Abstract
Background
Expression of programmed cell death ligand 1 (PD-L1) on tumor cells with or without immune cells is widely reported in clinical trials of programmed cell death receptor 1 (PD-1) blockade in metastatic non-small cell lung cancer. Various cutpoints have been studied.
Methods
We performed a systematic search of MEDLINE, EMBASE, and conference proceedings up to December 2019 for randomized and nonrandomized clinical trials of anti-PD-1 or anti-PD-L1 monotherapy in metastatic non-small cell lung cancer. We retrieved data on objective response rate (ORR), 1-year and 2-year progression-free survival (PFS), and 2-year and 3-year overall survival (OS) in various PD-L1 subgroups. Results were pooled and analyzed based on different cutpoints, with nonrandomized comparisons made with pooled chemotherapy outcomes.
Results
A total of 9810 patients in 27 studies were included. In treatment-naïve patients, benefits with PD-1 blockade over chemotherapy were seen in ORR in patients having PD-L1 50% or greater, in 2-year OS for PD-L1 1% or greater, and in 1-year PFS, 2-year PFS, and 3-year OS for unselected patients. First-line PD-1 blockade compared with chemotherapy demonstrated higher ORR, 2-year PFS, and 3-year OS if PD-L1 was 50% or greater; lower ORR, higher 2-year PFS, and similar 3-year OS if PD-L1 was 1%-49%; and lower ORR, similar 1-year PFS, and lower 2-year OS if PD-L1 was less than 1%. In previously treated patients, PD-1 blockade demonstrated similar or superior outcomes to chemotherapy in all PD-L1 subgroups.
Conclusions
PD-L1 should guide the choice of PD-1 blockade vs chemotherapy in treatment-naïve patients. In previously treated patients, PD-1 blockade provides a favorable outcome profile to chemotherapy in all PD-L1 subgroups.
The most common cause of cancer death is lung cancer, with an estimated global incidence of 1.8 million and mortality of 1.6 million per year (1). Non-small cell lung cancer (NSCLC) without oncogenic driver mutations harbors a high burden of somatic mutations (2), which act as antigenic targets for host immunity. Evasion of immune destruction is a key mechanism of lung cancer development and was described as an emerging hallmark of cancer by Hanahan and Weinberg in 2011 (3). The relationship between tumor cells and host immunity usually favors tumor elimination or an equilibrium between immune destruction and tumor growth, but when tumor cells progress to immune escape, these cancer cells overcome host immunity, protect themselves with an immunosuppressive environment, and metastasize with fatal consequences (4).
Interaction between programmed cell death receptor 1 (PD-1) on T cells and programmed cell death ligand 1 (PD-L1) on tumor cells plays an important role in immune evasion. PD-1 and PD-L1 inhibitors, herein referred to as PD-1 blockade, have been established as standard of care in both treatment-naïve and previously treated patients with advanced NSCLC (5,6). Some patients experience durable tumor responses, but others derive no clinical benefit, highlighting the importance of identifying biomarkers to improve patient selection. PD-L1 expression on tumor cells with or without its expression on immune cells remains the most reported association with antitumor activity of PD-1 blockade.
PD-L1 expression is a continuous variable ranging from 0% to 100% based on the percentage of tumor cells in a tissue specimen that display partial or complete PD-L1 membrane staining of any intensity (7,8). In some assays, the percentage of tumor-infiltrating immune cells with PD-L1 staining is also measured (7,8). PD-L1 is often reported in a dichotomous fashion, where tumors are defined as either “PD-L1 high” or “PD-L1 low” based on a selected PD-L1 cutpoint. The uncertainty regarding which cutpoint should be chosen is demonstrated by the variation in cutpoints used in different studies, from 1% in some trials to 90% in others (9,10). Another important consideration is heterogeneity among different PD-L1 assays. Various pharmaceutical companies have each developed PD-1/PD-L1–inhibiting drugs with different corresponding PD-L1 assays. Results from the Phase I and II BluePrint Study, which compared the different assays, suggested that the Dako 22–8, Dako 22C3, and Ventana SP263 assays used in nivolumab, pembrolizumab, and durvalumab studies, respectively, had high concordance with each other (11). However, the Ventana SP142 assay used in atezolizumab studies had fainter staining on tumor cells and therefore produced lower scores, but in turn supplemented its scoring by including immune cell staining. The Dako 73–10 assay used in avelumab trials had stronger staining on tumor cells and therefore produced higher scores (11).
The efficacy of different PD-1–blocking agents has not been compared head to head. Nevertheless, pembrolizumab, nivolumab, and atezolizumab have all displayed similar superior efficacy over docetaxel in previously treated NSCLC patients (9,12–15), and meta-analysis has found no statistically significant observable difference between PD-1 inhibitors and PD-L1 inhibitors (16).
There are accumulating published data with longer follow-up on the relationship of PD-L1 expression with response rate, and progression-free survival (PFS) and overall survival (OS) at key time points in NSCLC patients. We conducted this meta-analysis in advanced NSCLC patients treated with PD-1 blockade, focusing on the impact of PD-L1 cutpoint choice on objective response rate (ORR), 1-year PFS, 2-year PFS, 2-year OS, and 3-year OS compared with the outcomes from chemotherapy. The aim of this meta-analysis was to identify the cutpoint at which PD-1 blockade provided superior outcomes to chemotherapy, and specifically compare PD-1 blockade with chemotherapy in the subgroups of PD-L1 less than 1%, 1%-49%, and 50% or greater.
Methods
Search Strategy and Risk of Bias
A systematic search of MEDLINE and EMBASE databases from January 1, 2010, to December 2, 2019, was performed (see the Supplementary Methods for the search strategy, available online) by the first author (J.M.) and checked by another author (J.Mi.). The search was supplemented with a hand search of abstracts and conference proceedings with the cutoff date of December 2, 2019. Studies meeting the following criteria were included: 1) phase I, II, or III human clinical trial; 2) reported results about stage IV NSCLC alone; 3) inclusion of PD-1 or PD-L1 inhibitor monotherapy arm; 4) reporting of PD-L1 subgroup analysis; 5) reporting of first- and second-line patients separately; 6) exclusion of studies limited only to patients with EGFR or ALK mutations; and 7) exclusion of studies limited to only patients with brain metastases.
Data were extracted by the first author (J.M.) and independently checked by 2 other authors (J.Mi. and A.M.), with any discrepancies resolved by consensus. In the presence of multiple publications of the same trial, the most updated data were chosen. Risk of bias was assessed by Cochrane Risk of Bias (17) for randomized trials and ROBINS-I Risk of Bias (18) for nonrandomized trials.
Statistical Analysis
Analyses were performed using Microsoft Excel v15.32. We extracted data on the primary outcomes of ORR, 1-year PFS, 2-year PFS, 2-year OS, and 3-year OS in different PD-L1 subgroups of patients on PD-1 blockade monotherapy or chemotherapy. Pooled estimates and their 95% confidence intervals were calculated for the primary outcomes in each PD-L1 subgroup on each therapy. The pooled estimates were obtained as a weighted average of individual study estimates, where the study weights were determined by the inverse of the variance of the estimate in each study. This is a widely used method for pooling the results of studies and details are given in Neyeloff et al. (19). Because many studies were single arm, an analysis based on hazard ratios was not practical. Heterogeneity in the pooled estimates for each staining group was calculated by Cochran’s Q test. A fixed effect method for the variance of the pooled estimates was used.
