Abstract
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer. Several treatment options are available for managing HCC patients, classified roughly as local, local-regional, and systemic therapies. The high post-monotherapy recurrence rate of HCC urges the need for the use of combined modalities to increase tumor control and patient survival. Different international guidelines offer treatment recommendations based on different points of view and classification systems. Radiotherapy (RT) is a well-known local-regional treatment modality for managing many types of cancers, including HCC. However, only some of these treatment guidelines include RT, and the role of combined modalities is rarely mentioned. Hence, the present study reviewed clinical evidence for the use of different combined modalities in managing HCC, focusing on modern RT's role. Modern RT has an increased utility in managing HCC patients, mainly due to two driving forces. First, technological advancement (e.g., stereotactic body radiotherapy and advanced proton-beam therapy) enables precise delivery of radiation to increase tumor control and reduce side effects in the surrounding normal tissue. Second, the boom in developing target therapies and checkpoint-blockade immunotherapy prolongs overall survival in HCC patients, re-emphasizing the importance of local tumor control. Remarkably, RT combines with systemic therapies to generate the systemic therapy augmented by radiotherapy effect, a benefit now being actively investigated.
Keywords: Hepatocellular carcinoma, Stereotactic body radiotherapies, Radiotherapy, Guideline, Combined treatment, Immunotherapy
Core Tip: Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer and treatments outcome are often unsatisfactory. The high post-monotherapy recurrence rate points to the need to combine modalities, including radiotherapy (RT). The international guidelines from North America, Europe and Asia offer treatment recommendations based on different classification systems. However, not all of these treatment guidelines include RT and the role of combined modalities. Hence, the present study reviewed clinical evidence of different combined modalities in managing HCC, focusing on RT's role and especially modern advanced RT techniques and the interaction with game-changing immunotherapy.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver, ranking as the sixth leading cause of cancer incidence and the fourth most common etiology of cancer death worldwide; overall, around 953000 cases were diagnosed in 2017 globally[1]. The majority of HCC patients are found in the Asia–Pacific region, and around 75%-90% of cases are associated with chronic viral hepatitis (mainly hepatitis B and C) infection[2-4]. Therefore, the management of HCC requires considering detailed information from three main domains together: Cancer characteristics, liver function reserve, and overall patient condition.
Several treatment modalities are available for managing HCC patients; these modalities are generally classified as local, local-regional, and systemic therapies. Local therapy includes surgical resection, ablative therapies, and radiotherapy (RT). Ablative therapies include radiofrequency ablation (RFA)[5-7], percutaneous ethanol injection therapy[8], cryoablation[9], and microwave[10]; these modalities can be performed through percutaneous, laparoscopic, or open approaches. Local-regional or so-called arterially directed therapies include trans-arterial embolization (TAE), trans-arterial chemoembolization (TACE)[5], drug-eluting beads (DEB)-TACE[11-13], trans-arterial radioembolization (TARE) with yttrium 90[14,15], and hepatic arterial infusion chemotherapy (HAIC)[16-18]. Systemic therapy includes target therapy (e.g., sorafenib[19], lenvatinib[20], and regorafenib[21]) and immunotherapy (e.g., nivolumab[22]); more notably, the use of several such types of agents has been investigated aggressively.
The preferred curative surgical modalities include liver resection and liver transplantation, generating a 5-year overall survival rate of up to 70%-75%[23,24]. RFA is another frequently used curative modality, with a 5-year overall survival rate of around 40%-70%[24]. However, local control of RFA is decreased in patients with large tumors, especially those larger than 3 cm[25,26]. It is recognized that only 10%-30% of HCC patients are candidates for curative surgical options because most HCCs are diagnosed at an intermediate or advanced stage[27,28]. Even though many treatment options can be chosen for HCC patients and each treatment modality has seen advancement in past decades, 5-year overall survival rates are still unsatisfactory, being less than 20%[29].
A high treatment failure (i.e., recurrence) rate is reported in the heterogeneous group of patients with intermediate- to advanced-stage HCC. Five-year tumor recurrence rates are more than 50% after liver resection and may be up to 80% after RFA[30]. This high recurrence rate includes patients who underwent TACE or some other singular local treatment modality. Of note, intrahepatic recurrence is the most common recurrence pattern. This unsatisfactory tumor control rate suggests that combining different locoregional therapies may improve treatment outcomes[31-33]. This review focuses mainly on RT's current role in managing HCC, not only as a monotherapy but also as an essential part of combined modalities, especially stereotactic body radiotherapy (SBRT)/stereotactic ablative radiotherapy (SABR).
CURRENT TREATMENT GUIDELINES FOR HCC
Currently, useful treatment guidelines for managing HCC include recommendations from the National Comprehensive Cancer Network (NCCN)[34], the American Association for the Study of Liver Diseases (AASLD)[35], the European Association for the Study of the Liver (EASL)[36], the Japan Society of Hepatology (JSH)-HCC[37], the Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC)[38], the National Health and Family Planning Commission (NHFPC) of the People’s Republic of China HCC guideline[39], and the Taiwan Liver Cancer Association (TLCA)[40].
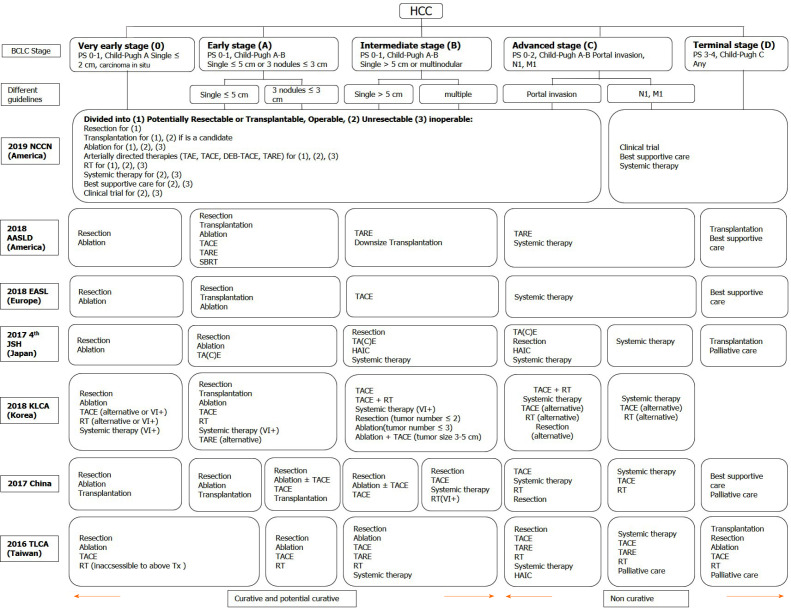
In practice, these guidelines implemented different classification systems to stratify HCC patients for appropriate management. For example, the NCCN grouped patients as potentially resectable/transplantable vs unresectable/inoperable to guide treatment options. The AASLD and EASL guidelines used the Barcelona clinic liver cancer (BCLC) stage to lead management recommendations. The KLCA and NCC guidelines used a modified Union for International Cancer staging system adapted from the Liver Cancer Study Group of Japan[41,42]. Both the JSH-HCC and TLCA guidelines adopted hepatic functional reserve, extra-hepatic metastasis, vascular invasion, tumor number, and tumor size to guide treatment choice using a step-by-step manner and the Chinese guideline added general health status to the previously-mentioned risk factors[37,39,40]. Due to the variety of classification systems used in these different guidelines, we summarized treatment recommendations according to the BCLC stage in Figure 1.
Figure 1.
Treatment recommendations modified in different guidelines according to the Barcelona clinic liver cancer stage. HCC: Hepatocellular carcinoma; BCLC: Barcelona clinic liver cancer; PS: Performance status; TA(C)E: Trans-arterial (chemo)embolization; TARE: Trans-arterial radioembolization; DEB-TACE: Trans-arterial chemoembolisation with drug-eluting beads; RT: Radiotherapy; SBRT: Stereotactic body radiation therapy; HAIC: Hepatic arterial infusion chemotherapy; NCCN: National Comprehensive Cancer Network; AASLD: American Association for the Study of Liver Diseases; EASL: European Association for the Study of the Liver; JSH: Japan Society of Hepatology; KLCA: Korean Liver Cancer Association; TLCA: Taiwan Liver Cancer Association; Tx: Treatments; VI+: Positive vascular or bile duct invasion.
COMBINED MODALITY FOR HCC
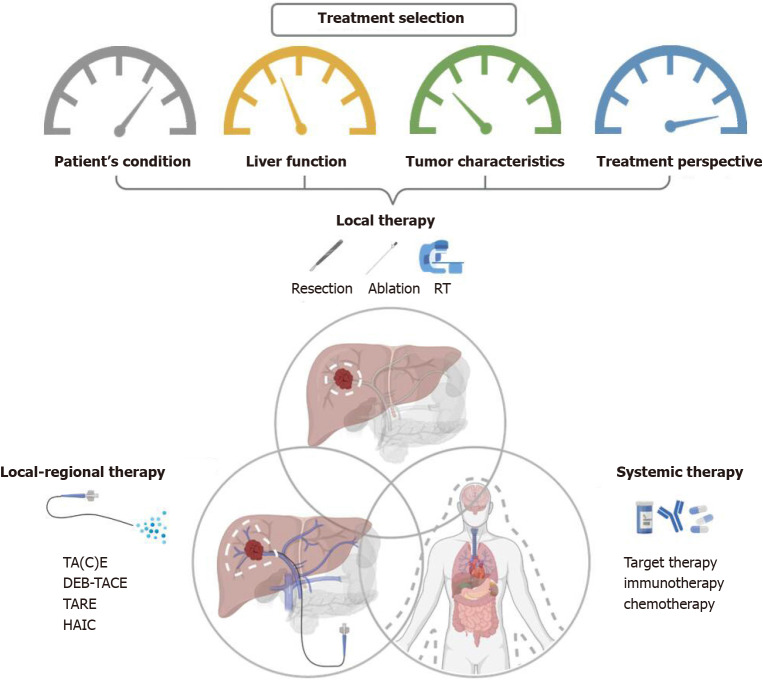
None of the guidelines mentioned above well declare the role of the combined treatment strategies used frequently in daily practice. Clinically, the choice of different combined modalities is based not only on guidelines or evidence, but also on the individual patient’s condition, the liver function preservation, the tumor characteristics, and the treatment perspective including the availability of resources within the facility and the therapeutic ratio. Overview of therapeutic options and the consideration behind the combination of different modalities for liver cancer are illustrated in Figure 2.
Figure 2.
Overview of therapeutic options and the consideration behind the combination of different combined modalities for liver cancer. RT: Radiotherapy; TA(C)E: Trans-arterial (chemo)embolization; TARE: Trans-arterial radioembolization; DEB-TACE: Trans-arterial chemoembolisation with drug-eluting beads; HAIC: Hepatic arterial infusion chemotherapy.
For very early and early stage HCC
Some anatomic locations of HCC, such as tumors adjacent to the gallbladder, liver hilum, bowel, stomach, and other critical organs, may limit the use of RFA as an intervention[43]. On the other hand, RT can be delivered to tumors that arise from any location, so that it can compensate for or combine with RFA.
For intermediate stage HCC
Many patients receive TACE as their first local-regional therapy. However, TACE alone seldom achieves satisfactory tumor control[44]. Therefore, several combined modalities have been reported to increase treatment outcomes, and they are subdivided into three main categories, as follows.
Local-regional plus local therapy: TACE combined with RFA achieves a complete response (CR) rate of 55-65% at the time of the first or second post-treatment check[31,32]. TACE combined with conventionally fractionated radiotherapy (CFRT) demonstrates a better 1-year survival rate than TACE alone[45]. Remarkably, TACE combined with SBRT shows promising results[46]. In contrast, the role of HAIC in conjunction with RT is still under investigation[47].
Local-regional plus another local-regional therapy: TACE combined with TARE has been reported as a safe and effective treatment modality for bi-lobar HCC[48].
Local-regional plus systemic therapy: This type of combination includes such options as TACE combined with target therapy[44,49,50], TARE combined with target therapy[51-53], TACE combined with immunotherapy[54-56], and TARE combined with immunotherapy[57].
For advanced HCC
Only limited HCC patients are responsive to immune checkpoint inhibitors, and a combination of these with RT may enhance the immune response; this phenomenon is named the systemic therapy augmented by radiotherapy (STAR) effect[58]. Overall, RT or SBRT combined with other treatment modalities has been applied increasingly in HCC patients.
THE ROLE OF RT IN HCC
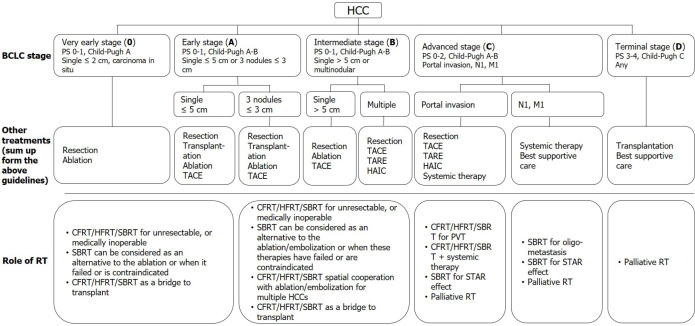
RT was used as a salvage or palliative treatment in the past, and only a few guidelines mention the role of RT. However, in the modern era, RT is indicated across all stages (i.e., from very early to end-stage HCC)[34,35,37,40]. Notably, RT can be used as a single therapy or as an essential component of a combined modality. Current treatment recommendations based on the BCLC stage and RT's potential roles are summarized in Figure 3.
