Abstract
The critical role of acetylcholine (ACh) in the basal ganglia is evident from the effect of cholinergic agents in patients suffering from several related neurological disorders such as Parkinson’s disease, Tourette syndrome or dystonia. The striatum possesses the highest density of ACh markers in the basal ganglia underlying the importance of ACh in this structure. Striatal cholinergic interneurons (CINs) are responsible for the bulk of striatal ACh, although extrinsic cholinergic afferents from brainstem structures may also play a role. CINs are tonically active, and synchronized pause in their activity occurs following the presentation of salient stimuli during behavioral conditioning. However, the synaptic mechanisms involved are not fully understood in this physiological response. ACh modulates striatal circuits by acting on muscarinic and nicotinic receptors existing in several combinations both presynaptically and postsynaptically. While the effects of ACh in the striatum through muscarinic receptors have received a particular attention, nicotinic receptors function has been less studied. Here, after briefly reviewing relevant results regarding muscarinic receptors expression and function, I will focus on striatal nicotinic receptor expressed presynaptically on glutamatergic and dopaminergic afferents and postsynaptically on diverse striatal interneurons populations. I will also review recent evidence suggesting the involvement of different GABAergic sources in two distinct nicotinic-receptor mediated striatal circuits: the disynaptic inhibition of striatal projection neurons and the recurrent inhibition among CINs. A better understanding of striatal nicotinic receptors expression and function may help developing targeted pharmacological interventions to treat brain disorders such as Parkinson’s disease, Tourette syndrome, dystonia or nicotine addiction.
Keywords: cholinergic interneurons, acetylcholine, nicotinic receptors, muscarinic receptors, GABAergic interneurons, dopamine, glutamate, cognitive flexibility, electrophysiology
INTRODUCTION.
The Basal Ganglia (BG) are a group of interconnected subcortical nuclei that are involved in a variety of functions ranging for sensorimotor, cognitive, and reward related behaviors (Alexander et al., 1986). Anatomical, electrophysiological and/or neurochemical alterations in several BG structures are associated with some of the most prevalent neurodegenerative and neuropsychiatric disorders. The striatum is the main input structure of the BG receiving massive excitatory innervation from almost the entire cortical mantle as well as several thalamic nuclei such as the parafascicular nucleus (PfN), (Yeterian & Van Hoesen, 1978; Smith & Parent, 1986; Berendse & Groenewegen, 1990; Francois et al., 1991; Sadikot et al., 1992; Flaherty & Graybiel, 1993; Smith et al., 2004; Haber et al., 2006; Smith et al., 2014; Haber, 2016). It also receives very dense dopaminergic projections from midbrain structures such as the substantia nigra pars compacta (SNc) and the ventral tegmental area (VTA) (Bolam et al., 2000; Gerfen, 2000; Gerdeman et al., 2003; Kreitzer & Malenka, 2008; Gerfen & Surmeier, 2011). While less studied, GABAergic structures such as the globus pallidus (GPe) and the midbrain or brainstem cholinergic nuclei such as the pedunculopontine nucleus (PPN) and the laterodorsal tegmental nucleus (LDT) also send significant projections to the striatum (Bevan et al., 1998; Mallet et al., 2012; Dautan et al., 2014; Gittis et al., 2014; Hegeman et al., 2016; Assous et al., 2019).
The striatum is an interesting structure where nearly all neurons are GABAergic (~99%). In rodents, 90–95% of striatal neurons are GABAergic spiny projection neurons (SPNs), equivalent to the principal cells of the striatum (Kemp & Powell, 1971; Chang et al., 1982; Graveland & DiFiglia, 1985; Luk & Sadikot, 2001). These cells constitute the only efferents of the striatum and can be subdivided into 2 populations based on the expression of dopamine receptors subtypes (D1 or D2) as well as their projection to downstream BG structures. While D1-expressing “direct pathway” SPNs (dSPNs) principally project monosynaptically to the output structures of the BG (internal segment of the GP, GPi and substantia nigra pars reticulata, SNr), D2-expressing “indirect pathway” SPNs (iSPNs) mostly project to the output nuclei of the BG via two relays, the GPe and the subthalamic nucleus (STN, (Alexander et al., 1986; Albin et al., 1989; DeLong, 1990; Gerfen & Surmeier, 2011)). Via these pathways, dSPNs and iSPNs may have opposite influence on BG output structures and on movement generation.
The remaining striatal neurons (5–10%) are composed of different classes of interneurons. Most striatal interneurons are GABAergic and are essential to modulate striatal excitability and striatal output. At least 7 populations of striatal GABAergic interneurons have been identified (Tepper et al., 2010; Tepper et al., 2018). The most studied populations include parvalbumin (PV)-expressing fats-spiking interneurons (FSIs), the neuropeptide Y (NPY)/somatostatin (SOM)/nitric oxide synthase (NOS)-expressing low threshold spike (LTS) interneurons and the calretinin-expressing interneurons (CR) (Kawaguchi, 1993; Kawaguchi et al., 1995). Additionally, other populations of striatal GABAergic interneurons have been identified and characterized such as the tyrosine hydroxylase-expressing interneurons (THINs, (Ibañez-Sandoval et al., 2010; Xenias et al., 2015)), a second population of NPY-expressing interneurons called neurogliaform interneurons (NGF, (Ibañez-Sandoval et al., 2011)) as well as at least two populations targeted in the Htr3a-Cre transgenic mice; the fast adapting interneurons (FAIs, (Faust et al., 2015)) and the spontaneously active bursty interneurons (SABIs) (Assous et al., 2018). It is also worth noting that the classification of these cell types may not be complete yet and some of these striatal GABAergic interneurons such as the FSIs or the THINs could be further subdivided into several subpopulations ((Ibañez-Sandoval et al., 2010; Garas, 2016; Bengtsson Gonzales et al., 2020).
Only one striatal interneuron population is not GABAergic, the cholinergic interneurons (CINs), which release acetylcholine (ACh, ~ 1% of striatal neurons (Wilson et al., 1990), but see (Lozovaya et al., 2018)). Despite their low number, the striatum possesses the highest density of cholinergic varicosities and cholinergic markers in the basal ganglia and one of the highest in the brain (Mesulam et al., 1984; Zhou et al., 2002). CINs constitute the principal source of ACh in the striatum although extrinsic sources of ACh in the striatum originate from the PPN and LDT region (Dautan et al., 2014). Such a high density of cholinergic markers underscores the importance of ACh neurotransmission in the striatum. Its particular interest comes partly from the effects of cholinergic and anticholinergic agents in patients with movement disorders, as well as in animal models of basal ganglia–dependent behaviors, suggesting a critical role for acetylcholine in the normal function of the basal ganglia (Pisani et al., 2007; Bonsi et al., 2011; Schulz & Reynolds, 2013; Girasole & Nelson, 2015; Gonzales & Smith, 2015; Mallet et al., 2019).
Different sources of ACh in the striatum
CINs are anatomically characterized as giant aspiny neurons (Wilson et al., 1990) and may present different dendritic profile and properties depending on the species or the location in specific striatal territories (Gonzales & Smith, 2015; Lozovaya et al., 2018; Ahmed et al., 2019). Electrophysiologically, CINs are believed to correspond to most of the tonically active neurons recorded in the primate and rodent striatum. They fire spontaneously at 2–10Hz both in vivo and in brain slices (Wilson et al., 1990; Aosaki et al., 1995; Bennett & Wilson, 1999; Bennett et al., 2000; Reynolds et al., 2004; Goldberg & Wilson, 2005; Wilson, 2005; Schulz & Reynolds, 2013), and importantly CINs show synchronized pauses in response to sensory salient events during behavioral conditioning (Kimura et al., 1984; Aosaki et al., 1994b; Aosaki et al., 1995; Apicella et al., 1996; 1997; Apicella, 2002; Morris et al., 2004; Joshua et al., 2008; Apicella, 2017). This “conditioned pause response”, which often consists of an initial pause in firing rate (~200 ms), occasionally preceded by a short burst of firing and followed by a rebound increase in firing rate, is modulated by dopamine and thalamic afferents from PfN (Matsumoto et al., 2001; Morris et al., 2004; Joshua et al., 2008). It has been suggested that the pause, which may regulate striatal plasticity conveys attention-related signals, possibly contributing to the interruption of ongoing behavior when a stimulus eliciting an orienting reaction is detected (Ding et al., 2010; Deffains & Bergman, 2015; Zhang & Cragg, 2017). However, the circuits mechanisms responsible for these physiological responses are still debated. Further, cholinergic signaling in the striatum is important for reward learning and behavioral flexibility, a capacity that involves the updating of action-outcome associations that is commonly tested through reversal learning paradigms (Ragozzino et al., 2002; Potter et al., 2006; Ragozzino et al., 2009; Powell & Ragozzino, 2017; Prado et al., 2017). While these functions have been solely attributed to CINs, extrinsic cholinergic inputs to the striatum originating from the PPN and the LDT may also play an important role (Coimbra et al., 2019; Dautan et al., 2020). Anatomically, the PPN mostly innervates the dorsolateral striatum whereas the LDT innervates the dorsomedial striatum and the ventral striatum (Dautan et al., 2014). Behaviorally, the activation of cholinergic input from LDT to the ventral striatum plays an important role in motivated behaviors and positive reinforcement (Coimbra et al., 2019). Further, similar to the inhibition or lesion of striatal CINs, inhibition of ACh release from these extrinsic sources impairs contingencies association and habit formation in an instrumental task (Dautan et al., 2020).
However, while it is clear that cholinergic signaling is essential for striatal functioning, a major gap remains in understanding the circuits underlying these function as well as the involvement of the different subtypes of ACh receptors.
Muscarinic receptors expression and function in striatal circuits.
The effect of ACh in the striatum through muscarinic receptors (mAChRs) have received a particular attention in part due to their wide cellular distribution. Indeed, in contrast to nicotinic receptors (nAChRs), mAChRs are expressed on the axon terminals of most striatal afferents as well as by all examined striatal neurons including the SPNs (for review see (Goldberg et al., 2012)). The expression of different subtypes of mAChRs in striatal glutamatergic afferents and striatal neurons is briefly reviewed below. For more information, readers are referred to some excellent recent reviews (Goldberg et al., 2012; Tanimura et al., 2018; Abudukeyoumu et al., 2019). The mAChRs are G protein-coupled receptors (GPCR) subdivided into two classes. The M1 receptors class (comprising M1, M3, and M5 receptors) are coupled to Gq/11 G proteins and activate protein kinase C and phospholipase C. The M2 class receptors (comprising M2 and M4 type receptors) are coupled to Gi/o G proteins and inhibit adenylyl cyclase.
Muscarinic receptors on striatal glutamatergic afferents
The majority of glutamatergic afferents to the dorsal striatum originate from almost every cortical area and several thalamic nuclei as well as other structures such as the PPN and the STN (see (Assous & Tepper, 2019) for review). While it has been shown that mAChRs are expressed on striatal glutamatergic afferents, the selective expression of mAChRs subtypes on specific glutamatergic afferents needs further investigation. Presynaptic mAChRs expressed on glutamatergic afferents are mostly M2 and M4 mAChRs that likely mediate presynaptic inhibition (Hersch et al., 1994; Levey et al., 1994; Hersch & Levey, 1995; Smolders et al., 1997; Calabresi et al., 1998a; Pancani et al., 2014). The activation of these receptors diminishes glutamate release and reduces the excitatory drive onto SPNs (Akaike et al., 1988; Malenka & Kocsis, 1988; Calabresi et al., 1998a; Barral et al., 1999; Pakhotin & Bracci, 2007; Higley et al., 2009). At the level of a single glutamatergic synapse, mAChRs activation decreases both the probability of release and the concentration of glutamate in the synaptic cleft. This may be an important mechanism to reduce the duration of cortex-evoked excitatory post synaptic potentials which could limit the temporal summation of excitatory inputs (Higley et al., 2009). Interestingly, using a paired recording approach, it was demonstrated that a single action potential in one CIN is able to reduce the evoked glutamatergic EPSC in a substantial proportion of SPNs and CINs located nearby (Pakhotin & Bracci, 2007).
Additionally, it has been demonstrated that CINs-induced reduction of corticostriatal activity could be engaged after the activation of thalamostriatal projections. Indeed, thalamostriatal projections especially originating from the PfN robustly innervate striatal CINs (Meredith & Wouterlood, 1990; Wilson et al., 1990; Lapper & Bolam, 1992; Ding et al., 2010; Doig et al., 2014; Assous et al., 2017; Assous & Tepper, 2019). Activation of this pathway evokes a transient suppression of excitatory cortical input to both classes of SPNs (Ding et al., 2010). This was due to presynaptic inhibition of corticostriatal terminals via M2 mAChRs. This temporary suppression of corticostriatal activity via the thalamic activation of CINs might be involved in the suppression of ongoing behavior after the occurrence of salient stimuli (Matsumoto et al., 2001; Minamimoto & Kimura, 2002; Ding et al., 2010).
