Abstract
Simple Summary
Mutations in the isocitrate dehydrogenase 1 (IDH1) gene occur in high-grade chondrosarcoma, high-grade glioma and intrahepatic cholangiocarcinoma. Due to the lack of effective treatment options, these aggressive types of cancer have a dismal outcome. The metabolism of IDH1-mutated cancer cells is reprogrammed in order to produce the oncometabolite D-2-hydroxyglutarate (D-2HG). In this clinical trial, we used the oral antidiabetic drug metformin and the oral antimalarial drug chloroquine to disrupt the vulnerable metabolism of IDH1-mutated solid tumors. We found that the combination regimen of metformin and chloroquine is well tolerated, but the combination did not induce a clinical response in this patient population. Secondly, we confirmed the clinical usefulness of D/L-2HG ratios in serum as a biomarker and the ddPCR-facilitated detection of an IDH1 mutation in circulating DNA from peripheral blood.
Abstract
Background: Mutations in isocitrate dehydrogenase 1 (IDH1) occur in 60% of chondrosarcoma, 80% of WHO grade II-IV glioma and 20% of intrahepatic cholangiocarcinoma. These solid IDH1-mutated tumors produce the oncometabolite D-2-hydroxyglutarate (D-2HG) and are more vulnerable to disruption of their metabolism. Methods: Patients with IDH1-mutated chondrosarcoma, glioma and intrahepatic cholangiocarcinoma received oral combinational treatment with the antidiabetic drug metformin and the antimalarial drug chloroquine. The primary objective was to determine the occurrence of dose-limiting toxicities (DLTs) and the maximum tolerated dose (MTD). Radiological and biochemical tumor responses to metformin and chloroquine were investigated using CT/MRI scans and magnetic resonance spectroscopy (MRS) measurements of D-2HG levels in serum. Results: Seventeen patients received study treatment for a median duration of 43 days (range: 7–74 days). Of twelve evaluable patients, 10 patients discontinued study medication because of progressive disease and two patients due to toxicity. None of the patients experienced a DLT. The MTD was determined to be 1500 mg of metformin two times a day and 200 mg of chloroquine once a day. A serum D/L-2HG ratio of ≥4.5 predicted the presence of an IDH1 mutation with a sensitivity of 90% and a specificity of 100%. By utilization of digital droplet PCR on plasma samples, we were able to detect tumor-specific IDH1 hotspot mutations in circulating tumor DNA (ctDNA) in investigated patients. Conclusion: Treatment of advanced IDH1-mutated solid tumors with metformin and chloroquine was well tolerated but did not induce a clinical response in this phase Ib clinical trial.
Keywords: metformin, chloroquine, cancer, isocitrate dehydrogenase, pharmacokinetics, glioblastoma, intrahepatic cholangiocarcinoma, chondrosarcoma
1. Introduction
Somatic heterozygous mutations in IDH1 and IDH2 occur in up to 60% of central conventional chondrosarcoma, 80% of WHO grade II–IV glioma, 20% of intrahepatic cholangiocarcinoma and 10% of acute myeloid leukemias (AML) [1]. In glioma, patients with an IDH1 mutation have a relatively prolonged survival, whereas prognosis of other IDH1/2-mutated tumors remains poor. IDH1 mutations are present in a large fraction or even all cancer cells in glioma [2], rendering IDH1 mutations an interesting target for treatment, because the high molecular homogeneity diminishes the risk of therapy resistance [3]. It has been shown that IDH1 mutations sensitize cancer cells in vitro to therapies that involve oxidative stress, such as radiotherapy, cisplatin and carmustine [4,5,6]. Appreciation of the causative role of IDH1/2 mutations in oncogenesis led to the development of the IDH1-mutation inhibitor agent ivosidenib (AG-120) [7] and the IDH2-mutation inhibitor enasidenib (AG-221). Both are FDA-approved for the treatment of relapsed or refractory IDH1-mutated or IDH2-mutated AML [8,9].
IDH1 and IDH2 are homodimeric enzymes that reversibly convert isocitrate to α-ketoglutarate (αKG) in the cytoplasm and mitochondria, respectively. Hotspot mutations in IDH1, of which IDH1R132H is the most prevalent in glioma and chondrosarcoma, lead to heterodimeric enzymes (IDH1WT/MUT), loss of wild-type IDH1 enzyme function and a neomorphic IDH1 activity that converts αKG into the oncometabolite D-2-hydroxyglutarate (D-2HG) [10]. D-2HG exerts its oncogenic effects via competitive inhibition of αKG-dependent dioxygenases [11,12], that are essential for epigenetic regulation of gene expression, including that of metabolic genes [13,14,15]. Moreover, D-2HG serum concentrations may serve as a surrogate biomarker of the presence of an IDH1/2 mutation [8,16,17,18].
Reprogramming of cellular metabolism is one of the hallmarks of cancer [14,19,20]. IDH1/2 mutations affect carbohydrate and NADP+/NADPH metabolism by causing loss of wild-type IDH1/2 function. Metabolic pathways are rewired and carbon metabolites are redirected away from the tricarboxylic acid (TCA) cycle for production of D-2HG [14,21,22,23,24]. IDH1-mutated cancer cells are more vulnerable to inhibition of metabolism with inhibitors of the electron transport chain (ETC), such as metformin that inhibits complex I of the ETC [4,25]. In vitro, we previously showed that IDH1-mutated cancer cells are more sensitive to metformin compared to IDH1 wild-type cancer cells [4]. However, sensitivity to metformin in chondrosarcoma cell lines was independent of the presence of an IDH1 mutation [26]. Moreover, metformin has attracted interest as an anti-cancer drug [27], since an association between metformin use in type 2 diabetes mellitus (T2DM) patients and a reduced risk of breast, colon, pancreas and prostate cancer was found [28,29,30,31,32,33], as well as a reduced risk of mortality, as compared with patients treated with insulin or sulfonylureas [34].
A second consequence of rewired metabolism in IDH1-mutated cells is the dependence on the glutaminolysis pathway, which provides anaplerosis to the TCA cycle at the level of αKG for generation of the oncometabolite D-2HG [6,14,21,35]. In this alternative pathway, the final step of glutaminolysis can be inhibited by chloroquine, an antimalarial drug, as well as metformin targeting glutamate dehydrogenase [20,36,37,38]. In addition, IDH1-mutated glioma cells in metabolic stress show increased levels of autophagy in order to provide substrates for energy production [39]. These anti-cancer properties of chloroquine in combination with inhibition of autophagy by chloroquine may thus be selective for IDH1-mutated cells because it inhibits glutaminolysis and autophagy on which these cells are dependent [40]. Chondrosarcoma cell lines are sensitive to chloroquine because it suppresses autophagy, irrespective of the IDH1-mutation status [26]. Both chloroquine and metformin are cheap, widely used and readily available drugs with a safety profile that is favorable comparable to most chemotherapeutic agents.
