrospective study. Diameters of all nodules were measured as the long-axis measurement on lung window settings in the axial plane on CT. Data review and collection was performed to confirm patient demographics (age, gender), previous cancer history, lesion location, lesion size, presence of lesion necrosis, presence of emphysema on CT, patient position, biopsy needle size, number of needle passes, histopathology reports and post-procedure complications.
US-Guided Biopsy Techniques and Procedures
All patients received CECT examinations in two weeks before the biopsy procedure. US-guided biopsies were performed using a MyLab Twice machine (Esoate, Genoa, Italy) equipped with a convex array probe CA541 (frequency range from 1 to 8 MHz) by a sonographer with 10 years’ experience in thoracic and interventional ultrasound. Biopsy was performed using needles with a core of 18-gauge or 16-gauge (Bard, Arizona, USA). The protruding needle (22 mm) with biopsy notch (16 mm length) was the same in all cases. Biopsy needle size and biopsy technique were dictated by operator discretion.
According to the location of PPLs, all patients were managed in supine, lateral or prone positions. After administration of local antiseptics and anesthetic, the biopsy needle was inserted with a freehand approach. Real-time color Doppler imaging was used to avoid vessels. The needle could be clearly seen throughout its whole course. Needle insertion angle along linear path was kept as close to 90° as possible. Biopsies were performed during patient’s breath-holding. Samples adequacy were assessed visually, and biopsy was repeated until adequate specimens were achieved. Biopsies were performed 1 to 4 times as tolerated by the patient. The tissue was conserved in formalin and sent for pathological analysis ( Figure 2 ).
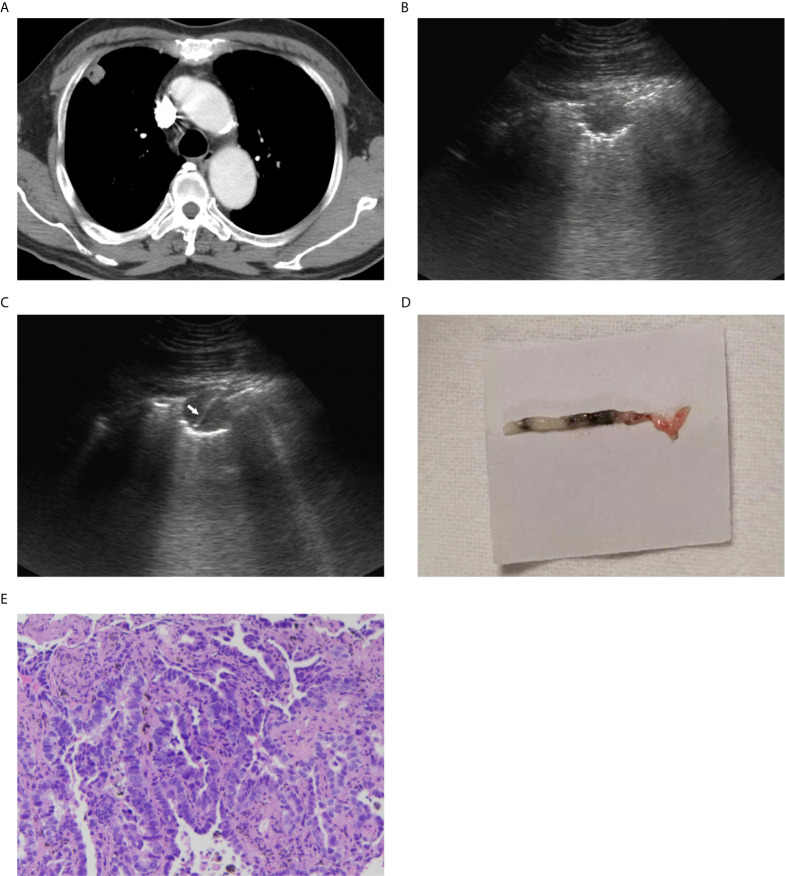
Figure 2.
A 67-year-old man with a peripheral lung lesion. Ultrasound-guided percutaneous core needle biopsy was performed. (A) Contrast-enhanced CT image showed a lung lesion located in right upper lobe with pleural contact. The diameter of the lesion was 19 mm. (B) The lesion manifested as low-echoic on baseline ultrasound. (C) The 16-gauge needle track during the biopsy procedure was shown on the image (white arrow). (D) Color photograph of the biopsy sample. (E) Pathological evaluation showed that heterotypic cells were arranged in tubular or adenoid structures (HE staining; 10×).
