Abstract
Orofacial pain is a universal predicament, afflicting millions of individuals worldwide. Research on the molecular mechanisms of orofacial pain has predominately focused on the role of neurons underlying nociception. However, aside from neural mechanisms, non-neuronal cells, such as Schwann cells and satellite ganglion cells in the peripheral nervous system, and microglia and astrocytes in the central nervous system, are important players in both peripheral and central processing of pain in the orofacial region. This review highlights recent molecular and cellular findings of the glia involvement and glia–neuron interactions in four common orofacial pain conditions such as headache, dental pulp injury, temporomandibular joint dysfunction/inflammation, and head and neck cancer. We will discuss the remaining questions and future directions on glial involvement in these four orofacial pain conditions.
Keywords: Schwann cell, satellite ganglia cells, microglia, astrocytes, trigeminal ganglia, headache, toothache, dental pulp injury, head and neck cancer
1. Introduction
Chronic orofacial pain is a major healthcare issue, affecting approximately 22% of Americans in adulthood and is associated with high morbidity and health service utilization [1,2]. Chronic orofacial pain conditions represent a challenge to clinicians because of the disease complexity and unclear etiology. Pain in the soft and hard tissues of the head, neck, and face is generally regarded as orofacial pain [2,3]. Orofacial structures include many unique end organs such as teeth, facial skin, intraoral mucosa, intracranial meninges, and temporomandibular joint (TMJ) with highly specialized anatomy and innervation [2,3,4]. Nerves and blood vessels heavily supply the orofacial region. Orofacial pain can be musculoskeletal, inflammatory, neuropathic, and vascular [3,4,5]. Cellular and molecular insights of orofacial pain are largely derived from animal models, yet behavioral phenotyping in the orofacial region is more challenging than other body regions (e.g., hind limb). The neuronal involvement of orofacial pain includes peripheral sensitization of the primary afferent neurons, central sensitization in the spinal cord and brain neurons, as well as the loss of inhibitory synaptic transmission and enhanced descending facilitation in the pain pathway, transitioning acute pain to chronic pain [6,7,8,9]. The non-neuronal mechanisms in pain, such as the contribution of glial cells, are not as well studied in the orofacial region compared to the other body regions [6]. Glial cells in the central nervous system (CNS) consist of three major types: astrocytes, microglia, and oligodendrocytes [4]. Glial cells in the peripheral nervous system (PNS) consist of satellite glial cells (SGCs) in the dorsal root ganglia (DRGs) and trigeminal ganglia (TGs), and Schwann cells in the peripheral nerve [4]. In response to tissue/nerve injury, bacterial infection, or tumor infiltration glial cells become reactivated and release mediators that could contribute to both peripheral and central sensitization (Figure 1). The most commonly used glial markers include calcium-binding protein beta (S100B) and glial fibrillary acidic protein (GFAP) that are expressed in Schwann cells, SGCs, and astrocytes, and ionized calcium-binding adaptor molecule 1 (Iba1), which is a microglia/macrophage-specific calcium-binding protein. CD11b or OX42 is often used to mark microglia as well.
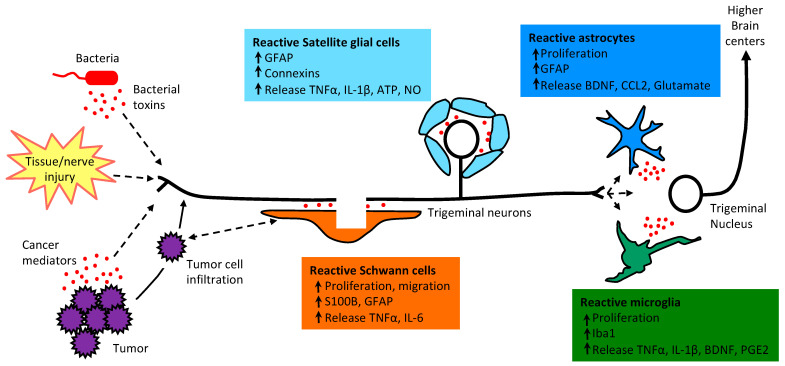
Figure 1.
Functional alterations of glial cells, including Schwann cells, SGCs, astrocytes and microglia in response to rofacial tumors, bacterial toxins, dental pulp injury or tissue/nerve injury. Peripheral injury/inflammation induces an increase in glial cell proliferation and hypertrophy, changes in glial activation markers, and the release of pronociceptive mediators that can contribute to neuronal sensitization and pain. TNFα: tumor necrosis factor alpha, IL: interlukin; NO: nitrogen oxide; BDNF: brain derived neurotrophic factor; CCL2: C-C motif chemokine ligand 2; PGE2: prostaglandin E2.
Headaches, toothaches, TMJ dysfunction (TMD)/inflammation, and head neck cancer (HNC) pain are a few common orofacial pain conditions that can drastically impact the patient’s quality of life [5]. This review will provide an update on recent advances on glial cell involvement in these four orofacial pain conditions. Although dental pulpal injury and HNC are known to have a neuropathic component [10,11], existing studies do not distinguish glial activation by neuropathic vs. non-neuropathic mechanisms. For a focused review on glial mechanisms in orofacial neuropathic pain, please see a recent publication by Kuchukulla and Boison [12]. Model-specific glial responses induced by each of these pain conditions reviewed here are summarized in Table 1.
Table 1.
Animal models and model-specific glial responses.
| Pain Conditions | Animal Models | Glial Responses | |||
|---|---|---|---|---|---|
| Schwann Cells | SGCs | Astrocytes | Microglia | ||
| Headache | Acute/Chronic dural inflammatory soup | ND |
|
|
|
| Cortical Spreading Depression (CSD) | ND | ||||
| Nitroglycerin | ND |
|
|
||
| TMD | CFA injection in the TMJ | ND | |||
| Carrageenan injection in the TMJ | ND | ND | ND |
|
|
| Zymosan injection in the TMJ | ND | ND | ND |
|
|
| Capsaicin injection in the TMJ | ND | ND | ND | ||
| Formalin injection into the TMJ | ND | ND | ND |
|
|
| Masseter tendon ligation | ND | ND |
|
|
|
| Tooth movement | ND | ND |
|
|
|
| Chronic stress | ND |
|
|||
| Dental pulp injury | Acute pulp exposure followed by mustard oil application | ND | ND |
|
|
| Pulp exposure followed by CFA application | ND |
|
ND | ||
| Pulp exposure alone | ND | ND |
|
||
| HNC | Oral cancer cells inoculated into the rat gingiva | ND |
|
Not activated [48] |
|
| Oral cancer cells inoculated into the rat tongue | ND | ND | ND |
|
|
| Breast cancer cells inoculated into the rat vibrissa pad | ND |
|
|||
| Oral cancer cells inoculated into themouse sciatic nerve to mimic PNI |
|
ND | ND | ND | |
| Schwann cell supernatant injection |
|
ND | ND | ND | |
ND: No Data. Arrows: upregulation.
2. Headache
Primary headaches, such as migraines, can be chronic and disabling, affecting over a billion adults globally, with a huge socioeconomic burden [53]. Migraine is a neurovascular disorder where neural events happen in different peripheral structures, central areas of the brain, and brainstem [54,55,56]. The dura mater, meningeal blood vessels and their innervation provided by the ophthalmic branch of the trigeminal nerve, the afferent connection to the trigeminal nucleus caudalis (TNC), and a reflex connection from the TNC to the parasympathetic outflow to the cranial vasculature, including the dural meninges, are known as the trigeminovascular system [57,58]. The nociceptive afferent inputs from these structures transmit information to the TNC, brainstem, and higher processing centers [58]. Migraine and other primary headache disorders are present as a multi-symptom complex, where pain is not the only symptom. The symptomatology may be present with sensory symptoms (e.g., photophobia, phonophobia, cutaneous allodynia), autonomic symptoms (e.g., nausea, vomiting, rhinorrhea, lacrimation, ptosis), affective symptoms (e.g., irritability), and cognitive symptoms (e.g., transient amnesia, aphasia) [54]. In 30% of patients, migraine headache is also associated with fully reversible neurological symptoms called ‘migraine aura’ [59], which affect visual, sensory, speech and/or language, motor, brainstem or retinal systems. The symptoms of primary headaches are also influenced by environmental factors such as stress, fasting, hormonal fluctuations and sleep, suggesting the involvement of epigenetic mechanisms in primary headaches [60].
Glial involvement in headache disorders is evidenced by the ability of glial cells to modulate neuronal function by releasing mediators and phenotypic changes in response to headache [61]. Microglia release of inflammatory mediators, as well as astrocytic modulation of glutamatergic mechanisms, have been shown to likely influence the activation of multiple brain and brainstem regions relevant to migraine and other primary headache disorders [61,62,63,64,65]. The neuron–glia communication via gap junctions within the TG from in vitro studies provides evidence that neuronal calcitonin gene-related peptide (CGRP), a key neuropeptide that is released during migraine attacks and a target for newly approved migraine therapeutics, could cause the activation of the adjacent SGCs to release cytokines and chemokines [36,66,67].
Glial involvement has also been hypothesized in the transformation of migraines to chronic daily headaches and medication overuse headache (MOH) [64,65]; the use of glial modulators for the management of these headaches has therefore been proposed. However, in a small double-blind, randomized, placebo-controlled trial in patients with chronic migraine, ibudilast, a cAMP phosphodiesterase (PDE) inhibitor and glial cell modulator that has shown to decrease the production of pro-inflammatory cytokines and gliosis [68,69], did not improve migraine symptomatology or decrease frequency [70]. In addition, in another small study, ibudilast did not improve headache or reduce opioid use in patients with MOH [71].
The strongest evidence of glial involvement in headache disorders comes from studies in astrocytes [55,58]. Serum levels of S100B, a calcium protein produced primarily in astrocytes but also in Schwann cells, are increased in children and adults with migraine [72,73,74]. It has been shown in familial hemiplegic migraine 2 (FHM2), a rare subtype of migraine with aura, that mutations in the expression of Atp1a2 cause ‘loss of function’ of the Na+/K+ ATPase pump in astrocytes, therefore affecting glutamate clearance [75]. Cortical spreading depression (CSD) is believed to be the underlying mechanism of migraine aura [76,77]. FHM2-knockin mice exhibit increased glutamate levels, which facilitate the induction of CSD [78]. FHM2-knockin mice also exhibit increased dendritic excitability in cingulate cortex pyramidal neurons and increased sensitivities to head pain triggers, which can be rescued by re-expression of the Atp1a2 gene in astrocytes within the cingulate cortex [79]. Tonabersat, a gap-junction modulator that inhibits CSD in animal models [80], is also known to inhibit neuronal-SGC signaling [81]. In clinical trials for migraine prophylaxis, tonabersat has been shown to have some efficacy [82,83,84], particularly in migraine with aura. Neuronal activation may cause transient extracellular alkalosis, which is buffered by glia acid secretion via electrogenic Na+-HCO3−contransporter NBCe1; loss of NBCe1 activity in astrocytes causes dysregulation of synaptic pH [85]. Changes in pH affect neuronal excitability, which has been shown in familial hemiplegic migraine [86]. Furthermore, evidence has suggested that adenosine signaling may play a role in migraine [15]. Astrocyte-derived adenosine is critical for glutamate homeostasis; activation of the adenosine A2A receptor by adenosine regulates glutamate transport via glutamate transporter 1 in astrocytes [63]. In addition to neuron–astrocyte interactions, astrocyte interaction with the vasculature has been shown. Astrocytes are in close contact with vascular cells and have the ability to influence vascular tone and release mediators that could result in either vasoconstriction or vasodilation [87,88]. Lastly, in a comprehensive genome-wide association study (GWAS) that examined the contribution of synaptic genes and glial genes (astrocytes, microglia, and oligodendrocytes) in migraine patients with and without aura, genes found to be associated with migraine are predominantly those involved in signal transduction and protein modification in astrocyte and oligodendrocytes [89].