Pooled estimates of primary outcomes were then compared between the different therapies using χ2 tests. All comparisons were 2-tailed, with statistical significance defined as P less than or equal to .05. These were nonrandomized comparisons. No adjustments were made for multiple comparisons.
Cohorts of treatment-naïve and previously treated patients were analyzed separately. When extracting data for a cutpoint of interest, we used only outcomes reported in studies that were exactly consistent with the cutpoint used. For example, if data on the PD-L1 1% or greater subgroup were being pooled, outcome data reported for the PD-L1 50% or greater subgroup were not included because they may overestimate the potential benefit for the 1% or greater subgroup. When values for PFS rate or OS rate were not explicitly stated in the manuscript, these were estimated using the published Kaplan-Meier graphs. When integrating data based on the SP142 assay, which combines tumor and immune cell staining, TC0/IC0 was considered PD-L1 less than 1%, TC1/2/3 or IC1/2/3 was considered PD-L1 1% or greater, TC2/3 or IC2/3 was considered PD-L1 5% or greater, and TC3/IC3 was considered PD-L1 50% or greater.
Because chemotherapy is unlikely to be affected by PD-L1 status, comparisons of outcomes between PD-1 blockade and chemotherapy were performed between the specific PD-L1 subgroup for PD-1 blockade and all chemotherapy patients irrespective of PD-L1 status in order to optimize the power of the chemotherapy outcome estimate and allow comparisons to occur where chemotherapy arms were not reported by specific PD-L1 subgroups.
Meta-analysis was performed on all included studies. Prespecified sensitivity analyses were performed using only studies that used the Dako 22C3, Dako 28–8, and Ventana SP263 assays, which have the most comparable staining (11).
Results
Identified Trials
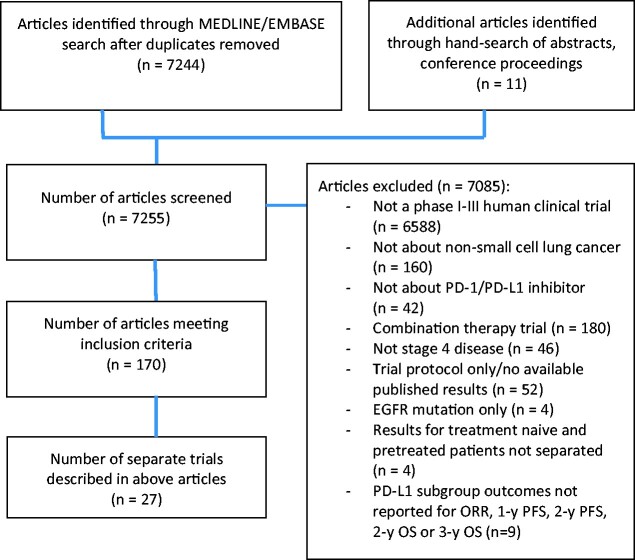
A total 7255 trials were identified by the search strategy for screening (Figure 1). A total of 170 articles or conference proceedings met the inclusion criteria, many of which were updates on the same trial. In total, 27 separate trials were included (Table 1). Risk of bias assessment of included trials is provided in Supplementary Tables 1 and 2 (available online).
Figure 1.
Flow diagram of included and excluded studies. EGFR = epidermal growth factor receptor; ORR = objective response rate; OS = overall survival; PD-1 = programmed cell death receptor 1; PD-L1 = programmed cell death ligand 1; PFS = progression-free survival.
Table 1.
Summary of included trials
| Study (trial name) | No.a | Randomization | Phase | Experimental arm | Control arm | Line | Assay | Selection | PD-L1 cutpointsb | Primary endpoint | Minimum follow-up for survival |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbone et al. 2017, (Checkmate 026) (20) | 541 | Yes | III | Nivolumab 3 mg/kg q14d | Physician’s choice chemotherapy | 1st | 28-8 | ≥1% | 1, 5, 50 | PFS | 13.7 mo (20) |
| Gettinger et al. 2017, (Checkmate 012) (21, 22) | 52 | No | I | Nivolumab 3 mg/kg q14d | Nil | 1st | 28-8 | All comers | 1, 5, 10, 25, 50 | Safety | Not specified (22) |
| Brahmer et al. 2015, (Checkmate 017) (12, 23–27) | 272 | Yes | III |
Nivolumab 3 mg/kg q14d |
Docetaxel 75 mg/m2 q21d | 2nd | 28-8 | All comers | 1, 5, 10 | OS | 51.6 mo (24) |
| Borghaei et al. 2015, (Checkmate 057) (23–27) | 582 | Yes | III |
Nivolumab 3 mg/kg q14d |
Docetaxel 75 mg/m2 q21d | 2nd | 28-8 | All comers | 1, 5, 10 | OS | 51.6 mo (24) |
| Rizvi et al. 2015, (Checkmate 063) (23, 28, 29) | 117 | No | II | Nivolumab 3 mg/kg q14d | Nil | ≥2nd | 28-8 | All comers | 1, 5, 10 | ORR | 56.3 mo (23) |
| Hida et al. 2017, (ONO-4538–05) (30, 31) | 35 | No | II |
Nivolumab 3 mg/kg q14d |
Nil | ≥2nd | 28-8 | All comers | 1, 5, 10, 50 | ORR | 3 y (31) |
| Nishio et al. 2016, (ONO-4538–06) (31, 32) | 76 | No | II |
Nivolumab 3 mg/kg q14d |
Nil | ≥2nd | 28-8 | All comers | 1, 5, 10, 50 | ORR | 3 y (31) |
| Gettinger et al. 2015, (Checkmate 003) (23, 33, 34) | 129 | No | I | Nivolumab 1 mg/kg, 3 mg/kg, or 10 mg/kg q14d | Nil | ≥2nd | 28–8 | All comers | 5 | ORR | 75.2 mo (23) |
| Mok et al. 2019, (KEYNOTE 042) (35, 36) | 1274 | Yes | III | Pembrolizumab 200 mg q21d | Physician’s choice chemotherapy | 1st | 22C3 | ≥1% | 1, 20, 50 | OS | Not specified (35) |
| Reck et al. 2016, (KEYNOTE 024) (37, 38) | 305 | Yes | III | Pembrolizumab 200 mg q21d | Physician’s choice chemotherapy | 1st | 22C3 | ≥50% | 50 | PFS | 20.4 mo (38) |
