Abstract
Plants from the Chrysanthemum genus are rich sources of chemical diversity and, in recent years, have been the focus of research on natural products chemistry. Sesquiterpenoids are one of the major classes of chemical constituents reported from this genus. To date, more than 135 sesquiterpenoids have been isolated and identified from the whole genus. These include 26 germacrane-type, 26 eudesmane-type, 64 guaianolide-type, 4 bisabolane-type, and 15 other-type sesquiterpenoids. Pharmacological studies have proven the biological potential of sesquiterpenoids isolated from Chrysanthemum species, reporting anti-inflammatory, antibacterial, antitumor, insecticidal, and antiviral activities for these interesting molecules. In this paper, we provide information on the chemistry and bioactivity of sesquiterpenoids obtained from the Chrysanthemum genus which could be used as the scientific basis for their future development and utilization.
Keywords: Chrysanthemum genus, sesquiterpenoids, biosynthetic pathway, pharmacological effects
1. Introduction
The Chrysanthemum genus belongs to the family Compostae and is comprised of more than 40 accepted species of flowering plants (http://www.theplantlist.org/ accessed on 22 March 2021), which are perennial herbs mainly distributed in East Asia; more than 20 species are found in China [1,2]. The flowers of several Chrysanthemum species, including ornamental cultivars and hybrids are economically the second most important floricultural crop after rose, and one of the most important sources of flower arrangement and potted plants around the world [3,4]. In addition, the flower is regularly consumed in many countries as both food and medicine. Traditionally, in China and Japan, some Chrysanthemum species, such as Chrysanthemum morifolium Ramat. (Juhua in Chinese) and Chrysanthemum indicum L. (Yejuhua in Chinese), are used as sedative, anti-inflammatory, antitussive, and a general tonic. C. morifolium and C. indicum are slightly cold in effect with a sweet and bitter taste. Chinese Pharmacopoeia (2015 edition) records their good effects on liver and heart. They are effective in reducing heat and detoxification, treating swollen furuncle carbuncle, swelling and eye pain, headache, and dizziness [5,6]. Modern pharmacological studies have shown that the Chrysanthemum genus has antitumor, antioxidant, anti-inflammatory and antibacterial properties. The species have been found effective against cardiovascular diseases and for reducing fat and cholesterol contents in the blood [7,8,9,10]. With the traditional Chinese Medicine Fufang, C. morifolium granule, C. indicum granule, and C. indicum injections are used to treat prostatitis, chronic pelvic inflammation, and upper respiratory tract infection [6]. In addition to sesquiterpenoids, the Chrysanthemum genus is also known to be a rich source of terpenoids, phenylpropanoids, and flavonoids [11,12,13].
Sesquiterpenoids are mainly found in essential oils, detected by GC-MS analysis [14,15]. In fact, most of these compounds have been purified from aqueous ethanolic extract of Chrysanthemum plants through chromatography on different adsorbents such as silica gel, C18 silica (reversed-phase column), Sephadex LH-20, and through semi-preparative HPLC. All the sesquiterpenoids have been isolated from air-dried flowers or aerial parts of the Chrysanthemum genus, except angeloylcumambrin B (53), cumambrin A (54), cumambrin-B (55), and handelin (104) which were obtained from fresh whole herbs (Table 1). C. morifolium, C. indicum, C lavandulifolium, C. zawadskii, and C. ornatum have been reported to be rich in sesquiterpenoids, especially the C. morifolium and C. indicum species [16,17,18,19]. To the best of our knowledge, there is not a single comprehensive review on the sesquiterpenoids of the Chrysanthemum genus. This review article is an attempt to summarize the sesquiterpenoids of the Chrysanthemum genus, highlight their possible biosynthetic pathway and pharmacological effects, and provide a rationale for future development and research on this important genus.
Table 1.
Sesquiterpenoids from the Chrysanthemum genus.
| No. | Compounds | Species | Parts Used | Identification Methods | Ref. |
|---|---|---|---|---|---|
| Germacrane | |||||
| 1 | 1β,3α,5β-trihydroxyl-7-isopropenyl-germacren-4(15),10(14)-diene | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; X-ray | [16] |
| 2 | 1β,3β,5α-trihydroxyl-7-isopropenyl-germacren-4(15),10(14)-diene | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [16] |
| 3 | 1β,3β,5β-trihydroxyl-7-isopropenyl-germacren-4(15),10(14)-diene | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [16] |
| 4 | chrysanthemumin C | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [21] |
| 5 | chrysanthemumin D | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [21] |
| 6 | chrysanthediacetate B | C. morifolium | Flowers | 1D, 2D NMR; EIMS | [17] |
| 7 | chrysanthediacetate C | C. morifolium | Flowers | 1D, 2D NMR; EIMS | [17] |
| 8 | (3R,7R,9R)-3,9-dihydroxygermacra-4(15),10(14),11(12)-triene | C. morifolium | Flowers | 1D, 2D NMR; ESIMS | [22] |
| 9 | chrysanthediol A | C. morifolium | Flowers | 1D, 2D NMR; EIMS | [17] |
| 10 | kikkanol D | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [23] |
| 11 | kikkanol D monoacetate | C. indicum | Flowers | 1D, 2D NMR; HRFABMS | [23] |
| 12 | kikkanol E | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; Mosher | [23] |
| 13 | 1β-hydroxy-4(15),5E,10(14)-germacratriene | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 14 | chrysanthemumin I | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 15 | chrysandiol | C. morifolium | [24] | ||
| 16 | chrysanthemumin H | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; X-ray; ECD | [11] |
| 17 | 1β,3β-dihydroxygermacra-4Z,10(14)-dien-6β,7α,11αH-12,6-olide | C lavandulifolium | Aerial parts | 1D, 2D NMR; EIMS | [18] |
| 18 | 1β-hydroperoxy-3β-hydroxygermacra-4Z,10(14)-dien-6β,7α,11αH-12,6-olide | C lavandulifolium | Aerial parts | 1D, 2D NMR; EIMS | [18] |
| 19 | zawadskinolide D | C. zawadskii | Aerial parts | 1D, 2D NMR; HRFABMS | [19] |
| 20 | zawadskinolide E | C. zawadskii | Aerial parts | 1D, 2D NMR; HRCIMS | [19] |
| 21 | zawadskinolide F | C. zawadskii | Aerial parts | 1D, 2D NMR; HRCIMS | [19] |
| 22 | zawadskinolide A | C. zawadskii | Aerial parts | 1D, 2D NMR; HREIMS | [19] |
| 23 | zawadskinolide B | C. zawadskii | Aerial parts | 1D, 2D NMR; HREIMS | [19] |
| 24 | zawadskinolide C | C. zawadskii | Aerial parts | 1D, 2D NMR; HREIMS | [19] |
| 25 | kikkanol F | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [23] |
| 26 | kikkanol F monoacetate | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [23] |
| Eudesmane | |||||
| 27 | chrysanthemumol I | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [21] |
| 28 | chrysanthemumol J | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [21] |
| 29 | eudesm-4(14)-ene-3α,11-diol | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [21] |
| 30 | (3β,5α,6β,7β,14β)-eudesmen-3,5,6,11-tetrol | C. indicum | Flowers | 1D NMR; X-ray | [25] |
| 31 | chrysanthemumin A | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [11] |
| 32 | 5α-hydroxy-β-eudesmol | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 33 | chrysanthemumin D | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [11] |
| 34 | chrysanthemumin E | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [11] |
| 35 | chrysanthemumin B | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [11] |
| 36 | 7-epi-1β-hydroxy-β-eudesmol | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [26] |
| 37 | chrysanthemol | C. indicum | Flowers | 1D, 2D NMR; HREIMS | [27] |
| 38 | chrysanthemumin F | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; ECD | [11] |
| 39 | ligucyperonol | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [11] |
| 40 | chrysanthemumol K | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [21] |
| 41 | canusesnol E | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [21] |
| 42 | chrysanthemumin C | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [11] |
| 43 | β-dictyopterol | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [17] |
| 44 | 7-epi-eudesm-4(15),11(13)-diene-1β,3β-diol | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [26] |
| 45 | intermedeol | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 46 | cyperusol C | C. morifolium | Flowers | 1D, 2D NMR; ESIMS | [22] |
| 47 | chrysantiloboside | C. zawadskii | Aerial parts | 1D, 2D NMR; FABMS | [19] |
| 48 | oplodiol 1-O-β-D-glucopyranoside | C. zawadskii | Aerial parts | 1D, 2D NMR; HREIMS | [19] |
| 49 | kikkanol C | C. indicum | Flowers | 1D, 2D NMR; HREIMS; Mosher | [28] |
| 50 | kikkanol B | C. indicum | Flowers | 1D, 2D NMR; HREIMS; Mosher | [28] |
| 51 | kikkanol A | C. indicum | Flowers | 1D, 2D NMR; HREIMS; Mosher | [28] |
| 52 | eudesm-4(15)-ene-1β,6α-diol | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| Guaianolide | |||||
| 53 | angeloylcumambrin B |
C. ornatum
C. indicum C. zawadskii |
Whole herbs Flowers Flowers |
1D, 2D NMR; ESIMS 1D, 2D NMR; ESIMS 1D, 2D NMR; ESIMS |
[29] [11] [30] |
| 54 | cumambrin A |
C.