3. TMD
TMD is a musculoskeletal dysfunction within the masticatory system comprising muscle pain and TMJ inflammation [90,91]. TMD pain commonly resides in the masseter muscle, preauricular area and anterior temporalis muscle region [90,91]. This disease, which is characterized by motion disorder, muscle pain, and inflammation of the joint, afflicts approximately 33% of the population and is highly prevalent in individuals between the ages of 20 and 40 years old [90]. Individuals with symptoms of TMD experience catching or locking of the joint, masseter pain, masticatory stiffness, a restricted mandibular range of motion, and TMJ dislocation, all of which considerably impair the quality of life [90]. The preceding examination has revealed that TMD symptoms can arise from numerous factors such as oral deformities, eating hard or chewy foods, yawning wide, clenching teeth, holding tension in the masticatory muscles and stress [90]. Pain symptoms that develop in the TMJ involve local inflammation associated with immune cell activation that induces neuronal sensitization through the release of pro-inflammatory mediators such as TNFα, IL1β, and IL6 [92,93,94]. In addition, it has been highlighted that both peripheral and central glial cells contribute to the physiopathology of TMD.
TMD has been largely studied using rodent models that consist of the injection of pro-inflammatory mediators, such as Complete Freund’s Adjuvant (CFA), carrageenan, or mustard oil in the TMJ [23,35,37,95]. Because CFA injection in the TMJ is the most used model to study TMD, this section will focus on glial cell dysregulation in models of CFA-induced TMJ inflammation.
3.1. Glial Cell Activation in the TG in Rodent Models of CFA-induced TMJ Inflammation
CFA injection into the TMJ increases the expression of SGC activation marker glial fibrillary acidic protein (GFAP) in the TG; SGC activation is observed within a few hours after the induction of the inflammation and lasts for at least one week [23,24]. SGC activation is associated with the increase of receptor expression that enhances SGC sensitivity to extracellular signals and parallels pain symptoms. Magni et al. [24] showed an overexpression of P2Y1 and P2Y2, two purinergic receptors important for SCG activation. The stimulation of SGCs with the neuropeptide CGRP potentiated calcium response induced by P2Y2 agonist UTP, contributing to downstream sensory neuron hyperactivity [96]. Moreover, the pharmacological blockade of P2Y2 reduced hyperalgesia in CFA-induced TMJ inflammation [96]. However, the administration of the P2Y1 antagonist failed to reduce hyperalgesia in the same model, suggesting a differential involvement of SGC P2Y1 and P2Y2 receptors in sensory neuron sensitization and hyperalgesia in the temporomandibular area [24].
The inflammation of the TMJ also affects SGC-sensory neuron communication through dysregulation of connexins, the gap junction-forming proteins that allow small molecules such as cAMP, glutamate and ions to directly activate neighboring cells. CFA injection into the TMJ induces overexpression of connexins 36 and 40 in neurons, connexin 43 in SGCs, and connexin 26 in both SGCs and neurons, increasing the permeability between gap junction-connected cells [22,37]. In addition, selective inhibition of connexin 43 was shown to reduce CFA-induced upregulation of GFAP, connexin 43, IL1β, Nav1.7, and mechanical hypersensitivity, indicating that connexin 43 can modify neuronal and SGC activity [22]. The increase of neuronal–glial cell signaling through gap junctions has been shown to increase neuronal excitability in various pain models [37,97,98], supporting the idea that SGCs contribute to sensory neuron sensitization through connexin expression/ function in TMJ inflammation.
Another characteristic of activated SGCs in TMD physiopathology is the production and release of pro-inflammatory mediators [25,26,34]. CFA injection in the TMJ increases COX2 expression and subsequent production of PGE2 by SGCs. PGE2 activates EP2 receptors to increase the expression of the voltage-dependent sodium channel Nav1.7 in TG neurons, leading to neuronal hyperexcitability [25,26,27]. COX2 inhibition or the blockade of SGC activation with fluorocitrate reduces Nav1.7 upregulation and hyperalgesia after CFA injection in the TMJ [25,26,27]. Similar to PGE2, IL1β expressed by SGCs can increase Nav1.7 expression in TG neurons [25].
3.2. Microglia and Astrocyte Activation in the TNC in Rodent Models of TMJ Inflammation
The injection of pro-inflammatory mediators into the TMJ induces central sensitization, which is accompanied by microglia and astrocyte activation [29,30,32,35,99]. In contrast to the microglia marker Iba1, which is upregulated as early as 24h after the injection of inflammatory mediators in the TMJ [32,33,35], upregulation of the astrocyte marker GFAP at early time points are not consistent [23,28,30,100]. However, all studies evaluating GFAP expression in the TNC later than 3 days after TMD induction showed an upregulation of GFAP, suggesting that astrocytes are more important in the persistence of CFA-induced TMJ inflammation [28,29,30].
Activated central glial cells contribute to CFA-induced neuronal sensitization through similar mechanisms as SGCs in the TG. CFA injection induces mechanical hyperalgesia, astrocyte activation and overexpression of connexin 43 and IL1β. Intracisternal injections of glial cell inhibitors reduce the expression of connexin 43, IL1β, the neuronal sensitization marker pNR1 in the TNC, and mechanical hyperalgesia induced by CFA [28,30]. CFA injection also activates microglia in the TNC, which subsequently overexpress TNFα, contributing to the development of pain symptoms [34].
3.3. Glial Cells Activation in Other Animal Models of TMD
Whereas CFA is typically used for modeling tonic/chronic pain, other pro-inflammatory agents, such as carrageenan, zymosan, capsaicin, and formalin, are used to model acute TMJ pain. Similar to CFA, carrageenan, zymosan, capsaicin, or formalin injections in the TMJ increase the expression of the microglial markers Iba1 and CD11b in the TNC [35,37,38,101]. Capsaicin and formalin injections into the TMJ induce increased p38 phosphorylation in microglia in the TNC and expression of connexin 26, 36, and 40 in SGCs in the TG [37,38,102].
Besides TMJ inflammation models, other TMD models that are more clinically relevant have been developed. In TMD models induced by sustained mouth opening, masseter tendon ligation, experimental tooth movement and stress, both peripheral and central glial cell activation have been shown [39,40,41,42,103]. The expression of astrocyte marker GFAP and microglia marker CD11b increased in both the TG and the TNC, and glial inhibitors reduced hyperalgesia in the temporomandibular area in these non-CFA induced TMD models [40,41]. Chronic stress increased the expression of IL1β in SGCs, and the local administration of the SGC inhibitor L-α-aminoadipate into the TG reduced the overexpression of IL1β and mechanical hyperalgesia [41]. Zhao et al. [42] showed that chronic stress induces persistent astrocyte activation but not microglia activation in the TNC that parallels the development of mechanical hyperalgesia. Moreover, whereas administration of the astrocyte inhibitor L-α-aminoadipate reduced stress-induced hyperalgesia, the microglia inhibitor minocycline was ineffective at reducing pain symptoms [42], suggesting a specific contribution of astrocytes in the central mechanism underlying stress-induced TMD in animal models.
4. Dental Pulp Injury
The dental pulp is a unique organ with complex neurobiology in which the afferents innervating pulp play a critical role in modulating immunological, repair, and regenerative functions [104,105]. Although there is much to be discovered about the physiological role of glial cells, specifically within the dental pulp, the importance of glial cells in the maintenance and repair of peripheral afferents is established, especially in the setting of injury. For example, cytokines and neurotrophic factors released from Schwann cells play a critical role in peripheral regeneration after cut or crush injury to peripheral nerves [106]. The dental pulp is densely innervated, and pulpal afferents exhibit a dynamic response upon different types of injury [107]. Although a direct causal effect of pulpal Schwann cells has not yet been demonstrated, elegant studies from Couve and colleagues have shown that their expression is dynamic during the physiological process of pulpal deafferentation that occurs when primary teeth are shed in humans [108]. A recent study demonstrated that pulpal Schwann cells interact with macrophages and are capable of inducing macrophages into the anti-inflammatory M2 phenotype [109]. This raises the intriguing possibility that pulpal Schwann cells play a protective role during inflammatory processes. Dental pulp Schwann cells also function as a reservoir of multipotent mesenchymal stem cells that can differentiate into odontoblasts, which are specialized pulp cells critical to hard tissue repair in teeth [110]. In addition to the dynamic role within the pulp, glial cell expression and activity within the TG and the TNC are responsive to dental pulp inflammation and injury and may play a role in persistent pain after pulp injury [8,9,10,111,112]. Interestingly, glial cell activation following pulp inflammation could lead to increased excitabilities of adjacent uninjured TG neurons, which could explain the clinical phenomena of ectopic tooth pain, in which pain originating from a single inflamed dental pulp, can be difficult to localize and produce pain in other non-injured orofacial structures [8,9,46,112,113]. Collectively, peripheral and central glial cells are important to the basic physiology of the dental pulp and the peripheral and central nervous system responses to injury and inflammation of the pulp.
4.1. Glial Cells within the Pulp
The primary glial cell types found within the dental pulp are myelinating and non-myelinating Schwann cells, and they surround the dense array of highly branched afferent fibers innervating pulpal tissue. Within the dental pulp, when evaluating the histological appearance of the afferents in cross-sections, the vast majority of fibers appear to be non-myelinated C-fibers [114]. However, many or most of these afferents are in actuality originating from medium and large diameter myelinated TG neurons [115,116]. It appears that as the afferents ascend through the root, into the crown, and further to the most peripheral aspect of the pulp, the afferents branch extensively, narrow, and lose their myelin [117,118]. There are several other unique aspects of pulp innervation, including the high density of the afferents within the tissue and the observation that despite many of the afferents having the phenotype of putative non-nociceptive Aβ fibers, nearly any activation of pulpal afferent elicits pain [119].
The presence of Schwann cell in the dental pulp has been evaluated using markers S100B and GFAP [108,118]. The non-myelinating Schwann cells are located adjacent to pulpal afferents in the most peripheral aspect of the pulp, where the afferents have branched extensively, and the soft pulp tissues interface with the hard tissue of dentin. The myelinating Schwann cells are located in the pulp tissues of the root, inferior to the more coronal branching location, and co-localize with markers of myelin protein such as myelin basic protein [118]. Interestingly, markers of myelinated afferents, such as neurofilament 200, do not always co-localize with the myelin basic protein in the peripheral pulp [118]. This is explained by the phenomenon described above whereby myelinated afferents branch and taper within the pulp tissue and lose their myelin sheath as they travel through the tooth.
Schwann cells within the pulp are dynamic, and their activation is affected by bacterial infection of the adjacent dentin, aging, and physiologic denervation that proceeds primary tooth exfoliation [120]. Studies evaluating Schwann cell markers in human teeth with and without caries have shown that the network of glia in the pulp below carious dentin is expanded and has altered morphology [120,121]. As caries encroaches into the pulp, the glial network breaks down [121]. This glial network might provide a biological barrier protecting the pulpal tissues from the bacterial pathogens and their by-products, as well as engage in the immune response. For example, Schwann cells may induce macrophages into the pro-healing M2 phenotype in the human dental pulp during the repairing process of carious infections [109]. This most peripheral glial network is also likely providing trophic support to the afferents, which are known to sprout into the area of dentinal injury [107]. Physiologic root resorption is the natural process by which primary teeth are shed in preparation for the eruption of the permanent dentition. The process includes coordinated die-back of the axons innervating the primary tooth pulp in a Wallerian-like manner [108]. As the root resorption progresses, the number of myelinated afferents decreases as Schwann cells proliferate and dedifferentiate in the process of clearing the myelin debris [108].