Muscarinic receptors on striatal neurons
SPNs
SPNs constitute the vast majority of striatal neurons (~90–95%) and express mAChRs. Accordingly, most of the CIN’s role in striatal circuits has been attributed to the modulation of SPNs excitability by mAChRs (Goldberg et al., 2012). Nonetheless, the modulation of SPNs by ACh through the activation of postsynaptic mAChRs is complex and needs to be further clarification.
At the cellular level, M1 mAChR mRNA is highly expressed in dSPNs and iSPNs (Goldberg et al., 2012; Gonzales & Smith, 2015). Although both types of SPNs express M4 mAChRs, these are expressed at higher level in dSPNs in comparison to iSPNs (Bernard et al., 1992; Yan et al., 2001). Activation of M1 mAChRs increases the excitability of SPNs (Dodt & Misgeld, 1986; Hsu et al., 1996; Galarraga et al., 1999; Lin et al., 2004; Shen et al., 2005; Pisani et al., 2007) and are involved in the second phase of the feedforward modulation of corticostriatal afferents to SPNs via the thalamostriatal activation of CINs by enhancing the dendritic excitability of iSPNs (Ding et al., 2010). Further, optogenetic activation of SPNs evokes inhibitory postsynaptic responses in CINs which can be reduced by activating presynaptic M1 mAChRs located on terminals of SPNs in a concentration dependent manner (Suzuki & Momiyama, 2020).
In contrast, activation of M4 receptors on SPNs leads to the inhibition of Ca2+ channels, a decreased collateral activity between SPNs, as well as a decrease in excitability and inhibition of long-term potentiation (Perez-Rosello et al., 2005; Yamamoto et al., 2013; Mamaligas & Ford, 2016; Mamaligas et al., 2019).
FSIs
Striatal FSIs are considered as the main source of feedforward inhibition to SPNs. They are strongly innervated by extrinsic excitatory afferents from the cortex and the thalamus and exert a powerful inhibitory effect on SPNs (Koos & Tepper, 1999; Mallet et al., 2005; Gittis et al., 2010; Szydlowski et al., 2013; Assous & Tepper, 2019). The precise regulation of FSIs by CINs is still not fully understood. It has been demonstrated that the synaptic connection between FSIs and SPNs is negatively regulated by presynaptic mAChRs (Koos & Tepper, 2002). The receptor involved in this inhibition has not been precisely identified, but may correspond to the M2-class (Grilli et al., 2009).
LTS
Immunocytochemical and in situ hybridization approaches have demonstrated that somatostatin-expressing striatal neurons, electrophysiologically identified as LTSIs express M1, M2 and, for a small percentage of them, M4 receptors (Bernard et al., 1992; Bernard et al., 1998). Pharmacological experiments suggest a dual role of mAChRs activation on LTSIs. Indeed, while mAChRs activation inhibits LTSIs directly, activation of these receptors concomitantly reduces their spontaneous inhibitory synaptic inputs (Elghaba et al., 2016). Importantly, the inhibitory effect of mAChRs on LTSIs has been confirmed after optogenetically-induced synaptic release of ACh by CINs (Melendez-Zaidi et al., 2019). Activation of CINs provokes a hyperpolarization of LTSIs mediated by the activation of M4 mAChRs. Further, the hyperpolarization was followed by rebound bursting activity that was maintained for several seconds after the single optogenetic stimulation of CINs (Melendez-Zaidi et al., 2019). This pathway could also be engaged after stimulation of thalamostriatal afferents from the PfN. In this case, the pause induced in LTSIs was a combination of GABAergic and M4 mAChR signaling (Assous et al., 2017; Melendez-Zaidi et al., 2019; Frost Nylen et al., 2020).
CINs
Anatomical studies have shown that M1, M2, and M4 receptors can act as autoreceptors in CINs (Bernard et al., 1992; Yan & Surmeier, 1996; Bernard et al., 1998; Alcantara et al., 2001). Application of muscarinic agonists can hyperpolarize and silence cholinergic interneurons via postsynaptic M2 and M4 autoreceptors (Calabresi et al., 1998b; Bonsi et al., 2008). However, in experiments investigating synaptic connectivity among striatal CINs, such muscarinic inhibition has not been reported (Sullivan et al., 2008; Dorst et al., 2020). This raises the possibility that these receptors could be selectively activated by brainstem cholinergic structures.
Nicotinic receptors
Presynaptic Nicotinic receptors
nAChRs are pentameric ligand-gated ion channels that consist of either heteromeric subunit combinations of α subunits (α2–10) and β subunits (β2–4; (Albuquerque et al., 1995; Exley & Cragg, 2008; Gotti et al., 2009)). The most common types of nAChRs in striatum are the homomeric α subunits (α7) and α4β2* (Exley & Cragg, 2008).
The main role of nAChRs in the central nervous system is to modulate the release of neurotransmitters via presynaptically located receptors. These presynaptic nAChRs have been shown to modulate the release of a large variety of neurotransmitters mainly via modifying presynaptic calcium permeability and intracellular calcium signaling (Dani, 2001; Dajas-Bailador & Wonnacott, 2004; Dani & Bertrand, 2007). Several subtypes of nAChRs with different subunit composition, and therefore different functional properties, are differentially located on diverse afferents.
However, given the large number of possible combinations of nAChR subunits, the specific function given by the expression of a distinct subtype of nAChR in a selective afferent is far from being understood. In addition, some terminals (or some neurons) co-express several subtypes of nAChRs which makes the comprehension of these functions even more complex. Given the involvement of nAChRs in a large number of neurological and neuropsychiatric disorders such as Parkinson’s disease or nicotine addiction (Dani, 2001; Gotti et al., 2006a; Gotti et al., 2006b; Quik & McIntosh, 2006; Dani & Bertrand, 2007; Exley & Cragg, 2008), this calls for a better characterization of 1) the expression of nAChRs subtype(s) presynaptically by different striatal afferents and postsynaptically by striatal interneurons and 2) determine the function of the selective nAChRs composition on neurotransmitter release, postsynaptic depolarization, influence in striatal microcircuits and physiologic and pathologic behaviors. This may allow better targeted drug strategies for the diseases listed above.
Glutamatergic afferents
Several studies using immunoprecipitation, microdialysis or radioligand binding have suggested that glutamate terminals in the striatum express α7-containing nAChRs (Kaiser & Wonnacott, 2000; Campos et al., 2010; Licheri et al., 2018). In comparison to α4β2 nAChRs which are the most abundantly expressed nAChRs subtype in the striatum, α7 nAChRs are more permeable to calcium (Bertrand et al., 1990; Dickinson et al., 2008) and show less desensitization (Keath et al., 2007). Their activation on corticostriatal terminals enhances glutamate release in the striatum (Kaiser & Wonnacott, 2000; Campos et al., 2010). In an experiment using glutamate biosensors to monitor nAChR-induced glutamate release, it was found that both α7 and α4β2 nAChRs were able to modulate glutamate release (Howe et al., 2016). However, while α7 nAChRs activation can directly enhance glutamate release, α4β2 nAChR activation acts as a brake on glutamate release. However, the effect of α4β2 nAChRs is indirect by enhancing dopamine release (see below), which inhibits glutamate release by acting on presynaptic D2 receptors (Cepeda et al., 2001; Surmeier et al., 2007; Xiao et al., 2009; Howe et al., 2016; Licheri et al., 2018).
While the studies above revealed the role of α7-containing nAChRs in enhancing glutamate release, they did not reveal whether this mechanism occurs differentially in specific extrinsic excitatory input. A recent study described the existence of a novel feed-forward excitatory circuit where stimulation of pyramidal tract corticostriatal neurons evokes biphasic excitation of SPNs (Morgenstern et al., 2020). Interestingly, the second phase of excitation can be blocked by a selective α7-containing nAChRs antagonist, suggesting the involvement of CINs in this response. Indeed, the authors demonstrated that pyramidal tract cortical neurons provide a strong suprathreshold activation of CINs (stronger than intratelencephalic neurons). In this circuit, ACh then activates presynaptic α7-containing corticostriatal terminals that form synapses onto SPNs. This subsequent glutamate release from corticostriatal terminals is responsible for the second phase of activation measured in SPNs which could be important for the integration of striatal afferents (Morgenstern et al., 2020).
Further, given the strong input from the PfN of the thalamus to the CINs (see Assous and Tepper, 2019), presynaptic nAChRs expressed on thalamostriatal afferents may carry an important function in striatal circuits. A study demonstrated that in parkinsonian models, the connectivity of a subset of PfN neurons with iSPNs was selectively enhanced (Tanimura et al., 2019). This effect was mediated by the suprathreshold activation of CINs and action through presynaptic nAChRs on PfN terminals. The increase of glutamate release by PfN synapse onto iSPNs was dependent upon presynaptic α6-containing nAChRs which are expressed by a subpopulation of PfN neurons (Tanimura et al., 2019). This is consistent with previous reports suggesting that thalamic neurons express α6β2 nAChRs (Hill et al., 1993; Parker et al., 2004; Bohr et al., 2005). Importantly, knocking down α6 subunit expression in the PfN attenuated the changes in electrophysiological connectivity and improved locomotor performance in parkinsonian mice ((Tanimura et al., 2019), Figure 1).
Figure 1. Nicotinic receptor expression in striatal circuits.
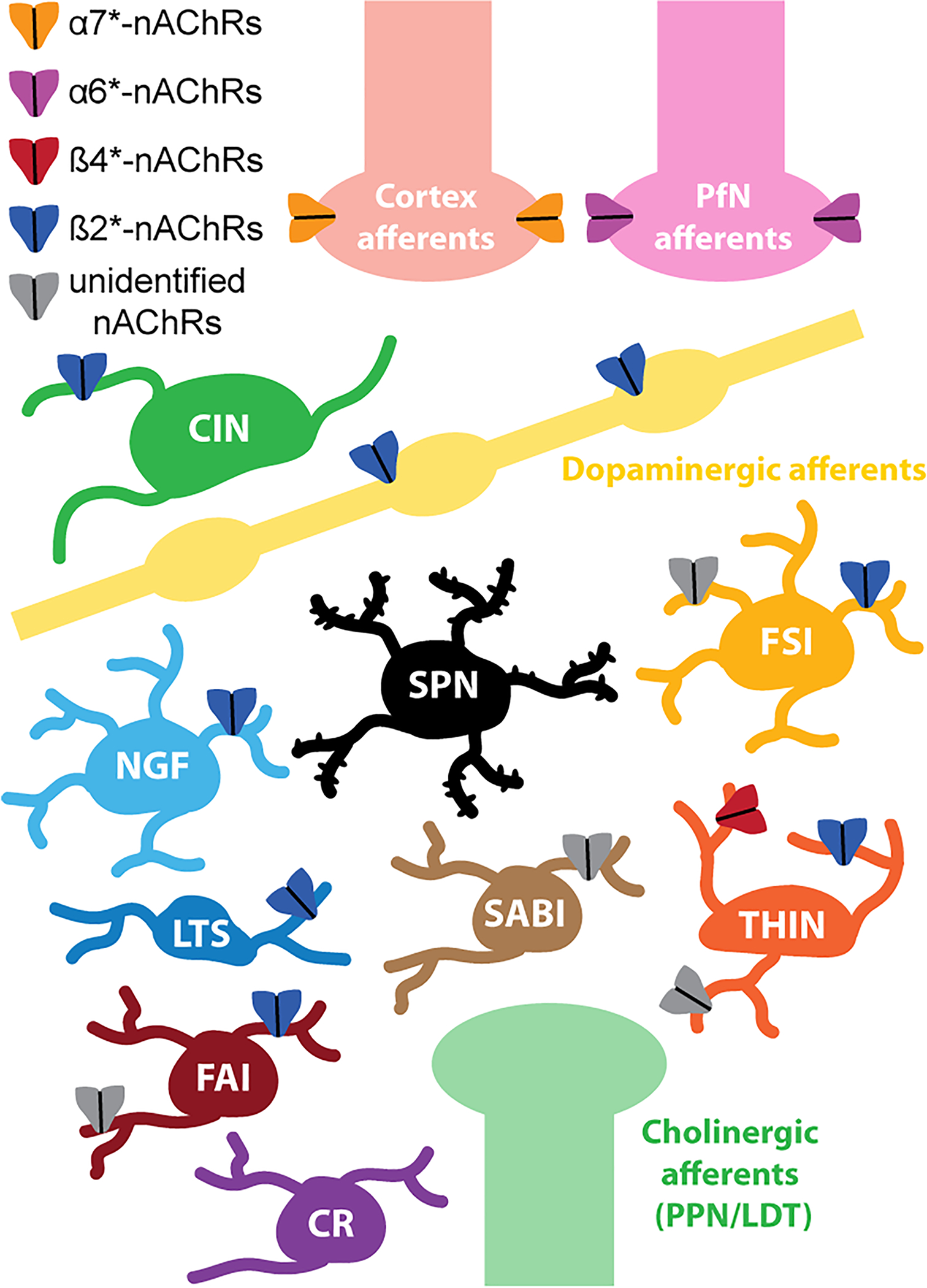
Schematic describing our current knowledge of nicotinic receptor (nAChRs) subtypes expression presynaptically on striatal afferents originating from the cortex, the parafascicular nucleus of the thalamus (PfN), dopaminergic neurons or brainstem cholinergic structures (pedunculopontine nucleus, PPN and laterodorsal tegmentum, LDT) as well as postsynaptically on striatal neurons. Note that while the majority of striatal interneurons express nicotinic receptors, spiny projection neurons (SPNs) do not seem to express any. Importantly, striatal GABAergic interneurons express various subtypes of nAChRs which may confer selective functions in striatal microcircuits discussed in this review. CINs: Cholinergic interneurons; NGF: Neurogliaform; FSI: Fast-spiking interneuron; LTS: Low threshold spike; SABI: Spontaneously active bursty interneuron; THIN: Tyrosine hydroxylase-expressing interneuron; FAI: Fast-adapting interneuron; CR: Calretinin-expressing interneurons.