ornatum
C. indicum C. zawadskii |
Whole herbs Flowers Flowers |
1D, 2D NMR; ESIMS 1D, 2D NMR; ESIMS 1D, 2D NMR; ESIMS |
[29] [11] [30] |
| 55 | cumambrin-B | C. ornatum | Whole herbs | 1D, 2D NMR; ESIMS | [29] |
| 56 | chrysanolide G | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 57 | tigloylcumambrin B | C. indicum | Aerial parts | 1D NMR | [31] |
| 58 | chrysanolide F | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 59 | 8-tigloylchrysanolide F | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 60 | chrysanolide B | C. indicum | Aerial parts | 1D NMR | [31] |
| 61 | 10α-hydroxy-8α-O-(β-D-glucopyranosyl)-1αH,5αH, 6βH,8βH,7αH,11βH,11α-methylguaia-3-enolide |
C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [32] |
| 62 | chrysanthemumin J | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; ECD | [11] |
| 63 | chrysanolide H | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [31] |
| 64 | 8-angeloylchrysanolide H | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 65 | chrysanthguaianolactone D | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [33] |
| 66 | chrysanthguaianolactone C | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [33] |
| 67 | 3α,4α,10β-trihydroxy-8α-acetoxyguai-1,11(13)-dien-6α,12-olide | C. morifolium | Flowers | 1D NMR | [33] |
| 68 | 3α,4α,10β-trihydroxy-8α-acetoxy-11βH-guai-1-en-6α,12-olide | C. morifolium | Flowers | 1D NMR | [33] |
| 69 | 1α,3α,4β-trihydroxy-8α-acetoxy-9-en-6α,12-olide | C. morifolium | Flowers | 1D NMR | [33] |
| 70 | chrysanthguaianolactone E | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [33] |
| 71 | 8α-(angelyloxy)-3β,4β-dihydroxy-5αH,6βH,7αH, 11αH-guai-1(10)-en-12,6-olide |
C. morifolium | Flowers | 1D NMR | [33] |
| 72 | indicumolide A | C. indicum | Flowers | 1D, 2D NMR; HREIMS | [34] |
| 73 | indicumolide B | C. indicum | Flowers | 1D, 2D NMR; HREIMS | [34] |
| 74 | chrysanolide I | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [31] |
| 75 | 3β,4α-dihydroxy-8α-angelyloxy-1(10),11(13)-dien-6β,12-olide | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [35] |
| 76 | 11,13-dehydrodesacetylmatricarin | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 77 | matricarin | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 78 | 8β-angeloyloxy-1β,4β,10β-trihydroxy-guai-2-en-6α,12-olide | C. indicum | Aerial parts | 1D, 2D NMR; EIMS | [36] |
| 79 | chrysanthguaianolactone B | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [37] |
| 80 | (3α,6α,8α)-8-tigloyl-3,4-epoxyguai-1(10)-eno-12,6-lactone | C. indicum | Flowers | 1D NMR | [37] |
| 81 | Angeloylajad | C. indicum | Aerial parts | 1D NMR; EIMS | [38] |
| 82 | arteglasin A | C. indicum | Aerial parts | 1D NMR; EIMS | [38] |
| 83 | guaianolide ajadin | C. indicum | Aerial parts | 1D NMR; EIMS | [38] |
| 84 | apressin | C. indicum | Flowers | 1D NMR | [37] |
| 85 | athanadregeolid | C. indicum | Flowers | 1D NMR | [37] |
| 86 | chrysanthemulide H | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 87 | 8-tigloyldesacetylezomontanin | C. indicum | Aerial parts | 1D NMR | [39] |
| 88 | 10α-hydroxy-1α,4α-endoperoxy-guaia-2-en-12,6α-olide | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [40] |
| 89 | chrysanthemulide F | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 90 | chrysanthemulide G | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 91 | 10-epiajafinin | C. indicum | Aerial parts | 1D NMR | [39] |
| 92 | chrysanthemulide A | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 93 | chrysanthemulide B | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 94 | chrysanthemulide C | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 95 | chrysanthemulide D | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 96 | chrysanthemulide E | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 97 | chrysanthguaianolactone A | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [37] |
| 98 | isoseco-tanapartholide | C. indicum | Aerial parts | 1D, 2D NMR; ECD | [41] |
| 99 | (-)-9-angeloyloxy-seco-tanapartholide B | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [41] |
| 100 | (-)-seco-tanapartholide B | C. indicum | Aerial parts | 1D, 2D NMR; ECD | [41] |
| 101 | (-)-seco-tanapartholide A | C. indicum | Aerial parts | 1D, 2D NMR; ECD | [41] |
| 102 | (+)-seco-tanapartholide A | C. indicum | Aerial parts | 1D, 2D NMR; ECD | [41] |
| 103 | (+)-seco-tanapartholide B | C. indicum | Aerial parts | 1D, 2D NMR; ECD | [41] |
| 104 | handelin |
C. ornatum
C. indicum |
Whole herbs Aerial parts |
1D, 2D NMR; ESIMS 1D NMR |
[29] [31] |
| 105 | chrysanolide D | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; X-ray | [31] |
| 106 | chrysanolide E | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [31] |
| 107 | 8′-tigloylchrysanolide D | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 108 | 8-angeloyl-8′-hydroxychrysanolide D | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 109 | 8,8′-ditigloylchrysanolide D | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 110 | 8-tigloylchrysanolide D | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 111 | artanomalide C | C. indicum | Aerial parts | 1D NMR | [31] |
| 112 | - | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; X-ray | [42] |
| 113 | chrysanthemulide I | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 114 | chrysanthemulide J | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 115 | chrysanolide C | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [43] |
| 116 | chrysanolide A | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; ECD | [43] |
| Other types | |||||
| 117 | jinsidajuol A | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [22] |
| 118 | jinsidajuol B | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [22] |
| 119 | chrysetuno | C. indicum | Aerial parts | 1D, 2D NMR; EIMS | [36] |
| 120 | tunefulin | C. indicum | Aerial parts | 1D, 2D NMR; EIMS | [36] |
| 121 | 11-hydroxy-1-oxo-4α,5α,7β,10β-eremophilane | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 122 | indicumolide C | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [34] |
| 123 | oplopanone | C. indicum | Flowers | 1D NMR; EIMS | [28] |
| 124 | spathulenol | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 125 | caryolane 1,9β-dio | C. indicum | Flowers | 1D NMR; EIMS | [28] |
| 126 | chrysanthemumin G | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 127 | 11(7→6)abeo-14-norcarbrane-4,7-dione | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 128 | 6,8-cycloeudesm-4(15)-en-1-ol | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 129 | (4R,5R)-4,5-dihydroxycaryophyll-8(13)-ene | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 130 | β-caryophyllene | C. indicum | Aerial parts | 1D NMR | [44] |
| 131 | grandiflorolide | C. grandiflora | Flowers | 1D, 2D NMR; HRESIMS | [45] |
| 132 | clovanediol | C. indicum | Flowers | 1D NMR; EIMS | [28] |
| 133 | - | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [42] |
| 134 | - | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; X-ray | [42] |
| 135 | chamazulene | C. indicum | Aerial parts | 1D NMR | [44] |
Note: NMR, nuclear magnetic resonance; HRESIMS, high resolution electrospray ionization mass spectroscopy; ESIMS, electron ionization mass spectrometry; X-ray, X-ray crystallography; ECD, electronic circular dichroism.
