Abstract
Purpose
To summarize the available evidence on the survival and pathologic outcomes after deferred radical prostatectomy (RP) in men with intermediate- and high-risk prostate cancer (PCa).
Methods
The PubMed database and Web of Science were searched in November 2020 according to the PRISMA statement. Studies were deemed eligible if they reported the survival and pathologic outcomes of patients treated with deferred RP for intermediate- and high-risk PCa compared to the control group including those patients treated with RP without delay.
Results
Overall, nineteen studies met our eligibility criteria. We found a significant heterogeneity across the studies in terms of definitions for delay and outcomes, as well as in patients’ baseline clinicopathologic features. According to the currently available literature, deferred RP does not seem to affect oncological survival outcomes, such as prostate cancer-specific mortality and metastasis-free survival, in patients with intermediate- or high-risk PCa. However, the impact of deferred RP on biochemical recurrence rates remains controversial. There is no clear association of deferring RP with any of the features of aggressive disease such as pathologic upgrading, upstaging, positive surgical margins, extracapsular extension, seminal vesicle invasion, and lymph node invasion. Deferred RP was not associated with the need for secondary treatments.
Conclusions
Owing to the different definitions of a delayed RP, it is hard to make a consensus regarding the safe delay time. However, the current data suggest that deferring RP in patients with intermediate- and high-risk PCa for at least around 3 months is generally safe, as it does not lead to adverse pathologic outcomes, biochemical recurrence, the need for secondary therapy, or worse oncological survival outcomes.
Keywords: Prostate cancer, Deferred, Radical prostatectomy, PCa, RP, COVID-19
Introduction
The rapid spread of coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has had a profound impact on the worldwide health care systems [1]. The clinical practice of healthcare workers and systems worldwide has substantially changed secondary to the present pandemic. Individual monitoring through precision diagnostics, interdisciplinary team boards, and therapy adjustments is an essential part of the adjustment of medical workers to the COVID-19 pandemic, urology being no exception [2, 3].
Delays in diagnosis and treatment of cancer patients can have an adverse impact on disease outcomes. In some cases, the benefit of ensuring a timely delivery of a definitive anti-cancer treatment outweighs the potential risk of a COVID-19 infection. Hence, several medical societies, including urologic societies, have introduced clinical guidelines to advise physicians about appropriate treatment decisions during the current pandemic [4]. General recommendations for surgical procedures should consider resource allocation as well as operation room (OR) and intensive care unit (ICU) capacities as well as the risk of COVID-19 transmission while minimizing its impact on disease outcomes. Therefore, evidence-based recommendations are needed to appropriately categorize a disease state as either high or low priority. In general, radical treatment can be deferred justifiable only if delays are unlikely to affect cancer outcomes [5]. The impact of treatment delay on disease outcome should be measurable to make the appropriate decision regarding the urgency and priority of a specific surgical/medical intervention.
However, it was reported that the number of radical prostatectomies (RP) in March–July 2020 was reduced by approximately 50% compared to the baseline period of March–July 2019 [6]. Although treatment delay in low-risk PCa is expected to have no or minimal impact on the disease outcomes, especially with the implementation of active surveillance, prolonged delays in treatment of intermediate or high risk PCa without regular monitoring could potentially result in disease progression [7]. Nevertheless, high level evidence on the subsequent risk of delaying RP during COVID-19 pandemic in patients with intermediate- and high-risk PCa is still lacking. We hypothesized that there is no evidence in the existing literature that a treatment delay of up to 3 months has a significant oncological impact in men affected by PCa. To test our hypothesis, we conducted a systematic review of the literature on the impact of deferring RP in intermediate- and high-risk PCa patients during the period before the COVID-19 pandemic.
This systematic review aimed to summarize the available evidence on the survival and pathologic outcomes after deferred RP in men with intermediate- and high-risk PCa.
Methods
Literature search
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [8]. The study protocol was registered a priori on the International Prospective Register of Systematic Reviews (PROSPERO; Registration ID CRD42020224178).
The PubMed and Web of Science databases were searched in November 2020 to identify studies reporting on the survival and pathologic outcomes after deferred RP in men with intermediate- and high-risk PCa. A comprehensive systematic literature search was independently performed by two authors. The keywords used in our search strategy included: "delayed" OR "deferred" AND "radical prostatectomy" AND "prostate cancer" AND "intermediate risk" AND "high risk". In addition, we searched the references of selected studies for potentially relevant articles. The main outcomes of interest were survival and pathologic outcomes.
After removing duplicates, two independent reviewers screened the titles and abstracts. Any citation which either reviewer thought should be included or unclear for inclusion was identified for full text screening. Subsequently, full texts of eligible articles were reviewed for final inclusion and data extraction. In cases of disagreement, the authors consulted with the co-authors, and final decisions were reached by consensus.
Inclusion and exclusion criteria
We included studies that reported on survival and pathologic outcomes after deferred RP in men with intermediate- and high-risk PCa. The PICO (population, intervention, control, and outcomes) in this study was the following: patients treated with deferred RP for intermediate- and high-risk PCa compared to control group including those patients treated with RP without delay. The outcomes were PCa recurrence, survival and pathologic outcomes.
We excluded reviews, letters, editorials, animal studies, study protocols, case reports, meeting abstracts, replies from authors, brief correspondence, and articles not published in English. Furthermore, we excluded studies that did not provide data regarding the oncologic outcomes. References of all papers included were scanned for additional studies of interest.
Data extraction
Data extracted from each study were independently extracted by two reviewers. Extracted data included the following: first author’s name, publication year, study design, demographics characteristics including age range, sample size, risk group, PSA level, median follow up, median time from diagnosis to RP, definition of RP delay. Subsequently, the hazard ratios (HR) or odds ratio (OR) and 95% confidence intervals (CI) associated with each outcome were retrieved.