Finally, with aging, the Schwann cell network at the dentin–pulp interface is reduced overall in numbers and complexity, with Schwann cells in aged pulp showing less density and arborization [122]. The diminished Schwann cell network could explain, in part, the reduced capacity for healing and regeneration of the dental pulp with aging [123]. Indeed, aged Schwann cells have a diminished capacity to repair damaged peripheral afferents relative to young Schwann cells [124]. The role of pulpal Schwann cells in regeneration is especially intriguing, as pulpal glial cells can become multipotent mesenchymal stem cells that can then differentiate into pulpal cells, including odontoblasts, the cell type unique to the pulp and responsible for secreting new dentin, the internal hard tissue of the tooth [110]. It is worth noting that in addition to glial cells dedifferentiating into dental pulp stem cells, mesenchymal stem cells from dental pulp also have a unique capacity to differentiate into Schwann cells. For this reason, there is a significant research interest in the therapeutic potential of dental pulp stem cells in the treatment of peripheral nerve regeneration, which hinges on their capacity to differentiate into Schwann cells [125,126]. In summary, pulpal Schwann cells are uniquely positioned to mediate pulpal repair and regeneration.
4.2. The Effect of Pulp Injury on Glial Changes in the TG and TN
It is now well known that activation, proliferation, and inflammatory signaling in microglia and astrocytes within the TN and SGCs within the TG are important mechanisms for the maintenance of pathological orofacial pain, as demonstrated using preclinical pain models [127]. More than 20 years ago, Byers and colleagues found a marked upregulation of GFAP staining in the TG after pulp injury, and the work has been replicated by other groups [45,128]. The increased expression of activated satellite glia cells in the TG, as measured by GFAP expression, can occur adjacent to the neurons innervating the injured or inflamed pulp, as well as neurons innervating adjacent, non-injured teeth [113,129]. The increase in GFAP expression occurs more often in small- and medium-sized TG neurons rather than large-sized neurons [129]. The activated SGCs can release an array of pro-inflammatory cytokines, of which IL-1β is important, which can act back on the neuronal cells, producing further activation and sensitization [113]. The connexin proteins, including connexin 43, appear to be important to this spreading phenomenon resulting in the activation of uninjured TG neurons and to ectopic hyperalgesia [46]. However, the impact of glial plasticity in the TG versus the TN on referred pain remains to be demonstrated.
Indeed, there are significant changes in glial architecture within the TN after pulp injury. After an injury to the dental pulp, an increase in GFAP staining occurs ipsilateral to the injury, in both the nucleus caudalis and the transition zone between the caudalis and interpolaris of the TN, suggesting astrocytic involvement [10]. Increased astrocyte glutamine synthetase activity initiates central sensitization and contributes to nociceptive behavior in rats with both acute and chronic pulp injury [43,44,47]. It is also observed that Iba1 is increased ipsilateral to tooth injury in the transition zone, suggestive of a role for microglia in central changes after dental pulp injury [10]; inhibitors of microglial p38MAPK activities blocks central sensitization in the TN [44]. These findings are consistent with those of Gobel and Brink, who described the involvement of glia in the removal of axonal debris from degraded pulpal afferent terminals, after pulp injury, in the TN [130].
Collectively, the demonstration of increased glial markers in the TN with dental pulp injury provides a plausible mechanism for persistent post-treatment pain after tooth infection and pulp injury. There is now good clinical evidence that persistent pain can occur after routine dental clinical interventions performed to address dental pulp inflammation, including root canal treatment and tooth extraction [111,131]. Although there is more work to be done to understand the biological mechanisms behind persistent dental pain, it is possible that pathological changes of glial cells within the TN or TG could be an important contributory mechanism.
5. HNC
HNC is the sixth most common cancer worldwide, causing significant morbidity and mortality [132]. HNC accounts for more than 650,000 cases and 330,000 deaths annually worldwide [132]. Pain is one of the most significant morbidities associated with HNC. Up to 85% of HNC patients report pain at the diagnosis, and most receive inadequate pain treatment [133,134]. Pain in the orofacial region due to cancer impairs an individual’s speech, swallowing, eating, drinking, and interpersonal skills, having a drastic impact on their quality of life [135]. Inflammation and nerve injury caused by nerve compression and tissue destruction contribute to HNC pain [11]. The cancer microenvironment produces mediators that activate and sensitize primary afferent neurons, contributing to the onset and sustainability of cancer pain in the orofacial region [136,137,138,139]. The role glial cells play in mediating the etiology of HNC pain remains poorly understood.
In animal models of HNC-induced pain, data on glial activation are mixed. In a face cancer model produced by Walker 256B breast cancer cells inoculated into the rat vibrissa pad, the upregulation of microglia and astrocyte markers but not SGC markers were found in the ipsilateral TNC, indicating that glial activations occurred only in the CNS [50]. Inhibiting central glial activation by propentofylline suppressed allodynia, hyperalgesia, and spontaneous pain induced by face cancers [50]. Using the same model, the same group of researchers examined the time course of microglia and astrocytes activation, as well as the effect of propentofylline on behavioral outcomes. The study demonstrated transient activation of microglia and astrocytes in the TNC, and propentofylline was more effective when treated early, suggesting central glial activation might contribute to the development but not the maintenance of pain [51]. In a tongue cancer model produced by oral squamous cell carcinoma cells (SCC-158) inoculation, rats developed local mechanical and heat sensitivity, accompanied by hypersensitivity in the spinal wide dynamic range (WDR) neurons, microglia activation, and P2Y12 expression in the TNC [49]. Administration of P2Y12 antagonist or minocycline reversed associated nociceptive behavior, microglial activation, and WDR neuron activity [49]. In contrast, in a rat gingiva SCC model using the same cell line SCC-158, the number of SGCs encircling the medium and large neurons was increased in the TG, accompanied by mechanical allodynia in the whisker-pad skin induced by the cancer growth [48]. However, microglia and astrocytes in the TNC were not activated in the gingiva cancer model [48]. These differences in glial activation patterns may reflect differences in anatomical location, the cell line used, or time course in tumor development and glial activation. Nevertheless, these studies suggest that glial activation could modulate neuronal activities and nociceptive behaviors in mice with HNC.
Perineural invasion, an active process that cancer invades into the nerve, occurs frequently in HNC patients and is associated with increased pain [11]. Emerging evidence highlights a critical role of Schwann cells in perineural invasion. Schwann cells are activated in the presence of pancreatic, colon, gastric, lung, skin, and oral cancer cells and promote cancer growth, invasion, and dispersion [11,52,140,141,142,143,144,145,146,147,148,149]. Cancer-activated Schwann cells secrete neurotrophic factors, chemokines/cytokines, proteases, and adhesion molecules [52,143,146,147,149,150], many of which could directly excite and sensitize primary afferent neurons [151,152,153]. We and others recently found that Schwann cells and oral cancer cells reciprocally interact to promote each other’s growth, migration, and invasion [52,147,152]. Besides, supernatant collected from oral cancer-activated Schwann cells produces a nociceptive response in mice [52]. In our in vivo model of perineural invasion, oral SCC cells invading into the sciatic nerve induced nerve injury and Schwann cell abnormalities, which were accompanied by mechanical hypersensitivities and spontaneous pain in mice [11]. These findings highlight the importance of Schwann cells in cancer progression and associated pain.
6. Remaining Questions and Future Directions
Glial activation mechanisms: Research in the field has focused on glial activation patterns using immunostaining of glial activation markers and examining glial cell hypertrophy or proliferation following inflammation or tissue injury [6]. The molecular mechanisms of glia activation and the time course of glial activation have not been explicitly studied in orofacial pain conditions. Elucidating such mechanisms might help hijack glial activation programs for better pain control.
Mediators released by glial cells: Understanding the synthesis and release of glial mediators (e.g., cytokines, chemokines, growth factors, and proteases) to the extracellular space under normal and pathophysiological conditions is key to understand glia–glia interaction, neuron–glia interaction, glia–immune cell interaction, and glia–cancer interaction. Chronic orofacial pain is a dynamic process; understanding the changes of glia mediators during the time course of the pathological pain states is also important.
The role of peripheral glia: The role of peripheral glia, especially Schwann cells, in orofacial pain remains poorly understood, particularly in headache and TMD conditions. Activated Schwann cells secrete cytokines, chemokines, and neurotrophic factors that can activate and recruit immune cells, disrupt the blood-nerve barrier, promote axonal growth, and contribute to abnormal pain states during inflammation and nerve injury [154,155,156,157]. Recently, studies have shown that specialized Schwann cells located at the skin terminals of C-nociceptive fibers can be directly activated by noxious stimuli [158]. TRPA1, an ion channel implicated in many orofacial pain conditions including TMD [91,159], is also expressed in Schwann cells, and the selective deletion of TRPA1 in Schwann cells reduces oxidative stress, macrophage infiltration, and sustained mechanical pain in sciatic nerve ligation and alcohol-induced neuropathic conditions [159,160,161].
Sex differences: Pain is more prominent in women with chronic orofacial pain conditions such as headache, TMD, pulpal injury, and HNC [6,162]. Sex differences in microglia in response to nerve injury have been nicely demonstrated in mice. Spinal microglial signaling inhibitors, such as minocycline and p38 inhibitors, reduce neuropathic pain primarily in male mice in a testosterone-dependent mechanism, with minimal effect on female mice [6,163]. In rodents with orofacial pain, the contribution of glial cells in sex dimorphism is still an open question. Whether other glial cells, such as astrocytes, SGCs, and Schwann cells, play a role in sex dimorphism in orofacial pain needs to be investigated.
Glia and pain resolution: Glia activation is not always bad. Activated glia also release anti-inflammatory mediators and neuroprotective factors that are important for nerve regeneration, repair, and pain resolution [164,165]. Understanding the molecular switch between the pro-nociceptive and the anti-nociceptive state of glial activation could be a powerful strategy to treat orofacial pain [164].
Body region/ pain type differences: The contribution of glial cells in pain is not as extensively studied in the orofacial region compared to other body regions [6]. Neurons and glial cells exhibit diverse functional and anatomical changes that are disease-specific [166]. While DRG and TG neurons share some functional and molecular similarities, they differ in embryonic origins, transcription patterns, signaling pathways, and responses to certain drugs [167,168,169]. A better understanding of the similarities and differences in glial mechanisms across different body regions and pain conditions will help develop targeted therapies to improve efficacy and minimize unwanted side effects.
Glial activation in higher brain centers: The majority of the studies so far have focused on glial activation in the TG and TNC in orofacial pain. The pain circuit is not complete without ascending pathways from the TNC to higher brain centers and the descending modulatory pathways from higher brain centers [9,168,170]. Chronic pain is associated with functional, structural plasticity and reorganization of the pain circuits [166]. Glial plasticity has been shown in the thalamus, the descending modulatory pathway as well as the limbic systems in a few orofacial pain conditions [8,31,170,171,172,173,174]. How glial cells modulate neuronal plasticity both functionally and structurally and their role in the organization of pain circuits at different disease states remains a daunting task in the orofacial pain field.
Glia–immune cell interactions: It is well known that the immune system plays a critical role in pain development, maintenance, and resolution [175]. Many types of pain conditions are associated with increased infiltration of immune cells at the site of tissue/nerve injury. The immune cells release autoantibodies, chemokines, and cytokines, influencing neuronal and glial activities at different levels of the pain circuitry [175]. Peripheral immune cells can infiltrate the CNS after injury and may directly alter microglia, astrocytes, and oligodendrocyte function [176]. Microglia are both glia and immune cells; they are resident macrophages of the CNS [127,177,178]. Other glial cells are also known to propagate inflammation, recruit immune cells, and exhibit immune-cell-like properties [127,157,177]. The heterogeneity of immune cell involvement and how they interact with neurons and glia in different orofacial pain conditions remain a considerable challenge for the future.