Complication Evaluations
After biopsy, patients were monitored for about 30 minutes. Patients were mandated to kept in bed without movement for at least 8 hours. Patients received chest radiograph and ultrasound examination 1 hour after biopsy to detect the presence of complications including pneumothorax and pleural effusion. Further follow-up radiographs were performed when needed (15, 16). A large pneumothorax is identified when the rim between the lung margin and inner chest wall more than 2 cm, which was measured on a chest radiograph at the level of the hilum (17). Hemorrhage could present as hemoptysis and pleural effusion. The severity of pleural effusion was based on the width of free fluid area on ultrasonography, and major pleural effusion was defined as width ≥2 cm. Chest tube insertion was required in cases with large pneumothorax and major pleural effusion, and removed when complication was relieved. Chest radiograph was taken before discharge.
Pathological Evaluations
Two pathologists with 10 years of experience evaluated the images together and made a final determination. Biopsy results were classified as malignant disease, benign disease, or nondiagnostic. Results were considered nondiagnostic when the tissue was deemed inadequate for diagnosis or showed few atypical cells. A malignant disease was confirmed on when the surgical pathology was the same as the biopsied one. A benign disease was confirmed when the surgical pathology was the same as the biopsied one or when the lesion regressed or remained stable for at least 2 years’ follow-up (18). Nondiagnostic cases were sent for re-biopsy or surgical biopsy.
Statistical Analysis
Data were reported as percentage or mean ± standard deviation, as appropriate. Differences in quantitative variables were detected with independent sample t-test or Mann–Whitney U-test. Differences in categorical variables were detected using χ2 -test or Fisher’s exact test. P<0.05 was considered to indicate a statistically significant difference.
A multivariate logistic regression model was built to identify independent influence factors associated with biopsy sample adequacy. An Enter model was used and all variables were sent for binary logistic regression analysis. As lesion location and patient position are unordered categorical variables, dummy variables were used in multivariate logistic regression analysis. For patient position, supine was used as reference to set up dummy variables (X1, lateral=1; X2, prone=1). For lesion location, left upper lobe was used as reference to set up dummy variables (X1, left lower lobe=1; X2, right upper lobe=1; X3, right middle lobe=1; X4, right lower lobe=1). A receiver operating characteristic (ROC) curve was plotted, and the area under the ROC curve was calculated to determine the predictive value of the logistic regression model. Statistical analyses were performed using the SPSS 16.0 software package (IBM, NY, USA).
Results
Patients and Lesions Profile
A total of 194 patients were included in the current study. There were 99 (51.0%) men and 95 (49.0%) women, with a mean age of 56.2 ± 13.7 years (range 18~80 years). The average diameter of the PPLs was 1.6 ± 0.4 cm (ranging from 0.6 cm to 2.0 cm). The detailed baseline characteristics and lesions profile were displayed in Table 1 .
Table 1.
Patients characteristics and lesion profiles (n = 194).
| Characteristics | n = 194 |
|---|---|
| Age (years) | |
| Mean ± SD (range) | 56.2 ± 13.7 (18~80) |
| Gender | |
| Male/female | 99/95 |
| Lesion size (cm) | |
| Mean ± SD (range) | 1.6 ± 0.4 (0.6~2.0) |
| Lesion location | |
| Left upper lobe | 31 |
| Left lower lobe | 49 |
| Right upper lobe | 47 |
| Right middle lobe | 14 |
| Right lower lobe | 53 |
| Presence of necrosis | |
| Yes/No | 40/154 |
| Patient position (%) | |
| Supine | 81 |
| Prone | 29 |
| Lateral | 84 |
| Emphysema on CT | |
| Yes/No | 17/177 |
| Needle size (gauge) | |
| 16/18 | 72/122 |
| Needle passes | |
| 1 time | 28 |
| 2 times | 117 |
| ≥3 times | 49 |
Diagnostic Performance of Biopsy and Influence Factors
The rate of adequate sample for pathological analysis was 83.0% (161/194). Among all biopsies, 74 lesions were identified as malignant disease: 48 adenocarcinoma, 12 metastatic cancer, 7 squamous cell carcinoma, 3 small cell lung cancer, 3 sarcoma and 1 poorly-differentiated carcinoma. On the other hand, 87 lesions were categorized as benign disease: 36 tuberculosis, 24 chronic inflammation, 9 pneumonia, 7 organizing pneumonia, 5 granulomatous inflammation, 4 cryptococcosis, 1 aspergillosis and 1 pneumoconiosis. The remaining 33 biopsy samples were categorized as non-diagnostic. Table 2 provides the pathologic diagnoses for the biopsies.
Table 2.