Figure 3.
Current treatment recommendations based on the Barcelona clinic liver cancer stage and the potential roles of radiotherapy. HCC: Hepatocellular carcinoma; BCLC: Barcelona clinic liver cancer; PS: Performance status; TACE: trans-arterial chemoembolization; TARE: Trans-arterial radioembolization; HAIC: Hepatic arterial infusion chemotherapy; RT: Radiotherapy; CFRT: Conventionally fractionated radiotherapy; HFRT: Hypo-fractionated radiotherapy; SBRT: Stereotactic body radiation therapy; PVT: Portal vein thrombosis; STAR effect: Systemic therapy augmented by radiotherapy effect.
Different RT techniques
Photon therapy: The most commonly available treatment beam of RT is photons. In managing patients with HCC, several photon-beam delivery systems of external-beam radiation therapy (EBRT) are clinical available, such as conventional fractionated RT (CFRT), hypo-fractionated RT (HFRT), and SBRT. CFRT is usually delivered with daily fractions of 1.8-2 Gy, and HFRT is characterized by a large daily dose (i.e., > 2 Gy) in the context of precise RT. Clinically, HFRT is a useful strategy for improving dose intensity and then tumor control. Both CFRT and HFRT can be delivered by using three-dimensional conformal radiotherapy (3DCRT), intensity-modulated radiotherapy (IMRT), and Volumetric-modulated Arc Therapy (VMAT).
Remarkably, SBRT, or so-called SABR, is an advanced technique of EBRT that delivers a very high dose of irradiation in a very precise way in a limited number of treatment fractions (i.e., usually 3-6 fractions and > 5 Gy per fraction) over a treatment course of 1-2 wk. For more focused and accurate delivery of SBRT, advancements across the whole RT department should be provided, including imaging, immobilization, target delineation, treatment planning, on-board image guidance, and respiratory motion management (RMM). Only advanced IMRT or VMAT with or without non-coplanar beam designs can be used for delivering SBRT. These advancements result in better dose distribution, deliver a higher dose within the tumor, and generate a rapid dose fall-off outside the target. Thus, SBRT can improve tumor control and reduce the irradiation dose to the surrounding normal tissue, to decrease RT toxicity. Owing to this double benefit of enhancing therapeutic gain, SBRT is highly recommended in managing HCC patients treated with curative intent.
For patients who cannot be treated successfully with SBRT, CFRT combined with two or more advanced irradiation techniques, such as combined VMAT and Simultaneously Integrated inner-Escalated Boost (SIEB), may be helpful to achieve a better therapeutic gain (i.e., better tumor control with minimal RT toxicity) compared to conventional CFRT, including for elderly HCC patients who have inoperable disease[59].
Proton therapy: Charged particle irradiations, including proton-beam therapy (PBT) and carbon-ion RT, have unique dosimetric characteristics. That is, they eliminate the low-dose bath volume distal to the target area that is associated with photons. This elimination is because the characteristic Bragg peak of charged particles deposits irradiation energy mainly within the targeted tumor area and results in a near-zero dose beyond the end of its path[60]. Therefore, charge particle irradiation represents an excellent option for improving normal liver sparing and minimizing side effects such as radiation-induced liver disease (RILD). It also makes possible dose escalation for curing unresectable huge HCC.
Park et al[61] reported that dose escalation could enhance HCC tumor control. Kim et al[62] further confirmed that proton dose escalation is safe and effective; they suggested an EQD2 ≥ 78 Gy-equivalents (GyE) could be delivered to achieve reasonable tumor control. According to the tumor location, the University of Tsukuba proton team developed different PBT dose protocols[63-65]. Extrapolating on the concept of a lung cancer SBRT “No Fly Zone”[66], peripheral liver tumors located at > 2 cm from the hepatic portal region or gastrointestinal (GI) tract can be treated with hypofractionated proton 66 GyE in 10 fractions. On the other hand, for tumors located within 2 cm adjacent to the hepatic portal region, small doses per fraction with 72.6 GyE in 22 fractions should be considered. For tumors located within 2 cm of the GI tract, 77.0 GyE in 35 fractions may be given[63-65].
Several studies report using PBT for localized HCC with excellent local control ranging from 80% to 100%, even for huge unresectable HCCs, due to dose escalation and sparing of more liver function[67-69]. Furthermore, Sanford et al[70] reported that the overall survival (OS) benefit of proton-RT over photon-RT might be due to decreasing the incidence of RILD. Hsieh et al[71] further identified the predictors of RILD in HCC patients treated with PBT beyond the conventional concept of minimizing the mean liver dose. A "volume-response" relationship between unirradiated liver volume (ULV)/standard liver volume (SLV) and RILD was found: For Child-Pugh A patients, it was < 50%; for Child-Pugh B patients, it was < 30%[71].
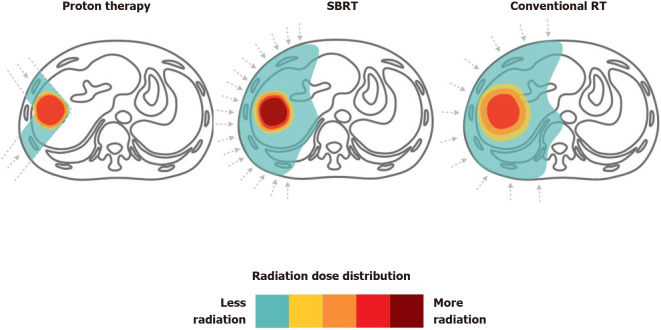
Both photon and PBT can achieve high rates of local control with acceptable toxicity. However, PBT has better potential to deliver a higher dose while maximizing the volume of unirradiated liver. Clinically, the reduced normal liver dose achieved by PBT is not critically required for all patients, since some may benefit from a smaller irradiated target volume when normal liver constraints can be met. The 2018 Miami Liver Proton Therapy Conference reached the consensus that the patients who should be strongly prioritized for PBT include those with: At least Child-Pugh B cirrhosis, a high tumor-to-liver ratio (i.e., larger tumor size or smaller uninvolved liver volume), a greater number of tumors, or prior RT to the liver[72]. The dose comparison of proton therapy vs SBRT vs conventional RT for liver tumors are illustrated in Figure 4.
Figure 4.
Proton therapy vs stereotactic body radiation therapy vs conventional radiotherapy for liver tumors. Dose distributions for a proton (left), stereotactic body radiation therapy (middle) and conventional radiotherapy (right) hepatocellular carcinoma radiotherapy plan are illustrated for comparison. RT: Radiotherapy; SBRT: Stereotactic body radiation therapy.
The role of RT in very early and early stage HCC
Very early and early stage HCCs include those with BCLC classification 0-A, as follows: Carcinoma in situ; a single tumor of ≤ 2 cm, a single tumor of ≤ 5 cm, or three tumors of < 3 cm; and tumor burden laying within the Milan criteria, with Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 as well as Child-Pugh classification A-B[73]. For these HCC patients, although the standard of care is still surgery and RFA, definitive SBRT is a potential third curative treatment modality for medically inoperable, technically unresectable, and difficult to RFA conditions; more notably, it can serve as a bridge to liver transplantation[26,74].
The role of RT in intermediate stage HCCs
Intermediate stage HCCs include those with BCLC classification B, as follows: Multi-tumors, a single tumor of > 5 cm, good patient condition (i.e., PS 0-1), as well as good liver reserve (i.e., Child-Pugh A-B). Tumor burden can be further subdivided, as follows: (1) Beyond Milan criteria but within the Up-to-7 criteria; and (2) Tumors beyond the Up-to-7 criteria[75]. For these patients, only limited cases can be treated by surgery or RFA. Several combined-modality approaches, including local therapy, local-regional therapy, and systemic therapy, have been reported in conjunction with CFRT and SBRT[76,77].
The role of RT in advanced stage HCCs
Advanced stage HCCs include those with BCLC classification C, with the criteria of portal vein invasion, inferior vena cava/heart invasion or thrombosis, lymph node metastasis, distant metastasis, and Child-Pugh A-B. SBRT or conventional RT may be applied in conjunction with other local, local-regional, and systemic therapies to serve as a potentially curative or palliative treatment[76-78].
The role of RT in terminal stage HCCs
Terminal stage HCCs include those with BCLC classification D, with the criteria of Child-Pugh C or ECOG PS 3-4. For these patients, SBRT with careful planning is safe as a bridge to liver transplantation in selected patients with a Child-Pugh score of ≥ 8. Additionally, SBRT or conventional RT can be used to treat symptoms[79-81].
THE ROLE OF SBRT IN MANAGING PATIENTS WITH HCC
SABR/SBRT
Recently, clinical evidence has rapidly grown for the use of SBRT in managing all stage HCC patients, with curative, potentially curative, or palliative intent[33,77,78,82]. Prospective clinical trials have demonstrated that SBRT effectively treats HCC, resulting in satisfactory local control, ranging from 75% to 100% at 1 year and 65% to 100% at 2 years[33,77,78]. Local control of HCC using SBRT is typically defined as no progression or no recurrent disease within the irradiated field according to the Response Evaluation Criteria in Solid Tumors (RECIST) or its modification (mRECIST)[83-85]. Moreover, SBRT showed a benefit of limited toxicity, with a severe late toxicity rate of < 15%; thus, SBRT is considered a safe modality for treating elderly patients[86]. In the literature, most patients treated with SBRT have Child-Pugh A disease and limited lesions (often < 3 tumors), and delivering SBRT in Child-Pugh B patients increases toxicity rates[80]. However, if dose modification is done to meet the more conservative (i.e., strict) normal tissue constraints, SBRT may be allowed for patients with small HCCs with Child-Pugh B or those with relatively larger tumors with a Child-Pugh score of 7[80].
SBRT as an alternative treatment modality to ablation therapy (i.e., RFA)
Ablative therapy is curative in treating small tumors (i.e., ≤ 3 cm) that locate in a feasible location, achieving excellent local control rates of around 70-90%; these results are similar to that of surgical resection[87]. Under such conditions, ablative therapy is considered an alternative treatment to surgical resection or liver transplantation.
Recently, SBRT showed comparable outcomes with RFA. Wahl et al[26] compared treatment outcomes between SBRT and RFA; they declared that both are effective for patients with inoperable HCC, with comparable freedom from local progression (FFLP) and comparable OS rates. The 1- and 2-year FFLP rates of SBRT were 97.4% and 83.8% vs 83.6% and 80.2% for RFA, respectively. The 1- and 2-year OS rates were also similar between the two treatment modalities. Remarkably, for larger tumors of ≥ 2 cm, SBRT demonstrated a better FFLP than that of RFA [hazard ratio (HR), 3.35; 95% confidence Interval (CI): 1.17-9.62; P = 0.025][26].
Hara et al[88] used propensity score matching (PSM) to assess the pre-treatment characteristics of BCLC stage, computed tomography (CT) status, and tumor size; they reported comparable 3-year OS rates between RFA and SBRT. Kim et al[89] also applied PSM to compare treatment results of SBRT and RFA. Two-year FFLP rates were 74.9% for the SBRT group and 64.9% for the RFA group. Taking these data together, SBRT demonstrates an emerging role as a curative treatment modality that is an alternative to ablative therapy for managing HCC patients.
SBRT as an alternative or adjuvant therapy to arterially directed therapies
Among arterially directed therapies, TACE is the most widely used treatment modality for managing HCC patients with an intermediate stage, applied in 50%-60% of patients[90]. However, TACE alone demonstrates unsatisfactory local control. This unacceptably low response rate suggests the use of local therapy, such as SBRT, as an alternative or adjuvant therapy to improve local control.
SBRT as an alternative therapy to TACE: Sapir et al[91] used PSM with inverse treatment weighting probability to compare SBRT and TACE in HCC patients with 1-2 tumors. They found that SBRT demonstrated better 1- and 2-year local control rates when compared with TACE: 97% and 91% vs 47% and 23% (HR, 66.5; 95%CI: 18.99-233.0; P< 0.001), respectively. However, the difference in OS did not significantly differ between the two treatment modalities.
SBRT combined with TACE: Several retrospective studies and reviews have demonstrated increased local control rates by adding conventional RT to TACE[45,92,93]. However, studies using SBRT to replace conventional RT are scarce because SBRT is a new technique.
