Abstract
Arrhythmogenic cardiomyopathy (ACM) is an inherited heart muscle disease characterized by loss of ventricular myocardium and fibrofatty replacement, which predisposes to scar-related ventricular arrhythmias and sudden cardiac death, particularly in the young and athletes. Although in its original description the disease was characterized by an exclusive or at least predominant right ventricle (RV) involvement, it has been demonstrated that the fibrofatty scar can also localize in the left ventricle (LV), with the LV lesion that can equalize or even overcome that of the RV. While the right-dominant form is typically associated with mutations in genes encoding for desmosomal proteins, other (non-desmosomal) mutations have been showed to cause the biventricular and left-dominant variants. This has led to a critical evaluation of the 2010 International Task Force criteria, which exclusively addressed the right phenotypic manifestations of ACM. An International Expert consensus document has been recently developed to provide upgraded criteria (“the Padua Criteria”) for the diagnosis of the whole spectrum of ACM phenotypes, particularly left-dominant forms, highlighting the use of cardiac magnetic resonance. This review aims to offer an overview of the current knowledge on the genetic basis, the phenotypic expressions, and the diagnosis of left-sided variants, both biventricular and left-dominant, of ACM.
Keywords: arrhythmogenic cardiomyopathy, left ventricular arrhythmogenic cardiomyopathy, genetics, mutation, differential diagnosis
1. Introduction
Arrhythmogenic cardiomyopathy (ACM) is an inherited heart muscle disease characterized by fibro-fatty replacement of the ventricular myocardium, which predisposes to scar-related ventricular arrhythmias (VAs) and sudden cardiac death (SCD), particularly in the young and athletes [1,2,3].
In its original description, the disease was characterized by a predominant involvement of the right ventricle (RV), with left ventricular (LV) lesions occurring later in the history of the disease [4,5]. However, autopsy investigations, studies reporting genotype-phenotype correlations, and the growing use of contrast-enhanced cardiac magnetic resonance (CMR), led to the discovery of biventricular and left-dominant variants, in which the myocardial scar was found earlier and even predominantly in the left ventricle (LV) [6,7,8].
The increased awareness that ACM could no longer be considered a disease limited to the RV led to a critical evaluation of the 2010 International Task Force (ITF) criteria, which exclusively addressed the right phenotypic manifestations of ACM [4]. The incomplete understanding of the genetic basis of ACM, the exclusion of tissue characterization findings by contrast-enhanced CMR, and the lack of specific criteria for left-sided ACM were considered the major limitations of the 2010 ITF criteria, that could result in disease underdiagnosis or misdiagnosis [9]. Accordingly, to improve the diagnostic approach to ACM, an International Expert consensus document was recently developed in order to provide upgraded criteria (“the Padua Criteria”) for the diagnosis of the entire spectrum of ACM phenotypes [10].
Especially for the left variants, the diagnostic specificity is of outmost importance due to the presence of overlapping phenotypes of other genetic disease, such as cardio-cutaneous syndromes and neuromuscular diseases (NMD), and non-genetic cardiomyopathies, such as dilated cardiomyopathy (DCM), myocarditis, and sarcoidosis [11].
This review aims to offer an overview of the current knowledge on the genetic basis, the phenotypic expressions, and the diagnosis of left-sided variants, both biventricular and left-dominant, of ACM.
2. Left Ventricular Involvement in Arrhythmogenic Cardiomyopathy
In a pathological study of SCD victims, LV involvement was observed to be predominant among those ACM cases diagnosed only at autopsy (87% of the examined hearts; isolated in 17% and with concomitant RV involvement in 70% of cases) [12]. This result underlies the intrinsic limitations of ITF 2010 criteria in diagnosing left ventricular forms of ACM before death and the consequent need of new diagnostic tools and criteria.
The fibro-fatty scar is the distinctive histological marker of ACM. Both pathological investigations and experimental studies on transgenic animal models demonstrated the genetic basis of the fibro-fatty scar [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Numerous chromosomal loci were shown, starting from the first linkage analysis report in 1994 [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Desmosomal alterations reproduced the ACM phenotype in transgenic mice [13,14,15,16]. The distinctive localization in the RV was traditionally labelled as the “triangle of dysplasia”, comprising the RV inflow, apex, and outflow. Transvenous endomyocardial biopsy (EMB) has been comprised of the diagnostic evaluation of ACM since 1994 [32] because it allows an in vivo histologic proof of the pathognomonic disease lesion, i.e., the myocardial necrosis and fibrofatty replacement. Indeed, EMB has been regarded as a major criterion for ACM diagnosis [4,33]. Then, genotype–phenotype studies and the growing use of CMR led to the discovery of biventricular and left-dominant variants. In particular, contrast-enhanced CMR, thanks to its tissue characterization capability resulting from late gadolinium enhancement (LGE) technique, allowed the demonstration of fibro-fatty scars involving the LV [9]. As for the RV, in the LV the fibro-fatty replacement wavefront proceeds from the subepicardial to the subendocardial LV layers, finally resulting in transmural lesion with localized or extensive wall thinning. It has been demonstrated that in left ACM, the scar tissue tends to localize in the inferolateral subepicardial LV wall [6,7,34]. The subendocardium, which predominantly contributes to myocardial thickening, is usually spared [35]. Consequently, differently from the classic right-dominant phenotype, the sensitivity of standard investigations, particularly echocardiography, for the diagnosis of left-dominant forms is low. In particular, the preserved global LV systolic function and the absence of regional wall motion abnormalities explain the normal results of echocardiography. In this scenario, contrast-enhanced CMR represents a fundamental tool for the detection of structural LV involvement. Indeed, while the systolic and regional function can still be unremarkable at CMR cine imaging, contrast-enhanced CMR imaging can show both fat infiltration and presence of fibrosis using T1-weighted sequences and LGE analysis, respectively. The combination of the signal enhancement at both sequences represents the equivalent of the fibro-fatty scar at the pathological study.
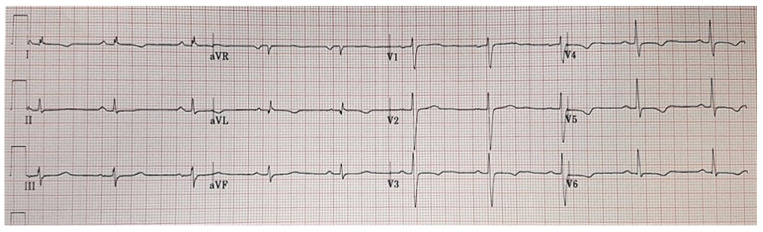
All these features outline an LV phenotype characterized by normal or mildly reduced LV systolic function with no or mild dilatation and extensive non-ischemic LGE predominantly involving the inferolateral segments. In addition to this, other distinctive characteristics are VAs with a right-bundle-branch-block (RBBB) morphology denoting the origin from the LV scar tissue and ECG anomalies such as negative T-waves or flattening in the lateral or inferolateral leads and low QRS voltages (peak-to-peak < 0.5 mV) in the limb leads (Figure 1 and Figure 2) [7,10,36].
Figure 1.
Electrocardiographic features of a patient affected by left-dominant arrhythmogenic cardiomyopathy. Basal electrocardiogram characterized by low QRS voltages in limb leads and negative T-waves in the lateral leads.
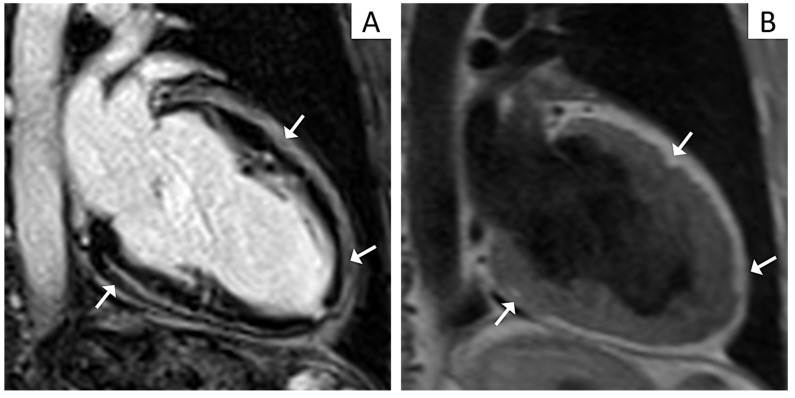
Figure 2.