The present clinical trial investigated the safety and maximum tolerated dose (MTD) of the combination therapy of metformin and chloroquine in patients with IDH1-mutated chondrosarcoma, glioma and intrahepatic cholangiocarcinoma. As secondary objectives, the pharmacokinetics of metformin and chloroquine combinational treatment and efficacy were assessed by measuring tumor size and/or levels of D-2HG and L-2HG in serum to determine their ratio.
2. Materials and Methods
2.1. Patient Population
Eligible patients were aged ≥18 years with IDH1/2-mutated newly-diagnosed, recurrent, relapsed or refractory grade II–III chondrosarcoma, WHO grade II–IV glioma or intrahepatic cholangiocarcinoma. The tumor material (obtained by surgery performed before or after this clinical trial (ClinicalTrials.gov NCT02496741) in the context of regular patient care or a tumor biopsy specifically for this clinical trial) had to carry a neomorphic D-2HG-generating mutation in IDH1 or IDH2 as determined by sequencing of tumor DNA or as shown by elevated D-2HG-to-L-2HG ratios in mass spectrometry (MS) of serum or magnetic resonance spectroscopy (MRS) imaging of the tumor. Other eligibility criteria included a WHO performance status ≤2, adequate renal function (creatinine <150 μmol/L or a creatinine clearance >60 mL/L), liver function (bilirubin <1.5 times the normal upper limit; ALAT and ASAT <2.5 times the normal upper limit), and bone marrow function (white blood cells >3.0 × 109/L, platelets >100 × 109/L). Patients were not eligible when they were concomitantly treated with other anti-cancer therapies (i.e., chemotherapy, targeted therapy, radiation therapy). Palliative therapy was permitted, such as local palliative radiotherapy, dexamethasone, and non-enzyme-inducing anti-epileptic drugs (with the exception of topiramate) in patients with glioma and epileptic seizures. Furthermore, patients were not eligible in case they were known to have a serious concomitant medical condition or used interacting medication that could not be replaced, a known hypersensitivity to either metformin or chloroquine, or when they had been treated with either metformin or chloroquine for another indication in the previous 6 months.
2.2. Study Design
This was a phase Ib, open-label, dose-escalation study to assess the safety, dose-limiting toxicities (DLTs), maximum tolerated dose (MTD) and the pharmacokinetic interactions of the combination of metformin and chloroquine in patients with IDH1/2-mutated chondrosarcoma, glioma and intrahepatic cholangiocarcinoma. The study was conducted at the Amsterdam University Medical Centers in The Netherlands. The study was executed in a standard 3 + 3 dose-escalation fashion: at least three patients per dose level were recruited, and the dose level was to be expanded to six patients when 1/3 patients experienced a DLT. Dose escalation to the next dose level was permitted when a DLT occurred in 0/3 or in ≤1/6 patients. In case of DLT(s) in ≥1/3 or in ≥2/6 patients, that dose level was declared intolerable. A DLT was defined as any of the following events related to study treatment occurred during the first treatment cycle, as defined by National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 (CTCAE): neutropenia grade 4 or febrile neutropenia grade 3 (fever ≥38.5 °C), grade ≥4 trombocytopenia or grade 3 trombocytopenia with bleeding, rash grade ≥2, diarrhea grade ≥3 or any other treatment-related toxicity grade ≥3, and missing >7 days of treatment for toxicity reasons, all despite optimal supportive care.
2.3. Study Treatment
To enable pharmacokinetic analyses between metformin and chloroquine, patients were treated with single-agent metformin in the first week before chloroquine was added to their treatment. In week 1, 500 mg of metformin was given once a day during the first 5 days. Subsequently, the metformin dose was escalated as outlined in Table 1. This escalation schedule is based on an earlier phase II clinical trial in pancreatic cancer [27]. The purpose of the lower metformin starting dose was to reduce gastrointestinal side effects of metformin and mimics recommended dosage schedules of metformin treatment in patients with T2DM. Treatment with chloroquine started on day 8. The chloroquine dose was fixed and was not escalated during the study.
Table 1.
Dose-escalation schedule for metformin and chloroquine.
| Dose Level | Dose of Metformin (Total Daily Dose) |
Dose of Chloroquine (Total Daily Dose) |
|---|---|---|
| −1 | 500 mg once a day (500 mg total) |
200 mg once a day |
| 1 | 500 mg two times a day (1000 mg total) |
200 mg once a day |
| 2 | 1000 mg two times a day (2000 mg total) |
200 mg once a day |
| 3 | 1500 mg two times a day (3000 mg total) |
200 mg once a day |
2.4. Recommended Phase II Dose
The MTD is the dose at which ≥2/3 or ≥2/6 patients experienced a DLT. One dose level below the MTD, or dose level 3 in case of 0/6 DLTs at that final dose level, was considered the recommended dose (RD) for follow-up phase II clinical trials. Three patients were observed for 4 weeks at a dose level before buildup to the next dose level started. When a patient was withdrawn from the study prior to completing 28 days of therapy without experiencing a DLT, an additional patient was added to that dose level. Patients missing 7 or more doses due to toxicity were not replaced since these patients were considered to have experienced a DLT.
2.5. Pharmacokinetics
Pharmacokinetics and pharmacokinetic interactions between metformin and chloroquine were monitored and assessed in order to evaluate a relationship between drug exposure, toxicity and/or efficacy. Predose plasma levels were determined in blood samples obtained prior to study medication ingestion on day 8 (week 2), day 29 (week 5) and at the end of the study. Since chloroquine administration started on day 8, plasma samples on that day contained a metformin plasma concentration that reflected metformin monotherapy. The pharmacokinetic interactions between metformin and chloroquine were evaluated by comparison of the metformin concentration on day 8 with the metformin concentration on subsequent time points. The relationship between exposure and toxicity was evaluated in all samples.
2.6. Detection of D-2HG Levels
Previous studies demonstrated that circulating total 2HG was a surrogate biomarker for an IDH1/2-mutation status. In order to distinguish D-2HG (which is more specific for IDH1/2 mutations) from the less specific L-2HG, we detected D-2HG and L-2HG levels in patient serum using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Our method uses a chiral derivatizing agent, (+)-di-O-acetyl-l-tartaric anhydride (DATAN), to modify the D and L-stereoisomers of 2HG, allowing separation and quantification by LC-MS/MS [41,42,43]. The D-2HG and L-2HG levels are shown as the 2HG enantiomeric ratio [41].
2.7. Therapy Response
Response was assessed using Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 guidelines [44] for chondrosarcoma and cholangiocarcinoma or Response Assessment in Neuro-Oncology (RANO) guidelines [45] for glioma on images obtained with CT or MRI scans. Scans were performed at screening and every 8 weeks after study inclusion.