2. Sesquiterpenoids
Sesquiterpenoids are compounds made up of three isoprene units, containing 15 carbons [20]. Some sesquiterpenoids with different skeleton types have been isolated and identified from the Chrysanthemum genus, including germacrane-type, eudesmane-type and guaianolide-type sesquiterpenoids. Interestingly, most of these compounds have been reported from just one species, except angeloylcumambrin B (53), cumambrin A (54), and handelin (104) which were reported from more than one species. The following is a classification of the sesquiterpenoids found in the Chrysanthemum genus. A complete profile of these compounds is given in Table 1.
2.1. Germacrane-Type Sesquiterpenoids
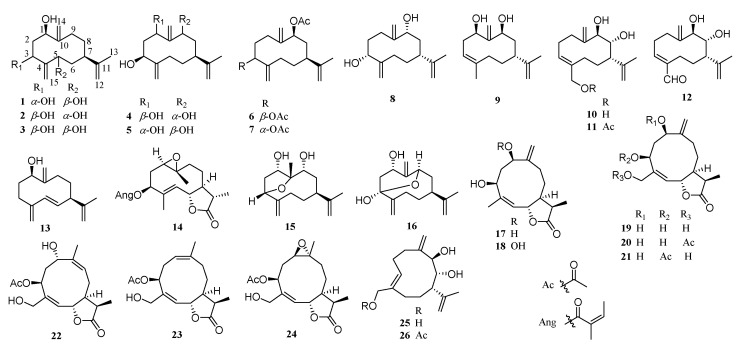
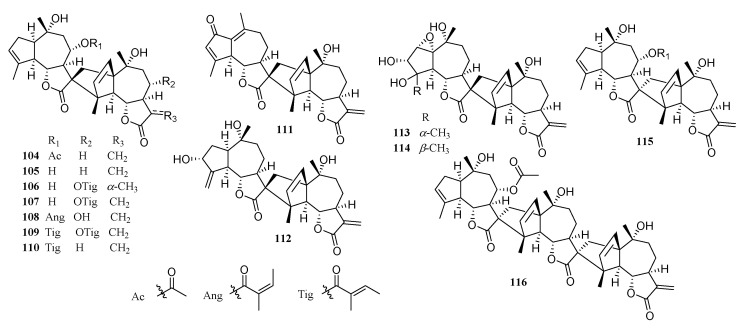
Germacrane-type sesquiterpenoids are monocyclic sesquiterpenoids, which are composed of a 10-membered carbon ring, a methyl group at C-4 and C-10, and an isopropyl group at C-7. Twenty-six compounds of this type have so far been reported from the genus. Compounds 1–13, 15, and 16 vary in the position and orientation of the oxygen-containing substituents. Compounds 14 and 17–24 form five-membered γ-lactone ring at C-6 and C-12. Compounds 25 and 26 form irregular rings due to the configuration of double bond in the ring. Examples of structures are shown in Figure 1.
Figure 1.
Germacrane-type sesquiterpenoids from Chrysanthemum genus.
2.2. Eudesmane-Type Sesquiterpenoids
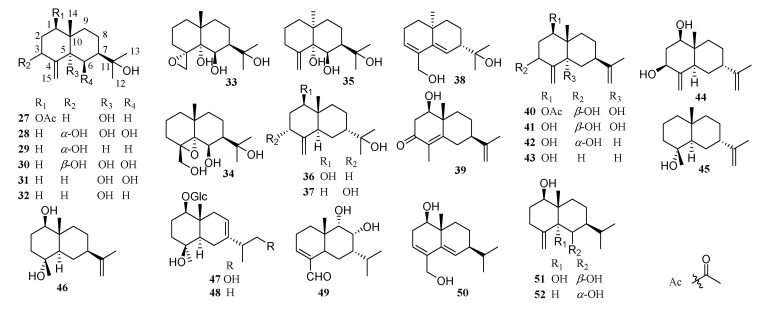
Eudesmane-type sesquiterpenoids are bicyclic compounds, composed of two six-membered carbon rings with methyl groups at C-4 and C-10 positions, and an isopropyl group at C-7. Twenty-six compounds with this skeleton have been isolated from this genus, however, no eudesmanolides have been reported so far. Compounds 35 and 38 are rare due to α-CH3 at C-10, others having a β-CH3 at the same position. Examples of structures are shown in Figure 2.
Figure 2.
Eudesmane-type sesquiterpenoids from Chrysanthemum genus.
2.3. Guaianolide-Type Sesquiterpenoids
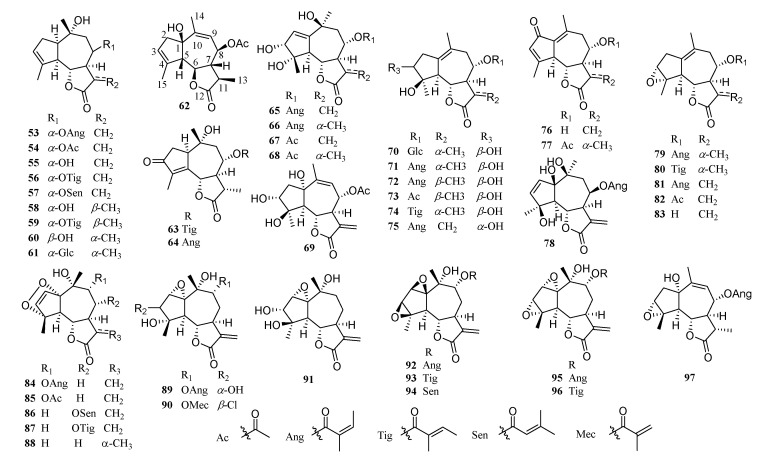
Guaianolide-type sesquiterpenoids have ternary rings consisting of a five-membered ring, a seven-membered ring, a five-membered γ-lactone ring, and methyl groups at C-4 and C-10 positions. The structures are quite complicated with up to nine stereocenters, mostly highly oxidized. Moreover, they are also the most abundant compounds in the Chrysanthemum genus. Compound 90 contains chlorine atoms. So far, 60 chlorine-containing guaianolide-type sesquiterpenoids have been isolated from Compositae [39,46,47]. Examples of structures are given in Figure 3.