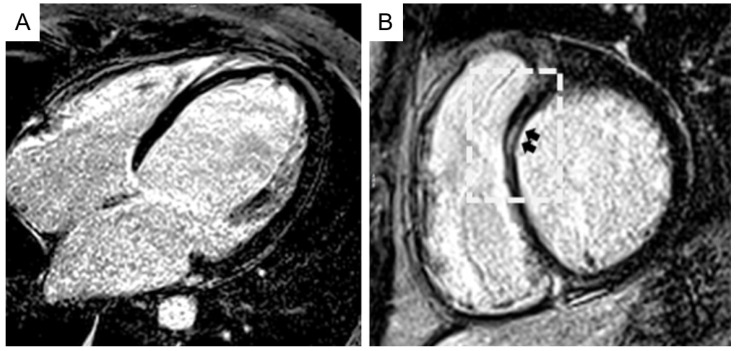
Cardiac magnetic resonance (CMR) imaging features of a patient affected by left-dominant arrhythmogenic cardiomyopathy. Post contrast CMR images in two-chamber view showing extensive late gadolinium enhancement in the form of stria proceeding from the epicardium towards the endocardium in the inferior and anterior walls (Panel A, arrows). T1 weighted CMR sequences in two-chamber view evidencing fatty infiltration in the same regions as in Panel A (Panel B, arrows).
3. Genetic Basis of Arrhythmogenic Cardiomyopathy
Arrhythmogenic cardiomyopathy generally has an autosomal dominant transmission with incomplete penetrance and variable expression, resulting in heterogeneous clinical manifestations. Less commonly, the disease can manifest with palmoplantar keratoderma and woolly hair and can be transmitted as an autosomal recessive trait (Naxos and Carvajal syndromes). Compound and digenic heterozygosity have been documented with a range of occurrences that varies widely depending on how many genes are sequenced and how missense variants are considered [37,38,39]. Homozygous mutations have also been reported [40]. It is important to note that additional genetic and/or environmental factors may act as modifiers, since the probability of being affected varies between siblings of the same index case. Thus, the role of genetic mutations in ACM pathogenesis should not be simplified as a linear cause–effect relationship in which a certain phenotype corresponds precisely to a particular genetic mutation as well as many other factors, either genetic or non-genetic, should be taken into account.
The most frequently involved genes in ACM are those encoding for desmosomal proteins, such as plakophilin-2 (PKP2), desmoplakin (DSP), desmoglein (DSG2), desmocollin (DSC2), and plakoglobin (JUP). Heterozygous mutations determining early protein termination and/or anomalous splicing in PKP2 are the most common [37].
Genes encoding for adherent junctional proteins, such as α-T-catenin (CTNNA3) and N-cadherin (CDH2), have also arisen as potentially relevant for the pathogenesis of ACM [18,20,22,26,31,41,42,43,44].
Desmosomes, adherent junctions, gap junctions, and ion channels make up the area composita, which is found at the intercalated disc level. Because this structure is essential both for cell–cell electromechanical connections and intracellular signaling pathways, mutations of its components not only affect mechanical matching among cardiomyocytes, favoring their separation and consequent cell death and scar formation, but also contribute to the arrhythmogenic pathogenesis of ACM by a purely electrical mechanism [14].
Besides the mentioned genes, other non-desmosomal ones have been recognized as pathogenic, such as lamin A/C (LMNA), desmin (DES), filamin C (FLNC), titin (TTN), sodium voltage-gated channel alpha subunit 5 (SCN5A), phospholamban (PLN), transmembrane protein 43 (TMEM 43), the cardiac ryanodine receptor-2 (RYR2), and transforming grow factor beta-3 (TGFβ-3) [24,27,28,29,31,45,46,47,48,49].
Lastly, gene-elusive cases with a typical lower prevalence of positive family history have also been reported [50]. It remains to be established whether they represent genetic variants with low penetrance and high environmental influence, or a primarily monogenic disease.
Overall, these heterogeneous mutations partially explain the clinical and genetic overlap between ACM and other genetic cardiomyopathies and channelopathies. Indeed, in patients affected by Brugada syndrome, PKP2 missense mutations have been documented [30].
3.1. Genotype-Phenotype Correlations in Left Arrhythmogenic Cardiomyopathy
3.1.1. Desmosomal Mutations
Desmosomal mutations are more common in subjects who fulfil an ACM diagnosis according to 2010 ITF criteria, supporting that the “classical” right-dominant ACM variant is mainly a disease of the desmosome. However, mutations in these genes have also been associated with “non classical” forms, both biventricular and left-dominant. Advanced stages of right-dominant ACM (often PKP2 carriers) can show LV involvement at the lateral wall with mildly or moderately reduced LV function [51]. DSG2 and DSC mutations have been related to biventricular variants of ACM [52,53]. DSP mutations have been associated to a peculiar phenotype characterized by episodes of acute myocardial injury (chest pain with troponin elevation in the presence of normal coronary arteries, “hot phases”), “burst”-induced LV fibrosis, progressive systolic dysfunction, and high incidence of VAs [54,55]. These forms are often diagnosed as acute myocarditis, thus underlying the poor diagnostic accuracy of classic ACM diagnostic criteria and the need for other diagnostic tools in such cases. Recurrent episodes of acute myocarditis among family members, or a personal history of acute myocarditis combined with a family history of cardiomyopathy or SCD, should raise the suspicion of LV variants of ACM, and tissue characterization and genetic testing should be advised [56,57].
3.1.2. Non Desmosomal-Mutations
On the other hand, left-dominant or biventricular forms, severe presentations, as well as atypical findings (e.g., altered conduction, early supraventricular arrhythmias, dystrophinopathy, polytopic VA, or arising from the LV), should suggest non-desmosomal mutations and encourage a broader genetic screening. Among non-desmosomal mutations, the fully penetrant mutation p.S358L in the gene TMEM43, endemic to Newfoundland, Canada, was the first to be identified. The p.S358L founder mutation in TMEM43 has been associated with the most aggressive heterozygous form of ACM, the arrhythmogenic right ventricular cardiomyopathy type V (ARVC-5), characterized by LV involvement and high risk of SCD [58]. Filamin C mutations were originally associated with skeletal myopathy, but isolated cardiac involvement has been documented. These mutations have been associated with LV involvement with systolic dysfunction, marked dilatation and wide fibrosis, and frequent Vas [59]. Furthermore, in FLNC as well as DES mutations carriers, extensive LV subepicardial circumferential late gadolinium enhancement (LGE)/fibrosis (ring-like pattern) and a higher incidence of SCD has been demonstrated [60,61]. Likewise, in patients with PLN-p.Arg14del variants, a distinctive tissue pattern at CMR has been shown with LGE mostly located at the LV postero-lateral wall, subsequent arrhythmias, and LV dysfunction [62].
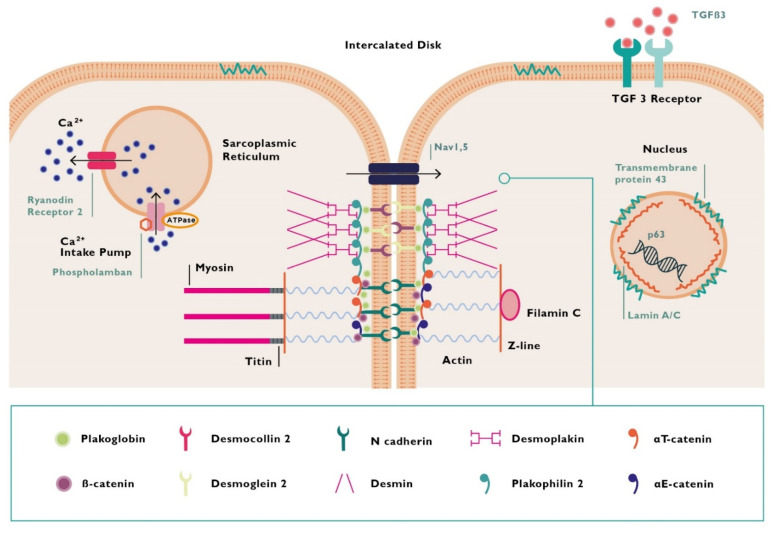
Figure 3 illustrates encoded proteins associated with ACM. Table 1 summarizes the genetic background of ACM and its correlation with different phenotypes of the disease.