2.8. ctDNA Analysis with the Digital Droplet Polymerase Chain Reaction
To quantify the variants in the ctDNA isolated from plasma, digital droplet polymerase chain reaction (ddPCR) assays with 20× primers with FAM- and HEX-labelled hydrolysis probes were used according to the manufacturer’s protocol. The ddPCR supermix for probes (no dUTP) and the assay were mixed in 20 μL with 20 ng ctDNA in a semi-skirted ddPCR 96-well plate. The droplets were generated using the QX200 droplet generator. The 96-well plate containing the droplets was sealed with pierceable heat seal and placed in the T100 Thermal Cycler. The PCR program was started with initial denaturation for 10 min at 95 °C followed by 40 cycles: 10 s at 94 °C and 30 s at 55 °C. The PCR program was ended by cooling down to 4 °C overnight. Positive and negative droplets were measured using a QX200 Droplet Reader (all reagents and machines for these measurements were purchased from Bio-Rad Laboratories, Veenendaal, The Netherlands).
2.9. Statistical Analysis
The occurrence of adverse events and clinical outcomes were described non-quantitatively. p Values were calculated as described in the figure legends with a significance level cutoff of α = 0.05. Significance levels are shown by * (p < 0.05), ** (p < 0.01), *** (p < 0.001) and **** (p < 0.0001). Data were processed in Excel version 2016 for Windows (Microsoft, Redmond, WA, USA) and using GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Characterization of the Study Cohort
The flowchart in Figure 1 shows that 38 patients with eligible tumor histologies were pre-screened for the presence of an IDH1/2 mutation between November 2015 and May 2019. In total, 32 cholangiocarcinoma, 3 glioma and 3 chondrosarcoma patients underwent the pre-screening. Twenty patients had an IDH1 mutation, including 14 cholangiocarcinoma, 3 glioma and 3 chondrosarcoma patients. Of these patients, 3 patients did not meet the study inclusion criteria; one patient with cholangiocarcinoma because of ongoing use of metformin for the treatment of T2DM, one patient with glioma because of participation in another clinical trial, and one cholangiocarcinoma patient because of unsolved hyperbilirubinemia (Figure 1). The high rate of 53% of the patients with an IDH1 mutation in the pre-screened group is an exaggeration of the true prevalence of the IDH1 mutation, since patients with IDH1/2 mutation were referred to enroll in the study. The most frequent IDH1 mutations found were R132C (50%), R132H (21%) and R132G (14%). We identified no patients with an IDH2 mutation. Table 2 summarizes the baseline characteristics of the 17 eligible patients that were enrolled in the study. All patients except one had received one or more lines of systemic treatment prior to study enrolment.
Figure 1.
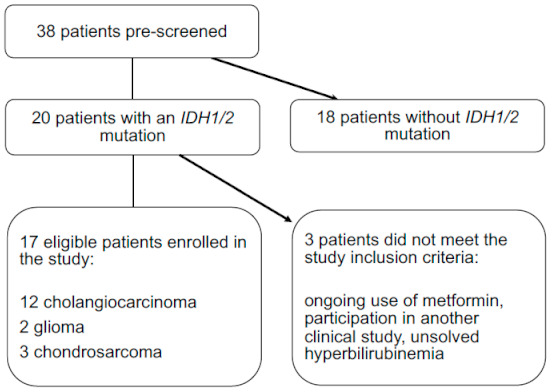
Flowchart showing that 38 patients were pre-screened for the presence of an IDH1/2 mutation. Twenty patients had an IDH1 mutation and 17 patients met the study inclusion criteria.
Table 2.
Patient demographics and disease characteristics. Chemotherapy regimens were given in an advanced setting, unless stated otherwise.
| Pt # | Gender | Age | WHO-PS | Primary Diagnosis | Earlier Surgery | Earlier Systemic Therapy | Earlier Radiotherapy | Time Since Initial Diagnosis |
|---|---|---|---|---|---|---|---|---|
| (in Years) | ||||||||
| 1 | Male | 54 | 0 | Chondrosarcoma | Resection of tumor of the right knee | None | No | 5.6 |
| 2 | Male | 49 | 1 | Cholangiocarcinoma | Right hemihepatectomy | Gemcitabine/cisplatin | No (during study) | 3.92 |
| 3 | Male | 51 | 2 | Chondrosarcoma | Resection of left pelvic tumor | Sirolimus/cyclophosphamide | Yes | 3.25 |
| 4 | Male | 64 | 0 | Cholangiocarcinoma | Exploratory laparotomy | Gemcitabine/cisplatin | No | 3.3 |
| 5 | Male | 58 | 1 | Cholangiocarcinoma | Right hemihepatectomy | Gemcitabine/cisplatin | No | 3.72 |
| 6 | Male | 57 | 1 | Cholangiocarcinoma | None | Gemcitabine/cisplatin | No | 2.84 |
| 7 | Male | 82 | 2 | Cholangiocarcinoma | None | Gemcitabine/cisplatin, pembrolizumab | Yes | 3.75 |
| 8 | Female | 53 | 1 | Cholangiocarcinoma | None | Gemcitabine/cisplatin | No | 2.33 |
| 9 | Male | 70 | 0 | Cholangiocarcinoma | Right hemihepatectomy | Gemcitabine/cisplatin, capecitabine/oxaliplatin | No | 2.58 |
| 10 | Male | 39 | 0 | Cholangiocarcinoma | Right hemihepatectomy | Gemcitabine/cisplatin, folfirinox | No | 3.82 |
| 11 | Male | 50 | 0 | Cholangiocarcinoma | None | Gemcitabine/cisplatin, gemcitabine/oxaliplatin | No | 3.25 |
| 12 | Male | 39 | 0 | Chondrosarcoma | Resection of right scapular tumor | Sirolimus/cyclophosphamide | No | 2.96 |
| 13 | Female | 42 | 0 | Cholangiocarcinoma | None | Gemcitabine/cisplatin | Yes | 1.53 |
| 14 | Male | 46 | 1 | Glioma | Tumor resection | Temozolomide | Yes | 6.43 |
| 15 | Male | 34 | 1 | Glioma | Tumor resection right frontal | Temozolomide, lomustine | Yes | 5.33 |
| 16 | Female | 63 | 0 | Cholangiocarcinoma | Right hemihepatectomy | Gemcitabine/cisplatin | No | 2.50 |
| 17 | Male | 64 | 0 | Cholangiocarcinoma | None | Gemcitabine/cisplatin, CAPOX | No | 1.50 |
Abbreviations: Pt #, patient number; WHO-PS, World Health Organization- Performance Status; CAPOX, Capecitabine/Oxaliplatin.