Figure 3.
Guaianolide-type sesquiterpenoids from the Chrysanthemum genus.
2.4. 1,10-Seco Guaianolide Sesquiterpenoids
1,10-Seco guaianolide sesquiterpenoids have a binary ring system. The seven-membered ring in the middle is disconnected at C-1 and C-10 of guaianolide-type sesquiterpenoids. Six compounds of this subclass have been reported from the genus under focus (Figure 4).
Figure 4.
1,10-Seco guaianolides from the Chrysanthemum genus.
2.5. Disesquiterpenoids and a Trisesquiterpenoid
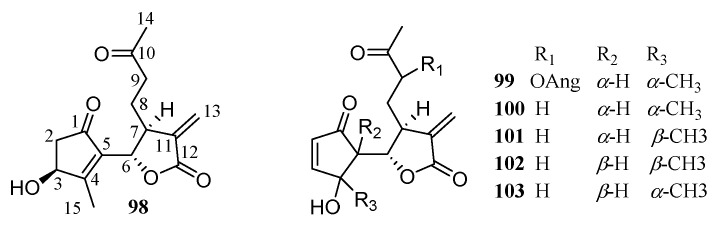
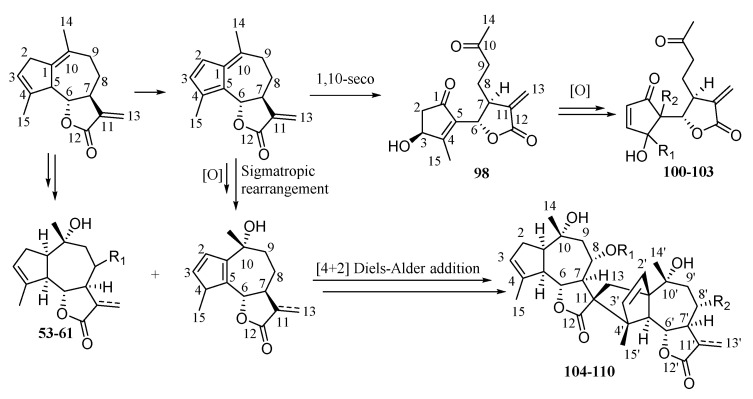
Disesquiterpenoids and a trisesquiterpenoid are composed of an electron donor conjugated diene and an electrophilic double bond fragment formed through [4 + 2] Diels–Alder reaction. An electron donor diene is a C5/C7/C5 guaianolide-type structure, while the electron deficient double bond is provided with the associated α-methylene-γ-lactone fragment [48]. Guaianolide-type compounds in the Chrysanthemum genus form dimers or trimers by cyclization to form five-membered ring. Examples of structures are given in Figure 5.
Figure 5.
Disesquiterpenoids and a trisesquiterpenoid from the Chrysanthemum genus.
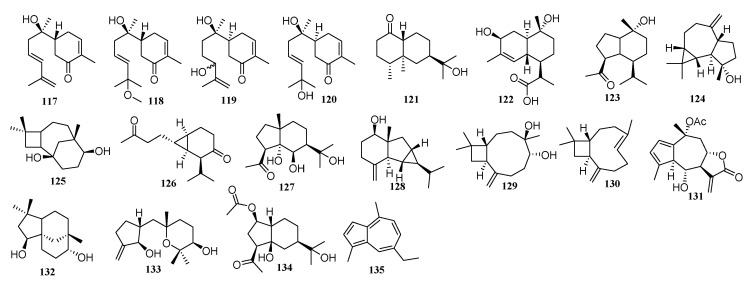
2.6. Other Types of Sesquiterpenoids
Nineteen other types of sesquiterpenoids have also been reported from this genus including, four bisabolane-type, one eremophilane-type, one cadinane-type, one oplopanane-type, one dodecane-type, and a carabranolide-type sesquiterpenoids (Figure 6).
Figure 6.
Other types of sesquiterpenoids from the Chrysanthemum genus.
3. Biosynthetic Pathway of Sesquiterpenoids
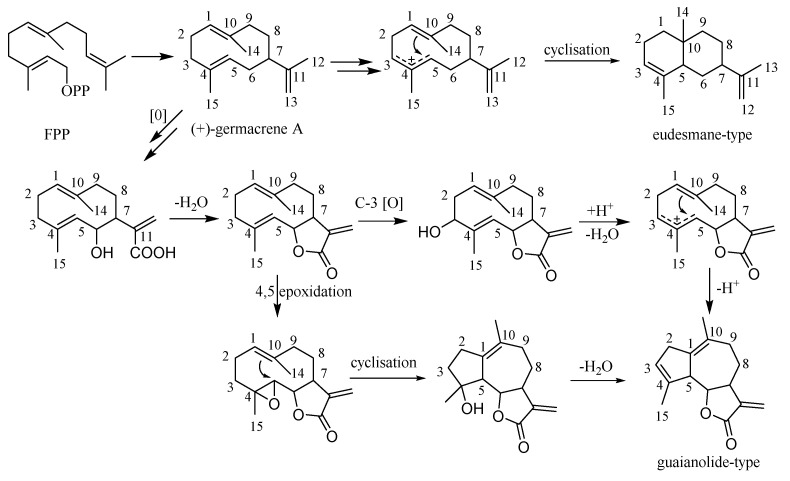
The main skeleton types of sesquiterpenoids in the Chrysanthemum genus are germacrane-type sesquiterpenoids, eudesmane-type sesquiterpenoids, and guaianolide-type sesquiterpenoids [49,50]. The proposed pathway to the sesquiterpenoids starts with the cyclization of farnesyl diphosphate (FPP) to germacrene A [50]. We consider (+)-germacrene A formation to be the first committed step in sesquiterpenoids biosynthesis. A direct cyclisation of FPP to a eudesmane or guaiane does not occur. We suggest that oxidations of the common (+)-germacrene A intermediate determine how additional cyclisation occurs, i.e., whether guaianolides or eudesmanes (plus germacranes) are biosynthesized (Figure 7) [51,52,53,54,55,56]. Furthermore, 1,10-seco guaianolides and disesquiterpenoids are special guaianolides which were isolated from the Chrysanthemum genus. A plausible biosynthetic pathway of 1,10-seco guaianolides and disesquiterpenoids is given in Figure 8 [57].
Figure 7.
The connection of carbon skeleton of three sesquiterpenoids.
Figure 8.
The plausible biosynthetic pathway of 1,10-seco guaianolides and disesquiterpenoids.