Figure 3.
Proteins encoded by mutant genes in arrhythmogenic cardiomyopathy. TGFB3 = transforming grow factor beta 3.
Table 1.
Summary table for the genetic background of arrhythmogenic cardiomyopathy and its correlation with different phenotypes of the disease.
| Gene | Encoded Protein | Chromosomal Locus | Mode of Transmission | Reference | Predominately Affected Ventricle | Notes |
|---|---|---|---|---|---|---|
| Desmosomal | ||||||
| JUP | Junction plakoglobin | 17q21.2 | AD/AR also reported | McKoy et al. [18] | RV, biventricular | AR form: Cardiocutaneous syndrome (Naxos) |
| DSP | Desmoplakin | 6p24.3 | AD/AR also reported | Norgett et al. [19] | LV, biventricular | AR form: Cardiocutaneous syndrome (Carvajal) |
| PKP2 | Plakophillin-2 | 12p11.21 | AD/AR also reported | Gerull et al. [20] | RV, biventricular | classic RV phenotype |
| DSG2 | Desmoglein-2 | 18q12.1 | AD/AR also reported | Awad et al. [21] | classic RV phenotype, biventricular | frequent LV involvement |
| DSC2 | Desmocollin-2 | 18q12.1 | AD/AR also reported | Syrris et al. [22] | RV, biventricular | AR Cardiocutaneous form |
| Non desmosomal | ||||||
| TMEM43 | Transmembrane protein 43 | 3p25.1 | AD | Merner et al. [23] | RV, biventricular | Newfoundland founder variant, SCD |
| LMNA | Lamin A/C | 1q22 | AD | Quarta et al. [24] | LV, biventricular | Overlapping syndrome (DCM, Lipodystrophies, Myopathies) |
| DES | Desmin | 2q35 | AD | Hedberg et al. [25] | LV, biventricular | Overlapping syndrome (DCM and HCM, early conduction disturbances) |
| CTNNA3 | Alpha T-catenin | 10q21.3 | AD | Van Hengel et al. [26] | RV, biventricular | Low penetrance |
| PLN | Phospholamban | 6q22.31 | AD | Van der Zwaag et al. [27] | LV, biventricular | Founder mutation in Netherlands. High SCD risk. |
| TGFB3 | Transforming grow factor beta 3 | 14q24.3 | AD | Beffagna et al. [28] | RV | |
| TTN | Titin | 2q31.2 | AD | Taylor et al. [29] | RV, LV, biventricular | Overlapping syndrome (early conduction disturbances, AF, DCM) |
| SCN5A | Sodium voltage-gated channel alpha subunit 5 | AD | Cerrone et al. [30] | LV, biventricular | Overlap syndrome (BrS, LQTS Type 3, AF) | |
| CDH2 | Cadherin C | AD | Mayosi et al. [31] | RV, biventricular | ||
AD = autosomal dominant; AF = atrial fibrillation; AR = autosomal recessive; BrS = Brugada syndrome; DCM = dilated cardiomyopathy; HCM = hypertrophic cardiomyopathy; LQTS = long QT Syndrome; LV = left ventricle; RV = right ventricle; SCD = sudden cardiac death.
4. New Diagnostic Criteria for Arrhythmogenic Cardiomyopathy
These new insights into the heterogeneous genetic mutations and phenotypic manifestations of ACM led to a critical revision of the 2010 ITF criteria, which exclusively targeted RV classical forms and did not include the tissue characterization by contrast enhanced CMR imaging [4,9]. Accordingly, an International Expert consensus document has been recently developed to provide upgraded criteria (“the Padua Criteria”) for the diagnosis of the whole spectrum of ACM phenotypes [10]. The principles behind the Padua Criteria are that:
-
(1)
As for RV forms, the diagnosis of LV variants is multiparametric, and comprises functional and structural ventricular alterations, tissue characterzation results, electrocardiographic (ECG) anomalies, VAs, and familial/genetic factors.
-
(2)
Structural abnormalities can be diagnosed by LGE at contrast enhanced CMR and represents a non-invasive imaging modality for the detection of fibro-fatty scar.
-
(3)
The diagnostic power of phenotypic criteria for left ventricular variants varies in accordance with the disease phenotype, whether biventricular or left-dominant.
-
(4)
When the criteria to diagnose the RV phenotype are met, “phenotypic criteria” such as morpho-functional and structural LV anomalies allows the diagnosis of biventricular variants.
-
(5)
When the RV is not involved, “phenotypic criteria” do not afford sufficient disease-specificity.
In light of this, in patients who meet the traditional 2010 ITF criteria for definite, borderline, or possible ACM, the concomitant presence of morpho-functional and structural LV abnormalities is sufficient to make diagnosis of biventricular forms. Differently, for patients with structural abnormalities (with or without morpho-functional alterations) in whom phenotypic RV alterations cannot be found, the documentation of a positive genotyping is mandatory for making diagnosis of left-dominant variants. Table 2 shows the “Padua criteria” for diagnosis of arrhythmogenic cardiomyopathy [10].
Table 2.
“Padua criteria” for diagnosis of arrhythmogenic cardiomyopathy.
| Category | Right Ventricle | Left Ventricle |
|---|---|---|
|
By echocardiography, CMR or angiography: Major
|
By echocardiography, CMR or angiography: Minor
|
|
By CE-CMR: Major
Major
|
By CE-CMR: Major
|
|
Major
|
Minor
|
|
Minor
|
Minor
|
|
Major
|
Minor
|
|
Major
|
|
ACM = arrhythmogenic cardiomyopathy; BSA = body surface area; EDV = end diastolic volume; EF = ejection fraction; ITF = International Task Force; LBBB = left bundle-branch block; LGE = late gadolinium enhancement; LV = left ventricle; RBBB = right bundle-branch block; RV = right ventricle; RVOT = right ventricular outflow tract. From Corrado et al. [10].
Especially for left-dominant variants, the diagnostic specificity is of outmost importance due to the presence of overlapping phenotypes of other genetic and non-genetic disorders that share both genetic and phenotypic features with ACM (i.e., “phenocopies”), such as DCM, myocarditis, sarcoidosis, cardio-cutaneous syndromes, and NMD.
4.1. Cardiac Magnetic Resonance Imaging
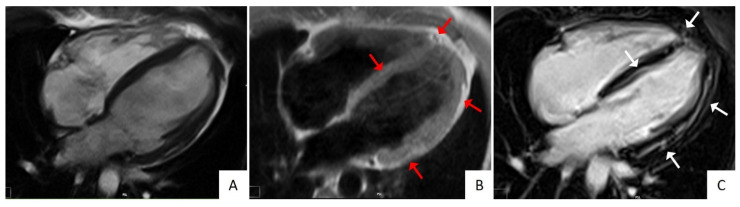
In pathological studies, LV involvement was reported in up to 87% of cases [12]. Fibro-fatty changes in ACM affect the LV either diffusely or regionally. Left ventricular structural abnormalities can be visualized as LGE by contrast enhanced CMR, often involving the inferolateral wall and, notably, in the absence of concomitant wall motion abnormalities at CMR cine imaging [6,7,34]. The septum is frequently involved in left-dominant variants, being found in up to 50% of cases, while it is an exceptional finding in right-dominant variants [6]. Fibro-fatty infiltration can be visualized by specific T1-weighted sequences, and results in wall thinning (Figure 4).
Figure 4.
Cardiac magnetic resonance (CMR) imaging features of a patient affected by biventricular arrhythmogenic cardiomyopathy. Cine CMR imaging showing a mildly dilated left ventricle (LV) (Panel A) with a slightly reduced systolic function (not showed). T1-weighted CMR images demonstrating fatty infiltration at the right ventricle apex, lateral, and septal LV walls (Panel B, red arrows). Post-contrast CMR images showing biventricular LGE in the same locations as in Panel B (Panel C, white arrows).
