Abstract
Introduction:
Tumor spread through air spaces (STAS) has prognostic significance in lung adenocarcinoma and squamous cell carcinoma. We sought to investigate the prognostic importance of STAS in lung neuroendocrine tumors (NETs).
Methods:
All tumor slides from patients with resected pathologic stage I-III lung NETs (overall, n=487; typical carcinoid [TC], n=299; atypical carcinoid [AC], n=38; large cell neuroendocrine carcinoma [LCNEC], n=93; small cell lung carcinoma [SCLC], n=57) treated between 1992 and 2012 were evaluated for presence of STAS. Cumulative incidence of recurrence (CIR) and lung cancer–specific cumulative incidence of death (LC-CID) were analyzed using a competing-risks approach.
Results:
STAS was identified in 26% of NETs (16% of TCs, 37% of ACs, 43% of LCNECs, and 46% of SCLCs). STAS was associated with distant metastasis as well as higher CIR and LC-CID in the overall cohort and the AC, LCNEC, and SCLC cohorts (owing to a small number of recurrences and deaths [<5], prognostic analysis was not performed in the TC cohort). In multivariable analysis stratified by stage, STAS was significantly associated with higher CIR (subhazard ratio [SHR], 2.85; 95% confidence interval [CI], 1.73–4.68 [p<0.001]) and LC-CID (SHR, 2.72; 95% CI, 1.57–4.70 [p<0.001]), independent of histologic subtype. STAS independently associated with CIR and LC-CID in the LCNEC cohort and LC-CID in the SCLC cohort.
Conclusions:
In patients with lung NETs, STAS is associated with early, distant metastasis and worse LC-CID. In patients with LCNEC or SCLC, STAS is an independent poor prognostic factor.
Keywords: Spread through air spaces (STAS), lung neuroendocrine tumor (NET), competing-risks analysis, recurrence, lung cancer–specific death
Introduction
Neuroendocrine tumors (NETs) of the lung constitute 15% to 25% of primary lung cancers.1–5 Lung NETs are classified into four histologic subtypes: typical carcinoid (TC), atypical carcinoid (AC), large cell neuroendocrine carcinoma (LCNEC), and small cell lung carcinoma (SCLC).5, 6 Although they share morphologic, immunohistochemical, molecular, and ultrastructural characteristics, the prognostic spectrum for the subtypes varies widely—it is accepted that TCs are low-grade, ACs are intermediate-grade, and LCNECs and SCLCs are high-grade NETs.5, 6 The treatment strategy for lung NETs depends on histologic subtype and tumor stage.7–9 Although mitotic rate has been shown to be prognostically important in within cohorts of AC,10 it has been very difficult to identify any histologic predictors of prognosis within the individual categories of SCLC11 and iLCNEC12, 13 so we sought to investigate whether STAS could be a prognostic factor in lung NET.
Spread through air spaces (STAS) is a newly identified pattern of tumor invasion, defined as tumor cells existing within air spaces in the lung parenchyma beyond the tumor edge.14 It has been demonstrated to have prognostic significance by multiple groups in lung adenocarcinoma,15–23 squamous cell carcinoma,17, 24, 25 and pleomorphic carcinoma.26 In addition, our group has recently shown that lobectomy is associated with better survival than sublobar resection in STAS-positive T1 lung adenocarcinomas.27 The importance of STAS in patients undergoing sublobar resection rather than lobectomy was also shown in an international multiinstitutional cohort from three continents.28 This has also been pointed out in a recent meta-analysis of over 3500 patients.22 Few studies have investigated STAS and its prognostic significance in patients with lung NETs.10, 29 To our knowledge, no study has investigated the prognostic significance of STAS in a comprehensive cohort of patients with lung NETs, including TC, AC, LCNEC, and SCLC.
In an attempt to identify whether STAS could be a clinically useful prognostic marker for lung NETs in general as well as within the individual NET histologic subtypes, we performed a comprehensive investigation of clinicopathologic prognostic factors in patients who underwent resection for lung NETs, focusing on the incidence and prognostic impact of STAS and on initial recurrence patterns following lung resection.
Materials and Methods
Patients
This retrospective study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSK; WA0219–09). The MSK Thoracic Surgery Service’s prospectively maintained lung cancer database was reviewed to identify consecutive patients surgically treated for pathologic stage I-III lung NETs between January 1, 1992, and December 31, 2012. Pathologic stage was based on the eighth edition of the American Joint Committee on Cancer Staging Manual.30 Exclusion criteria included positive surgical margin (R1 or R2), inadequate tissue available to review, and a diagnosis of combined histologic subtypes other than lung NET (e.g., SCLC with an adenocarcinoma component).
Histologic Evaluation
Tumors slides were reviewed by at least two experienced pathologists (R.G.A., W.D.T.) blinded to patient clinical outcomes, using an Olympus BX51 microscope (Olympus, Tokyo, Japan) with a standard 22-mm diameter eyepiece. Discrepancies in histologic evaluation between pathologists were later resolved by consensus using a multiheaded microscope.
All lung NETs were reviewed and classified into one of four histologic subtypes (TC, AC, LCNEC, and SCLC) in accordance with the 2015 World Health Organization classification criteria: number of mitotic figures per 2 mm2, presence of necrosis, and cytologic features.5 Ki-67 staining was performed on tissue microarray blocks using the standard avidin-biotin complex peroxidase technique (clone MIB-1 [Immunotech, Westbrook, ME] diluted at 1:100).
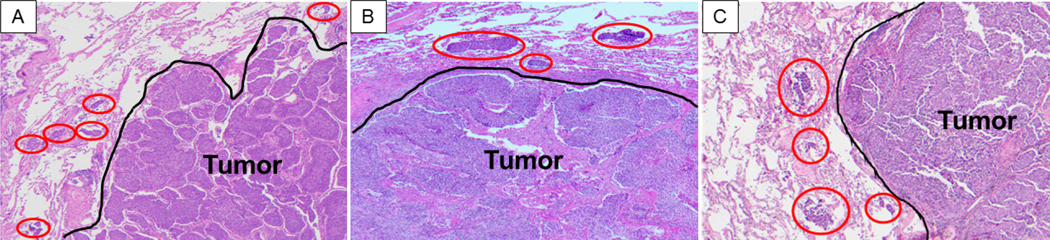
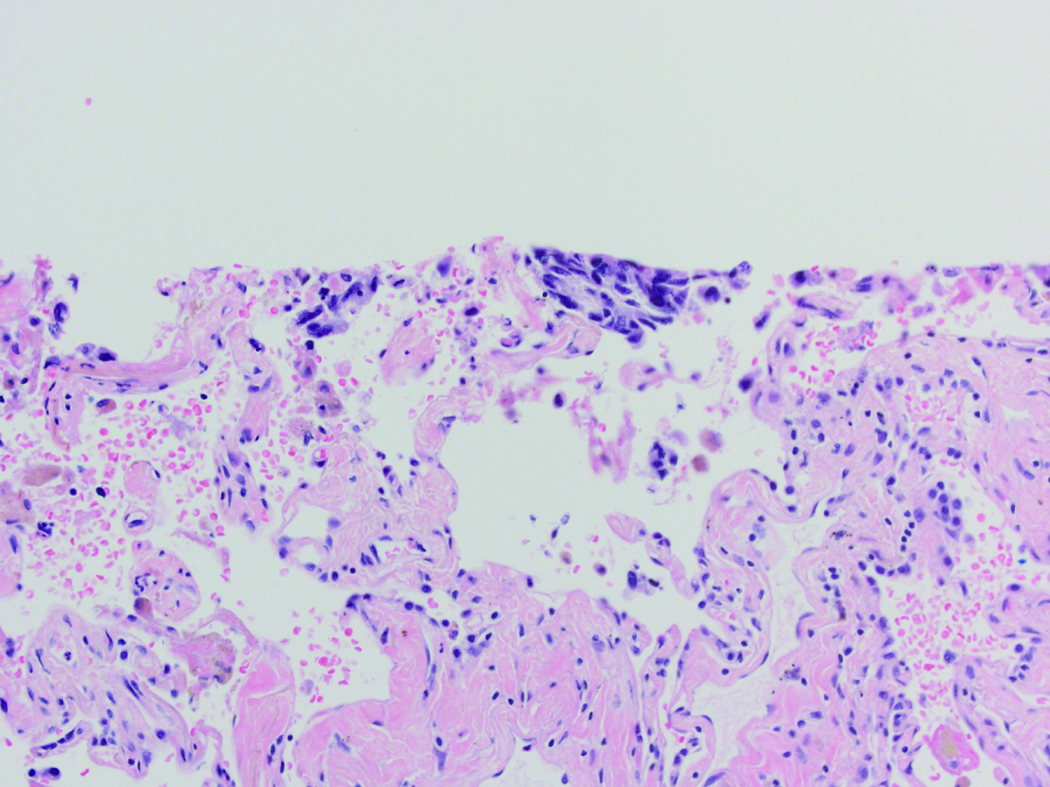
STAS was defined as the presence of tumor cells within air spaces in the lung parenchyma beyond the edge of the main tumor. STAS was identified in the form of solid nests among all four subtypes included in the study (TC, AC, LCNEC, and SCLC) (Figure 2). We required the following for STAS, we required more than one tumor cell nest, involvement at least one air space beyond the tumor edge and continuous alveolar space spread from the tumor edge to the furthest STAS. The tumor edge was determined by evaluation of the border of the tumor and the nonneoplastic lung at low-power microscopic magnification.
