Abstract
Older adults walk with greater metabolic energy consumption than younger for reasons that are not well understood. We suspect that a distal-to-proximal redistribution of leg muscle demand, from muscles spanning the ankle to those spanning the hip, contributes to greater metabolic energy costs. Recently, we found that when younger adults using biofeedback target smaller than normal peak propulsive forces (FP), they do so via a similar redistribution of leg muscle demand during walking. This alludes to an experimental paradigm that emulates characteristics of elderly gait independent of other age-related changes relevant to metabolic energy cost. Thus, our purpose was to quantify the metabolic and limb- and joint-level mechanical energy costs associated with modulating propulsive forces during walking in younger adults. Walking with larger FP increased net metabolic power by 47% (main effect, p=0.001), which was accompanied by small by relatively uniform increases in hip, knee, and ankle joint power and which correlated with total joint power (R2=0.151, p=0.019). Walking with smaller FP increased net metabolic power by 58% (main effect, p<0.001), which was accompanied by higher step frequencies and increased total joint power due to disproportionate increases in hip joint power. Increases in hip joint power when targeting smaller than normal FP accounted for more than 65% of the variance in the measured changes in net metabolic power. Our findings suggest that walking with a diminished push-off exacts a metabolic penalty because of higher step frequencies and more total limb work due to an increased demand on proximal leg muscles.
Keywords: Biomechanics, Oxygen Consumption, Aging, Elderly, Fatigue
1. Introduction
Compared to younger adults walking at the same speed, older adults exhibit ~20% greater metabolic energy costs for reasons that are poorly understood (Ortega and Farley, 2007). Simultaneously, older adults exert smaller peak anterior (i.e., propulsive) ground reaction forces (FP) and exhibit higher step frequencies and a characteristic distal-to-proximal redistribution of leg muscle demand (Browne and Franz, 2017; Cofré et al., 2011; DeVita and Hortobagyi, 2000; Franz and Kram, 2014; Kerrigan et al., 1998). Specifically, older adults tend to rely more on hip muscle-tendon units (MTU) for power generation with each step (DeVita and Hortobagyi, 2000), which likely serves to compensate for reduced mechanical output from their plantarflexor MTUs. Similar compensatory patterns are also evident in gait pathologies such as in Parkinson’s Disease (Skinner et al., 2015) and following stroke (Farris et al., 2015). We suspect that more proximal leg muscles, with longer fascicles and shorter tendons, are less economical for powering the mechanical demands of walking than the plantarflexor muscles with shorter fascicles and longer tendons (Farris et al., 2015; Franz, 2016; Huang et al., 2015; Zelik et al., 2014). Accordingly, a distal-to-proximal redistribution of leg muscle demand may contribute to increased metabolic energy cost. However, no empirical evidence exists to support this premise.
We are not the first to investigate the veracity of specific biomechanical explanations for the greater metabolic energy cost of walking in older adults. We know that the positive mechanical work used to support and accelerate the body’s center of mass is closely related to whole-body metabolic energy cost during bipedal locomotion (Donelan et al., 2001; Donelan et al., 2002a). However, Ortega and Farley (2007) found no age-related difference in total stance phase positive mechanical work, despite greater metabolic costs in older adults (Ortega and Farley, 2007). Those authors subsequently proposed an alternative explanation - an increased metabolic cost of lateral stability. Although plausible, they measured no difference in the change in metabolic energy cost between young and older adults when the demands for lateral stability were alleviated via lateral support (Ortega et al., 2008). Older adults do walk with greater levels of antagonist muscle coactivation than young adults - a neuromuscular consequence of aging that may serve to increase joint stiffness and stability (Franz and Kram, 2013; Hortobagyi et al., 2011; Mian et al., 2006; Peterson and Martin, 2010). Hortobagyi et al. (Hortobagyi et al., 2011) found that approximately one-third of the greater metabolic energy cost of walking in older adults can be explained by increased activation of antagonistic leg muscles. However, a significant amount of the variance in the greater metabolic energy cost in older adults is left unexplained.
The distal-to-proximal redistribution of leg muscle demand is one of the most common observations in studies of age-related differences in walking biomechanics. Although some exceptions do exist (Lim et al., 2013), reduced ankle joint kinetics in older adults are routinely accompanied by increases in leading leg hip extensor moment, angular impulses, and/or power generation in early stance (DeVita and Hortobagyi, 2000; Savelberg et al., 2007; Silder et al., 2008) and an increase in trailing leg hip flexor moments, angular impulses, and/or power generation during swing initiation (Cofré et al., 2011; Judge et al., 1996; Kerrigan et al., 1998). But, the extent to which these changes in joint power generation and the greater demand on proximal muscles contributes to greater metabolic energy cost has yet to be systematically explored. Indirect evidence from Huang et al. (2015) reveals that experimental reductions in ankle power output in walking are accompanied by larger mechanical output from proximal leg muscles and a dramatic increase in metabolic energy cost (Huang et al., 2015). However, while providing some support for these cause-and-effect relations, that study restricted ankle joint rotation and elicited exaggerated flexion across all leg joints which may confound our interpretations.
A major challenge for testing the basis of individual factors potentially explaining increased metabolic energy cost in older adults is the multi-factorial nature of age-related changes relevant to walking economy. One potential way to overcome such challenges is to use an experimental manipulation that, when applied in younger adults, elicits biomechanical changes representative of elderly gait. We recently used a real-time biofeedback paradigm to reveal that younger adults targeting smaller than normal FP do so by redistributing demand from distal to proximal leg muscles (Browne and Franz, 2017). Conversely, when targeting larger than normal FP, younger adults increase stance phase ankle, knee, and hip joint work proportionally. Similar experimental paradigms can be extended to study how altered patterns of joint power generation contribute to increased metabolic energy cost in the absence of other age-related changes.