While the combination of enhancement at both sequences increases the probability of ACM, the solely LV LGE enhancement is non-specific and present in numerous ischemic or non-ischemic diseases, including myocarditis and sarcoidosis (“phenocopies”). The difficulty in making a differential diagnosis is increased when the also RV is involved. Moreover, sequences for fatty infiltration are not usually included in standard protocols and the fibrofatty scar—the pathognomonic hallmark of the disease—can be missed. Finally, it is important to underline that, even in the presence of the aforementioned CMR alterations, the diagnosis of ACM, whether right, biventricular, or left-dominant, remains multiparametric [63]. Thus, an isolated although indicative CMR finding is not sufficient to make a diagnosis.
4.2. Differential Diagnosis
Myocarditis can represent a differential diagnostic dilemma with left-sided ACM forms. Subepicardial LV fibrosis can be found in ACM, causing healed myocarditis overdiagnosis. Since it has been reported an higher risk of malignant VAs and SCD in athletes with LV scar [64], this finding should not be considered benign, but it should prompt more in-depth investigations. Differential diagnosis between left ACM and myocarditis may be difficult because myocarditis can be a clinical presentation of some patients with ACM (“hot phases”) [65]. On the other hand, myocarditis can show VAs with a RBBB morphology, denoting the origin from the LV, and ECG anomalies such as negative T-waves and low QRS voltages [66]. In this context, molecular genetic testing with demonstration of AC-gene mutation—typically DSP mutations—is of outmost importance to exclude left-sided forms of ACM [67].
Dilated cardiomyopathy is another tricky mime of left ACM. The resemblance between the two cardiomyopathies encompasses etiological, clinical, and imaging aspects. Desmosomal gene mutations have been documented also in DCM patients [68]. Because DCM can express in milder forms, especially in relatives, the spectrum of LV involvement in DCM ranges from limited scars as assessed by CMR imaging to severely dilated and impaired LV [69]. In this regard, the “hypokinetic non-dilated cardiomyopathy”, which has been considered as a less expressed phenotype of DCM, characterized by an extensive scarring of the LV scarcely affecting the LV systolic function, seems to belong more to the ACM left variants rather than to the DCM spectrum. Indeed, at variance with ACM, in DCM the degree of LV dysfunction does not correlate with the amount of LGE (Figure 5) [70]. Finally, one-third of DCM cardiomyopathies display an arrhythmogenic propensity with a poorer prognosis and an increased SCD risk in analogy with ACM [71]. With the knowledge of these similarities, genetic testing is often needed to reach the correct diagnosis between the two entities.
Figure 5.
Cardiac magnetic resonance (CMR) imaging features of a patient affected by dilated cardiomyopathy. Post-contrast long-axis (A) and short-axis (B) CMR views evidencing a severely dilated left ventricle cavity and myocardial late gadolinium enhancement (black arrows), limited to the anteroseptal region (boxed area). Modified from ref [70].
Cardiac sarcoidosis is associated with life-threatening arrhythmias and heart failure due to the presence of infiltrative granulomas and fibrosis [72]. The RV free wall is interested in up to 40% of cases, while at the LV level, the septum and the free wall are the most common locations [73]. These features lead to difficulties in the differential diagnosis with biventricular and left-dominant ACM. However, some details can help the diagnostic assessment. First, in sarcoidosis cardiac disease is usually observed in the context of a multiorgan disorder interesting the lungs, skin, liver, and eyes, while isolated forms are rarer. Second, when the granulomas infiltrate the basal interventricular septum, bundle branch block and atrioventricular block can appear consequently, whereas they are usually absent in ACM. Finally, CMR imaging can offer some clues useful for distinguishing sarcoidosis from ACM, as described below. In sarcoidosis, myocardial granulomas can be evidenced at post-contrast images as intramural, patchy LGE, mostly located in the basal lateral wall, not related to a coronary distribution territory and responsive to immunosuppressive therapy. An intensive signal at RV insertion points extending into the septum and the RV (“the hook sign”) is also associated with a high probability of sarcoidosis. Moreover, extracardiac findings can be documented. The combination with positron emission tomography can finally highlight fluorodeoxyglucose uptake, which is indicative of active inflammatory lesions [74].
Cardiomyopathies associated with neuromuscular disorders (i.e., Duchenne and Becker distrophinopathies) can be undistinguishable from left-dominant ACM, especially when the cardiac disorder occurs in isolation. As it is observed in ACM, the myocardial scar as evidenced by LGE is typically located at the lateral wall with a subepicardial distribution, and acts as a substrate for ventricular tachyarrhythmias, which carry a risk of SCD [75,76]. There is also an overlap in the genetic background, since LMNA and FLNC gene mutations can occur not only in left ACM but also in muscular dystrophies, and the “hypokinetic, non-dilated cardiomyopathy” is the phenotypic appearance of the disease determined by these mutations [46,77].
5. Prognostic Role of LV Involvement and Genetic Mutations
Left ventricular involvement, both isolated and combined with a RV disease, demonstrated by clinical, echocardiographic, and invasive exploration, proved to be a predictor of adverse prognosis [78,79,80,81]. However, it has been demonstrated that if the aim is to detect the LV involvement in ACM, the sole estimation of the LV function is not enough. Conversely, contrast-enhanced CMR raises the diagnostic sensitivity allowing the documentation of LV fibrosis, which spare the subendocardial layers and, consequently, do not impair the regional or global LV function [35]. The identification of the LV involvement in the form of LGE has significant implications in terms of prognosis and thus of prophylactic treatment [82]. Indeed, the indication for implantable cardiac defibrillator (ICD) implantation may be considered even in the absence of a severely impaired LV function [78]. In particular, risk stratification in ACM distinguishes three risk categories (“high”, “moderate”, and “low”) with different levels of recommendation to ICD implantation [78]. “High-risk” subjects (class I recommendation for ICD implantation) are those who have a history of aborted SCD or hemodynamically unstable VT or who have severely depressed LV function, either right or left or of both ventricles [78]. Patients with an “intermediate risk” (class IIa recommendation for ICD implantation) are those with major risk factors, like syncope, non-sustained ventricular tachycardias, or moderate disfunction of the right or left or of both ventricles [78]. The inclusion of moderate levels of dysfunction into this category reflects the awareness that the risk of VAs and SCD cannot exclusively be estimated by the LV function since the presence of scars in ACM may not affect the LV performance, but can still trigger adverse arrhythmic events.
Genotyping is not only useful for the diagnostic workup, but it can also allow prognostic stratification of ACM patients, particularly in terms of the risk prediction of SCD or heart failure. As mentioned above, some genetic mutations have been associated with phenotypes with a higher risk of VAs and SCD. Mutations of FLNC, DSP, and PLN genes predispose to LV lesions and heart failure [6,37,46]. Male carriers of the TMEM43 p.S358L founder mutation show a higher disease penetrance and arrhythmic risk [23].
6. Conclusions
Left ventricular arrhythmogenic cardiomyopathy is an emerging entity that has probably been underestimated in the past. Clinical, electrocardiographic, and arrhythmic features should raise the diagnostic suspicion regardless normal echocardiography, and prompt more in-depth investigations. Cardiac magnetic resonance improves the diagnostic sensitivity because it allows the detection of subepicardial/mid-mural LV scars. Myocardial scar is a non-specific finding, and accurate medical history, genetic testing, and clinical family screening can highlight the genetic basis of the disease. The LV involvement as evidenced by cardiac magnetic resonance and/or genetic testing is valued not only for diagnostic purposes but also because it can stratify the arrhythmic risk of ACM patients. The National ARVC Data Registry and Bio Bank, (https://clinicaltrials.gov/ct2/show/record/NCT01804699, accessed on 13 April 2021) will provide data on phenotype–genotype correlations of heterogeneous genetic backgrounds.