Figure 2.
Representative images of spread through air spaces (STAS) (red circles) in atypical carcinoid (A), large cell neuroendocrine carcinoma (B), and small cell lung carcinoma (C). STAS is identified as solid nests in the air spaces in the lung parenchyma beyond the edge of the main tumor (black line) (original magnification, x100)
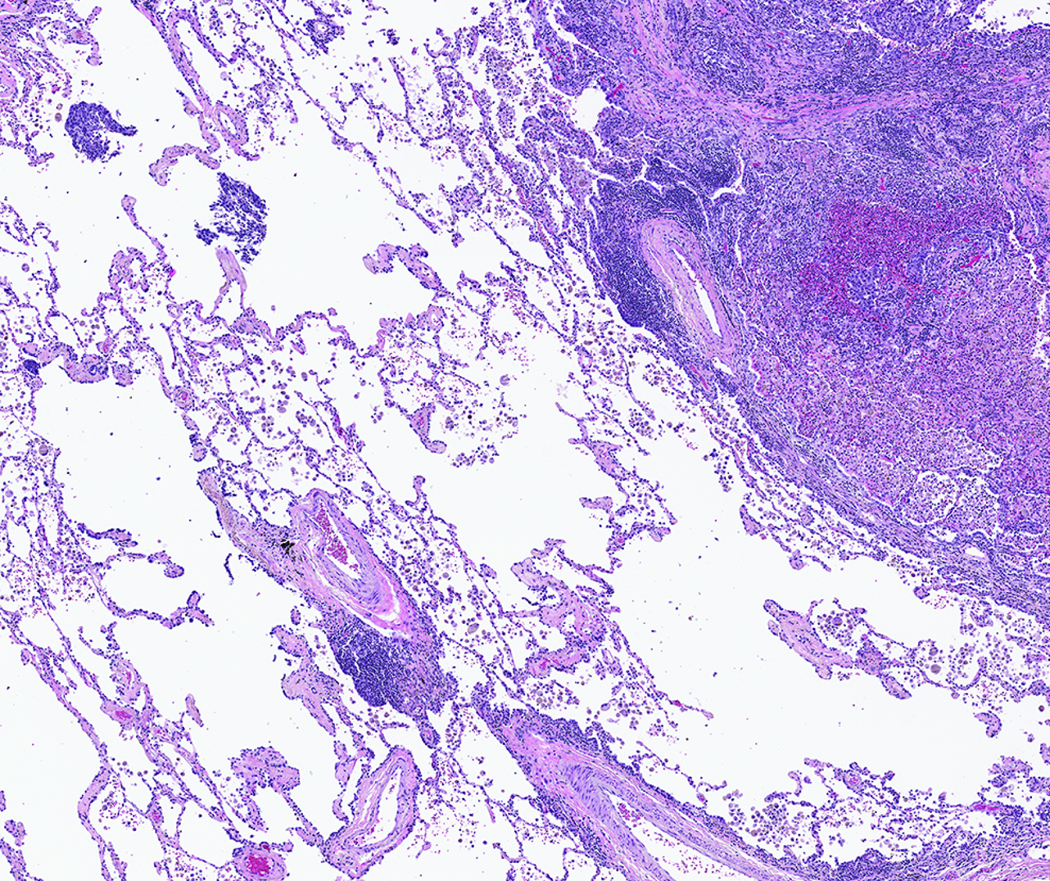
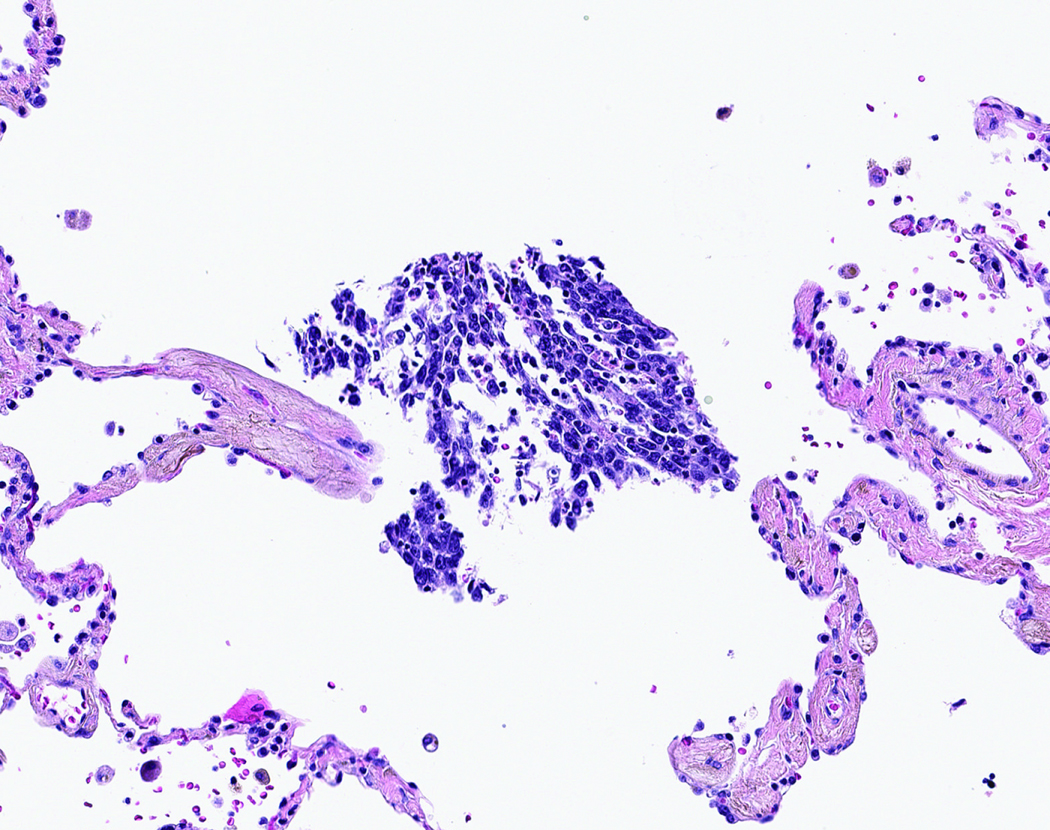
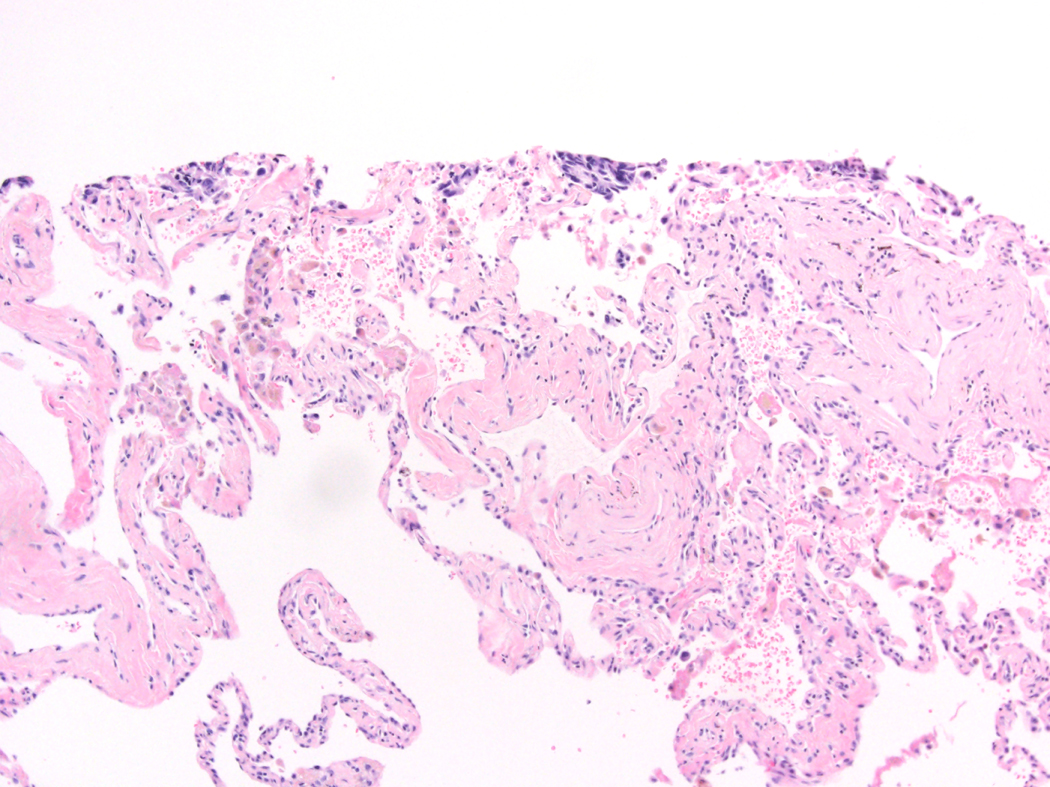
STAS was distinguished from artifacts that consisted of (1) mechanically dissociated tumor floaters, (2) normal benign pneumocytes or bronchial cells, (3) strips of tumor cells detached from alveolar walls or stroma because of poor preservation, and (4) isolated tumor clusters distantly situated away from the tumor rather than being present in a continuous manner from the tumor edge to the most distant location within adjacent air spaces. Tumor floaters were distinguished from STAS by 1) ragged-edged clusters of tumor cells (Figure 1A and B), 2) their random situation in tissue sections (Figure 1C and D), 3) location at the edge of the tissue section (Figure 1C and D) and 4) their appearance out of the plane of section. Normal benign pneumocytes or bronchial cells in air spaces in poorly fixed specimens were differentiated from STAS by their benign features, with or without cilia formation. Linear strips of cells that were lifted off alveolar walls favored an artifact. In addition, alveolar macrophages can be confused with STAS, but they are identified by their small uniform nuclei—inconspicuous nucleoli with foamy cytoplasm in nonsmokers and faint cytoplasmic pigments that ranged from brown and granular to black and particulate in smokers. In contrast, STAS tumor cells, typically consist of cohesive clusters with dense eosinophilic cytoplasm. In addition, tumor cells often have atypical hyperchromatic nuclei and prominent nucleoli.
Figure 1:
Artifacts: A) More than four air spaces away from the tumor edge are clusters of tumor cells with ragged edges. These tumor clusters are isolated distantly away from the tumor edge lacking spread in air spaces continuously from the tumor edge. B) Higher power shows this tumor cluster has jagged edges suggesting an artifact from a knife cut. C) These tumor cells are situated on the edge of the tissue and were located far away from the border of the tumor. D) Along the edge of the tumor clusters is a sharp flat edge that indicates a knife cut and the tumor cells appear compressed against the tissue rather than being contained within the tissue.
Data Collection and Endpoints
Patient demographic information was obtained from the MSK Thoracic Surgery Service’s prospectively maintained lung cancer database. Clinicopathologic data were obtained by reviewing patient medical records specifically for the purpose of this study, to determine clinical characteristics and follow-up status. Patient follow-up status was updated as of September 2017.