| Garon et al. 2015, (KEYNOTE 001) (39–42) | 550 | No | I | Pembrolizumab 2 mg/kg q21d, or 10 mg/kg q14d or 10 mg/kg q21d | Nil | ≥1st | 22C3 | All comersc | 1, 50 | Safety, side-effect profile, antitumor activity | 51.8 mo (39) |
| Hersbt et al. 2015, (KEYNOTE 010) (13, 43, 44) | 1033 | Yes | II/III | Pembrolizumab 2 mg/kg or 10 mg/kg q21d | Docetaxel 75 mg/m2 q21d | ≥2nd | 22C3 | ≥1% | 1, 25, 50, 75 | OS and PFS | 35.2 mo (44) |
| Theelen et al. 2019, (Pembro-RT control arm) (45) | 40 | Yes | II | Pembrolizumab 200 mg q21d + 8 Gy/3 Fr RT | Pembrolizumab 200 mg/kg q21d | ≥2nd | 22C3 | All comers | 1, 50 | ORR | 0.1 mo (45) |
| Nishio et al. 2019, (KEYNOTE 025) (46) | 38 | No | Ib | Pembrolizumab 10 mg/kg q21d | Nil | ≥2nd | 22C3 | ≥1% | 1, 50 | ORR, safety, tolerability | 1.9 mo (46) |
| Levy et al. 2017, (CC-486-NSCL-001) (47) | 49 | Yes | II | Pembrolizumab 200 mg q21d + oral azacitadine | Pembrolizumab 200 mg/kg q21d + placebo | 2nd | 22C3 | All comers | 1, 50 | PFS | Not specified (47) |
| Spigel et al. 2019, (IMpower110) (48) | 554 | Yes | III | Atezolizumab 1200 mg q21d | Platinum doublet chemotherapy | 1st | SP142 | ≥1% | 1d, 5e, 50f | OS | 0 mo (48) |
| Peters et al. 2017, (BIRCH) (49, 50) | 660 | No | II | Atezolizumab 1200 mg q21d | Nil | ≥1st | SP142 | ≥5% | 5e, 50f | ORR | 20 mo (50) |
| Spigel et al. 2018, (FIR) (51) | 137 | No | II | Atezolizumab 1200 mg q21d | Nil | ≥1st | SP142 | ≥5% | 5e, 50f | ORR | Not specified (51) |
| Rittmeyer et al. 2017, (OAK) (15, 52) | 1038 | Yes | III | Atezolizumab 1200 mg q21d | Docetaxel 75 mg/m2 q21d | ≥2nd | SP142 | All comers | 1d, 5e, 50f | OS | 21 mo (52) |
| Fehrenbacher et al. 2016 (POPLAR) (14, 53) | 287 | Yes | II | Atezolizumab 1200 mg q21d | Docetaxel 75 mg/m2 q21d | ≥2nd | SP142 | All comers | 1d, 5e, 50f | OS | 3 y (53) |
| Rizvi et al. 2018, (MYSTIC) (54) | 746 | Yes | II | Durvalumab 20 mg/kg q28d or durvalumab 20 mg/kg q28d + tremelimumab 1 mg/kg q28d for 4 doses | Platinum based chemotherapy | 1st | SP263 | All comers | 1, 25 | OS, PFS | Not specified (54) |
| Antonia et al. 2019, (NCT01693562) (55) | 279 | No | I/II | Durvalumab 10 mg/kg q14d | Nil | ≥1st | SP263 | All comers | 25 | Side-effect profile, antitumor activity | 0.3 mo (55) |
| Garassino et al. 2018, (ATLANTIC cohorts 2 and 3) (56) | 310 | No | II | Durvalumab 10 mg/kg q14d | Nil | ≥2nd | SP263 | All comers | 25, 90 | ORR | 3.4 mo (56) |
| Papadimitrakopoulou et al. 2017, (Lung-MAP SWOG S1400A) (57) | 68 | No | II | Durvalumab q14d | Nil | ≥2nd | SP263 | All comers | 25 | ORR | Not specified (57) |
| Jerusalem et al. 2017, JAVELIN Solid Tumor) (58–60) | 259 | No | Ib | Avelumab 10 mg/kg q14d | Nil | ≥1st | 73–10 | All comers | 1, 5, 25 | Safety, tolerability | 31 mo (59) |
| Barlesi et al. 2018, (JAVELIN Lung 200) (61) | 396 | Yes | III | Avelumab 10 mg/kg q14d | Docetaxel 75 mg/m2 q21d | ≥2nd | 73-10 | All comers | 1, 50, 80 | OS | Not specified (61) |
| Wu et al. 2019, (SHR-1210–201) (62) | 146 | No | II | Camrelizumab 200 mg q14d | Nil | ≥2nd | 22C3 | All comers | 1, 25, 50 | ORR | Not specified (62) |
Number of patients with published efficacy data. IC = immune cell staining; ORR = objective response rate; OS = overall survival; PD-L1 = programmed cell death ligand 1; PFS = progression-free survival; q2 wk = once every 2 weeks; q3 wk = once every 3 weeks.
PD-L1 cutpoints with published data either in the original article or subsequent updates.
Four cohorts enrolled PD-L1 1% or greater, 1 cohort enrolled all comers, 1 cohort enrolled PD-L1 less than 1%.
Or IC greater than or equal to 1%.
Or IC greater than or equal to 5%.
Or IC greater than or equal to 10%.
Outcome data were available for 9810 patients in total, including 6950 patients on PD-1 blockade (nivolumab, n = 1107; pembrolizumab, n = 2158; atezolizumab, n = 1830; durvalumab, n = 1054; avelumab, n = 655; camrelizumab, n = 146) and 2860 patients on chemotherapy (docetaxel, n = 1525; platinum doublet, n = 1335). Eleven trials reported on treatment-naïve patients, 21 trials reported on previously treated patients, and 5 trials reported on both. All studies enrolled patients with Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 or 1.
PD-L1 cutpoints used in first-line trials were 1% (7 trials), 5% (6 trials), 10% (1 trial), 20% (1 trial), 25% (4 trials), and 50% (8 trials), and cutpoints used in second- or later line trials were 1% (15 trials), 5% (12 trials), 10% (5 trials), 25% (6 trials), 50% (16 trials), 75% (1 trial), 80% (1 trial), and 90% (1 trial).
The full extracted dataset including statistical analysis can be viewed as Supplementary Material (available online for separate download).