Dopamine afferents
Perhaps the most studied role of nAChRs in striatal functions involve their expression on presynaptic dopamine afferents and their role in the regulation of local dopamine release (Zhou et al., 2001; Rice & Cragg, 2004; Zhang & Sulzer, 2004). Striatal dopamine terminals express α4β2-containing nAChRs at high levels as well as lower levels of other subunits (α5–7 and β3) whose function are less well described (Clarke & Pert, 1985; Le Novere et al., 1996; Sharples et al., 2000; Jones et al., 2001; Grady et al., 2007). Early pharmacological evidence suggests that activation of these presynaptic nAChRs by agonists can facilitate dopamine release (Wonnacott et al., 2000; Zhou et al., 2001; Dajas-Bailador & Wonnacott, 2004; Rice & Cragg, 2004; Zhang & Sulzer, 2004; Exley & Cragg, 2008). Other studies have indicated that ACh-induced dopamine release can take place following synaptically released ACh from CINs (Bennett & Wilson, 1999; Zhou et al., 2001; Zhou et al., 2003) and involve β2-containing nAChRs located on striatal dopamine terminals (Figure 1). Activation of this receptor subtype increases the probability of dopamine release (Zhou et al., 2001; Rice & Cragg, 2004; Exley & Cragg, 2008) as well as use-dependent short-term depression. Subsequent studies using optogenetics activation of populations of CINs in different striatal subregion (Cachope et al., 2012; Threlfell et al., 2012) demonstrated that a synchronous activation of CINs can elicit dopamine release. Importantly, this nAChR-dependent local control of dopamine release is independent of action potential firing in midbrain dopaminergic neurons (Cachope et al., 2012; Surmeier & Graybiel, 2012; Threlfell et al., 2012). Further, activation of extrinsic excitatory inputs from the thalamus (Zackheim & Abercrombie, 2005; Threlfell et al., 2012) or the cortex (Kosillo et al., 2016) can indirectly induce dopamine release via the activation of CINs.
Additionally, the question whether the activation of extrinsic sources of striatal ACh can also induce dopamine release was investigated (Brimblecombe et al., 2018). In this study, the authors indicated that surprisingly, activation of cholinergic brainstem afferents originating from the PPN and LDT do not elicit any dopamine release (Brimblecombe et al., 2018). The reasons for the lack of ACh-induced dopamine release in this context need further examination. Additionally, whether the activation of glutamatergic PPN neurons, which strongly innervate CINs (Assous et al., 2019), can elicit dopamine release, is yet to characterize.
Dopamine afferents can also co-release GABA in brain slices (Tritsch et al., 2012; Tritsch et al., 2014). The possibility of a cholinergic regulation of GABA release from these terminals through nAChRs is discussed below.
Nicotinic-mediated striatal GABAergic circuits
Recurrent Inhibition
Stimulation of intrastriatal cholinergic fibers evokes polysynaptic GABAA IPSCs in CINs (Sullivan et al., 2008). These polysynaptic GABAA IPSCs could be abolished both by a specific antagonist of nAChRs containing β2 subunits and a GABAA receptor antagonist (Figure 2). Interestingly, application of dopamine receptor antagonists or dopamine depletion failed to block polysynaptic IPSCs, indicating that phasic dopamine release does not directly mediate the polysynaptic transmission. In addition, dual recording from pairs of cholinergic interneurons revealed that activation of a single cholinergic interneuron is capable of eliciting polysynaptic GABAA IPSCs both in itself and in nearby CINs (Sullivan et al., 2008; Dorst et al., 2020). These results support the existence of a recurrent inhibitory circuit in the striatum where cholinergic interneurons are disynaptically connected to one another through GABAergic interneurons (Sullivan et al., 2008; Dorst et al., 2020). In terms of striatal microcircuits, these results suggest that one or several populations of GABAergic interneurons 1) express β2 nAChRs, 2) receive suprathreshold nAChRs-mediated cholinergic input from CINs and 3) provide a GABAergic innervation to CINs. Further, optogenetic activation of populations of CINs can also trigger large recurrent inhibition in CINs sensitive to β2 nAChRs antagonists and a GABAA receptor antagonist ((English et al., 2012), Figure 2).
Figure 2. Nicotinic-mediated striatal GABAergic circuits.
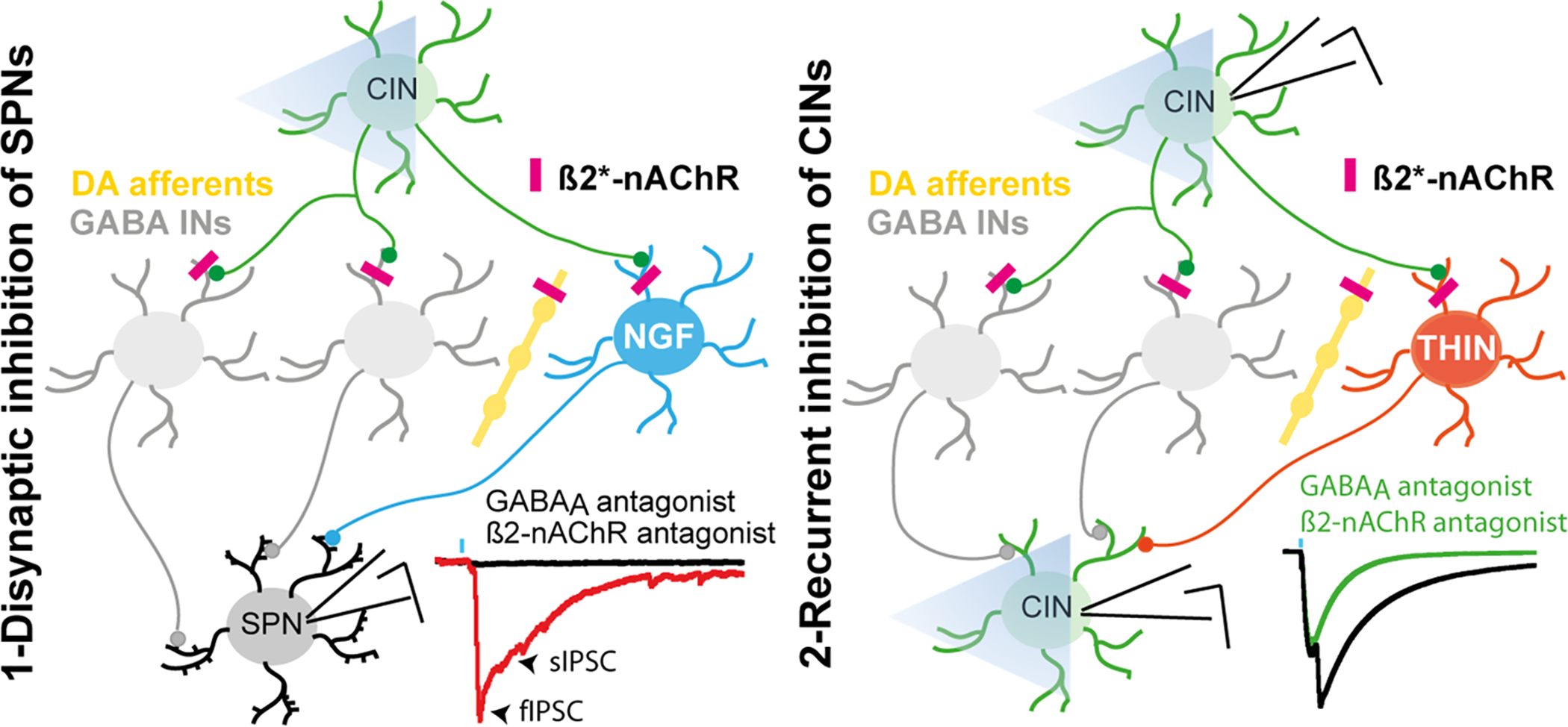
So far, two distinct GABAergic striatal microcircuits involving β2-subunit containing nicotinic receptor (β2*-nAChR) have been described. Left. Optogenetic activation of striatal cholinergic interneurons (CINs) evoke a disynaptic composite IPSC in SPNs. This inhibition can be subdivided into a fast and a slow component. While the slow component is mediated by CINs activation of neurogliaform interneurons (NGF), the source of the fast IPSC is still under investigation. Right. Single CIN activation or optogenetic stimulation of populations of CINs induces polysynaptic recurrent inhibition among CINs which can participate in synchronizing CINs activity. Part of this inhibition involves nicotinic receptors activation on THINs, which are reciprocally connected with CINs. The contribution of other subtypes of striatal GABAergic interneurons or extrinsic GABAergic afferents is yet to discover.
A recent study has investigated thoroughly this recurrent inhibitory circuit among CINs by performing several hundreds of pairs, triplets or quadruple recordings of CINs (Dorst et al., 2020). Findings of this study revealed that polysynaptic connections between CINs were common and exhibited a high degree of divergence and convergence. Single CINs can broadcast simultaneous pauses in postsynaptic CINs, sometimes followed by an increase in action potential discharge frequency. These results show that the robust polysynaptic inhibition between spontaneously active CINs can act as a mechanism for synchronizing their activity via an intrastriatal mechanism. It is then tempting to imagine that this circuit may contribute, at least partially, to the generation of synchronous reward-related pauses observed in CINs. Interestingly, optogenetic inhibition of all striatal GABAergic neurons (SPNs and interneurons) in vivo provoked an increase in the firing rate of putative CINs and slightly but significantly shortened the reward-related pause events (Dorst et al., 2020). This suggests that intrastriatal inhibition via GABAergic neurons, and most likely interneurons (given the absence of nAChRs expression on SPNs) may play an important role (in addition to extrinsic afferents) in shaping the activity patterns of putative CINs.
Disynaptic inhibition of SPNs
Another nicotinic-mediated striatal GABAergic microcircuit involves the disynaptic inhibition of SPNs. The existence of such circuit has first been suggested by de Rover and colleagues showing that nicotine can enhance GABAergic transmission onto SPNs. The hypothesis was that population(s) of GABA interneurons expressing nAChRs were activated by ACh which, in turn, would be responsible of the increase in inhibitory transmission onto SPNs (de Rover et al., 2002).
Subsequently, it was demonstrated that optogenetic activation of CINs can induce disynaptic GABAergic inhibition onto SPNs (Witten et al., 2010; English et al., 2012). These GABAergic IPSP/Cs are secondary to nAChRs activation (Witten et al., 2010; English et al., 2012). This phenomenon is very robust, and it is observed in all recorded SPNs and can efficiently delay or block spike generation. While smaller, such disynaptic inhibitory responses can also be evoked in SPNs in paired recording experiments after a single spike in CINs. The optically elicited IPSCs are multiphasic and can be separated into two components that are biophysically distinct, a fast and a slow component (fIPSC and sIPSC, respectively, English et al., 2012, Figure 2). The fIPSC kinetic is consistent with most GABAA-mediated transmission observed in the striatum. However, the sIPSC is similar to a slow GABAA current whose kinetics are likely due to a combination of an extrasynaptic location of the receptor, and the presence of the GABAA β3 subunit (Capogna & Pearce, 2011; Luo et al., 2013b). This is confirmed by the sensitivity of the sIPSC to GABA uptake inhibition (Faust et al., 2015). This observation suggests that striatal interneurons under cholinergic control may influence striatal information processing in a cortically-independent manner. In this case, this circuitry underlies that one, or more likely, several GABAergic interneurons populations 1) receive suprathreshold nicotinic input from CINs, 2) express β2-containing nAChRs and 3) provide a GABAA inhibition to SPNs (fast and slow, Figure 2).