The study endpoints were recurrence and lung cancer–specific death. In cases where a new tumor developed in the lung or pleura for which a biopsy specimen was available, the histologic profile was reviewed to determine whether the new tumor was a metachronous primary tumor, a recurrence, or a metastasis; this was completed in accordance with the method developed by our group and the 8th edition TNM classification.30–32 Lung cancer–specific death was defined as death due to recurrent disease associated with resected lung cancer.3325}
Statistical Analysis
Associations between variables and presence of STAS were analyzed using Fisher’s exact test (for categorical variables), the Wilcoxon rank sum test (for continuous variables compared between two groups), and the Kruskal-Wallis test (for continuous variables compared among more than two groups). Both recurrence and lung cancer–specific death were analyzed in the competing-risks framework. For recurrence, death from any cause without recurrence was considered a competing event. For lung cancer–specific death, death from causes other than lung cancer or death from an unknown cause were considered competing events. Cumulative incidence of recurrence (CIR) and lung cancer–specific cumulative incidence of death (LC-CID) were used to estimate the probability of recurrence or lung cancer–specific death following surgical resection with curative intent.34 Patients who did not have recurrence or die during the study were censored at the time of the last available follow-up. Differences in CIR or LC-CID between groups were tested using the Gray method.35 Associations between variables and CIR or LC-CID were estimated using Gray and Fine models.36 Multivariable models were constructed starting with variables with p<0.1 in the univariable analyses. In site–specific CIR analysis, the initial recurrence site (including overlap in patients with multiple recurrence sites at the initial recurrence) was analyzed. There were only 4 recurrences and 2 lung cancer–specific deaths in the TC cohort; therefore, we did not conduct any prognostic analysis in this cohort. Statistical analyses were conducted in R 3.5.1 (R Core Team, Vienna Austria) using the “survival” and “cmprsk” packages. All p values were 2-sided, and we used a 5% threshold for statistical significance.
Results
Patient Clinicopathologic and Demographic Characteristics and Comparison between STAS-Positive and STAS-Negative Cases
In total, 487 patients met the inclusion criteria: 299 patients with TC, 38 patients with AC, 93 patients with LCNEC, and 57 patients with SCLC (Supplementary Figure S1). Table 1 shows patient clinicopathologic and demographic characteristics by STAS status (absent or present). The incidence of STAS in the overall cohort was 26%. Only one case with a single focus of tumor cells beyond the tumor edge was excluded. A higher incidence of STAS was associated with higher-grade histologic subtypes (16% of TCs, 37% of ACs, 43% of LCNECs, and 46% of SCLCs [p<0.001]). A comparison of clinicopathologic variables between the four histologic subtypes is shown in Supplementary Table S1.