4. Pharmacological Activities
The main pharmacological activities of sesquiterpenoids are antitumor, anti-inflammatory, antibacterial and antiviral. Some of the monomers are very good antitumor and anti-inflammatory agents [58].
4.1. Antitumor Activity
Current studies have shown that sesquiterpenoids have low cytotoxic effects on normal cells while playing an anticancer role, and therefore more and more experts and scholars are focusing on the antitumor properties of sesquiterpenoids [59]. Among them, sesquiterpenoid lactone have strong antitumor activity. It has been found that α-methylene-γ-butyrolactone ring is the effective group with antitumor activity [60].
Six compounds, angeloylcumambrin B (53), chrysanolide G (56), tigloylcumambrin B (57), chrysanolide E (106), 8′-tigloylchrysanolide D (107), and 8,8′-ditigloylchrysanolide D (109) were isolated and identified from C. indicum by the Xu Jun research group. These six compounds showed multiple cytotoxic activities against four human nasopharyngeal carcinoma (NPC) cell lines (CNE1, CNE2, HONE-1, and SUNE-1) and one human intestinal epithelial cell line (HT-29). The IC50 value of some compounds was lower than that of the positive control drug, among which compound 109 had the strongest activity against cancer. It was found that compound 109 concentration-dependently induces G2/M cell arrest. Thus, investigation of the mechanism of action and structure-activity relation (SAR) for compound 109 is worth exploring further [31]. In addition, the Kong Lingyi research group found that the new compound Chrysanthemulide A (CA) (92) demonstrated significant anti-osteosarcoma potential; therefore, its mechanism was studied. The c-Jun N-terminal kinase (JNK) signaling pathway was activated by CA, and treatment with JNK siRNAs or inhibitor SP600125 significantly reduced CA-mediated autophagosome accumulation and DR5-mediated cell apoptosis. CA can induce apoptosis according to upregulated DR5 via JNK-mediated autophagosome accumulation and the combined treatment of CA and TRAIL might be a promising therapy for osteosarcoma [61].
4.2. Anti-Inflammatory Activity
The flower of C. morifolium and C. indicum is a common functional food and a well-known traditional Chinese medicine (TCM) for the treatment of inflammatory diseases [62]. The sesquiterpenoids isolated from the Chrysanthemum genus also showed strong anti-inflammatory activity. The anti-inflammatory effects on lipopolysaccharide (LPS)-induced nitric oxide (NO) were investigated in RAW 264.7 cells. Chrysanthemulide H (86), 8-tigloyldesacetylezomontanin (87), chrysanthemulide F (89), chrysanthemulide G (90), chrysanthemulide A (92), chrysanthemulide B (93), chrysanthemulide C (94), chrysanthemulide D (95), and chrysanthemulide E (96) and 98–103 displayed NO production inhibitory activities with IC50 values ranging from 1.4 to 9.7 μM. The IC50 value of the positive control drug L-NMMA was 25.8 μM [39,61]. These sesquiterpenoids have great anti-inflammatory potential. A mechanistic study revealed that the potential anti-inflammatory activity of compound chrysanthemulide A (92) appears to be mediated via suppression of an LPS-induced NF-κB pathway and downregulation of MAPK activation [39,61]. In addition, using H9c2 cardiocytes impaired by lipopolysaccharide (LPS), compounds chrysanthguaianolactone D (65), 3α,4α,10β-trihydroxy-8α-acetoxyguai-1,11(13)-dien-6α,12-olide (67), 3α,4α,10β-trihydroxy-8α-acetoxy-11βH-guai-1-en-6α,12-olide (68), 8α-(angelyloxy)-3β,4β-dihydroxy-5αH,6βH,7αH,11αH-guai-1(10)-en-12,6-olide (71), and 3β,4α-dihydroxy-8α-angelyloxy-1(10),11(13)-dien-6β,12-olide (75) exhibited anti-inflammatory activity [33].
4.3. Antibacterial Activity
Two sesquiterpenoids were isolated and purified from the flower of C zawadskii and identified as angeloycumambrin B (53) and cumambrin A (54). Compared to the positive control benzoic acid and sorbic acid which showed antibacterial activity against Bacillus subtilis, Staphylococcus aureus, and Vibrio parahemolyticus with 10–13 mm diameter of clear zone (500 μg/disk), compound 53 (100 μg/disk) showed antibacterial activity against B. subtilis, S. aureus, and V. parahemolyticus with diameter of clear zone 12, 10, and 11 mm, respectively, and compound 54 (100 μg/disk) exhibited the activity against B. subtilis and V. parahemolyticus with 13 and 12 mm diameters of clear zone, respectively. Compounds 53 and 54 showed about five-fold stronger antibacterial activity against B. subtilis and V. parahemolyticus [30].
4.4. Antiviral Activity
There have been some compounds from Chrysanthemum species showing selective antiviral activities. Ten sesquiterpenoids, chrysanthemumin A (31), chrysanthemumin B (35), chrysanthemumin C (42), chrysanthemumin D (33), chrysanthemumin E (34), 6,8-cycloeudesm-4(15)-en-1-ol (128), 11-hydroxy-1-oxo-4α,5α,7β,10β-eremophilane (121), chrysanthediol A (9), 1β-hydroxy-4(15),5E,10(14)-germacratriene (13), and eudesm-4(15)-ene-1β,6α-diol (52) were isolated from C. indicum by the Shi Yanping research group. These compounds inhibited the porcine epidemic diarrhea virus (PEDV) protein expression, which showed that these compounds increased cell viability against cell death in PEDV-injected cells, among which compounds 13, 35, and 128 can also dose-dependently inhibite PEDV replication at concentrations ranging from 20 to 90 μM. The antiviral mechanism of compound 35 was studied. The results indicated that compound 35 exhibited potential inhibition of the viral protein synthesis in a dose-dependent manner [11]. In addition, chrysanolide B (60), chrysanolide C (115), and chrysanolide A (116) were evaluated for their anti-HBV activities, and 3TC (lamivudine, a frequently used clinical anti-HBV agent) was used as the positive control. These three compounds exhibited potent inhibitory activities against the secretion of HBsAg (IC50 = 131.28, 33.91, and 6.67 μM) and HBeAg (IC50 = 144.48, 30.09, and 6.23 μM), respectively. The positive control drug lamivudine had an IC50 value of 14.85 μM against HBsAg and 42.36 μM against HBeAg, respectively. More interestingly the anti-HBV activities increased with the increasing degree of aggregation. Compound 116 is a third guaianolide-type sesquiterpenoid trimer found in plant, its activity is better than that of the positive control drug, and its mechanism of anti-HBV is worthy of further exploration [43].
4.5. Antidiabetic and Antiobesity Activity
10α-Hydroxy-1α,4α-endoperoxy-guaia-2-en-12,6α-olide (88) showed strong inhibitory effects against α-glucosidase and lipase activities, with IC50 values of 229.3 and 161.0 μM, respectively. The positive control drug acarbose inhibited α-glucosidase with IC50 value of 1907 μM, and orlistat inhibited lipase with IC50 value of 108.3 μM [40].