Interestingly, while this circuit also involves β2-containing nAChR activation, the GABAergic neurons involved in this disynaptic circuit seem to be (at least partially) non overlapping with interneuron(s) responsible for the recurrent inhibition of CINs. Indeed, the feedforward inhibition in SPNs and the recurrent inhibition in CINs recorded simultaneously exhibit distinct temporal patterns with significantly different onset latencies. Further, it has been shown that these two circuits can be activated independently using different stimulation paradigms (English et al., 2012).
Involvement of nAChR expressed on midbrain GABAergic afferents
The interplay between CINs and midbrain dopaminergic afferents is well documented ((Howe et al., 2019) and see (Exley & Cragg, 2008; Aosaki et al., 2010) for review). Reward-related pauses in CINs are correlated with an increase in the activity of dopaminergic neurons and as mentioned above, activation of CINs can induce dopamine release independently of dopaminergic neuron somatic firing (Cachope et al., 2012; Threlfell et al., 2012). However, the specific contribution of midbrain dopamine neurons in the synchronization of CINs activity is still not fully understood (Zhang & Cragg, 2017).
Recent literature has demonstrated that nigrostriatal dopamine neurons can co-release GABA under certain conditions in vitro. This non-canonical release of GABA can efficiently inhibit SPNs with slow decay kinetics and is able to prevent action potential firing (Tritsch et al., 2012; Tritsch et al., 2014). However, direct evidence demonstrating nicotinic-induced GABAergic release by nigrostriatal terminals following activation of CINs (using for example GABA sensors, (Marvin et al., 2019)) is still lacking. Nonetheless, it has been proposed that such a circuit may exist in brain slices and would be responsible for the majority of the nicotinic-induced inhibition of SPNs ((Nelson et al., 2014), Figure 2). Indeed, both treatment with vesicular monoamine transport inhibitors or nigrostriatal dopamine lesion dramatically reduced both the fast and the slow component of the compound IPSC measured in SPNs after optogenetic stimulation of CINs. These results suggested that GABA release from dopamine terminals may be the major source of the disynaptic inhibition of SPNs (Nelson et al., 2014). It is worth noting that the importance of GABA release by nigrostriatal terminals in this circuit has been recently challenged ((Faust et al., 2016); see below). Another possibility discussed below would be that nigrostriatal dopamine deletion or depletion alters the strength of the disynaptic interneuronal circuits in a rapid manner, similar to that observed in SPN–SPN connections (Taverna et al., 2008) or in the recurrent inhibitory circuit existing among CINs (Dorst et al., 2020).
CINs located in the ventral and dorsal striatum receive strong perisomatic GABAergic innervation from midbrain neurons (Brown et al., 2012; Dorst et al., 2020). To investigate the contribution of these afferents as well as different striatal neurons in the nicotinic-mediated recurrent inhibition of CINs, the authors combined the expression of both ChR2 and an inhibitory Designer Receptors Exclusively Activated by Designer Drugs (DREADD) receptor in selective afferents or populations of striatal neurons in a Cre-dependent manner. They tested: 1) the attenuation in polysynaptic inhibition between neighboring CINs following an optogenetic interference protocol (obtained after a train of optogenetic stimulation of Cre-expressing cells) that would indicate that the transduced afferent or striatal interneuron population (expressing Cre) is involved in the regulation of this polysynaptic pathway, 2) the repetition of this protocol in the presence of Clozapine N-oxide (CNO) would confirm the involvement of specific interneurons population or GABAergic afferents in mediating the recurrent inhibition (Dorst et al., 2020). In VGAT-Cre and DAT-Cre mice, chemogenetic silencing of midbrain GABAergic afferents did not reduce or abolish the nicotinic-mediated recurrent inhibition between CINs, suggesting that extrinsic GABAergic inputs to CINs from the midbrain do not mediate it. Consistent with the lack of participation from midbrain neurons in this circuit, the proportion of CINs expressing polysynaptic inhibition was unaffected after lesion of dopaminergic neurons. However, this pathway seems to be significantly modulated by dopamine as the interference protocol in DAT-Cre mice (but not VGAT-Cre) reduces the polysynaptic inhibition. Pharmacology experiments have demonstrated that the attenuated effect of dopamine involves D2 receptors present on cholinergic terminals synapsing onto striatal GABAergic interneurons and reducing ACh release (Dorst et al., 2020).
Involvement of postsynaptic nAChRs expressed by striatal GABAergic interneurons
In contrast to SPNs, which do not express nAChRs, many striatal interneurons exhibit robust nAChR-mediated cholinergic responses (Figure 1). CINs express nAChRs autoreceptors containing α7 or β2 subunits (Azam et al., 2003) which function is still unknown. Regarding striatal GABAergic interneurons, findings over the past decade demonstrated that their diversity and associated networks is far more complex than previously envisaged (Tepper et al., 2018; Assous & Tepper, 2019). Whether these GABAergic interneuron populations receive uniform innervation from CINs or express specific functional subtypes of nAChRs and differentially participate in the two nicotinic-mediated striatal GABAergic circuits described above is not fully appreciated (Figure 2). A better grasp of the nicotinic innervation of striatal GABAergic interneurons will lead to a better understanding of the organization of striatal circuits and activity, as well as developing more targeted drug strategy to selectively act on specific synapses.
FSIs
Pharmacological experiments performed in rat striatal slices showed that acetylcholine exerted two distinct effects on FSIs. As mentioned above, presynaptic mAChRs activation on FSIs reduced the GABAergic inhibition on SPNs. Additionally, ACh led to large depolarization and AP firing in FSI by acting on nondesensitizing soma-dendritic nAChRs (Koos & Tepper, 2002). The nicotinic excitation appears to be a direct postsynaptic effect, independent of glutamatergic afferents. Excitatory post synaptic responses are blocked by a general nicotinic antagonist, but not by a selective antagonist of α7-containing nAChRs (Koos & Tepper, 2002). ACh agonists consistently evoked a slight depolarization in PV+ FSIs (Luo et al., 2013a) which could be blocked by an α4β2 nAChRs antagonist (Figure 1). However, using other transgenic mice lines (5HT3aEGFP and Lhx6EGFP) no postsynaptic responses were measured in FSIs after applying nicotine possibly revealing difference in nicotine receptor expression in subpopulations of FSIs (Munoz-Manchado et al., 2016).
Given that FSIs are a major source of inhibition of SPNs, it has been tested whether FSIs could be implicated in the disynaptic inhibition of SPNs after stimulation of CINs. However, optogenetic stimulation failed to elicit any substantial depolarization or action potential firing in the recorded FSIs despite the presence of large fIPSC components in nearby SPNs (English et al., 2012). Furthermore, the ablation of PV+ FSIs does not alter the disynaptic ISPCs in SPNs (Nelson et al., 2014). Altogether, these results indicate that GABAergic interneurons other than FSIs are involved in the feedforward inhibition of SPNs. Additionally, given that FSIs do not seem to innervate CINs (Szydlowski et al., 2013), it is unlikely that this cell population participate in the CINs recurrent inhibitory circuit (Figure 2).
NGFs
Simultaneous paired-recording between CINs and NPY-NGF interneurons revealed that a postsynaptic response could be elicited in the NPY NGF neurons by single action potentials in the presynaptic CIN (English et al., 2012). The response was a type-2 receptor–mediated nicotinic excitatory postsynaptic potential (containing the β2 subunit, Figure 1). Furthermore, optogenetic stimulation of the CINs interneuron population elicited large-amplitude depolarizing postsynaptic potentials in all NPY-NGF neurons and can even trigger action potential firing in some of them (English et al., 2012). Interestingly, the optogenetically elicited postsynaptic response in NPY-NGF interneurons consisted of an early excitatory and a delayed inhibitory component. The IPSC component, which itself was secondary to nAChR activation was mediated by GABAA receptors (English et al., 2012). This inhibitory response may be important for limiting the nicotinic activation of NPY-NGF neurons. Further testing local application of cholinergic agonists in a variety of striatal GABAergic neurons (LTSIs, THINs, FSIs and NGFs), demonstrated that NGFs exhibits the most robust nicotinic response among the interneurons tested (Luo et al., 2013a).
NGFs form synapses onto SPNs with a very high connection probability in brain slices (~85%; (Ibañez-Sandoval et al., 2011; Tepper et al., 2018)). Furthermore, the NGF-evoked synaptic response in SPNs exhibits slow kinetics similar to a GABAA slow current (Ibañez-Sandoval et al., 2011). Consistent with their circuit connectivity as well as synaptic properties, it has been shown that these interneurons are responsible (at least in part) for the slow disynaptic IPSC measured in SPNs after optogenetic activation of CINs ((English et al., 2012; Faust et al., 2016), Figure 2).
THINs
Application of ACh receptor agonists have shown that THINs express somato-dendritic nAChRs (Luo et al., 2013a; Ibañez-Sandoval et al., 2015). THINs are present in 4 subtypes in the mouse striatum with different anatomical, electrophysiological and synaptic characteristics (Ibañez-Sandoval et al., 2010). While it seems that all subtypes of THINs express nAChRs, the size of the response induced by the application of nAChRs agonists varies. Indeed, Type I THINs, the most common subtype, responded to carbachol application with strong depolarizations and action potential firing. Similarly, type IV THINs, the second most abundant THIN subtype, also responded robustly to carbachol. In contrast, Type II THINs responded with small-amplitude, brief depolarizations that rarely elicited action potentials (Ibañez-Sandoval et al., 2015). In all cases, the response was shown to be due to direct activation of a nAChRs distinct from the α4β2-type and α7 nAChRs ((Luo et al., 2013a; Ibañez-Sandoval et al., 2015), Figure 1). Furthermore, application of cystine, a selective partial agonist of β2 and full agonist of β4 subunit-containing nAChRs (Zoli et al., 1998), evoked large responses in all tested THINs, suggesting the involvement of α3β4 receptor in these neurons (Luo et al., 2013a).
A recent study performing paired recording between CINs and THINs demonstrated that part of the nAChR-mediated cholinergic effects on THINs comes from CINs (Dorst et al., 2020). Whether the THINs are also the target of extrinsic cholinergic inputs is still unknown. Further, THINs provide a GABAergic innervation to SPNs (Ibañez-Sandoval et al., 2010; Xenias et al., 2015), and pharmacological activation of nAChRs on THINs can induce a GABAA-mediated current in recorded SPNs (Luo et al., 2013a). However, direct evidence regarding the participation of THINs in the disynaptic inhibition of SPNs after the stimulation of CINs is still lacking, but is under investigation in our lab (Figure 2).
Interestingly, THINs are reciprocally connected with CINs as they are with SPNs (Ibañez-Sandoval et al., 2010). Both paired recording experiments as well as optogenetic stimulation revealed that stimulation of THINs elicit GABAA-mediated IPSCs in CINs (Dorst et al., 2020). Interestingly, the polysynaptic inhibition between CINs was modestly, but significantly, reduced after the chemogenetic silencing of THINs. This suggest that the THINs are at least partially involved in mediating the polysynaptic pathway between CINs (Figure 2). However, these important results need to be reconciled with other studies described above suggesting that THINs do not express β2-containing nAChRs (Luo et al., 2013b; Ibañez-Sandoval et al., 2015), which are mediating the polysynaptic recurrent inhibition between CINs. It is then possible that at least one or several subtypes of THINs, such as the type III (not tested in Ibañez-Sandoval et al., 2015) express this subtype of receptor and may selectively be involved in the polysynaptic inhibition between CINs. Another possibility would be that THINs express a variety of nAChRs such as α3β4 in combination with β2-containing nAChRs and the effect of selective nicotinic blockers after puffing ACh agonists (Luo et al., 2013b; Ibañez-Sandoval et al., 2015) was masked due to the rapid desensitization of β2-containing nAChRs. This scenario would be supported by our own preliminary data (Assous and Tepper 2019) showing that optogenetic stimulation of CINs elicits large depolarization in all THINs and a small but significant portion of the excitatory response involve the activation of β2-containing nAChRs in THINs (Figure 1).
