Abstract
Muscimol is a psychoactive isoxazole derived from the mushroom Amanita muscaria. As a potent GABAA receptor agonist, muscimol suppresses the activity of the central nervous system, reduces anxiety and induces sleep. We investigated the effects of muscimol on Drosophila behavior. Drosophila behavioral assays are powerful tools that are used to assess neural functions by focusing on specific changes in selected behavior, with the hypothesis that this behavioral change is due to alteration of the underlying neural function of interest. In this study, we developed a comparatively simple and cost-effective method for feeding adult flies muscimol, a pharmacologically active compound, and for quantifying the phenotypes of “resting” and “grooming+walking”. This protocol may provide researchers with a convenient method to characterize small molecule-induced behavioral output in flies.
Keywords: Drosophila, Behavior, Anxiety, GABAA, Muscimol, Neural function
Background
There is increasing evidence that small molecules can be used for the treatment of neuronal disorders (Rissman and Mobley, 2011). As such, knowledge of the neurobiology of small molecule-induced behavioral changes and elucidation of their underlying mechanisms will further our understanding of the functional mechanisms of neural circuits. However, most previous behavioral studies used mice or rats as a model, and a significant amount of resources is required to complete such studies. Drosophila have been used as a tractable genetic model to investigate many genetic disorders (Mackay and Anholt, 2006), and in recent years their use as a model for behavioral studies has become increasingly common ( Zhukovskaya et al., 2013 ; Chen et al., 2014 ; Hampel et al., 2015 ; Kaur et al., 2015 ; Pitmon et al., 2016 ; Hidalgo et al., 2017 ).
Muscimol is a selective agonist of GABAA receptors, which are responsible for the induction of rapid inhibitory neurotransmission in mammals. Thus, the administration of muscimol causes sedative hypnotic-like effects (Lancel, 1999; Yoshimoto et al., 1999 ; Kumar and Kalonia, 2008; Zhang et al., 2016 ). In rats, intravenous injection of muscimol causes a dose-dependent decrease in motor activity, and high doses lead to cataleptic-like effects ( Biggio et al., 1977 ).
We developed a novel approach for testing and quantifying the effects of the potent GABAA agonist muscimol on fly behaviors, including “grooming”, “walking”, and “resting” phenotypes. “Grooming” and “walking” together were considered to represent the active state of the fly, and “resting” implied no movement or a cataleptic phenotype. Direct observation of these active and non-active states of fly behavior can be achieved easily by visual observation and manual recording of the duration of individual behavioral events.
We used a 5-ml Eppendorf tube to design a basic feeding chamber, into which a glass capillary filled with a target solution was inserted. Thus, the flies were able to drink easily while traveling down the capillary drain. For the behavioral assay, a 96-well plate half-filled with 1% agar solution enabled multiple parallel samples to be run simultaneously. A standard period of 9–10 hours of starvation was utilized to induce thirst and rapid consumption of the target solution. We observed in our laboratory that the flies were generally less thirsty on rainy days, so we generally avoided performing these experiments on such days. This method enables screening of the effects of neuro-modulating peptides and natural compounds, which may act in flies through different subsets of neurons; therefore, the underlying functions of neurons may be determined. Karim MR et al. (2018) recently conducted an experiment using this method to explore the role of soy β-conglycinin-derived peptide bCGα(323-333), which regulates fly grooming behavior through effects on dopamine receptors. The significance of this approach is that it allows the study of a broad range of bioactive peptides and their neuro-modular functions with a simple and cost-effective method.
Materials and Reagents
Drosophila vial (Chemglass Life Sciences, catalog number: CGE-4789-001)
NuncTM 96-well polystyrene round-bottom microwell plates (ThermoFisher Scientific, catalog number: 168136)
5-ml microcentrifuge tubes (Thomas Scientific, catalog number: 1149Y05)
Thin-walled glass capillary (Narishige, Japan, catalog number: G-100)
Feather edge file (Hardwick & Sons, catalog number: HWXH952X)
Thin wall needle 18G × 1 1/2" (Terumo, catalog number: NN-1838R)
Ultra-thin wall needle 22G × 1 1/2" (Terumo, catalog number: NN-2238R)
Transparent plastic sheet.