Table 1.
Patient clinicopathologic and demographic characteristics and comparison between patients with and without spread through air spaces
| STAS |
||||
|---|---|---|---|---|
| Characteristic | All patients (n=487) | Absent (n=358 [74]) | Present (n=129 [26]) | p |
| Age, years | 64 (54–72) | 63 (53–71) | 67 (59–72) | 0.006 |
| Sex | 0.4 | |||
| Female | 315 [65] | 236 (75) | 79 (25) | |
| Male | 172 [35] | 122 (71) | 50 (29) | |
| Smoking | 0.001 | |||
| Never | 174 [36] | 144 (83) | 30 (17) | |
| Former | 262 [54] | 182 (69) | 80 (31) | |
| Current | 51 [10] | 32 (63) | 19 (37) | |
| Smoking pack-years | 13 (0–42) | 8 (0–40) | 32 (0.5–50) | <0.001 |
| Neoadjuvant therapy | 0.3 | |||
| No | 464 [95] | 343 (74) | 121 (26) | |
| Yes | 23 [5] | 15 (65) | 8 (35) | |
| Surgery | 0.8 | |||
| Lobectomy or greater | 378 [78] | 279 (74) | 99 (26) | |
| Sublobar resection | 109 [22] | 79 (72) | 30 (28) | |
| Adjuvant therapy | <0.001 | |||
| No | 428 [88] | 330 (77) | 98 (23) | |
| Yes | 59 [12] | 28 (47) | 31 (53) | |
| Tumor size, cm | 2.0 (1.4–3.0) | 2.0 (1.3–3.0) | 2.0 (1.5–3.5) | 0.13 |
| T factor | 0.6 | |||
| T1a | 64 [13] | 51 (80) | 13 (20) | |
| T1b | 192 [39] | 139 (72) | 53 (28) | |
| T1c | 94 [19] | 73 (78) | 21 (22) | |
| T2a | 72 [15] | 51 (71) | 21 (29) | |
| T2b | 20 [4] | 12 (60) | 8 (40) | |
| T3 | 36 [7] | 25 (69) | 11 (31) | |
| T4 | 9 [2] | 7 (78) | 2 (22) | |
| N factor | <0.001 | |||
| N0 | 403 [83] | 311 (77) | 92 (23) | |
| N1 | 42 [9] | 22 (52) | 20 (48) | |
| N2 | 42 [9] | 25 (60) | 17 (40) | |
| Pathologic stage | 0.002 | |||
| I | 358 [74] | 278 (78) | 80 (22) | |
| II | 75 [15] | 45 (60) | 30 (40) | |
| III | 54 [11] | 35 (65) | 19 (35) | |
| Lymphatic invasion | <0.001 | |||
| Absent | 347 [71] | 280 (81) | 67 (19) | |
| Present | 140 [29] | 78 (56) | 62 (44) | |
| Vascular invasion | <0.001 | |||
| Absent | 380 [78] | 304 (80) | 76 (20) | |
| Present | 107 [22] | 54 (50) | 53 (50) | |
| Pleural invasion | 0.024 | |||
| Absent | 449 [92] | 337 (75) | 112 (25) | |
| Present | 34 [7] | 19 (56) | 15 (44) | |
| Necrosis percentage | 0 (0–3) | 0 (0–0) | 1 (0–10) | <0.001 |
| Mitotic count | 0 (0–28) | 0 (0–5) | 9 (0–50) | <0.001 |
| Ki-67 labeling index (n=317) | 1.3 (1–70) | 1 (1–5) | 38 (1–90) | <0.001 |
| Histologic subtype | <0.001 | |||
| TC | 299 [61] | 250 (84) | 49 (16) | |
| AC | 38 [8] | 24 (63) | 14 (37) | |
| LCNEC | 93 [19] | 53 (57) | 40 (43) | |
| SCLC | 57 [12] | 31 (54) | 26 (46) | |
Data are shown as number [% among total patients] or (% among the number of patients in each covariate) or median (25–75 percentile). Significant p values are shown in bold. AC, atypical carcinoid; LCNEC, large cell neuroendocrine carcinoma; SCLC, small cell carcinoma; TC, typical carcinoid.
Presence of STAS was associated with smoking history; adjuvant therapy; lymph node metastasis; higher stage; lymphatic, vascular, and pleural invasion; higher percentage of necrosis; higher mitotic count; and higher Ki-67 index, compared with STAS absent tumors (Table 1). Following the observation that incidence of STAS was strongly associated with histologic subtype, we further compared patients according to STAS status (absent or present) in the individual TC, AC, LCNEC, and SCLC cohorts. We found that clinicopathologic variables did not differ significantly according to STAS status (absent or present), with the following exceptions: the presence of STAS was associated with higher N factor and more-frequent lymphatic and vascular invasion in the TC cohort, higher Ki-67 index in the AC cohort, and higher mitotic count in the LCNEC cohort (Supplementary Tables S2, S3, S4, and S5).
In the AC and LCNEC cohorts, 16% of patients underwent either adjuvant chemotherapy or radiotherapy, and no patients underwent prophylactic cranial irradiation (PCI). In the SCLC cohort, 65% of patients underwent adjuvant therapy, and 21% (n=12) underwent PCI following adjuvant chemotherapy (5 stage I, 4 stage II, 3 stage III)—of these, 3 experienced brain metastases as their initial recurrence.
Recurrence Pattern by Histologic Subtype and STAS
Table 2 demonstrates site-specific risk of recurrence and recurrence-free interval by histologic subtype and STAS status. Among the overall cohort (AC, LCNEC, and SCLC), 5-year CIR for any recurrence was 37%. Site-specific 5-year CIR was highest for liver (11%), followed by brain (10%), bone (10%), regional lymph node (7%), lung (6%), pleura (3%), and adrenal gland (2%). The majority of recurrences were distant: 5-year CIR for only locoregional, only distant, and both locoregional and distant were 6%, 30%, and 5%, respectively. Half of recurrences occurred within 1 year of surgery (raw percentage of recurrence-free interval <1 year vs. ≥1 year after surgery, 19% vs. 20%).
Table 2.