LTSIs
Striatal LTSIs have also been shown to express nAChRs (Luo et al., 2013b). Interestingly, the effect of nAChRs activation on these cells seems to be dual. For instance, nAChRs activation has a direct excitatory effect through β2-containing nAChRs (Figure 1) in a significant proportion of LTSIs, but could also decrease the spontaneous firing activity in others (Luo et al., 2013b; Elghaba et al., 2016, but see Muñoz-Manchado et al., 2016). This dual effect may be explained by voltage clamp recordings where pharmacological activation of LTSIs nAChRs increased both the holding current as well as the occurrence GABAA receptors-dependent sIPSCs (Luo et al., 2013b; Elghaba et al., 2016). This suggests that presynaptic activation of some populations of GABAergic interneurons by nicotinic agonists could result in increasing synaptic inhibition in other populations of GABAergic interneurons such as the LTSIs. Indeed, we have demonstrated that THINs provide inhibitory innervation to the LTSIs (Assous et al., 2017) which receive strong suprathreshold nicotinic input from CINs (Luo et al., 2013b; Ibañez-Sandoval et al., 2015). Hence, activation of CINs or application of nicotinic agonists could elicit an increase in inhibitory synaptic events in LTSIs through the activation of THINs (Assous et al., 2017; Assous and Tepper 2019; Melendez-Zaidi et al., 2019). This suggests that the effect of CINs activation and nicotinic agonists on GABAergic interneurons may depend on the balance between the presence of nAChR and the interconnection with other nAChR-expressing striatal GABAergic interneurons. However, it is worth noting that such direct nicotinic activation of LTSIs has not been found in studies using direct optogenetic stimulation of CINs (Melendez-Zaidi et al., 2019; English et al., 2012). This may imply that nAChRs present on LTSIs are mostly activated by brainstem cholinergic sources.
In early paired-recording experiments, it was suggested that LTSIs provide a sparse (or even non-existent) innervation to SPNs (Gittis et al., 2010; Ibañez-Sandoval et al., 2011; Assous et al., 2018). However, more recent studies using optogenetic stimulation of a population of LTSIs have shown that the inhibitory response evoked in SPNs is very large (Straub et al., 2016). Therefore, it was tested whether LTSIs could participate in the disynaptic inhibition of SPNs after optogenetic activation of CINs. However, while optogenetic stimulation of CINs evoked large disynaptic IPSCs in SPNs, this was not accompanied by any detectable postsynaptic currents in LTSIs recorded in the same preparation. Hence, the involvement of LTSIs in this circuit can be excluded ((English et al., 2012), Figure 2).
Further, the participation of LTSIs in the recurrent inhibitory circuit among CINs has been tested using a similar strategy as described above combining optogenetics and DREADD. The authors observed that optogenetic train stimulation of LTSIs modulates the polysynaptic inhibition between CINs (Dorst et al., 2020). However, the blockade of synaptic transmission between LTSIs and CINs using DREADD did not alter the recurrent inhibition circuit suggesting that LTSIs may gate the polysynaptic input by depressing distal dendrites on cholinergic neurons (Holley et al., 2015; Straub et al., 2016; Dorst et al., 2020) but are not directly involved in the generation of the recurrent inhibition (Figure 2).
5HT3a Interneurons
There are two principal transgenic models in which neurons expressing the 5HT3a receptor subunit can be identified, the Htr3a-Cre mouse and the 5HT3a-EGFP mouse. In each, the transduced neuronal populations in the striatum are slightly different, but do show significant overlap. While 3 neuronal subtypes were characterized in the 5HT3a-EGFP mice (Muñoz-Manchado et al., 2016), at least 4 different populations of striatal GABAergic interneurons were identified and characterized in the Htr3a-Cre mice; some of them being non-overlapping in the two lines (Muñoz-Manchado et al., 2016; Faust et al., 2015). For example, while NGF interneurons targeted in the EGFP line do not express NPY (Muñoz-Manchado et al., 2016) and do not seem to respond robustly to nicotine, NGFs targeted in Htr3a-Cre express NPY and are strongly activated by CINs (Faust et al., 2015; Faust et al., 2016). A rather heterogenous group of neurons, characterized in the 5HT3a-EGFP as type III neurons, present some LTS-like properties, but do not express the classic markers of LTSIs (SOM, NOS, NPY). Furthermore, in contrast to LTSIs, type III cells in the 5HT3a-EGFP mice responded with a robust depolarization and action potential firing following a brief nicotine puff (Muñoz-Manchado et al., 2016). This divergence is not currently understood, and for the most part, does not seem to occur with other mouse models commonly used to identify FSIs, THINs, LTSIs or CINs.
In the Htr3a-Cre mice, we identified and characterized 2 novel populations of interneurons, i.e., the FAIs and the SABIs in addition to NGFs and FSIs (Faust et al., 2015; Assous et al., 2018). We took advantage from the variety of striatal GABAergic interneurons targeted in the Htr3a-Cre mice to determine the contribution of local striatal GABAergic interneurons to the cholinergic-induced disynaptic inhibition of SPNs (Figure 2). We used ChAT-ChR2 x Htr3a-Cre mice injected in the striatum with a Cre-dependent eNpHR 3.0 AAV (Faust et al., 2016). This allowed us to activate ChR2 and eNpHR 3.0, individually or simultaneously. Thus, we were able to optogenetically disconnect the participation of Htr3a-Cre transduced interneurons in the disynaptic inhibition of SPNs following activation of CINs on a trial-by-trial basis. We showed that the CIN-induced inhibition of SPNs was significantly reduced after hyperpolarizing the Htr3a-Cre populations of striatal interneurons with eNpHR 3.0. Interestingly, yellow light pulses were able to significantly reduce, or in some cases nearly eliminate both the fast and slow components of cholinergic-mediated GABAergic inhibition in SPNs in a fast and reversible manner causally demonstrating an interneuronal source of this inhibition (Faust et al., 2016). While we attributed the reduction of the slow component to the presence of NGFs, the reduction of the fast component still needs further investigation.
We examined the cholinergic input to FAIs and assessed whether FAIs could be a good candidate for mediating the fIPSCs in SPNs (Faust et al., 2015). We demonstrated that optogenetic stimulation of CINs evoked large excitatory nicotinic responses in all recorded FAIs. This response was sufficient to elicit action potential firing in the majority of them. Interestingly, the nicotinic response was pharmacologically heterogeneous. Indeed, in the vast majority of FAIs, the response was not reduced or blocked by a β2-subunit containing nAChR antagonist possibly revealing some heterogeneity in the nAChR subunit composition among FAIs ((Faust et al., 2015), Figure 1). In this study we also demonstrated that FAIs exhibit a high connectivity probability with nearby SPNs. However, in contrast to other striatal GABAergic synapses, the synaptic connection between FAIs and SPNs exhibits short-term facilitation. The facilitation was so marked that in some cases, the initial FAI spike in a train failed to produce any response in the postsynaptic SPN while later spikes evoked larger IPSCs (Faust et al., 2015). Therefore, given the pharmacology of the nicotinic response measured in FAIs together with the low initial release probability and strong facilitation of the FAI to SPN synapse, FAIs are unlikely to participate in the fIPSC observed in SPNs as well as in the recurrent inhibition of CINs.
The CINs input to SABIs is under current investigation by our lab. Our preliminary results suggest that SABIs receive a strong nAChR-mediated cholinergic input from CINs that does not involve α4β2 subunits- or α7 subunits-containing nAChRs (Figure 1). We also demonstrated that SABIs do not significantly innervate SPNs and thus appear to represent the first example of interneuron-selective interneurons in the mouse striatum (Assous et al., 2018). For these reasons, the participation of SABIs to the CIN-mediated disynaptic inhibition of SPNs as well as recurrent inhibition to CINs is ruled out.
If all major populations of interneurons targeted in the Htr3a-Cre mice are unlikely to participate in the fast disynaptic IPSCs measured in SPNs either because of synaptic connectivity or nAChR subunit composition, then why did we observe such a reduction in our experiments? While we attributed such reduction to either a polysynaptic disinhibitory circuit or electrical synapses between interneurons, this needs to be further examined (Figure 2).
In addition, as mentioned above, another study had suggested that GABA release from nigrostriatal afferents may be involved in this circuit. Indeed, following either nigrostriatal lesion or depletion of dopaminergic vesicles, a significant proportion of the nicotinic-induced disynaptic inhibition onto SPNs is reduced after optogenetic stimulation of CINs (Nelson et al., 2014). However, while the rate of reduction was highly variable among SPNs in our experiments obtained in Htr3a-Cre mice (Faust et al., 2016), some exhibited 80–90% reduction, making it unlikely that GABAergic projections from midbrain sources play a major role in the disynaptic inhibition of SPNs.
The involvement of 5HT3a interneuron population in the recurrent inhibition of CINs has been explored but consistent with their synaptic connectivity, these interneurons do not seem to play a major role in this circuit ((Dorst et al., 2020), Figure 2).
Functional implications
Striatal cholinergic transmission is essential for higher behavioral functions such as cognitive flexibility defined as the ability to adapt behavior in response to new and unexpected circumstances in the environment. This behavior has commonly been assessed using a reversal learning paradigm or attentional set-shifting tasks where, in both cases, animals have to suppress a previously learned stimulus-reward association (See (Nilsson et al., 2015; Powell & Ragozzino, 2017; Prado et al., 2017)).
Early electrophysiological recordings in monkeys showed that tonically active neurons (TANs) (putative CINs) exhibit a phasic decrease in their activity or “pause” associated with a change in the contingencies of the task (Apicella et al., 1991; Ravel et al., 2001). Further, ACh levels are significantly affected in reversal-learning tasks (Ragozzino & Choi, 2004; Ragozzino et al., 2009). Finally, CINs modulation or ablation evokes alterations in attentional set-shifting and habit formation (Okada et al., 2014; Aoki et al., 2015; Aoki et al., 2018; Okada et al., 2018).
However, while these observations support the involvement of CINs in cognitive flexibility, the exact circuits engaged in this modulation are still not fully understood. Specifically, what are the contribution of specific inputs in these functions? What are the transduction mechanisms? Are extrinsic sources of ACh involved?
In terms of synaptic inputs, dopamine is necessary for the pause response to develop following behavioral training (Aosaki et al., 1994a) and the pause in TANs coincides with the phasic firing of dopaminergic neurons in a classical conditioning task (Morris et al., 2004). Further, inactivation of centre-median-PfN has been shown to attenuate the pause of TANs following the presentation of salient sensory cues (Matsumoto et al., 2001). Functionally, caudal intralaminar thalamic afferents to CINs are involved in the establishment of new selected strategies in reversal learning tasks (Bradfield et al., 2013).
The transduction mechanism involves activation of M1 mAChR which are important in specific aspects of reversal learning ((Ragozzino et al., 2002; Tzavos et al., 2004; McCool et al., 2008; Prado et al., 2017), but see (Okada et al., 2014)). However, nAChRs activation may also play an important underestimated role. Indeed, the thalamic-evoked pause in CINs can be reduced after blockade of nAChRs or D2 receptors (Ding et al., 2010). As reviewed here, nAChRs are present in striatal circuits at strategic locations both presynaptically on dopamine and glutamatergic afferents as well as postsynaptically on the majority of striatal GABAergic interneurons. Particularly, the demonstration that activation of CINs can evoke dopamine release locally through presynaptic nAChRs certainly underscores critically important learning function (Cachope et al., 2012; Threlfell et al., 2012).
Additionally, some evidence reviewed above suggests that nAChR-mediated intrastriatal GABAergic inhibition could play an important role in broadcasting a reward-related pause in CINs via polysynaptic recurrent inhibition (Sullivan et al., 2008; Dorst et al., 2020). This would be the first evidence that nAChRs expressed postsynaptically on striatal interneurons significantly participate in shaping the activity of CINs in vivo and, potentially, in reward-related learning (Dorst et al., 2020). Further, the disynaptic inhibition of SPNs via nAChR activation of several classes of GABAergic interneurons represent a circuit allowing CINs to exert a rapid inhibitory control over SPNs (English et al., 2012). While the function of this circuit is yet to identify, it could contribute to the interruption of an ongoing behavior and reorientation after the encounter of a salient stimuli.
Despite their rarity, we and others have demonstrated that striatal GABAergic interneurons receiving nicotinic inputs are important in the regulation striatal related behaviors such as prepulse-inhibition (Assous et al., 2017) or goal-directed behaviors (Holly et al., 2019; Kaminer et al., 2019; Assous, 2020). However, the role of their interconnections with cholinergic interneurons has yet to be explored.
Increasing our knowledge on the synaptic organization of nAChR-mediated striatal circuits may lead to the development of better targeted strategies for neurological disorders such as nicotine addiction, Parkinson’s disease, Tourette syndrome or dystonia.
Acknowledgements
This work was supported by a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation to Maxime Assous, a Rutgers Busch Biomedical Research Grant to Maxime Assous and Michael Shiflett and National Institutes of Health NS034865 to James M. Tepper. I thank Dr. Tepper for insightful comments on the manuscript.