Results
Search results
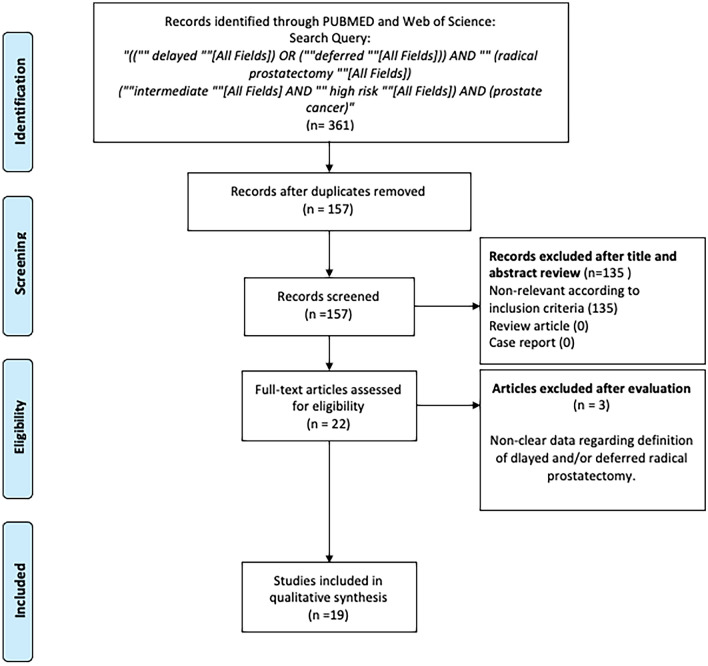
The literature search identified 361 unique references. Among them, 204 records were removed due to duplication, and 135 articles were excluded due to unrelated outcomes during the screening process (Fig. 1). Of the 22 full-text articles assessed for eligibility, three were excluded based on the selection criteria.
Fig. 1.
Selection process of the articles to assess the survival and pathologic outcomes after deferred radical prostatectomy in men with intermediate- and high-risk PCa
Nineteen studies were finally included in the present systematic review [9–27]. Table 1 summarizes the studies and their main findings. A few studies included low-risk patients along with intermediate and high-risk patients without performing separate analyses in terms of risk groups [11, 13, 17, 18, 24, 27]. We made the decision to include these studies in the qualitative analysis.
Table 1.
Characteristics of included studies
| Author, publication year | Study design | Number of patients | Age, years, median, range or IQR) | Risk group, stage | PSA, ng/ml, median (range or IQR) | Follow-up, median (range or IQR) | Median time from diagnosis to RP (range) | Definition of RP delay | Main results |
|---|---|---|---|---|---|---|---|---|---|
| Aas 2018 [25] | R | 5163 | 62 (39–77) | Low (28.2%); intermediate (42.9%); high (28.9%) | NR | 7.9 years (0–15) | 93 days (1–180) | Stratified by interval: ≤ 60 days (16.5%); 61–90 days (30.7%); 91–120 days (25.5%); 121–180 days (27.3%) |
No association was found between RP-interval and PCSM in the intermediate-or high-risk groups Increasing RP-interval did not increase the rate of adverse histological outcomes (upgrading, upstaging, PSM) or incidence of RP-failure |
| Abern 2012 [9] | R | 1561 | NR | Low (52%); intermediate (48%) | NR | 53 months (IQR 25–86) | NR | Stratified by interval: ≤ 3 months (60%); 3–6 months (30%); 6–9 months (6%); > 9 months (4%) | For intermediate-risk, delays > 9 months were significantly related to BCR (HR: 2.10, p = 0.01), PSM (OR: 4.08, p < 0.01), and ECE (OR: 6.68, p = 0.045) |
| Anil 2018 [23] | R | 248 | 64.2 | Low (49.2%); intermediate (25.4%); high (25.4%) | 9.96 | 16.1 months (2–72) | NR |
Stratified by interval: ≤ 60 days (43.1%); 61–120 days (45.6%); ≥ 120 days (11.3%) |
No significant difference between the groups in terms of BCR (p = 0.06) or additional treatment (p = 0.1) ECE increased significantly in the intermediate-risk group with longer surgical delay (p = 0.044) |
| Balakrishnan 2019 [19] | P | 1916 | 61 (IQR 56–65) | Low and intermediate risk:GG1 (86.6%), GG2 with 1 high grade core (6.9%), GG2 with 2 or more high grade cores(6.5%) | 5.50 (IQR 4.31–7.38) | 66 (IQR 42–93) | 27 months (IQR 15.5–46.5) | After AS | GG2 with 2 or more high grade cores at diagnosis was associated with an increased risk of recurrence compared to GG1 disease (HR 3.29, 95% CI 1.49–7.26, p < 0.01). GG2 disease with 1 high grade core did not significantly differ from GG1 |
| Berg 2015 [10] | R | 2,212 | Mean 60.77 (SD 7.2) | Low (34.9%); intermediate (54.6%); high (10.4%) | NR | 39 months (IQR 12–82) | 64 days (48–90) | Stratified by interval: ≤ 90 days (75.7%); 91–180 days (24.3%); > 180 days (2.7%) | Significant increases in the proportion of adverse pathological outcomes were found beyond 60 days for patients with Gleason 7 and PSA > 20 (p = 0.032), and 30 days for patients with Gleason 8–10 and PSA 11–20 (p = 0.041) |
| Cooperberg 2011 [17] | P | 466 | NR | Low and intermediate CAPRA risk | NR | 37.5 (IQR 27–60) | 19.5 (IQR 14–36) | After AS | No statistically significant differences regarding adverse histological outcomes (upstaging, PSM, ECE) (all p > 0.05) |
| Diamand 2020 [26] | R | 926 | 66 (IQR 61–70) | Intermediate (67%); high (33%) |
8.2 (5–12) |
26 months (IQR 10–40) |
3 months (2–5) |
3 months—cutoff | Delay was not significantly associated with upgrading (OR 0.98, 95% CI 0.94–1.02, p = 0.3), nor to LNI (OR 0.88, 95% CI 0.77–1.01, p = 0.07), pathological locally advanced disease (OR 1, 95% CI 0.97–1.03, p = 0.8), or need for adjuvant therapy (OR 0.96, 95% CI 0.84–1.11, p = 0.6), or BCR (HR 0.97, 95% CI 0.91–1.04, p = 0.6) |
| Filippou 2015 [11] | R | 3,372 | Mean 60.6 (SD 6.9) | Low and intermediate | 4.9 (IQR 4.0–6.1) |
40 months (7–166) |
20 months (6–148) |
RP > 6 months after diagnostic biopsy | Immediate RP had lower probability of adverse pathology than delayed RP (OR 0.34, 95% CI 0.21–0.55) |
| Fossati 2016 [14] | R | 2,653 |
66 (60–70) |
Low (35%); intermediate (50%); high (15%) |
6.5 (4.8–9.8) |