Muscimol (Sigma-Aldrich, catalog number: M1523)
Drosophila melanogaster (Canton-S strain). Flies were purchased from the “Kyoto Stock Center”
Formula 4-24® Instant Drosophila Medium (Caroline Biological Supply Company, catalog number: 173120)
Drosophila Yeast Active (SciMart, catalog number: DR-840-25LB)
Agarose ME (Wako, catalog number: 010-13975)
Brilliant Blue FCF (TCI, catalog number: B0790)
100 mM muscimol solution (see Recipes)
1 mg/ml Brilliant Blue FCF (see Recipes)
5 mM/2.5 mM/1 mM muscimol solution (see Recipes)
1% agarose solution (see Recipes)
Equipment
Olympus SZ40 microscope
Tripod Stands (Science Equip, catalog number: TSR13.00.1541)
iPad mini (Apple)
Incubator
Software
Excel 2013 (Microsoft)
Free Stopwatch Software
Procedure
-
Preparation of reagents
-
Prepare muscimol solution
Dissolve muscimol in Milli-Q to prepare a 100 mM stock solution.
Dilute the stock solution to the target concentrations of 5 mM, 2.5 mM, and 1 mM using Milli-Q water and 1 mg/ml of Brilliant Blue (working concentration 0.125 mg/ml).
Brilliant Blue is used to monitor flies that consumed muscimol solution (the fly stomach turns blue). Control flies are only fed Milli-Q water with Brilliant Blue (0.125 mg/ml).
-
Prepare a 96-well agar plate
Add 0.5 g of agar into 50 ml of H2O to prepare 1% agar solution.
Microwave the solution and leave at room temperature (RT) until it is sufficiently cool to pour 250 μl of agar into each well of a 96-well plate.
Prepare the plate for 1 h before the behavioral test and leave the plate at RT.
-
Capillary tube preparation
Break a glass capillary into two pieces using a hand saw (feather edge file).
-
-
Preparation of 5-ml tube and plastic sheet
Make a hole in the center of the lid of a 5-ml Eppendorf tube using a syringe needle (18G × 1 1/2’’). Next, insert the glass capillary filled with muscimol halfway into the hole in the lid. Make 2 more small holes in the vicinity of the glass capillary using a 22G × 1 1/2’’ needle to facilitate airflow into the tube (Figure 1A).
Using scissors, cut a sheet (8.0 cm × 7.0 cm) from a thin, soft plastic folder and use it to cover the 96-well plate to prevent flies from flying away as they are placed in the plate (Figure 1B). The plastic sheet is reusable.
-
Behavioral assay
-
Collect approximately 50 male and 50 female one-day-old flies (Canton-S) under carbon dioxide anesthesia and place them in a culture vial for individual assay. Raise the flies for 4 days in the incubator on a 12 h:12 h day:night cycle (lights on at 07:00 and off at 19:00), at 25 °C and 40-60% humidity. Commercially available Drosophila food is recommended for this stage of the assay.
Cautionary note: This behavioral test requires constant temperature, humidity, and light within the fly rearing incubator. In addition, it is important to maintain a quiet environment during the behavioral assay, including constant light, temperature, humidity, and time of day.
On the day of the experiment, separate 35-40 male flies (5 days old) from females under a microscope using carbon dioxide anesthesia and leave them in a fresh empty vial for starvation for 9-10 h in the incubator. Male flies are identified by observing the genital organ under a microscope.
-
After 10 h, the starved flies are ready for the behavioral assay. Hold the fly vial in ice to apply cold-induced anesthesia, and move 5 flies from the fly vial to each 5-ml tube using a chill unit, which consists of a yellow tip box cover that is filled with ice and covered with a convex glass plate. The chill unit is used to facilitate the movement of flies from one location to another and should be kept dry. Ice is used for anesthesia to avoid the potential effects of CO2 on the behavioral assay. Use a brush to transfer the flies within 1 to 2 min after removing the vial from the ice, because the behavior of the flies will return to normal after this period. Constant room temperature (25 °C) and humidity (40-60%) should be maintained strictly until the behavioral experiment.
Cautionary note: Following starvation, we used cold anesthesia to transfer flies from one chamber to another. It is necessary to keep the chill unit dry by wiping off the glass plate with a tissue while transferring flies from the culture vial to the 5-ml tube. Likewise, when moving flies from the 5-ml tube to the 96-well plate, the chill unit should be kept dry.