Therefore, our purpose was to quantify the metabolic and limb- and joint-level mechanical energy costs associated with modulating propulsive forces during walking in young adults. We first hypothesized that when walking with larger than normal FP, metabolic energy costs would increase in a manner explained by (i.e., accompanied by) proportional increases hip, knee, and ankle joint positive leg joint work. Conversely, second, we hypothesized that when walking with smaller than normal FP, metabolic energy costs would increase in a manner explained by (i.e., accompanied by) a redistribution to muscles spanning the hip for power generation. We would interpret support for this final hypothesis as evidence that the distal-to-proximal redistribution during walking, a hallmark biomechanical feature of elderly gait, exacts a metabolic penalty. We include joint angular impulses as a complementary measure of cumulative muscle demand considered relevant in associating the mechanics and energetics of walking (Helseth et al., 2008). Moreover, because of the important interplay between positive and negative mechanical work during walking, we include both in our attempt to present a more comprehensive description of biofeedback-induced changes to motivate continued study.
2. Methods
2.1. Participants
Twelve unimpaired young adults (8F/4M) participated after providing written, informed consent according to the University of North Carolina Biomedical Sciences IRB. Subjects had a mean ± standard deviation age of 23±3 years, height of 1.74±0.12 m, body mass of 74.7±14.3 kg, and preferred overground walking speed of 1.37±0.15 m/s. Subjects were free of neurologic impairments and had no musculoskeletal injuries within the prior 6 months.
2.2. Experimental Protocol
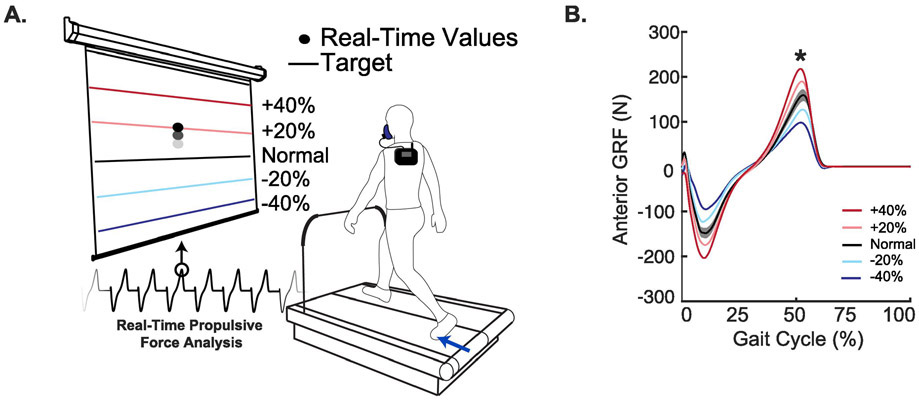
Using a photo cell timing system, we determined each subjects’ preferred overground walking speed (i.e., PWS) as the average of 3 times taken to walk 30 m. After completing a 5-minute standing trial (details below), subjects walked on a dual-belt, force-measuring treadmill (Bertec, Columbus, OH) for 5 minutes at their PWS while we collected ground reaction force (GRF) data using a custom Matlab (Mathworks, Natick, MA) script. We identified individual steps using a 20 N vertical GRF threshold as a reference for heel-strikes. For each step, we recorded the peak anterior (i.e., propulsive) ground reaction force (FP) and the trial average was determined to be the subject’s habitual FP. Using another custom Matlab script, subjects viewed their step-by-step FP as a dot projected on a screen in front of the treadmill and completed an exploration trial of at least 3 minutes to become familiar with the biofeedback paradigm. To elicit naturally emerging biomechanical patterns associated with modulating FP, we explained the timing of push-off and that if they pushed harder or softer with their foot, the dot would rise or fall, respectively. Subjects then completed a series of trials where they were asked to match their instantaneous FP data to targets representing ±20%, and ±40% of their habitual value in randomized order (Fig. 1). Subjects also targeted their own habitual FP in this randomized order to test the effects of biofeedback alone. Subjects were given at least 2 minutes of seated rest between conditions.
Figure 1.
(A) Real-time peak anterior ground reaction force (FP) biofeedback paradigm. Subjects walked on a dual-belt treadmill while receiving visual feedback of their FP on a step-by-step basis. A custom Matlab script displayed the participant’s ground reaction force on a screen in front of the participant while they walked. (B) Group average FP stride profiles for each biofeedback trial are shown. Asterisks (*) represent significant main effects of speed on reduced FP.
2.3. Measurement and Analysis
A 16-camera motion capture system (Motion Analysis Corporation, Santa Rosa, CA) operating at 100 Hz recorded pelvis and lower extremity kinematics via 17 anatomical markers and an additional 14 tracking markers affixed using rigid clusters. A standing trial, as well two unilateral hip circumduction trials used to estimate functional hip joint centers (Piazza et al., 2001), also included markers placed on the left and right medial knee and malleoli. During all trials, subjects wore a portable metabolic system (COSMED, Rome, Italy) that collected oxygen consumption () and carbon dioxide production () on a breath-by-breath basis. The flow rates were normalized to body mass (i.e., mL*kg−1*min−1).