PD-L1 Cutpoint Required for Superiority of PD-1 Blockade to Chemotherapy
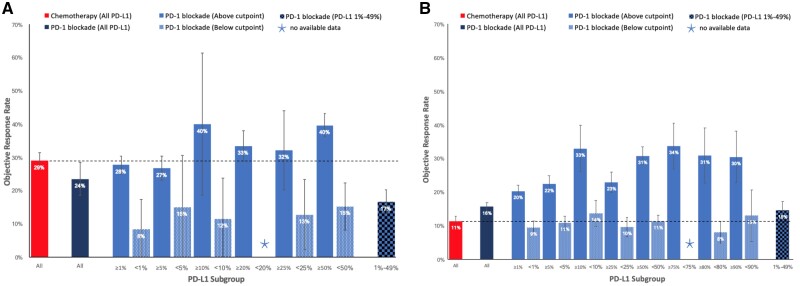
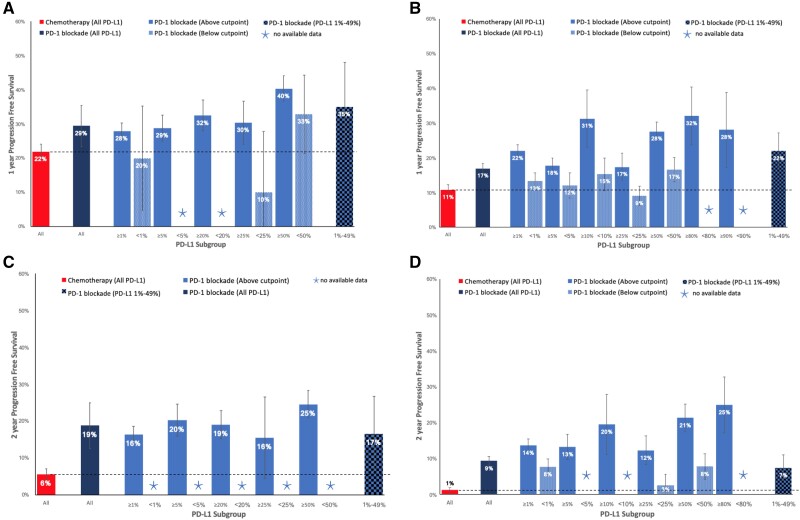
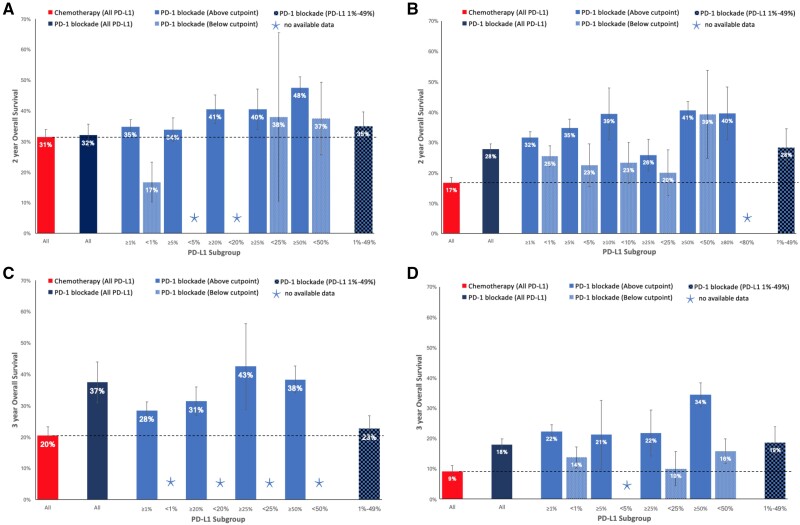
In the first-line setting, a 50% cutpoint was required to select patients, above which PD-1 blockade demonstrated higher ORR compared with platinum-based chemotherapy (39.7% vs 29.0%, P < .001) (Figure 2, A). Patients not selected by their PD-L1 status demonstrated higher 1-year PFS (29.4% vs 21.8%, P = .002), 2-year PFS (18.8% vs 5.6%, P < .001), and 3-year OS (37.4% vs 20.4%, P < .001) rates than chemotherapy (Figures 3, A and C, and 4C). Similar patterns were seen in populations selected above other specific PD-L1 cutpoints (Figures 3, A and C, and 4C). Treatment-naïve patients selected above the 1%, 20%, 25%, and 50% cutpoints experienced a higher 2-year OS rate with PD-1 blockade compared with chemotherapy (Figure 4, A and C).
Figure 2.
Objective response rate (ORR) of chemotherapy, or programmed cell death receptor 1 (PD-1) blockade in different programmed cell death ligand 1 (PD-L1) subgroups. A) ORR in treatment-naïve patients. B) ORR in previously treated patients. Error bars represent 95% confidence intervals for the pooled estimate.
Figure 3.
One-year and 2-year progression-free survival (PFS) rates of chemotherapy, or programmed cell death receptor 1 (PD-1) blockade in different programmed cell death ligand 1 (PD-L1) subgroups. A) One-year PFS in treatment-naïve patients. B) One-year PFS in previously treated patients. C) Two-year PFS in treatment-naïve patients. D) Two-year PFS in previously treated patients. Error bars represent 95% confidence intervals for the pooled estimate.
Figure 4.
Two-year and 3-year overall survival (OS) rates of chemotherapy, or programmed cell death receptor 1 (PD-1) blockade in different programmed cell death ligand 1 (PD-L1) subgroups. A) Two-year OS in treatment naïve patients. B) Two-year OS in previously treated patients. C) Three-year OS in treatment-naïve patients. D) Three-year OS in previously treated patients. Error bars represent 95% confidence intervals for the pooled estimate. PD-1 = programmed cell death receptor 1; PD-L1 = programmed cell death ligand 1.
For patients receiving second- or later line therapy, those receiving PD-1 blockade, irrespective of the level of PDL-1 expression, demonstrated better outcomes compared with docetaxel in ORR (15.7% vs 11.3%, P < .001), 1-year PFS (16.9% vs 10.8%, P < .001), 2-year PFS (9.3% vs 1.3%, P < .001), 2-year OS (27.8% vs 16.7%, P < .001), and 3-year OS (18.0% vs 9.1%, P < .001) rates, with larger benefits seen with increasing PDL-1 level (Figures 2, B; 3, B and D; and 4, B and D).
Outcomes of PD-L1 Less Than 1%, 1%-49%, and 50% or Greater Subgroups
In treatment-naïve patients, outcomes for PD-L1 subgroups of less than 1%, 1%-49%, and 50% or greater were reported in 3 trials (36 patients), 3 trials (409 patients), and 8 trials (759 patients), respectively. In previously treated patients, outcomes for PD-L1 subgroups of less than 1%, 1%-49%, and 50% or greater were reported in 14 trials (10 404 patients), 10 trials (696 patients), and 15 trials (1132 patients), respectively.
ORR in PD-L1 Less Than 1%, 1%-49%, and 50% or Greater Subgroups
As first-line therapy, PD-1 blockade resulted in objective response in 39.7% (95% confidence interval [CI] = 36.2% to 43.1%) of patients with PD-L1 50% or greater, 16.6% (95% CI = 13.0% to 20.2%) of patients with PD-L1 1%-49%, and 8.3% (95% CI = 0.0% to 17.4%) of patients with PD-L1 less than 1% (Figure 2, A). When comparing with a response rate of 29.0% (95% CI = 26.5% to 31.5%) with first-line platinum doublet chemotherapy, the ORR was statistically significantly higher in the 50% or greater subgroup (P < .001) and statistically significantly lower in the 1%-49% (P < .001) and less than 1% (P = .01) subgroups.
PD-1 blockade used as a second- or later line of therapy was associated with objective response in 30.7% (95% CI = 28.1% to 33.4%) of patients with PD-L1 50% or greater, 14.5% (95% CI = 11.9% to 17.1%) of patients with PD-L1 1%-49%, and 9.4% (95% CI = 7.5% to 11.3%) of patients with PD-L1 less than 1% (Figure 2, B). Compared with docetaxel in previously treated patients, which was associated with a response rate of 11.3% (95% CI = 9.9% to 12.7%), the ORR was higher with PD-1 blockade in the PD-L1 1%-49% (P = .03) and 50% or greater (P < .001) subgroups, whereas comparison between these treatment options in the PD-L1 less than 1% subgroup did not reach statistical significance.