5. Conclusions
Sesquiterpenoids are one of the main chemical constituents of the Chrysanthemum genus which have various chiral centers and configurations. At present, 135 sesquiterpenes have been reported from the Chrysanthemum genus (most of them were isolated from C. morifolium and C. indicum), mainly including germacrane-type sesquiterpenoids, eudesmane-type sesquiterpenoids, and guaianolide-type sesquiterpenoids. The compounds have been found to exhibit wide spectrum pharmacological activities such as anti-inflammatory, antitumor, antibacterial, antiviral, antidiabetic, and anti-obesity activities. The activity of some compounds has been found to be better than that of positive control drugs in vitro. Some compounds, especially guaianolide-type sesquiterpenoids, showed better anticancer and anti-inflammatory activity, and their mechanism of action was explained from the aspects of signal pathway and target, which was consistent with the traditional function of clearing heat and removing toxicity exhibited by the Chrysanthemum genus, which is widely distributed in China and has unique advantages in number and species. Therefore, further research and development of the Chrysanthemum genus based on sesquiterpenoids need to be conducted.
At present, some progress has been made in the study of the sesquiterpenoids of the Chrysanthemum genus, but there are still some species to be explored. Most of the sesquiterpenoids have complicated structures, in which there are many chiral centers, conformers, and configurational isomers, and therefore it is difficult to separate and identify them. In terms of pharmacological activities, studies have mainly focused on the in vitro activity screening of pure compounds, while studies on pharmacodynamic evaluation and structure-activity relationship in vivo are almost nonexistent. However, it may be related to the insufficient yield and configuration of isolated compounds.
Therefore, for further research and development of the Chrysanthemum genus the following suggestions should be considered: Firstly, focus on other species of the Chrysanthemum genus that have not been fully studied, especially those whose chemical composition has not been studied. Secondly, try to obtain more sesquiterpenoids from Chrysanthemum genus plants by using more advanced methods. Thirdly, screen the sesquiterpenoids with strong activity and carry out synthesis and structural modification to study structure-activity relationships, efficacy evaluation, and mechanism targets in vivo, and to develop drugs for clinical use.
Author Contributions
Writing—original draft preparation, S.J. and M.W.; writing—review and editing, Z.J., S.Z., Q.X., Y.Y., Y.L. and Y.J.; funding acquisition, H.Y. and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by (1) The National Natural Science Foundation of China (grant no. 81874369); (2) the Natural Science Foundation of Hunan Province (grant no. 2020JJ4463); (3) the Natural Science Foundation of Hunan Province (grant no. 2020JJ4064); (4) the Pharmaceutical Open Fund of Domestic First-Class Disciplines (cultivation) of Hunan Province (grant no. 2020YX10); (5) the Changsha Municipal Natural Science Foundation (grant no. kq2014086).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu P.L., Wan Q., Guo Y.P., Yang J., Rao G.Y. Phylogeny of the genus Chrysanthemum L.: Evidence from single-copy nuclear gene and chloroplast DNA sequences. PLoS ONE. 2012;7:e48970. doi: 10.1371/journal.pone.0048970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao H.E., Liu Z.H., Hu X., Yin J.L., Li W., Rao G.Y., Zhang X.H., Huang C.L., Anderson N., Zhang Q.X., et al. Chrysanthemum genetic resources and related genera of Chrysanthemum collected in China. Genet. Resour. Crop. Evol. 2009;56:937–946. doi: 10.1007/s10722-009-9412-8. [DOI] [Google Scholar]
- 3.Ja T.D.S. Chrysanthemum: advances in tissue culture, cryopreservation, postharvest technology, genetics and transgenic biotechnology. Biotechnol. Adv. 2003;21:715–766. doi: 10.1016/s0734-9750(03)00117-4. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee J., Mandal A., Ranade S., Da Silva J.A.T., Datta S. Molecular systematics in Chrysanthemum×grandiflorum (Ramat.) Kitamura. Sci. Hortic. 2006;110:373–378. doi: 10.1016/j.scienta.2006.09.004. [DOI] [Google Scholar]
- 5.Zhou H.P., Ren M.X., Guan J.Q., Liu Y.L., Xiong Y.X., Zhong Q.F., Jiang S., Wu S.X. Research progress on chemical constituents and pharmacological effects of Chrysanthemum morifolium and predictive analysis on quality markers. Chin. Tradit. Herbal Drugs. 2019;50:4785–4795. [Google Scholar]
- 6.Liu L.L., Xiao Z.B. Chemical constituents from flowers of Chrysanthemum indicum. Chin. Tradit. Herbal Drugs. 2018;49:29–33. [Google Scholar]
- 7.Jin J.Z., Wen M., Shen T.C. Research progress in chemical components in Chrysanthemum morifolium. Sci. Tech. Food Ind. 2014;35:386–389. [Google Scholar]
- 8.Cai H.F. The research progression of flos Chrysanthemi indici on chemical constituents and medicial application. Nat. Med. Front. China. 2007;2:118–120. [Google Scholar]
- 9.Li Y., Yang P., Luo Y., Gao B., Sun J., Lu W., Liu J., Chen P., Zhang Y., Yu L.L. Chemical compositions of chrysanthemum teas and their anti-inflammatory and antioxidant properties. Food Chem. 2019;286:8–16. doi: 10.1016/j.foodchem.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Gao T., Zhu Z.Y., Zhou X., Xie M.L. Chrysanthemum morifolium extract improves hypertension-induced cardiac hypertrophy in rats by reduction of blood pressure and inhibition of myocardial hypoxia inducible factor-1alpha expression. Pharm. Biol. 2016;54:2895–2900. doi: 10.1080/13880209.2016.1190764. [DOI] [PubMed] [Google Scholar]
- 11.Liu L.L., Ha T.K.Q., Ha W., Oh W.K., Yang J.L., Shi Y.P. Sesquiterpenoids with various carbocyclic skeletons from the flowers of Chrysanthemum indicum. J. Nat. Prod. 2017;80:298–307. doi: 10.1021/acs.jnatprod.6b00694. [DOI] [PubMed] [Google Scholar]
- 12.Yang P.F., Feng Z.M., Yang Y.N., Jiang J.S., Zhang P.C. Neuroprotective caffeoylquinic acid derivatives from the flowers of Chrysanthemum morifolium. J. Nat. Prod. 2017;80:1028–1033. doi: 10.1021/acs.jnatprod.6b01026. [DOI] [PubMed] [Google Scholar]