56 months (IQR 26–92) | 2.8 months (IQR 1.6–4.7) | NR | Time from biopsy to RP was significantly associated with an increased risk of BCR (HR 1.02, p = 0.0005) and CR (HR 1.03, p = 0.0002) |
| Ginsburg 2020 [20] | R | 128,062 | 63 (IQR 58–67) | Intermediate and high risk | 6.3 (IQR 4.8–9.7) | NR |
3 months (IQR 2–4) |
Stratified by interval: 0–3 months (73.2%), 4–6 months (23.7%), 7–9 months (2.5%), 10–12 months (0.6%) | Delay up to 12 months was not significantly associated with odds of adverse pathology, upgrading, node positive disease or post-RP secondary treatments |
| Godtman, 2018 [18] | R | 132 | 64 (IQR 61–67) | Low (85%); intermediate (14%); high (1%) | 4.1 (IQR 3.4–5.5) | 10.9 years (IQR 7.5–14.5) | 1.9 years (IQR 1.2–4.2) | After AS | 39% experienced at least 1 unfavorable pathology feature at RP. The 10-year prostate specific antigen relapse-free survival was 79.5% |
| Gupta 2019 [22] | R | 2303 | 62 (IQR 57–66) | Intermediate, high: GG3 (54%), GG4 (26%), GG5 (20%) | 6.0 (IQR 4.5–9.1) | 3 years (IQR 2–5) | NR | < 3 months (72%), 3–6 (28%) |
There was no significant difference in rates of adjuvant therapy, PSM, EPE, SVI, or LNI There was no significant difference in 2- and 5-year BCRFS There was no significant difference in 2-, 5-, and 10-year MFS |
| Korets 2011 [24] | R | 1568 | 60 (IQR 56–66) | Low, intermediate, high risk | NR | NR | 45 days (36–847) |
Stratified by interval: ≤ 60 days (70%); 61–90 days (19.3%); > 90 days (10.7%) |
A delay of > 60 days was not associated with adverse pathological findings at surgery or worse BCR |
| Morini, 2017 [21] | R | 908 | 61.5 | Low (47.4%); intermediate (40.8%); high (11.9%) | Mean 7.88 (0.02–53.55) | Mean 4 years | Mean 191.0 (30.0–941.0) |
Stratified by interval: ≤ 6 months (56.5%); 6–12 months (38.5%); > 12 months (5.1%) |
No time interval correlated with poor oncological outcomes (including BCR) in all risk groups |
| Nesbitt 2020 [27] | R | 332 | Mean 63.1 (SD 6.4) | Low, intermediate, high risk | Mean 7.2 (SD 5.6) | NR | Mean 125.7 days (SD 70.0) | > 90 days | Time between biopsy and surgery was not associated with adverse outcome except for pathological ECE or pT3 disease (p = 0.04) |
| Patel 2019 [12] | R | 2728 | Mean 60.2 (SD 6.9) | GG1 (39%), GG2 (45%), GG3 (10%), GG4 (3%), GG5 (2.4%) | 6.0 (IQR 4.7–8.1) | NR | 83 days (61–109) | < 2 months and then at monthly intervals of up to 6 months | Delays of up to 6 months were not associated with an increased risk of upgrading, ECE, SVI, PSM, or LNI |
| Zakaria, 2020 [16] | R | 1057 | Mean 60.9 (SD 6.5) | Low (36.0%); intermediate (53.6%); high (10.3%) UCSF-CAPRA risk | Mean 6.8 (SD 3.6) | NR | NR | NR | Cohort analysis showed correlation between CAPRA-score difference and wait time (Pearson correlation: r = − 0.062; p = 0.044) |
| Zanaty 2017 [15] | R | 619 | NR | Low (35%); intermediate (50%); high (15%) | NR | 22 months | 153 days | NR | Surgical wait time was positively correlated to BCR for high-risk group (p = 0.001). On threshold analysis, cutoff was found to be 90 days |
| Westerman 2019 [13] | R | 7350 | Mean 61.5 ± 7.1 | NCCN Risk Group: low (53.6%); intermediate (37.7%); high (8.7%) | Mean 7.1 ± 7.3 | 7.1 years (IQR 4.2–11.7) | 61 days (IQR 37–84) | ≤ 3 weeks, 4–6 weeks, 7–12 weeks, 12–26 weeks, and > 26 weeks | High risk men waiting more than 6 months had higher rates of BCR (HR: 3.38, p = 0.05) |
BCR biochemical recurrence; BCRFS biochemical recurrence-free survival; CI confidential interval; CR clinical recurrence; ECE extracapsular extension; HR hazard ratio; GG Gleason grade; IQR interquartile range; LNI lymph node invasion; MFS metastasis-free survival; NCCN National Comprehensive Cancer Network; OR odds ratio; P prospective; PCa prostate cancer, PCSM prostate cancer-specific mortality; PSM positive surgical margins; R retrospective; RP radical prostatectomy; SD standard deviation; SVI seminal vesicle invasion
Definition of delay
We found a significant heterogeneity across the studies in terms of delayed RP definitions. Most of the studies used 3-month intervals between diagnosis and RP [9, 10, 20–22]; three studies used 2-month intervals [23–25]. Diamand et al. and Nesbitt et al. used a cutoff of 3 months [26, 27], while Filippou et al. used a delay of > 6 months after diagnostic biopsy [11]; Patel et al. used an interval < 2 months and then monthly intervals up to 6 months [12]. Westerman et al. used the following intervals: ≤ 3, 4–6, 7–12, 12–26, and > 26 weeks [13]. Fossati et al., Zakaria et al., and Zanaty et al. used time between diagnosis and RP as a continuous variable [14–16]. Three studies reported that the delay groups were followed with an AS protocol [17–19]. Korets et al. excluded men on AS [24].