Site-specific 5-year cumulative incidence of recurrence and recurrence-free interval
| Overall (AC+LCNEC+SCLC)* (n=188) | Histologic subtype | STAS | ||||||
|---|---|---|---|---|---|---|---|---|
| AC (n=38) | LCNEC (n=93) | SCLC (n=57) | p | Absent (n=108) | Present (n=80) | p | ||
| 5-year CIR (any) | 37 (31, 45) | 37 (24, 57) | 40 (31, 51) | 33 (22, 48) | 0.7 | 24 (17, 34) | 54 (43, 66) | <0.001 |
| 5-year CIR (site-specific)¶ | ||||||||
| Liver | 11 (7, 17) | 20 (10, 39) | 5 (2, 13) | 15 (8, 28) | 0.020 | 8 (4, 15) | 15 (9, 26) | 0.098 |
| Brain | 10 (7, 16) | 0 (N/A) | 13 (8, 22) | 13 (6, 26) | 0.060 | 5 (2, 11) | 18 (11, 29) | 0.010 |
| Bone | 10 (7, 16) | 14 (6, 32) | 10 (5, 18) | 9 (4, 21) | 0.5 | 8 (4, 15) | 14 (8, 24) | 0.3 |
| Lymph node | 7 (4, 12) | 8 (3, 25) | 4 (2, 12) | 11 (5, 24) | 0.4 | 4 (1, 10) | 12 (6, 22) | 0.032 |
| Lung | 6 (3, 10) | 3 (0, 21) | 7 (3, 15) | 5 (2, 17) | 0.9 | 5 (2, 12) | 6 (3, 15) | 0.3 |
| Pleural/chest wall | 3 (2, 7) | 3 (0, 21) | 4 (2, 12) | 2 (0, 13) | 0.7 | 2 (0, 8) | 5 (2, 13) | 0.2 |
| Adrenal | 2 (1, 6) | 0 (N/A) | 1 (0, 8) | 5 (2, 17) | 0.14 | 0 (N/A) | 5 (2, 13) | 0.020 |
| Other | 3 (2, 7) | 6 (1, 24) | 3 (1, 10) | 2 (0, 13) | 0.8 | 3 (1, 9) | 4 (1, 12) | 1.0 |
| 5-year CIR by recurrence pattern | ||||||||
| Only locoregional | 6 (4, 12) | 6 (1, 23) | 8 (3, 17) | 5 (1, 20) | 0.8 | 6 (3, 14) | 7 (3, 17) | 0.8 |
| Only distant | 30 (24, 38) | 31 (18, 53) | 33 (24, 45) | 26 (16, 42) | 0.8 | 21 (14, 32) | 42 (32, 56) | 0.006 |
| Both | 5 (3, 10) | 3 (0, 23) | 4 (1, 11) | 9 (3, 23) | 0.6 | 2 (1, 10) | 8 (4, 18) | 0.027 |
| Recurrence-free interval# | ||||||||
| <1 year | 35 (19) | 2 (5) | 22 (24) | 11 (19) | — | 12 (11) | 23 (29) | — |
| ≥1 year | 37 (20) | 13 (34) | 16 (17) | 8 (14) | — | 16 (15) | 21 (26) | — |
Data are shown as percentage of 5-year cumulative incidence of recurrence (95% confidence interval) or number (raw %).
The typical carcinoid cohort is not included in prognostic analysis because of only 4 recurrences.
Initial recurrence site (including overlap in patients with multiple recurrence sites at the initial recurrence) was analyzed. Significant p values are shown in bold. AC, atypical carcinoid; CI, confidence interval; CIR, cumulative incidence of recurrence; LCNEC, large cell neuroendocrine carcinoma; N/A, not applicable; SCLC, small cell lung carcinoma; STAS, spread through air spaces.
The 5-year CIR for any recurrence was 37% in the AC cohort, 40% in the LCNEC cohort, and 33% in the SCLC cohort. In the AC cohort, the most common site of initial recurrence was liver (5-year CIR, 20%), followed by bone (14%) and lymph node (8%)—there were no cases of brain as the initial site of recurrence in this cohort. In the LCNEC cohort, brain was the most common site of initial recurrence (5-year CIR, 13%), followed by bone (10%), lung (7%), and liver (5%). In the SCLC cohort, liver was the most common site of initial recurrence (5-year CIR, 15%), followed by brain (13%), lymph node (11%), and bone (9%). In the LCNEC and SCLC cohorts, more than half of recurrences occurred within 1 year of surgery (LCNEC, 22/38 [58%]; SCLC, 11/19 [58%]), whereas in the AC cohort only 13% of recurrences (2/15) occurred within 1 year of surgery.
Presence of STAS was associated with a significantly higher risk of recurrence than absence of STAS (5-year CIR for any recurrence: STAS present: 54%; STAS absent: 24% [p<0.001]). STAS was associated with a significantly higher risk of recurrence at the brain, adrenal gland, and regional lymph nodes. Specifically, STAS was associated with a 3-fold higher risk of brain metastasis (5-year CIR for brain metastasis: STAS present: 18%; STAS absent: 5% [p=0.010]). STAS was also associated with risk of distant recurrence (5-year CIR for only locoregional recurrence: STAS present: 7%; STAS absent: 6%; [p=0.8] only distant recurrence: STAS, 42%; no STAS, 21% [p=0.006]; both locoregional and distant recurrence: STAS present: 8%; STAS absent: 2% [p=0.027]). Nearly one-third of patients with STAS present (29%) had recurrence within 1 year of surgery; in contrast, only 11% of patients STAS absent had recurrence within 1 year of surgery.
CIR and LC-CID Curves
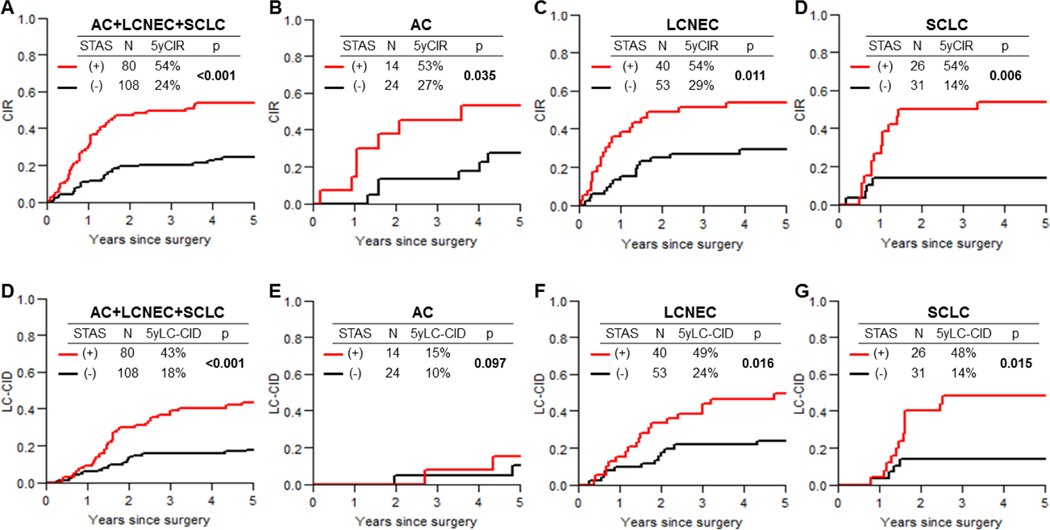
CIR and LC-CID curves by STAS status (presence versus absence), for the overall cohort and the subtype cohorts, are shown in Figure 3. STAS status was associated with a higher risk of recurrence in all cohorts (overall cohort, STAS present: 54% vs. absent: 24% [p<0.001]; AC, STAS present: 53% vs. absent: 27% [p=0.035]; LCNEC, present: 54% vs. absent: 29% [p=0.011]; SCLC, STAS present:54% vs. absent:14% [p=0.006]).
Figure 3.
Cumulative incidence of recurrence (CIR) and lung cancer–specific cumulative incidence of death (LC-CID) curves for patients with and without spread through air spaces (STAS) in the overall cohort (A and E) and the atypical carcinoid (AC; B and F), large cell neuroendocrine carcinoma (LCNEC; C and G), and small cell lung carcinoma (SCLC; D and H) cohorts.
STAS was associated with a worse LC-CID in the overall (STAS present:43% vs. absent:18% [p<0.001]), LCNEC (present:49% vs. absent:24% [p=0.016]), and SCLC (STAS present: 48% vs. absent: 14% [p=0.015]) cohorts. In the AC cohort, STAS showed a nonsignificant trend for higher risk of lung cancer–specific death (5-year LC-CID, STAS present:15% vs. absent:10% [p=0.097]).
Cumulative incidence of brain metastasis was investigated in patients with LCNEC and SCLC (Supplementary Figure S2). STAS was associated with a higher risk of brain metastasis in the combined LCNEC and SCLC cohort (5-year cumulative incidence of brain metastasis: STAS present: 22% vs. STAS absent: 6% [p=0.013]) and the LCNEC cohort (5-year cumulative incidence of brain metastasis: STAS present: 23% vs. STAS absent: 6% [p=0.035]). In the SCLC cohort, STAS showed a nonsignificant trend for higher risk of brain metastasis (5-year cumulative incidence of brain metastasis: STAS present: 19% vs. STAS absent: 7% [p=0.195]).