Fill 90% of the glass capillary (Narishige, G-100 thin-walled glass capillary) with muscimol solution at the target concentration and mark the capillary wall at the solution level. Insert the glass capillary halfway through the center hole in the lid of the 5-ml tube containing the flies. The presence of exactly 5 flies in each 5-ml tube allows each fly to consume the same dosage of the target solution, which allows the behavioral assay to be performed five times for a single concentration of muscimol solution.
Allow the flies to drink the muscimol (5 mM, 2.5 mM, or 1 mM) or control solution (Brilliant Blue-containing Milli-Q water) for 20-30 min. Specific glass capillaries are used to feed different dosages of muscimol solution to specific groups of flies (5 flies in a 5-ml tube). When a fly consumes the target solution, its stomach turns blue because of the presence of Brilliant Blue. Thus, consumption of the target solution by the fly can be confirmed.
Remove the glass capillary and use slide calipers to measure the total amount (μl) of target solution consumed by the flies based on the capillary markings. Immediately anesthetize the flies in the 5-ml tube by holding the tube in ice for a few minutes. Use the chill unit to carefully move one fly into each well of the 96-well plate prepared as described above. After transferring all 5 flies using forceps (soft forceps suitable for holding flies), cover the wells of the 96-well plate with a transparent plastic sheet to prevent them from flying away (Figure 2).
Allow the flies to recover from anesthesia for 10 min after placing them into the 96-well plate. Place the 96-well plate on a piece of white paper, set up a tripod in an appropriate location with a good view of the 96-well plate, and place an iPad on top of the tripod to record video for 3 min.
Afterwards, leave the fly-containing 96-well plate at -30 °C for 2 h to euthanize the flies. Note each fly that consumed the target solution by checking for the presence of Brilliant Blue in the stomach using a light microscope. Exclude flies that did not drink any of the target solution from the behavioral analysis. The average amount of the target solution consumed by the flies may be calculated by dividing the total solution intake by the number of flies that consumed the target solution (fly behavior may vary based on the amount (μl) they drink).
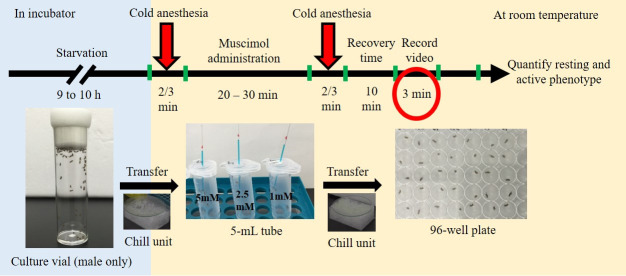
The entire process of the behavioral assay is presented schematically in Figure 3. Video 1 and Video 2 were used to visualize both the reagent preparation and the behavioral assay. Video 3 was used to show the fly movements recorded using the iPad.
-
Figure 1. Experimental setup for 5-ml tube and plastic sheet.
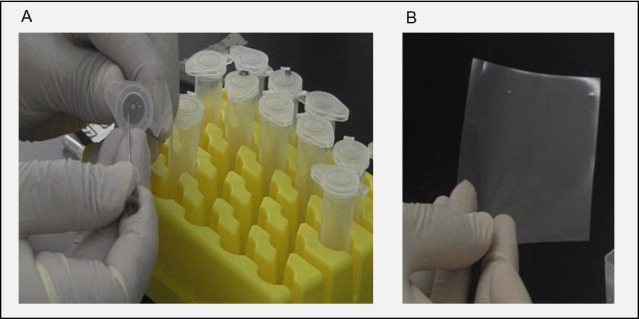
A. Holes are made in a 5-ml tube using a syringe needle. B. Prepare a transparent plastic sheet (8.0 cm × 7.0 cm).
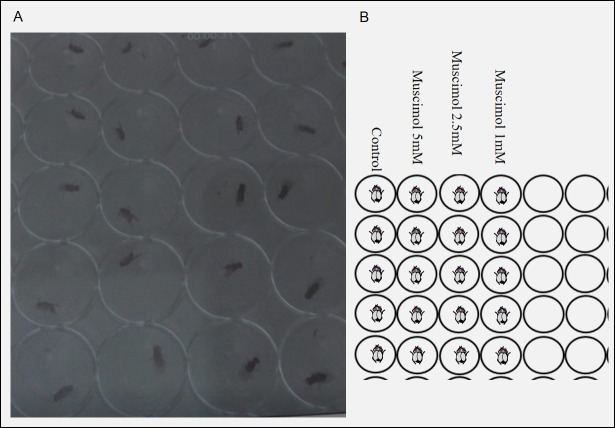
Figure 2. Flies in a 96-well plate for the behavioral assay.