Definition of outcomes
Two studies reported data on survival outcomes, particularly on prostate cancer-specific mortality (PCSM) [25] and metastasis-free survival (MFS) [22]. Nine studies reported data on biochemical recurrence (BCR) [9, 13, 15, 21–24, 26]. Fourteen studies reported data on adverse pathologic outcomes such as upgrading, upstaging, positive surgical margins (PSM), extracapsular extension (ECE), seminal vesicle invasion (SVI), lymph node invasion (LNI), etc.[9–12, 16, 20–27]. Five studies reported data on the need of secondary therapy after surgery [20, 22, 23, 26, 27].
Observations of outcome
Biochemical recurrence
Five studies found no significant impact of treatment delay on BCR [21–24, 26], while four studies found that treatment delay had an unfavorable impact in intermediate- [9] and high-risk [13–15] PCa patients (Table 2).
Table 2.
Biochemical recurrence along the studies
| Author, publication year | Results according to definition of delay | ||||
|---|---|---|---|---|---|
| Anil 2018 [23] |
≤ 60 days (2 months) 27.6% |
61–120 days (2–4 months) 30.6% |
≥ 120 days (4 months) 0% |
p = 0.06 | |
| Korets 2011 [24] |
≤ 60 days (2 months) HR 1 |
61–90 days (2–3 months) HR 1.26; 95% CI 0.94–1.70; p = 0.12 |
> 90 days (3 months) HR 1.13; 95% CI 0.73–1.31; p = 0.43 |
||
| Abern 2012 [9] |
≤ 3 months HR 1 |
3–6 months HR 1.00; 95% CI 0.76–1.33; p = 0.989 |
6–9 months HR 0.72; 95% CI 0.40–1.28; p = 0.262 |
> 9 months HR 2.19; 95% CI 1.24–3.87; p = 0.01 |
|
| Morini 2017 [21] |
≤ 6 months HR 1 |
6–12 months HR 0.691; 95% CI 0.466–1.026; p = 0.067 |
> 12 months HR 0.416; 95% CI 0.139–1.243; p = 0.116 |
||
| Westerman 2019 [13] |
≤ 3 weeks IR: HR 1 High risk: HR 1 |
4–6 weeks IR: HR 1.07; 95% CI.88–1.31; p = 0.51 High risk: HR 1.15; 95% CI 0.86–1.54; p = 0.34 |
7–12 weeks IR: HR 1.11; 95% CI 0.91–1.36; p = 0.31 High risk: HR 1.35; 95% CI 0.98–1.87; p = 0.07 |
12–26 weeks IR: HR 0.98; 95% CI 0.75–1.29; p = 0.9 High risk: HR 1.16; 95% CI 0.71–1.91; p = 0.55 |
> 26 weeks IR: HR 0.99; 95% CI 0.58–1.68; p = 0.97 High risk: HR 3.03; 95% CI 1.05–8.78; p = 0.04 |
| Diamand 2020 [26] | 3-month delay was not significantly associated with BCR (HR 0.97, 95% CI 0.91–1.04, p = 0.6) | ||||
| Fossati 2016 [14] |
Time from biopsy to RP was significantly associated with an increased risk of BCR (HR 1.02, p = 0.0005) A significant increased risk of BCR after approximately 12 months was observed in high-risk group |
||||
| Zanaty 2017 [15] |
Surgical wait time was positively correlated to BCR for high-risk group (p = 0.001) On threshold analysis, cutoff was found to be 90 days |
||||
| Gupta 2019 [22] |
There was no significant difference in 2- and 5-year BCRFS between both intermediate- and high-risk patients who had RP < 3 months vs. 3–6 months after diagnosis in terms of GG: GG3: 78% vs. 83% and 69% vs. 66%, respectively, p = 0.6; GG4: 68% vs. 74% and 51% vs. 57%, respectively, p = 0.4; GG5: 58% vs. 74% and 48% vs. 54%, respectively, p = 0.2 |
||||
BCR biochemical recurrence; BCRFS biochemical recurrence-free survival; CI confidential interval; GG Gleason score; HR hazard ratio; IR intermediate risk; RP radical prostatectomy
Abern et al. suggested 9 months as a threshold for association with BCR (HR 2.10, p = 0.01) among men with intermediate-risk PCa [9]. For high-risk patients, Westerman et al. found that higher rates of BCR were observed with more than 6-month delay in RP (HR 3.38, p = 0.05) [13], while Fossati et al. reported significantly increased risk of BCR after approximately 12 months [14]. At the same time, Zanaty et al. reported a cutoff surgical waiting time of 90 days (3 months) for an increase in the rate of BCR (p = 0.001) [15].
In contrast, Diamand et al. observed no association between RP delay of more than 3 months after diagnosis with BCR (HR 0.97, 95% CI 0.91–1.04, p = 0.6) in both intermediate- and high-risk patients [26]. In agreement with these results, Gupta et al. reported that there was no significant difference in 2- and 5-year biochemical recurrence-free survival (BCRFS) in both intermediate- and high-risk patients who had RP < 3 months vs. those who had between 3 and 6 months after diagnosis [22].
In summary, owing to inconsistencies in findings among studies, the impact of deferred RP, in patients with intermediate-or high-risk PCa, on BCR is still controversial. However, most of the studies reported that around 3-month delay was not significantly associated with BCR, especially in intermediate-risk PCa patients (Table 2).