A pilot study and preliminary results of prospective studies also confirmed the safety and efficacy of SBRT in patients with HCC that failed to respond to TACE[94-96]. Kang et al[46] published a phase-II trial that enrolled 47 patients who received TACE 1 to 5 times before SBRT. The result showed a good response, with a 2-year local control rate of 94.6%. Moreover, 38.3% of patients achieved a complete response within 6 mo. However, there is still no published data from head-to-head trials that compare TACE plus SBRT with TACE alone. Several clinical trials are ongoing (ClinicalTrials.gov Identifier: NCT02762266, NCT02323360, NCT02323360, NCT02794337, NCT02921139).
SBRT combined with TARE: Previously, there has been much concern over increasing radiation-related toxicity when using SBRT after TARE. Hardy-Abeloos et al[97] recently reported that TARE followed by SBRT has comparable safety and efficacy to TACE followed by SBRT.
SBRT as a bridge to liver transplantation
One of the preferred gold-standard treatments for managing HCC patients is liver transplantation, but only a few patients have a chance to receive a transplant due to an insufficient supply of donor livers[23]. Therefore, several local and local-regional therapies for HCC have been used to bridge care in patients seeking a transplant, to delay tumor progression[24]. Several studies have shown that SBRT may be an excellent alternative to conventional therapies as a bridge to transplantation[81,98]. Sapisochin et al[98] reported 1-, 3-, and 5-year actuarial patient survival rates from the time of listing of 83%, 61%, and 61% in the SBRT group, respectively, rates which were comparable with those of the TACE or RFA groups.
SBRT for macroscopic vascular invasion or portal vein thrombosis
In the past decades, two landmark randomized trials revealed that sorafenib yielded modest survival prolongation in patients with portal vein thrombosis (PVT)[99,100]. However, the response rate was unsatisfactory (only 2%). CFRT has been widely used in advanced HCC with macroscopic vascular invasion or PVT because it can be delivered regardless of tumor location, and major vessels demonstrate high radiation tolerance[24,78,79].
Rim et al[101] published a meta-analysis and systematic review which compared 3DCRT, TARE, and SBRT in patients with PVT. No significant differences in OS were observed among the three treatment modalities, but SBRT demonstrated the highest response rate of around 70%. More notably, toxicities of more than grade 3 were rare in the SBRT group[101]. These data revealed that SBRT could be safely applied in patients with PVT, with a better response rate than CFRT.
SBRT and sorafenib
SBRT compared with sorafenib: Bettinger et al[102] used PSM to compare SBRT and sorafenib. SBRT showed a superior survival benefit to that of sorafenib: median overall survival was 17.0 mo (range, 10.8-23.2) vs 9.6 mo (range, 8.6-10.7), respectively, with or without adjusting for different baseline characteristics. A cost-effectiveness study also reported that SBRT had better cost-effectiveness than sorafenib for patients with advanced HCC[103].
SBRT combined with sorafenib: Brade et al[104] initiated a phase-I trial to evaluate the combination of sorafenib and SBRT. Sorafenib was delivered before, during, and after SBRT. The researchers found that concurrent use of SBRT with sorafenib significantly increased side effects, e.g., grade 3+ bowel toxicity and tumor rupture. Thus, they did not recommend using this combination outside of a clinical trial. A clinical trial (RTOG 1112) is ongoing to compare SBRT followed by sorafenib with sorafenib alone in patients with advanced HCC (ClinicalTrials.gov Identifier: NCT01730937).
SBRT combined with immunotherapy
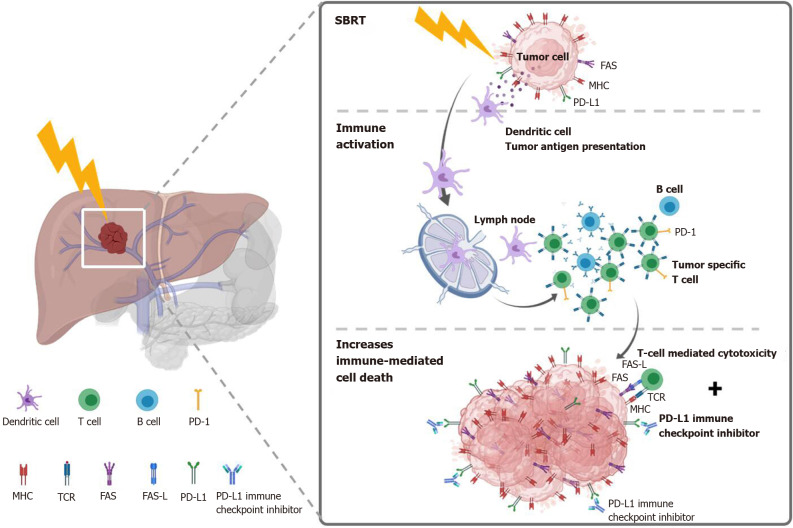
SBRT as an immunostimulator: Preclinical data demonstrated that RT could augment the intra-tumor cell surface expression of immunogenicity markers (e.g., dendritic cells) and enhance therapeutic efficacy[105-107]. Recently, immunotherapy combined with RT is an active field in managing HCC and has shown promising results[106,108,109]. SBRT may stimulate the release of tumor antigens and increase antigen-presenting cells to enhance the immune response to cancer cells provided by the immunotherapy[110]. This effect is also termed STAR[58] or “immunotherapy and stereotactic ablative radiotherapy (ISABR)”[111]. Investigations exploring in detail the underlying mechanisms of these effects are ongoing aggressively. Potential mechanism of SBRT combined with systemic therapy to induce the STAR effect (ISABR) for liver tumors is illustrated in Figure 5.
Figure 5.
Potential mechanism of stereotactic body radiation therapy combined with systemic therapy to induce the systemic therapy augmented by radiotherapy effect (also known as immunotherapy and stereotactic ablative radiotherapy) for liver tumors. Stereotactic body radiation therapy (SBRT) induces antigen release and immunogenic cell death, activation of several transcription factors and signal pathways, as well as dendritic cell antigen presentation and maturation, resulting in proliferation of tumor-specific T cells and immune-mediated cytotoxicity. SBRT combined with Immune-checkpoint inhibitors augmented the tumoricidal effect by upregulates major histocompatibility complex and FAS on tumor cells, increasing susceptibility to T-cell-mediated cell death. MHC: Major histocompatibility complex; TCR: T cell receptor; FAS-L: FAS ligand.
SBRT is more immunogenic than conventional RT: SBRT is more immunogenic and has a beneficial outcome than the conventionally fractionated RT[112,113]. By using ablative SBRT in a mouse model, Lee et al[113] observed that a 20-Gy single fraction dose leads to a reduction in the tumor burden in both primary and distant metastases in a CD8+ T-cell–dependent fashion. However, conventionally fractionated irradiation showed a CD8+-depleted condition. In another preclinical study that compared soluble PD-L1 (sPD-L1) between the SBRT and conventional RT groups[114], Kim and colleagues found that the sPD-L1 Level increases persistently for 1 mo in the SBRT group. In comparison, it increased initially after irradiation but decreased after 1 mo in the conventional RT group.
Potential optimal SBRT dose and treatment sequences for induced immunogenicity: The optimal window of radiation immunogenicity is determined by the levels of double-strand DNA (dsDNA) vs Trex1[115,116]. SBRT doses up to 10-12 Gy (e.g., 8 Gy x 3 fractions) can up-regulate dsDNA accumulation in cancer cells via the cGAS/STING pathway, turning on RT-driven immune responses. However, higher doses above 12–18 Gy, such as 20-30 Gy in a single fraction, may induce the exonuclease TREX1, which down-regulates the immunogenicity by degrading cytosolic DNA, turning off RT-driven immune responses[115]. Otherwise, increasing the dose to higher than 10 Gy per fraction can rapidly reduce the tumor blood perfusion, leading to a tumoricidal effect via severe vascular damage[117].
The optimal sequence of SBRT and immunotherapy is still unclear. Both concurrent and sequential combination have been applied[118-120]. In other cancers, Wegner et al[121] found that OS was improved when immunotherapy was given more than 3 wk after initiating SBRT/SRS in patients with stage IV non-small-cell lung cancer. In a study designed by Tang et al[120], SBRT was given either concurrently or sequentially with ipilimumab in patients with advanced solid tumors. However, there are limited studies specifically focused on HCC. Chiang et al[122] added nivolumab at 2 wk after completed SBRT and continuously applied every 2 wk until disease progression. Currently, the optimal timing of SBRT and immunotherapy remains under active investigation.
Clinical data and ongoing trials for SBRT combined with immunotherapy: Recently, two phase-II studies, CheckMate 040[123] and KEYNOTE-224[124], have shown that the anti-PD-1 immune checkpoint inhibitors of nivolumab and pembrolizumab demonstrate favorable tumor responses (15%-20%) in managing HCC patients. The two agents have been approved by the Food and Drug Administration (FDA) for patients previously treated with sorafenib. A phase-III randomized trial, KEYNOTE-240[125], then compared pembrolizumab and placebo in 413 patients previously treated with sorafenib. A median OS was 13.9 mo for pembrolizumab vs 10.6 mo for placebo (HR, 0.781; 95%CI: 0.611-0.998; P = 0.0238) and the median PFS was 3.0 vs 2.8 mo (HR, 0.718; 95%CI: 0.570-0.904; P = 0.0022). However, neither study endpoint reached statistical significance according to the trial-specified criteria (one-sided significance threshold, P = 0.0174, for the final analysis). Another phase-III trial, CheckMate 459, comparing nivolumab vs sorafenib as first-line treatment in advanced HCC, is ongoing (ClinicalTrials.gov Identifier: NCT02576509). Abstracts of CheckMate 459 in the 44th European Society for Medical Oncology Congress revealed that the primary endpoint of OS was statistically insignificant, but the objective response rate was double in the nivolumab group (15% vs 7%, respectively)[126].
Although the immune checkpoint inhibitor's objective response rate was higher than that of sorafenib, 15%-20% is still relatively insufficient[123-125]. Hence, the combination of immunotherapy with SBRT has been proposed to improve the efficacy of immunotherapy, to combine the direct tumoricidal effects with the immunogenic STAR effect[110,111,127,128]. As a novel combined treatment modality, only one published HCC study is currently available, revealing encouraging results. Chiang et al[122] reported 5 retrospective cases with unresectable HCC treated with SBRT 27.5-35 Gy in 5 fractions, followed by nivolumab. The median follow-up time was 14.9 mo. An objective response rate of 100% was reported, with 2 complete response and 3 partial responses. The 1-year OS and LC rates were both 100%. In addition, several phase-I or -II trials that combine SBRT and immunotherapy are ongoing (ClinicalTrials.gov Identifier: NCT03817736, NCT03316872, NCT03482102, and NCT03203304).
SBRT for metastatic disease
SBRT becomes much more important in managing cancer patients with oligometastatic disease[129-134], including those with HCC[76]. Recently, a landmark trial applied SBRT to manage oligometastatic cancers, defined as primary controlled tumors with only 1-5 metastatic lesions, PS ≤ 1, and life expectancy > 6 mo[135]. Median OS of the SBRT group was better than that of the control group (41 vs 28 mo; HR, 0.57; 95%CI: 0.30-1.10; P = 0.090)[135]. Therefore, it is reasonable to treat more aggressively those HCC patients with oligometastasis, oligo-progression, and oligo-recurrence, i.e., treat with potentially curative intent by using SBRT if individual conditions allow.
How to combine SBRT?
Clinically, managing HCC patients with a combination of different treatment modalities, including SBRT, remains a challenge. Information from several domains should be judged together carefully, such as the individual patient's condition, the tumor location/characteristics, liver function reservation, facility resources, and the irradiation techniques available. A multidisciplinary evaluation before initiating treatment is crucial[34]. Moreover, to individualize the treatment combination into the best available option, clinicians must discuss options with patients and their families, using shared decision making (SDM)[136].
COMBINED MODERN RT TECHNIQUES FOR PATIENTS IN WHOM SBRT CANNOT BE DONE SAFELY
As mentioned above, for vulnerable patients with unresectable bulky HCCs (i.e., > 5 cm), clinicians may hesitate to use SBRT because of the potentially greater risk of severe non-classic RILD or GI tract toxicity[61,137]. Under such conditions, a combination of modern irradiation techniques, such as VMAT, RMM[138], and modified simultaneously integrated boost (SIB)[139-142], has been reported to be useful.