1-Year and 2-Year PFS in PD-L1 Less Than 1%, 1%-49%, and 50% or Greater Subgroups
First-line PD-1 blockade was associated with 1-year PFS and 2-year PFS rates of 40.3% (95% CI = 36.6% to 44.0%) and 24.6% (95% CI = 20.8% to 28.3%), respectively, if PD-L1 was equal to 50% or greater; rates of 35.0% (95% CI = 22.0% to 48.0%) and 16.6% (95% CI = 6.5% to 26.7%) if PD-L1 was equal to 1%-49%; and 19.9% (95% CI = 4.7% to 35.2%) and no data for 2-year PFS if PD-L1 was less than 1% (Figure 3, A and C). Compared with pooled data for first-line platinum doublet chemotherapy, which results in a 1-year PFS rate of 21.8% (95% CI = 19.6% to 24.0%), and a 2-year PFS rate of 5.6% (95% CI = 4.1% to 7.1%), PD-1 blockade demonstrated higher 1-year PFS (P < .001) and 2-year PFS (P < .001) rates if PD-L1 was 50% or greater; and higher 1-year PFS (P = .04) and 2-year PFS (P = .003) rates if PD-L1 was equal to 1%-49%. If PD-L1 was less than 1%, there was little difference in the 1-year PFS rate (P = .80) between the 2 treatment groups.
In previously treated patients, PD-1 blockade demonstrated 1-year PFS and 2-year PFS rates of 27.5% (95% CI = 24.9% to 30.2%) and 21.3% (95% CI = 17.4% to 25.2%) if PD-L1 was 50% or greater; 21.9% (95% CI = 16.7% to 27.1%) and 7.4% (95% CI = 3.8% to 11.0%) if PD-L1 was equal to 1%-49%; and 13.2% (95% CI = 10.8% to 15.7%) and 7.7% (95% CI = 5.5% to 9.8%) if PD-L1 was less than 1% (Figure 3, B and D). Compared with second- or later-line docetaxel, which resulted in a 1-year PFS rate of 10.8% (95% CI = 9.3% to 12.2%) and 2-year PFS rate of 1.3% (95% CI = 0.6% to 1.9%), PD-1 blockade was statistically superior in each of the less than 1%, 1%-49%, and 50% or greater subgroups.
2-Year and 3-Year OS in PD-L1 Less Than 1%, 1%-49%, and 50% or Greater Subgroups
In patients receiving PD-1 blockade as first-line therapy, 2-year OS and 3-year OS rates were 47.5% (95% CI = 44.0% to 51.0%) and 38.3% (95% CI = 34.1% to 42.6%), respectively, if PD-L1 was 50% or greater; and 34.9% (95% CI = 30.2% to 39.7%) and 22.6% (95% CI = 18.5% to 26.8%), respectively, if PD-L1 was equal to 1%-49%. In the PD-L1 less than 1% subgroup, the 2-year OS rate was 16.7% (95% CI = 10.2% to 23.2%) and no data were available to inform the 3-year OS rate (Figure 4, A and C). Compared with patients receiving platinum doublet chemotherapy as first-line therapy, which resulted in a 2-year OS rate of 31.4% (95% CI = 29.0% to 33.9%) and 3-year OS rate of 20.4% (95% CI = 17.6% to 23.2%), PD-1 blockade demonstrated higher 2-year OS (P < .001) and 3-year OS (P < .001) rates in the PD-L1 50% or greater subgroup, similar 2-year OS (P = .23) and 3-year OS (P = .38) rates in the PD-L1 1%-49% subgroup, and a lower 2-year OS rate (P = .003) in the PD-L1 less than 1% subgroup.
In second- or later-line therapy, treatment with PD-1 blockade resulted in 2-year OS and 3-year OS rates of 40.5% (95% CI = 37.6% to 43.5%) and 34.4% (95% CI = 30.5% to 38.3%), respectively, if PD-L1 was 50% or greater; 28.3% (95% CI = 22.1% to 34.5%) and 18.5% (95% CI = 13.2% to 23.9%), respectively, if PD-L1 was 1%-49%; and 25.4% (95% CI = 22.0% to 28.8%) and 13.7% (95% CI = 10.2% to 17.1%), respectively, if PD-L1 was less than 1% (Figure 4, B and D). Compared with docetaxel chemotherapy, which resulted in 2-year OS and 3-year OS rates of 16.7% (95% CI = 15.0% to 18.4%) and 9.1% (95% CI = 7.3% to 11.0%), respectively, PD-1 blockade in previously treated patients demonstrated statistically significantly higher 2-year OS and 3-year OS if PD-L1 was 50% or greater, 1%-49%, or less than 1%.
Heterogeneity Testing
Because the number of studies being pooled for each estimate ranged from 1 to 18, and given that the fixed-effects approach yields consistent estimates of the underlying effect, the fixed-effect was the method of choice in terms of both numerical stability and interpretability given that heterogeneity was absent in the majority (>75% as calculated by Cochran’s Q test) of the pooled estimates (data not shown).
Sensitivity Analyses Using Dako 22C3, Dako 28–8, or Ventana SP263 Studies Only
There was no difference in results when excluding studies that used SP142 and 73–10 assays and only including studies that used the 22C3, 28–8, and SP263 assays, which have the most comparable staining (Supplementary Figures 2-4, available online).
Discussion
This meta-analysis provides a comprehensive summary of response rate and survival at key time points for treatment with PD-1 blockade monotherapy or chemotherapy in advanced NSCLC patients with various PD-L1 expressions. An increase in publications with longer term follow-up data from trials of PD-1 blockade in advanced NSCLC has provided the foundation for this meta-analysis, which builds on the work of previous meta-analyses on this topic (63–67) by including a number of newly published phase I-III trials, updated survival data from older trials, and survival outcomes based on survival at key time points rather than median survival.
A number of key practice points are suggested by the findings of this meta-analysis. Although there is increasing evidence in the first-line setting that combination PD-1 blockade plus chemotherapy with or without the antiangiogenesis agent bevacizumab or with or without a CTLA-4 inhibitor ipilimumab is the optimal treatment for patients with PD-L1 less than 50% or those with PD-L1 50% or greater with a high burden of disease (35,68–77), for patients unsuitable for combination therapy or those who prefer to spare chemotherapy toxicity, the choice between PD-1 blockade monotherapy and chemotherapy remains important. This meta-analysis supports the choice of PD-1 blockade over platinum doublet chemotherapy in patients with PD-L1 50% or greater, yielding higher ORR (39.7% vs 29.0%), 2-year PFS (24.6% vs 5.6%), and 3-year OS (38.3% vs 20.4%) rates. Patients with PD-L1 1%-49% are less likely to experience tumor response with PD-1 blockade than chemotherapy (16.6% vs 29.0%), but any clinical benefit is more likely to be sustained, demonstrated by higher 1-year PFS (35.0% vs 21.8%) and 2-year PFS (16.6% vs 5.6%) rates. This is an important distinction between a patient in whom urgent tumor shrinkage for symptom improvement is the primary goal of treatment compared with a patient who is asymptomatic and the chance of sustained disease control with minimal toxicity is the primary goal of treatment. The similar 2-year OS and 3-year OS rates between PD-1 blockade and chemotherapy in this group of patients, as shown in Figure 4, may be due to crossover of treatment with PD-1 blockade in later lines of therapy. In patients with PD-L1 less than 1%, most patients would benefit from chemotherapy rather than monotherapy PD-1 blockade because response rate (29.0% vs 8.3%) and 2-year OS rate (31.4% vs 16.7%) with chemotherapy are higher, and 1-year PFS rate is not any worse (21.8% vs 19.9%).