Abbreviations:
- ACh
Acetylcholine
- BG
Basal ganglia
- CINs
Cholinergic interneurons
- PfN
Parafscicular nucleus of the thalamus
- SNc
Substantia nigra pars compacta
- VTA
Ventral tegmental area
- GPe
Golbus pallidus external segment
- PPN
Pedunculopontine nucleus
- LDT
Laterodorsal tegmentum
- dSPNs
Direct pathway spiny projection neurons
- GPi
Globus pallidus internal segment
- SNr
Substantia nigra pars reticulata
- iSPNs
Indirect pathway spiny projection neurons
- PV
Parvalbumin
- FSIs
Fast-spiking interneurons
- NPY
Neuropeptide Y
- SOM
Somatostatin
- NOS
Nitrix oxide synthase
- LTS
Low threshold spike
- CR
Calretinin-expressing interneurons
- THINs
Tyrosine hydroxylase-expressing interneurons
- NGF
Neurogliaform
- FAIs
Fast adapting interneurons
- SABIs
Spontaneously active bursty interneurons
- mAChRs
muscarinic receptors
- nAChRs
nicotinic receptors
- GPCR
G protein coupled receptors
- IPSC/P
Inhibitory postsynaptic current/potential
- CNO
Clozapine-N-oxide
- DREADD
Designer receptors exclusively activated by designer drugs
- TANs
Tonically active neurons
Footnotes
Conflict of interest
The author declares no conflict of interest.
Data sharing statement
All the data are available in the main text.
References
- Abudukeyoumu N, Hernandez-Flores T, Garcia-Munoz M & Arbuthnott GW (2019) Cholinergic modulation of striatal microcircuits. Eur J Neurosci, 49, 604–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed NY, Knowles R & Dehorter N (2019) New Insights Into Cholinergic Neuron Diversity. Front Mol Neurosci, 12, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike A, Sasa M & Takaori S (1988) Muscarinic inhibition as a dominant role in cholinergic regulation of transmission in the caudate nucleus. J Pharmacol Exp Ther, 246, 1129–1136. [PubMed] [Google Scholar]
- Albin RL, Young AB & Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci, 12, 366–375. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Castro NG, Alkondon M, Reinhardt S, Schroder H & Maelicke A (1995) Nicotinic receptor function in the mammalian central nervous system. Ann N Y Acad Sci, 757, 48–72. [DOI] [PubMed] [Google Scholar]
- Alcantara AA, Mrzljak L, Jakab RL, Levey AI, Hersch SM & Goldman-Rakic PS (2001) Muscarinic m1 and m2 receptor proteins in local circuit and projection neurons of the primate striatum: anatomical evidence for cholinergic modulation of glutamatergic prefronto-striatal pathways. J Comp Neurol, 434, 445–460. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR & Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci, 9, 357–381. [DOI] [PubMed] [Google Scholar]
- Aoki S, Liu AW, Akamine Y, Zucca A, Zucca S & Wickens JR (2018) Cholinergic interneurons in the rat striatum modulate substitution of habits. Eur J Neurosci, 47, 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S, Liu AW, Zucca A, Zucca S & Wickens JR (2015) Role of Striatal Cholinergic Interneurons in Set-Shifting in the Rat. J Neurosci, 35, 9424–9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosaki T, Graybiel AM & Kimura M (1994a) Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science, 265, 412–415. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Kimura M & Graybiel AM (1995) Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J Neurophysiol, 73, 1234–1252. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Miura M, Suzuki T, Nishimura K & Masuda M (2010) Acetylcholine-dopamine balance hypothesis in the striatum: an update. Geriatr Gerontol Int, 10 Suppl 1, S148–157. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM & Kimura M (1994b) Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci, 14, 3969–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P (2002) Tonically active neurons in the primate striatum and their role in the processing of information about motivationally relevant events. Eur J Neurosci, 16, 2017–2026. [DOI] [PubMed] [Google Scholar]
- Apicella P (2017) The role of the intrinsic cholinergic system of the striatum: What have we learned from TAN recordings in behaving animals? Neuroscience, 360, 81–94. [DOI] [PubMed] [Google Scholar]
- Apicella P, Legallet E, Nieoullon A & Trouche E (1991) Neglect of contralateral visual stimuli in monkeys with unilateral striatal dopamine depletion. Behav Brain Res, 46, 187–195. [DOI] [PubMed] [Google Scholar]
- Apicella P, Legallet E & Trouche E (1996) Responses of tonically discharging neurons in monkey striatum to visual stimuli presented under passive conditions and during task performance. Neurosci Lett, 203, 147–150. [DOI] [PubMed] [Google Scholar]
- Apicella P, Legallet E & Trouche E (1997) Responses of tonically discharging neurons in the monkey striatum to primary rewards delivered during different behavioral states. Exp Brain Res, 116, 456–466. [DOI] [PubMed] [Google Scholar]
- Assous M (2020) Emergence of novel functions in striatal low-threshold spike interneurons (Commentary on Gazan et al., 2019). Eur J Neurosci, 52, 3490–3492. [DOI] [PubMed] [Google Scholar]
- Assous M, Dautan D, Tepper JM & Mena-Segovia J (2019) Pedunculopontine Glutamatergic Neurons Provide a Novel Source of Feedforward Inhibition in the Striatum by Selectively Targeting Interneurons. J Neurosci, 39, 4727–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assous M, Faust TW, Assini R, Shah F, Sidibe Y & Tepper JM (2018) Identification and Characterization of a Novel Spontaneously Active Bursty GABAergic Interneuron in the Mouse Striatum. J Neurosci, 38, 5688–5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assous M, Kaminer J, Shah F, Garg A, Koos T & Tepper JM (2017) Differential processing of thalamic information via distinct striatal interneuron circuits. Nat Commun, 8, 15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assous M & Tepper JM (2019) Excitatory extrinsic afferents to striatal interneurons and interactions with striatal microcircuitry. Eur J Neurosci, 49, 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan U & Leslie FM (2003) Co-expression of alpha7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience, 119, 965–977. [DOI] [PubMed] [Google Scholar]
- Barral J, Galarraga E & Bargas J (1999) Muscarinic presynaptic inhibition of neostriatal glutamatergic afferents is mediated by Q-type Ca2+ channels. Brain Res Bull, 49, 285–289. [DOI] [PubMed] [Google Scholar]
- Bengtsson Gonzales C, Hunt S, Munoz-Manchado AB, McBain CJ & Hjerling-Leffler J (2020) Intrinsic electrophysiological properties predict variability in morphology and connectivity among striatal Parvalbumin-expressing Pthlh-cells. Sci Rep, 10, 15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Callaway JC & Wilson CJ (2000) Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci, 20, 8493–8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD & Wilson CJ (1999) Spontaneous activity of neostriatal cholinergic interneurons in vitro. J Neurosci, 19, 5586–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW & Groenewegen HJ (1990) Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol, 299, 187–228. [DOI] [PubMed] [Google Scholar]
- Bernard V, Laribi O, Levey AI & Bloch B (1998) Subcellular redistribution of m2 muscarinic acetylcholine receptors in striatal interneurons in vivo after acute cholinergic stimulation. J Neurosci, 18, 10207–10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard V, Normand E & Bloch B (1992) Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci, 12, 3591–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Ballivet M & Rungger D (1990) Activation and blocking of neuronal nicotinic acetylcholine receptor reconstituted in Xenopus oocytes. Proc Natl Acad Sci U S A, 87, 1993–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Booth PA, Eaton SA & Bolam JP (1998) Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. J Neurosci, 18, 9438–9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr IJ, Ray MA, McIntosh JM, Chalon S, Guilloteau D, McKeith IG, Perry RH, Clementi F, Perry EK, Court JA & Piggott MA (2005) Cholinergic nicotinic receptor involvement in movement disorders associated with Lewy body diseases. An autoradiography study using [(125)I]alpha-conotoxinMII in the striatum and thalamus. Exp Neurol, 191, 292–300. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA & Bevan MD (2000) Synaptic organisation of the basal ganglia. J Anat, 196 (Pt 4), 527–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, Martella G, Madeo G, Schirinzi T, Puglisi F, Ponterio G & Pisani A (2011) Centrality of striatal cholinergic transmission in Basal Ganglia function. Front Neuroanat, 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsi P, Martella G, Cuomo D, Platania P, Sciamanna G, Bernardi G, Wess J & Pisani A (2008) Loss of muscarinic autoreceptor function impairs long-term depression but not long-term potentiation in the striatum. J Neurosci, 28, 6258–6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimblecombe KR, Threlfell S, Dautan D, Kosillo P, Mena-Segovia J & Cragg SJ (2018) Targeted Activation of Cholinergic Interneurons Accounts for the Modulation of Dopamine by Striatal Nicotinic Receptors. eNeuro, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Tan KR, O’Connor EC, Nikonenko I, Muller D & Luscher C (2012) Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature, 492, 452–456. [DOI] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM & Cheer JF (2012) Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep, 2, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A & Bernardi G (1998a) Blockade of M2-like muscarinic receptors enhances long-term potentiation at corticostriatal synapses. Eur J Neurosci, 10, 3020–3023. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Pisani A, Sancesario G, North RA & Bernardi G (1998b) Muscarinic IPSPs in rat striatal cholinergic interneurones. J Physiol, 510 (Pt 2), 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos F, Alfonso M & Duran R (2010) In vivo modulation of alpha7 nicotinic receptors on striatal glutamate release induced by anatoxin-A. Neurochem Int, 56, 850–855. [DOI] [PubMed] [Google Scholar]
- Capogna M & Pearce RA (2011) GABA A,slow: causes and consequences. Trends Neurosci, 34, 101–112. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Altemus KL, Flores-Hernandez J, Calvert CR, Jokel ES, Grandy DK, Low MJ, Rubinstein M, Ariano MA & Levine MS (2001) Facilitated glutamatergic transmission in the striatum of D2 dopamine receptor-deficient mice. J Neurophysiol, 85, 659–670. [DOI] [PubMed] [Google Scholar]
- Chang HT, Wilson CJ & Kitai ST (1982) A Golgi study of rat neostriatal neurons: light microscopic analysis. J Comp Neurol, 208, 107–126. [DOI] [PubMed] [Google Scholar]
- Clarke PB & Pert A (1985) Autoradiographic evidence for nicotine receptors on nigrostriatal and mesolimbic dopaminergic neurons. Brain Res, 348, 355–358. [DOI] [PubMed] [Google Scholar]
- Coimbra B, Soares-Cunha C, Vasconcelos NAP, Domingues AV, Borges S, Sousa N & Rodrigues AJ (2019) Role of laterodorsal tegmentum projections to nucleus accumbens in reward-related behaviors. Nat Commun, 10, 4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas-Bailador F & Wonnacott S (2004) Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci, 25, 317–324. [DOI] [PubMed] [Google Scholar]
- Dani JA (2001) Nicotinic receptor activity alters synaptic plasticity. ScientificWorldJournal, 1, 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA & Bertrand D (2007) Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol, 47, 699–729. [DOI] [PubMed] [Google Scholar]
- Dautan D, Huerta-Ocampo I, Gut NK, Valencia M, Kondabolu K, Kim Y, Gerdjikov TV & Mena-Segovia J (2020) Cholinergic midbrain afferents modulate striatal circuits and shape encoding of action strategies. Nat Commun, 11, 1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautan D, Huerta-Ocampo I, Witten IB, Deisseroth K, Bolam JP, Gerdjikov T & Mena-Segovia J (2014) A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J Neurosci, 34, 4509–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rover M, Lodder JC, Kits KS, Schoffelmeer AN & Brussaard AB (2002) Cholinergic modulation of nucleus accumbens medium spiny neurons. Eur J Neurosci, 16, 2279–2290. [DOI] [PubMed] [Google Scholar]
- Deffains M & Bergman H (2015) Striatal cholinergic interneurons and cortico-striatal synaptic plasticity in health and disease. Mov Disord, 30, 1014–1025. [DOI] [PubMed] [Google Scholar]
- DeLong MR (1990) Primate models of movement disorders of basal ganglia origin. Trends Neurosci, 13, 281–285. [DOI] [PubMed] [Google Scholar]