Survival outcomes
Two studies found no significant impact of treatment delay on survival outcomes (PCSM and MFS) [22, 25]. Aas et al. reported no association between RP-interval and PCSM in the intermediate-or high-risk groups in a study of 5163 patients with time from diagnosis to treatment stratified by intervals: ≤ 60, 61–90, 91–120 and 121–180 days [25]. Among 2303 intermediate- and high-risk patients, Gupta et al. reported that there was no significant difference in 2-, 5-, and 10-year MFS between patients who had RP < 3 months vs. 3–6 months after diagnosis and according to Gleason grade group (GG3: 98, 92, and 84% vs. 97, 95, and 91%, respectively, p = 0.4; GG4: 97, 90, and 72% vs. 94, 91, and 81%, respectively, p = 0.8; GG5: 89 and 81% vs. 91 and 71%, respectively, p = 0.9) [22]. Taken together, at least 3-month deferred RP does not seem to affect the oncologic survival outcomes in patients with intermediate- and/or high-risk PCa, but only two studies with different definition of delay assessed these outcomes.
Pathologic outcomes
Seven studies found no significant impact of treatment delay on pathologic findings [12, 20–22, 24–26], while five studies found that treatment delay had an unfavorable impact in intermediate- [9–11, 16, 23] and high-risk [10] PCa patients (Table 3). One study reported inconsistent correlations between time from diagnosis to RP and different pathologic outcomes [27].
Table 3.
Pathologic outcomes along the studies
| Author, publication year | Results according to definition of delay | |||||
|---|---|---|---|---|---|---|
| Anil 2018 [23] | ≤ 60 days (2 months) | 61–120 days (2–4 months) | ≥ 120 days (4 months) | |||
| EPE | Ref | OR 2.250; 95% CI 1.029–4.918; p = 0.042 | OR 0.694; 95% CI 0.206–2.341; p = 0.556 | |||
| SVI | Ref | OR 0.396; 95% CI 0.143–1.092; p = 0.073 | OR 0.162; 95% CI 0.024–1.111; p = 0.064 | |||
| PSM | Ref | OR 1.569; 95% CI 0.735–3.351; p = 0.244 | OR 1.674; 95% CI 0.509–5.513; p = 0.397 | |||
| LVI | Ref | OR 1.500; 95% CI 0.362–6.213; p = 0.576 | OR 1.640; 95% CI 0.110–24.540; p = 0.720 | |||
| Korets 2011 [24] | ≤ 60 days (2 months) | 61–90 days (2–3 months) | > 90 days (3 months) | |||
| ECE | Ref | OR 1.03; 95% CI 0.78–1.35; p = 0.84 | OR 0.95; 95% CI 0.69–1.04; p = 0.07 | |||
| SVI | Ref | OR 0.91; 95% CI 0.69–1.20; p = 0.49 | OR 0.91; 95% CI 0.62–1.11; p = 0.45 | |||
| PSM | Ref | OR 1.13; 95% CI 0.85–1.51; p = 0.38 | OR 1.06; 95% CI 0.66–1.41; p = 0.86 | |||
| LVI | Ref | OR 0.87; 95% CI 0.56–1.33; p = 0.53 | OR 0.99; 95% CI 0.59–1.68; p = 0.68 | |||
| UG | Ref | OR 1.11; 95% CI 0.83–1.48; p = 0.48 | OR 1.08; 95% CI 0.78–1.44; p = 0.96 | |||
| Aas, 2018 [25] | PSM |
≤ 60 days (2 months) IR: 29.6 HR: 27.7 |
61–90 days (2–3 months) IR: 30.4 HR: 34.4 |
91–120 days (3–4 months) IR: 23.1 HR: 33.9 |
121–180 days (4–6 months) IR: 21.1 HR: 35.7 |
p = 0.02 p = 0.46 |
| Ginsburg 2020 [20] | ≤ 3 months | 4–6 months | 7–9 months | 10–12 months | ||
| AP | Ref | OR 0.98; 95% CI 0.94–1.02; p = 0.31 | OR 1.02; 95% CI 0.91–1.13; p = 0.773 | OR 1.00; 95% CI 0.80–1.26; p = 0.98 | ||
| LVI | Ref | OR 1.02; 95% CI 0.93–1.12; p = 0.608 | OR 0.91; 95% CI 0.68–1.22; p = 0.533 | OR 1.06; 95% CI 0.65–1.74; p = 0.814 | ||
| UG | Ref | OR 1.00; 95% CI 0.95–1.05; p = 0.922 | OR 1.09; 95% CI 0.95–1.24; p = 0.228 | OR 1.06; 95% CI 0.82–1.37; p = 0.649 | ||
| Abern 2012 [9] | ≤ 3 months | 3–6 months | 6–9 months | > 9 months | ||
| ECE | Ref | NR | NR | OR 6.68, 95% CI 1.04–42.77, p = 0.045 | ||
| PSM | Ref | OR 1.01; 95% CI 0.71–1.44; p = 0.941 | OR 1.03; 95% CI 0.53–1.99; p = 0.929 | OR 4.08; 95% CI 1.52–10.91; p = 0.005 | ||
| Morini 2017 [21] | ≤ 6 months | 6–12 months | > 12 months | |||
| ECE | 9.9% | 12.1% | 10.6% | |||
| SVI | 6% | 3.8% | 2.1% | |||
| PSM | 34.1% | 33.5% | 31.2% | |||
| LVI | 2.7% | 4.3% | 2.1% | |||
| UG | 35.2% | 40.2% | 35.4% | |||
| Berg 2015 [10] | Significant increases in the proportion of adverse pathological outcomes were found beyond 60 days for patients with Gleason 7 and PSA > 20 (p = 0.032), and 30 days for patients with Gleason 8–10 and PSA 11–20 (p = 0.041) | |||||
| Diamand | 3-month delay was not significantly associated with upgrading (OR 0.98, 95% CI 0.94–1.02, p = 0.3), LNI (OR 0.88, 95% CI 0.77–1.01, p = 0.07), pathological locally advanced disease (OR 1, 95% CI 0.97–1.03, p = 0.8) | |||||
| Filippou 2015 [11] |
Immediate RP had a lower probability of adverse pathology than delayed RP > 6 months after diagnostic biopsy (OR 0.34, 95% CI 0.21–0.55) The rate of adverse pathology did not differ between immediate and delayed RP > 6 months in patients matched for pretreatment characteristics (OR 0.79, 95% CI 0.27–2.28) |
|||||
| Gupta 2019 [22] |