Competing-Risks Regression Analysis of Recurrence and Lung Cancer–Specific Death
Univariable analysis of recurrence and lung cancer–specific death in the overall cohort is shown in Table 3. Male sex, adjuvant chemotherapy, lymph node metastasis, higher stage, lymphatic invasion, vascular invasion, and STAS were associated with a higher risk of recurrence. Male sex, higher smoking pack-year index, adjuvant chemotherapy, lymph node metastasis, higher stage, lymphatic invasion, vascular invasion, higher Ki-67 index, and STAS were associated with a higher risk of lung cancer–specific death. In the final multivariable model, which was adjusted by pathologic stage, for the overall cohort (Table 4, top), male sex, lymphatic invasion, and STAS were independently associated with a higher risk of recurrence, and male sex, higher smoking pack-year index, lymphatic invasion, and STAS were independently associated with a higher risk of lung cancer–specific death. Specifically, STAS was significantly associated with both recurrence and lung cancer–specific death (recurrence: subhazard ratio [SHR], 2.85; 95% confidence interval [CI], 1.73–4.68 [p<0.001]; lung cancer– specific death: SHR, 2.72; 95% CI, 1.57–4.70 [p<0.001]).
Table 3.
Univariable competing-risks regression analysis for recurrence and lung cancer–specific death
| Variable | Recurrence | Lung cancer–specific death | ||
|---|---|---|---|---|
| SHR (95% CI) | p | SHR (95% CI) | p | |
| Histologic subtype (vs AC) | ||||
| LCNEC | 1.15 (0.66, 2.00) | 0.6 | 1.66 (0.87, 3.14) | 0.12 |
| SCLC | 0.92 (0.48, 1.74) | 0.8 | 1.36 (0.66, 2.82) | 0.4 |
| Age (per 1-year increase) | 0.99 (0.96, 1.01) | 0.2 | 0.99 (0.96, 1.02) | 0.4 |
| Male sex (vs female) | 1.88 (1.18, 2.98) | 0.008 | 2.11 (1.28, 3.49) | 0.004 |
| Smoking (vs former) | ||||
| Never | 0.79 (0.38, 1.66) | 0.5 | 0.58 (0.19, 1.73) | 0.3 |
| Current | 0.80 (0.43, 1.49) | 0.5 | 1.20 (0.63, 2.28) | 0.6 |
| Smoking pack-years (per 1 pack/year increase) | 1.01 (1.00, 1.01) | 0.066 | 1.01 (1.00, 1.01) | 0.01 |
| Neoadjuvant therapy (vs no neoadjuvant) | 1.01 (0.47, 2.19) | 1 | 1.07 (0.47, 2.44) | 0.9 |
| Adjuvant therapy (vs no adjuvant) | 1.97 (1.23, 3.15) | 0.005 | 1.94 (1.18, 3.21) | 0.009 |
| Sublobar resection (vs lobectomy) | 1.18 (0.69, 2.03) | 0.5 | 1.18 (0.64, 2.17) | 0.6 |
| Tumor size (per 1 cm increase) | 1.09 (0.97, 1.22) | 0.2 | 1.11 (0.98, 1.26) | 0.1 |
| N-factor (vs N0) | ||||
| N1 | 1.76 (0.94, 3.30) | 0.078 | 1.67 (0.89, 3.13) | 0.11 |
| N2 | 2.02 (1.11, 3.65) | 0.021 | 1.66 (0.83, 3.32) | 0.2 |
| Pathologic stage (vs stage 1) | ||||
| Stage 2 | 1.93 (1.14, 3.24) | 0.014 | 1.97 (1.13, 3.43) | 0.016 |
| Stage 3 | 2.15 (1.18, 3.92) | 0.012 | 1.90 (0.98, 3.68) | 0.056 |
| Lymphatic invasion (vs absent) | 2.77 (1.66, 4.63) | <0.001 | 3.09 (1.75, 5.44) | <0.001 |
| Vascular invasion (vs absent) | 2.04 (1.28, 3.25) | 0.003 | 2.31 (1.38, 3.86) | 0.001 |
| Pleural invasion (vs absent) | 1.64 (0.93, 2.89) | 0.089 | 1.72 (0.90, 3.28) | 0.1 |
| Necrosis percentage (per 1% increase) | 1.01 (0.99, 1.02) | 0.3 | 1.01 (1.00, 1.03) | 0.093 |
| Mitotic count (per 1 count increase) | 1.00 (0.99, 1.01) | 0.8 | 1.00 (0.99, 1.01) | 0.5 |
| Ki-67 index (per 1 percent increase) | 1.00 (1.00, 1.01) | 0.5 | 1.01 (1.00, 1.02) | 0.045 |
| STAS (vs absent) | 2.69 (1.68, 4.30) | <0.001 | 2.89 (1.72, 4.86) | <0.001 |
Significant p values are shown in bold. AC, atypical carcinoid; CI, confidence interval; LCNEC, large cell neuroendocrine carcinoma; SHR, subhazard ratio; STAS, spread through air spaces.
Table 4.
Final multivariable competing-risk regression models, adjusted by stage, for recurrence and lung cancer–specific death in the overall, large cell neuroendocrine carcinoma, and small cell lung carcinoma cohorts
| Any recurrence | Lung cancer-specific death | |||
|---|---|---|---|---|
| Cohort, Variable | SHR (95% CI) | p | SHR (95% CI) | p |
| MODEL 1 Overall (n=188) | ||||
| Histologic subtype (vs LCNEC) | ||||
| AC | 0.81 (0.45, 1.46) | 0.480 | 0.55 (0.27, 1.11) | 0.094 |
| SCLC | 0.47 (0.23, 0.98) | 0.019 | 0.59 (0.32, 1.11) | 0.1 |
| Sex: male (vs. female) | 2.05 (1.24, 3.38) | 0.005 | 1.96 (1.09, 3.53) | 0.024 |
| Smoking pack-years (per 1 pack/year increase) | — | — | 1.01 (1.00, 1.01) | 0.003 |
| Adjuvant therapy (vs no adjuvant) | 1.72 (0.96, 3.08) | 0.068 | 1.29 (0.69, 2.42) | 0.4 |
| Lymphatic invasion (vs absent) | 2.21 (1.20, 4.09) | 0.011 | 2.85 (1.36, 5.98) | 0.005 |
| STAS (present vs absent) | 2.85 (1.73, 4.68) | <0.001 | 2.72 (1.57, 4.70) | <0.001 |
| MODEL 2: LCNEC (n=93) | ||||
| Sex: male (vs. female) | 2.13 (1.10, 4.12) | 0.026 | 2.20 (1.10, 4.40) | 0.025 |
| Adjuvant therapy (vs no adjuvant) | 1.51 (0.69, 3.3) | 0.3 | 0.91 (0.45, 1.86) | 0.8 |
| Lymphatic invasion (vs absent) | 2.49 (1.18, 5.27) | 0.017 | 3.17 (1.38, 7.28) | 0.006 |
| STAS (present vs absent) | 2.39 (1.26, 4.54) | 0.008 | 2.42 (1.21, 4.84) | 0.012 |
| MODEL 3: SCLC (n=57) | ||||
| Smoking pack-years (per 1 pack/year increase) | 1.02 (1.00, 1.04) | 0.017 | — | — |
| Adjuvant therapy (vs no adjuvant) | 6.61 (1.68, 25.92) | 0.007 | — | — |
| Lymphatic invasion (vs absent) | 4.14 (0.82, 20.87) | 0.086 | — | — |
| Pleural invasion (vs absent) | — | — | 3.67 (1.19, 11.34) | 0.024 |
| Necrosis percentage (per 1% increase) | 1.03 (1.01, 1.05) | 0.007 | 1.04 (1.01, 1.07) | 0.003 |
| STAS (present vs absent) | 2.63 (0.86, 8.03) | 0.090 | 4.06 (1.33, 12.35) | 0.014 |
Significant p values are shown in bold. AC, atypical carcinoid; CI, confidence interval; LCNEC, large cell neuroendocrine carcinoma; SCLC, small cell carcinoma; SHR, subhazard ratio; STAS, spread through air spaces.