A. Flies are transferred to a 96-well plate after consuming the desired target solution. B. A 96-well plate is drawn to demonstrate the fly location and the target solution consumed by each fly.
Figure 3. Outline of the experimental procedure.
Male flies are starved for 9-10 h, after which they are carefully transferred into 5-ml tubes following brief anesthesia by chilling. The flies are allowed to drink muscimol solution for 20-30 min, after which they are transferred to a 96-well plate for recording of behavioral activity for 3 min. This figure was modified from Karim et al. (2018) .
Video 1. Preparation of reagents.
This video shows the preparation of 96-well agar plates and muscimol solution. The detailed protocol is described in Procedure A.
Video 2. Fly behavioral assay.

This video shows the step-by-step procedure of the fly behavioral assay in detail. The protocol is described in Procedure C.
Video 3. Fly movements recorded using the iPad.
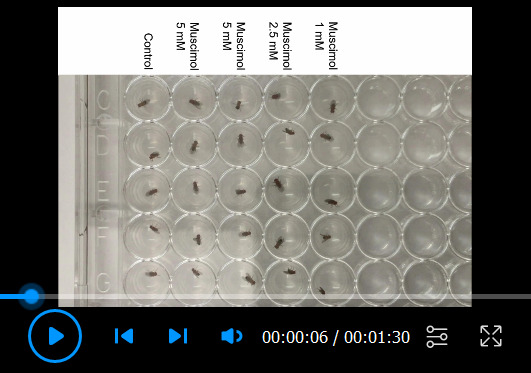
This video shows the tracked movements of control flies and the effects of different concentrations of muscimol.
Data analysis
Fly behavior was described as “resting”, “grooming”, and “walking”. “Grooming” and “walking” together are considered to be the active state. “Resting” indicates no fly movement. In our experiment, flies became motionless immediately upon consuming the high concentration (5 mM) of muscimol solution, which may affect a specific set of fly neurons.
In our experiment, an individual fly consumed a specific concentration of muscimol solution, after which we quantified its phenotypic behavior manually from the recorded 3-min video based on the time each fly spent “resting” or “grooming+walking” (Figure 4). Free stopwatch software is available from Google to help quantify these behavioral activities (https://free-stopwatch.com/).
All values are expressed as the mean ± S.E.M. Student’s t-test was used to assess differences between the active and resting phenotypes.
Figure 4. Muscimol causes a dose-dependent cataleptic phenotype.

Flies are fed either Milli-Q water or muscimol at a dose ranging from 1 mM to 5 mM. Compared with control flies, a high dose of muscimol induces complete loss of movement and a cataleptic-like phenotype.
Limitations
Since the fly olfactory system is relatively strong, it is often difficult to make the flies drink the target solution of interest. Patience is needed to manually quantify the behavioral activities from the recorded video. The experimenters are thoroughly trained to minimize bias in interpreting the results. If the target solution has an impact, it should be reflected clearly in fly behavior.
Fly age, size and sex are important factors that affect the reproducibility of results. It is possible to measure, on average, how much of the target solution was consumed by the flies during the behavioral assay. However, the effects of the target solution are proportional to its dosage and volume, and the amount of the target solution that must be consumed by each fly is dependent on its size and mode. In addition, larger flies are generally less active than smaller flies. When rearing the flies in an incubator for 4 days, the numbers of male and female flies should be equivalent, because the presence of a lower number of females or males during rearing causes abnormally increased fly activity.
Conclusion
We report a simple Drosophila behavioral assay for assessing the impact of muscimol in a standard laboratory setting. This technique is useful for rapidly screening small molecules, peptides, and plant extracts in vivo using a fly model to observe whether these compounds act through specific sets of neurons.