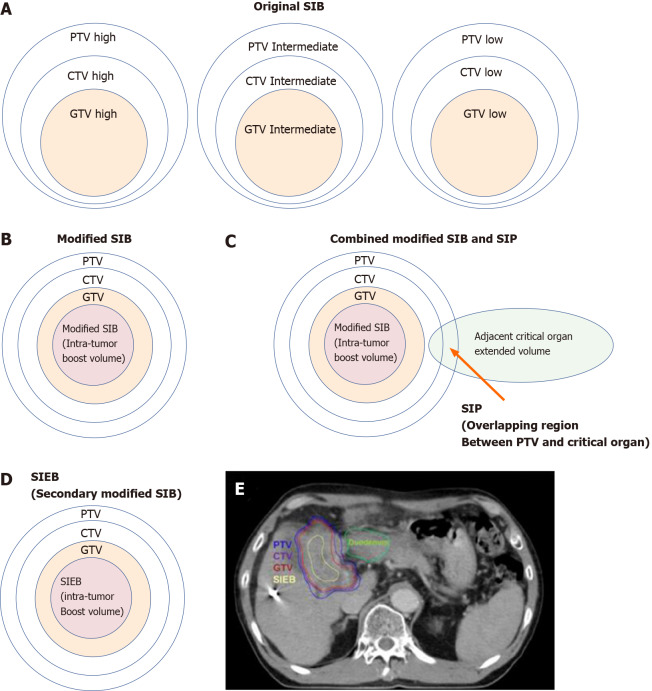
In the literature, SIB dose prescription has been recommended for dose escalation[143]. The original SIB prescription delivers different homogenous doses to separate target regions in the same fraction numbers[144]. That is, the high-, intermediate-, and low-risk target volumes are simultaneously irradiated with high, intermediate, and low doses, respectively, per fraction (Figure 6A).
Figure 6.
Cartoon and case illustrations of simultaneously integrated boost, modified simultaneously integrated boost, combined simultaneously integrated boost and simultaneously integrated protection, and simultaneously integrated inner-escalated boost dose-prescription techniques. A: A simple cartoon figure representing original simultaneously integrated boost (SIB) dose prescription. Original SIB is prescribed in different doses per fraction to different target regions according to the risk levels of recurrence. For example, uniform doses per fraction may be given at planning target volume (PTV) high, intermediate, and low with 240 centigray (cGy) (simultaneously integrated boost), 180-200 cGy (traditional dose per fraction), and 160 cGy (inferior to the traditional dose) in the same fractions, respectively; B: A simple cartoon figure representing a modified SIB dose prescription. Traditionally, radiotherapy is prescribed as a uniform dose per fraction (e.g., 200 cGy) on PTV, which provides a homogeneous dose to cover clinical target volume (CTV) and gross target volume (GTV). Recently, to maximally enhance the possibility of tumor control, a modified SIB technique is applied using a planned non-homologous dose distribution, i.e., escalating a simultaneous intra-tumor boost (e.g., 220-240 cGy per fraction) in addition to a traditional covering dose to the PTV (e.g., 200 cGy per fraction) in the same treatment fractions; C: A simple cartoon figure representing combined modified SIB and simultaneously integrated protection (SIP) dose prescription. To reduce treatment toxicities to adjacent critical organs/tissue, SIP was developed in conjunction with modified SIB. SIP prescribes an inferior-to-traditional dose per fraction, e.g., 150 cGy, to the overlapping region between the PTV and the extended critical organ volume (as shown by the long arrow); D: A simple cartoon figure representing simultaneously integrated inner-escalated boost (SIEB) dose prescription. For further enhanced therapeutic gain (i.e., increased tumor control and decreased treatment toxicity) in managing patients with unresectable liver tumors, we applied a secondary modified SIB (also termed SIEB). SIEB further escalates the intra-tumor boost (e.g., 240-260 cGy per fraction) based on a planned generally attenuated peri-tumor dose (e.g., 120-150 cGy per fraction delivered to the PTV), administered simultaneously. The intra-tumor SIEB boost volume is delineated as a uniform-inner-shrinkage area from the GTV with a margin of 1-10 mm (i.e., a geometrically central zone), depending on the tumor size, the intensity of the dose escalation, the level of liver preservation, the closeness of the gastrointestinal organs to the irradiation targets, and the patient's condition. Note that an additional most-inner SIEB boost volume with the highest dose per fraction (e.g., 260-300 cGy or higher) might be considered for highly selected patients with very bulky tumors. E: A clinical case with SIEB dose prescription. The blue, purple, and red lines show the PTV, CTV, and GTV of the irradiating target, respectively. In this case, based on the physician’s choice and the patient’s condition, a dose per fraction of 120 cGy was prescribed to the PTV, 150 cGy to the CTV, and 200 cGy to the GTV. Finally, in the yellow-outlined region, 280 cGy per fraction was simultaneously delivered to the SIEB boost volume. A total of 30 fractions were given, generating total doses levels of 3600 cGy, 4500 cGy, 6000 cGy, and 7400 cGy to the PTC, CTV, GTV, and SIEB boost volume, respectively. Note that the most peripheral dose per fraction of 120 cGy was chosen mainly due to a very close distance between the PTV and an adjacent critical organ, i.e., the duodenum. This short-distance closeness could easily lead irradiation to harm the duodenum under the context of daily organ motion. SIB: Simultaneously integrated boost; SIP: Simultaneously integrated protection; SIEB: Simultaneously integrated inner-escalated boost; PTV: Planning target volume; CTV: Clinical target volume; GTV: Gross target volume.
Recently, a modified SIB technique simultaneously applied a heterogeneous dose per fraction on peripheral (lower dose per fraction, e.g., traditional 200 centigray [cGy]) and intra-tumor zones (escalated higher dose per fraction, e.g., 240 cGy) (Figure 6B)[139-143]. Modified SIB simultaneously delivers an intra-tumor boost dose to the irradiated tumor's inner region to maximally enhance the possibility of tumor control in treating bulky tumors. This type of planned intra-tumor heterogenous dose distribution of modified SIB differs from the original SIB. This modified SIB has shown a potential role in managing bulky tumors, such as bulky pelvic tumor[139], retroperitoneal mass[140], breast cancer[141], and liver tumor[142,143,145]. Recently, higher doses per fraction, e.g., 2.4 Gy on peripheral and 3 Gy on intra-tumor boost zones, have been prescribed for managing very bulky tumors[145].
To gain better tumor control without the cost of increasing irradiation-associated critical organ toxicity, a type of combined SIB and simultaneously integrated protection (SIP) was developed (Figure 6C)[146,147]. In conjunction with an SIB to the intra-tumor volume, SIP includes an attenuated dose per fraction on the overlapping sites of PTV and the extended critical organ volume, to gain a double benefit from clinical irradiation. Several unresectable bulky cancers have benefitted from this modern RT dose prescription technique, including HCC[142,146,148].
Following this line of dose prescription, a secondary-modified SIB technique (i.e., SIEB; Figure 6D) was developed[59]. SIEB secondly remodeled the modified SIB to further expand the double benefits of SIB in managing unresectable bulky tumors. That is, SIEB simultaneously maintained an intra-tumor escalated boost of the modified SIB to gain maximal tumor control (e.g., 220-240 cGy per fraction). Moreover, SIEB includes prescribing a planned attenuated peri-gross-tumor dose to all adjacent normal tissues (e.g., 120-150 cGy per fraction). Note that this intended protection is not limited only to critical organs/structures[59]. As a result, in theory, SIEB's therapeutic gain is further enhanced even over that of modified SIB (Figure 6B) or combined SIB and SIP (Figure 6C). However, prospective randomized clinical trials should be conducted to confirm the effectiveness of SIEB in managing patients with unresectable HCC.
One topic for further investigation in modified SIB and SIEB is whether the intra-tumor boost volume should be guided by using metabolic images, such as positron emission tomography (PET)[149,150]. For example, F-18-labeled fluoromisonidazole PET/CT images have been used to guide an SIB-escalated boost to the hypoxia tumor region[150]. However, using metabolic images to guide the intra-tumor boost volume has some limitations in daily clinical use, such as resource availability and the dynamic change in the PET-guided increased-uptake region during the treatment course of irradiation. As a result, multiple sessions of PET images and adaptive RT treatment planning may be required. Hence, in practice, it is feasible to deliver an intra-tumor boost to the geometric-central zone to maintain treatment efficacy, enhance tumor control, and minimize irradiation toxicity[59,143,145].
CONCLUSION
Currently, two driving forces have come together to improve the treatment efficacy of RT in HCC. One is technological advancement, which enables the precise delivery of SBRT, increasing tumor control and reducing the side effects to the surrounding normal tissue. The other is the boom in the development of target therapies and checkpoint-blockade immunotherapy, which prolongs HCC patients' survival and re-emphasizes the importance of local tumor control. Currently, the role of RT in HCC treatment is actively being investigating in combination with systemic therapies to generate the STAR effect.
Remarkably, the development and use of combined modalities to increase liver reserve and patient tolerance may have the best chance to improve treatment outcomes. For HCC patients with any stage disease, RT plays a crucial role, whether delivered alone or in combination with other treatment modalities, because it is not limited by tumor location. Currently, a lack of level-III evidence is the main barrier to recommending SBRT as a standard of care in most international treatment guidelines. In this regard, several clinical trials of SBRT for HCC are ongoing. In the modern era, the role of photon- and proton-based precise RT in managing patients with HCC is shifting continuously from palliative to curative intent.
ACKNOWLEDGEMENTS
We gratefully acknowledge all RT staff members of the Buddhist Dalin Tzu Chi Hospital and Tzu Chi Medication Foundation. We also thank the contribution of all RT members, including Buddhist Hualien Tzu Chi Hospital, Far Eastern Memorial Hospital and China Medical University Hospital who involved in inter-hospital HCC research program TASABR trial registered prospectively on ClinicalTrials.gov (Trial Identifier: NCT02921139).
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: January 26, 2021
First decision: March 29, 2021
Article in press: April 26, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ali Shah SI S-Editor: Gong ZM L-Editor: A P-Editor: Liu JH
Contributor Information
Liang-Cheng Chen, Department of Radiation Oncology, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Dalin, Chia-Yi 62247, Taiwan.
Hon-Yi Lin, Department of Radiation Oncology, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Dalin, Chia-Yi 62247, Taiwan; School of Medicine, Buddhist Tzu Chi University, Hualien 970, Taiwan; Institute of Molecular Biology, National Chung Cheng University, Min-Hsiung, Chia-Yi 62102, Taiwan.
Shih-Kai Hung, Department of Radiation Oncology, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Dalin, Chia-Yi 62247, Taiwan; School of Medicine, Buddhist Tzu Chi University, Hualien 970, Taiwan. oncology158@yahoo.com.tw.
Wen-Yen Chiou, Department of Radiation Oncology, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Dalin, Chia-Yi 62247, Taiwan; School of Medicine, Buddhist Tzu Chi University, Hualien 970, Taiwan.
Moon-Sing Lee, Department of Radiation Oncology, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Dalin, Chia-Yi 62247, Taiwan; School of Medicine, Buddhist Tzu Chi University, Hualien 970, Taiwan.