The glial mechanism in patients: Using Positron Emission Tomography with a radioligand (11)C-PBR28 that binds to the translocator protein (TSPO), a protein unregulated in activated microglia and astrocytes in the brain, studies have probed microglia and astrocyte activation in patients with lower back pain, fibromyalgia, and rheumatoid arthritis [177,178,179,180]. Similar techniques can be applied to patients with chronic orofacial pain to image glial activation in the brain. Peripheral nerve tissue biopsies and postmortem analysis of TG and brain tissues from patients with chronic orofacial pain conditions will also provide insights into glial involvement in chronic orofacial pain. Currently, there are no approved drugs that specifically target glial cells. A few non-selective agents that can either inhibit glia activities or toll-like receptor 4 or glial p38 signaling were explored in clinical trials as potential analgesics. While glial modulators show some promise in certain disease conditions, most clinical studies fail to demonstrate efficacy (Table 2). To improve the efficacy, future works need to resolve the selectivity issues of purported glial modulators. A better understanding of the type of glial cells involved, the timing, and sex dependence of glial contributions to persistent pain in human pain conditions are needed [165].
Table 2.
Clinical trials using glial modulators.
| Drug Name | Target | Indication | Efficacy | Reference |
|---|---|---|---|---|
| Minocycline | Microglia inhibitor | Third molar surgery | Yes | Gelesko et al., 2011 [181] |
| Lumbar discectomy | No | Martinez et al., 2013 [182] | ||
| Diabetic peripheral neuropathy | No | Syngle et al., 2014 [183] | ||
| Carpal tunnel and trigger finger release | No; longer pain in a patient subgroup | Curtin et al., 2017 [184] | ||
| Lumbar radiculopathy | Yes | Vanelderen et al., 2015 [185] | ||
| Unilateral sciatica | No | Sumracki et al., 2012 [186] | ||
| Propentofylline | Microglia and astrocytes modulator | Post-herpetic neuralgia | No | Landry et al., 2012 [187] |
| Ibudilast | cAMP phosphodiesterase inhibitor | Chronic migraine | No | Kwok et al., 2016 [70] |
| Medication overuse headache | No | Loggia et al., 2015 [71] | ||
| Tonabersat | Gap-junction modulator | Migraine prophylaxis | Yes, in migraine patients with aura | Hauge et al., 2009 [84] |
| Naltrexone | Toll-like receptor 4 antagonist | Fibromyalgia | Yes | Younger et al., 2009, 2013 [188,189] |
| Amitriptyline | P38 mitogen-activated protein kinase inhibitor | Lumbar radiculopathy | Yes | Vanelderen et al., 2015 [185] |
| Losmapimod | P38 mitogen-activated protein kinase inhibitor | Traumatic peripheral nerve injury | No | Ostenfeld et al., 2013 [190] |
| Lumbosacral radiculopathies | No | Ostenfeld et al., 2015 [191] | ||
| Dilmapimod | P38 mitogen-activated protein kinase inhibitor | Mixed neuropathic pain | Yes | Anand et al., 2011 [192] |
Author Contributions
Conceptualization, Y.Y.; Writing-Original Draft Preparation, Y.Y., E.S. (Elizabeth Salvo), M.R.-R., S.A., B.M., J.G.; Writing-Review & Editing, Y.Y., E.S. (Elizabeth Salvo), M.R.-R., S.A., E.S. (Emi Shimizu), Y.K., B.M., J.G; Visualization, B.M., Y.Y.; Supervision, Y.Y.; Project Administration, Y.Y.; Funding Acquisition, Y.Y., E.S. (Elizabeth Salvo) All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by NIH grants R03DE027777 (Y. Ye), R01DE029493 (Y. Ye) and R01DE025885 E.S. (Emi Shimizu).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Araujo-Filho H.G., Pereira E.W.M., Campos A.R., Quintans-Junior L.J., Quintans J.S.S. Chronic orofacial pain animal models—Progress and challenges. Expert Opin. Drug Discov. 2018;13:949–964. doi: 10.1080/17460441.2018.1524458. [DOI] [PubMed] [Google Scholar]
- 2.Hargreaves K.M. Orofacial pain. Pain. 2011;152(Suppl. 3):S25–S32. doi: 10.1016/j.pain.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Rossi S.S. Orofacial pain: A primer. Dent. Clin. N. Am. 2013;57:383–392. doi: 10.1016/j.cden.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Chiang C.Y., Dostrovsky J.O., Iwata K., Sessle B.J. Role of glia in orofacial pain. Neuroscientist. 2011;17:303–320. doi: 10.1177/1073858410386801. [DOI] [PubMed] [Google Scholar]
- 5.Romero-Reyes M., Uyanik J.M. Orofacial pain management: Current perspectives. J. Pain Res. 2014;7:99–115. doi: 10.2147/JPR.S37593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berta T., Qadri Y.J., Chen G., Ji R.R. Microglial Signaling in Chronic Pain with a Special Focus on Caspase 6, p38 MAP Kinase, and Sex Dependence. J. Dent. Res. 2016;95:1124–1131. doi: 10.1177/0022034516653604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hucho T., Levine J.D. Signaling pathways in sensitization: Toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Iwata K., Sessle B.J. The Evolution of Neuroscience as a Research Field Relevant to Dentistry. J. Dent. Res. 2019;98:1407–1417. doi: 10.1177/0022034519875724. [DOI] [PubMed] [Google Scholar]
- 9.Shinoda M., Kubo A., Hayashi Y., Iwata K. Peripheral and Central Mechanisms of Persistent Orofacial Pain. Front. Neurosci. 2019;13:1227. doi: 10.3389/fnins.2019.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee C., Ramsey A., De Brito-Gariepy H., Michot B., Podborits E., Melnyk J., Gibbs J.L.G. Molecular, cellular and behavioral changes associated with pathological pain signaling occur after dental pulp injury. Mol. Pain. 2017;13:1744806917715173. doi: 10.1177/1744806917715173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salvo E., Campana W.M., Scheff N.N., Nguyen T.H., Jeong S.H., Wall I., Wu A.K., Zhang S., Kim H., Bhattacharya A., et al. Peripheral nerve injury and sensitization underlie pain associated with oral cancer perineural invasion. Pain. 2020;161:2592–2602. doi: 10.1097/j.pain.0000000000001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuchukulla M., Boison D. Are glia targets for neuropathic orofacial pain therapy? J. Am. Dent. Assoc. 2020;9 doi: 10.1016/j.adaj.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukacs M., Haanes K.A., Majlath Z., Tajti J., Vecsei L., Warfvinge K., Edvinsson L. Dural administration of inflammatory soup or Complete Freund’s Adjuvant induces activation and inflammatory response in the rat trigeminal ganglion. J. Headache Pain. 2015;16:564. doi: 10.1186/s10194-015-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried N.T., Maxwell C.R., Elliott M.B., Oshinsky M.L. Region-specific disruption of the blood-brain barrier following repeated inflammatory dural stimulation in a rat model of chronic trigeminal allodynia. Cephalalgia Int. J. Headache. 2018;38:674–689. doi: 10.1177/0333102417703764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried N.T., Elliott M.B., Oshinsky M.L. The Role of Adenosine Signaling in Headache: A Review. Brain Sci. 2017;7:30. doi: 10.3390/brainsci7030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su M., Ran Y., He Z., Zhang M., Hu G., Tang W., Zhao D., Yu S. Inhibition of toll-like receptor 4 alleviates hyperalgesia induced by acute dural inflammation in experimental migraine. Mol. Pain. 2018;14:1744806918754612. doi: 10.1177/1744806918754612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charles A., Brennan K. Cortical spreading depression-new insights and persistent questions. Cephalalgia Int. J. Headache. 2009;29:1115–1124. doi: 10.1111/j.1468-2982.2009.01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karatas H., Erdener S.E., Gursoy-Ozdemir Y., Lule S., Eren-Kocak E., Sen Z.D., Dalkara T. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013;339:1092–1095. doi: 10.1126/science.1231897. [DOI] [PubMed] [Google Scholar]
- 19.Aizawa H., Sun W., Sugiyama K., Itou Y., Aida T., Cui W., Toyoda S., Terai H., Yanagisawa M., Tanaka K. Glial glutamate transporter GLT-1 determines susceptibility to spreading depression in the mouse cerebral cortex. Glia. 2020;68:2631–2642. doi: 10.1002/glia.23874. [DOI] [PubMed] [Google Scholar]
- 20.Qin G., Gui B., Xie J., Chen L., Chen L., Cui Z., Zhou J., Tan G. Tetrandrine Alleviates Nociception in a Rat Model of Migraine via Suppressing S100B and p-ERK Activation in Satellite Glial Cells of the Trigeminal Ganglia. J. Mol. Neurosci. 2018;64:29–38. doi: 10.1007/s12031-017-0999-5. [DOI] [PubMed] [Google Scholar]
- 21.Long T., He W., Pan Q., Zhang S., Zhang Y., Liu C., Liu Q., Qin G., Chen L., Zhou J. Microglia P2X4 receptor contributes to central sensitization following recurrent nitroglycerin stimulation. J. Neuroinflamm. 2018;15:245. doi: 10.1186/s12974-018-1285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Y.Z., Zhang P., Hao T., Wang L.M., Guo M.D., Gan Y.H. Connexin 43 contributes to temporomandibular joint inflammation induced-hypernociception via sodium channel 1.7 in trigeminal ganglion. Neurosci. Lett. 2019;707:134301. doi: 10.1016/j.neulet.2019.134301. [DOI] [PubMed] [Google Scholar]
- 23.Villa G., Ceruti S., Zanardelli M., Magni G., Jasmin L., Ohara P.T., Abbracchio M.P. Temporomandibular joint inflammation activates glial and immune cells in both the trigeminal ganglia and in the spinal trigeminal nucleus. Mol. Pain. 2010;6:89. doi: 10.1186/1744-8069-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magni G., Merli D., Verderio C., Abbracchio M.P., Ceruti S. P2Y2 receptor antagonists as anti-allodynic agents in acute and sub-chronic trigeminal sensitization: Role of satellite glial cells. Glia. 2015;63:1256–1269. doi: 10.1002/glia.22819. [DOI] [PubMed] [Google Scholar]
- 25.Zhang P., Bi R.Y., Gan Y.H. Glial interleukin-1beta upregulates neuronal sodium channel 1.7 in trigeminal ganglion contributing to temporomandibular joint inflammatory hypernociception in rats. J. Neuroinflamm. 2018;15:117. doi: 10.1186/s12974-018-1154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang P., Gan Y.H. Prostaglandin E2 Upregulated Trigeminal Ganglionic Sodium Channel 1.7 Involving Temporomandibular Joint Inflammatory Pain in Rats. Inflammation. 2017;40:1102–1109. doi: 10.1007/s10753-017-0552-2. [DOI] [PubMed] [Google Scholar]
- 27.Bi R.Y., Kou X.X., Meng Z., Wang X.D., Ding Y., Gan Y.H. Involvement of trigeminal ganglionic Nav 1.7 in hyperalgesia of inflamed temporomandibular joint is dependent on ERK1/2 phosphorylation of glial cells in rats. Eur. J. Pain. 2013;17:983–994. doi: 10.1002/j.1532-2149.2012.00262.x. [DOI] [PubMed] [Google Scholar]