In previously treated patients, the chemotherapy tested in trials was docetaxel, which is considerably less effective than platinum doublet chemotherapy used in the first-line setting and is therefore a weaker comparator with PD-1 blockade. This meta-analysis concludes that PD-1 blockade provides equivalent or higher ORR, higher 1- and 2-year PFS rates, and higher 2- and 3-year OS rates than chemotherapy regardless of PD-L1 status. Even in patients with PD-L1 less than 1%, clinicians can expect a similar ORR to chemotherapy of approximately 10% but superior 1- and 2-year PFS rates and 2- and 3-year OS rates with PD-1 blockade. Therefore, our meta-analysis supports current clinical practice guidelines that recommend the use of nivolumab and atezolizumab irrespective of PD-L1 and the use of pembrolizumab for patients with PD-L1 1% or greater, based on randomized controlled trials that demonstrate superiority of these agents over docetaxel in their respective PD-L1 populations (5,12–15,78,79).
The findings from this meta-analysis demonstrate the continuous relationship between PD-L1 expression and outcomes from PD-1 blockade. There does not seem to be a natural cutpoint above which PD-1 blockade is definitely effective and below which PD-1 blockade is ineffective. This meta-analysis therefore does not support the use of a single cutpoint to classify patients as either “PD-L1 high” or “PD-L1 low” because it can falsely separate patients close to but on either side of a cutpoint, and falsely group on the same side of a cutpoint patients who may have very different PD-L1 expression. For example, to treat a patient with PD-L1 of 40% very differently from a patient with PD-L1 of 60%, when in fact they likely would have similar responses to PD-1 blockade, can be a consequence of dichotomizing patients according to a 50% cutpoint. Throughout other branches of medicine, it has been shown that when a continuous variable is interpreted as a dichotomous variable, there can be loss of information, unproven assumption of linearity, and reduction in statistical power to determine a true relationship between the variable and its outcome (80). If more studies were to report outcomes by PD-L1 expression as a continuous variable, or at least by centiles rather than overlapping subgroups, our understanding of the true relationship between PD-L1 expression and outcomes from PD-1 blockade would be more precise. This may also create better opportunity to compare outcomes against other treatment options across the whole spectrum of the continuous PD-L1 scale without being restricted if data have only been reported by a few selected cutpoints.
This meta-analysis demonstrates the benefit of reporting survival at key time points, which can inform clinical decisions that are of direct benefit to patients. The chance of prolonged survival is an important motivator for clinicians and patients to choose immunotherapy (81), and therefore reporting the proportion of patients in the “tail of the survival curve” provides key information for clinical decision making. As more studies report survival at key time points, future meta-analyses will become more powerful.
One limitation of this meta-analysis is the inclusion of nonrandomized trials including single-arm studies to achieve an adequate sample size when comparing PD-1 blockade and chemotherapy across various PD-L1 cutpoints. Comparison of outcomes between different arms of nonrandomized trials can be affected by different baseline patient characteristics and inter-trial protocol variability. Another limitation is the lack of uniformity of follow-up across trials that introduce variation in data maturity at 2-year and 3-year time points. Furthermore, publication bias is a risk, because trials showing poorer results from PD-1 blockade may be less likely to be published and updated. This meta-analysis also does not make comparison with the combinations of PD-1 blockade with chemotherapy, PD-1 blockade with cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors, or PD-1 blockade with chemotherapy and vascular endothelial growth factor (VEGF) inhibitors, which are important treatment options in the current landscape.
This study does not take into consideration a variety of other biomarkers that may also affect the efficacy of PD-1 blockade. The complexity of the immune oncology landscape is becoming increasingly evident, including the potential value of tumor mutational burden (82), immune-related gene expression signature (83,84), tumor-infiltrating lymphocyte density (85), programmed cell death ligand-2 (86), and many others.
Although this meta-analysis focuses on treatment efficacy, PD-1 blockade and chemotherapy also have different toxicity profiles, which are crucial in clinical decision making between the 2 treatment modalities. Previous meta-analyses have summarized these toxicity differences and contribute to informed treatment choices (87–89). Another area of further research includes the effect of PD-L1 expression on toxicity from PD-1–blocking drugs.
Evidently, PD-L1 expression is not a perfect predictor of outcomes from PD-1 blockade in advanced NSCLC but remains an important, most studied, and validated biomarker that has clear practical implications for treatment decisions.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Notes
Role of the funder: Not applicable.
Disclosures: Johnathan Man’s disclosures are: Speaker honorarium: Novartis. Rina Hui’s disclosures are: Advisory Board member for: AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novartis, Roche. Speaker honorarium from: Merck Sharp & Dohme, Novartis, Roche. The remaining authors have no disclosures.
Author contributions: Johnathan Man: Conceptualization, Methodology, Formal analysis, Investigation, Data Curation, Writing—Original draft, Writing—Review and editing, Visualization. Jared Millican: Conceptualization, Verification, Data curation, Writing—Review and editing. Arthur Mulvey: Conceptualization, Verification, Data curation, Writing—Review and editing. Val Gebski: Conceptualization, Methodology, Formal analysis, Writing—Original draft, Writing—Review and editing. Rina Hui: Conceptualization, Methodology, Writing—Review and editing, Supervision.
Data Availability
No new data were generated or analyzed in support of this research.
Supplementary Material
References
- 1. Cheng T-YD, Crumb SM, Baade PD, et al. The international epidemiology of lung cancer: latest trends, disparities, and tumor characteristics. J Thorac Oncol. 2016;11(10):1653–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. ; Australian Pancreatic Cancer Genome Initiative. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 4. Mittal D, Gubin MM, Schreiber RD, Smyth MJ.. New insights into cancer immunoediting and its three component phases—elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanna N, Johnson D, Temin S, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2017;35(30):3484–3515. [DOI] [PubMed] [Google Scholar]
- 6. Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v1–v27. [DOI] [PubMed] [Google Scholar]
- 7. Dolled-Filhart M, Ebbinghaus S, Roach C, et al. Development of a clinical trial assay for PD-L1 immunohistochemistry (IHC) for use with pembrolizumab (pembro) clinical trials in melanoma. J Clin Oncol. 2016;34(15_suppl):9571–9571. [Google Scholar]
- 8. International Study for the Study of Lung Cancer. IASLC Atlas of PD-L1. In: Tsao MS, Kerr K, Dacic S, et al. , eds. Immunohistochemistry for PD-L1. North Fort Myers, FL: Editorial Rx Press; 2017. [Google Scholar]