We also performed analyses of recurrence and lung cancer–specific death for the LCNEC and SCLC cohorts separately. As there were a small number of events among AC patients, we did not conduct separate analyses in the AC cohort. Univariable analyses of the LCNEC and SCLC cohorts are shown in Supplementary Tables S6 and S7, respectively. In the LCNEC cohort, STAS was a significant risk factor for both recurrence and lung cancer–specific death (recurrence: SHR, 2.39; 95% CI, 1.26–4.54 [p=0.008]; lung cancer–specific death: SHR, 2.42; 95% CI, 1.21–4.84 [p=0.012]), independently of sex, adjuvant chemotherapy, and lymphatic invasion (Table 4, middle). In the SCLC cohort, STAS was a significant risk factor for lung cancer–specific death (SHR, 4.06; 95% CI, 1.33–12.35 [p=0.014]) (Table 4, bottom).
Discussion
The strength and novelty of our study are demonstrated by the following: (1) a large cohort of patients with lung NETs, including all histologic subtypes (TC, AC, LCNEC, and SCLC), was investigated; (2) a detailed recurrence pattern for each lung NET subtype was observed; (3) STAS was identified in 26% of lung NETs, with a higher incidence in higher-grade subtypes (43% in LCNECs and 46% in SCLCs); (4) STAS was associated with more distant-site recurrences, especially brain metastasis; (5) in multivariable analysis stratified by stage, STAS was an independently associated with lung cancer–specific death in the overall NET cohort as well as in the LCNEC and SCLC cohorts.
In previous investigations of STAS in lung adenocarcinoma and squamous cell carcinoma from our group and others using independent cohorts, STAS was associated with larger tumor size, higher-grade subtypes, more-frequent lymphovascular invasion, and higher stages.14, 25, 26, 37 After adjustment for these variables in multivariable analyses, STAS remained significantly associated with prognosis. These findings suggest that STAS reflects a novel histologic marker that surgical pathologists can recognize by routine light microscopy that helps predict prognosis for tumors such as SCLC and LCNEC. Therefore, the histologic finding of STAS indicates a clinically relevant biological phenomenon that reflects invasive behavior that occurred in vivo where pathologists can provide clinically useful prognostic information beyond the usual staging information by recording its presence in pathology reports. To recognize this clinically relevant histologic finding requires application of the criteria to distinguish STAS from artifacts as defined in this manuscript and previous papers and utilized by numerous other investigative groups from many institutions around the world who have also demonstrated this to be a predictor of poor prognosis in virtually all histologic types of lung cancer including multiple studies demonstrating this is an independent predictor in multivariate analysis.14–21, 24–26.22, 23, 27, 28 In this paper we excluded one case where there was only a single nest of tumor cells in the alveolar parenchyma beyond the tumor edge and therefore required at least two foci of tumor cells beyond the tumor edge. The rarity of this finding is unlikely to significantly impact comparison with prior published studies in other tumor types.
Interestingly, in the LCNEC and SCLC cohorts in the present study, STAS was not associated with pathologic factors of prognostic importance commonly recognized in lung cancer, such as stage, tumor size, and lymphovascular invasion. However, STAS was significantly associated with worse outcomes—in particular, a higher risk of brain metastasis and lung cancer–specific death. The argument that the only reason STAS is associated with poor prognosis is because of other confounding poor prognostic histologic features is contradicted by the current study where it is independently prognostic in SCLC and LCNEC where there are no reliable histologic predictors of poor outcome. Because patients with SCLC and LCNEC die rapidly, the finding that STAS is associated with poor outcome indicates it is a clinically relevant and important histologic finding rather than an incidental artifact to be ignored.
“Aerogenous spread” in lung NETs has previously been reported.10, 38, 39 Beasley and colleagues investigated clinicopathologic features in 106 patients with AC. Aerogenous spread was defined as “tumor growth, which appears to spread into the alveolar spaces without accompanying destruction of the septa”—which is consistent with our definition of STAS, although it was not clear whether this was observed within the tumor or the adjacent air spaces.10 In this study, aerogenous spread was identified in 27% of patients with AC similar to the frequency of STAS found in the current study, with associated worse overall survival.10 Huang and colleagues investigated the histologic features of 50 resected lung NETs.38 They observed aerogenous spread in very few tumors classified as well- to moderately differentiated NETs (2/20, likely representing TC and AC) but in almost all tumors classified as poorly differentiated to undifferentiated NETs (28/30, including LCNECs and SCLCs). Unfortunately, the authors of this study did not provide a definition of “aerogenous spread,” nor did they perform an analysis of its prognostic significance.38Suzuki K, et al reported a case of bronchial carcinoid with intrapulmonary dissemination which appeared to represent aerogenous implanted foci.39 In this case tumor cells were seen away from the primary tumor in the peripheral lung and some seemed to be floating within alveoli but others were attached to alveolar walls.39 Recently, Toyokawa and colleagues investigated the prognostic importance of STAS in 30 patients with resected SCLC: STAS had an incidence of 83% and in contrast to our findings was not associated with prognosis.29 Although the concept of aerogenous spread was used long before STAS was defined, these studies provide support for our findings that STAS can be found in all histologic subtypes of lung NETs and provide rationale to further investigate the clinical importance of STAS in patients with these tumors.
In the present study, recurrence occurred slightly less often in the SCLC cohort (33%) than in the AC (37%) or LCNEC (40%) cohorts. In addition, brain as the initial site of recurrence was higher in SCLC (23%) than in LCNEC (14%)(Table 2). The slightly lower risk of recurrence in the SCLC cohort, compared with the LCNEC and AC cohorts, may be attributable to differences in the percentage of patients who underwent adjuvant therapy and PCI (adjuvant therapy/PCI: AC, 16%/0%; LCNEC, 16%/0%; SCLC, 65%/21%, respectively) as well as selection bias favoring surgical treatment for higher-grade histologic subtypes (SCLC tumors were lower stage and smaller than LCNEC and AC tumors). We also observed a higher risk of distant recurrence and lung cancer–specific death according to the presence versus absence of STAS, which raises the consideration whether adjuvant therapy should be considered following resection of lung NETs, whether SCLC, LCNEC, or AC, especially in patients with tumor STAS.
During the study period (1992–2012), our surgical resection cohort underwent varying regimens of chemotherapy, as the treatment strategy for NETs evolved, including improved diagnosis and treatment and perioperative patient care. This might have affected patient prognosis and the results of our analysis. However, our data provide rational to investigate the clinical significance of STAS and further validation studies in worldwide cohorts of lung NET are warranted.
In conclusion, we have demonstrated that the presence of STAS in patients with lung NETs is significantly associated with worse prognosis. STAS is associated with distant recurrence—in particular, brain metastasis—and lung cancer–specific death following resection of early-stage, intermediate- to high-grade lung NETs, independently of tumor stage. Routine inclusion of STAS status in pathology reports may help clinicians identify high-risk patients who could benefit from additional interventions.
Supplementary Material
Acknowledgments
We thank David B. Sewell of the Memorial Sloan Kettering Thoracic Surgery Service for his editorial assistance.