- 28.Guo W., Wang H., Watanabe M., Shimizu K., Zou S., LaGraize S.C., Wei F., Dubner R., Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J. Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S.M., Cho Y.S., Kim T.H., Jin M.U., Ahn D.K., Noguchi K., Bae Y.C. An ultrastructural evidence for the expression of transient receptor potential ankyrin 1 (TRPA1) in astrocytes in the rat trigeminal caudal nucleus. J. Chem. Neuroanat. 2012;45:45–49. doi: 10.1016/j.jchemneu.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Wang S., Song L., Tan Y., Ma Y., Tian Y., Jin X., Lim G., Zhang S., Chen L., Mao J. A functional relationship between trigeminal astroglial activation and NR1 expression in a rat model of temporomandibular joint inflammation. Pain Med. 2012;13:1590–1600. doi: 10.1111/j.1526-4637.2012.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nascimento G.C., De Paula B.B., Gerlach R.F., Leite-Panissi C.R.A. Temporomandibular inflammation regulates the matrix metalloproteinases MMP-2 and MMP-9 in limbic structures. J. Cell Physiol. 2021 doi: 10.1002/jcp.30341. [DOI] [PubMed] [Google Scholar]
- 32.Cady R.J., Denson J.E., Sullivan L.Q., Durham P.L. Dual orexin receptor antagonist 12 inhibits expression of proteins in neurons and glia implicated in peripheral and central sensitization. Neuroscience. 2014;269:79–92. doi: 10.1016/j.neuroscience.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 33.Koop L.K., Hawkins J.L., Cornelison L.E., Durham P.L. Central Role of Protein Kinase A in Promoting Trigeminal Nociception in an In Vivo Model of Temporomandibular Disorders. J. Oral Facial Pain Headache. 2017;31:264–274. doi: 10.11607/ofph.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai Q., Liu S., Shu H., Tang Y., George S., Dong T., Schmidt B.L., Tao F. TNFalpha in the Trigeminal Nociceptive System Is Critical for Temporomandibular Joint Pain. Mol. Neurobiol. 2019;56:278–291. doi: 10.1007/s12035-018-1076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Araujo J.C.B., Gondim D.V., Cavalcante A.L.C., Lisboa M.R.P., de Castro Brito G.A., Vale M.L. Inflammatory pain assessment in the arthritis of the temporomandibular joint in rats: A comparison between two phlogistic agents. Pt 1J. Pharmacol. Toxicol. Methods. 2017;88:100–108. doi: 10.1016/j.vascn.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Thalakoti S., Patil V.V., Damodaram S., Vause C.V., Langford L.E., Freeman S.E., Durham P.L. Neuron-glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garrett F.G., Durham P.L. Differential expression of connexins in trigeminal ganglion neurons and satellite glial cells in response to chronic or acute joint inflammation. Neuron Glia Biol. 2008;4:295–306. doi: 10.1017/S1740925X09990093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Won K.A., Kang Y.M., Lee M.K., Park M.K., Ju J.S., Bae Y.C., Ahn D.K. Participation of microglial p38 MAPK in formalin-induced temporomandibular joint nociception in rats. J. Orofac. Pain. 2012;26:132–141. [PubMed] [Google Scholar]
- 39.Guo W., Wang H., Zou S., Wei F., Dubner R., Ren K. Long lasting pain hypersensitivity following ligation of the tendon of the masseter muscle in rats: A model of myogenic orofacial pain. Mol. Pain. 2010;6:40. doi: 10.1186/1744-8069-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X.D., Wang J.J., Sun L., Chen L.W., Rao Z.R., Duan L., Cao R., Wang M.Q. Involvement of medullary dorsal horn glial cell activation in mediation of masseter mechanical allodynia induced by experimental tooth movement. Arch Oral. Biol. 2009;54:1143–1150. doi: 10.1016/j.archoralbio.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Y.J., Liu Y., Zhao Y.H., Li Q., Zhang M., Chen Y.J. Activation of satellite glial cells in the trigeminal ganglion contributes to masseter mechanical allodynia induced by restraint stress in rats. Neurosci. Lett. 2015;602:150–155. doi: 10.1016/j.neulet.2015.06.048. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y.J., Liu Y., Li Q., Zhao Y.H., Wang J., Zhang M., Chen Y.J. Involvement of trigeminal astrocyte activation in masseter hyperalgesia under stress. Physiol. Behav. 2015;142:57–65. doi: 10.1016/j.physbeh.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Chiang C.Y., Wang J., Xie Y.F., Zhang S., Hu J.W., Dostrovsky J.O., Sessle B.J. Astroglial glutamate-glutamine shuttle is involved in central sensitization of nociceptive neurons in rat medullary dorsal horn. J. Neurosci. 2007;27:9068–9076. doi: 10.1523/JNEUROSCI.2260-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie Y.F., Zhang S., Chiang C.Y., Hu J.W., Dostrovsky J.O., Sessle B.J. Involvement of glia in central sensitization in trigeminal subnucleus caudalis (medullary dorsal horn) Brain Behav. Immun. 2007;21:634–641. doi: 10.1016/j.bbi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Filippini H.F., Scalzilli P.A., Costa K.M., Freitas R.D.S., Campos M.M. Activation of trigeminal ganglion satellite glial cells in CFA-induced tooth pulp pain in rats. PLoS ONE. 2018;13:e0207411. doi: 10.1371/journal.pone.0207411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komiya H., Shimizu K., Ishii K., Kudo H., Okamura T., Kanno K., Shinoda M., Ogiso B., Iwata K. Connexin 43 expression in satellite glial cells contributes to ectopic tooth-pulp pain. J. Oral Sci. 2018;60:493–499. doi: 10.2334/josnusd.17-0452. [DOI] [PubMed] [Google Scholar]
- 47.Tsuboi Y., Iwata K., Dostrovsky J.O., Chiang C.Y., Sessle B.J., Hu J.W. Modulation of astroglial glutamine synthetase activity affects nociceptive behaviour and central sensitization of medullary dorsal horn nociceptive neurons in a rat model of chronic pulpitis. Eur. J. Neurosci. 2011;34:292–302. doi: 10.1111/j.1460-9568.2011.07747.x. [DOI] [PubMed] [Google Scholar]
- 48.Hironaka K., Ozaki N., Hattori H., Nagamine K., Nakashima H., Ueda M., Sugiura Y. Involvement of glial activation in trigeminal ganglion in a rat model of lower gingival cancer pain. Nagoya J. Med. Sci. 2014;76:323–332. [PMC free article] [PubMed] [Google Scholar]
- 49.Tamagawa T., Shinoda M., Honda K., Furukawa A., Kaji K., Nagashima H., Akasaka R., Chen J., Sessle B.J., Yonehara Y., et al. Involvement of Microglial P2Y12 Signaling in Tongue Cancer Pain. J. Dent. Res. 2016;95:1176–1182. doi: 10.1177/0022034516647713. [DOI] [PubMed] [Google Scholar]
- 50.Hidaka K., Ono K., Harano N., Sago T., Nunomaki M., Shiiba S., Nakanishi O., Fukushima H., Inenaga K. Central glial activation mediates cancer-induced pain in a rat facial cancer model. Neuroscience. 2011;180:334–343. doi: 10.1016/j.neuroscience.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Sago T., Ono K., Harano N., Furuta-Hidaka K., Hitomi S., Nunomaki M., Yoshida M., Shiiba S., Nakanishi O., Matsuo K., et al. Distinct time courses of microglial and astrocytic hyperactivation and the glial contribution to pain hypersensitivity in a facial cancer model. Brain Res. 2012;1457:70–80. doi: 10.1016/j.brainres.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 52.Salvo E., Saraithong P., Curtin J.G., Janal M.N., Ye Y. Reciprocal interactions between cancer and Schwann cells contribute to oral cancer progression and pain. Heliyon. 2019;5:e01223. doi: 10.1016/j.heliyon.2019.e01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart W.F., Ricci J.A., Chee E., Morganstein D., Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443–2454. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 54.Burstein R., Noseda R., Borsook D. Migraine: Multiple processes, complex pathophysiology. J. Neurosci. 2015;35:6619–6629. doi: 10.1523/JNEUROSCI.0373-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goadsby P.J., Holland P.R., Martins-Oliveira M., Hoffmann J., Schankin C., Akerman S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017;97:553–622. doi: 10.1152/physrev.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vila-Pueyo M., Hoffmann J., Romero-Reyes M., Akerman S. Brain structure and function related to headache: Brainstem structure and function in headache. Cephalalgia Int. J. Headache. 2019;39:1635–1660. doi: 10.1177/0333102418784698. [DOI] [PubMed] [Google Scholar]
- 57.Kemper R.H., Meijler W.J., Korf J., Ter Horst G.J. Migraine and function of the immune system: A meta-analysis of clinical literature published between 1966 and 1999. Cephalalgia Int. J. Headache. 2001;21:549–557. doi: 10.1046/j.1468-2982.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 58.Akerman S., Holland P.R., Goadsby P.J. Diencephalic and brainstem mechanisms in migraine. Nat. Rev. Neurosci. 2011;12:570–584. doi: 10.1038/nrn3057. [DOI] [PubMed] [Google Scholar]
- 59.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd ed. Cephalalgia Int. J. Headache. 2018;38:1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 60.Eising E., Datson N.A., van den Maagdenberg A.M., Ferrari M.D. Epigenetic mechanisms in migraine: A promising avenue? BMC Med. 2013;11:26. doi: 10.1186/1741-7015-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartley J. Could glial activation be a factor in migraine? Med. Hypotheses. 2009;72:255–257. doi: 10.1016/j.mehy.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 62.Hoffmann J., Charles A. Glutamate and Its Receptors as Therapeutic Targets for Migraine. Neurother. J. Am. Soc. Exp. Neurother. 2018;15:361–370. doi: 10.1007/s13311-018-0616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vermeiren C., Najimi M., Vanhoutte N., Tilleux S., de Hemptinne I., Maloteaux J.M., Hermans E. Acute up-regulation of glutamate uptake mediated by mGluR5a in reactive astrocytes. J. Neurochem. 2005;94:405–416. doi: 10.1111/j.1471-4159.2005.03216.x. [DOI] [PubMed] [Google Scholar]
- 64.Johnson J.L., Kwok Y.H., Sumracki N.M., Swift J.E., Hutchinson M.R., Johnson K., Williams D.B., Tuke J., Rolan P.E. Glial Attenuation With Ibudilast in the Treatment of Medication Overuse Headache: A Double-Blind, Randomized, Placebo-Controlled Pilot Trial of Efficacy and Safety. Headache. 2015;55:1192–1208. doi: 10.1111/head.12655. [DOI] [PubMed] [Google Scholar]
- 65.Meng I.D., Cao L. From migraine to chronic daily headache: The biological basis of headache transformation. Headache. 2007;47:1251–1258. doi: 10.1111/j.1526-4610.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 66.Maasumi K., Michael R.L., Rapoport A.M. CGRP and Migraine: The Role of Blocking Calcitonin Gene-Related Peptide Ligand and Receptor in the Management of Migraine. Drugs. 2018;78:913–928. doi: 10.1007/s40265-018-0923-5. [DOI] [PubMed] [Google Scholar]
- 67.Edvinsson L. The CGRP Pathway in Migraine as a Viable Target for Therapies. Headache. 2018;58(Suppl. 1):33–47. doi: 10.1111/head.13305. [DOI] [PubMed] [Google Scholar]
- 68.Rodgers K.M., Deming Y.K., Bercum F.M., Chumachenko S.Y., Wieseler J.L., Johnson K.W., Watkins L.R., Barth D.S. Reversal of established traumatic brain injury-induced, anxiety-like behavior in rats after delayed, post-injury neuroimmune suppression. J. Neurotrauma. 2014;31:487–497. doi: 10.1089/neu.2013.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suzumura A., Ito A., Yoshikawa M., Sawada M. Ibudilast suppresses TNFalpha production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain Res. 1999;837:203–212. doi: 10.1016/S0006-8993(99)01666-2. [DOI] [PubMed] [Google Scholar]