For all outcomes, we analyzed the last 2 minutes of each 5 minute trial (Hortobagyi et al., 2011). First, we calculated net (i.e., walking-standing) rates of oxygen consumption and used standard procedures to estimate net metabolic power (Brockway, 1987). Marker trajectories and GRF data were filtered using 4th order low-pass Butterworth filters with cutoff frequencies of 6 Hz and 20 Hz, respectively. We used the static standing calibration and functional hip joint centers to scale a seven segment, 18 degree-of-freedom model of the pelvis and right and left legs (Arnold et al., 2010). We used the filtered marker and force data to estimate joint angles, moments, and powers of the lower limb joints using an inverse dynamics routine described in detail previously (Browne and Franz, 2019). We extracted our kinetic and kinematic outcome measures of interest from the bilateral, stride-averaged profiles. Positive and negative joint work and angular impulses were calculated by integrating the respective area under the joint power and moment profiles, respectively. We normalized those values by average stride time for each trial to estimate the cumulative muscular demand per unit time. Accordingly, we report average mechanical powers (i.e., work rates) in W/kg and angular impulses in Nm/kg/s. Finally, we calculated total leg joint power and angular impulse as the sum of the respective ankle, knee, and hip joint values. Negative joint work and angular impulse outcomes are summarized in our appendix.
2.4. Statistical Analysis
Shapiro-Wilks tests confirmed normal distributions. A paired sample t-test evaluated the effects of biofeedback alone (i.e., targeting normal FP) on metabolic cost. We used a repeated-measures analysis of variance (ANOVA) to test for significant main effects of biofeedback on FP; net metabolic power; and stance phase hip, knee, and ankle joint powers and angular impulses. In addition, we calculated Pearson correlations between positive joint powers and net metabolic power. Consistent with our hypotheses, statistical tests were performed separately for targeting larger than normal FP (i.e., Norm, +20%, +40%) and targeting smaller than normal FP (i.e., Norm, −20%, −40%). When a significant main effect was found, we focused pairwise post-hoc comparisons versus normal walking values. Here, we defined significance using a Bonferroni-adjusted level of significance to account for those two comparisons (α=0.025). We report effect sizes as partial eta square and Cohen’s d for the ANOVA and t-tests, respectively, and interpret 0.2, 0.5, and 0.8 effect sizes as small, medium, and large, respectively (Cohen, 1988).
3. Results
3.1. Targeting larger than normal peak propulsive forces
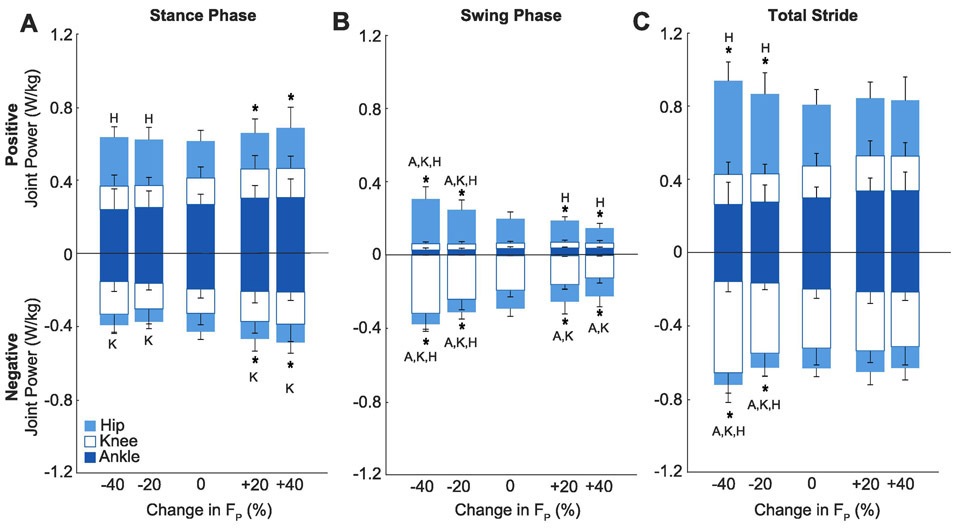
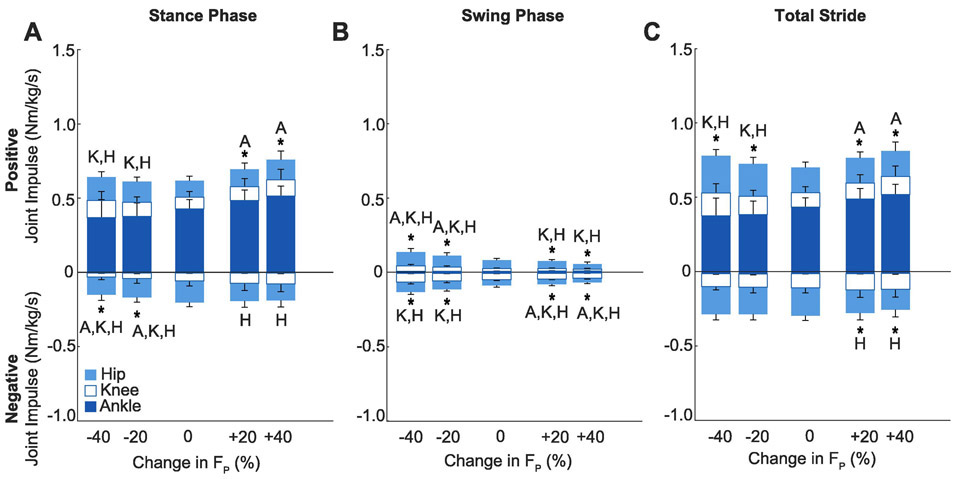
Subjects walked normally with a net metabolic cost of 3.92±0.87 W/kg, which was invariant when targeting their normal FP via biofeedback (p=0.990, d<0.01; Fig. 2). Compared to that when walking normally, net metabolic power increased by an average of up to 47% (+1.83 W/kg) when targeting larger than normal FP (main effect, p<0.001, ηp2=0.93; Fig. 2). During stance, this metabolic response was accompanied by up to an 13% increase in total positive leg joint power (main effect, p=0.017, ηp2=0.31; Fig. 3) and increases in positive angular impulse at the ankle and in total (main effects, p<0.001, ηp2≥0.60; Fig. 4). During leg swing, positive leg joint power decreased at the hip and in total compared to walking normally (main effects, p<0.001, ηp2≥0.57; Fig. 3), as did positive hip, knee, and total joint angular impulses (main effects, p<0.001, ηp2≥0.59; Fig. 4). Over the entire stride, we found no significant changes in positive or negative joint powers when targeting larger than normal FP (main effects, p≥0.082, ηp2≤0.20; Fig. 3). However, over the same period, positive angular impulse at the ankle in total increased significantly compared to walking normally (main effects, p≤0.003, ηp2≥0.52). Subjects also decreased their stride frequency by up to 10% compared to walking normally (Table 2).