3.2. Safety and Dose Adjustments
Seventeen patients started the study treatment (Figure 1). Twelve patients received at least 4 weeks of study treatment and were thus evaluable for toxicity assessments (Table 3). Patients remained in the study for a median duration of 43 days (range: 7–74 days). Five patients discontinued study participation during the first 4 weeks of treatment, in 4 cases because of clinical progression and in one case due to toxicity. Of the 12 evaluable patients who received metformin and chloroquine, 10 patients discontinued because of progressive disease and 2 patients due to toxicity. None of the patients experienced a DLT. The treatment-related adverse events per dose level are listed in Table 4. All observed treatment-related adverse events were CTCAE-grade ≤2 toxicities. The most frequently reported clinical toxicities of any grade included nausea (28%), anorexia (23%), fatigue (16%), diarrhea (13%) and vomiting (10%). In two patients, the metformin dose was de-escalated from 2000 mg per day to 1000 mg per day; in one due to toxicity without meeting DLT criteria; and in one due to abdominal pain, which was related to progressive disease. One patient with back pain due to a bone metastasis underwent local palliative radiotherapy during study participation. Regarding serious adverse events (SAE), one patient with glioma had to be hospitalized due to hydrocephalus and one patient with cholangiocarcinoma had to be hospitalized due to cholangitis (treated with antibiotics, no intervention). Both SAEs were considered to be unrelated to the study medication. The MTD was determined as 1500 mg metformin two times a day and 200 mg chloroquine once a day. The study protocol specified that this highest dose level was the MTD as well as the RD. According to protocol, we expanded this dose level to six patients. Because all patients showed clinical or radiological progression after eight weeks of study treatment, we considered it unethical to enroll three additional patients at this dose level. We determined the RD for future clinical trials with this combination to be 1500 mg metformin two times a day and 200 mg chloroquine once a day.
Table 3.
Description of administered doses, dose-limiting toxicities and serious adverse events.
| Pt # | Metformin Dose (mg) | Chloroquine Dose (mg) | DLT (Grade) | SAE (Grade) | Days on Study | Reason for Study Termination | Overall Survival |
|---|---|---|---|---|---|---|---|
| (Days after Start of Study) | |||||||
| 1 | 1000 | 200 | - | - | 61 | Progressive disease (CT) | 818 |
| 2 | 1000 | 200 | - | - | 33 | Patient decision (toxicity) | 66 |
| 3 | 1000 | - | - | - | 7 | Progressive disease (clinical) | 29 |
| 4 | 1000 | 200 | - | - | 56 | Progressive disease | 426 |
| 5 | 2000 | 200 | - | - | 33 | Progressive disease (clinical and CT) | 951 |
| 6 | 2000 | 200 | - | - | 43 | Patient decision (toxicity) | 351 |
| 7 | 1000 | 200 | - | - | 14 | Progressive disease (clinical) | 680 |
| 8 | 2000 | 200 | - | - | 17 | Patient decision (toxicity) | 322 |
| 9 | 2000 | 200 | - | - | 62 | Progressive disease (CT) | 194 |
| 10 | 2000 | 200 | - | - | 58 | Progressive disease (CT) | 108 |
| 11 | 2000 | 200 | - | - | 67 | Progressive disease (CT) | 323 |
| 12 | 3000 | 200 | - | - | 59 | Progressive disease (CT) | 330 |
| 13 | 2000 | 200 | - | - | 59 | Progressive disease (CT) | 255 |
| 14 | 3000 | 200 | - | Hydrocephalus (4) | 28 | Progressive disease (clinical) | 154 |
| 15 | 3000 | - | - | - | 13 | Progressive disease (clinical) | 42 |
| 16 | 3000 | 200 | - | Bile duct stenosis (3) | 43 | Progressive disease (CT) | 92 |
| 17 | 3000 | 200 | - | - | 74 | Progressive disease (CT) | 102 |
Table 4.
Possible, probable or definitive treatment-related adverse event.
| Dose Level 1 | Dose Level 2 | Dose Level 3 | ||||
|---|---|---|---|---|---|---|
| Number of Patients: | n = 3 | n = 6 | n = 3 | |||
| CTCAE Grade: | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 |
| Fatigue | 1 | 3 | 2 | |||
| Anorexia | 1 | 6 | 2 | |||
| Nausea | 3 | 6 | 2 | |||
| Vomiting | 2 | 2 | ||||
| Diarrhea | 4 | 1 | ||||
| Constipation | 1 | 1 | ||||
| Weight loss | 1 | |||||
| Abdominal pain | 1 | 1 | ||||
Numbers represent number of patients. Abbreviation: CTCAE, Common Terminology Criteria for Adverse Events version 4.0.
3.3. Pharmacokinetics
Blood concentrations of metformin and chloroquine were obtained from all evaluable patients and are shown in Figure 2. We observed a dose-level dependent increase of the plasma metformin concentration; 0.86 ± 0.32 mg/L, 1.86 ± 0.24 mg/L and 2.38 ± 0.38 mg/L, after administration of dose levels 1, 2 and 3, respectively (p = 0.0015). As expected with non-escalating chloroquine doses, whole-blood chloroquine concentrations were stable at subsequent time points with 520.7 ± 306.3 μg/L at week 4 and 462.5 ± 194.5 μg/L at week 8. The plasma metformin concentration was comparable between single-agent administration and co-administration with chloroquine (Supplementary Materials Figure S1).
Figure 2.
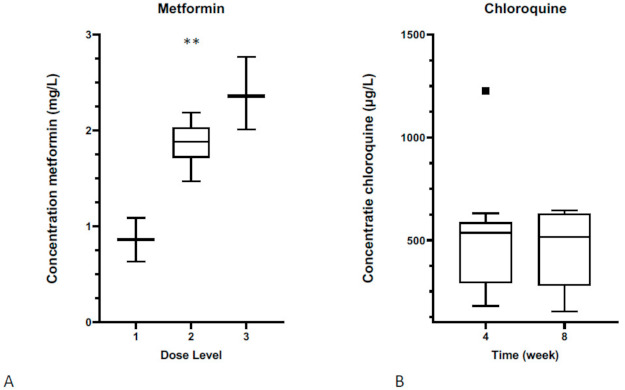
Serum concentrations of metformin and chloroquine. (A) A dose level-dependent increase of the plasma metformin concentration is shown; (** p < 0.01, one-way ANOVA test). (B) Non-escalating chloroquine doses in time, resulting in stable plasma concentrations at subsequent time points.
3.4. Plasma D-2HG Concentrations
Previous studies demonstrated that circulating total 2HG was a surrogate biomarker for an IDH1/2 mutation status. In order to distinguish D-2HG (which is more specific for IDH1/2 mutations) from the less specific L-2HG, we calculated the D/L-2HG ratio in serum to determine a cutoff marker value for the presence of an IDH1 mutation [41]. Analyzing the metabolite concentration in a subset of the screened 38 patients, the median value of total 2HG and D-2HG serum concentration was 5.6 ± 1.3 µmol/L and 5.3 ± 1.2 µmol/L in patients with an IDH1-mutated tumor and 1.0 ± 0.2 µmol/L and 0.6 ± 0.1 µmol/L in patients with an IDH1 wild-type tumor, respectively (p = 0.0008 and p = 0.0006; Figure 3). The D/L-2HG ratio was significantly higher in patients with an IDH1-mutated tumor compared to patients with an IDH1 wild-type tumor; 20.6 (95% confidence interval [CI] 8.6–32.5) versus 1.83 (95% CI 1.4–2.2; p < 0.0001; Figure 4). As illustrated in Figure 4, the optimal cutoff value of the D/L-2HG ratio was 4.5 for the presence of an IDH1 mutation. This cutoff value predicted the presence of an IDH1 mutation with a sensitivity of 90% and a specificity of 100%. Two patients with an IDH1-mutated tumor had a D/L-2HG ratio lower than 4.5.