- Dickinson JA, Kew JN & Wonnacott S (2008) Presynaptic alpha 7- and beta 2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol Pharmacol, 74, 348–359. [DOI] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA & Surmeier DJ (2010) Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron, 67, 294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodt HU & Misgeld U (1986) Muscarinic slow excitation and muscarinic inhibition of synaptic transmission in the rat neostriatum. J Physiol, 380, 593–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig NM, Magill PJ, Apicella P, Bolam JP & Sharott A (2014) Cortical and thalamic excitation mediate the multiphasic responses of striatal cholinergic interneurons to motivationally salient stimuli. J Neurosci, 34, 3101–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorst MC, Tokarska A, Zhou M, Lee K, Stagkourakis S, Broberger C, Masmanidis S & Silberberg G (2020) Polysynaptic inhibition between striatal cholinergic interneurons shapes their network activity patterns in a dopamine-dependent manner. Nat Commun, 11, 5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elghaba R, Vautrelle N & Bracci E (2016) Mutual Control of Cholinergic and Low-Threshold Spike Interneurons in the Striatum. Front Cell Neurosci, 10, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsaki G, Deisseroth K, Tepper JM & Koos T (2012) GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci, 15, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R & Cragg SJ (2008) Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol, 153 Suppl 1, S283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust TW, Assous M, Shah F, Tepper JM & Koos T (2015) Novel fast adapting interneurons mediate cholinergic-induced fast GABAA inhibitory postsynaptic currents in striatal spiny neurons. Eur J Neurosci, 42, 1764–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust TW, Assous M, Tepper JM & Koos T (2016) Neostriatal GABAergic Interneurons Mediate Cholinergic Inhibition of Spiny Projection Neurons. J Neurosci, 36, 9505–9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty AW & Graybiel AM (1993) Output architecture of the primate putamen. J Neurosci, 13, 3222–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois C, Percheron G, Parent A, Sadikot AF, Fenelon G & Yelnik J (1991) Topography of the projection from the central complex of the thalamus to the sensorimotor striatal territory in monkeys. J Comp Neurol, 305, 17–34. [DOI] [PubMed] [Google Scholar]
- Frost Nylen J, Carannante I, Grillner S & Hellgren Kotaleski J (2020) Reciprocal interaction between striatal cholinergic and low-threshold spiking interneurons - A computational study. Eur J Neurosci. [DOI] [PubMed] [Google Scholar]
- Galarraga E, Hernandez-Lopez S, Reyes A, Miranda I, Bermudez-Rattoni F, Vilchis C & Bargas J (1999) Cholinergic modulation of neostriatal output: a functional antagonism between different types of muscarinic receptors. J Neurosci, 19, 3629–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garas FNS, R. S; Kormann E; Doig NM; Vinciati F; Nakamura KC; Dorst MC; Smith Y; Magill PJ; Sharott A (2016) Secretagogin expression delineates functionally-specialized populations of striatal parvalbumin-containing interneurons. Elife, 5, e16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR & Lovinger DM (2003) It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci, 26, 184–192. [DOI] [PubMed] [Google Scholar]
- Gerfen CR (2000) Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci, 23, S64–70. [DOI] [PubMed] [Google Scholar]
- Gerfen CR & Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci, 34, 441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girasole AE & Nelson AB (2015) Probing striatal microcircuitry to understand the functional role of cholinergic interneurons. Mov Disord, 30, 1306–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Berke JD, Bevan MD, Chan CS, Mallet N, Morrow MM & Schmidt R (2014) New roles for the external globus pallidus in basal ganglia circuits and behavior. J Neurosci, 34, 15178–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Nelson AB, Thwin MT, Palop JJ & Kreitzer AC (2010) Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci, 30, 2223–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Ding JB & Surmeier DJ (2012) Muscarinic modulation of striatal function and circuitry. Handb Exp Pharmacol, 223–241. [DOI] [PubMed] [Google Scholar]
- Goldberg JA & Wilson CJ (2005) Control of spontaneous firing patterns by the selective coupling of calcium currents to calcium-activated potassium currents in striatal cholinergic interneurons. J Neurosci, 25, 10230–10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales KK & Smith Y (2015) Cholinergic interneurons in the dorsal and ventral striatum: anatomical and functional considerations in normal and diseased conditions. Ann N Y Acad Sci, 1349, 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L & Zoli M (2009) Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol, 78, 703–711. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Bohr I, Ziabreva I, Vailati S, Longhi R, Riganti L, Gaimarri A, McKeith IG, Perry RH, Aarsland D, Larsen JP, Sher E, Beattie R, Clementi F & Court JA (2006a) Selective nicotinic acetylcholine receptor subunit deficits identified in Alzheimer’s disease, Parkinson’s disease and dementia with Lewy bodies by immunoprecipitation. Neurobiol Dis, 23, 481–489. [DOI] [PubMed] [Google Scholar]
- Gotti C, Riganti L, Vailati S & Clementi F (2006b) Brain neuronal nicotinic receptors as new targets for drug discovery. Curr Pharm Des, 12, 407–428. [DOI] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC & Marks MJ (2007) The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol, 74, 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveland GA & DiFiglia M (1985) The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res, 327, 307–311. [DOI] [PubMed] [Google Scholar]
- Grilli M, Zappettini S, Raiteri L & Marchi M (2009) Nicotinic and muscarinic cholinergic receptors coexist on GABAergic nerve endings in the mouse striatum and interact in modulating GABA release. Neuropharmacology, 56, 610–614. [DOI] [PubMed] [Google Scholar]
- Haber SN (2016) Corticostriatal circuitry. Dialogues Clin Neurosci, 18, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P & Calzavara R (2006) Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci, 26, 8368–8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman DJ, Hong ES, Hernandez VM & Chan CS (2016) The external globus pallidus: progress and perspectives. Eur J Neurosci, 43, 1239–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Gutekunst CA, Rees HD, Heilman CJ & Levey AI (1994) Distribution of m1–m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci, 14, 3351–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM & Levey AI (1995) Diverse pre- and post-synaptic expression of m1–m4 muscarinic receptor proteins in neurons and afferents in the rat neostriatum. Life Sci, 56, 931–938. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Soler-Llavina GJ & Sabatini BL (2009) Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. Nat Neurosci, 12, 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA Jr., Zoli M, Bourgeois JP & Changeux JP (1993) Immunocytochemical localization of a neuronal nicotinic receptor: the beta 2-subunit. J Neurosci, 13, 1551–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley SM, Joshi PR, Parievsky A, Galvan L, Chen JY, Fisher YE, Huynh MN, Cepeda C & Levine MS (2015) Enhanced GABAergic Inputs Contribute to Functional Alterations of Cholinergic Interneurons in the R6/2 Mouse Model of Huntington’s Disease. eNeuro, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EN, Davatolhagh MF, Choi K, Alabi OO, Vargas Cifuentes L & Fuccillo MV (2019) Striatal Low-Threshold Spiking Interneurons Regulate Goal-Directed Learning. Neuron, 103, 92–101 e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe M, Ridouh I, Allegra Mascaro AL, Larios A, Azcorra M & Dombeck DA (2019) Coordination of rapid cholinergic and dopaminergic signaling in striatum during spontaneous movement. Elife, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Young DA, Bekheet G & Kozak R (2016) Nicotinic receptor subtypes differentially modulate glutamate release in the dorsal medial striatum. Neurochem Int, 100, 30–34. [DOI] [PubMed] [Google Scholar]
- Hsu KS, Yang CH, Huang CC & Gean PW (1996) Carbachol induces inward current in neostriatal neurons through M1-like muscarinic receptors. Neuroscience, 73, 751–760. [DOI] [PubMed] [Google Scholar]
- Ibañez-Sandoval O, Tecuapetla F, Unal B, Shah F, Koos T & Tepper JM (2010) Electrophysiological and morphological characteristics and synaptic connectivity of tyrosine hydroxylase-expressing neurons in adult mouse striatum. J Neurosci, 30, 6999–7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez-Sandoval O, Tecuapetla F, Unal B, Shah F, Koos T & Tepper JM (2011) A novel functionally distinct subtype of striatal neuropeptide Y interneuron. J Neurosci, 31, 16757–16769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez-Sandoval O, Xenias HS, Tepper JM & Koos T (2015) Dopaminergic and cholinergic modulation of striatal tyrosine hydroxylase interneurons. Neuropharmacology, 95, 468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones IW, Bolam JP & Wonnacott S (2001) Presynaptic localisation of the nicotinic acetylcholine receptor beta2 subunit immunoreactivity in rat nigrostriatal dopaminergic neurones. J Comp Neurol, 439, 235–247. [DOI] [PubMed] [Google Scholar]
- Joshua M, Adler A, Mitelman R, Vaadia E & Bergman H (2008) Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J Neurosci, 28, 11673–11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S & Wonnacott S (2000) alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)H]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol, 58, 312–318. [DOI] [PubMed] [Google Scholar]
- Kaminer J, Espinoza D, Bhimani S, Tepper JM, Koos T & Shiflett MW (2019) Loss of striatal tyrosine-hydroxylase interneurons impairs instrumental goal-directed behavior. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y (1993) Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci, 13, 4908–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ & Emson PC (1995) Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci, 18, 527–535. [DOI] [PubMed] [Google Scholar]
- Keath JR, Iacoviello MP, Barrett LE, Mansvelder HD & McGehee DS (2007) Differential modulation by nicotine of substantia nigra versus ventral tegmental area dopamine neurons. J Neurophysiol, 98, 3388–3396. [DOI] [PubMed] [Google Scholar]
- Kemp JM & Powell TP (1971) The termination of fibres from the cerebral cortex and thalamus upon dendritic spines in the caudate nucleus: a study with the Golgi method. Philos Trans R Soc Lond B Biol Sci, 262, 429–439. [DOI] [PubMed] [Google Scholar]
- Kimura M, Rajkowski J & Evarts E (1984) Tonically discharging putamen neurons exhibit set-dependent responses. Proc Natl Acad Sci U S A, 81, 4998–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T & Tepper JM (1999) Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci, 2, 467–472. [DOI] [PubMed] [Google Scholar]
- Koos T & Tepper JM (2002) Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci, 22, 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosillo P, Zhang YF, Threlfell S & Cragg SJ (2016) Cortical Control of Striatal Dopamine Transmission via Striatal Cholinergic Interneurons. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC & Malenka RC (2008) Striatal plasticity and basal ganglia circuit function. Neuron, 60, 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapper SR & Bolam JP (1992) Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience, 51, 533–545. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Zoli M & Changeux JP (1996) Neuronal nicotinic receptor alpha 6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur J Neurosci, 8, 2428–2439. [DOI] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Heilman CJ, Desmond TJ & Frey KA (1994) Localization of muscarinic m3 receptor protein and M3 receptor binding in rat brain. Neuroscience, 63, 207–221. [DOI] [PubMed] [Google Scholar]
- Licheri V, Lagstrom O, Lotfi A, Patton MH, Wigstrom H, Mathur B & Adermark L (2018) Complex Control of Striatal Neurotransmission by Nicotinic Acetylcholine Receptors via Excitatory Inputs onto Medium Spiny Neurons. J Neurosci, 38, 6597–6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Chung KK, de Castro D, Funk GD & Lipski J (2004) Effects of muscarinic acetylcholine receptor activation on membrane currents and intracellular messengers in medium spiny neurones of the rat striatum. Eur J Neurosci, 20, 1219–1230. [DOI] [PubMed] [Google Scholar]
- Lozovaya N, Eftekhari S, Cloarec R, Gouty-Colomer LA, Dufour A, Riffault B, Billon-Grand M, Pons-Bennaceur A, Oumar N, Burnashev N, Ben-Ari Y & Hammond C (2018) GABAergic inhibition in dual-transmission cholinergic and GABAergic striatal interneurons is abolished in Parkinson disease. Nat Commun, 9, 1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC & Sadikot AF (2001) GABA promotes survival but not proliferation of parvalbumin-immunoreactive interneurons in rodent neostriatum: an in vivo study with stereology. Neuroscience, 104, 93–103. [DOI] [PubMed] [Google Scholar]
- Luo R, Janssen MJ, Partridge JG & Vicini S (2013a) Direct and GABA-mediated indirect effects of nicotinic ACh receptor agonists on striatal neurones. J Physiol, 591, 203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, Partridge JG & Vicini S (2013b) Distinct roles of synaptic and extrasynaptic GABAAreceptors in striatal inhibition dynamics. Front Neural Circuits, 7, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC & Kocsis JD (1988) Presynaptic actions of carbachol and adenosine on corticostriatal synaptic transmission studied in vitro. J Neurosci, 8, 3750–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Le Moine C, Charpier S & Gonon F (2005) Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci, 25, 3857–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Leblois A, Maurice N & Beurrier C (2019) Striatal Cholinergic Interneurons: How to Elucidate Their Function in Health and Disease. Front Pharmacol, 10, 1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Micklem BR, Henny P, Brown MT, Williams C, Bolam JP, Nakamura KC & Magill PJ (2012) Dichotomous organization of the external globus pallidus. Neuron, 74, 1075–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamaligas AA, Barcomb K & Ford CP (2019) Cholinergic Transmission at Muscarinic Synapses in the Striatum Is Driven Equally by Cortical and Thalamic Inputs. Cell Rep, 28, 1003–1014 e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamaligas AA & Ford CP (2016) Spontaneous Synaptic Activation of Muscarinic Receptors by Striatal Cholinergic Neuron Firing. Neuron, 91, 574–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin JS, Shimoda Y, Magloire V, Leite M, Kawashima T, Jensen TP, Kolb I, Knott EL, Novak O, Podgorski K, Leidenheimer NJ, Rusakov DA, Ahrens MB, Kullmann DM & Looger LL (2019) A genetically encoded fluorescent sensor for in vivo imaging of GABA. Nat Methods, 16, 763–770. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Minamimoto T, Graybiel AM & Kimura M (2001) Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant sensory events. J Neurophysiol, 85, 960–976. [DOI] [PubMed] [Google Scholar]