Author Contributions
G.M. contributed to the design of the work and drafted the manuscript. A.C., B.B., I.R., A.Z., and D.C. revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
To be excluded because our review article does not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Corrado D., Cristina B., Judge D.P. Arrhythmogenic cardiomyopathy. Circ. Res. 2017;121:784–802. doi: 10.1161/CIRCRESAHA.117.309345. [DOI] [PubMed] [Google Scholar]
- 2.Corrado D., Basso C., Thiene G. Essay: Sudden Death in Young Athletes. Lancet Lond. Engl. 2005;366(Suppl. 1):S47–S48. doi: 10.1016/S0140-6736(05)67847-6. [DOI] [PubMed] [Google Scholar]
- 3.Corrado D., Migliore F., Basso C., Thiene G. Exercise and the Risk of Sudden Cardiac Death. Herz. 2006;31:553–558. doi: 10.1007/s00059-006-2885-8. [DOI] [PubMed] [Google Scholar]
- 4.Marcus F.I., McKenna W.J., Sherrill D., Basso C., Bauce B., Bluemke D.A., Calkins H., Corrado D., Cox M.G.P.J., Daubert J.P., et al. Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: Proposed Modification of the Task Force Criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiene G., Nava A., Corrado D., Rossi L., Pennelli N. Right Ventricular Cardiomyopathy and Sudden Death in Young People. N. Engl. J. Med. 1988;318:129–133. doi: 10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- 6.Sen-Chowdhry S., Syrris P., Ward D., Asimaki A., Sevdalis E., McKenna W.J. Clinical and Genetic Characterization of Families with Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Provides Novel Insights into Patterns of Disease Expression. Circulation. 2007;115:1710–1720. doi: 10.1161/CIRCULATIONAHA.106.660241. [DOI] [PubMed] [Google Scholar]
- 7.Sen-Chowdhry S., Syrris P., Prasad S.K., Hughes S.E., Merrifield R., Ward D., Pennell D.J., McKenna W.J. Left-Dominant Arrhythmogenic Cardiomyopathy: An under-Recognized Clinical Entity. J. Am. Coll. Cardiol. 2008;52:2175–2187. doi: 10.1016/j.jacc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Pelliccia A., Caselli S., Sharma S., Basso C., Bax J.J., Corrado D., D’Andrea A., D’Ascenzi F., Di Paolo F.M., Edvardsen T., et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) Joint Position Statement: Recommendations for the Indication and Interpretation of Cardiovascular Imaging in the Evaluation of the Athlete’s Heart. Eur. Heart J. 2018;39:1949–1969. doi: 10.1093/eurheartj/ehx532. [DOI] [PubMed] [Google Scholar]
- 9.Corrado D., van Tintelen P.J., McKenna W.J., Hauer R.N.W., Anastastakis A., Asimaki A., Basso C., Bauce B., Brunckhorst C., Bucciarelli-Ducci C., et al. Arrhythmogenic Right Ventricular Cardiomyopathy: Evaluation of the Current Diagnostic Criteria and Differential Diagnosis. Eur. Heart J. 2020;41:1414–1429. doi: 10.1093/eurheartj/ehz669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corrado D., Perazzolo Marra M., Zorzi A., Beffagna G., Cipriani A., Lazzari M.D., Migliore F., Pilichou K., Rampazzo A., Rigato I., et al. Diagnosis of Arrhythmogenic Cardiomyopathy: The Padua Criteria. Int. J. Cardiol. 2020;319:106–114. doi: 10.1016/j.ijcard.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Cipriani A., Perazzolo Marra M., Bariani R., Mattesi G., Vio R., Bettella N., De Lazzari M., Motta R., Bauce B., Zorzi A., et al. Differential Diagnosis of Arrhythmogenic Cardiomyopathy: Phenocopies vs Disease Variants. Minerva Med. 2020 doi: 10.23736/S0026-4806.20.06782-8. [DOI] [PubMed] [Google Scholar]
- 12.Miles C., Finocchiaro G., Papadakis M., Gray B., Westaby J., Ensam B., Basu J., Parry-Williams G., Papatheodorou E., Paterson C., et al. Sudden Death and Left Ventricular Involvement in Arrhythmogenic Cardiomyopathy. Circulation. 2019;139:1786–1797. doi: 10.1161/CIRCULATIONAHA.118.037230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirchhof P., Fabritz L., Zwiener M., Witt H., Schäfers M., Zellerhoff S., Paul M., Athai T., Hiller K.-H., Baba H.A., et al. Age- and Training-Dependent Development of Arrhythmogenic Right Ventricular Cardiomyopathy in Heterozygous Plakoglobin-Deficient Mice. Circulation. 2006;114:1799–1806. doi: 10.1161/CIRCULATIONAHA.106.624502. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Gras E., Lombardi R., Giocondo M.J., Willerson J.T., Schneider M.D., Khoury D.S., Marian A.J. Suppression of Canonical Wnt/Beta-Catenin Signaling by Nuclear Plakoglobin Recapitulates Phenotype of Arrhythmogenic Right Ventricular Cardiomyopathy. J. Clin. Investig. 2006;116:2012–2021. doi: 10.1172/JCI27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Z., Bowles N.E., Scherer S.E., Taylor M.D., Kearney D.L., Ge S., Nadvoretskiy V.V., DeFreitas G., Carabello B., Brandon L.I., et al. Desmosomal Dysfunction Due to Mutations in Desmoplakin Causes Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Circ. Res. 2006;99:646–655. doi: 10.1161/01.RES.0000241482.19382.c6. [DOI] [PubMed] [Google Scholar]
- 16.Pilichou K., Remme C.A., Basso C., Campian M.E., Rizzo S., Barnett P., Scicluna B.P., Bauce B., van den Hoff M.J.B., de Bakker J.M.T., et al. Myocyte Necrosis Underlies Progressive Myocardial Dystrophy in Mouse Dsg2-Related Arrhythmogenic Right Ventricular Cardiomyopathy. J. Exp. Med. 2009;206:1787–1802. doi: 10.1084/jem.20090641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rampazzo A., Nava A., Danieli G.A., Buja G., Daliento L., Fasoli G., Scognamiglio R., Corrado D., Thlene G. The Gene for Arrhythmogenic Right Ventricular Cardiomyopathy Maps to Chromosome 14q23–Q24. Hum. Mol. Genet. 1994;3:959–962. doi: 10.1093/hmg/3.6.959. [DOI] [PubMed] [Google Scholar]
- 18.McKoy G., Protonotarios N., Crosby A., Tsatsopoulou A., Anastasakis A., Coonar A., Norman M., Baboonian C., Jeffery S., McKenna W.J. Identification of a Deletion in Plakoglobin in Arrhythmogenic Right Ventricular Cardiomyopathy with Palmoplantar Keratoderma and Woolly Hair (Naxos Disease) Lancet Lond. Engl. 2000;355:2119–2124. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- 19.Norgett E.E., Hatsell S.J., Carvajal-Huerta L., Cabezas J.C., Common J., Purkis P.E., Whittock N., Leigh I.M., Stevens H.P., Kelsell D.P. Recessive Mutation in Desmoplakin Disrupts Desmoplakin-Intermediate Filament Interactions and Causes Dilated Cardiomyopathy, Woolly Hair and Keratoderma. Hum. Mol. Genet. 2000;9:2761–2766. doi: 10.1093/hmg/9.18.2761. [DOI] [PubMed] [Google Scholar]