References
- 1.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, Abdulle ASM, Abebe ND, Abraha HN, Abu-Raddad LJ, Abualhasan A, Adedeji IA, Advani SM, Afarideh M, Afshari M, Aghaali M, Agius D, Agrawal S, Ahmadi A, Ahmadian E, Ahmadpour E, Ahmed MB, Akbari ME, Akinyemiju T, Al-Aly Z, AlAbdulKader AM, Alahdab F, Alam T, Alamene GM, Alemnew BTT, Alene KA, Alinia C, Alipour V, Aljunid SM, Bakeshei FA, Almadi MAH, Almasi-Hashiani A, Alsharif U, Alsowaidi S, Alvis-Guzman N, Amini E, Amini S, Amoako YA, Anbari Z, Anber NH, Andrei CL, Anjomshoa M, Ansari F, Ansariadi A, Appiah SCY, Arab-Zozani M, Arabloo J, Arefi Z, Aremu O, Areri HA, Artaman A, Asayesh H, Asfaw ET, Ashagre AF, Assadi R, Ataeinia B, Atalay HT, Ataro Z, Atique S, Ausloos M, Avila-Burgos L, Avokpaho EFGA, Awasthi A, Awoke N, Ayala Quintanilla BP, Ayanore MA, Ayele HT, Babaee E, Bacha U, Badawi A, Bagherzadeh M, Bagli E, Balakrishnan S, Balouchi A, Bärnighausen TW, Battista RJ, Behzadifar M, Bekele BB, Belay YB, Belayneh YM, Berfield KKS, Berhane A, Bernabe E, Beuran M, Bhakta N, Bhattacharyya K, Biadgo B, Bijani A, Bin Sayeed MS, Birungi C, Bisignano C, Bitew H, Bjørge T, Bleyer A, Bogale KA, Bojia HA, Borzì AM, Bosetti C, Bou-Orm IR, Brenner H, Brewer JD, Briko AN, Briko NI, Bustamante-Teixeira MT, Butt ZA, Carreras G, Carrero JJ, Carvalho F, Castro C, Castro F, Catalá-López F, Cerin E, Chaiah Y, Chanie WF, Chattu VK, Chaturvedi P, Chauhan NS, Chehrazi M, Chiang PP, Chichiabellu TY, Chido-Amajuoyi OG, Chimed-Ochir O, Choi JJ, Christopher DJ, Chu DT, Constantin MM, Costa VM, Crocetti E, Crowe CS, Curado MP, Dahlawi SMA, Damiani G, Darwish AH, Daryani A, das Neves J, Demeke FM, Demis AB, Demissie BW, Demoz GT, Denova-Gutiérrez E, Derakhshani A, Deribe KS, Desai R, Desalegn BB, Desta M, Dey S, Dharmaratne SD, Dhimal M, Diaz D, Dinberu MTT, Djalalinia S, Doku DT, Drake TM, Dubey M, Dubljanin E, Duken EE, Ebrahimi H, Effiong A, Eftekhari A, El Sayed I, Zaki MES, El-Jaafary SI, El-Khatib Z, Elemineh DA, Elkout H, Ellenbogen RG, Elsharkawy A, Emamian MH, Endalew DA, Endries AY, Eshrati B, Fadhil I, Fallah Omrani V, Faramarzi M, Farhangi MA, Farioli A, Farzadfar F, Fentahun N, Fernandes E, Feyissa GT, Filip I, Fischer F, Fisher JL, Force LM, Foroutan M, Freitas M, Fukumoto T, Futran ND, Gallus S, Gankpe FG, Gayesa RT, Gebrehiwot TT, Gebremeskel GG, Gedefaw GA, Gelaw BK, Geta B, Getachew S, Gezae KE, Ghafourifard M, Ghajar A, Ghashghaee A, Gholamian A, Gill PS, Ginindza TTG, Girmay A, Gizaw M, Gomez RS, Gopalani SV, Gorini G, Goulart BNG, Grada A, Ribeiro Guerra M, Guimaraes ALS, Gupta PC, Gupta R, Hadkhale K, Haj-Mirzaian A, Hamadeh RR, Hamidi S, Hanfore LK, Haro JM, Hasankhani M, Hasanzadeh A, Hassen HY, Hay RJ, Hay SI, Henok A, Henry NJ, Herteliu C, Hidru HD, Hoang CL, Hole MK, Hoogar P, Horita N, Hosgood HD, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hussen MM, Ileanu B, Ilic MD, Innos K, Irvani SSN, Iseh KR, Islam SMS, Islami F, Jafari Balalami N, Jafarinia M, Jahangiry L, Jahani MA, Jahanmehr N, Jakovljevic M, James SL, Javanbakht M, Jayaraman S, Jee SH, Jenabi E, Jha RP, Jonas JB, Jonnagaddala J, Joo T, Jungari SB, Jürisson M, Kabir A, Kamangar F, Karch A, Karimi N, Karimian A, Kasaeian A, Kasahun GG, Kassa B, Kassa TD, Kassaw MW, Kaul A, Keiyoro PN, Kelbore AG, Kerbo AA, Khader YS, Khalilarjmandi M, Khan EA, Khan G, Khang YH, Khatab K, Khater A, Khayamzadeh M, Khazaee-Pool M, Khazaei S, Khoja AT, Khosravi MH, Khubchandani J, Kianipour N, Kim D, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Komaki H, Koyanagi A, Krohn KJ, Bicer BK, Kugbey N, Kumar V, Kuupiel D, La Vecchia C, Lad DP, Lake EA, Lakew AM, Lal DK, Lami FH, Lan Q, Lasrado S, Lauriola P, Lazarus JV, Leigh J, Leshargie CT, Liao Y, Limenih MA, Listl S, Lopez AD, Lopukhov PD, Lunevicius R, Madadin M, Magdeldin S, El Razek HMA, Majeed A, Maleki A, Malekzadeh R, Manafi A, Manafi N, Manamo WA, Mansourian M, Mansournia MA, Mantovani LG, Maroufizadeh S, Martini SMS, Mashamba-Thompson TP, Massenburg BB, Maswabi MT, Mathur MR, McAlinden C, McKee M, Meheretu HAA, Mehrotra R, Mehta V, Meier T, Melaku YA, Meles GG, Meles HG, Melese A, Melku M, Memiah PTN, Mendoza W, Menezes RG, Merat S, Meretoja TJ, Mestrovic T, Miazgowski B, Miazgowski T, Mihretie KMM, Miller TR, Mills EJ, Mir SM, Mirzaei H, Mirzaei HR, Mishra R, Moazen B, Mohammad DK, Mohammad KA, Mohammad Y, Darwesh AM, Mohammadbeigi A, Mohammadi H, Mohammadi M, Mohammadian M, Mohammadian-Hafshejani A, Mohammadoo-Khorasani M, Mohammadpourhodki R, Mohammed AS, Mohammed JA, Mohammed S, Mohebi F, Mokdad AH, Monasta L, Moodley Y, Moosazadeh M, Moossavi M, Moradi G, Moradi-Joo M, Moradi-Lakeh M, Moradpour F, Morawska L, Morgado-da-Costa J, Morisaki N, Morrison SD, Mosapour A, Mousavi SM, Muche AA, Muhammed OSS, Musa J, Nabhan AF, Naderi M, Nagarajan AJ, Nagel G, Nahvijou A, Naik G, Najafi F, Naldi L, Nam HS, Nasiri N, Nazari J, Negoi I, Neupane S, Newcomb PA, Nggada HA, Ngunjiri JW, Nguyen CT, Nikniaz L, Ningrum DNA, Nirayo YL, Nixon MR, Nnaji CA, Nojomi M, Nosratnejad S, Shiadeh MN, Obsa MS, Ofori-Asenso R, Ogbo FA, Oh IH, Olagunju AT, Olagunju TO, Oluwasanu MM, Omonisi AE, Onwujekwe OE, Oommen AM, Oren E, Ortega-Altamirano DDV, Ota E, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakhale S, Pakpour AH, Pana A, Park EK, Parsian H, Pashaei T, Patel S, Patil ST, Pennini A, Pereira DM, Piccinelli C, Pillay JD, Pirestani M, Pishgar F, Postma MJ, Pourjafar H, Pourmalek F, Pourshams A, Prakash S, Prasad N, Qorbani M, Rabiee M, Rabiee N, Radfar A, Rafiei A, Rahim F, Rahimi M, Rahman MA, Rajati F, Rana SM, Raoofi S, Rath GK, Rawaf DL, Rawaf S, Reiner RC, Renzaho AMN, Rezaei N, Rezapour A, Ribeiro AI, Ribeiro D, Ronfani L, Roro EM, Roshandel G, Rostami A, Saad RS, Sabbagh P, Sabour S, Saddik B, Safiri S, Sahebkar A, Salahshoor MR, Salehi F, Salem H, Salem MR, Salimzadeh H, Salomon JA, Samy AM, Sanabria J, Santric Milicevic MM, Sartorius B, Sarveazad A, Sathian B, Satpathy M, Savic M, Sawhney M, Sayyah M, Schneider IJC, Schöttker B, Sekerija M, Sepanlou SG, Sepehrimanesh M, Seyedmousavi S, Shaahmadi F, Shabaninejad H, Shahbaz M, Shaikh MA, Shamshirian A, Shamsizadeh M, Sharafi H, Sharafi Z, Sharif M, Sharifi A, Sharifi H, Sharma R, Sheikh A, Shirkoohi R, Shukla SR, Si S, Siabani S, Silva DAS, Silveira DGA, Singh A, Singh JA, Sisay S, Sitas F, Sobngwi E, Soofi M, Soriano JB, Stathopoulou V, Sufiyan MB, Tabarés-Seisdedos R, Tabuchi T, Takahashi K, Tamtaji OR, Tarawneh MR, Tassew SG, Taymoori P, Tehrani-Banihashemi A, Temsah MH, Temsah O, Tesfay BE, Tesfay FH, Teshale MY, Tessema GA, Thapa S, Tlaye KG, Topor-Madry R, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tsadik AG, Ullah I, Uthman OA, Vacante M, Vaezi M, Varona Pérez P, Veisani Y, Vidale S, Violante FS, Vlassov V, Vollset SE, Vos T, Vosoughi K, Vu GT, Vujcic IS, Wabinga H, Wachamo TM, Wagnew FS, Waheed Y, Weldegebreal F, Weldesamuel GT, Wijeratne T, Wondafrash DZ, Wonde TE, Wondmieneh AB, Workie HM, Yadav R, Yadegar A, Yadollahpour A, Yaseri M, Yazdi-Feyzabadi V, Yeshaneh A, Yimam MA, Yimer EM, Yisma E, Yonemoto N, Younis MZ, Yousefi B, Yousefifard M, Yu C, Zabeh E, Zadnik V, Moghadam TZ, Zaidi Z, Zamani M, Zandian H, Zangeneh A, Zaki L, Zendehdel K, Zenebe ZM, Zewale TA, Ziapour A, Zodpey S, Murray CJL. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol . 2019;5:1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang CJ, Yang YW, Chen JD, You SL, Yang HI, Lee MH, Lai MS, Chen CJ. Significant reduction in end-stage liver diseases burden through the national viral hepatitis therapy program in Taiwan. Hepatology . 2015;61:1154–1162. doi: 10.1002/hep.27630. [DOI] [PubMed] [Google Scholar]
- 3.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int . 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuen MF, Hou JL, Chutaputti A Asia Pacific Working Party on Prevention of Hepatocellular Carcinoma. Hepatocellular carcinoma in the Asia pacific region. J Gastroenterol Hepatol . 2009;24:346–353. doi: 10.1111/j.1440-1746.2009.05784.x. [DOI] [PubMed] [Google Scholar]
- 5.Yun BY, Lee HW, Min IK, Kim SU, Park JY, Kim DY, Ahn SH, Kim BK. Prognosis of Early-Stage Hepatocellular Carcinoma: Comparison between Trans-Arterial Chemoembolization and Radiofrequency Ablation. Cancers (Basel) . 2020;12 doi: 10.3390/cancers12092527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu LH, Mei J, Kan A, Ling YH, Li SH, Wei W, Chen MS, Zhang YF, Guo RP. Treatment optimization for recurrent hepatocellular carcinoma: Repeat hepatic resection versus radiofrequency ablation. Cancer Med . 2020;9:2997–3005. doi: 10.1002/cam4.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y, Song J, Zheng J, Jiang L, Yan L, Yang J, Zeng Y, Wu H. Comparison of Hepatic Resection Combined with Intraoperative Radiofrequency Ablation, or Hepatic Resection Alone, for Hepatocellular Carcinoma Patients with Multifocal Tumors Meeting the University of California San Francisco (UCSF) Criteria: A Propensity Score-Matched Analysis. Ann Surg Oncol . 2020;27:2334–2345. doi: 10.1245/s10434-020-08231-0. [DOI] [PubMed] [Google Scholar]
- 8.Yu SJ, Yoon JH, Lee JM, Lee JY, Kim SH, Cho YY, Yoo JJ, Lee M, Lee DH, Cho Y, Cho EJ, Lee JH, Kim YJ, Kim CY. Percutaneous ethanol injection therapy is comparable to radiofrequency ablation in hepatocellular carcinoma smaller than 1.5 cm: A matched case-control comparative analysis. Medicine (Baltimore) . 2016;95:e4551. doi: 10.1097/MD.0000000000004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi C, Gao H, Zhao Q, Zhang L. Computed Tomography-Guided Percutaneous Cryoablation for Subcardiac Hepatocellular Carcinoma: Safety, Efficacy, Therapeutic Results and Risk Factors for Survival Outcomes. Cancer Manag Res . 2020;12:3333–3342. doi: 10.2147/CMAR.S250652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi C, Li S, Zhang L. Development and Validation of a Clinicopathological-Based Nomogram to Predict the Survival Outcome of Patients with Recurrent Hepatocellular Carcinoma After Hepatectomy Who Underwent Microwave Ablation. Cancer Manag Res . 2020;12:7589–7600. doi: 10.2147/CMAR.S266052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peisen F, Maurer M, Grosse U, Nikolaou K, Syha R, Ketelsen D, Artzner C, Bitzer M, Horger M, Grözinger G. Predictive performance of the mHAP-II score in a real-life western cohort with hepatocellular carcinoma following trans-arterial chemoembolisation with drug-eluting beads (DEB-TACE) Eur Radiol . 2020;30:3782–3792. doi: 10.1007/s00330-020-06734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano MM, Yamamoto A, Nishida N, Hamuro M, Hamamoto S, Jogo A, Sohgawa E, Kageyama K, Minami T, Miki Y. Risk factors for local recurrence of hepatocellular carcinoma after transcatheter arterial chemoembolization with drug-eluting beads (DEB-TACE) Jpn J Radiol . 2019;37:543–548. doi: 10.1007/s11604-019-00840-4. [DOI] [PubMed] [Google Scholar]
- 13.Vesselle G, Quirier-Leleu C, Velasco S, Charier F, Silvain C, Boucebci S, Ingrand P, Tasu JP. Predictive factors for complete response of chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma. Eur Radiol . 2016;26:1640–1648. doi: 10.1007/s00330-015-3982-y. [DOI] [PubMed] [Google Scholar]
- 14.Adcock CS, Puneky LV, Campbell GS. Favorable Response of Metastatic Hepatocellular Carcinoma to Treatment with Trans-arterial Radioembolization Followed by Sorafenib and Nivolumab. Cureus . 2019;11:e4083. doi: 10.7759/cureus.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rognoni C, Ciani O, Sommariva S, Bargellini I, Bhoori S, Cioni R, Facciorusso A, Golfieri R, Gramenzi A, Mazzaferro V, Mosconi C, Ponziani F, Sacco R, Trevisani F, Tarricone R. Trans-arterial radioembolization for intermediate-advanced hepatocellular carcinoma: a budget impact analysis. BMC Cancer . 2018;18:715. doi: 10.1186/s12885-018-4636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fornaro L, Vivaldi C, Lorenzoni G, Masi G, Bargellini I. Moving beyond sorafenib alone in advanced hepatocellular carcinoma: is hepatic arterial infusion chemotherapy the best option? Ann Oncol . 2017;28:667. doi: 10.1093/annonc/mdw664. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda M, Shimizu S, Sato T, Morimoto M, Kojima Y, Inaba Y, Hagihara A, Kudo M, Nakamori S, Kaneko S, Sugimoto R, Tahara T, Ohmura T, Yasui K, Sato K, Ishii H, Furuse J, Okusaka T. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin vs sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol . 2016;27:2090–2096. doi: 10.1093/annonc/mdw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa T, Kubota T, Abe S, Watanabe Y, Sugano T, Inoue R, Iwanaga A, Seki K, Honma T, Yoshida T. Hepatic arterial infusion chemotherapy with cisplatin before radical local treatment of early hepatocellular carcinoma (JIS score 0/1) improves survival. Ann Oncol . 2014;25:1379–1384. doi: 10.1093/annonc/mdu155. [DOI] [PubMed] [Google Scholar]