Figure 2.
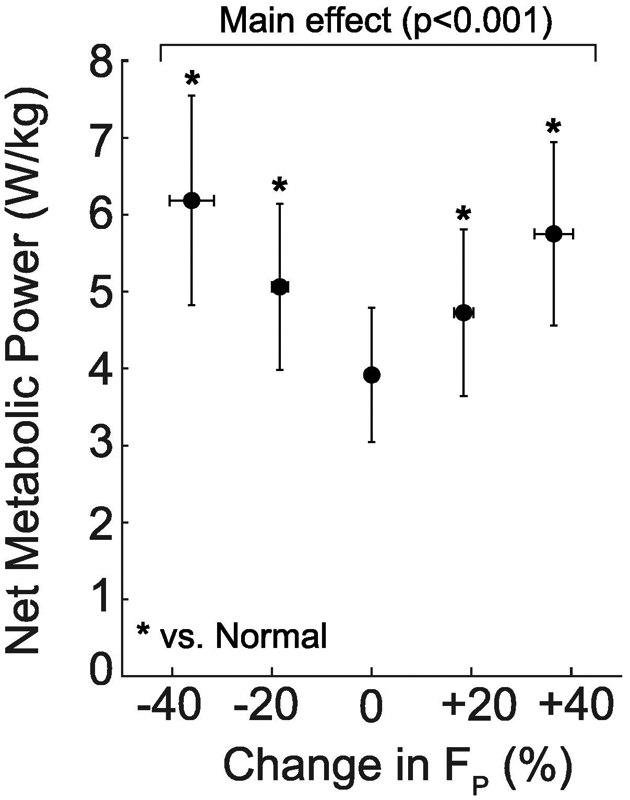
Group average (standard deviation) net metabolic power. Conditions included biofeedback trials at targeted −40%, −20%, 0%, +20%, +40% peak trailing limb propulsion (FP). Asterisks (*) indicate a significant pairwise comparison versus normal walking (p<0.05).
Figure 3.
Group average (standard deviation) positive and negative joint powers (i.e., work rates) for the ankle, knee, and hip. Data were calculated from the time integral of the instantaneous power curves normalized by average stance time to estimate the cumulative muscular demand per unit time. Conditions included biofeedback trials at targeted −40%, −20%, 0%, +20%, +40% peak trailing limb propulsion (FP). Letters represent a significant pairwise comparison from the normal walking condition (p<0.05) for ankle (A), knee (K), and hip (H). Asterisks (*) indicate a significant pairwise comparison for total (i.e., Ankle + Knee + Hip) joint power versus normal walking (p<0.05).
Figure 4.
Group average (standard deviation) positive and negative joint angular impulse. Conditions included biofeedback trials at targeted −40%, −20%, 0%, +20%, +40% peak trailing limb propulsion (FP). Letters represent a significant pairwise comparison from the normal walking condition (p<0.05) for ankle (A), knee (K), and hip (H). Asterisks (*) indicate a significant pairwise comparison for total (i.e., Ankle + Knee + Hip) angular impulse versus normal walking (p<0.05).
Table 2.
Average stride length and frequency across experimental conditions
| Experimental Condition | |||||
|---|---|---|---|---|---|
| −40% | −20% | Norm | 20% | 40% | |
| Stride Length (m) | 1.07 | 1.22 | 1.41 | 1.47 | 1.59 |
| Stride Frequency (Hz) | 1.29 | 1.13 | 0.97 | 0.94 | 0.87 |
3.2. Targeting smaller than normal peak propulsive forces
Conversely, when targeting smaller than normal FP, net metabolic power increased by up to 58% (2.26 W/kg) (main effect, p<0.001, ηp2=0.58; Fig. 2) but without accompanying changes in total positive leg joint power (main effect, p=0.693, ηp2=0.02; Fig. 3) or total positive leg joint angular impulse (main effect, p=0.37, ηp2=0.09, Fig.4) during the stance phase. Rather, this metabolic response was accompanied by up to an 31% increase in positive stance phase hip joint power (main effect, p=0.001, ηp2=0.58; Fig. 3) and significant increases in positive stance phase angular impulses at the knee and hip (main effect, p≤0.015, ηp2≥0.32; Fig. 4). During leg swing, positive leg joint power increased at the hip and in total (main effects, p≤0.001, ηp2≥0.83, Fig. 3) but decreased at the ankle and knee (main effects, p≤0.039, ηp2≥0.25, Fig. 3). Conversely, all positive joint angular impulse outcomes increased during leg swing compare to walking normally (main effect, p≤0.012, ηp2≥0.33; Fig. 4). Over the entire stride, positive leg joint power (main effects, p≤0.022, ηp2≥0.43, Fig. 3) and positive angular impulse (main effect, p≤0.029, ηp2≥0.34; Fig. 4) increased significantly in total, due to disproportionate increases at the hip. Subjects also increased their stride frequency by up to 33% (Table 2).