Figure 3.
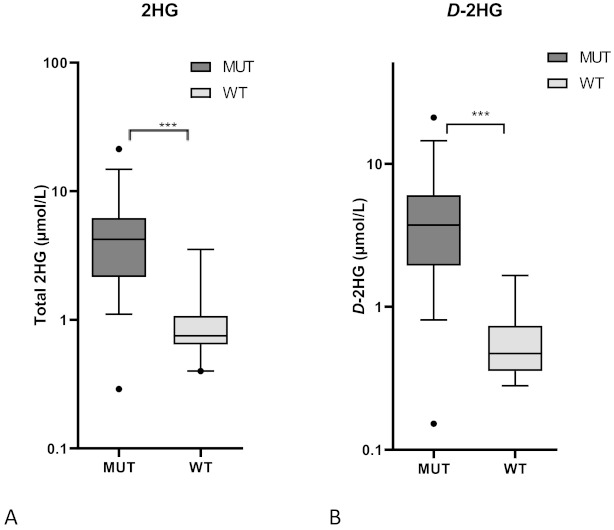
Serum concentrations of (A) total 2HG and (B) D-2HG was 5.6 ± 1.3 µmol/L and 5.3 ± 1.2 µmol/L in patients with an IDH1-mutated tumor and 1.0 ± 0.2 µmol/L and 0.6 ± 0.1 µmol/L in patients with an IDH1 wild-type tumor, respectively. (*** p < 0.001, two-way Mann–Whitney test).
Figure 4.
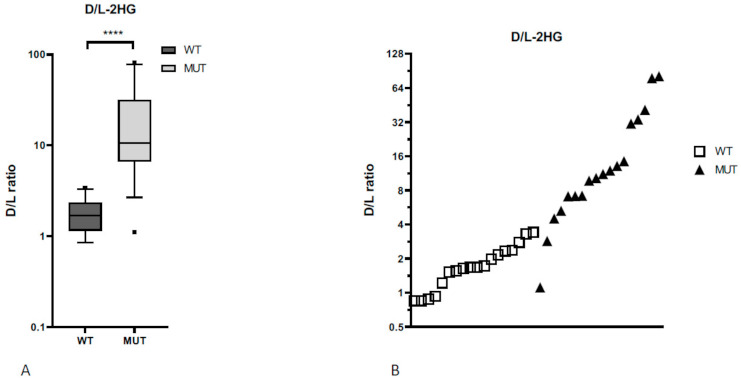
The D/L-2HG ratio (A) was significantly higher in patients with an IDH1 mutation compared to patients without an IDH1 mutation (**** p < 0.0001, two-way Mann–Whitney test). (B) The optimal cutoff value of 4.5 for the presence of an IDH1 mutation is shown.
3.5. ctDNA
In order to detect IDH1/2 mutation status in serum, we investigated serum on ctDNA. Since the most prevalent hotspot mutations in IDH1 are IDH1R132H and IDH1R132C, we used these mutations to design digital droplet PCR. Serum of 3 random patients were investigated to detect ctDNA of these IDH1 mutations. In all the samples, we successfully detected the IDH1 mutations (Figure 5).
Figure 5.
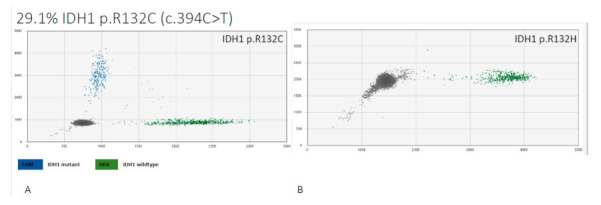
Serum analysis of ctDNA by NGS to detect hotspots mutations IDH1R132C and IDH1R132H. Analysis of one patient sample (A) with blue spots indicating detection of IDH1R132C ctDNA that consists of 29% ctDNA, (B) IDH1R132H was not detected.
3.6. Tumor Responses
At first radiological evaluation after eight weeks of treatment, all patients had progressive disease and discontinued study treatment (Table 3). Since D-2HG serves as a surrogate biomarker of progression, we monitored serial D-2HG serum concentrations in order to investigate possible biochemical treatment responses. As illustrated in Figure 6, patients treated with dose level 1 and 2 had an increasing D-2HG serum concentration over time (not significant, p = 0.1 and p = 0.23, respectively). However, patients treated with dose level 3 had a stable D-2HG serum concentration (no significant difference between doses, p = 0.1) but progressive disease at radiological evaluation.
Figure 6.
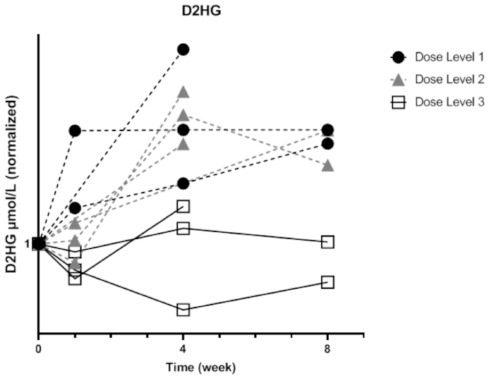
Serial D-2HG serum concentrations of patients treated with dose levels 1, 2 and 3. D-2HG serum concentrations were increased with time in dose levels 1 and 2 (not significant, respectively p = 0.1 and p = 0.23, Kruskal–Wallis test), whereas with dose level 3, stable or lower D-2HG serum concentrations were found (Not significant, p = 0.1, one-way ANOVA test).
4. Discussion
This is the first clinical trial to describe the toxicity profile, safety and pharmacokinetics of the combination of metformin and chloroquine in patients with IDH1-mutated chondrosarcoma, glioma and intrahepatic cholangiocarcinoma. We found that the combination regimen of metformin and chloroquine is well tolerated, but the combination did not induce a clinical response in this patient population. On the other hand, our results confirm the clinical usefulness of D/L-2HG ratios in serum as a biomarker for the presence of an IDH1-mutated solid tumor and the ddPCR-facilitated detection of an IDH1 mutation in ctDNA from peripheral blood.
The rationale of using metformin and chloroquine in order to disrupt the metabolism of IDH1-mutated solid tumors and to inhibit tumor growth was not supported by our clinical data, since ten out of twelve patients showed tumor progression during study treatment. After we published the first randomized controlled trial studying metformin in pancreatic cancer with a survival endpoint [27], dozens of negative clinical trials with metformin in cancer have been published. So far, only two clinical trials have shown a benefit of metformin on progression-free survival in cancer, and they both concerned non-small cell lung cancer [46,47]. The mechanism and pathophysiological background of the sensitivity of metformin in especially non-small cell lung cancer are currently unknown. High intracellular metformin concentrations are needed in order to induce profound metabolic inhibition and this may be unattainable using oral administration. Since metformin failed to show any metabolic or anti-tumor effect in this trial, phenformin should be considered as an alternative, since phenformin is the lipophilic analog of metformin and may have a better intratumoral bioavailability.