Pathological results of ultrasound-guided biopsy for 194 PPLs.
| Biopsy results | No. (%) of patients |
|---|---|
| Malignant disease | 74 (38.2) |
| Squamous cell carcinoma | 7 (3.6) |
| Adenocarcinoma | 48 (24.7) |
| Small cell carcinoma | 3 (1.6) |
| Metastasis | 12 (6.2) |
| Sarcoma | 3 (1.6) |
| Poorly-differentiated carcinoma | 1 (0.5) |
| Benign disease | 87 (44.8) |
| Tuberculosis | 36 (18.6) |
| Pneumonia | 9 (4.6) |
| Chronic inflammation | 24 (12.4) |
| Organizing pneumonia | 7 (3.6) |
| Pneumoconiosis | 1 (0.5) |
| Aspergillosis | 1 (0.5) |
| Cryptococcosis | 4 (2.0) |
| Granulomatous inflammation | 5 (2.6) |
| Nondiagnostic | 33 (17.0) |
PPLs, peripheral pulmonary lesions.
Of the 74 lesions diagnosed as malignant, 62 (83.8%) were confirmed by surgical resection. Twelve (16.2%) were confirmed based on the results of biopsy for PPLs and the biopsies for other sites, and both presented as the same histological results. Of the 87 lesions diagnosed as benign, 2 lesions (2.3%) were confirmed through surgical resection. Overall, 34 lesions (39.1%) were confirmed as benign based on biopsy results and regression or disappearance of the lesion at follow-up. Fifty-one lesions (58.6%) were confirmed as benign owing to absence of change in size for more than 2 years.
Seventy-four malignant lesions and 84 of the 87 benign lesions were diagnosed correctly. Three cases were diagnosed as benign based on biopsy result but were confirmed as malignant after a surgical biopsy or a bronchoscopy biopsy. The overall diagnostic accuracy was 81.4% (158/194). Of the 33 lesions diagnosed as non-diagnostic, 12 (36.4%) were confirmed by surgical resection, 18 (54.5%) were confirmed by surgical biopsy and the remaining 3 (9.1%) were confirmed by bronchoscopy biopsy.
Univariate analysis showed that the rate of sample adequacy was significantly higher in 16-gauge-needle group than that of the 18-gauge-needle group (90.3% vs 78.7%, P=0.038). There was no significant difference in age, gender, lesion location, lesion size, presence of lesion necrosis, patient position, presence of emphysema on CT and times of needle passes between sample-adequate group and sample-inadequate group (all P>0.05) ( Table 3 ).
Table 3.
Univariate analysis of influence factors for sample adequacy of ultrasound-guided percutaneous core needle biopsy for PPLs ≤ 2 cm.
| Variables | Sample adequacy for pathological diagnosis | P value | |
|---|---|---|---|
| Confirmed cases (n = 161) | Unconfirmed cases (n = 33) | ||
| Age (years) | 56.5 ± 13.9 | 54.7 ± 12.7 | 0.491 |
| Gender | |||
| Male/female | 80/81 | 19/14 | 0.409 |
| Lesion size (cm) | 1.6 ± 0.4 | 1.5 ± 0.4 | 0.708 |
| Lesion location | |||
| Left upper lobe | 27 | 4 | 0.086 |
| Left lower lobe | 35 | 14 | |
| Right upper lobe | 40 | 7 | |
| Right middle lobe | 14 | 0 | |
| Right lower lobe | 45 | 8 | |
| Presence of necrosis | |||
| Yes/No | 36/125 | 4/29 | 0.185 |
| Patient position (%) | |||
| Supine/Lateral/ Prone |
71/21/69 | 10/8/15 | 0.166 |
| Emphysema on CT | |||
| Yes/No | 16/145 | 1/32 | 0.347 |
| Needle size (gauge) | |||
| 16/18 | 65/96 | 7/26 | 0.038 |
| Needle passes | |||
| 1 time | 23 | 5 | 0.842 |
| 2 times | 96 | 21 | |
| ≥3 times | 42 | 7 | |
PPLs, peripheral pulmonary lesions.
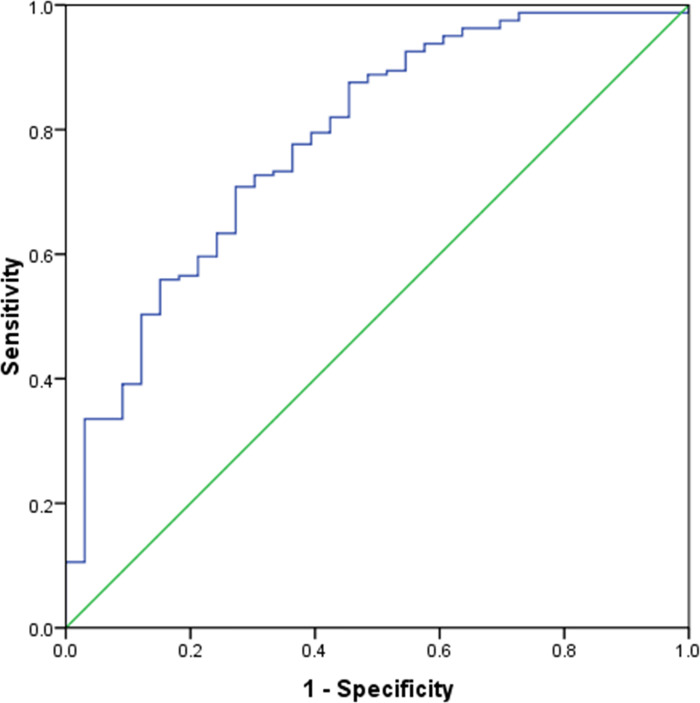
All variables were sent for binary multivariate logistic regression analysis. Multivariate logistic regression analysis revealed that only needle size (16-gauge vs 18-gauge) (P=0.015, OR = 3.419, 95% CI = 1.264-9.249) was an independent influence factor of sample adequacy. Details of multivariate logistic regression analysis results are summarized in Table 4 . An ROC curve ( Figure 3 ) was plotted to evaluate the predictive value of the logistic regression model. The area under the ROC curve was 0.774 (P<0.001, 95% CI = 0.686–0.862).