3.3. Correlations between joint mechanics and metabolic outcomes.
When targeting larger than normal FP, net metabolic power exhibited a modest but significant correlation with total limb positive power (R2=0.151, p=0.019) due in large part to positive correlations during stance (R2=0.216, p=0.004) (Table 1). Conversely, when targeting smaller than normal FP, net metabolic power exhibited moderate to strong correlations with hip (R2=0.652, p<0.001) and total limb (R2=0.436, p<0.001) positive power due to positive correlations during both during stance and leg swing (Table 1).
Table 1.
Correlations Between Mechanics and Metabolic Outcomes
| Stance | Swing | Total Stride | ||||
|---|---|---|---|---|---|---|
| Targeting Larger FP | R2 | P-Value | R2 | P-Value | R2 | P-Value |
| Total | 0.216 | 0.004 | <0.001 | 0.879 | 0.151 | 0.019 |
| Hip | 0.141 | 0.024 | 0.009 | 0.574 | 0.078 | 0.100 |
| Knee | 0.028 | 0.326 | 0.005 | 0.684 | 0.021 | 0.405 |
| Ankle | 0.068 | 0.125 | 0.212 | 0.005 | 0.087 | 0.08 |
| Targeting Smaller FP | R2 | P-Value | R2 | P-Value | R2 | P-Value |
| Total | 0.255 | 0.002 | 0.618 | <0.001 | 0.436 | <0.001 |
| Hip | 0.403 | <0.001 | 0.684 | <0.001 | 0.652 | <0.001 |
| Knee | 0.002 | 0.795 | 0.029 | 0.318 | <0.001 | 0.999 |
| Ankle | 0.102 | 0.057 | 0.024 | 0.368 | 0.101 | 0.059 |
FP: Propulsive Forces
4. Discussion
Reduced ankle joint moment and power generation, for example due to aging , are routinely accompanied by higher step frequencies and compensatory increases in leading leg hip extensor moment, angular impulses, and/or power generation in early stance (DeVita and Hortobagyi, 2000; Savelberg et al., 2007; Silder et al., 2008) and an increase in trailing leg hip flexor moments, angular impulses, and/or power generation during swing initiation (Cofré et al., 2011; Judge et al., 1996; Kerrigan et al., 1998). Consistent with our first hypothesis, walking with larger than normal FP increased net metabolic power in a manner consistent with small but relatively uniform increases in hip, knee, and ankle joint positive power generation. Moreover, in support of the important role that ankle extensor muscles play in generating push-off power, we found that walking with larger than normal FP increased positive ankle and thus total joint angular impulses. Consistent with our second hypothesis, we found that when walking with smaller than normal FP, subjects increased net metabolic power with increases in total limb power due to disproportionate increases in positive hip joint power and positive hip and knee joint angular impulses. Stride-average profiles suggest that the timing of those more proximal effects coincided with that of diminished peak ankle power contributions to push-off and swing initiation. Indeed, increases in hip joint mechanical power when targeting smaller than normal FP accounted for more than 65% of the variance in the measured changes in net metabolic power. Ultimately, we interpret our outcomes to suggest that walking with a diminished push-off exacts a metabolic penalty because of higher step frequencies and more total limb work due to an increased demand on proximal leg muscles.
Our metabolic measurements during normal walking at an average speed of 1.37 m/s agree with published values (Donelan et al., 2001; Donelan et al., 2002b). Moreover, net metabolic power was at its minimum when subjects walked normally – namely, when producing their preferred FP magnitude while walking at their preferred speed. Humans are known to walk at their preferred speed with a combination of step lengths, frequencies, and widths that minimize net metabolic energy cost (Bertram, 2005; Bertram and Ruina, 2001; Donelan et al., 2001; Sutherland, 1997). We add that metabolic cost may also be shaped by modulating propulsive force in younger adults. Furthermore, and perhaps surprisingly, the increase in net metabolic power when targeting smaller than normal FP was comparable, if not modestly greater in magnitude than that when targeting larger FP.
The biomechanical mechanisms by which subjects increased their net metabolic power differed between targeting larger and smaller than usual FP. Consistent with our previously published work (Browne and Franz, 2018), targeting larger than normal FP elicited significant and predictable increases in total stance phase positive leg joint power and, presumably because of the fixed treadmill speed, in negative knee joint power. Those changes were accompanied by larger positive (i.e., extensor) ankle and positive total leg joint angular impulses. These positive joint angular impulse changes are comparable to those reported by Lewis and Ferris (2008), who asked participants to increase push-off intensity via verbal encouragement (Lewis and Ferris, 2008). During leg swing, joint powers and angular impulses decreased in a manner consistent with those reported by Lewis and Ferris (2008) and which surprisingly served to offset the contribution of stance phase increases to stride-averaged values. These findings underscore the complex associations between walking mechanics and energetics, and may arise from differences in the metabolic cost of operating muscles during phases of isometric, eccentric, or concentric action. Ultimately, at least from a joint mechanics perspective, the metabolic cost of walking with larger than normal FP appears relatively well shared among muscles spanning the hip, knee, and ankle. This interpretation is also supported by our finding that, only total limb mechanical power rather than that at individual joints correlated with net metabolic power when walking with larger than normal FP.