- 20.Gerull B., Heuser A., Wichter T., Paul M., Basson C.T., McDermott D.A., Lerman B.B., Markowitz S.M., Ellinor P.T., MacRae C.A., et al. Mutations in the Desmosomal Protein Plakophilin-2 Are Common in Arrhythmogenic Right Ventricular Cardiomyopathy. Nat. Genet. 2004;36:1162–1164. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 21.Awad M.M., Dalal D., Cho E., Amat-Alarcon N., James C., Tichnell C., Tucker A., Russell S.D., Bluemke D.A., Dietz H.C., et al. DSG2 Mutations Contribute to Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Am. J. Hum. Genet. 2006;79:136–142. doi: 10.1086/504393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Syrris P., Ward D., Evans A., Asimaki A., Gandjbakhch E., Sen-Chowdhry S., McKenna W.J. Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Associated with Mutations in the Desmosomal Gene Desmocollin-2. Am. J. Hum. Genet. 2006;79:978–984. doi: 10.1086/509122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merner N.D., Hodgkinson K.A., Haywood A.F.M., Connors S., French V.M., Drenckhahn J.-D., Kupprion C., Ramadanova K., Thierfelder L., McKenna W., et al. Arrhythmogenic Right Ventricular Cardiomyopathy Type 5 Is a Fully Penetrant, Lethal Arrhythmic Disorder Caused by a Missense Mutation in the TMEM43 Gene. Am. J. Hum. Genet. 2008;82:809–821. doi: 10.1016/j.ajhg.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quarta G., Syrris P., Ashworth M., Jenkins S., Zuborne Alapi K., Morgan J., Muir A., Pantazis A., McKenna W.J., Elliott P.M. Mutations in the Lamin A/C Gene Mimic Arrhythmogenic Right Ventricular Cardiomyopathy. Eur. Heart J. 2012;33:1128–1136. doi: 10.1093/eurheartj/ehr451. [DOI] [PubMed] [Google Scholar]
- 25.Hedberg C., Melberg A., Kuhl A., Jenne D., Oldfors A. Autosomal Dominant Myofibrillar Myopathy with Arrhythmogenic Right Ventricular Cardiomyopathy 7 Is Caused by a DES Mutation. Eur. J. Hum. Genet. EJHG. 2012;20:984–985. doi: 10.1038/ejhg.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Hengel J., Calore M., Bauce B., Dazzo E., Mazzotti E., De Bortoli M., Lorenzon A., Li Mura I.E.A., Beffagna G., Rigato I., et al. Mutations in the Area Composita Protein AT-Catenin Are Associated with Arrhythmogenic Right Ventricular Cardiomyopathy. Eur. Heart J. 2013;34:201–210. doi: 10.1093/eurheartj/ehs373. [DOI] [PubMed] [Google Scholar]
- 27.van der Zwaag P.A., van Rijsingen I.A.W., Asimaki A., Jongbloed J.D.H., van Veldhuisen D.J., Wiesfeld A.C.P., Cox M.G.P.J., van Lochem L.T., de Boer R.A., Hofstra R.M.W., et al. Phospholamban R14del Mutation in Patients Diagnosed with Dilated Cardiomyopathy or Arrhythmogenic Right Ventricular Cardiomyopathy: Evidence Supporting the Concept of Arrhythmogenic Cardiomyopathy. Eur. J. Heart Fail. 2012;14:1199–1207. doi: 10.1093/eurjhf/hfs119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beffagna G., Occhi G., Nava A., Vitiello L., Ditadi A., Basso C., Bauce B., Carraro G., Thiene G., Towbin J.A., et al. Regulatory Mutations in Transforming Growth Factor-Beta3 Gene Cause Arrhythmogenic Right Ventricular Cardiomyopathy Type 1. Cardiovasc. Res. 2005;65:366–373. doi: 10.1016/j.cardiores.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Taylor M., Graw S., Sinagra G., Barnes C., Slavov D., Brun F., Pinamonti B., Salcedo E.E., Sauer W., Pyxaras S., et al. Genetic Variation in Titin in Arrhythmogenic Right Ventricular Cardiomyopathy-Overlap Syndromes. Circulation. 2011;124:876–885. doi: 10.1161/CIRCULATIONAHA.110.005405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerrone M., Delmar M. Desmosomes and the Sodium Channel Complex: Implications for Arrhythmogenic Cardiomyopathy and Brugada Syndrome. Trends Cardiovasc. Med. 2014;24:184–190. doi: 10.1016/j.tcm.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayosi B.M., Fish M., Shaboodien G., Mastantuono E., Kraus S., Wieland T., Kotta M.-C., Chin A., Laing N., Ntusi N.B.A., et al. Identification of Cadherin 2 (CDH2) Mutations in Arrhythmogenic Right Ventricular Cardiomyopathy. Circ. Cardiovasc. Genet. 2017;10 doi: 10.1161/CIRCGENETICS.116.001605. [DOI] [PubMed] [Google Scholar]
- 32.McKenna W.J., Thiene G., Nava A., Fontaliran F., Blomstrom-Lundqvist C., Fontaine G., Camerini F. Diagnosis of Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br. Heart J. 1994;71:215–218. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angelini A., Basso C., Nava A., Thiene G. Endomyocardial Biopsy in Arrhythmogenic Right Ventricular Cardiomyopathy. Am. Heart J. 1996;132:203–206. doi: 10.1016/S0002-8703(96)90416-0. [DOI] [PubMed] [Google Scholar]
- 34.Igual B., Zorio E., Maceira A., Estornell J., Lopez-Lereu M.P., Monmeneu J.V., Quesada A., Navarro J., Mas F., Salvador A. Arrhythmogenic cardiomyopathy. Patterns of ventricular involvement using cardiac magnetic resonance. Rev. Esp. Cardiol. 2011;64:1114–1122. doi: 10.1016/j.recesp.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Rademakers F.E., Rogers W.J., Guier W.H., Hutchins G.M., Siu C.O., Weisfeldt M.L., Weiss J.L., Shapiro E.P. Relation of Regional Cross-Fiber Shortening to Wall Thickening in the Intact Heart. Three-Dimensional Strain Analysis by NMR Tagging. Circulation. 1994;89:1174–1182. doi: 10.1161/01.CIR.89.3.1174. [DOI] [PubMed] [Google Scholar]
- 36.De Lazzari M., Zorzi A., Cipriani A., Susana A., Mastella G., Rizzo A., Rigato I., Bauce B., Giorgi B., Lacognata C., et al. Relationship Between Electrocardiographic Findings and Cardiac Magnetic Resonance Phenotypes in Arrhythmogenic Cardiomyopathy. J. Am. Heart Assoc. 2018;7:e009855. doi: 10.1161/JAHA.118.009855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhonsale A., Groeneweg J.A., James C.A., Dooijes D., Tichnell C., Jongbloed J.D.H., Murray B., te Riele A.S.J.M., van den Berg M.P., Bikker H., et al. Impact of Genotype on Clinical Course in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy-Associated Mutation Carriers. Eur. Heart J. 2015;36:847–855. doi: 10.1093/eurheartj/ehu509. [DOI] [PubMed] [Google Scholar]
- 38.Lodder E.M., Bezzina C.R. Arrhythmogenic Right Ventricular Cardiomyopathy: Growing Evidence for Complex Inheritance. Circ. Cardiovasc. Genet. 2013;6:525–527. doi: 10.1161/CIRCGENETICS.113.000367. [DOI] [PubMed] [Google Scholar]
- 39.Rigato I., Bauce B., Rampazzo A., Zorzi A., Pilichou K., Mazzotti E., Migliore F., Marra M.P., Lorenzon A., De Bortoli M., et al. Compound and Digenic Heterozygosity Predicts Lifetime Arrhythmic Outcome and Sudden Cardiac Death in Desmosomal Gene-Related Arrhythmogenic Right Ventricular Cardiomyopathy. Circ. Cardiovasc. Genet. 2013;6:533–542. doi: 10.1161/CIRCGENETICS.113.000288. [DOI] [PubMed] [Google Scholar]
- 40.Lorenzon A., Pilichou K., Rigato I., Vazza G., De Bortoli M., Calore M., Occhi G., Carturan E., Lazzarini E., Cason M., et al. Homozygous Desmocollin-2 Mutations and Arrhythmogenic Cardiomyopathy. Am. J. Cardiol. 2015;116:1245–1251. doi: 10.1016/j.amjcard.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 41.Bauce B., Basso C., Rampazzo A., Beffagna G., Daliento L., Frigo G., Malacrida S., Settimo L., Danieli G., Thiene G., et al. Clinical Profile of Four Families with Arrhythmogenic Right Ventricular Cardiomyopathy Caused by Dominant Desmoplakin Mutations. Eur. Heart J. 2005;26:1666–1675. doi: 10.1093/eurheartj/ehi341. [DOI] [PubMed] [Google Scholar]
- 42.Pilichou K., Nava A., Basso C., Beffagna G., Bauce B., Lorenzon A., Frigo G., Vettori A., Valente M., Towbin J., et al. Mutations in Desmoglein-2 Gene Are Associated with Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation. 2006;113:1171–1179. doi: 10.1161/CIRCULATIONAHA.105.583674. [DOI] [PubMed] [Google Scholar]