- 9. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garassino MC, Barlesi F, Chaft J, et al. Phase 2 study of medi4736 in patients with PD-l1+ locally advanced or metastatic stage IIIb-IV NSCLC treated with > 2 prior regimens (Atlantic). J Thorac Oncol. 2015;2:S429. [Google Scholar]
- 11. Tsao M, Kerr K, Yatabe Y, et al. PL 03.03 blueprint 2: PD-L1 immunohistochemistry comparability study in real-life, clinical samples. J Thorac Oncol. 2017;12(11):S1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. [DOI] [PubMed] [Google Scholar]
- 14. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. [DOI] [PubMed] [Google Scholar]
- 15. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peng TR, Wu TW.. Efficacy of PD-1/PD-L1 inhibitors in patients with advanced non-small cell lung cancer: a meta-analysis of randomized clinical trials. Thorac Cancer. 2019;10(5):1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neyeloff JL, Fuchs SC, Moreira LB.. Meta-analyses and Forest plots using a Microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gettinger S, Rizvi N, Chow L, et al. First-line nivolumab monotherapy and nivolumab plus ipilimumab in patients with advanced NSCLC: long-term outcomes from checkmate 012. J Thorac Oncol. 2017;12(1):S250–S251. [Google Scholar]
- 22. Goldman JW, Antonia SJ, Gettinger SN, et al. Nivolumab (N) plus ipilimumab (I) as first-line (1L) treatment for advanced (adv) NSCLC: 2-yr OS and long-term outcomes from CheckMate 012. J Clin Oncol. 2017;35(15_suppl):9093. [Google Scholar]
- 23. Antonia SJ, Borghaei H, Ramalingam SS, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol. 2019;20(10):1395–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brahmer J, Borghaei H, Ramalingam SS, et al. Long-term survival outcomes with nivolumab (NIVO) in pts with previously treated advanced non-small cell lung cancer (NSCLC): impact of early disease control and response. Cancer Research Conference: American Association for Cancer Research Annual Meeting; March 29-April 3, 2019; 79; Atlanta, GA. Conference Abstract. [Google Scholar]
- 25. Felip Font E, Gettinger SN, Burgio MA, et al. Three-year follow-up from CheckMate 017/057: nivolumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer (NSCLC). Ann Oncol. 2017;28:v462. [DOI] [PubMed] [Google Scholar]
- 26. Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non–small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. 2018;29(4):959–965. [DOI] [PubMed] [Google Scholar]
- 28. Lena H, Rizvi NA, Wolf J, et al. 137O: nivolumab in patients (pts) with advanced refractory squamous (SQ) non-small cell lung cancer (NSCLC): 2-year follow-up from CheckMate 063 and exploratory cytokine profiling analyses. J Thorac Oncol. 2016;11(4):S115–S116. [Google Scholar]
- 29. Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hida T, Nishio M, Nogami N, et al. Efficacy and safety of nivolumab in Japanese patients with advanced or recurrent squamous non-small cell lung cancer. Cancer Sci. 2017;108(5):1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horinouchi H, Nishio M, Hida T, et al. Three-year follow-up results from phase II studies of nivolumab in Japanese patients with previously treated advanced non-small cell lung cancer: pooled analysis of ONO-4538-05 and ONO-4538-06 studies. Cancer Med. 2019;8(11):5183–5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nishio M, Hida T, Atagi S, et al. Multicentre phase II study of nivolumab in Japanese patients with advanced or recurrent non-squamous non-small cell lung cancer. Esmo Open. 2017;2:e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gettinger S, Horn L, Jackman D, et al. Five-year follow-up of nivolumab in previously treated advanced non–small-cell lung cancer: results from the CA209-003 study. J Clin Oncol. 2018;36(17):1675–1684. [DOI] [PubMed] [Google Scholar]
- 34. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mok TSK, Wu YL, Kowalski DM, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. [DOI] [PubMed] [Google Scholar]
- 36. Lopes G, Wu YL, Kudaba I, et al. Pembrolizumab (pembro) versus platinumbased chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) >= 1%: Open-label, phase 3 KEYNOTE-042 study. J Clin Oncol. 2018;36(18_suppl):LBA4. [Google Scholar]
- 37. Brahmer J, Rodriguez-Abreu D, Robinson A, et al. Updated analysis of keynote-024: pembrolizumab vs platinum-based chemotherapy for advanced NSCLC with PD-L1 TPS >=50%. J Thorac Oncol. 2017;12(11):S1793–S1794. [Google Scholar]
- 38. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537–546. [DOI] [PubMed] [Google Scholar]
- 39. Garon EB, Hellmann MD, Rizvi NA, et al. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 Study. J Clin Oncol. 2019;37(28):2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 41. Hui R, Garon EB, Goldman JW, et al. Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non-small cell lung cancer: a phase 1 trial. Ann Oncol. 2017;28(4):874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leighl NB, Hellmann MD, Hui R, et al. KEYNOTE-001: 3-year overall survival for patients with advanced NSCLC treated with pembrolizumab. J Clin Oncol. 2017;35(15_suppl):9011–9011. [Google Scholar]
- 43. Baas P, Garon EB, Herbst RS, et al. Relationship between level of PD-L1 expression and outcomes in the KEYNOTE-010 study of pembrolizumab vs docetaxel for previously treated, PD-Ll-Positive NSCLC. J Clin Oncol. 2016;34(15_suppl):9015. [Google Scholar]
- 44. Herbst RS, Garon EB, Kim DW, et al. Long-term follow-up in the KEYNOTE-010 study of pembrolizumab (pembro) for advanced NSCLC, including in patients (pts) who completed 2 years of pembro and pts who received a second course of pembro. Ann Oncol. 2018;29:x42–x41. [Google Scholar]
- 45. Theelen W, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5(9):1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nishio M, Takahashi T, Yoshioka H, et al. KEYNOTE-025: phase 1b study of pembrolizumab in Japanese patients with previously treated programmed death ligand 1-positive advanced non-small-cell lung cancer. Cancer Sci. 2019;110(3):1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Levy B, Giaccone G, Besse B, et al. Phase 2 study of pembrolizumab plus CC-486 vs pembrolizumab plus placebo in previously treated patients with advanced NSCLC. J Thorac Oncol. 2017;12(11):S1803–S1804. [Google Scholar]
- 48. Spigel D, de Marinis F, Giaccone G, et al. IMpower110: interim overall survival (OS) analysis of a phase III study of atezolizumab (atezo) vs platinum-based chemotherapy (chemo) as first-line (1L) treatment (tx) in PD-L1–selected NSCLC. Ann Oncol. 2019;30:v915. [Google Scholar]
- 49. Carcereny E, Felip E, Reck M, et al. Updated efficacy results from the birch study: first-line atezolizumab therapy in PD-L1-selected patients with advanced NSCLC. J Thorac Oncol. 2017;12(11):S1791–S1792. [Google Scholar]
- 50. Peters S, Gettinger S, Johnson ML, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol. 2017;35(24):2781–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Spigel DR, Chaft JE, Gettinger S, et al. FIR: efficacy, safety, and biomarker analysis of a phase ii open-label study of atezolizumab in PD-L1-selected patients with NSCLC. J Thorac Oncol. 2018;13(11):1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fehrenbacher L, von Pawel J, Park K, et al. Updated efficacy analysis including secondary population results for OAK: a randomized phase III study of atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2018;13(8):1156–1170. [DOI] [PubMed] [Google Scholar]