Although walking with smaller than normal FP elicited a similar whole-body metabolic response to that of walking with larger than normal FP, doing so had a different biomechanical basis at the limb and joint levels. Our data show that younger adults walk with smaller than normal FP with higher step frequencies and a disproportionate demand on muscle-tendon units spanning the hip, in particular during the periods of push-off and swing initiation. We know that older adults often compensate for insufficient ankle push-off power not only via increased demand on contralateral hip extensors during early stance, but also on ipsilateral hip flexors to facilitate swing initiation with concomitant increases in step frequencies (Cofré et al., 2011). Compared to walking normally, subjects that walked with smaller FP were compelled to increase positive hip joint power and thus total joint power along with positive hip, knee, and total joint angular impulses – changes that were contrasted by more modest reductions at the ankle that did not reach significance. Thus, the higher step frequencies and greater mechanical output from more proximal leg muscles to power walking with smaller than normal FP provide likely explanations for the measured and significant increases in whole-body net metabolic power. First, it is well established that the rate of cyclic force generation that would accompany higher step frequencies can increase metabolic cost (Doke et al., 2005). Second, we (Fickey et al., 2018) and others before us (Farris et al., 2015; Huang et al., 2015; Roberts et al., 1997; Zelik et al., 2014) have suspected that differences in anatomical architecture between muscle-tendon units spanning the ankle and those spanning the hip could play a role in the economy of force generation within the leg. Architecturally, the plantarflexor MTUs have relatively short muscle fascicles and long series elastic tendons. The Achilles tendon, for example, exhibits substantial elongation during the stance phase, which facilitates the storage and return of elastic energy as well as economical, relatively isometric muscle action during force generation (Ishikawa et al., 2005). Conversely, MTUs spanning the hip have relatively longer fascicles and shorter series elastic tendons. Those muscles are likely to undergo larger fascicle excursions while performing greater positive mechanical work and less economical muscle actions with a greater ATP utilization to produce a given force (Huang et al., 2015; Roberts et al., 1997). Because a redistribution to more proximal leg muscles in older adults can present even in older adults that walk with step frequencies indistinguishable from those in young adults, these anatomical differences between lower limb MTUs provided at least some of the motivation for our hypotheses and certainly guided our interpretations. However, disproportionate increases in hip joint power output also elicited larger total limb mechanical power for these conditions. Thus, we are unable to objectively partition the extent to which these anatomical differences serve to explain the observed results in this study.
Individuals who habitually walk with diminished push-off intensity walk with higher step frequencies and disproportionate demand on proximal leg muscles for powering locomotion, both of which are accompanied by higher metabolic costs. Indeed, older adults and many individuals with gait pathology (e.g., Parkinson’s, stroke) exhibit greater metabolic energy costs during walking that reduce independence and quality of life (Farris et al., 2015; Ortega and Farley, 2007). Biomechanically, compared to young adults or healthy controls who exhibit an effective and robust ankle push-off, older adults also often exhibit higher step frequencies and an increased demand on more proximal leg muscles for push-off and swing initiation during walking (Cofré et al., 2011; DeVita and Hortobagyi, 2000; Judge et al., 1996; Kerrigan et al., 1998; Silder et al., 2008). Accordingly, our results should inspire continued research into the independent effects of disproportionately operating those proximal leg muscles on metabolic energy cost. Though, such efforts may benefit from inclusion of paradigms that also control for step frequency.
There are several opportunities for continued study. For example, it is not possible to quantify individual muscle metabolic energy costs in human subjects during functional activities such as walking. Moreover, integrating joint power curves has been shown to underestimate mechanical power at the muscle-tendon unit level (Sasaki et al., 2009). Fortunately, advancements in musculoskeletal modeling and bioenergetic simulation (Song and Geyer, 2018; Umberger and Rubenson, 2011) can be leveraged to overcome these challenges. In addition, the accumulation of research implicating the distal-to-proximal redistribution as a basis for greater metabolic energy costs during walking could inform the development and targeted prescription of wearable assistive technologies (e.g., exoskeletons). Moreover, biofeedback systems, such as that used here, have translational potential through the use of wearable sensors for daily use. Indeed, Browne and Franz (2019) found that use of ankle power biofeedback can reverse the distal-to-proximal redistribution in older adults; which lends itself well to low-cost sensor solutions (Pieper et al., 2020).
We acknowledge several limitations. First, we did not ask participants to fast prior to their laboratory visit, and eating can alter metabolic cost. However, those effects peak roughly 60 minutes after consumption (Visser et al., 1995), a time period surpassed for all subjects prior to data collection. The randomized trial order in our repeated-measures design further mitigated those effects. We also report effects in younger adults which controlled for other age- and disease-related factors known to influence metabolic cost. Nevertheless, it is unclear whether our results will generalize to other cohorts. Analogous measurements in older adults are fully warranted. Another limitation is our lack of electromyographic recordings. Muscle activity would have provided supplementary evidence for changes relevant to metabolic cost.
Taken together, our experimental manipulations and metabolic measurements in young adults provide novel biomechanical insight to explain associations between walking with altered push-off intensity and changes in metabolic energy cost. With specific translational implications for older adults or those with gait pathology, our findings suggest that walking with a diminished push-off exacts a metabolic penalty because of higher step frequencies and more total limb work due to increased demand on proximal leg muscles. Ultimately, this research has the potential to inform the design of physical therapy interventions and the prescription of targeted wearable assistive devices to reduce metabolic energy cost and thus enhance independence and quality of life.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01AG058615) and the Parkinson’s Foundation (PF-VSA-SFW-1908).