- 43.Rampazzo A., Nava A., Malacrida S., Beffagna G., Bauce B., Rossi V., Zimbello R., Simionati B., Basso C., Thiene G., et al. Mutation in Human Desmoplakin Domain Binding to Plakoglobin Causes a Dominant Form of Arrhythmogenic Right Ventricular Cardiomyopathy. Am. J. Hum. Genet. 2002;71:1200–1206. doi: 10.1086/344208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sen-Chowdhry S., Syrris P., McKenna W.J. Desmoplakin Disease in Arrhythmogenic Right Ventricular Cardiomyopathy: Early Genotype-Phenotype Studies. Eur. Heart J. 2005;26:1582–1584. doi: 10.1093/eurheartj/ehi343. [DOI] [PubMed] [Google Scholar]
- 45.van Tintelen J.P., Van Gelder I.C., Asimaki A., Suurmeijer A.J.H., Wiesfeld A.C.P., Jongbloed J.D.H., van den Wijngaard A., Kuks J.B.M., van Spaendonck-Zwarts K.Y., Notermans N., et al. Severe Cardiac Phenotype with Right Ventricular Predominance in a Large Cohort of Patients with a Single Missense Mutation in the DES Gene. Heart Rhythm. 2009;6:1574–1583. doi: 10.1016/j.hrthm.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 46.Ortiz-Genga M.F., Cuenca S., Dal Ferro M., Zorio E., Salgado-Aranda R., Climent V., Padrón-Barthe L., Duro-Aguado I., Jiménez-Jáimez J., Hidalgo-Olivares V.M., et al. Truncating FLNC Mutations Are Associated With High-Risk Dilated and Arrhythmogenic Cardiomyopathies. J. Am. Coll. Cardiol. 2016;68:2440–2451. doi: 10.1016/j.jacc.2016.09.927. [DOI] [PubMed] [Google Scholar]
- 47.Yu J., Hu J., Dai X., Cao Q., Xiong Q., Liu X., Liu X., Shen Y., Chen Q., Hua W., et al. SCN5A Mutation in Chinese Patients with Arrhythmogenic Right Ventricular Dysplasia. Herz. 2014;39:271–275. doi: 10.1007/s00059-013-3998-5. [DOI] [PubMed] [Google Scholar]
- 48.Te Riele A.S.J.M., Agullo-Pascual E., James C.A., Leo-Macias A., Cerrone M., Zhang M., Lin X., Lin B., Sobreira N.L., Amat-Alarcon N., et al. Multilevel Analyses of SCN5A Mutations in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Suggest Non-Canonical Mechanisms for Disease Pathogenesis. Cardiovasc. Res. 2017;113:102–111. doi: 10.1093/cvr/cvw234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiso N., Stephan D.A., Nava A., Bagattin A., Devaney J.M., Stanchi F., Larderet G., Brahmbhatt B., Brown K., Bauce B., et al. Identification of Mutations in the Cardiac Ryanodine Receptor Gene in Families Affected with Arrhythmogenic Right Ventricular Cardiomyopathy Type 2 (ARVD2) Hum. Mol. Genet. 2001;10:189–194. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- 50.Groeneweg J.A., Bhonsale A., James C.A., te Riele A.S., Dooijes D., Tichnell C., Murray B., Wiesfeld A.C.P., Sawant A.C., Kassamali B., et al. Clinical Presentation, Long-Term Follow-Up, and Outcomes of 1001 Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Patients and Family Members. Circ. Cardiovasc. Genet. 2015;8:437–446. doi: 10.1161/CIRCGENETICS.114.001003. [DOI] [PubMed] [Google Scholar]
- 51.Te Riele A.S.J.M., Bhonsale A., Burt J.R., Zimmerman S.L., Tandri H. Genotype-Specific Pattern of LV Involvement in ARVD/C. JACC Cardiovasc. Imaging. 2012;5:849–851. doi: 10.1016/j.jcmg.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Fressart V., Duthoit G., Donal E., Probst V., Deharo J.-C., Chevalier P., Klug D., Dubourg O., Delacretaz E., Cosnay P., et al. Desmosomal Gene Analysis in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy: Spectrum of Mutations and Clinical Impact in Practice. EP Eur. 2010;12:861–868. doi: 10.1093/europace/euq104. [DOI] [PubMed] [Google Scholar]
- 53.Wong J.A., Duff H.J., Yuen T., Kolman L., Exner D.V., Weeks S.G., Gerull B. Phenotypic Analysis of Arrhythmogenic Cardiomyopathy in the Hutterite Population: Role of Electrocardiogram in Identifying High-Risk Desmocollin-2 Carriers. J. Am. Heart Assoc. 2014;3:e001407. doi: 10.1161/JAHA.114.001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bariani R., Cipriani A., Rizzo S., Celeghin R., Bueno Marinas M., Giorgi B., De Gaspari M., Rigato I., Leoni L., Zorzi A., et al. “Hot Phase” Clinical Presentation in Arrhythmogenic Cardiomyopathy. EP Eur. 2020 doi: 10.1093/europace/euaa343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith E.D., Lakdawala N.K., Papoutsidakis N., Aubert G., Mazzanti A., McCanta A.C., Agarwal P.P., Arscott P., Dellefave-Castillo L.M., Vorovich E.E., et al. Desmoplakin Cardiomyopathy, a Fibrotic and Inflammatory Form of Cardiomyopathy Distinct From Typical Dilated or Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation. 2020;141:1872–1884. doi: 10.1161/CIRCULATIONAHA.119.044934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piriou N., Marteau L., Kyndt F., Serfaty J.M., Toquet C., Le Gloan L., Warin-Fresse K., Guijarro D., Le Tourneau T., Conan E., et al. Familial Screening in Case of Acute Myocarditis Reveals Inherited Arrhythmogenic Left Ventricular Cardiomyopathies. ESC Heart Fail. 2020;7:1520–1533. doi: 10.1002/ehf2.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poller W., Haas J., Klingel K., Kühnisch J., Gast M., Kaya Z., Escher F., Kayvanpour E., Degener F., Opgen-Rhein B., et al. Familial Recurrent Myocarditis Triggered by Exercise in Patients With a Truncating Variant of the Desmoplakin Gene. J. Am. Heart Assoc. 2020;9:e015289. doi: 10.1161/JAHA.119.015289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dominguez F., Zorio E., Jimenez-Jaimez J., Salguero-Bodes R., Zwart R., Gonzalez-Lopez E., Molina P., Bermúdez-Jiménez F., Delgado J.F., Braza-Boïls A., et al. Clinical Characteristics and Determinants of the Phenotype in TMEM43 Arrhythmogenic Right Ventricular Cardiomyopathy Type 5. Heart Rhythm. 2020;17:945–954. doi: 10.1016/j.hrthm.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 59.Begay R.L., Graw S.L., Sinagra G., Asimaki A., Rowland T.J., Slavov D.B., Gowan K., Jones K.L., Brun F., Merlo M., et al. Filamin C Truncation Mutations Are Associated With Arrhythmogenic Dilated Cardiomyopathy and Changes in the Cell-Cell Adhesion Structures. JACC Clin. Electrophysiol. 2018;4:504–514. doi: 10.1016/j.jacep.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Augusto J.B., Eiros R., Nakou E., Moura-Ferreira S., Treibel T.A., Captur G., Akhtar M.M., Protonotarios A., Gossios T.D., Savvatis K., et al. Dilated Cardiomyopathy and Arrhythmogenic Left Ventricular Cardiomyopathy: A Comprehensive Genotype-Imaging Phenotype Study. Eur. Heart J. Cardiovasc. Imaging. 2020;21:326–336. doi: 10.1093/ehjci/jez188. [DOI] [PubMed] [Google Scholar]
- 61.Segura-Rodríguez D., Bermúdez-Jiménez F.J., Carriel V., López-Fernández S., González-Molina M., Oyonarte Ramírez J.M., Fernández-Navarro L., García-Roa M.D., Cabrerizo E.M., Durand-Herrera D., et al. Myocardial Fibrosis in Arrhythmogenic Cardiomyopathy: A Genotype-Phenotype Correlation Study. Eur. Heart J. Cardiovasc. Imaging. 2020;21:378–386. doi: 10.1093/ehjci/jez277. [DOI] [PubMed] [Google Scholar]