Table 4.
Multivariate logistic regression analysis of influence factors for sample adequacy of ultrasound-guided percutaneous core needle biopsy for PPLs ≤ 2 cm.
| Variables | B | OR | 95%CI | P value |
|---|---|---|---|---|
| Age (y) | 0.004 | 1.004 | 0.972-1.036 | 0.828 |
| Gender (female vs male) | 0.366 | 1.442 | 0.621-3.351 | 0.395 |
| Lesion location | NA | NA | NA | 0.323 |
| X1 (left lower lobe=1) | -1.174 | 0.309 | 0.080-1.203 | 0.090 |
| X2 (right upper lobe=1) | -0.670 | 0.512 | 0.112-2.343 | 0.388 |
| X3 (right middle lobe=1) | 19.698 | 3.59×108 | NA | 0.998 |
| X4 (right lower) | -0.158 | 0.853 | 0.210-3.460 | 0.824 |
| Lesion size (cm) | 0.023 | 1.023 | 0.909-1.151 | 0.708 |
| Emphysema by CT (no vs yes) | -1.809 | 0.164 | 0.018-1.497 | 0.109 |
| Presence of necrosis (no vs yes) | -0.896 | 0.408 | 0.124-1.340 | 0.139 |
| Patient position | NA | NA | NA | 0.129 |
| X1 (lateral=1) | -1.336 | 0.263 | 0.071-1.274 | 0.065 |
| X2 (prone=1) | -0.440 | 0.644 | 0.236-1.756 | 0.390 |
| Needle size (16-gauge vs 18-gauge) | 1.229 | 3.419 | 1.264-9.249 | 0.015* |
| Needle passes (times) | 0.368 | 1.445 | 0.771-2.705 | 0.250 |
PPLs, peripheral pulmonary lesions; OR, odds ratio; CI, confidence interval; NA, not available.
*P < 0.05.
Figure 3.
Receiver operating characteristic curve analysis of predictive value of the multivariate logistic regression model. The area under the curve was 0.774.
Complications of Biopsy and Influence Factors
The overall post-procedure complication rate was 8.8% (17/194). None of these incidents resulted in permanent severe sequelae or death. The overall rate of hemorrhage was 6.7% (13/194), including 10 cases of hemoptysis and 3 cases of pleural effusion. No major hemorrhage occurred. All cases were self-limited and recovered after conservative treatment including rest, placement in a puncture-site-down position and use of Tranexamic acid. No significant difference was detected in respect to age, gender, lesion location, presence of lesion necrosis, patient position, presence of emphysema on CT, needle size and times of needle passes between complication group and non-complication group, hemoptysis group and non-hemoptysis group, pleural-effusion group and non-pleural-effusion group (all P>0.05).
Totally, pneumothorax occurred in 4 out of 194 patients (2.1%), and all cases were recorded as minor pneumothorax. The patients were monitored in a puncture-site-down position and received nasal oxygen. The rate of pneumothorax in the 16-gauge-needle group were significantly higher than that of the 18-gauge-needle group (5.6% vs 0%, P=0.018), while no significant difference was detected in respect to age, gender, lesion location, presence of lesion necrosis, patient position, presence of emphysema on CT and times of needle passes between pneumothorax group and non-pneumothorax group (all P>0.05) ( Table 5 ).
Table 5.