There was no significant difference in rates of PSM, EPE, SVI, or LNI in men who had RP < 3 months vs. 3–6 months after diagnosis in terms of GG: GG3: PSM: 22% vs. 21%, p = 0.7; EPE: 50% vs. 48%, p = 0.6; SVI: 13% vs. 11%, p = 0.3; LNI: 6% vs. 4%, p = 0.3; GG4: PSM: 19% vs. 21%, p = 0.7; EPE: 53% vs. 46%, p = 0.2; SVI: 13% vs. 13%, p = 1.0; LNI: 7% vs. 5%, p = 0.4; GG5: PSM: 34% vs. 32%, p = 0.7; EPE: 72% vs. 74%, p = 0.8; SVI: 32% vs. 33%, p = 0.8; LNI: 19% vs. 16%, p = 0.5 |
|||||
| Nesbitt 2020 [27] | Time between biopsy and surgery more than 90 days (3 months) was not associated with adverse outcomes (upgrading, PSM) except for pathological ECE or pT3 disease (p = 0.04) | |||||
| Patel 2019 [12] | Delays of up to 6 months were not associated with an increased risk of upgrading, ECE, SVI, PSM, or LNI | |||||
| Zakaria 2020 [16] | Cohort analysis showed correlation between CAPRA-score difference and wait time (Pearson correlation: r = − 0.062; p = 0.044) | |||||
Ap adverse pathology; CI confidential interval; ECE extracapsular extension; EPE exctraprostatic extension; GG Gleason score; HR high risk; IR intermediate risk; LNI lymph node invasion; OR odds ratio; PSM positive surgical margins; RP radical prostatectomy; SVI seminal vesicle invasion; UG upgrade
Diamand et al. found that 3 months as a threshold of RP delay was neither associated with upgrading (OR 0.98, 95% CI 0.94–1.02, p = 0.3), LNI (OR 0.88, 95% CI 0.77–1.01, p = 0.07), nor upstaging (OR 1, 95% CI 0.97–1.03, p = 0.8) [26]. In agreement with these results, Gupta et al. indicated that there was no significant difference in rates of PSM, ECE, SVI, or LNI in men with intermediate- and high-risk PCa who had RP < 3 months vs. 3–6 months after diagnosis [22]. Moreover, Ginsburg et al. reported that RP delay even up to 12 months was not significantly associated with adverse pathologic outcomes in patients with intermediate- and high-risk disease [20].
Some studies obtained controversial results. Abern et al. noted that if RP was delayed > 9 months, PSM (OR 4.08, p < 0.01) and ECE (OR 6.68, p = 0.045) rates were higher among men with intermediate-risk PCa [9]. More strict cutoff was obtained by Berg et al. in a study with median time from diagnosis to treatment of 64 days (48–90); the risk of adverse pathologic findings increased beyond 60 days for patients with intermediate-risk disease (p = 0.032) and 30 days for patients with high-risk disease (p = 0.041) [10]. Interestingly, Nesbitt et al. reported that a time between biopsy and surgery of more than 90 days (3 months) was not significantly associated with pathologic upgrading or higher rate of PSM. However, they found a higher proportion of patients with ≥ pT3 disease in the group who had RP > 90 days after the biopsy (p = 0.04) [27].
Summing up, according to the currently available literature, deferring RP in patients with intermediate- or high-risk PCa does not seem to lead to a worsening in pathologic outcomes. Ten of twelve studies agreed that around 3-month delay is unlikely to affect pathologic outcomes (Table 3).
Adjuvant therapy
Five studies found no significant impact of treatment delay on need for adjuvant therapy [20, 22, 23, 26, 27]. Diamand et al. found that RP delay of more than 3 months after diagnosis was not significantly associated with the need for adjuvant therapy (OR 0.96, 95% CI 0.84–1.11, p = 0.6) in intermediate- and high-risk patients [26]. Ginsburg et al. reported that RP delay up to 12 months was not significantly associated with post-RP secondary treatments in patients with both intermediate- and high-risk disease [20]. Gupta et al. reported that there was no significant difference in rates of adjuvant therapy in men with intermediate- and high-risk PCa who had RP < 3 months vs. 3–6 months after diagnosis [22]. Nesbitt et al. reported that waiting more than 90 days from biopsy to surgery did not put men at an increased risk of the need for postoperative treatment such as radiation therapy [27]. Anil et al. did not report a significant difference between the intervals between prostate biopsy and RP (≤ 60, 61–120, ≥ 120 days) in terms of additional treatment (p = 0.1) [23]. Thereby, deferred RP does not seem to be associated with the need for secondary treatment in patients with intermediate- or high-risk PCa. Although definition of a delayed surgery was different among all above studies, a delay around 3 months was shown to be safe in all of them in terms of the need for adjuvant therapy.
Clinical implications and discussion
During the current COVID-19 pandemic, it is critical for clinicians to consider the impact of delayed RP for individual PCa patients [28]. COVID-19 should not result in missing the ideal time frame for treatment, especially in men with high-risk disease. For this reason, we conducted a systematic review summarizing the available evidence on the impact of deferring RP in men with intermediate- and high-risk PCa.