- McCool MF, Patel S, Talati R & Ragozzino ME (2008) Differential involvement of M1-type and M4-type muscarinic cholinergic receptors in the dorsomedial striatum in task switching. Neurobiol Learn Mem, 89, 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez-Zaidi AE, Lakshminarasimhah H & Surmeier DJ (2019) Cholinergic modulation of striatal nitric oxide-producing interneurons. Eur J Neurosci, 50, 3713–3731. [DOI] [PubMed] [Google Scholar]
- Meredith GE & Wouterlood FG (1990) Hippocampal and midline thalamic fibers and terminals in relation to the choline acetyltransferase-immunoreactive neurons in nucleus accumbens of the rat: a light and electron microscopic study. J Comp Neurol, 296, 204–221. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI & Wainer BH (1984) Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Neuroscience, 12, 669–686. [DOI] [PubMed] [Google Scholar]
- Minamimoto T & Kimura M (2002) Participation of the thalamic CM-Pf complex in attentional orienting. J Neurophysiol, 87, 3090–3101. [DOI] [PubMed] [Google Scholar]
- Morgenstern NA, Isidro AF, Israely I & Costa RM (2020) Pyramidal tract neurons drive feed-forward excitation of striatum through cholinergic interneurons. bioRxiv, 2020.2012.2014.422716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E & Bergman H (2004) Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron, 43, 133–143. [DOI] [PubMed] [Google Scholar]
- Munoz-Manchado AB, Foldi C, Szydlowski S, Sjulson L, Farries M, Wilson C, Silberberg G & Hjerling-Leffler J (2016) Novel Striatal GABAergic Interneuron Populations Labeled in the 5HT3a(EGFP) Mouse. Cereb Cortex, 26, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Hammack N, Yang CF, Shah NM, Seal RP & Kreitzer AC (2014) Striatal cholinergic interneurons Drive GABA release from dopamine terminals. Neuron, 82, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson SR, Alsio J, Somerville EM & Clifton PG (2015) The rat’s not for turning: Dissociating the psychological components of cognitive inflexibility. Neurosci Biobehav Rev, 56, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Nishizawa K, Fukabori R, Kai N, Shiota A, Ueda M, Tsutsui Y, Sakata S, Matsushita N & Kobayashi K (2014) Enhanced flexibility of place discrimination learning by targeting striatal cholinergic interneurons. Nat Commun, 5, 3778. [DOI] [PubMed] [Google Scholar]
- Okada K, Nishizawa K, Setogawa S, Hashimoto K & Kobayashi K (2018) Task-dependent function of striatal cholinergic interneurons in behavioural flexibility. Eur J Neurosci, 47, 1174–1183. [DOI] [PubMed] [Google Scholar]
- Pakhotin P & Bracci E (2007) Cholinergic interneurons control the excitatory input to the striatum. J Neurosci, 27, 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancani T, Bolarinwa C, Smith Y, Lindsley CW, Conn PJ & Xiang Z (2014) M4 mAChR-mediated modulation of glutamatergic transmission at corticostriatal synapses. ACS Chem Neurosci, 5, 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SL, Fu Y, McAllen K, Luo J, McIntosh JM, Lindstrom JM & Sharp BM (2004) Up-regulation of brain nicotinic acetylcholine receptors in the rat during long-term self-administration of nicotine: disproportionate increase of the alpha6 subunit. Mol Pharmacol, 65, 611–622. [DOI] [PubMed] [Google Scholar]
- Perez-Rosello T, Figueroa A, Salgado H, Vilchis C, Tecuapetla F, Guzman JN, Galarraga E & Bargas J (2005) Cholinergic control of firing pattern and neurotransmission in rat neostriatal projection neurons: role of CaV2.1 and CaV2.2 Ca2+ channels. J Neurophysiol, 93, 2507–2519. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bernardi G, Ding J & Surmeier DJ (2007) Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci, 30, 545–553. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA & Bucci DJ (2006) Central nicotinic cholinergic systems: a role in the cognitive dysfunction in attention-deficit/hyperactivity disorder? Behav Brain Res, 175, 201–211. [DOI] [PubMed] [Google Scholar]
- Powell EM & Ragozzino ME (2017) Cognitive flexibility: Development, disease and treatment. Neuroscience, 345, 1–2. [DOI] [PubMed] [Google Scholar]
- Prado VF, Janickova H, Al-Onaizi MA & Prado MA (2017) Cholinergic circuits in cognitive flexibility. Neuroscience, 345, 130–141. [DOI] [PubMed] [Google Scholar]
- Quik M & McIntosh JM (2006) Striatal alpha6* nicotinic acetylcholine receptors: potential targets for Parkinson’s disease therapy. J Pharmacol Exp Ther, 316, 481–489. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME & Choi D (2004) Dynamic changes in acetylcholine output in the medial striatum during place reversal learning. Learn Mem, 11, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Jih J & Tzavos A (2002) Involvement of the dorsomedial striatum in behavioral flexibility: role of muscarinic cholinergic receptors. Brain Res, 953, 205–214. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Mohler EG, Prior M, Palencia CA & Rozman S (2009) Acetylcholine activity in selective striatal regions supports behavioral flexibility. Neurobiol Learn Mem, 91, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel S, Sardo P, Legallet E & Apicella P (2001) Reward unpredictability inside and outside of a task context as a determinant of the responses of tonically active neurons in the monkey striatum. J Neurosci, 21, 5730–5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI & Wickens JR (2004) Modulation of an afterhyperpolarization by the substantia nigra induces pauses in the tonic firing of striatal cholinergic interneurons. J Neurosci, 24, 9870–9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME & Cragg SJ (2004) Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci, 7, 583–584. [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A, Smith Y & Bolam JP (1992) Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a light and electron microscopic study of the thalamostriatal projection in relation to striatal heterogeneity. J Comp Neurol, 320, 228–242. [DOI] [PubMed] [Google Scholar]
- Schulz JM & Reynolds JN (2013) Pause and rebound: sensory control of cholinergic signaling in the striatum. Trends Neurosci, 36, 41–50. [DOI] [PubMed] [Google Scholar]
- Sharples CG, Kaiser S, Soliakov L, Marks MJ, Collins AC, Washburn M, Wright E, Spencer JA, Gallagher T, Whiteaker P & Wonnacott S (2000) UB-165: a novel nicotinic agonist with subtype selectivity implicates the alpha4beta2* subtype in the modulation of dopamine release from rat striatal synaptosomes. J Neurosci, 20, 2783–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Hamilton SE, Nathanson NM & Surmeier DJ (2005) Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci, 25, 7449–7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Galvan A, Ellender TJ, Doig N, Villalba RM, Huerta-Ocampo I, Wichmann T & Bolam JP (2014) The thalamostriatal system in normal and diseased states. Front Syst Neurosci, 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y & Parent A (1986) Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus). Neuroscience, 18, 347–371. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF & Sidibe M (2004) The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci, 27, 520–527. [DOI] [PubMed] [Google Scholar]
- Smolders I, Bogaert L, Ebinger G & Michotte Y (1997) Muscarinic modulation of striatal dopamine, glutamate, and GABA release, as measured with in vivo microdialysis. J Neurochem, 68, 1942–1948. [DOI] [PubMed] [Google Scholar]
- Straub C, Saulnier JL, Begue A, Feng DD, Huang KW & Sabatini BL (2016) Principles of Synaptic Organization of GABAergic Interneurons in the Striatum. Neuron, 92, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MA, Chen H & Morikawa H (2008) Recurrent inhibitory network among striatal cholinergic interneurons. J Neurosci, 28, 8682–8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z & Shen W (2007) D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci, 30, 228–235. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ & Graybiel AM (2012) A feud that wasn’t: acetylcholine evokes dopamine release in the striatum. Neuron, 75, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E & Momiyama T (2020) M1 muscarinic acetylcholine receptor-mediated inhibition of GABA release from striatal medium spiny neurons onto cholinergic interneurons. Eur J Neurosci. [DOI] [PubMed] [Google Scholar]
- Szydlowski SN, Pollak Dorocic I, Planert H, Carlen M, Meletis K & Silberberg G (2013) Target selectivity of feedforward inhibition by striatal fast-spiking interneurons. J Neurosci, 33, 1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Du Y, Kondapalli J, Wokosin DL & Surmeier DJ (2019) Cholinergic Interneurons Amplify Thalamostriatal Excitation of Striatal Indirect Pathway Neurons in Parkinson’s Disease Models. Neuron. [DOI] [PubMed] [Google Scholar]
- Tanimura A, Pancani T, Lim SAO, Tubert C, Melendez AE, Shen W & Surmeier DJ (2018) Striatal cholinergic interneurons and Parkinson’s disease. Eur J Neurosci, 47, 1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S, Ilijic E & Surmeier DJ (2008) Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson’s disease. J Neurosci, 28, 5504–5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Ibanez-Sandoval O, Tecuapetla F, Faust TW & Assous M (2018) Heterogeneity and Diversity of Striatal GABAergic Interneurons: Update 2018. Front Neuroanat, 12, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Tecuapetla F, Koos T & Ibanez-Sandoval O (2010) Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat, 4, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K & Cragg SJ (2012) Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron, 75, 58–64. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB & Sabatini BL (2012) Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature, 490, 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Oh WJ, Gu C & Sabatini BL (2014) Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA, not synthesis. Elife, 3, e01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavos A, Jih J & Ragozzino ME (2004) Differential effects of M1 muscarinic receptor blockade and nicotinic receptor blockade in the dorsomedial striatum on response reversal learning. Behav Brain Res, 154, 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ (2005) The mechanism of intrinsic amplification of hyperpolarizations and spontaneous bursting in striatal cholinergic interneurons. Neuron, 45, 575–585. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT & Kitai ST (1990) Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci, 10, 508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C & Deisseroth K (2010) Cholinergic interneurons control local circuit activity and cocaine conditioning. Science, 330, 1677–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L & Jones IW (2000) Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur J Pharmacol, 393, 51–58. [DOI] [PubMed] [Google Scholar]
- Xenias HS, Ibanez-Sandoval O, Koos T & Tepper JM (2015) Are striatal tyrosine hydroxylase interneurons dopaminergic? J Neurosci, 35, 6584–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Nashmi R, McKinney S, Cai H, McIntosh JM & Lester HA (2009) Chronic nicotine selectively enhances alpha4beta2* nicotinic acetylcholine receptors in the nigrostriatal dopamine pathway. J Neurosci, 29, 12428–12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Ebihara K, Koshikawa N & Kobayashi M (2013) Reciprocal regulation of inhibitory synaptic transmission by nicotinic and muscarinic receptors in rat nucleus accumbens shell. J Physiol, 591, 5745–5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Flores-Hernandez J & Surmeier DJ (2001) Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience, 103, 1017–1024. [DOI] [PubMed] [Google Scholar]
- Yan Z & Surmeier DJ (1996) Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J Neurosci, 16, 2592–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH & Van Hoesen GW (1978) Cortico-striate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain Res, 139, 43–63. [DOI] [PubMed] [Google Scholar]
- Zackheim J & Abercrombie ED (2005) Thalamic regulation of striatal acetylcholine efflux is both direct and indirect and qualitatively altered in the dopamine-depleted striatum. Neuroscience, 131, 423–436. [DOI] [PubMed] [Google Scholar]
- Zhang H & Sulzer D (2004) Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci, 7, 581–582. [DOI] [PubMed] [Google Scholar]
- Zhang YF & Cragg SJ (2017) Pauses in Striatal Cholinergic Interneurons: What is Revealed by Their Common Themes and Variations? Front Syst Neurosci, 11, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Liang Y & Dani JA (2001) Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci, 4, 1224–1229. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson C & Dani JA (2003) Muscarinic and nicotinic cholinergic mechanisms in the mesostriatal dopamine systems. Neuroscientist, 9, 23–36. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ & Dani JA (2002) Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol, 53, 590–605. [DOI] [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto MR & Changeux JP (1998) Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J Neurosci, 18, 4461–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]