- 70.Kwok Y.H., Swift J.E., Gazerani P., Rolan P. A double-blind, randomized, placebo-controlled pilot trial to determine the efficacy and safety of ibudilast, a potential glial attenuator, in chronic migraine. J. Pain Res. 2016;9:899–907. doi: 10.2147/JPR.S116968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loggia M.L., Chonde D.B., Akeju O., Arabasz G., Catana C., Edwards R.R., Hill E., Hsu S., Izquierdo-Garcia D., Ji R.R., et al. Evidence for brain glial activation in chronic pain patients. Pt 3Brain. 2015;138:604–615. doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teepker M., Munk K., Mylius V., Haag A., Möller J.C., Oertel W.H., Schepelmann K. Serum concentrations of s100b and NSE in migraine. Headache. 2009;49:245–252. doi: 10.1111/j.1526-4610.2008.01228.x. [DOI] [PubMed] [Google Scholar]
- 73.Papandreou O., Soldatou A., Tsitsika A., Kariyannis C., Papandreou T., Zachariadi A., Papassotiriou I., Chrousos G.P. Serum S100beta protein in children with acute recurrent headache: A potentially useful marker for migraine. Headache. 2005;45:1313–1316. doi: 10.1111/j.1526-4610.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 74.Yilmaz S., Serum N.O. S100B, NSE concentrations in migraine and their relationship. J. Clin. Neurosci. 2020;82:32–35. doi: 10.1016/j.jocn.2020.10.046. [DOI] [PubMed] [Google Scholar]
- 75.Isaksen T.J., Lykke-Hartmann K. Insights into the Pathology of the α2-Na(+)/K(+)-ATPase in Neurological Disorders; Lessons from Animal Models. Front. Physiol. 2016;7:161. doi: 10.3389/fphys.2016.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leao A.A. Further observations on the spreading depression of activity in the cerebral cortex. J. Neurophysiol. 1947;10:409–414. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- 77.Olesen J., Larsen B., Lauritzen M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann. Neurol. 1981;9:344–352. doi: 10.1002/ana.410090406. [DOI] [PubMed] [Google Scholar]
- 78.Capuani C., Melone M., Tottene A., Bragina L., Crivellaro G., Santello M., Casari G., Conti F., Pietrobon D. Defective glutamate and K+ clearance by cortical astrocytes in familial hemiplegic migraine type 2. Embo Mol. Med. 2016;8:967–986. doi: 10.15252/emmm.201505944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Romanos J., Benke D., Pietrobon D., Zeilhofer H.U., Santello M. Astrocyte dysfunction increases cortical dendritic excitability and promotes cranial pain in familial migraine. Sci. Adv. 2020;6:eaaz1584. doi: 10.1126/sciadv.aaz1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simons C.T., Dessirier J.M., Jinks S.L., Carstens E. An animal model to assess aversion to intra-oral capsaicin: Increased threshold in mice lacking substance p. Chem. Senses. 2001;26:491–497. doi: 10.1093/chemse/26.5.491. [DOI] [PubMed] [Google Scholar]
- 81.Damodaram S., Thalakoti S., Freeman S.E., Garrett F.G., Durham P.L. Tonabersat inhibits trigeminal ganglion neuronal-satellite glial cell signaling. Headache. 2009;49:5–20. doi: 10.1111/j.1526-4610.2008.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silberstein S.D., Schoenen J., Göbel H., Diener H.C., Elkind A.H., Klapper J.A., Howard R.A. Tonabersat, a gap-junction modulator: Efficacy and safety in two randomized, placebo-controlled, dose-ranging studies of acute migraine. Cephalalgia Int. J. Headache. 2009;29(Suppl. 2):17–27. doi: 10.1111/j.1468-2982.2009.01974.x. [DOI] [PubMed] [Google Scholar]
- 83.Goadsby P.J., Ferrari M.D., Csanyi A., Olesen J., Mills J.G., Tonabersat T.O.N.S.G. Randomized, double-blind, placebo-controlled, proof-of-concept study of the cortical spreading depression inhibiting agent tonabersat in migraine prophylaxis. Cephalalgia Int. J. Headache. 2009;29:742–750. doi: 10.1111/j.1468-2982.2008.01804.x. [DOI] [PubMed] [Google Scholar]
- 84.Hauge A.W., Asghar M.S., Schytz H.W., Christensen K., Olesen J. Effects of tonabersat on migraine with aura: A randomised, double-blind, placebo-controlled crossover study. Lancet Neurol. 2009;8:718–723. doi: 10.1016/S1474-4422(09)70135-8. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki M., Van Paesschen W., Stalmans I., Horita S., Yamada H., Bergmans B.A., Legius E., Riant F., De Jonghe P., Li Y., et al. Defective membrane expression of the Na(+)-HCO(3)(-) cotransporter NBCe1 is associated with familial migraine. Proc. Natl. Acad. Sci. USA. 2010;107:15963–15968. doi: 10.1073/pnas.1008705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weir G.A., Cader M.Z. New directions in migraine. BMC Med. 2011;9:116. doi: 10.1186/1741-7015-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Epstein J.B., Hong C., Logan R.M., Barasch A., Gordon S.M., Oberle-Edwards L., McGuire D., Napenas J.J., Elting L.S., Spijkervet F.K., et al. A systematic review of orofacial pain in patients receiving cancer therapy. Support Care Cancer. 2010;18:1023–1031. doi: 10.1007/s00520-010-0897-7. [DOI] [PubMed] [Google Scholar]
- 88.Gooz M. ADAM-17: The enzyme that does it all. Crit. Rev. Biochem. Mol. Biol. 2010;45:146–169. doi: 10.3109/10409231003628015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eising E., de Leeuw C., Min J.L., Anttila V., Verheijen M.H., Terwindt G.M., Dichgans M., Freilinger T., Kubisch C., Ferrari M.D., et al. Involvement of astrocyte and oligodendrocyte gene sets in migraine. Cephalalgia Int. J. Headache. 2016;36:640–647. doi: 10.1177/0333102415618614. [DOI] [PubMed] [Google Scholar]
- 90.Wright E.F., North S.L. Management and treatment of temporomandibular disorders: A clinical perspective. J. Man. Manip. 2009;17:247–254. doi: 10.1179/106698109791352184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chung M.K., Ro J.Y. Peripheral glutamate receptor and transient receptor potential channel mechanisms of craniofacial muscle pain. Mol. Pain. 2020;16:1744806920914204. doi: 10.1177/1744806920914204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Damlar I., Esen E., Tatli U. Effects of glucosamine-chondroitin combination on synovial fluid IL-1β, IL-6, TNF-α and PGE2 levels in internal derangements of temporomandibular joint. Med. Oralpatologia Oral Y Cir. Bucal. 2015;20:e278–e283. doi: 10.4317/medoral.20242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Güven O., Tekin U., Salmanoğlu B., Kaymak E. Tumor necrosis factor-alpha levels in the synovial fluid of patients with temporomandibular joint internal derangement. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2015;43:102–105. doi: 10.1016/j.jcms.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 94.Ibi M., Horie S., Kyakumoto S., Chosa N., Yoshida M., Kamo M., Ohtsuka M., Ishisaki A. Cell-cell interactions between monocytes/macrophages and synoviocyte-like cells promote inflammatory cell infiltration mediated by augmentation of MCP-1 production in temporomandibular joint. Biosci. Rep. 2018;38 doi: 10.1042/BSR20171217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okamoto K., Bereiter D.F., Thompson R., Tashiro A., Bereiter D.A. Estradiol replacement modifies c-fos expression at the spinomedullary junction evoked by temporomandibular joint stimulation in ovariectomized female rats. Neuroscience. 2008;156:729–736. doi: 10.1016/j.neuroscience.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rozanski G.M., Li Q., Stanley E.F. Transglial transmission at the dorsal root ganglion sandwich synapse: Glial cell to postsynaptic neuron communication. Eur. J. Neurosci. 2013;37:1221–1228. doi: 10.1111/ejn.12132. [DOI] [PubMed] [Google Scholar]
- 97.Yang H., Yan H., Li X., Liu J., Cao S., Huang B., Huang D., Wu L. Inhibition of Connexin 43 and Phosphorylated NR2B in Spinal Astrocytes Attenuates Bone Cancer Pain in Mice. Front. Cell Neurosci. 2018;12:129. doi: 10.3389/fncel.2018.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choi H.S., Roh D.H., Yoon S.Y., Choi S.R., Kwon S.G., Kang S.Y., Moon J.Y., Han H.J., Beitz A.J., Lee J.H. Differential involvement of ipsilateral and contralateral spinal cord astrocyte D-serine in carrageenan-induced mirror-image pain: Role of σ1 receptors and astrocyte gap junctions. Br. J. Pharm. 2018;175:558–572. doi: 10.1111/bph.14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou Q., Imbe H., Dubner R., Ren K. Persistent Fos protein expression after orofacial deep or cutaneous tissue inflammation in rats: Implications for persistent orofacial pain. J. Comp. Neurol. 1999;412:276–291. doi: 10.1002/(SICI)1096-9861(19990920)412:2<276::AID-CNE7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 100.Bartolucci M.L., Marini I., Bortolotti F., Impellizzeri D., Di Paola R., Bruschetta G., Crupi R., Portelli M., Militi A., Oteri G., et al. Micronized palmitoylethanolamide reduces joint pain and glial cell activation. Inflamm. Res. 2018;67:891–901. doi: 10.1007/s00011-018-1179-y. [DOI] [PubMed] [Google Scholar]
- 101.Manuel Munoz-Lora V.R., Abdalla H.B., Del Bel Cury A.A., Clemente-Napimoga J.T. Modulatory effect of botulinum toxin type A on the microglial P2X7/CatS/FKN activated-pathway in antigen-induced arthritis of the temporomandibular joint of rats. Toxicon. 2020;187:116–121. doi: 10.1016/j.toxicon.2020.08.027. [DOI] [PubMed] [Google Scholar]
- 102.Kiyomoto M., Shinoda M., Honda K., Nakaya Y., Dezawa K., Katagiri A., Kamakura S., Inoue T., Iwata K. p38 phosphorylation in medullary microglia mediates ectopic orofacial inflammatory pain in rats. Mol. Pain. 2015;11:48. doi: 10.1186/s12990-015-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang G.Y.F., Shi X.Q., Wu W., Gueorguieva M., Yang M., Zhang J. Sustained and repeated mouth opening leads to development of painful temporomandibular disorders involving macrophage/microglia activation in mice. Pain. 2018;159:1277–1288. doi: 10.1097/j.pain.0000000000001206. [DOI] [PubMed] [Google Scholar]
- 104.Michot B., Casey S.M., Gibbs J.L. Effects of Calcitonin Gene-related Peptide on Dental Pulp Stem Cell Viability, Proliferation, and Differentiation. J. Endod. 2020;46:950–956. doi: 10.1016/j.joen.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 105.Austah O.N., Ruparel N.B., Henry M.A., Fajardo R.J., Schmitz J.E., Diogenes A. Capsaicin-sensitive Innervation Modulates the Development of Apical Periodontitis. J. Endod. 2016;42:1496–1502. doi: 10.1016/j.joen.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 106.Jessen K.R., Mirsky R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016;594:3521–3531. doi: 10.1113/JP270874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taylor P.E., Byers M.R., Redd P.E. Sprouting of CGRP nerve fibers in response to dentin injury in rat molars. Brain Res. 1988;461:371–376. doi: 10.1016/0006-8993(88)90270-3. [DOI] [PubMed] [Google Scholar]
- 108.Suzuki K., Lovera M., Schmachtenberg O., Couve E. Axonal Degeneration in Dental Pulp Precedes Human Primary Teeth Exfoliation. J. Dent. Res. 2015;94:1446–1453. doi: 10.1177/0022034515593055. [DOI] [PubMed] [Google Scholar]
- 109.Yoshiba N., Edanami N., Ohkura N., Maekawa T., Takahashi N., Tohma A., Izumi K., Maeda T., Hosoya A., Nakamura H., et al. M2 Phenotype Macrophages Colocalize with Schwann Cells in Human Dental Pulp. J. Dent. Res. 2020;99:329–338. doi: 10.1177/0022034519894957. [DOI] [PubMed] [Google Scholar]