- 13.Yang P.F., Yang Y.N., Feng Z.M., Jiang J.S., Zhang P.C. Six new compounds from the flowers of Chrysanthemum morifolium and their biological activities. Bioorganic Chem. 2019;82:139–144. doi: 10.1016/j.bioorg.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Fan S., Chang J., Zong Y., Hu G., Jia J. GC-MS analysis of the composition of the essential oil from Dendranthema indicum var. Aromaticum using three extraction methods and two columns. Molecules. 2018;23:576. doi: 10.3390/molecules23030576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han X.B., Zhao J., Cao J.M., Zhang C.S. Essential oil of Chrysanthemum indicum L.: potential biocontrol agent against plant pathogen Phytophthora nicotianae. Environ. Sci. Pollut. Res. 2019;26:7013–7023. doi: 10.1007/s11356-019-04152-y. [DOI] [PubMed] [Google Scholar]
- 16.Wang J.S., Zhou J., Kong L.Y. Three new germacrane-type sesquiterpene stereoisomers from the flowers of Chrysanthemum indicum. Fitoterapia. 2012;83:1675–1679. doi: 10.1016/j.fitote.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Hu L., Chen Z. Sesquiterpenoid alcohols from Chrysanthemum morifolium. Phytochemistry. 1997;44:1287–1290. doi: 10.1016/s0031-9422(96)00690-5. [DOI] [Google Scholar]
- 18.Mareo J.A., Sanz J.F., Jakupovic J., Huneck S. New sesquiterpene lactones and acetylenes from Chrysanthemum lavandulifolium. Tetrahedron. 1990;46:6931–6938. doi: 10.1016/S0040-4020(01)87880-7. [DOI] [Google Scholar]
- 19.Shin H.J., Lee S.Y., Kim J.S., Lee S., Choi R.J., Chung H.S., Kim Y.S., Kang S.S. Sesquiterpenes and other constituents from Dendranthema zawadskii var. latilobum. Chem. Pharm. Bull. 2012;60:306–314. doi: 10.1248/cpb.60.306. [DOI] [PubMed] [Google Scholar]
- 20.Guo L.M., Lyu J.L., Zhang L.B. Research progress on anti-inflammatory mechanism of natural sesquiterpenoids. China J. Chin. Mater. Med. 2018;43:3989–3999. doi: 10.19540/j.cnki.cjcmm.20180726.013. [DOI] [PubMed] [Google Scholar]
- 21.Liu L., Wang R., Yang J., Shi Y. Five new sesquiterpenoids from Chrysanthemum indicum. Chin. J. Chem. 2012;30:1255–1260. doi: 10.1002/cjoc.201200184. [DOI] [Google Scholar]
- 22.Zhao F.Q., Zhang Q.Q., Yan Y., Jia H.Y., Zhao X.R., Li X.Y., Zheng L.H., Han G. Antioxidant constituents of Chrysanthemum ‘jinsidaju’ cultivated in Kaifeng. Fitoterapia. 2019;134:39–43. doi: 10.1016/j.fitote.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Yoshikawa M., Morikawa T., Toguchida I., Harima S., Matsuda H. Medicinal flowers. II. Inhibitors of nitric oxide production and absolute stereostructures of five new germacrane-type sesquiterpenes, kikkanols D, D monoacetate, E, F, and F monoacetate from the flowers of Chrysanthemum indicum L. Chem. Pharm. Bull. 2000;48:651–656. doi: 10.1248/cpb.48.651. [DOI] [PubMed] [Google Scholar]
- 24.Toshihiko O., Akinori S., Saburo T. Molecular structure and stereochemistry of chrysandiol, a novel sesquiterpene diol from Chrysanthemum morifolium. Tetrahedron Lett. 1974;17:1569–1572. [Google Scholar]
- 25.Wang X.L., Peng S.L., Liang J., Yu K.B. (3β,5α,6β,7β,14β)-Eudesmen-3,5,6,11-tetrol methanol solvate: a new sesquiterpenoid from Chrysanthemum indicum L. Acta. Crystallogr. E. 2010;62:o3570–o3571. doi: 10.1107/S1600536806028522. [DOI] [Google Scholar]
- 26.Yang J.L., Liu L.L., Shi Y.P. Two new eudesmane sesquiterpenoids from the flowers of Chrysanthemum indicum. Nat. Prod. Bioprospecting. 2019;9:145–148. doi: 10.1007/s13659-019-0199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu D.Q., Xie F.Z. Chemical constituents from flowers of Chrysanthemum indicum. Acta Pharm. Sin. 1987;22:837–840. [PubMed] [Google Scholar]
- 28.Yoshikawa M., Morikawa T., Murakami T., Toguchida I., Harima S., Matsuda H. Medicinal flowers. I. Aldose reductase inhibitors and three new eudesmane-type sesquiterpenes, kikkanols A, B, and C, from the flowers of Chrysanthemum indicum L. Chem. Pharm. Bull. 1999;47:340–345. doi: 10.1248/cpb.47.340. [DOI] [PubMed] [Google Scholar]
- 29.Haruna M., Kato M., Ito K., Nikai T., Sugihara H., Muratat H. Angeloylcumambrin-B, an antimicrobial sesquiterpene lactone from Chrysanthemum ornatum var. Spontaneum. Phytochemistry. 1981;20:2583–2584. doi: 10.1016/0031-9422(81)83100-7. [DOI] [Google Scholar]
- 30.Jang D.S., Park K.H., Choi S.U., Nam S.H., Yang M.S. Antibacterial substances of the flower of Chrysanthemum zawadskii Herbich var. latilobum Kitamura. Agr. Chem. Biotechnol. 1997;40:85–88. [Google Scholar]
- 31.Luo P., Cheng Y., Yin Z., Li C., Xu J., Gu Q. Monomeric and dimeric cytotoxic guaianolide-type sesquiterpenoids from the aerial parts of Chrysanthemum indicum. J. Nat. Prod. 2019;82:349–357. doi: 10.1021/acs.jnatprod.8b00863. [DOI] [PubMed] [Google Scholar]
- 32.Chen J., Yang X., Li B., Yang K., Wang Y., Sun K., Zhang Y., Zhu W. A new sesquiterpenoid from Chrysanthemum indicum. Chem. Nat. Compd. 2019;55:1076–1079. doi: 10.1007/s10600-019-02898-y. [DOI] [Google Scholar]
- 33.Chen W., Zeng M., Li M., Li F., Zhao X., Fan H., Zheng X., Feng W. Four new sesquiterpenoids from Dendranthema morifolium (Ramat.) kitam flowers. Phytochem. Lett. 2018;23:52–56. doi: 10.1016/j.phytol.2017.11.009. [DOI] [Google Scholar]
- 34.Feng Z.M., Song S., Xia P.F., Jiang J.S., Zhang P.C. Three new sesquiterpenoids from Chrysanthemum indicum L. Helv. Chim. Acta. 2009;92:1823–1828. doi: 10.1002/hlca.200900017. [DOI] [Google Scholar]
- 35.Zhang B., Zeng M., Li M., Chen W., Li B., Kan Y., Feng W., Zheng X. Guaiane-type sesquiterpenoids from Dendranthema morifolium (Ramat.) S. Kitam flowers protect H9c2 cardiomyocyte from LPS-induced injury. Nat. Prod. Commun. 2019;14:1–8. doi: 10.1177/1934578X19864179. [DOI] [Google Scholar]
- 36.Mladenova K., Tsankova E., Van Hung D. New sesquiterpenoids from Chrysanthemum indicum var. tuneful. Planta Med. 1988;54:553–555. doi: 10.1055/s-2006-962548. [DOI] [PubMed] [Google Scholar]
- 37.Bi Y.F., Jia L., Shi S.P., Sun X.L., Chen Y.Y., Zhang Y.B. New sesquiterpenes from the flowers of Chrysanthemum indicum L. Helv. Chim. Acta. 2010;93:1953–1959. doi: 10.1002/hlca.201000003. [DOI] [Google Scholar]