APPENDIX
The following summarized our findings for negative joint powers and angular impulses. Because of the important interplay between positive and negative mechanical work during walking, we include both in our attempt to present a more comprehensive description of biofeedback-induced changes to motivate continued study.
Targeting larger than normal peak propulsive forces
Negative joint powers and angular impulse outcomes generally mirrored changes in their respective positive power and angular impulse outcomes. Compared to that during the stance phase of normal walking, we found significant increases in negative joint powers at the knee and in total (main effects, p≤0.022, ηp2≥0.29; Fig. 3) and significant decreases in negative hip joint angular impulse (main effect, p<0.001, ηp2≤0.50; Fig. 4). During leg swing, negative joint powers at the ankle, knee, and in total decreased compared to normal walking (main effects, p≤0.029, ηp2≥0.28; Fig. 3), as did all negative angular impulse outcomes (main effect, p≤0.040, ηp2≥0.25). Over the entire stride, negative hip and total limb angular impulse decreased compared to walking normally (main effect, p≤0.005, ηp2≥0.46). Joint moment and power curves across all conditions from which these joint mechanics were derived are presented in Figure 5.
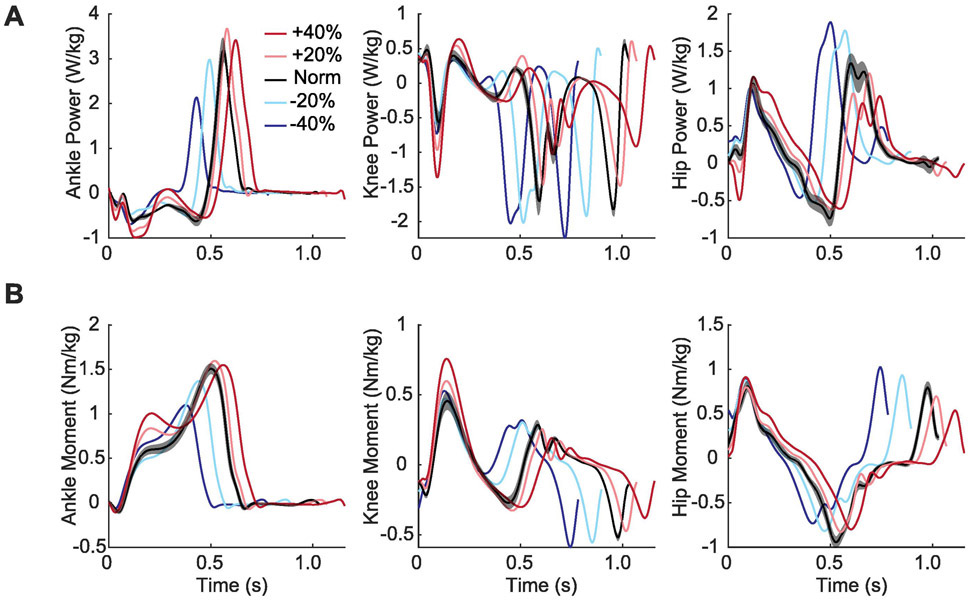
Figure 5.
Group average profiles for (A) joint power generation and (B) joint moments for each experimental condition plotted across an averaged gait cycle, from heel-strike to heel-strike. Conditions included biofeedback trials at targeted −40%, −20%, 0%, +20%, +40% peak trailing limb propulsion values in comparison to normal walking.
Targeting smaller than normal peak propulsive forces
Compared to those when targeting larger than normal FP, negative joint power and negative angular impulse responses to walking with smaller than normal FP were more varied and summarized in Figures 3 and 4 and in our supplementary data document.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold EM, Ward SR, Lieber RL, Delp SL, 2010. A model of the lower limb for analysis of human movement. Annals of biomedical engineering 38, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram JEA, 2005. Constrained optimization in human walking: cost minimization and gait plasticity. Journal of Experimental Biology 208, 979–991. [DOI] [PubMed] [Google Scholar]
- Bertram JEA, Ruina A, 2001. Multiple Walking Speed-frequency Relations are Predicted by Constrained Optimization. Journal of theoretical biology 209, 445–453. [DOI] [PubMed] [Google Scholar]
- Brockway JM, 1987. Derivation of formulae used to calculate energy expenditure in man. Hum Nutr Clin Nutr 41, 463–471. [PubMed] [Google Scholar]
- Browne M, Franz JR, 2017. The independent effects of speed and propulsive force on joint power generation in walking. Journal of biomechanics 55, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne M, Franz JR, 2018. More push from your push-off: Joint-level modifications to modulate propulsive forces in old age. PloS one 13, e0201407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne MG, Franz JR, 2019. Ankle power biofeedback attenuates the distal-to-proximal redistribution in older adults. Gait & posture 71, 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofré LE, Lythgo N, Morgan D, Galea MP, 2011. Aging modifies joint power and work when gait speeds are matched. Gait & Posture 33, 484–489. [DOI] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical power analysis for the behavioral sciences, 2nd ed. Erlbaum Associates L, Hillsdale NJ [Google Scholar]
- DeVita P, Hortobagyi T, 2000. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol 88, 1804–1811. [DOI] [PubMed] [Google Scholar]
- Doke J, Donelan JM, Kuo AD, 2005. Mechanics and energetics of swinging the human leg. Journal of Experimental Biology 208, 439–445. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Kram R, Arthur D K, 2001. Mechanical and metabolic determinants of the preferred step width in human walking. Proceedings of the Royal Society of London. Series B: Biological Sciences 268, 1985–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelan JM, Kram R, Kuo AD, 2002a. Mechanical work for step-to-step transitions is a major determinant of the metabolic cost of human walking. The Journal of experimental biology 205, 3717–3727. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Kram R, Kuo AD, 2002b. Simultaneous positive and negative external mechanical work in human walking. Journal of biomechanics 35, 117–124. [DOI] [PubMed] [Google Scholar]