Univariate analysis of influence factors for safety of ultrasound-guided percutaneous core needle biopsy for peripheral pulmonary lesions ≤ 2 cm.
| Characteristics | Total No. (%) | Complication | Pneumothorax | Hemoptysis | Pleural effusion | ||||
|---|---|---|---|---|---|---|---|---|---|
| 194 (100) | n (%) | P | n (%) | P | n (%) | P value | n (%) | P | |
| Age (years) | |||||||||
| ≤65 | 52 (26.8) | 5 (9.6) | 0.799 | 2 (3.8) | 0.292 | 3 (5.8) | 1.000 | 2 (3.8) | 1.000 |
| >65 | 142 (73.2) | 12 (8.5) | 2 (1.4) | 7 (4.9) | 1 (0.7) | ||||
| Gender | |||||||||
| Male | 99 (51.0) | 5 (5.1) | 0.062 | 1 (1.0) | 0.361 | 2 (2.0) | 0.091 | 1 (1.0) | 0.615 |
| Female | 95 (49.0) | 12 (12.6) | 3 (3.2) | 8 (8.4) | 2 (2.1) | ||||
| Lesion location | |||||||||
| Left upper lobe | 31 (15.9) | 2 (6.5) | 0.843 | 1 (3.2) | 0.972 | 0 (0) | 0.242 | 1 (3.2) | 0.402 |
| Left lower lobe | 49 (25.3) | 3 (6.1) | 1 (2.0) | 2 (4.0) | 0 (0) | ||||
| Right upper lobe | 47 (24.2) | 5 (10.6) | 1 (2.1) | 4 (8.5) | 0 (0) | ||||
| Right middle lobe | 14 (7.2) | 2 (14.3) | 0 (0) | 2 (14.3) | 0 (0) | ||||
| Right lower lobe | 53 (27.4) | 5 (9.4) | 1 (1.9) | 2 (3.8) | 2 (3.8) | ||||
| Presence of necrosis | |||||||||
| Yes | 30 (15.5) | 2 (6.7) | 0.528 | 2 (6.7) | 0.189 | 0 (0) | 0.210 | 0 (0) | 1.000 |
| No | 164 (84.5) | 15 (9.1) | 2 (1.2) | 10 (6.1) | 3 (1.8) | ||||
| Patient position (%) | |||||||||
| Supine | 81 (41.8) | 5 (6.2) | 0.191 | 0 (0) | 0.069 | 5 (6.2) | 0.213 | 0 (0) | 0.310 |
| Lateral | 29 (14.9) | 5 (17.2) | 0 (0) | 3 (10.3) | 1 (3.4) | ||||
| Prone | 84 (43.3) | 7 (8.3) | 4 (4.8) | 2 (2.4) | 2 (2.4) | ||||
| Emphysema by CT | |||||||||
| Yes | 17 (8.8) | 0 (0) | 0.374 | 0 (0) | 1.000 | 0 (0) | 0.666 | 0 (0) | 1.000 |
| No | 177 (91.2) | 17 (9.6) | 4 (2.3) | 10 (5.6) | 3 (1.7) | ||||
| Needle size (gauge) | |||||||||
| 16 | 72 (37.1) | 9 (12.5) | 0.157 | 4 (5.6) | 0.018* | 3 (4.2) | 0.887 | 1 (1.4) | 0.286 |
| 18 | 122 (62.9) | 8 (6.6) | 0 (0) | 7 (5.7) | 2 (1.6) | ||||
| Needle passes | |||||||||
| 1 time | 28 (14.4) | 3 (10.8) | 0.492 | 0 (0) | 0.438 | 1 (3.6) | 0.539 | 1 (3.6) | 0.462 |
| 2 times | 117 (60.3) | 8 (6.8) | 2 (1.7) | 5 (4.3) | 2 (1.7) | ||||
| ≥3 times | 49 (25.3) | 6 (12.2) | 2 (4.1) | 4 (8.2) | 0 (0) | ||||
*P < 0.05.
Discussion
The current study demonstrated that US-guided percutaneous needle biopsy for small PPLs is feasible and safe, and needle size is the independent influence factor of sample adequacy and post-procedure pneumothorax. In other words, sixteen-gauge needle has the advantage of achieving adequate sample for pathological analysis compared with 18-gauge needle, though the increasing risk of pneumothorax should be alerted.
How to manage patients with PPLs that are 2 cm or smaller is the ultimate question for radiologists and physicians. When physicians consider adopting US-guided percutaneous core needle biopsy for small PPLs, there is no doubt that they will consider the lesion size inevitably lower the diagnostic performance, which has been proved by researchers (5, 11). Some scholars attributed the lower diagnostic performance in small lesions to missed sampling and insufficient sampling (5). Therefore, to acquire adequate tissue sample for pathological diagnosis plays a crucial role in improving diagnostic performance. As we know, repeatability and a larger number of samples may increase the success rate of biopsy. In our study, the only influence factor that affected the sample adequacy of US-guided percutaneous biopsy for small PPLs in univariate and multivariate analyses was the needle size. In our series, 16-gauge needle was applied in 37.1% of cases, yielding a larger tissue sample and improving the success rate of pathological evaluation to about 3.4 folds compared with using an 18-gauge needle. Certainly, cutting groove volume of 16-gauge needle is naturally greater than that of 18-gauge needle. Besides, when minor pneumothorax occurs during biopsy, the gas might effuse to the pleural cavity from the needle tract. As a result, the PPL would be obscured by the gas and affect further sampling. In this situation, a large needle will take advantage over a smaller one in acquiring adequate tissue sample. Many studies have demonstrated that US-guided percutaneous biopsy for PPLs is effective and the accuracy has ranged from 82%~96% in confirmative pathological diagnoses (7, 19–24). In terms of diagnostic accuracy, the overall rate was 81.4% in this study, which was comparable to those of previous studies.