- 19.Labeur TA, Hofsink Q, Takkenberg RB, van Delden OM, Mathôt RAA, Schinner R, Malfertheiner P, Amthauer H, Schütte K, Basu B, Kuhl C, Mayerle J, Ricke J, Klümpen HJ. The value of sorafenib trough levels in patients with advanced hepatocellular carcinoma - a substudy of the SORAMIC trial. Acta Oncol . 2020;59:1028–1035. doi: 10.1080/0284186X.2020.1759826. [DOI] [PubMed] [Google Scholar]
- 20.Briggs A, Daniele B, Dick K, Evans TRJ, Galle PR, Hubner RA, Lopez C, Siebert U, Tremblay G. Covariate-adjusted analysis of the Phase 3 REFLECT study of lenvatinib versus sorafenib in the treatment of unresectable hepatocellular carcinoma. Br J Cancer . 2020;122:1754–1759. doi: 10.1038/s41416-020-0817-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung AS, Tam VC, Meyers DE, Sim HW, Knox JJ, Zaborska V, Davies J, Ko YJ, Batuyong E, Samawi H, Cheung WY, Lee-Ying R. Second-line treatment of hepatocellular carcinoma after sorafenib: Characterizing treatments used over the past 10 years and real-world eligibility for cabozantinib, regorafenib, and ramucirumab. Cancer Med . 2020;9:4640–4647. doi: 10.1002/cam4.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi WM, Choi J, Lee D, Shim JH, Lim YS, Lee HC, Chung YH, Lee YS, Park SR, Ryu MH, Ryoo BY, Lee SJ, Kim KM. Regorafenib Versus Nivolumab After Sorafenib Failure: Real-World Data in Patients With Hepatocellular Carcinoma. Hepatol Commun . 2020;4:1073–1086. doi: 10.1002/hep4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffy JP, Vardanian A, Benjamin E, Watson M, Farmer DG, Ghobrial RM, Lipshutz G, Yersiz H, Lu DS, Lassman C, Tong MJ, Hiatt JR, Busuttil RW. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg . 2007;246:502–509; discussion 509. doi: 10.1097/SLA.0b013e318148c704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT, Shiina S, Cheng AL, Jia JD, Obi S, Han KH, Jafri W, Chow P, Lim SG, Chawla YK, Budihusodo U, Gani RA, Lesmana CR, Putranto TA, Liaw YF, Sarin SK. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int . 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, Goto T, Yoshida H, Omata M, Koike K. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol . 2012;107:569–77; quiz 578. doi: 10.1038/ajg.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, Schipper MJ, Feng M. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol . 2016;34:452–459. doi: 10.1200/JCO.2015.61.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delis SG, Dervenis C. Selection criteria for liver resection in patients with hepatocellular carcinoma and chronic liver disease. World J Gastroenterol . 2008;14:3452–3460. doi: 10.3748/wjg.14.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol . 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 29.McMasters KM, Vauthey JN. Hepatocellular Carcinoma: Targeted Therapy and Multidisciplinary Care. New York: Springer, 2011. [Google Scholar]
- 30.Chan AC, Chan SC, Chok KS, Cheung TT, Chiu DW, Poon RT, Fan ST, Lo CM. Treatment strategy for recurrent hepatocellular carcinoma: salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transpl . 2013;19:411–419. doi: 10.1002/lt.23605. [DOI] [PubMed] [Google Scholar]
- 31.Veltri A, Moretto P, Doriguzzi A, Pagano E, Carrara G, Gandini G. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC) Eur Radiol . 2006;16:661–669. doi: 10.1007/s00330-005-0029-9. [DOI] [PubMed] [Google Scholar]
- 32.Guo W, He X, Li Z, Li Y. Combination of Transarterial Chemoembolization (TACE) and Radiofrequency Ablation (RFA) vs Surgical Resection (SR) on Survival Outcome of Early Hepatocellular Carcinoma: A Meta-Analysis. Hepatogastroenterology . 2015;62:710–714. [PubMed] [Google Scholar]
- 33.Doi H, Beppu N, Kitajima K, Kuribayashi K. Stereotactic Body Radiation Therapy for Liver Tumors: Current Status and Perspectives. Anticancer Res . 2018;38:591–599. doi: 10.21873/anticanres.12263. [DOI] [PubMed] [Google Scholar]
- 34.Benson AB, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, Anders R, Are C, Brown D, Chang DT, Cloyd J, Covey AM, Hawkins W, Iyer R, Jacob R, Karachristos A, Kelley RK, Kim R, Palta M, Park JO, Sahai V, Schefter T, Sicklick JK, Singh G, Sohal D, Stein S, Tian GG, Vauthey JN, Venook AP, Hammond LJ, Darlow SD. Guidelines Insights: Hepatobiliary Cancers, Version 2.2019. J Natl Compr Canc Netw . 2019;17:302–310. doi: 10.6004/jnccn.2019.0019. [DOI] [PubMed] [Google Scholar]
- 35.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology . 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 36.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol . 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, Kudo M, Iijima H, Genda T, Tateishi R, Torimura T, Igaki H, Kobayashi S, Sakurai H, Murakami T, Watadani T, Matsuyama Y. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res . 2019;49:1109–1113. doi: 10.1111/hepr.13411. [DOI] [PubMed] [Google Scholar]
- 38.Korean Liver Cancer Association National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver . 2019;13:227–299. doi: 10.5009/gnl19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition) Liver Cancer . 2018;7:235–260. doi: 10.1159/000488035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surveillance group ; Diagnosis group; Staging group; Surgery group; Local ablation group; TACE/TARE/HAI group; Target therapy/systemic therapy group; Radiotherapy group; Prevention group; Drafting group. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc . 2018;117:381–403. doi: 10.1016/j.jfma.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Ueno S, Tanabe G, Nuruki K, Hamanoue M, Komorizono Y, Oketani M, Hokotate H, Inoue H, Baba Y, Imamura Y, Aikou T. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res . 2002;24:395–403. doi: 10.1016/s1386-6346(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 42.Kudo M, Kitano M, Sakurai T, Nishida N. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, Nationwide Follow-Up Survey and Clinical Practice Guidelines: The Outstanding Achievements of the Liver Cancer Study Group of Japan. Dig Dis . 2015;33:765–770. doi: 10.1159/000439101. [DOI] [PubMed] [Google Scholar]
- 43.Künzli BM, Abitabile P, Maurer CA. Radiofrequency ablation of liver tumors: Actual limitations and potential solutions in the future. World J Hepatol . 2011;3:8–14. doi: 10.4254/wjh.v3.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Wang K, Wang M, Yang G, Ye X, Wu M, Cheng S. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. Oncotarget . 2017;8:29416–29427. doi: 10.18632/oncotarget.15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huo YR, Eslick GD. Transcatheter Arterial Chemoembolization Plus Radiotherapy Compared With Chemoembolization Alone for Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol . 2015;1:756–765. doi: 10.1001/jamaoncol.2015.2189. [DOI] [PubMed] [Google Scholar]
- 46.Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, Bae SH, Jung DH, Kim KB, Lee DH, Han CJ, Kim J, Park SC, Kim YH. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer . 2012;118:5424–5431. doi: 10.1002/cncr.27533. [DOI] [PubMed] [Google Scholar]
- 47.Li MF, Leung HW, Chan AL, Wang SY. Network meta-analysis of treatment regimens for inoperable advanced hepatocellular carcinoma with portal vein invasion. Ther Clin Risk Manag . 2018;14:1157–1168. doi: 10.2147/TCRM.S162898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon JH, Kim GM, Han K, Won JY, Kim MD, Lee DY, Lee J, Choi W, Kim YS, Kim DY, Han KH. Safety and Efficacy of Transarterial Radioembolization Combined with Chemoembolization for Bilobar Hepatocellular Carcinoma: A Single-Center Retrospective Study. Cardiovasc Intervent Radiol . 2018;41:459–465. doi: 10.1007/s00270-017-1826-7. [DOI] [PubMed] [Google Scholar]
- 49.Qu XD, Chen CS, Wang JH, Yan ZP, Chen JM, Gong GQ, Liu QX, Luo JJ, Liu LX, Liu R, Qian S. The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer . 2012;12:263. doi: 10.1186/1471-2407-12-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pan T, Li XS, Xie QK, Wang JP, Li W, Wu PH, Zhao M. Safety and efficacy of transarterial chemoembolization plus sorafenib for hepatocellular carcinoma with portal venous tumour thrombus. Clin Radiol . 2014;69:e553–e561. doi: 10.1016/j.crad.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Salman A, Simoneau E, Hassanain M, Chaudhury P, Boucher LM, Valenti D, Cabrera T, Nudo C, Metrakos P. Combined sorafenib and yttrium-90 radioembolization for the treatment of advanced hepatocellular carcinoma. Curr Oncol . 2016;23:e472–e480. doi: 10.3747/co.23.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ricke J, Bulla K, Kolligs F, Peck-Radosavljevic M, Reimer P, Sangro B, Schott E, Schütte K, Verslype C, Walecki J, Malfertheiner P SORAMIC study group. Safety and toxicity of radioembolization plus Sorafenib in advanced hepatocellular carcinoma: analysis of the European multicentre trial SORAMIC. Liver Int . 2015;35:620–626. doi: 10.1111/liv.12622. [DOI] [PubMed] [Google Scholar]
- 53.Lorenzin D, Pravisani R, Leo CA, Bugiantella W, Soardo G, Carnelutti A, Umberto B, Risaliti A. Complete Remission of Unresectable Hepatocellular Carcinoma After Combined Sorafenib and Adjuvant Yttrium-90 Radioembolization. Cancer Biother Radiopharm . 2016;31:65–69. doi: 10.1089/cbr.2015.1905. [DOI] [PubMed] [Google Scholar]
- 54.Floudas CS, Brar G, Greten TF. Immunotherapy: Current Status and Future Perspectives. Dig Dis Sci . 2019;64:1030–1040. doi: 10.1007/s10620-019-05516-7. [DOI] [PubMed] [Google Scholar]
- 55.Okusaka T, Ikeda M. Immunotherapy for hepatocellular carcinoma: current status and future perspectives. ESMO Open . 2018;3:e000455. doi: 10.1136/esmoopen-2018-000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ikeda M, Morizane C, Ueno M, Okusaka T, Ishii H, Furuse J. Chemotherapy for hepatocellular carcinoma: current status and future perspectives. Jpn J Clin Oncol . 2018;48:103–114. doi: 10.1093/jjco/hyx180. [DOI] [PubMed] [Google Scholar]
- 57.Wehrenberg-Klee E, Goyal L, Dugan M, Zhu AX, Ganguli S. Y-90 Radioembolization Combined with a PD-1 Inhibitor for Advanced Hepatocellular Carcinoma. Cardiovasc Intervent Radiol . 2018;41:1799–1802. doi: 10.1007/s00270-018-1993-1. [DOI] [PubMed] [Google Scholar]
- 58.Torok JA, Salama JK. Combining immunotherapy and radiotherapy for the STAR treatment. Nat Rev Clin Oncol . 2019;16:666–667. doi: 10.1038/s41571-019-0277-2. [DOI] [PubMed] [Google Scholar]
- 59.Lin YH, Hung SK, Chiou WY, Lee MS, Shen BJ, Chen LC, Liu DW, Tsai WT, Lin PH, Shih YT, Hsu FC, Tsai SJ, Chan MWY, Lin HY. Significant symptoms alleviation and tumor volume reduction after combined simultaneously integrated inner-escalated boost and volumetric-modulated arc radiotherapy in a patient with unresectable bulky hepatocellular carcinoma: A care-compliant case report. Medicine (Baltimore) . 2016;95:e4717. doi: 10.1097/MD.0000000000004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frank SJ, Zhu XR. Proton Therapy: Indications, Techniques, and Outcomes. Elsevier Health Sciences, 2020. [Google Scholar]
- 61.Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys . 2002;54:150–155. doi: 10.1016/s0360-3016(02)02864-x. [DOI] [PubMed] [Google Scholar]