- Farris D, Hampton A, Lewek MD, Sawicki GS, 2015. Revisiting the mechanics and energetics of walking in individuals with chronic hemiparesis following stroke: from individual limbs to lower limb joints. Journal of neuroengineering and rehabilitation 12, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickey SN, Browne MG, Franz JR, 2018. Biomechanical effects of augmented ankle power output during human walking. The Journal of experimental biology 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, 2016. The age-associated reduction in propulsive power generation in walking. Exercise and sport sciences reviews 44, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Kram R, 2013. How does age affect leg muscle activity/coactivity during uphill and downhill walking? Gait & posture 37, 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Kram R, 2014. Advanced age and the mechanics of uphill walking: a joint-level, inverse dynamic analysis. Gait & posture 39, 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helseth J, Hortobagyi T, Devita P, 2008. How do low horizontal forces produce disproportionately high torques in human locomotion? J Biomech 41, 1747–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortobagyi T, Finch A, Solnik S, Rider P, Devita P, 2011. Association Between Muscle Activation and Metabolic Cost of Walking in Young and Old Adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 66A, 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TP, Shorter KA, Adamczyk PG, Kuo AD, 2015. Mechanical and energetic consequences of reduced ankle plantarflexion in human walking. The Journal of experimental biology 218, 3541–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Komi PV, Grey MJ, Lepola V, Bruggemann GP, 2005. Muscle-tendon interaction and elastic energy usage in human walking. J Appl Physiol 99, 603–608. [DOI] [PubMed] [Google Scholar]
- Judge JO, Davis RB 3rd, Ounpuu S, 1996. Step length reductions in advanced age: the role of ankle and hip kinetics. The journals of gerontology. Series A, Biological sciences and medical sciences 51, M303–312. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ, 1998. Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Archives of physical medicine and rehabilitation 79, 317–322. [DOI] [PubMed] [Google Scholar]
- Lewis CL, Ferris DP, 2008. Walking with increased ankle pushoff decreases hip muscle moments. Journal of biomechanics 41, 2082–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YP, Lin YC, Pandy MG, 2013. Muscle function during gait is invariant to age when walking speed is controlled. Gait Posture 38, 253–259. [DOI] [PubMed] [Google Scholar]
- Mian OS, Thom JM, Ardigo LP, Narici MV, Minetti AE, 2006. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta physiologica 186, 127–139. [DOI] [PubMed] [Google Scholar]
- Ortega JD, Farley CT, 2007. Individual limb work does not explain the greater metabolic cost of walking in elderly adults. Journal of applied physiology 102, 2266–2273. [DOI] [PubMed] [Google Scholar]
- Ortega JD, Fehlman LA, Farley CT, 2008. Effects of aging and arm swing on the metabolic cost of stability in human walking. Journal of biomechanics 41, 3303–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DS, Martin PE, 2010. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait & posture 31, 355–359. [DOI] [PubMed] [Google Scholar]
- Piazza SJ, Okita N, Cavanagh PR, 2001. Accuracy of the functional method of hip joint center location: effects of limited motion and varied implementation. 34, 967–973. [DOI] [PubMed] [Google Scholar]
- Pieper NL, Lewek MD, Franz JR, 2020. Can shank acceleration provide a clinically feasible surrogate for individual limb propulsion during walking? Journal of Biomechanics 98, 109449. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Marsh RL, Weyand PG, Taylor CR, 1997. Muscular Force in Running Turkeys: The Economy of Minimizing Work. Science 275, 1113–1115. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Neptune RR, Kautz SA, 2009. The relationships between muscle, external, internal and joint mechanical work during normal walking. Journal of Experimental Biology 212, 738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelberg HHCM, Verdijk LB, Willems PJB, Meijer K, 2007. The robustness of age-related gait adaptations: Can running counterbalance the consequences of ageing? Gait & Posture 25, 259–266. [DOI] [PubMed] [Google Scholar]
- Silder A, Heiderscheit B, Thelen DG, 2008. Active and passive contributions to joint kinetics during walking in older adults. Journal of Biomechanics 41, 1520–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner JW, Lee HK, Roemmich RT, Amano S, Hass CJ, 2015. Execution of Activities of Daily Living in Persons with Parkinson Disease. Medicine & Science in Sports & Exercise 47, 1906–1912. [DOI] [PubMed] [Google Scholar]
- Song S, Geyer H, 2018. Predictive neuromechanical simulations indicate why walking performance declines with ageing. J Physiol 596, 1199–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D, 1997. The development of mature gait. Gait & Posture 6, 163–170. [Google Scholar]
- Umberger BR, Rubenson J, 2011. Understanding muscle energetics in locomotion: new modeling and experimental approaches. Exerc Sport Sci Rev 39, 59–67. [DOI] [PubMed] [Google Scholar]
- Visser M, Deurenberg P, Van Staveren WA, Hautvast JG, 1995. Resting metabolic rate and diet-induced thermogenesis in young and elderly subjects: relationship with body composition, fat distribution, and physical activity level. The American Journal of Clinical Nutrition 61, 772–778. [DOI] [PubMed] [Google Scholar]
- Zelik KE, Huang TW, Adamczyk PG, Kuo AD, 2014. The role of series ankle elasticity in bipedal walking. Journal of theoretical biology 346, 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.