The other consideration is the safety of this invasion biopsy procedure. The common complications are pneumothorax and hemorrhage (including hemoptysis or pleural effusion). In a study of CT-guided needle biopsy for lung lesions, the rates of pneumothorax and bleeding were 17-fold and 6-fold higher in pulmonary lesions ≤ 2 cm (25). On the other hand, US-guided biopsies for PPLs≤ 2 cm were safe with a pneumothorax rate of 2.1% and a hemorrhage rate of 6.7% in the present study. There were no mortalities in all procedures. The results were comparable to previous studies that included various lesion size and reported rates of pneumothorax and hemorrhage ranging from 1%~6% and 3.3%~5.1%, respectively (2, 6, 12). Influence factors regarding patient characteristics, tumor profiles, needle size and needle passage were analysed. In our series, the incidence of pneumothorax was higher using 16-gauge needle (5.6%) rather than 18-gauge needle (0%). For small PPLs, lesion-pleura contact arc length that proposed by Jeon et al. (26) is short. Consequently, when the needles penetrate into visceral pleura, the possibilities of injuring gas-containing pulmonary tissue increase, especially if larger size needles are employed in the biopsy. Larger needle size tends to injure a larger part of lung parenchyma and lead to expanding in the amount of air leakage. As a result, gas escapes from the damaged pleura to form a pneumothorax. Similarly, Geraghty et al. reported needle size had a significant effect on pneumothorax rate in CT-guided biopsy (27). However, the pneumothorax induced by 16-gauge needle in our study were all recognized as minor, and all were discharged after conservation therapy.
Hemorrhage is another concerned issue. Hemoptysis and hemothorax presenting as pleural effusion are two important urgent complications that will interrupt air exchanges in the lungs (28, 29). Except for lesion-related, patient-related and operator-related-factors, procedure-related factors should be paid more attention. Procedure-related factors including needle size, needle pass times and needle insertion angle are well within operator control. In our series, choosing the shortest distances to the PPLs and penetrate the pleural as vertically as possible by a single experienced operator resulted in 5.2% hemoptysis and 1.5% pleural effusion among all cases. The occurrence of hemoptysis and pleural effusion with the use of 16-gauge needle was approximate to that using 18-gauge needle (4.2% vs 5.7% and 1.4% vs 1.6%, respectively). At present, 18-gauge needles are typically used. For patients, 16-gauge needle could increase the tissue yield without increasing the risk of bleeding compared with 18-guage needle, which was very praiseworthy and valuable. This reason might be that the blood supply of small PPLs is not so abundant, and the risk of penetrating large blood vessels and causing severe hemorrhage is low. In respect to needle passes, no significant correlation between number of needle passes and hemorrhage rate was found in a meta-analysis of influence factor of CT-guided core needle biopsy for lung lesions (30).
Several limitations of this study should be taken into account. First, it is a retrospective study and selection bias was inevitable. Second, the lack of surgical pathology in some cases is an important limitation. In this respect, we used reference standards accepted in clinical practice and routinely used for management decisions. Third, the study was performed in a single center, and some unavailable potential influence factors could not be evaluated, such as relationship between lesions and respiration, the depth of penetration, pulmonary function and smoking history of the patients. Therefore, further validation of results of this study is still warranted.