- 62.Kim TH, Park JW, Kim YJ, Kim BH, Woo SM, Moon SH, Kim SS, Koh YH, Lee WJ, Park SJ, Kim JY, Kim DY, Kim CM. Phase I dose-escalation study of proton beam therapy for inoperable hepatocellular carcinoma. Cancer Res Treat . 2015;47:34–45. doi: 10.4143/crt.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mizumoto M, Tokuuye K, Sugahara S, Nakayama H, Fukumitsu N, Ohara K, Abei M, Shoda J, Tohno E, Minami M. Proton beam therapy for hepatocellular carcinoma adjacent to the porta hepatis. Int J Radiat Oncol Biol Phys . 2008;71:462–467. doi: 10.1016/j.ijrobp.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 64.Fukumitsu N, Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Abei M, Shoda J, Thono E, Tsuboi K, Tokuuye K. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys . 2009;74:831–836. doi: 10.1016/j.ijrobp.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 65.Nakayama H, Sugahara S, Fukuda K, Abei M, Shoda J, Sakurai H, Tsuboi K, Matsuzaki Y, Tokuuye K. Proton beam therapy for hepatocellular carcinoma located adjacent to the alimentary tract. Int J Radiat Oncol Biol Phys . 2011;80:992–995. doi: 10.1016/j.ijrobp.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 66.Chang JY, Li QQ, Xu QY, Allen PK, Rebueno N, Gomez DR, Balter P, Komaki R, Mehran R, Swisher SG, Roth JA. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: how to fly in a "no fly zone". Int J Radiat Oncol Biol Phys . 2014;88:1120–1128. doi: 10.1016/j.ijrobp.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 67.Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, Kwak EL, Allen JN, Clark JW, Goyal L, Murphy JE, Javle MM, Wolfgang JA, Drapek LC, Arellano RS, Mamon HJ, Mullen JT, Yoon SS, Tanabe KK, Ferrone CR, Ryan DP, DeLaney TF, Crane CH, Zhu AX. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol . 2016;34:460–468. doi: 10.1200/JCO.2015.64.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakayama H, Sugahara S, Tokita M, Fukuda K, Mizumoto M, Abei M, Shoda J, Sakurai H, Tsuboi K, Tokuuye K. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer . 2009;115:5499–5506. doi: 10.1002/cncr.24619. [DOI] [PubMed] [Google Scholar]
- 69.Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, Nagase M, Nihei K, Ogino T. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol . 2005;23:1839–1846. doi: 10.1200/JCO.2005.00.620. [DOI] [PubMed] [Google Scholar]
- 70.Sanford NN, Pursley J, Noe B, Yeap BY, Goyal L, Clark JW, Allen JN, Blaszkowsky LS, Ryan DP, Ferrone CR, Tanabe KK, Qadan M, Crane CH, Koay EJ, Eyler C, DeLaney TF, Zhu AX, Wo JY, Grassberger C, Hong TS. Protons vs Photons for Unresectable Hepatocellular Carcinoma: Liver Decompensation and Overall Survival. Int J Radiat Oncol Biol Phys . 2019;105:64–72. doi: 10.1016/j.ijrobp.2019.01.076. [DOI] [PubMed] [Google Scholar]
- 71.Hsieh CE, Venkatesulu BP, Lee CH, Hung SP, Wong PF, Aithala SP, Kim BK, Rao A, Tung-Chieh Chang J, Tsang NM, Wang CC, Lee CC, Lin CC, Tseng JH, Chou WC, Wang YC, Krishnan S, Hong JH. Predictors of Radiation-Induced Liver Disease in Eastern and Western Patients With Hepatocellular Carcinoma Undergoing Proton Beam Therapy. Int J Radiat Oncol Biol Phys . 2019;105:73–86. doi: 10.1016/j.ijrobp.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 72.Chuong MD, Kaiser A, Khan F, Parikh P, Ben-Josef E, Crane C, Brunner T, Okumura T, Schreuder N, Bentzen SM, Gutierrez A, Mendez Romero A, Yoon SM, Sharma N, Kim TH, Kishi K, Moeslein F, Hoffe S, Schefter T, Hanish S, Scorsetti M, Apisarnthanarax S. Consensus Report From the Miami Liver Proton Therapy Conference. Front Oncol . 2019;9:457. doi: 10.3389/fonc.2019.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med . 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 74.Rajyaguru DJ, Borgert AJ, Smith AL, Thomes RM, Conway PD, Halfdanarson TR, Truty MJ, Kurup AN, Go RS. Radiofrequency Ablation Versus Stereotactic Body Radiotherapy for Localized Hepatocellular Carcinoma in Nonsurgically Managed Patients: Analysis of the National Cancer Database. J Clin Oncol . 2018;36:600–608. doi: 10.1200/JCO.2017.75.3228. [DOI] [PubMed] [Google Scholar]
- 75.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol . 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 76.Burkoň P, Slavik M, Kazda T, Pospíšil P, Prochazka T, Vrzal M, Šlampa P. Stereotactic Body Radiotherapy - Current Indications. Klin Onkol . 2019;32:10–24. doi: 10.14735/amko201910. [DOI] [PubMed] [Google Scholar]
- 77.Murray LJ, Dawson LA. Advances in Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Semin Radiat Oncol . 2017;27:247–255. doi: 10.1016/j.semradonc.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 78.Ohri N, Dawson LA, Krishnan S, Seong J, Cheng JC, Sarin SK, Kinkhabwala M, Ahmed MM, Vikram B, Coleman CN, Guha C. Radiotherapy for Hepatocellular Carcinoma: New Indications and Directions for Future Study. J Natl Cancer Inst . 2016;108 doi: 10.1093/jnci/djw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen CP. Role of Radiotherapy in the Treatment of Hepatocellular Carcinoma. J Clin Transl Hepatol . 2019;7:183–190. doi: 10.14218/JCTH.2018.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Culleton S, Jiang H, Haddad CR, Kim J, Brierley J, Brade A, Ringash J, Dawson LA. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol . 2014;111:412–417. doi: 10.1016/j.radonc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 81.Gresswell S, Tobillo R, Hasan S, Uemura T, Machado L, Thai N, Kirichenko A. Stereotactic body radiotherapy used as a bridge to liver transplant in patients with hepatocellular carcinoma and Child-Pugh score ≥8 cirrhosis. J Radiosurg SBRT . 2018;5:261–267. [PMC free article] [PubMed] [Google Scholar]
- 82.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers. [cited 7 July 2020]. Available from: http://www.nccn.org/professionals/physician_gls . [DOI] [PMC free article] [PubMed]
- 83.Ronot M, Bouattour M, Wassermann J, Bruno O, Dreyer C, Larroque B, Castera L, Vilgrain V, Belghiti J, Raymond E, Faivre S. Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist . 2014;19:394–402. doi: 10.1634/theoncologist.2013-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu M, Lin MX, Lu MD, Xu ZF, Zheng KG, Wang W, Kuang M, Zhuang WQ, Xie XY. Comparison of contrast-enhanced ultrasound and contrast-enhanced computed tomography in evaluating the treatment response to transcatheter arterial chemoembolization of hepatocellular carcinoma using modified RECIST. Eur Radiol . 2015;25:2502–2511. doi: 10.1007/s00330-015-3611-9. [DOI] [PubMed] [Google Scholar]
- 85.Edeline J, Boucher E, Rolland Y, Vauléon E, Pracht M, Perrin C, Le Roux C, Raoul JL. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer . 2012;118:147–156. doi: 10.1002/cncr.26255. [DOI] [PubMed] [Google Scholar]
- 86.Teraoka Y, Kimura T, Aikata H, Daijo K, Osawa M, Honda F, Nakamura Y, Morio K, Morio R, Hatooka M, Kobayashi T, Nakahara T, Murakami E, Nagaoki Y, Kawaoka T, Tsuge M, Hiramatsu A, Imamura M, Kawakami Y, Nagata Y, Chayama K. Clinical outcomes of stereotactic body radiotherapy for elderly patients with hepatocellular carcinoma. Hepatol Res . 2018;48:193–204. doi: 10.1111/hepr.12916. [DOI] [PubMed] [Google Scholar]
- 87.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg . 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hara K, Takeda A, Tsurugai Y, Saigusa Y, Sanuki N, Eriguchi T, Maeda S, Tanaka K, Numata K. Radiotherapy for Hepatocellular Carcinoma Results in Comparable Survival to Radiofrequency Ablation: A Propensity Score Analysis. Hepatology . 2019;69:2533–2545. doi: 10.1002/hep.30591. [DOI] [PubMed] [Google Scholar]
- 89.Kim N, Kim HJ, Won JY, Kim DY, Han KH, Jung I, Seong J. Retrospective analysis of stereotactic body radiation therapy efficacy over radiofrequency ablation for hepatocellular carcinoma. Radiother Oncol . 2019;131:81–87. doi: 10.1016/j.radonc.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 90.Elshaarawy O, Gomaa A, Omar H, Rewisha E, Waked I. Intermediate stage hepatocellular carcinoma: a summary review. J Hepatocell Carcinoma . 2019;6:105–117. doi: 10.2147/JHC.S168682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sapir E, Tao Y, Schipper MJ, Bazzi L, Novelli PM, Devlin P, Owen D, Cuneo KC, Lawrence TS, Parikh ND, Feng M. Stereotactic Body Radiation Therapy as an Alternative to Transarterial Chemoembolization for Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys . 2018;100:122–130. doi: 10.1016/j.ijrobp.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu L, Zeng J, Wen Z, Tang C, Xu N. Transcatheter arterial chemoembolisation followed by three-dimensional conformal radiotherapy versus transcatheter arterial chemoembolisation alone for primary hepatocellular carcinoma in adults. Cochrane Database Syst Rev . 2019;2:CD012244. doi: 10.1002/14651858.CD012244.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xie H, Yu H, Tian S, Yang X, Wang X, Yang Z, Wang H, Guo Z. What is the best combination treatment with transarterial chemoembolization of unresectable hepatocellular carcinoma? Oncotarget . 2017;8:100508–100523. doi: 10.18632/oncotarget.20119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paik EK, Kim MS, Jang WI, Seo YS, Cho CK, Yoo HJ, Han CJ, Park SC, Kim SB, Kim YH. Benefits of stereotactic ablative radiotherapy combined with incomplete transcatheter arterial chemoembolization in hepatocellular carcinoma. Radiat Oncol . 2016;11:22. doi: 10.1186/s13014-016-0597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jacob R, Turley F, Redden DT, Saddekni S, Aal AK, Keene K, Yang E, Zarzour J, Bolus D, Smith JK, Gray S, White J, Eckhoff DE, DuBay DA. Adjuvant stereotactic body radiotherapy following transarterial chemoembolization in patients with non-resectable hepatocellular carcinoma tumours of ≥ 3 cm. HPB (Oxford) . 2015;17:140–149. doi: 10.1111/hpb.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lo CH, Huang WY, Lee MS, Lin KT, Lin TP, Chang PY, Fan CY, Jen YM. Stereotactic ablative radiotherapy for unresectable hepatocellular carcinoma patients who failed or were unsuitable for transarterial chemoembolization. Eur J Gastroenterol Hepatol . 2014;26:345–352. doi: 10.1097/MEG.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 97.Hardy-Abeloos C, Lazarev S, Ru M, Kim E, Fischman A, Moshier E, Rosenzweig K, Buckstein M. Safety and Efficacy of Liver Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma After Segmental Transarterial Radioembolization. Int J Radiat Oncol Biol Phys . 2019;105:968–976. doi: 10.1016/j.ijrobp.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 98.Sapisochin G, Barry A, Doherty M, Fischer S, Goldaracena N, Rosales R, Russo M, Beecroft R, Ghanekar A, Bhat M, Brierley J, Greig PD, Knox JJ, Dawson LA, Grant DR. Stereotactic body radiotherapy vs TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol . 2017;67:92–99. doi: 10.1016/j.jhep.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 99.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med . 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 100.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol . 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 101.Rim CH, Kim CY, Yang DS, Yoon WS. Comparison of radiation therapy modalities for hepatocellular carcinoma with portal vein thrombosis: A meta-analysis and systematic review. Radiother Oncol . 2018;129:112–122. doi: 10.1016/j.radonc.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 102.Bettinger D, Pinato DJ, Schultheiss M, Sharma R, Rimassa L, Pressiani T, Burlone ME, Pirisi M, Kudo M, Park JW, Buettner N, Neumann-Haefelin C, Boettler T, Abbasi-Senger N, Alheit H, Baus W, Blanck O, Gerum S, Guckenberger M, Habermehl D, Ostheimer C, Riesterer O, Tamihardja J, Grosu AL, Thimme R, Brunner TB, Gkika E. Stereotactic Body Radiation Therapy as an Alternative Treatment for Patients with Hepatocellular Carcinoma Compared to Sorafenib: A Propensity Score Analysis. Liver Cancer . 2019;8:281–294. doi: 10.1159/000490260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leung HW, Liu CF, Chan AL. Cost-effectiveness of sorafenib versus SBRT for unresectable advanced hepatocellular carcinoma. Radiat Oncol . 2016;11:69. doi: 10.1186/s13014-016-0644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brade AM, Ng S, Brierley J, Kim J, Dinniwell R, Ringash J, Wong RR, Cho C, Knox J, Dawson LA. Phase 1 Trial of Sorafenib and Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys . 2016;94:580–587. doi: 10.1016/j.ijrobp.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 105.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, Ito F, Davis MA, McGinn CJ, Chang AE. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res . 2003;63:8466–8475. [PubMed] [Google Scholar]