Recipes
-
100 mM muscimol solution
1.14 mg of muscimol
Add 100 μl of Milli-Q water
-
1 mg/ml Brilliant Blue FCF
1 mg Brilliant Blue
Add 1 ml of Milli-Q water
-
5 mM muscimol solution
2.5 μl of muscimol (stock 100 mM)
6.25 μl of Brilliant Blue FCF (stock 1 mg/ml)
Add 41.25 μl of Milli-Q water
-
1% agarose solution
0.5 g of agarose
Add 50 ml of Milli-Q water
Acknowledgments
We wish to thank Juhelee Naznin for her kind assistance in recording the video. We also thank Md. Tanvir Rahman for video editing. This study was supported by the Japan Society for the Promotion of Science (JSPS) scholarship (P15394). This protocol was adopted from previous work (Karim MR et al., 2018).
Competing interests
The author declares no conflicts of interest.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Biggio G., Casu M., Corda M. G., Vernaleone F. and Gessa G. L.(1977). Effect of muscimol, a GABA-mimetic agent, on dopamine metabolism in the mouse brain. Life Sci 21(4): 525-531. [DOI] [PubMed] [Google Scholar]
- 2. Chen A. Y., Wilburn P., Hao X. and Tully T.(2014). Walking deficits and centrophobism in an alpha-synuclein fly model of Parkinson's disease. Genes Brain Behav 13(8): 812-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hampel S., Franconville R., Simpson J. H. and Seeds A. M.(2015). A neural command circuit for grooming movement control. Elife 4: e08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hidalgo S., Molina-Mateo D., Escobedo P., Zarate R. V., Fritz E., Fierro A., Perez E. G., Iturriaga-Vasquez P., Reyes-Parada M., Varas R., Fuenzalida-Uribe N. and Campusano J. M.(2017). Characterization of a novel Drosophila SERT mutant: insights on the contribution of the serotonin neural system to behaviors . ACS Chem Neurosci 8(10): 2168-2179. [DOI] [PubMed] [Google Scholar]
- 5. Kaur K., Simon A. F., Chauhan V. and Chauhan A.(2015). Effect of bisphenol A on Drosophila melanogaster behavior– a new model for the studies on neurodevelopmental disorders . Behav Brain Res 284: 77-84. [DOI] [PubMed] [Google Scholar]
- 6. Karim M. R., Yanagawa A. and Ohinata K.(2018). Soy undecapeptide induces Drosophila hind leg grooming via domapmine receptor . Biochem Biophys Res Commun 499(3):454-458. [DOI] [PubMed] [Google Scholar]
- 7. Kumar A. and Kalonia H.(2008). Effect of withania somnifera on sleep-wake cycle in sleep-disturbed rats: possible GABAergic mechanism. Indian J Pharm Sci 70(6): 806-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lancel M.(1999). Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists . Sleep 22(1): 33-42. [DOI] [PubMed] [Google Scholar]
- 9. Mackay T. F. and Anholt R. R.(2006). Of flies and man: Drosophila as a model for human complex traits . Annu Rev Genomics Hum Genet 7: 339-367. [DOI] [PubMed] [Google Scholar]
- 10. Pitmon E., Stephens G., Parkhurst S. J., Wolf F. W., Kehne G., Taylor M. and Lebestky T.(2016). The D1 family dopamine receptor, DopR, potentiates hind leg grooming behavior in Drosophila . Genes Brain Behav 15(3): 327-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rissman R. A. and Mobley W. C.(2011). Implications for treatment: GABAA receptors in aging, Down syndrome and Alzheimer's disease . J Neurochem 117(4): 613-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshimoto M., Higuchi H., Kamata M., Yoshida K., Shimizu T. and Hishikawa Y.(1999). The effects of benzodiazepine(triazolam), cyclopyrrolone(zopiclone) and imidazopyridine(zolpidem) hypnotics on the frequency of hippocampal theta activity and sleep structure in rats. Eur Neuropsychopharmacol 9(1-2): 29-35. [DOI] [PubMed] [Google Scholar]
- 13. Zhang X., Yao N. and Chergui K.(2016). The GABAA receptor agonist muscimol induces an age- and region-dependent form of long-term depression in the mouse striatum . Learn Mem 23(9): 479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhukovskaya M., Yanagawa A. and Forschler B. T.(2013). Grooming behavior as a mechanism of insect disease defense. Insects 4(4): 609-630. [DOI] [PMC free article] [PubMed] [Google Scholar]