Conclusions
In conclusion, US-guided percutaneous needle biopsy for small PPLs is a feasible and safe technique. Needle size is the independent influence factor of sample adequacy and post-procedure pneumothorax. Therefore, 16-gauge needle has the advantage of achieving adequate sample for pathological analysis compared with 18-gauge needle, though the risk of pneumothorax should be alerted.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of First People’s Hospital of Foshan. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YDQ, WWP, NHL, TH, YHO, and XYD participated in literature search, data acquisition, data analysis, or data interpretation. WJH, JYY, and YJL conceived and designed the study, and critically revised the manuscript, performed the research, wrote the first draft, collected and analyzed the data. WJH and JYY participated in paper writing and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Foshan Engineering Technology Research Center Project [Grant No. 2020001004115].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor Characteristics. J Thorac Oncol (2016) 11:1653–71. 10.1016/j.jtho.2016.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamamoto N, Watanabe T, Yamada K, Nakai T, Suzumura T, Sakagami K, et al. Efficacy and Safety of Ultrasound (US) Guided Percutaneous Needle Biopsy for Peripheral Lung or Pleural Lesion: Comparison With Computed Tomography (CT) Guided Needle Biopsy. J Thorac Dis (2019) 11:936–43. 10.21037/jtd.2019.01.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laursen CB, Graumann O, Moller TV, Davidsen JR. Contrast-Enhanced Ultrasound-guided Transthoracic Lung Biopsy. Am J Respir Crit Care Med (2016) 194:e5–6. 10.1164/rccm.201603-0500IM [DOI] [PubMed] [Google Scholar]
- 4. Bai C, CM C, CM C, Anantham D, Chung-Man Ho J, AZ K, et al. Evaluation of Pulmonary Nodules: Clinical Practice Consensus Guidelines for Asia. Chest (2016) 150:877–93. 10.1016/j.chest.2016.02.650 [DOI] [PubMed] [Google Scholar]
- 5. Guo YQ, Liao XH, Li ZX, Chen YY, Wang SD, Wang JH, et al. Ultrasound-Guided Percutaneous Needle Biopsy for Peripheral Pulmonary Lesions: Diagnostic Accuracy and Influencing Factors. Ultrasound Med Biol (2018) 44:1003–11. 10.1016/j.ultrasmedbio.2018.01.016 [DOI] [PubMed] [Google Scholar]
- 6. Zhang H, Guang Y, He W, Cheng L, Yu T, Tang Y, et al. Ultrasound-Guided Percutaneous Needle Biopsy Skill for Peripheral Lung Lesions and Complications Prevention. J Thorac Dis (2020) 12:3697–705. 10.21037/jtd-2019-abc-03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sconfienza LM, Mauri G, Grossi F, Truini M, Serafini G, Sardanelli F, et al. Pleural and Peripheral Lung Lesions: Comparison of US- and CT-guided Biopsy. Radiology (2013) 266:930–5. 10.1148/radiol.12112077 [DOI] [PubMed] [Google Scholar]
- 8. Rivera MP, Mehta AC, Wahidi MM. Establishing the Diagnosis of Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest (2013) 143:e142S–65S. 10.1378/chest.12-2353 [DOI] [PubMed] [Google Scholar]
- 9. Wang Y, Xu Z, Huang H, Zhou X, Xian M. Application of Quantitative Contrast-Enhanced Ultrasound for Evaluation and Guiding Biopsy of Peripheral Pulmonary Lesions: A Preliminary Study. Clin Radiol (2020) 75:79.e19–24. 10.1016/j.crad.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 10. Tekin AF, Turgut B, Oncu F. Should We Perform Transthoracic Trucut Lung Biopsies of Pleural-Based Lung Masses Under Ultrasound Guidance or Computed Tomography Guidance? Ultrasound Q (2019) 36:49–53. 10.1097/ruq.0000000000000435 [DOI] [PubMed] [Google Scholar]
- 11. Lee MH, Lubner MG, Hinshaw JL, Pickhardt PJ. Ultrasound Guidance Versus CT Guidance for Peripheral Lung Biopsy: Performance According to Lesion Size and Pleural Contact. AJR Am J Roentgenol (2018) 210:W110–7. 10.2214/ajr.17.18014 [DOI] [PubMed] [Google Scholar]
- 12. Fu Y, Zhang Y-Y, Cui L-G, Tan S, Sun Y. Ultrasound-Guided Biopsy of Pleural-Based Pulmonary Lesions by Injection of Contrast-Enhancing Drugs. Front Pharmacol (2019) 10:960. 10.3389/fphar.2019.00960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahn SY, Park CM, Jeon YK, Kim H, Lee JH, Hwang EJ, et al. Predictive CT Features of Visceral Pleural Invasion by T1-Sized Peripheral Pulmonary Adenocarcinomas Manifesting as Subsolid Nodules. AJR Am J Roentgenol (2017) 209:561–6. 10.2214/AJR.16.17280 [DOI] [PubMed] [Google Scholar]