- 110.Kaukua N., Shahidi M.K., Konstantinidou C., Dyachuk V., Kaucka M., Furlan A., An Z., Wang L., Hultman I., Ahrlund-Richter L., et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513:551–554. doi: 10.1038/nature13536. [DOI] [PubMed] [Google Scholar]
- 111.Nixdorf D.R., Law A.S., Lindquist K., Reams G.J., Cole E., Kanter K., Nguyen R.H.N., Harris D.R., National Dental P.C.G. Frequency, impact, and predictors of persistent pain after root canal treatment: A national dental PBRN study. Pain. 2016;157:159–165. doi: 10.1097/j.pain.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Iwata K., Katagiri A., Shinoda M. Neuron-glia interaction is a key mechanism underlying persistent orofacial pain. J. Oral Sci. 2017;59:173–175. doi: 10.2334/josnusd.16-0858. [DOI] [PubMed] [Google Scholar]
- 113.Komiya H., Shimizu K., Noma N., Tsuboi Y., Honda K., Kanno K., Ohara K., Shinoda M., Ogiso B., Iwata K. Role of Neuron-Glial Interaction Mediated by IL-1β in Ectopic Tooth Pain. J. Dent. Res. 2018;97:467–475. doi: 10.1177/0022034517741253. [DOI] [PubMed] [Google Scholar]
- 114.Naftel J.P., Bernanke J.M., Qian X.B. Quantitative study of the apical nerve fibers of adult and juvenile rat molars. Anat. Rec. 1994;238:507–516. doi: 10.1002/ar.1092380410. [DOI] [PubMed] [Google Scholar]
- 115.Sugimoto T., Takemura M. Tooth pulp primary neurons: Cell size analysis, central connection, and carbonic anhydrase activity. Brain Res. Bull. 1993;30:221–226. doi: 10.1016/0361-9230(93)90247-9. [DOI] [PubMed] [Google Scholar]
- 116.Gibbs J.L., Melnyk J.L., Basbaum A.I. Differential TRPV1 and TRPV2 channel expression in dental pulp. J. Dent. Res. 2011;90:765–770. doi: 10.1177/0022034511402206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paik S.K., Lee D.S., Kim J.Y., Bae J.Y., Cho Y.S., Ahn D.K., Yoshida A., Bae Y.C. Quantitative ultrastructural analysis of the neurofilament 200-positive axons in the rat dental pulp. J. Endod. 2010;36:1638–1642. doi: 10.1016/j.joen.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 118.Henry M.A., Luo S., Levinson S.R. Unmyelinated nerve fibers in the human dental pulp express markers for myelinated fibers and show sodium channel accumulations. BMC Neurosci. 2012;13:29. doi: 10.1186/1471-2202-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fried K., Sessle B.J., Devor M. The paradox of pain from tooth pulp: Low-threshold “algoneurons”? Pain. 2011;152:2685–2689. doi: 10.1016/j.pain.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Couve E., Schmachtenberg O. Schwann Cell Responses and Plasticity in Different Dental Pulp Scenarios. Front. Cell Neurosci. 2018;12:299. doi: 10.3389/fncel.2018.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Houshmandi M., Ye P., Hunter N. Glial network responses to polymicrobial invasion of dentin. Caries Res. 2014;48:534–548. doi: 10.1159/000360610. [DOI] [PubMed] [Google Scholar]
- 122.Couve E., Lovera M., Suzuki K., Schmachtenberg O. Schwann Cell Phenotype Changes in Aging Human Dental Pulp. J. Dent. Res. 2018;97:347–355. doi: 10.1177/0022034517733967. [DOI] [PubMed] [Google Scholar]
- 123.Iohara K., Murakami M., Nakata K., Nakashima M. Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp. Gerontol. 2014;52:39–45. doi: 10.1016/j.exger.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 124.Painter M.W., Brosius Lutz A., Cheng Y.C., Latremoliere A., Duong K., Miller C.M., Posada S., Cobos E.J., Zhang A.X., Wagers A.J., et al. Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron. 2014;83:331–343. doi: 10.1016/j.neuron.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Omi M., Hata M., Nakamura N., Miyabe M., Ozawa S., Nukada H., Tsukamoto M., Sango K., Himeno T., Kamiya H., et al. Transplantation of dental pulp stem cells improves long-term diabetic polyneuropathy together with improvement of nerve morphometrical evaluation. Stem Cell Res. 2017;8:279. doi: 10.1186/s13287-017-0729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martens W., Sanen K., Georgiou M., Struys T., Bronckaers A., Ameloot M., Phillips J., Lambrichts I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014;28:1634–1643. doi: 10.1096/fj.13-243980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Grace P.M., Hutchinson M.R., Maier S.F., Watkins L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stephenson J.L., Byers M.R. GFAP immunoreactivity in trigeminal ganglion satellite cells after tooth injury in rats. Exp. Neurol. 1995;131:11–22. doi: 10.1016/0014-4886(95)90003-9. [DOI] [PubMed] [Google Scholar]
- 129.Liu H., Zhao L., Gu W., Liu Q., Gao Z., Zhu X., Wu Z., He H., Huang F., Fan W. Activation of satellite glial cells in trigeminal ganglion following dental injury and inflammation. J. Mol. Histol. 2018;49:257–263. doi: 10.1007/s10735-018-9765-4. [DOI] [PubMed] [Google Scholar]
- 130.Gobel S., Binck J.M. Degenerative changes in primary trigeminal axons and in neurons in nucleus caudalis following tooth pulp extirpations in the cat. Brain Res. 1977;132:347–354. doi: 10.1016/0006-8993(77)90427-9. [DOI] [PubMed] [Google Scholar]
- 131.Vena D.A., Collie D., Wu H., Gibbs J.L., Broder H.L., Curro F.A., Thompson V.P., Craig R.G., Group P.N. Prevalence of persistent pain 3 to 5 years post primary root canal therapy and its impact on oral health-related quality of life: PEARL Network findings. J. Endod. 2014;40:1917–1921. doi: 10.1016/j.joen.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 132.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 133.Luckett T., Davidson P.M., Boyle F., Liauw W., Agar M., Green A., Lovell M., ImPaCct. PoCoG Australian survey of current practice and guideline use in adult cancer pain assessment and management: Perspectives of oncologists. Asia Pac. J. Clin. Oncol. 2014;10:e99–e107. doi: 10.1111/ajco.12040. [DOI] [PubMed] [Google Scholar]
- 134.Macfarlane T.V., Wirth T., Ranasinghe S., Ah-See K.W., Renny N., Hurman D. Head and neck cancer pain: Systematic review of prevalence and associated factors. J. Oral Maxillofac. Res. 2012;3:e1. doi: 10.5037/jomr.2012.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Romero-Reyes M., Teruel A., Ye Y. Cancer and referred facial pain. Curr. Pain Headache Rep. 2015;19:37. doi: 10.1007/s11916-015-0512-1. [DOI] [PubMed] [Google Scholar]
- 136.Schmidt B.L. The neurobiology of cancer pain. Neuroscientist. 2014;20:546–562. doi: 10.1177/1073858414525828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Scheff N.N., Ye Y., Bhattacharya A., MacRae J., Hickman D.N., Sharma A.K., Dolan J.C., Schmidt B.L. Tumor necrosis factor alpha secreted from oral squamous cell carcinoma contributes to cancer pain and associated inflammation. Pain. 2017;158:2396–2409. doi: 10.1097/j.pain.0000000000001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ye Y., Ono K., Bernabe D.G., Viet C.T., Pickering V., Dolan J.C., Hardt M., Ford A.P., Schmidt B.L. Adenosine triphosphate drives head and neck cancer pain through P2X2/3 heterotrimers. Acta Neuropathol. Commun. 2014;2:62. doi: 10.1186/2051-5960-2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kolokythas A., Cox D.P., Dekker N., Schmidt B.L. Nerve growth factor and tyrosine kinase A receptor in oral squamous cell carcinoma: Is there an association with perineural invasion? J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2010;68:1290–1295. doi: 10.1016/j.joms.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 140.Demir I.E., Kujundzic K., Pfitzinger P.L., Saricaoglu O.C., Teller S., Kehl T., Reyes C.M., Ertl L.S., Miao Z., Schall T.J., et al. Early pancreatic cancer lesions suppress pain through CXCL12-mediated chemoattraction of Schwann cells. Proc. Natl. Acad. Sci. USA. 2017;114:e85–e94. doi: 10.1073/pnas.1606909114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Demir I.E., Tieftrunk E., Schorn S., Saricaoglu O.C., Pfitzinger P.L., Teller S., Wang K., Waldbaur C., Kurkowski M.U., Wormann S.M., et al. Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut. 2016;65:1001–1014. doi: 10.1136/gutjnl-2015-309784. [DOI] [PubMed] [Google Scholar]
- 142.Shurin G.V., Kruglov O., Ding F., Lin Y., Hao X., Keskinov A.A., You Z., Lokshin A.E., LaFramboise W.A., Falo L.D., et al. Melanoma-induced reprogramming of Schwann cell signaling aids tumor growth. Cancer Res. 2019;79:2736–2747. doi: 10.1158/0008-5472.CAN-18-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhou Y., Shurin G.V., Zhong H., Bunimovich Y.L., Han B., Shurin M.R. Schwann Cells Augment Cell Spreading and Metastasis of Lung Cancer. Cancer Res. 2018;78:5927–5939. doi: 10.1158/0008-5472.CAN-18-1702. [DOI] [PubMed] [Google Scholar]
- 144.Bunimovich Y.L., Keskinov A.A., Shurin G.V., Shurin M.R. Schwann cells: A new player in the tumor microenvironment. Cancer Immunol. Immunother. 2017;66:959–968. doi: 10.1007/s00262-016-1929-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Deborde S., Wong R.J. How Schwann cells facilitate cancer progression in nerves. Cell. Mol. Life Sci. CMLS. 2017;74:4405–4420. doi: 10.1007/s00018-017-2578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Deborde S., Omelchenko T., Lyubchik A., Zhou Y., He S., McNamara W.F., Chernichenko N., Lee S.-Y., Barajas F., Chen C.-H., et al. Schwann cells induce cancer cell dispersion and invasion. J. Clin. Investig. 2016;126:1538–1554. doi: 10.1172/JCI82658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Salvo E., Tu N.H., Scheff N.N., Dubeykovskaya Z.A., Chavan S.A., Aouizerat B.E., Ye Y. TNFalpha promotes oral cancer growth, pain, and Schwann cell activation. Sci. Rep. 2021;11:1840. doi: 10.1038/s41598-021-81500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ein L., Mei C., Bracho O., Bas E., Monje P., Weed D., Sargi Z., Thomas G., Dinh C. Modulation of BDNF-TRKB Interactions on Schwann Cell-induced Oral Squamous Cell Carcinoma Dispersion In Vitro. Anticancer Res. 2019;39:5933–5942. doi: 10.21873/anticanres.13798. [DOI] [PubMed] [Google Scholar]
- 149.Huang T., Fan Q., Wang Y., Cui Y., Wang Z., Yang L., Sun X., Wang Y. Schwann Cell-Derived CCL2 Promotes the Perineural Invasion of Cervical Cancer. Front. Oncol. 2020;10:19. doi: 10.3389/fonc.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Demir I.E., Boldis A., Pfitzinger P.L., Teller S., Brunner E., Klose N., Kehl T., Maak M., Lesina M., Laschinger M., et al. Investigation of Schwann Cells at Neoplastic Cell Sites Before the Onset of Cancer Invasion. JNCI J. Natl. Cancer Inst. 2014;106:dju184. doi: 10.1093/jnci/dju184. [DOI] [PubMed] [Google Scholar]