- 38.Mladenova K., Tsankova E., Stoianova-Ivanova B. Sesquiterpene lactones from Chrysanthemum indicum. Planta Med. 1985;51:284–285. doi: 10.1055/s-2007-969486. [DOI] [PubMed] [Google Scholar]
- 39.Xue G.M., Li X.Q., Chen C., Chen K., Wang X.B., Gu Y.C., Luo J.G., Kong L.Y. Highly oxidized guaianolide sesquiterpenoids with potential anti-inflammatory activity from Chrysanthemum indicum. J. Nat. Prod. 2018;81:378–386. doi: 10.1021/acs.jnatprod.7b00867. [DOI] [PubMed] [Google Scholar]
- 40.Luyen N.T., Tram L.H., Hanh T.T.H., Binh P.T., Dang N.H., Minh C.V., Dat N.T. Inhibitors of α-glucosidase, α-amylase and lipase from Chrysanthemum morifolium. Phytochem. Lett. 2013;6:322–325. doi: 10.1016/j.phytol.2013.03.015. [DOI] [Google Scholar]
- 41.Xue G.M., Xue J.F., Zhao C.G., Zhao Z.Z., Zhi Y.L., Du K., Li H.W., Sun Y.J., Feng W.S. 1,10-seco guaianolide-type sesquiterpenoids from Chrysanthemum indicum. J. Asian Nat. Prod. Res. 2020:1–7. doi: 10.1080/10286020.2020.1787388. [DOI] [PubMed] [Google Scholar]
- 42.Zhou J., Wang J.S., Zhang Y., Wang P.R., Guo C., Kong L.Y. Disesquiterpenoid and sesquiterpenes from the flos of Chrysanthemum indicum. Chem. Pharm. Bull. 2012;60:1067–1071. doi: 10.1248/cpb.c12-00163. [DOI] [PubMed] [Google Scholar]
- 43.Gu Q., Chen Y., Cui H., Huang D., Zhou J., Wu T., Chen Y., Shi L., Xu J. Chrysanolide A, an unprecedented sesquiterpenoid trimer from the flowers of Chrysanthemum indicum L. RSC Adv. 2013;3:10168–10172. doi: 10.1039/c3ra23172k. [DOI] [Google Scholar]
- 44.Zhang W.J., You C.X., Yang K., Wang Y., Su Y., Geng Z.F., Du S.S., Wang C.F., Deng Z.W., Wang Y.Y. Bioactivity and chemical constituents of the essential oil from Dendranthema indicum (L.) Des Moul. against two stored insects. J. Oleo Sci. 2015;64:553–560. doi: 10.5650/jos.ess14231. [DOI] [PubMed] [Google Scholar]
- 45.Ragasa C.Y., Si M., Tan M.C.S., Pelobello D.H., Don M.J., Shen C.C. A new sesquiterpene from Dendranthema grandiflora flowers. Chem. Nat. Compd. 2020;56:436–439. doi: 10.1007/s10600-020-03057-4. [DOI] [Google Scholar]
- 46.Engvild K.C. Chlorine-containing natural compounds in higher plants. Phytochemistry. 1986;25:781–791. doi: 10.1016/0031-9422(86)80002-4. [DOI] [Google Scholar]
- 47.Youssef D., Frahm A. Constituents of the Egyptian Centaurea scoparia; Chlorinated guaianolides of the aerial parts. Planta Med. 1994;60:267–271. doi: 10.1055/s-2006-959473. [DOI] [PubMed] [Google Scholar]
- 48.Xuan G.M., Xuan J.F., Du K. Research progress on sesquiterpenoid dimers from Compositae. Nat. Prod. Res. Dev. 2019;31:2189–2196. [Google Scholar]
- 49.Adio A.M. Germacrenes A–E and related compounds: thermal, photochemical and acid induced transannular cyclizations. Tetrahedron. 2009;65:1533–1552. doi: 10.1016/j.tet.2008.11.050. [DOI] [Google Scholar]
- 50.Ramirez A.M., Saillard N., Yang T., Franssen M.C.R., Bouwmeester H.J., Jongsma M.A. Biosynthesis of sesquiterpene lactones in Pyrethrum (Tanacetum cinerariifolium) PLoS ONE. 2013;8:e65030. doi: 10.1371/journal.pone.0065030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kraker J.W.d., Bouwmeester H.J., Franssen M.C.R., Groot A. (+)-Germacrene A synthesis in chicory (Cichorium intybus L.); the first step in sesquiterpene lactone biosynthesis. Acta Bot. Gallica. 1999;146:111–115. doi: 10.1080/12538078.1999.10515807. [DOI] [Google Scholar]
- 52.Xi F.M., Ma S.G., Liu Y.B., Li L., Yu S.S. Artaboterpenoids A and B, bisabolene-derived sesquiterpenoids from Artabotrys hexapetalus. Org. Lett. 2016;47:3374–3377. doi: 10.1021/acs.orglett.6b01519. [DOI] [PubMed] [Google Scholar]
- 53.Phan C.S., Li H., Kessler S., Solomon P.S., Piggott A.M., Chooi Y.H. Bipolenins K–N: New sesquiterpenoids from the fungal plant pathogen Bipolaris sorokiniana. Beilstein J. Org. Chem. 2019;15:2020–2028. doi: 10.3762/bjoc.15.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song Y.P., Liu X.H., Shi Z.Z., Miao F.P., Fang S.T., Ji N.Y. Bisabolane, cyclonerane, and harziane derivatives from the marine-alga-endophytic fungus Trichoderma asperellum cf44-2. Phytochemistry. 2018;152:45–52. doi: 10.1016/j.phytochem.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 55.Drew D.P., Krichau N., Reichwald K., Simonsen H.T. Guaianolides in apiaceae: perspectives on pharmacology and biosynthesis. Phytochem. Rev. 2009;8:581–599. doi: 10.1007/s11101-009-9130-z. [DOI] [Google Scholar]
- 56.Andreas S., Oliver R. Synthesis of biologically active guaianolides with a trans-annulated lactone moiety. Eur. J. Org. Chem. 2008;14:2253–2264. [Google Scholar]
- 57.Xue G.M., Han C., Chen C., Li L.N., Wang X.B., Yang M.H., Gu Y.C., Luo J.G., Kong L.Y. Artemisians A–D, diseco-guaianolide involved heterodimeric [4 + 2] adducts from Artemisia argyi. Org. Lett. 2017;19:5410–5413. doi: 10.1021/acs.orglett.7b02681. [DOI] [PubMed] [Google Scholar]
- 58.Salazar-Gómez A., Ontiveros-Rodríguez J.C., Pablo-Pérez S.S., Vargas-Díaz M.E., Garduo-Siciliano L. The potential role of sesquiterpene lactones isolated from medicinal plants in the treatment of the metabolic syndrome - A review. S. Afr. J. Bot. 2020;135:240–251. doi: 10.1016/j.sajb.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu Y., Wang J.L. Research progress on anticancer effects and mechanisms of three natural sesquiterpenoids. J. Jinzhou Med. Univ. 2020;041:109–112. [Google Scholar]
- 60.Zhu H.Y., Pu H.S. Research progress on anti-cancer mechanism of natural sesquiterpenoids. West China J. Pharm. Sci. 2015;30:381–383. [Google Scholar]
- 61.Zhuo F., Zhang C., Xia Y., Xue G., Yang L., Kong L. Chrysanthemulide A induces apoptosis through DR5 upregulation via JNK-mediated autophagosome accumulation in human osteosarcoma cells. J. Cell. Physiol. 2019;234:13191–13208. doi: 10.1002/jcp.27991. [DOI] [PubMed] [Google Scholar]
- 62.Tian D., Yang Y., Yu M., Han Z.Z., Wei M., Zhang H.W., Jia H.M., Zou Z.M. Anti-inflammatory chemical constituents of Flos Chrysanthemi Indici determined by UPLC-MS/MS integrated with network pharmacology. Food Funct. 2020;11:6340–6351. doi: 10.1039/D0FO01000F. [DOI] [PubMed] [Google Scholar]