- 53. Mazieres J, Park K, Lewanski C, et al. 3-year survival and duration of response in randomized phase II study of atezolizumab (atezo) vs docetaxel (doc) in 2L+ NSCLC (POPLAR.). J Thorac Oncol. 2018;13(4):S79. [Google Scholar]
- 54. Rizvi NA, Chul Cho B, Reinmuth N, et al. Durvalumab with or without tremelimumab vs platinum-based chemotherapy as first-line treatment for metastatic non-small cell lung cancer: MYSTIC. Ann Oncol. 2018;29:x40–x48. [Google Scholar]
- 55. Antonia SJ, Balmanoukian A, Brahmer J, et al. Clinical activity, tolerability, and long-term follow-up of durvalumab in patients with advanced NSCLC. J Thorac Oncol. 2019;14(10):1794–1806. [DOI] [PubMed] [Google Scholar]
- 56. Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Papadimitrakopoulou V, Redman MW, Borghaei H, et al. A phase II study of durvalumab (MEDI4736) for previously treated patients with stage IV squamous NSCLC (SqNSCLC): Lung-MAP Substudy SWOG S1400A. Ann Oncol. 2017;28:ii29. [Google Scholar]
- 58. Jerusalem G, Chen F, Spigel D, et al. Javelin solid tumor: safety and clinical activity of avelumab (anti-PD-l1) as first-line treatment in patients with advanced NSCLC. J Thorac Oncol. 2017;12(1):S252. [Google Scholar]
- 59. Rajan A, Gulley JL, Spigel DR, et al. Avelumab (anti-PD-L1) in patients with platinum-treated advanced NSCLC: 2.5-year follow-up from the JAVELIN Solid Tumor trial. J Clin Oncol. 2018;36(15_suppl):9090. [Google Scholar]
- 60. Verschraegen CF, Chen F, Spigel DR, et al. Avelumab (MSB0010718C; anti-PD-L1) as a first-line treatment for patients with advanced NSCLC from the JAVELIN Solid Tumor phase 1b trial: safety, clinical activity, and PD-L1 expression. J Clin Oncol. 2016;34(15_suppl):9036. [Google Scholar]
- 61. Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19(11):1468–1479. [DOI] [PubMed] [Google Scholar]
- 62. Wu Y, Huang C, Fan Y, et al. JCSE01.09 a phase ii umbrella study of camrelizumab in different PD-L1 expression cohorts in pre-treated advanced/metastatic non-small cell lung cancer. J Thorac Oncol. 2019;14(10):S128. [Google Scholar]
- 63. Abdel-Rahman O. Correlation between PD-L1 expression and outcome of NSCLC patients treated with anti-PD-1/PD-L1 agents: a meta-analysis. Crit Rev Oncol Hematol. 2016;101:75–85. [DOI] [PubMed] [Google Scholar]
- 64. Gandini S, Massi D, Mandala M.. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016;100:88–98. Review. [DOI] [PubMed] [Google Scholar]
- 65. Passiglia F, Bronte G, Rizzo S, et al. PD-L1 expression as predictive biomarker in patients with NSCLC: a pooled analysis. J Thorac Oncol. 2015;2:S232–S233. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Khunger M, Hernandez AV, Pasupuleti V, et al. Programmed cell death 1 (PD-1) Ligand (PD-L1) expression in solid tumors as a predictive biomarker of benefit from PD-1/PD-L1 axis inhibitors: a systematic review and meta-analysis. J Clin Oncol Precis Oncol. 2017;1(1):1–15. [DOI] [PubMed] [Google Scholar]
- 67. Kim J, Cho J, Lee MH, et al. Relative efficacy of checkpoint inhibitors for advanced NSCLC according to programmed death-ligand-1 expression: a systematic review and network meta-analysis. Sci Rep. 2018;8(1):11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang C, Qiao W, Jiang Y, et al. The landscape of immune checkpoint inhibitor plus chemotherapy versus immunotherapy for advanced non-small-cell lung cancer: a systematic review and meta-analysis. J Cell Physiol. 2020;235(5):4913–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tun AM, Thein KZ, Thein WL, et al. Checkpoint inhibitors plus chemotherapy for first-line treatment of advanced non-small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Future Sci OA. 2019;5(9):FSO421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhou Y, Lin Z, Zhang X, et al. First-line treatment for patients with advanced non-small cell lung carcinoma and high PD-L1 expression: pembrolizumab or pembrolizumab plus chemotherapy. J Immunother Cancer. 2019;7(1):120–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Doherty M, Delos Santos S, Rahmadian AP, et al. Pembrolizumab alone or with chemotherapy for PD-L1 positive NSCLC: a network meta-analysis of randomized trials. J Clin Oncol. 2019;37(15_suppl):9087. doi: 10.1200/J Clin Oncol.2019.37.15_suppl.9087. [Google Scholar]
- 72. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. [DOI] [PubMed] [Google Scholar]
- 73. Reck M, Ciuleanu T-E, Dols MC, et al. Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol. 2020;38(15_suppl):9501. [Google Scholar]
- 74. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. [DOI] [PubMed] [Google Scholar]
- 75. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. [DOI] [PubMed] [Google Scholar]
- 76. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. [DOI] [PubMed] [Google Scholar]
- 77. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. [DOI] [PubMed] [Google Scholar]
- 78. Borghaei H, Brahmer JR, Horn L, et al. Nivolumab (nivo) vs docetaxel (doc) in patients (pts) with advanced NSCLC: CheckMate 017/057 2-y update and exploratory cytokine profile analyses. J Clin Oncol Conf. 2016;34: Abstract 9025. [Google Scholar]
- 79. ESMO Guidelines Committee. Appendix 8: metastatic non-small-cell lung cancer (2): eUpdate published online 28 June 2017. Ann Oncol. 2017;28(suppl_4):iv162–iv164. [DOI] [PubMed] [Google Scholar]
- 80. Altman DG, Royston P.. The cost of dichotomising continuous variables. BMJ. 2006;332(7549):1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Steffen L, Lycan T, Levine BJ, et al. Prognostic understanding and barriers to palliative care in patients with metastatic lung cancer on immunotherapy. J Clin Oncol. 2018;36(34_suppl):36. [Google Scholar]
- 82. Peters S, Creelan B, Hellman M. Impact of tumor mutation burden on the efficacy of first-line nivolumab in stage IV or recurrent non-small cell lung cancer: an exploratory analysis of CheckMate 026. American Association for Cancer Research Annual Meeting 2017. Apr 1-5, 2017; Washington, DC; Philadelphia, PA.
- 83. Prat A, Navarro A, Paré L, et al. Immune-related gene expression profiling after PD-1 blockade in non–small cell lung carcinoma, head and neck squamous cell carcinoma, and melanoma. Cancer Res. 2017;77(13):3540–3550. [DOI] [PubMed] [Google Scholar]
- 84. Voong KR, Feliciano J, Becker D, et al. Beyond PD-L1 testing-emerging biomarkers for immunotherapy in non-small cell lung cancer. Ann Transl Med. 2017;5(18):376–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bremnes RM, Busund L-T, Kilvær TL, et al. The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non–small cell lung cancer. J Thorac Oncol. 2016;11(6):789–800. [DOI] [PubMed] [Google Scholar]
- 86. Yearley JH, Gibson C, Yu N, et al. PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin Cancer Res. 2017;23(12):3158–3167. [DOI] [PubMed] [Google Scholar]
- 87. Wang DY, Johnson DB, Davis EJ.. Toxicities associated with PD-1/PD-L1 blockade. Cancer J. 2018;24(1):36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Man J, Ritchie G, Links M, et al. Treatment-related toxicities of immune checkpoint inhibitors in advanced cancers: a meta-analysis. Asia-Pac J Clin Oncol. 2018;14(3):141–152. [DOI] [PubMed] [Google Scholar]
- 89. Sun L, Zhang L, Yu J, et al. Clinical efficacy and safety of anti-PD-1/PD-L1 inhibitors for the treatment of advanced or metastatic cancer: a systematic review and meta-analysis. Sci Rep. 2020;10(1):2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analyzed in support of this research.