According to the currently available literature, there is no clear, reproducible, and significant difference in oncologic and survival outcomes, including PCSM and MFS, between patients who underwent immediate RP and those who underwent deferred RP after approximately 3 months. Although it seems that the delaying of RP for 3–6 months after diagnosis in patients with intermediate- and high-risk PCa was not associated with worsening of the survival outcomes, the current data are not sufficient enough to reliably consider this as an evidence. This is mainly due to the limited numbers of studies assessing the impact of this delay on the disease outcome. Furthermore, BCR and adverse pathologic findings were assessed in almost all the studies included in this systematic review. However, the impact of delayed surgery on these outcomes is still controversial, at best, owing to the inconsistency in study populations, methods, and endpoints. Some studies suggest that the delaying RP may even be safe for up to 12 months, while most of the studies reported shorter safe delay periods (i.e., 3–6 months). Indeed, different definitions of delay in the literature led to inconsistency in the results. Consequently, any decision to delay the definitive treatment of patients with intermediate- and/or high-risk PCa will tend to bias in terms of the safe delay time. Moreover, it is important to note that the retrospective nature of most of the selected studies may have led to a selection bias with regards to comorbidities of patients who underwent immediate RP and those who underwent deferred RP. The majority of the studies did not report the reason for the delay. While the delay of a definitive treatment in patients with intermediate- and/or high-risk PCa should be done with caution and based on a rational decision, a 3-month delay may be safe and acceptable. Due to selection bias, elderly patients with more comorbidities could be most likely to be selected for the delayed management than younger patients without comorbidities (possible attrition bias).
The Prostate Cancer EAU Guidelines Panel, which is applicable in the current COVID-19 pandemic, recommends postponing RP until after the pandemic in intermediate- and high-risk PCa patients and does not advocate for treatment with neoadjuvant ADT in most cases [4]. On the other hand, recently, there was an international accelerated consensus on the management of intermediate-risk patients using close surveillance, while high-risk prostate cancer patients may be managed using either immediate surgery in the absence of COVID-19 risk features or alternatively treated using ADT until it is safe to proceed with surgery [29]. According to the present systematic review findings, we could recommend a 3-month delay as a safe delay time in intermediate-risk PCa patients and the same delay with caution for high-risk PCa patients until safe surgical conditions are possible.
Our systematic review is not devoid of limitations. The main limitation is the retrospective and heterogeneous nature of most of the included studies. We found significant heterogeneity across the studies in terms of delay definitions, definitions of outcomes (endpoints), and baseline clinicopathologic features of patients. We were also unable to elaborate on the effect of treatment delay in different PCa risk groups due to the fact that most of the included studies did not report separate analyses based on risk groups. Moreover, performing a quantitative synthesis was not feasible due to different outcomes of interest and heterogeneity in the definition of delayed RP. Therefore, well-designed controlled comparative studies are required to clarify our findings.
Conclusions
The COVID-19 pandemic is likely to continue affecting the health care system for the foreseeable future. Consequently, it is important to prioritize the timely care of patients with PCa for whom delays are most likely to result in worse oncologic outcomes. According to the present systematic review in patients with intermediate- and high-risk PCa, a delay of RP for up to 3 months is likely to be safe, as it is not associated with biochemical recurrence, worse oncological survival outcomes, other adverse pathologic outcomes, or the need for adjuvant therapy. In addition, because of the long duration of the current pandemic and the fact that it could continue for a longer time period, we recommend further studies to prospectively assess the outcomes secondary to delays in PCa patients care during the current pandemic. This could help elucidate the oncologic impact of delays and prepare for future events that could result in prolonged delays in definitive care in patients with intermediate- and high-risk PCa.
Acknowledgements
Ekaterina Laukhtina and Victor M. Schuettfort are supported by the EUSP Scholarship of the European Association of Urology (EAU). Nico C. Grossmann is supported by the Zurich Cancer League.
Author's contribution
EL and RSM: data analysis and manuscript writing; KM, FQ, VMS, HM, SK, NCG, GP, PIK, AB, MA, DE, and BP: manuscript editing; SFS: manuscript editing and project development.
Funding
Open access funding provided by Medical University of Vienna. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
All authors state that they have no conflict of interest that might bias this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ekaterina Laukhtina and RezaSari Motlagh are co-first authors.
References
- 1.Guan W, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallis CJD, et al. The impact of the COVID-19 pandemic on genitourinary cancer care: re-envisioning the future. Eur Urol. 2020 doi: 10.1016/j.eururo.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teoh JYC, et al. A global survey on the impact of COVID-19 on urological services. Eur Urol. 2020 doi: 10.1016/j.eururo.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribal MJ, Cornford P, Briganti A, Knoll T, Gravas S, Babjuk M, Harding C, Breda A, Bex A, GORRG Group, Rassweiler JJ, Gözen AS, Pini G, Liatsikos E, Giannarini G, Mottrie A, Subramaniam R, Sofikitis N, Rocco BMC, Xie LP, Witjes JA, Mottet N, Ljungberg B, Rouprêt M, Laguna MP, Salonia A, Bonkat G, Blok BFM, Türk C, Radmayr C, Kitrey ND, Engeler DS, Lumen N, Hakenberg OW, Watkin N, Hamid R, Olsburgh J, Darraugh J, Shepherd R, Smith EJ, Chapple CR, Stenzl A, Van Poppel H, Wirth M, Sønksen J, N'Dow J EAU section offices and the EAU guidelines panels. European Association of Urology guidelines office rapid reaction group: An organisation-wide collaborative effort to Adapt the European Association of Urology guidelines recommendations to the coronavirus disease 2019 era. Eur Urol. 2020;78(1):21–28. doi: 10.1016/j.eururo.2020.