- 14. Yankelevitz DF, Gupta R, Zhao B, Henschke CI. Small Pulmonary Nodules: Evaluation With Repeat CT–preliminary Experience. Radiology (1999) 212:561–6. 10.1148/radiology.212.2.r99au33561 [DOI] [PubMed] [Google Scholar]
- 15. Perlmutt LM, Braun SD, Newman GE, Oke EJ, Dunnick NR. Timing of Chest Film Follow-Up After Transthoracic Needle Aspiration. AJR Am J Roentgenol (1986) 146:1049–50. 10.2214/ajr.146.5.1049 [DOI] [PubMed] [Google Scholar]
- 16. Manhire A, Charig M, Clelland C, Gleeson F, Miller R, Moss H, et al. Guidelines for Radiologically Guided Lung Biopsy. Thorax (2003) 58:920–36. 10.1136/thorax.58.11.920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Christiansen IS, Clementsen PF, Bodtger U, Naur TMH, Pietersen PI, Laursen CB. Transthoracic Ultrasound-Guided Biopsy in the Hands of Chest Physicians - a Stepwise Approach. Eur Clin Respir J (2019) 6:1579632. 10.1080/20018525.2019.1579632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology (2017) 284:228–43. 10.1148/radiol.2017161659 [DOI] [PubMed] [Google Scholar]
- 19. Yang PC, Luh KT, Sheu JC, Kuo SH, Yang SP. Peripheral Pulmonary Lesions: Ultrasonography and Ultrasonically Guided Aspiration Biopsy. Radiology (1985) 155:451–6. 10.1148/radiology.155.2.3885310 [DOI] [PubMed] [Google Scholar]
- 20. Liao WY, Chen MZ, Chang YL, Wu HD, Yu CJ, Kuo PH, et al. US-Guided Transthoracic Cutting Biopsy for Peripheral Thoracic Lesions Less Than 3 Cm in Diameter. Radiology (2000) 217:685–91. 10.1148/radiology.217.3.r00dc21685 [DOI] [PubMed] [Google Scholar]
- 21. Sagar P, Gulati M, Gupta SK, Gupta S, Shankar S, Joshi K, et al. Ultrasound-Guided Transthoracic Co-Axial Biopsy of Thoracic Mass Lesions. Acta Radiol (Stockholm Sweden 1987) (2000) 41:529–32. 10.1080/028418500127346117 [DOI] [PubMed] [Google Scholar]
- 22. Diacon AH, Theron J, Schubert P, Brundyn K, Louw M, Wright CA, et al. Ultrasound-Assisted Transthoracic Biopsy: Fine-Needle Aspiration or Cutting-Needle Biopsy? Eur Respir J (2007) 29:357–62. 10.1183/09031936.00077706 [DOI] [PubMed] [Google Scholar]
- 23. Zhou X, Jiang P, Huan X, Li W, Chen Y, Gao H, et al. Ultrasound-Guided Versus Thoracoscopic Pleural Biopsy for Diagnosing Tuberculous Pleurisy Following Inconclusive Thoracentesis: A Randomized, Controlled Trial. Med Sci Monit (2018) 24:7238–48. 10.12659/MSM.912506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Capalbo E, Peli M, Lovisatti M, Cosentino M, Mariani P, Berti E, et al. Trans-Thoracic Biopsy of Lung Lesions: FNAB or CNB? Our Experience and Review of the Literature. Radiol Med (2014) 119:572–94. 10.1007/s11547-013-0360-1 [DOI] [PubMed] [Google Scholar]
- 25. Yeow K-M, Su IH, Pan K-T, Tsay P-K, Lui K-W, Cheung Y-C, et al. Risk Factors of Pneumothorax and Bleeding: Multivariate Analysis of 660 CT-guided Coaxial Cutting Needle Lung Biopsies. Chest (2004) 126:748–54. 10.1378/chest.126.3.748 [DOI] [PubMed] [Google Scholar]
- 26. Jeon KN, Bae K, Park MJ, Choi HC, Shin HS, Shin S, et al. US-Guided Transthoracic Biopsy of Peripheral Lung Lesions: Pleural Contact Length Influences Diagnostic Yield. Acta Radiol (2014) 55:295–301. 10.1177/0284185113494984 [DOI] [PubMed] [Google Scholar]
- 27. Geraghty PR, Kee ST, McFarlane G, Razavi MK, Sze DY, Dake MD. CT-Guided Transthoracic Needle Aspiration Biopsy of Pulmonary Nodules: Needle Size and Pneumothorax Rate. Radiology (2003) 229:475–81. 10.1148/radiol.2291020499 [DOI] [PubMed] [Google Scholar]
- 28. Tai R, Dunne RM, Trotman-Dickenson B, Jacobson FL, Madan R, Kumamaru KK, et al. Frequency and Severity of Pulmonary Hemorrhage in Patients Undergoing Percutaneous CT-Guided Transthoracic Lung Biopsy: Single-Institution Experience of 1175 Cases. Radiology (2016) 279:287–96. 10.1148/radiol.2015150381 [DOI] [PubMed] [Google Scholar]
- 29. Huo YR, Chan MV, Habib AR, Lui I, Ridley L. Pneumothorax Rates in CT-Guided Lung Biopsies: A Comprehensive Systematic Review and Meta-Analysis of Risk Factors. Br J Radiol (2020) 93:20190866. 10.1259/bjr.20190866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heerink WJ, de Bock GH, de Jonge GJ, Groen HJM, Vliegenthart R, Oudkerk M. Complication Rates of CT-guided Transthoracic Lung Biopsy: Meta-Analysis. Eur Radiol (2017) 27:138–48. 10.1007/s00330-016-4357-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors. Further inquiries can be directed to the corresponding author.