Funding Support
P.S.A’s laboratory work is supported by grants from the National Institutes of Health [R01 CA217169 and P30 CA008748], the U.S. Department of Defense [LC160212], the Joanne and John DallePezze Foundation, the Derfner Foundation, and the Mr. William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center.
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. JClinOncol 2008;26:3063–72. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD. Advances in neuroendocrine lung tumors. AnnOncol 2010;21 Suppl 7:vii65-vii71. [DOI] [PubMed] [Google Scholar]
- 3.Skuladottir H, Hirsch FR, Hansen HH, Olsen JH. Pulmonary neuroendocrine tumors: incidence and prognosis of histological subtypes. A population-based study in Denmark. Lung Cancer 2002;37:127–35. [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5–21. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon: International Agency for Research on Cancer; 2015. [DOI] [PubMed] [Google Scholar]
- 6.Travis WD, Nicholson AG, Geisinger K, Brambilla E. Tumors of the Lower Respiratory Tract. Sliver Spring, MD: American Registry of Pathology; 2019. [Google Scholar]
- 7.Kalemkerian GP, Loo BW, Akerley W, et al. NCCN Guidelines: Small Cell Lung Cancer. In: National Comprehensive Cancer Network. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2019:1–225. [Google Scholar]
- 8.Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines: Non-small cell lung cancer. In: National Comprehensive Cancer Network. Plymouth Meeting, PS: National Comprehensive Cancer Network; 2019:1–2225. [DOI] [PubMed] [Google Scholar]
- 9.Rudin CM, Ismaila N, Hann CL, et al. Treatment of Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol 2015;33:4106–11. [DOI] [PubMed] [Google Scholar]
- 10.Beasley MB, Thunnissen FB, Brambilla E, et al. Pulmonary atypical carcinoid: Predictors of survival in 106 cases. HumPathol 2000;31:1255–65. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson SA, Beasley MB, Brambilla E, et al. Small Cell Lung Carcinoma (SCLC): A Clinicopathologic Study of 100 Cases With Surgical Specimens. AmJSurgPathol 2002;26:1184–97. [DOI] [PubMed] [Google Scholar]
- 12.Rossi G, Cavazza A, Marchioni A, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and met in large-cell neuroendocrine carcinoma of the lung. JClinOncol 2005;23:8774–85. [DOI] [PubMed] [Google Scholar]
- 13.Sarkaria IS, Iyoda A, Roh MS, et al. Neoadjuvant and Adjuvant Chemotherapy in Resected Pulmonary Large Cell Neuroendocrine Carcinomas: A Single Institution Experience. AnnThoracSurg 2011;92:1180–7. [DOI] [PubMed] [Google Scholar]
- 14.Kadota K, Nitadori J, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang YK, Song YS, Cho S, et al. Prognostic stratification model for patients with stage I non-small cell lung cancer adenocarcinoma treated with surgical resection without adjuvant therapies using metabolic features measured on F-18 FDG PET and postoperative pathologic factors. Lung Cancer 2018;119:1–6. [DOI] [PubMed] [Google Scholar]
- 16.Toyokawa G, Yamada Y, Tagawa T, et al. Significance of Spread Through Air Spaces in Resected Pathological Stage I Lung Adenocarcinoma. Ann Thorac Surg 2018;105:1655–63. [DOI] [PubMed] [Google Scholar]
- 17.Yanagawa N, Shiono S, Endo M, Ogata SY. Tumor spread through air spaces is a useful predictor of recurrence and prognosis in stage I lung squamous cell carcinoma, but not in stage II and III. Lung Cancer 2018;120:14–21. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Yang Y, Ma P, et al. Spread through air spaces predicts a worse survival in patients with stage I adenocarcinomas >2 cm after radical lobectomy. Journal of thoracic disease 2018;10:5308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi E, Bae MK, Cho S, Chung JH, Jheon S, Kim K. Pathological prognostic factors of recurrence in early stage lung adenocarcinoma. ANZ journal of surgery 2018;88:327–31. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto J, Nakajima T, Suzuki H, et al. Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J Thorac Cardiovasc Surg 2016;152:64–72 e1. [DOI] [PubMed] [Google Scholar]
- 21.Warth A, Muley T, Harms A, et al. Clinical Relevance of Different Papillary Growth Patterns of Pulmonary Adenocarcinoma. The American journal of surgical pathology 2016;40:818–26. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Yin Q, Yang G, Qie P. Prognostic Impact of Tumor Spread Through Air Spaces in Non-small Cell Lung Cancers: a Meta-Analysis Including 3564 Patients. Pathol Oncol Res 2019. [DOI] [PubMed] [Google Scholar]
- 23.Liu A, Hou F, Qin Y, et al. Predictive value of a prognostic model based on pathologic features in lung invasive adenocarcinoma. Lung Cancer 2019;131:14–22. [DOI] [PubMed] [Google Scholar]
- 24.Kadota K, Kushida Y, Katsuki N, et al. Tumor Spread Through Air Spaces Is an Independent Predictor of Recurrence-free Survival in Patients With Resected Lung Squamous Cell Carcinoma. The American journal of surgical pathology 2017;41:1077–86. [DOI] [PubMed] [Google Scholar]
- 25.Lu S, Tan KS, Kadota K, et al. Spread through Air Spaces (STAS) Is an Independent Predictor of Recurrence and Lung Cancer-Specific Death in Squamous Cell Carcinoma. J Thorac Oncol 2017;12:223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokoyama S, Murakami T, Tao H, et al. Tumor Spread Through Air Spaces Identifies a Distinct Subgroup With Poor Prognosis in Surgically Resected Lung Pleomorphic Carcinoma. Chest 2018;154:83847. [DOI] [PubMed] [Google Scholar]
- 27.Eguchi T, Kameda K, Lu S, et al. Lobectomy Is Associated with Better Outcomes than Sublobar Resection in Spread through Air Spaces (STAS)-Positive T1 Lung Adenocarcinoma: A Propensity Score-Matched Analysis. J Thorac Oncol 2019;14:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bains S, Eguchi T, Warth A, et al. Procedure-Specific Risk Prediction for Recurrence in Patients Undergoing Lobectomy or Sublobar Resection for Small (</=2 cm) Lung Adenocarcinoma: An International Cohort Analysis. J Thorac Oncol 2019;14:72–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toyokawa G, Yamada Y, Tagawa T, et al. High Frequency of Spread Through Air Spaces in Resected Small Cell Lung Cancer. Anticancer Res 2018;38:1821–5. [DOI] [PubMed] [Google Scholar]
- 30.Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung. In: Amin MB, ed. AJCC Cancer Staging Manual 8th ed. Chicago: American Joint Committee on Cancer; 2017:431–56. [DOI] [PubMed] [Google Scholar]
- 31.Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA: a cancer journal for clinicians 2017;67:138–55. [DOI] [PubMed] [Google Scholar]
- 32.Girard N, Deshpande C, Lau C, et al. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. AmJSurgPathol 2009;33:1752–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eguchi T, Bains S, Lee MC, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J Clin Oncol 2017;35:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res 2012;18:2301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat 1988;16:1141–54. [Google Scholar]
- 36.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 37.Warth A, Muley T, Kossakowski CA, et al. Prognostic Impact of Intra-alveolar Tumor Spread in Pulmonary Adenocarcinoma. The American journal of surgical pathology 2015;39:793–801. [DOI] [PubMed] [Google Scholar]
- 38.Huang Q, Muzitansky A, Mark EJ. Pulmonary neuroendocrine carcinomas. A review of 234 cases and a statistical analysis of 50 cases treated at one institution using a simple clinicopathologic classification. ArchPatholLab Med 2002;126:545–53. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki K, Kimula Y, Ogata T, Nakagawa H. Bronchial carcinoid with multiple aerogenous implanted foci. J Surg Oncol 1987;34:211–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.