- 62.Sepehrkhouy S., Gho J.M.I.H., van Es R., Harakalova M., de Jonge N., Dooijes D., van der Smagt J.J., Buijsrogge M.P., Hauer R.N.W., Goldschmeding R., et al. Distinct Fibrosis Pattern in Desmosomal and Phospholamban Mutation Carriers in Hereditary Cardiomyopathies. Heart Rhythm. 2017;14:1024–1032. doi: 10.1016/j.hrthm.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 63.te Riele A.S.J.M., Bhonsale A., James C.A., Rastegar N., Murray B., Burt J.R., Tichnell C., Madhavan S., Judge D.P., Bluemke D.A., et al. Incremental Value of Cardiac Magnetic Resonance Imaging in Arrhythmic Risk Stratification of Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy-Associated Desmosomal Mutation Carriers. J. Am. Coll. Cardiol. 2013;62:1761–1769. doi: 10.1016/j.jacc.2012.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zorzi A., Perazzolo Marra M., Rigato I., De Lazzari M., Susana A., Niero A., Pilichou K., Migliore F., Rizzo S., Giorgi B., et al. Nonischemic Left Ventricular Scar as a Substrate of Life-Threatening Ventricular Arrhythmias and Sudden Cardiac Death in Competitive Athletes. Circ. Arrhythm. Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.116.004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mattesi G., Zorzi A., Corrado D., Cipriani A. Natural History of Arrhythmogenic Cardiomyopathy. J. Clin. Med. 2020;9:878. doi: 10.3390/jcm9030878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caforio A.L.P., Pankuweit S., Arbustini E., Basso C., Gimeno-Blanes J., Felix S.B., Fu M., Helio T., Heymans S., Jahns R., et al. Current State of Knowledge on Aetiology, Diagnosis, Management, and Therapy of Myocarditis: A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 67.Scheel P.J., Murray B., Tichnell C., James C.A., Tandri H., Calkins H., Chelko S.P., Gilotra N.A. Arrhythmogenic Right Ventricular Cardiomyopathy Presenting as Clinical Myocarditis in Women. Am. J. Cardiol. 2021 doi: 10.1016/j.amjcard.2020.12.090. [DOI] [PubMed] [Google Scholar]
- 68.Sen-Chowdhry S., Morgan R.D., Chambers J.C., McKenna W.J. Arrhythmogenic Cardiomyopathy: Etiology, Diagnosis, and Treatment. Annu. Rev. Med. 2010;61:233–253. doi: 10.1146/annurev.med.052208.130419. [DOI] [PubMed] [Google Scholar]
- 69.Pinto Y.M., Elliott P.M., Arbustini E., Adler Y., Anastasakis A., Böhm M., Duboc D., Gimeno J., de Groote P., Imazio M., et al. Proposal for a Revised Definition of Dilated Cardiomyopathy, Hypokinetic Non-Dilated Cardiomyopathy, and Its Implications for Clinical Practice: A Position Statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2016;37:1850–1858. doi: 10.1093/eurheartj/ehv727. [DOI] [PubMed] [Google Scholar]
- 70.Cipriani A., Bauce B., De Lazzari M., Rigato I., Bariani R., Meneghin S., Pilichou K., Motta R., Aliberti C., Thiene G., et al. Arrhythmogenic Right Ventricular Cardiomyopathy: Characterization of Left Ventricular Phenotype and Differential Diagnosis with Dilated Cardiomyopathy. J. Am. Heart Assoc. 2020;9:e014628. doi: 10.1161/JAHA.119.014628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spezzacatene A., Sinagra G., Merlo M., Barbati G., Graw S.L., Brun F., Slavov D., Di Lenarda A., Salcedo E.E., Towbin J.A., et al. Arrhythmogenic Phenotype in Dilated Cardiomyopathy: Natural History and Predictors of Life-Threatening Arrhythmias. J. Am. Heart Assoc. 2015;4:e002149. doi: 10.1161/JAHA.115.002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Philips B., Madhavan S., James C.A., te Riele A.S.J.M., Murray B., Tichnell C., Bhonsale A., Nazarian S., Judge D.P., Calkins H., et al. Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy and Cardiac Sarcoidosis: Distinguishing Features When the Diagnosis Is Unclear. Circ. Arrhythm. Electrophysiol. 2014;7:230–236. doi: 10.1161/CIRCEP.113.000932. [DOI] [PubMed] [Google Scholar]
- 73.Yatsynovich Y., Dittoe N., Petrov M., Maroz N. Cardiac Sarcoidosis: A Review of Contemporary Challenges in Diagnosis and Treatment. Am. J. Med. Sci. 2018;355:113–125. doi: 10.1016/j.amjms.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 74.Vita T., Okada D.R., Veillet-Chowdhury M., Bravo P.E., Mullins E., Hulten E., Agrawal M., Madan R., Taqueti V.R., Steigner M., et al. Complementary Value of Cardiac Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography in the Assessment of Cardiac Sarcoidosis. Circ. Cardiovasc. Imaging. 2018;11:e007030. doi: 10.1161/CIRCIMAGING.117.007030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Florian A., Ludwig A., Engelen M., Waltenberger J., Rösch S., Sechtem U., Yilmaz A. Left Ventricular Systolic Function and the Pattern of Late-Gadolinium-Enhancement Independently and Additively Predict Adverse Cardiac Events in Muscular Dystrophy Patients. J. Cardiovasc. Magn. Reson. 2014;16:81. doi: 10.1186/s12968-014-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van der Bijl P., Delgado V., Bootsma M., Bax J.J. Risk Stratification of Genetic, Dilated Cardiomyopathies Associated With Neuromuscular Disorders: Role of Cardiac Imaging. Circulation. 2018;137:2514–2527. doi: 10.1161/CIRCULATIONAHA.117.031110. [DOI] [PubMed] [Google Scholar]
- 77.Hasselberg N.E., Haland T.F., Saberniak J., Brekke P.H., Berge K.E., Leren T.P., Edvardsen T., Haugaa K.H. Lamin A/C Cardiomyopathy: Young Onset, High Penetrance, and Frequent Need for Heart Transplantation. Eur. Heart J. 2018;39:853–860. doi: 10.1093/eurheartj/ehx596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Corrado D., Wichter T., Link M.S., Hauer R.N.W., Marchlinski F.E., Anastasakis A., Bauce B., Basso C., Brunckhorst C., Tsatsopoulou A., et al. Treatment of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: An International Task Force Consensus Statement. Circulation. 2015;132:441–453. doi: 10.1161/CIRCULATIONAHA.115.017944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hulot J.-S., Jouven X., Empana J.-P., Frank R., Fontaine G. Natural History and Risk Stratification of Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Circulation. 2004;110:1879–1884. doi: 10.1161/01.CIR.0000143375.93288.82. [DOI] [PubMed] [Google Scholar]
- 80.Lemola K., Brunckhorst C., Helfenstein U., Oechslin E., Jenni R., Duru F. Predictors of Adverse Outcome in Patients with Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy: Long Term Experience of a Tertiary Care Centre. Heart Br. Card. Soc. 2005;91:1167–1172. doi: 10.1136/hrt.2004.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peters S. Long-Term Follow-up and Risk Assessment of Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy: Personal Experience from Different Primary and Tertiary Centres. J. Cardiovasc. Med. 2007;8:521–526. doi: 10.2459/01.JCM.0000278450.35107.b3. [DOI] [PubMed] [Google Scholar]
- 82.Migliore F., Viani S., Bongiorni M.G., Zorzi A., Silvetti M.S., Francia P., D’Onofrio A., De Franceschi P., Sala S., Donzelli S., et al. Subcutaneous Implantable Cardioverter Defibrillator in Patients with Arrhythmogenic Right Ventricular Cardiomyopathy: Results from an Italian Multicenter Registry. Int. J. Cardiol. 2019;280:74–79. doi: 10.1016/j.ijcard.2019.01.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available in a publicly accessible repository.