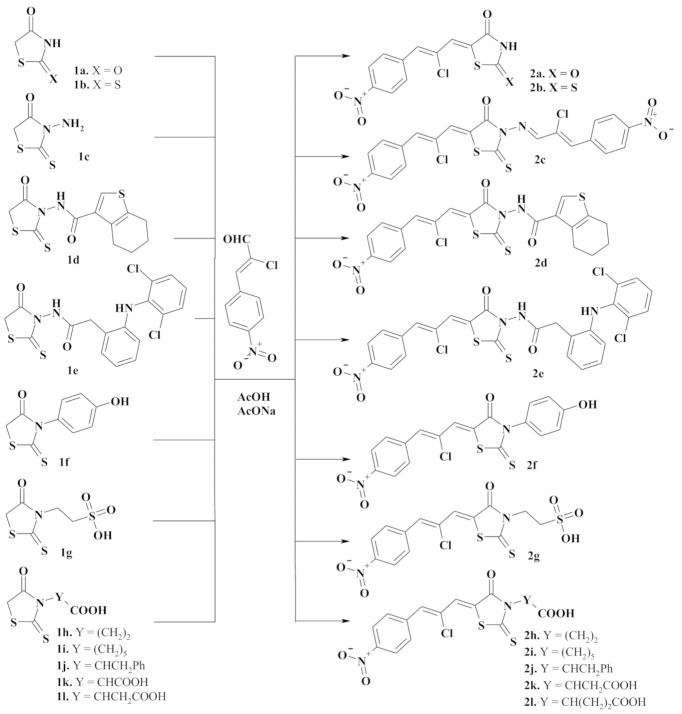
Figure 2.
Synthesis of 5-[(Z,2Z)-2-chloro-3-(4-nitrophenyl)-2-propenylidene]-4-thiazolidinones 2a-m. Reagents and conditions: appropriate 4-thiazolidinone 1a-l (0.01 mol), (2Z)-2-chloro-3-(4-nitrophenyl)prop-2-enal (0.010 mol, in the case of 3-aminorhodanine 1e 0.02 mol), AcONa (0.01 mol), AcOH (20 mL), reflux, 3 h, 68–83%.