- 151.Gosselin R.D., Suter M.R., Ji R.R., Decosterd I. Glial cells and chronic pain. Neuroscientist. 2010;16:519–531. doi: 10.1177/1073858409360822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ji R.R., Chamessian A., Zhang Y.Q. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354:572–577. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Scholz J., Woolf C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 154.Ji R.R., Berta T., Nedergaard M. Glia and pain: Is chronic pain a gliopathy? Pain. 2013;154(Suppl. 1):S10–S28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Campana W.M. Schwann cells: Activated peripheral glia and their role in neuropathic pain. Brain Behav. Immun. 2007;21:522–527. doi: 10.1016/j.bbi.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Armati P.J., Mathey E.K. An update on Schwann cell biology—Immunomodulation, neural regulation and other surprises. J. Neurol. Sci. 2013;333:68–72. doi: 10.1016/j.jns.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 157.Ydens E., Lornet G., Smits V., Goethals S., Timmerman V., Janssens S. The neuroinflammatory role of Schwann cells in disease. Neurobiol. Dis. 2013;55:95–103. doi: 10.1016/j.nbd.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 158.Abdo H., Calvo-Enrique L., Lopez J.M., Song J., Zhang M.D., Usoskin D., El Manira A., Adameyko I., Hjerling-Leffler J., Ernfors P. Specialized cutaneous Schwann cells initiate pain sensation. Science. 2019;365:695–699. doi: 10.1126/science.aax6452. [DOI] [PubMed] [Google Scholar]
- 159.Souza Monteiro de Araujo D., Nassini R., Geppetti P., De Logu F. TRPA1 as a therapeutic target for nociceptive pain. Expert Opin. Ther. Targets. 2020;24:997–1008. doi: 10.1080/14728222.2020.1815191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.De Logu F., Li Puma S., Landini L., Portelli F., Innocenti A., de Araujo D.S.M., Janal M.N., Patacchini R., Bunnett N.W., Geppetti P., et al. Schwann cells expressing nociceptive channel TRPA1 orchestrate ethanol-evoked neuropathic pain in mice. J. Clin. Investig. 2019;129:5424–5441. doi: 10.1172/JCI128022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.De Logu F., Nassini R., Materazzi S., Carvalho Goncalves M., Nosi D., Rossi Degl’Innocenti D., Marone I.M., Ferreira J., Li Puma S., Benemei S., et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat. Commun. 2017;8:1887. doi: 10.1038/s41467-017-01739-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Horst O.V., Cunha-Cruz J., Zhou L., Manning W., Mancl L., DeRouen T.A. Prevalence of pain in the orofacial regions in patients visiting general dentists in the Northwest Practice-based REsearch Collaborative in Evidence-based DENTistry research network. J. Am. Dent. Assoc. 2015;146:721–728.e3. doi: 10.1016/j.adaj.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sorge R.E., Mapplebeck J.C., Rosen S., Beggs S., Taves S., Alexander J.K., Martin L.J., Austin J.S., Sotocinal S.G., Chen D., et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Milligan E.D., Watkins L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen G., Zhang Y.Q., Qadri Y.J., Serhan C.N., Ji R.R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron. 2018;100:1292–1311. doi: 10.1016/j.neuron.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kuner R., Flor H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2017;18:113. doi: 10.1038/nrn.2017.5. [DOI] [PubMed] [Google Scholar]
- 167.Messlinger K., Balcziak L.K., Russo A.F. Cross-talk signaling in the trigeminal ganglion: Role of neuropeptides and other mediators. J. Neural Transm. (Vienna) 2020;127:431–444. doi: 10.1007/s00702-020-02161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Megat S., Ray P.R., Tavares-Ferreira D., Moy J.K., Sankaranarayanan I., Wanghzou A., Fang Lou T., Barragan-Iglesias P., Campbell Z.T., Dussor G., et al. Differences between Dorsal Root and Trigeminal Ganglion Nociceptors in Mice Revealed by Translational Profiling. J. Neurosci. 2019;39:6829–6847. doi: 10.1523/JNEUROSCI.2663-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lopes D.M., Denk F., McMahon S.B. The Molecular Fingerprint of Dorsal Root and Trigeminal Ganglion Neurons. Front. Mol. Neurosci. 2017;10:304. doi: 10.3389/fnmol.2017.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ren K., Dubner R. The role of trigeminal interpolaris-caudalis transition zone in persistent orofacial pain. Int. Rev. Neurobiol. 2011;97:207–225. doi: 10.1016/B978-0-12-385198-7.00008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Okada S., Katagiri A., Saito H., Lee J., Ohara K., Iinuma T., Bereiter D.A., Iwata K. Differential activation of ascending noxious pathways associated with trigeminal nerve injury. Pain. 2019;160:1342–1360. doi: 10.1097/j.pain.0000000000001521. [DOI] [PubMed] [Google Scholar]
- 172.Chichorro J.G., Porreca F., Sessle B. Mechanisms of craniofacial pain. Cephalalgia Int. J. Headache. 2017;37:613–626. doi: 10.1177/0333102417704187. [DOI] [PubMed] [Google Scholar]
- 173.Saito K., Hitomi S., Suzuki I., Masuda Y., Kitagawa J., Tsuboi Y., Kondo M., Sessle B.J., Iwata K. Modulation of trigeminal spinal subnucleus caudalis neuronal activity following regeneration of transected inferior alveolar nerve in rats. J. Neurophysiol. 2008;99:2251–2263. doi: 10.1152/jn.00794.2007. [DOI] [PubMed] [Google Scholar]
- 174.Kawamura J., Kaneko T., Kaneko M., Sunakawa M., Kaneko R., Chokechanachaisakul U., Okiji T., Suda H. Neuron-immune interactions in the sensitized thalamus induced by mustard oil application to rat molar pulp. J. Dent. Res. 2010;89:1309–1314. doi: 10.1177/0022034510377202. [DOI] [PubMed] [Google Scholar]
- 175.Totsch S.K., Sorge R.E. Immune System Involvement in Specific Pain Conditions. Mol. Pain. 2017;13:1744806917724559. doi: 10.1177/1744806917724559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Greenhalgh A.D., David S., Bennett F.C. Immune cell regulation of glia during CNS injury and disease. Nat. Rev. Neurosci. 2020;21:139–152. doi: 10.1038/s41583-020-0263-9. [DOI] [PubMed] [Google Scholar]
- 177.Hou Z., Wang Q., Guo Z., Wang T., Wu H., Ma C., Wang W., Su F., Zhang H., Su X. Gadolinium-conjugated CB86: A novel TSPO-targeting MRI contrast agent for imaging of rheumatoid arthritis. J. Drug Target. 2020;28:398–407. doi: 10.1080/1061186X.2019.1669040. [DOI] [PubMed] [Google Scholar]
- 178.Forsberg A., Lampa J., Estelius J., Cervenka S., Farde L., Halldin C., Lekander M., Olgart Hoglund C., Kosek E. Disease activity in rheumatoid arthritis is inversely related to cerebral TSPO binding assessed by [(11)C]PBR28 positron emission tomography. J. Neuroimmunol. 2019;334:577000. doi: 10.1016/j.jneuroim.2019.577000. [DOI] [PubMed] [Google Scholar]
- 179.de Groot M., Patel N., Manavaki R., Janiczek R.L., Bergstrom M., Ostor A., Gerlag D., Roberts A., Graves M.J., Karkera Y., et al. Quantifying disease activity in rheumatoid arthritis with the TSPO PET ligand (18)F-GE-180 and comparison with (18)F-FDG and DCE-MRI. EJNMMI Res. 2019;9:113. doi: 10.1186/s13550-019-0576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Albrecht D.S., Forsberg A., Sandstrom A., Bergan C., Kadetoff D., Protsenko E., Lampa J., Lee Y.C., Hoglund C.O., Catana C., et al. Brain glial activation in fibromyalgia—A multi-site positron emission tomography investigation. Brain Behav. Immun. 2019;75:72–83. doi: 10.1016/j.bbi.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gelesko S., Long L., Faulk J., Phillips C., Dicus C., White R.P., Jr. Cryotherapy and topical minocycline as adjunctive measures to control pain after third molar surgery: An exploratory study. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2011;69:e324–e332. doi: 10.1016/j.joms.2011.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Martinez V., Szekely B., Lemarie J., Martin F., Gentili M., Ben Ammar S., Lepeintre J.F., Garreau de Loubresse C., Chauvin M., Bouhassira D., et al. The efficacy of a glial inhibitor, minocycline, for preventing persistent pain after lumbar discectomy: A randomized, double-blind, controlled study. Pain. 2013;154:1197–1203. doi: 10.1016/j.pain.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 183.Syngle A., Verma I., Krishan P., Garg N., Syngle V. Minocycline improves peripheral and autonomic neuropathy in type 2 diabetes: MIND study. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2014;35:1067–1073. doi: 10.1007/s10072-014-1647-2. [DOI] [PubMed] [Google Scholar]
- 184.Darcey E., Boyle T. Tobacco smoking and survival after a prostate cancer diagnosis: A systematic review and meta-analysis. Cancer Treat. Rev. 2018;70:30–40. doi: 10.1016/j.ctrv.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 185.Vanelderen P., Van Zundert J., Kozicz T., Puylaert M., De Vooght P., Mestrum R., Heylen R., Roubos E., Vissers K. Effect of minocycline on lumbar radicular neuropathic pain: A randomized, placebo-controlled, double-blind clinical trial with amitriptyline as a comparator. Anesthesiology. 2015;122:399–406. doi: 10.1097/ALN.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 186.Sumracki N.M., Hutchinson M.R., Gentgall M., Briggs N., Williams D.B., Rolan P. The effects of pregabalin and the glial attenuator minocycline on the response to intradermal capsaicin in patients with unilateral sciatica. PLoS ONE. 2012;7:e38525. doi: 10.1371/journal.pone.0038525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Landry R.P., Jacobs V.L., Romero-Sandoval E.A., DeLeo J.A. Propentofylline, a CNS glial modulator does not decrease pain in post-herpetic neuralgia patients: In vitro evidence for differential responses in human and rodent microglia and macrophages. Exp. Neurol. 2012;234:340–350. doi: 10.1016/j.expneurol.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 188.Younger J., Noor N., McCue R., Mackey S. Low-dose naltrexone for the treatment of fibromyalgia: Findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65:529–538. doi: 10.1002/art.37734. [DOI] [PubMed] [Google Scholar]
- 189.Younger J., Mackey S. Fibromyalgia symptoms are reduced by low-dose naltrexone: A pilot study. Pain Med. 2009;10:663–672. doi: 10.1111/j.1526-4637.2009.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ostenfeld T., Krishen A., Lai R.Y., Bullman J., Baines A.J., Green J., Anand P., Kelly M. Analgesic efficacy and safety of the novel p38 MAP kinase inhibitor, losmapimod, in patients with neuropathic pain following peripheral nerve injury: A double-blind, placebo-controlled study. Eur. J. Pain. 2013;17:844–857. doi: 10.1002/j.1532-2149.2012.00256.x. [DOI] [PubMed] [Google Scholar]
- 191.Ostenfeld T., Krishen A., Lai R.Y., Bullman J., Green J., Anand P., Scholz J., Kelly M. A randomized, placebo-controlled trial of the analgesic efficacy and safety of the p38 MAP kinase inhibitor, losmapimod, in patients with neuropathic pain from lumbosacral radiculopathy. Clin. J. Pain. 2015;31:283–293. doi: 10.1097/AJP.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 192.Anand P., Shenoy R., Palmer J.E., Baines A.J., Lai R.Y., Robertson J., Bird N., Ostenfeld T., Chizh B.A. Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury. Eur. J. Pain. 2011;15:1040–1048. doi: 10.1016/j.ejpain.2011.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.