In line with previous studies, this study confirms that 2HG serum concentrations serve as a surrogate biomarker of the presence of an IDH1 mutation. The prediction of the presence of an IDH1/2 mutation by 2HG measurement is well established in AML [16]. A study by Borger et al. of 18 patients with IDH1-mutated intrahepatic cholangiocarcinoma showed a sensitivity of 83% and a specificity of 90% at a cutoff of 2HG serum levels ≥1.15 mmol/L [48]. Our results confirmed this observation and since our method distinguishes the IDH1 mutation-specific D-2HG from the less specific L-2HG, we suggest that the D/L-2HG ratio performs even better in a more heterogeneous patient population, with a sensitivity of 90% and a specificity of 100% at cutoff of 4.5 for the presence of an IDH1 mutation. This is in accordance with a report by Delahousse et al. including 8 patients with IDH1-mutated intrahepatic cholangiocarcinoma and 9 patients with wild-type IDH1 intrahepatic cholangiocarcinoma, which proposed a D/L-2HG ratio cutoff of 4.9 [49]. With three independent studies supporting the use of D-2HG measurement as a pre-screening tool for IDH1 mutational status with high accuracy and precision, this technique fulfils the criteria for implementation in routine clinical use.
Since the presence of IDH1 mutations is relevant for prognosis and treatment, 2HG may serve as a surrogate marker of treatment efficacy. Levels of 2HG in serum and urine of patients with IDH1/2-mutated AML decreased throughout conventional therapy, concordant with a decrease in blast counts. However, the concentration of serum 2HG is substantially lower in patients with an IDH1-mutated solid tumors compared with patients with IDH1/2-mutated AML (5.6 µmol/L in our study versus 21.2 µmol/L in AML) [16]. We observed increasing D-2HG serum concentrations in low dose-level treatment, but a stable concentration of D-2HG in the highest dose-level of metformin in combination with chloroquine. Future studies are needed to determine how well D-2HG correlates with changes in tumor volume during treatment.
We were able to confirm the clinical utility of ctDNA in IDH1-mutated cancers as described earlier [50]. The use of plasma-derived ctDNA is a promising tool for treatment decision-making based on predictive testing, detection of resistance mechanisms, and monitoring tumor response. By utilization of digital droplet PCR, we were able to detect tumor-specific IDH1 hotspot mutations in ctDNA, which may facilitate the monitoring of tumor response during therapy. In addition, our data generate the hypothesis that the longitudinal evaluation of D/L-2HG ratios can be used to determine effects of anti-cancer treatment, although the stable D/L-2HG ratios in the highest dose level did not corroborate with clinical responses and the number of studied patients was small. Necessary steps for the translation of these minimally invasive measurements to clinical practice is subject for future research.
5. Conclusions
Results from this prospective, open-label, phase Ib study show that the combination of metformin and chloroquine has a favorable toxicity profile but no clinical activity in patients with IDH1-mutated chondrosarcoma, glioma and intrahepatic cholangiocarcinoma. In addition, our data confirm and support the use of D-2HG measurements as a screening tool for IDH1 mutational status in routine clinical use for these tumors. Although our analyses of tumor responses and overall survival are based on very small numbers and late-stage cancer patients, alternative combination regimens disrupting the metabolism in IDH1/2-mutated cancers should be investigated in future studies.
Abbreviations
| αKG | alpha-ketoglutarate |
| AML | acute myeloid leukemia |
| b.i.d. | bis in die, two times a day |
| CTCAE | common terminology criteria for adverse events |
| ctDNA | circulating tumor DNA |
| D-2HG | D-2-hydroxyglutarate |
| DLT | dose-limiting toxicity |
| ETC | electron transport chain |
| IDH1/2 | isocitrate dehydrogenase 1 or 2 |
| IDH1/2WT | IDH1/2 wild-type |
| IDH1/2MT | IDH1/2 mutant |
| MAD | maximum administered dose |
| MRS | magnetic resonance spectroscopy |
| MS | mass spectrometry |
| MTD | maximum tolerated dose |
| NGS | next-generation sequencing |
| q.d. | quaque die, one a day |
| RANO | response assessment in neuro-oncology |
| RD | recommended dose (for a phase II clinical trial) |
| RECIST | response evaluation criteria in solid tumors |
| SAE | serious adverse events |
| T2DM | type 2 diabetes mellitus |
| TCA cycle | tricarboxylic acid cycle |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13102474/s1, Figure S1: Serial metformin serum concentrations of patients in time.
Author Contributions
Conceptualization, R.J.M., C.J.F.v.N., J.V.M.G.B. and J.W.W.; methodology, M.K., R.J.M., J.V.M.G.B. and J.W.W.; formal analysis, M.K., R.A.M., T.v.W. and E.A.S.; patient care, M.K., R.J.M., M.E.v.L., H.-J.K. and J.W.W.; investigation, M.K., R.J.M., T.v.W. and J.W.W.; data curation, M.K. and T.v.W.; writing—original draft preparation, M.K.; writing—review and editing, M.K., R.J.M., C.J.F.v.N. and J.W.W.; supervision, J.W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Academic Medical Center. R.J.M. was supported by an AMC PhD Scholarship. M.K., R.J.M., C.J.F.v.N. and J.W.W. were supported by the Dutch Cancer Society (KWF grant 10460). C.J.F.v.N. is supported by the Slovenian Research Agency (research project J3-2526).