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallis CJD, et al. Risks from deferring treatment for genitourinary cancers: a collaborative review to aid triage and management during the COVID-19 pandemic [formula presented] Eur Urol. 2020 doi: 10.1016/j.eururo.2020.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patt D, et al. The Impact of COVID-19 on Cancer Care: how the pandemic is delaying cancer diagnosis and treatment for American Seniors. JCO Clin Cancer Informatics. 2020 doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enikeev D, et al. Active surveillance for intermediate risk prostate cancer: a systematic review and meta-analysis of current protocols and outcomes. Clin Genitourin Cancer. 2020 doi: 10.1016/j.clgc.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abern MR, et al. Delayed radical prostatectomy for intermediate-risk prostate cancer is associated with biochemical recurrence: possible implications for active surveillance from the SEARCH database. Prostate. 2013;73(4):409–417. doi: 10.1002/pros.22582. [DOI] [PubMed] [Google Scholar]
- 10.Berg WT, et al. Delay from biopsy to radical prostatectomy influences the rate of adverse pathologic outcomes. Prostate. 2015;75(10):1085–1091. doi: 10.1002/pros.22992. [DOI] [PubMed] [Google Scholar]
- 11.Filippou P, Welty CJ, Cowan JE, Perez N, Shinohara K, Carroll PR. Immediate versus delayed radical prostatectomy: updated outcomes following active surveillance of prostate cancer. Eur Urol. 2015;68(3):458–463. doi: 10.1016/j.eururo.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Patel P, Sun R, Shiff B, Trpkov K, Gotto GT. The effect of time from biopsy to radical prostatectomy on adverse pathologic outcomes. Res Reports Urol. 2019 doi: 10.2147/RRU.S187950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westerman ME, et al. Impact of time from biopsy to surgery on complications, functional and oncologic outcomes following radical prostatectomy. Int Braz J Urol. 2019 doi: 10.1590/S1677-5538.IBJU.2018.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fossati N, et al. Evaluating the effect of time from prostate cancer diagnosis to radical prostatectomy on cancer control: Can surgery be postponed safely? Urol Oncol Orig Investig. 2017;35(4):150.e9. doi: 10.1016/j.urolonc.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Zanaty M, et al. Does surgical delay for radical prostatectomy affect biochemical recurrence? A retrospective analysis from a Canadian cohort. World J Urol. 2018;36(1):1–6. doi: 10.1007/s00345-017-2105-6. [DOI] [PubMed] [Google Scholar]
- 16.Zakaria AS, et al. Impact of surgical wait times during summer months on the oncological outcomes following robotic-assisted radical prostatectomy: 10 years’ experience from a large Canadian academic center. World J Urol. 2020 doi: 10.1007/s00345-020-03496-2. [DOI] [PubMed] [Google Scholar]
- 17.Cooperberg MR, et al. Outcomes of active surveillance for men with intermediate-risk prostate cancer. J Clin Oncol. 2011;29(2):228–234. doi: 10.1200/JCO.2010.31.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godtman RA, Schafferer M, Pihl C-G, Stranne J, Hugosson J. Long-term outcomes after deferred radical prostatectomy in men initially treated with active surveillance. J Urol. 2018;200(4):779–785. doi: 10.1016/j.juro.2018.04.078. [DOI] [PubMed] [Google Scholar]
- 19.Balakrishnan SA, Cowan EJ, Cooperberg RM, Katsuto S, Nguyen GH, Carroll RP. Evaluating the safety of active surveillance: outcomes of deferred radical prostatectomy after an initial period of surveillance. J Urol. 2019;202(3):506–510. doi: 10.1097/JU.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 20.Ginsburg KB, Curtis GL, Timar RE, George AK, Cher ML. Delayed radical prostatectomy is not associated with adverse oncologic outcomes: implications for men experiencing surgical delay due to the COVID-19 pandemic. J Urol. 2020;204(4):720–725. doi: 10.1097/JU.0000000000001089. [DOI] [PubMed] [Google Scholar]
- 21.Morini MA, Muller RL, de Castro Junior PCB, de Souza RJ, Faria EF. Time between diagnosis and surgical treatment on pathological and clinical outcomes in prostate cancer: does it matter? World J Urol. 2018;36(8):1225–1231. doi: 10.1007/s00345-018-2251-5. [DOI] [PubMed] [Google Scholar]
- 22.Gupta N, et al. Evaluating the impact of length of time from diagnosis to surgery in patients with unfavourable intermediate-risk to very-high-risk clinically localised prostate cancer. BJU Int. 2019 doi: 10.1111/bju.14659. [DOI] [PubMed] [Google Scholar]
- 23.Anıl H, et al. Impact of delay from biopsy to surgery on the rate of adverse pathologic and oncologic outcomes for clinically localized prostate cancer. Bull Urooncol. 2018;17(4):133–137. doi: 10.4274/uob.1084. [DOI] [Google Scholar]
- 24.Korets R, Seager CM, Pitman MS, Hruby GW, Benson MC, McKiernan JM. Effect of delaying surgery on radical prostatectomy outcomes: a contemporary analysis. Bju Int. 2012;110(2):211–216. doi: 10.1111/j.1464-410X.2011.10666.x. [DOI] [PubMed] [Google Scholar]
- 25.Aas K, et al. Is time from diagnosis to radical prostatectomy associated with oncological outcomes? World J Urol. 2019;37(8):1571–1580. doi: 10.1007/s00345-018-2570-6. [DOI] [PubMed] [Google Scholar]
- 26.Diamand R, et al. Timing and delay of radical prostatectomy do not lead to adverse oncologic outcomes: results from a large European cohort at the times of COVID-19 pandemic. World J Urol. 2020 doi: 10.1007/s00345-020-03402-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nesbitt AL, Smith PG, Antoniou S, Evans GA, Pridgeon SW. Delay to radical prostatectomy: who, why and does it matter? J Clin Urol. 2020 doi: 10.1177/2051415820945933. [DOI] [Google Scholar]
- 28.Obek C, Doganca T, Argun OB, Kural AR. Management of prostate cancer patients during COVID-19 pandemic. Prostate Cancer Prostatic Dis. 2020 doi: 10.1038/s41391-020-0258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tandogdu Z, et al. Management of patients who opt for radical prostatectomy during the COVID-19 pandemic: An International Accelerated Consensus Statement. BJU Int. 2020 doi: 10.1111/bju.15299. [DOI] [PubMed] [Google Scholar]