- 106.Chi KH, Liu SJ, Li CP, Kuo HP, Wang YS, Chao Y, Hsieh SL. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother . 2005;28:129–135. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 107.Teitz-Tennenbaum S, Li Q, Okuyama R, Davis MA, Sun R, Whitfield J, Knibbs RN, Stoolman LM, Chang AE. Mechanisms involved in radiation enhancement of intratumoral dendritic cell therapy. J Immunother . 2008;31:345–358. doi: 10.1097/CJI.0b013e318163628c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rai V, Abdo J, Alsuwaidan AN, Agrawal S, Sharma P, Agrawal DK. Cellular and molecular targets for the immunotherapy of hepatocellular carcinoma. Mol Cell Biochem . 2018;437:13–36. doi: 10.1007/s11010-017-3092-z. [DOI] [PubMed] [Google Scholar]
- 109.Choi C, Yoo GS, Cho WK, Park HC. Optimizing radiotherapy with immune checkpoint blockade in hepatocellular carcinoma. World J Gastroenterol . 2019;25:2416–2429. doi: 10.3748/wjg.v25.i20.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kreidieh M, Zeidan YH, Shamseddine A. The Combination of Stereotactic Body Radiation Therapy and Immunotherapy in Primary Liver Tumors. J Oncol . 2019;2019:4304817. doi: 10.1155/2019/4304817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol . 2016;13:516–524. doi: 10.1038/nrclinonc.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese TL, Drake CG. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res . 2015;3:345–355. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee Y, Auh SL, Wang Y, Burnette B, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, Fu YX. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood . 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim HJ, Park S, Kim KJ, Seong J. Clinical significance of soluble programmed cell death ligand-1 (sPD-L1) in hepatocellular carcinoma patients treated with radiotherapy. Radiother Oncol . 2018;129:130–135. doi: 10.1016/j.radonc.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 115.Vanpouille-Box C, Formenti SC, Demaria S. TREX1 dictates the immune fate of irradiated cancer cells. Oncoimmunology . 2017;6:e1339857. doi: 10.1080/2162402X.2017.1339857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun . 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS) Radiat Res . 2012;177:311–327. doi: 10.1667/rr2773.1. [DOI] [PubMed] [Google Scholar]
- 118.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, Jones H, Wilkinson RW, Honeychurch J, Illidge TM. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res . 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 119.Tian S, Switchenko JM, Buchwald ZS, Patel PR, Shelton JW, Kahn SE, Pillai RN, Steuer CE, Owonikoko TK, Behera M, Curran WJ, Higgins KA. Lung Stereotactic Body Radiation Therapy and Concurrent Immunotherapy: A Multicenter Safety and Toxicity Analysis. Int J Radiat Oncol Biol Phys . 2020;108:304–313. doi: 10.1016/j.ijrobp.2019.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang C, Welsh JW, de Groot P, Massarelli E, Chang JY, Hess KR, Basu S, Curran MA, Cabanillas ME, Subbiah V, Fu S, Tsimberidou AM, Karp D, Gomez DR, Diab A, Komaki R, Heymach JV, Sharma P, Naing A, Hong DS. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clin Cancer Res . 2017;23:1388–1396. doi: 10.1158/1078-0432.CCR-16-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wegner RE, Abel S, Hasan S, White RJ, Finley G, Monga D, Colonias A, Verma V. Time from stereotactic radiotherapy to immunotherapy is a predictor for outcome in stage IV non-small cell lung cancer. J Immunol Sci . 2019;3:6–13. [Google Scholar]
- 122.Chiang CL, Chan ACY, Chiu KWH, Kong FS. Combined Stereotactic Body Radiotherapy and Checkpoint Inhibition in Unresectable Hepatocellular Carcinoma: A Potential Synergistic Treatment Strategy. Front Oncol . 2019;9:1157. doi: 10.3389/fonc.2019.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet . 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol . 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 125.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol . 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 126.Yau T, Park JW, Finn RS, Cheng A-L, Mathurin P, Edeline J, Kudo M, Han K-H, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Begic D, Chen G, Neely J, Anderson J, Sangro B. LBA38_PR - CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) Ann Oncol . 2019;30:v874–v875. [Google Scholar]
- 127.Waidmann O. Recent developments with immunotherapy for hepatocellular carcinoma. Expert Opin Biol Ther . 2018;18:905–910. doi: 10.1080/14712598.2018.1499722. [DOI] [PubMed] [Google Scholar]
- 128.Choi SH, Seong J. Stereotactic Body Radiotherapy: Does It Have a Role in Management of Hepatocellular Carcinoma? Yonsei Med J . 2018;59:912–922. doi: 10.3349/ymj.2018.59.8.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Salama JK, Milano MT. Radical irradiation of extracranial oligometastases. J Clin Oncol . 2014;32:2902–2912. doi: 10.1200/JCO.2014.55.9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kissel M, Martel-Lafay I, Lequesne J, Faivre JC, Le Péchoux C, Stefan D, Barraux V, Loiseau C, Grellard JM, Danhier S, Lerouge D, Chouaid C, Gervais R, Thariat J. Stereotactic ablative radiotherapy and systemic treatments for extracerebral oligometastases, oligorecurrence, oligopersistence and oligoprogression from lung cancer. BMC Cancer . 2019;19:1237. doi: 10.1186/s12885-019-6449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kam TY, Chan OSH, Hung AWM, Yeung RMW. Utilization of stereotactic ablative radiotherapy in oligometastatic & oligoprogressive skeletal metastases: Results and pattern of failure. Asia Pac J Clin Oncol . 2019;15 Suppl 2:14–19. doi: 10.1111/ajco.13115. [DOI] [PubMed] [Google Scholar]
- 132.Cheung P. Stereotactic body radiotherapy for oligoprogressive cancer. Br J Radiol . 2016;89:20160251. doi: 10.1259/bjr.20160251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Berghen C, Joniau S, Ost P, Poels K, Everaerts W, Decaestecker K, Haustermans K, Devos G, De Meerleer G. Progression-directed Therapy for Oligoprogression in Castration-refractory Prostate Cancer. Eur Urol Oncol . 2021;4:305–309. doi: 10.1016/j.euo.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 134.Wray J, Hawamdeh RF, Hasija N, Dagan R, Yeung AR, Lightsey JL, Okunieff P, Daily KC, George TJ, Zlotecki RA, Trevino J, Dang LH. Stereotactic body radiation therapy for oligoprogression of metastatic disease from gastrointestinal cancers: A novel approach to extend chemotherapy efficacy. Oncol Lett . 2017;13:1087–1094. doi: 10.3892/ol.2016.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP, Schellenberg D, Ahmad B, Griffioen G, Senthi S, Swaminath A, Kopek N, Liu M, Moore K, Currie S, Bauman GS, Warner A, Senan S. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet . 2019;393:2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 136.Molinari M, De Coutere S, Krahn M, Helton S, Urbach DR. Patients' preferences and trade-offs for the treatment of early stage hepatocellular carcinoma. J Surg Res . 2014;189:57–67. doi: 10.1016/j.jss.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 137.Liang SX, Zhu XD, Xu ZY, Zhu J, Zhao JD, Lu HJ, Yang YL, Chen L, Wang AY, Fu XL, Jiang GL. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys . 2006;65:426–434. doi: 10.1016/j.ijrobp.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 138.Gargett M, Haddad C, Kneebone A, Booth JT, Hardcastle N. Clinical impact of removing respiratory motion during liver SABR. Radiat Oncol . 2019;14:93. doi: 10.1186/s13014-019-1300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nomiya T, Akamatsu H, Harada M, Ota I, Hagiwara Y, Ichikawa M, Miwa M, Kawashiro S, Hagiwara M, Chin M, Hashizume E, Nemoto K. Modified simultaneous integrated boost radiotherapy for an unresectable huge refractory pelvic tumor diagnosed as a rectal adenocarcinoma. World J Gastroenterol . 2014;20:18480–18486. doi: 10.3748/wjg.v20.i48.18480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nomiya T, Akamatsu H, Harada M, Ota I, Hagiwara Y, Ichikawa M, Miwa M, Mizutani M, Kato T, Nagaoka A, Tomita Y, Nemoto K. Modified simultaneous integrated boost radiotherapy for large retroperitoneal malignant tumor: A case report. Oncol Lett . 2015;9:2520–2524. doi: 10.3892/ol.2015.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nomiya T, Akamatsu H, Harada M, Ota I, Hagiwara Y, Ichikawa M, Miwa M, Suzuki A, Nemoto K. Modified simultaneous integrated boost radiotherapy for unresectable locally advanced breast cancer: preliminary results of a prospective clinical trial. Clin Breast Cancer . 2015;15:161–167. doi: 10.1016/j.clbc.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 142.Crane CH, Koay EJ. Solutions that enable ablative radiotherapy for large liver tumors: Fractionated dose painting, simultaneous integrated protection, motion management, and computed tomography image guidance. Cancer . 2016;122:1974–1986. doi: 10.1002/cncr.29878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Byun HK, Kim HJ, Im YR, Kim DY, Han KH, Seong J. Dose escalation in radiotherapy for incomplete transarterial chemoembolization of hepatocellular carcinoma. Strahlenther Onkol . 2020;196:132–141. doi: 10.1007/s00066-019-01488-9. [DOI] [PubMed] [Google Scholar]
- 144.Mohan R, Wu Q, Manning M, Schmidt-Ullrich R. Radiobiological considerations in the design of fractionation strategies for intensity-modulated radiation therapy of head and neck cancers. Int J Radiat Oncol Biol Phys . 2000;46:619–630. doi: 10.1016/s0360-3016(99)00438-1. [DOI] [PubMed] [Google Scholar]
- 145.Byun HK, Kim HJ, Im YR, Kim DY, Han KH, Seong J. Dose escalation by intensity modulated radiotherapy in liver-directed concurrent chemoradiotherapy for locally advanced BCLC stage C hepatocellular carcinoma. Radiother Oncol . 2019;133:1–8. doi: 10.1016/j.radonc.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 146.Simoni N, Micera R, Paiella S, Guariglia S, Zivelonghi E, Malleo G, Rossi G, Addari L, Giuliani T, Pollini T, Cavedon C, Salvia R, Milella M, Bassi C, Mazzarotto R. Hypofractionated Stereotactic Body Radiation Therapy With Simultaneous Integrated Boost and Simultaneous Integrated Protection in Pancreatic Ductal Adenocarcinoma. Clin Oncol (R Coll Radiol) . 2021;33:e31–e38. doi: 10.1016/j.clon.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 147.Oehlke O, Wucherpfennig D, Fels F, Frings L, Egger K, Weyerbrock A, Prokic V, Nieder C, Grosu AL. Whole brain irradiation with hippocampal sparing and dose escalation on multiple brain metastases: Local tumour control and survival. Strahlenther Onkol . 2015;191:461–469. doi: 10.1007/s00066-014-0808-9. [DOI] [PubMed] [Google Scholar]
- 148.Mazzola R, Ruggieri R, Figlia V, Rigo M, Giaj Levra N, Ricchetti F, Nicosia L, Corradini S, Alongi F. Stereotactic body radiotherapy of central lung malignancies using a simultaneous integrated protection approach : A prospective observational study. Strahlenther Onkol . 2019;195:719–724. doi: 10.1007/s00066-018-01419-0. [DOI] [PubMed] [Google Scholar]
- 149.Li QS, Liang N, Ouyang WW, Su SF, Ma Z, Geng YC, Yang WG, Hu YX, Li HQ, Lu B. Simultaneous integrated boost of intensity-modulated radiation therapy to Stage II-III non-small cell lung cancer with metastatic lymph nodes. Cancer Med . 2020;9:8364–8372. doi: 10.1002/cam4.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Qiu J, Lv B, Fu M, Wang X, Zheng X, Zhuo W. 18 F-Fluoromisonidazole positron emission tomography/CT-guided volumetric-modulated arc therapy-based dose escalation for hypoxic subvolume in nasopharyngeal carcinomas: A feasibility study. Head Neck . 2017;39:2519–2527. doi: 10.1002/hed.24925. [DOI] [PubMed] [Google Scholar]