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. The study protocol was approved by the Medical Ethics Committee of the Academic Medical Center (reference number NL53150.018.15).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Molenaar R.J., Radivoyevitch T., Maciejewski J.P., van Noorden C.J., Bleeker F.E. The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation. Biochim. Biophys. Acta. 2014;1846:326–341. doi: 10.1016/j.bbcan.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Johnson B.E., Mazor T., Hong C., Barnes M., Aihara K., McLean C.Y., Fouse S.D., Yamamoto S., Ueda H., Tatsuno K., et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189–193. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molenaar R.J., Thota S., Nagata Y., Patel B., Clemente M., Przychodzen B., Hirsh C., Viny A.D., Hosano N., Bleeker F.E., et al. Clinical and biological implications of ancestral and non-ancestral IDH1 and IDH2 mutations in myeloid neoplasms. Leukemia. 2015;29:2134–2142. doi: 10.1038/leu.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molenaar R.J., Botman D., Smits M.A., Hira V.V., van Lith S.A., Stap J., Henneman P., Khurshed M., Lenting K., Mul A.N., et al. Radioprotection of IDH1-mutated cancer cells by the IDH1-mutant inhibitor AGI-5198. Cancer Res. 2015;75:4790–4802. doi: 10.1158/0008-5472.CAN-14-3603. [DOI] [PubMed] [Google Scholar]
- 5.Mohrenz I.V., Antonietti P., Pusch S., Capper D., Balss J., Voigt S., Weissert S., Mukrowsky A., Frank J., Senft C., et al. Isocitrate dehydrogenase 1 mutant R132H sensitizes glioma cells to BCNU-induced oxidative stress and cell death. Apoptosis. 2013;18:1416–1425. doi: 10.1007/s10495-013-0877-8. [DOI] [PubMed] [Google Scholar]
- 6.Khurshed M., Aarnoudse N., Hulsbos R., Hira V.V.V., van Laarhoven H.W.M., Wilmink J.W., Molenaar R.J., van Noorden C.J.F. IDH1-mutant cancer cells are sensitive to cisplatin and an IDH1-mutant inhibitor counteracts this sensitivity. FASEB J. 2018;32:6344–6352. doi: 10.1096/fj.201800547R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amatangelo M.D., Quek L., Shih A., Stein E.M., Roshal M., David M.D., Marteyn B., Farnoud N.R., de Botton S., Bernard O.A., et al. Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response. Blood. 2017;130:732–741. doi: 10.1182/blood-2017-04-779447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohle D., Popovici-Muller J., Palaskas N., Turcan S., Grommes C., Campos C., Tsoi J., Clark O., Oldrini B., Komisopoulou E., et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein E.M., DiNardo C.D., Pollyea D.A., Fathi A.T., Roboz G.J., Altman J.K., Stone R.M., DeAngelo D.J., Levine R.L., Flinn I.W., et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722–731. doi: 10.1182/blood-2017-04-779405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan H., Parsons D.W., Jin G., McLendon R., Rasheed B.A., Yuan W., Kos I., Batinic-Haberle I., Jones S., Riggins G.J., et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu W., Yang H., Liu Y., Yang Y., Wang P., Kim S.H., Ito S., Yang C., Wang P., Xiao M.T., et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang L., White D.W., Gross S., Bennett B.D., Bittinger M.A., Driggers E.M., Fantin V.R., Jang H.G., Jin S., Keenan M.C., et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury R., Yeoh K.K., Tian Y.M., Hillringhaus L., Bagg E.A., Rose N.R., Leung I.K., Li X.S., Woon E.C., Yang M., et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. Embo Rep. 2011;12:463–469. doi: 10.1038/embor.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khurshed M., Molenaar R.J., Lenting K., Leenders W.P., van Noorden C.J.F. In silico gene expression analysis reveals glycolysis and acetate anaplerosis in IDH1 wild-type glioma and lactate and glutamate anaplerosis in IDH1-mutated glioma. Oncotarget. 2017;8:49165–49177. doi: 10.18632/oncotarget.17106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Y.F., Li B.Z., Li Z., Liu P., Wang Y., Tang Q., Ding J., Jia Y., Chen Z., Li L., et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–1307. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janin M., Mylonas E., Saada V., Micol J.B., Renneville A., Quivoron C., Koscielny S., Scourzic L., Forget S., Pautas C., et al. Serum 2-hydroxyglutarate production in IDH1- and IDH2-mutated de novo acute myeloid leukemia: A study by the Acute Leukemia French Association Group. J. Clin. Oncol. 2014;32:297–305. doi: 10.1200/JCO.2013.50.2047. [DOI] [PubMed] [Google Scholar]
- 17.Wang F., Travins J., DeLaBarre B., Penard-Lacronique V., Schalm S., Hansen E., Straley K., Kernytsky A., Liu W., Gliser C., et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340:622–626. doi: 10.1126/science.1234769. [DOI] [PubMed] [Google Scholar]
- 18.Tap W.D., Villalobos V.M., Cote G.M., Burris H., Janku F., Mir O., Beeram M., Wagner A.J., Jiang L., Wu B., et al. Phase I study of the mutant IDH1 inhibitor Ivosidenib: Safety and clinical activity in patients with advanced chondrosarcoma. J. Clin. Oncol. 2020;38:1693–1701. doi: 10.1200/JCO.19.02492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 20.van Lith S.A., Navis A.C., Verrijp K., Niclou S.P., Bjerkvig R., Wesseling P., Tops B., Molenaar R., van Noorden C.J., Leenders W.P. Glutamate as chemotactic fuel for diffuse glioma cells: Are they glutamate suckers? Biochim. Biophys. Acta. 2014;1846:66–74. doi: 10.1016/j.bbcan.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Lenting K., Khurshed M., Peeters T.H., van den Heuvel C., van Lith S.A.M., de Bitter T., Hendriks W., Span P.N., Molenaar R.J., Botman D., et al. Isocitrate dehydrogenase 1-mutated human gliomas depend on lactate and glutamate to alleviate metabolic stress. FASEB J. 2019;33:557–571. doi: 10.1096/fj.201800907RR. [DOI] [PubMed] [Google Scholar]
- 22.Grassian A.R., Parker S.J., Davidson S.M., Divakaruni A.S., Green C.R., Zhang X., Slocum K.L., Pu M., Lin F., Vickers C., et al. IDH1 mutations alter citric acid cycle metabolism and increase dependence on oxidative mitochondrial metabolism. Cancer Res. 2014;74:3317–3331. doi: 10.1158/0008-5472.CAN-14-0772-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Lith S.A., Molenaar R., van Noorden C.J., Leenders W.P. Tumor cells in search for glutamate: An alternative explanation for increased invasiveness of IDH1 mutant gliomas. Neuro Oncol. 2014;16:1669–1670. doi: 10.1093/neuonc/nou152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khurshed M., Molenaar R.J., van Noorden C.J. A simple in silico approach to generate gene-expression profiles from subsets of cancer genomics data. Biotechniques. 2019;67:172–176. doi: 10.2144/btn-2018-0179. [DOI] [PubMed] [Google Scholar]
- 25.Cuyas E., Fernandez-Arroyo S., Corominas-Faja B., Rodriguez-Gallego E., Bosch-Barrera J., Martin-Castillo B., De Llorens R., Joven J., Menendez J.A. Oncometabolic mutation IDH1 R132H confers a metformin-hypersensitive phenotype. Oncotarget. 2015;6:12279–12296. doi: 10.18632/oncotarget.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterse E.F.P., Niessen B., Addie R.D., de Jong Y., Cleven A.H.G., Kruisselbrink A.B., van den Akker B., Molenaar R.J., Cleton-Jansen A.M., Bovee J. Targeting glutaminolysis in chondrosarcoma in context of the IDH1/2 mutation. Br. J. Cancer. 2018;118:1074–1083. doi: 10.1038/s41416-018-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kordes S., Pollak M.N., Zwinderman A.H., Mathot R.A., Weterman M.J., Beeker A., Punt C.J., Richel D.J., Wilmink J.W. Metformin in patients with advanced pancreatic cancer: A double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16:839–847. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z.J., Bi Y., Li S., Zhang Q., Zhao G., Guo Y., Song Q. Reduced risk of lung cancer with metformin therapy in diabetic patients: A systematic review and meta-analysis. Am. J. Epidemiol. 2014;180:11–14. doi: 10.1093/aje/kwu124. [DOI] [PubMed] [Google Scholar]
- 29.Bodmer M., Meier C., Krahenbuhl S., Jick S.S., Meier C.R. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33:1304–1308. doi: 10.2337/dc09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans J.M., Donnelly L.A., Emslie-Smith A.M., Alessi D.R., Morris A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadeghi N., Abbruzzese J.L., Yeung S.C., Hassan M., Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin. Cancer Res. 2012;18:2905–2912. doi: 10.1158/1078-0432.CCR-11-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin H.C., Kachingwe B.H., Lin H.L., Cheng H.W., Uang Y.S., Wang L.H. Effects of metformin dose on cancer risk reduction in patients with type 2 diabetes mellitus: A 6-year follow-up study. Pharmacotherapy. 2014;34:36–45. doi: 10.1002/phar.1334. [DOI] [PubMed] [Google Scholar]
- 33.Mazurek M., Litak J., Kamieniak P., Kulesza B., Jonak K., Baj J., Grochowski C. Metformin as potential therapy for high-grade glioma. Cancers (Basel) 2020;12:210. doi: 10.3390/cancers12010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin M., Zhou J., Gorak E.J., Quddus F. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: A systematic review and meta-analysis. Oncologist. 2013;18:1248–1255. doi: 10.1634/theoncologist.2013-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molenaar R.J., Maciejewski J.P., Wilmink J.W., van Noorden C.J.F. Wild-type and mutated IDH1/2 enzymes and therapy responses. Oncogene. 2018;37:1949–1960. doi: 10.1038/s41388-017-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi M.M., Kim E.A., Choi S.Y., Kim T.U., Cho S.W., Yang S.J. Inhibitory properties of nerve-specific human glutamate dehydrogenase isozyme by chloroquine. J. Biochem. Mol. Biol. 2007;40:1077–1082. doi: 10.5483/BMBRep.2007.40.6.1077. [DOI] [PubMed] [Google Scholar]
- 37.Jarzyna R., Kiersztan A., Lisowa O., Bryla J. The inhibition of gluconeogenesis by chloroquine contributes to its hypoglycaemic action. Eur. J. Pharmacol. 2001;428:381–388. doi: 10.1016/S0014-2999(01)01221-3. [DOI] [PubMed] [Google Scholar]
- 38.Jarzyna R., Lenarcik E., Bryla J. Chloroquine is a potent inhibitor of glutamate dehydrogenase in liver and kidney-cortex of rabbit. Pharmacol. Res. 1997;35:79–84. doi: 10.1006/phrs.1996.0108. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert M.R., Liu Y., Neltner J., Pu H., Morris A., Sunkara M., Pittman T., Kyprianou N., Horbinski C. Autophagy and oxidative stress in gliomas with IDH1 mutations. Acta Neuropathol. 2014;127:221–233. doi: 10.1007/s00401-013-1194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubinsztein D.C., Gestwicki J.E., Murphy L.O., Klionsky D.J. Potential therapeutic applications of autophagy. Nat. Rev. Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 41.Wiseman D.H., Struys E.A., Wilks D.P., Clark C.I., Dennis M.W., Jansen E.E., Salomons G.S., Somervaille T.C. Direct comparison of quantitative digital PCR and 2-hydroxyglutarate enantiomeric ratio for IDH mutant allele frequency assessment in myeloid malignancy. Leukemia. 2015;29:2421–2423. doi: 10.1038/leu.2015.151. [DOI] [PubMed] [Google Scholar]
- 42.Struys E.A., Jansen E.E., Verhoeven N.M., Jakobs C. Measurement of urinary D- and L-2-hydroxyglutarate enantiomers by stable-isotope-dilution liquid chromatography-tandem mass spectrometry after derivatization with diacetyl-L-tartaric anhydride. Clin. Chem. 2004;50:1391–1395. doi: 10.1373/clinchem.2004.033399. [DOI] [PubMed] [Google Scholar]
- 43.Jones P.M., Boriack R., Struys E.A., Rakheja D. Measurement of oncometabolites D-2-hydroxyglutaric acid and L-2-hydroxyglutaric acid. Methods Mol. Biol. 2017;1633:219–234. doi: 10.1007/978-1-4939-7142-8_14. [DOI] [PubMed] [Google Scholar]
- 44.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 45.Wen P.Y., Macdonald D.R., Reardon D.A., Cloughesy T.F., Sorensen A.G., Galanis E., Degroot J., Wick W., Gilbert M.R., Lassman A.B., et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 46.Arrieta O., Barron F., Padilla M.S., Aviles-Salas A., Ramirez-Tirado L.A., Arguelles Jimenez M.J., Vergara E., Zatarain-Barron Z.L., Hernandez-Pedro N., Cardona A.F., et al. Effect of metformin plus tyrosine kinase inhibitors compared with tyrosine kinase inhibitors alone in patients with epidermal growth factor receptor-mutated lung adenocarcinoma: A phase 2 randomized clinical trial. JAMA Oncol. 2019:e192553. doi: 10.1001/jamaoncol.2019.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marrone K.A., Zhou X., Forde P.M., Purtell M., Brahmer J.R., Hann C.L., Kelly R.J., Coleman B., Gabrielson E., Rosner G.L., et al. A Randomized phase II study of metformin plus paclitaxel/carboplatin/bevacizumab in patients with chemotherapy-naive advanced or metastatic nonsquamous non-small cell lung cancer. Oncologist. 2018;23:859–865. doi: 10.1634/theoncologist.2017-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borger D.R., Goyal L., Yau T., Poon R.T., Ancukiewicz M., Deshpande V., Christiani D.C., Liebman H.M., Yang H., Kim H., et al. Circulating oncometabolite 2-hydroxyglutarate is a potential surrogate biomarker in patients with isocitrate dehydrogenase-mutant intrahepatic cholangiocarcinoma. Clin. Cancer Res. 2014;20:1884–1890. doi: 10.1158/1078-0432.CCR-13-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delahousse J., Verlingue L., Broutin S., Legoupil C., Touat M., Doucet L., Ammari S., Lacroix L., Ducreux M., Scoazec J.Y., et al. Circulating oncometabolite D-2-hydroxyglutarate enantiomer is a surrogate marker of isocitrate dehydrogenase-mutated intrahepatic cholangiocarcinomas. Eur. J. Cancer. 2018;90:83–91. doi: 10.1016/j.ejca.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 50.Gutteridge A., Rathbone V.M., Gibbons R., Bi M., Archard N., Davies K.E.J., Brown J., Plagnol V., Pillay N., Amary F., et al. Digital PCR analysis of circulating tumor DNA: A biomarker for chondrosarcoma diagnosis, prognostication, and residual disease detection. Cancer Med. 2017;6:2194–2202. doi: 10.1002/cam4.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.


