Abstract
Axons extend for tremendously long distances from the neuronal soma and make use of localized mRNA translation to rapidly respond to different extracellular stimuli and physiological states. The locally synthesized proteins support many different functions in both developing and mature axons, raising questions about the mechanisms by which local translation is organized to ensure the appropriate responses to specific stimuli. Publications over the past few years have uncovered new mechanisms for regulating the axonal transport and localized translation of mRNAs, with several of these pathways converging on the regulation of cohorts of functionally related mRNAs — known as RNA regulons — that drive axon growth, axon guidance, injury responses, axon survival and even axonal mitochondrial function. Recent advances point to these different regulatory pathways as organizing platforms that allow the axon’s proteome to be modulated to meet its physiological needs.
Localization and translation of mRNAs in subcellular regions is now recognized as a critical mechanism through which polarized cells establish and maintain the specialized domains that are needed for their polarized functions. This is especially the case for neurons, which have long cytoplasmic projections (dendrites and axons) that are needed for communication between neurons (that is, for neural circuit function) and with target tissues. Formative ultrastructural analyses of the mature rodent hippocampus advanced the idea that translation of dendritic mRNAs promotes synaptic function in this subcellular compartment1. This local translation provides the dendrite with the ability to rapidly respond to trans-synaptic stimuli that contribute to synaptic plasticity2. Recent reviews have nicely outlined the various functions served by and the mechanisms regulating dendritic protein synthesis3,4.
Dendrites extend only a few millimetres at most from the neuronal soma, whereas an axon can extend anywhere from a few centimetres in rodents to a metre or more in humans. Thus, it was appealing to speculate that axons might also contain mRNAs and be capable of locally synthesizing proteins in order to rapidly change their localized protein composition (that is, the local proteome) in the same manner as dendrites. Although early ultrastructural work failed to detect ribosomes in axons of the mature CNS1, a handful of laboratories reported the identification of mRNAs and ribosomes in axons of different organisms and neuron types between the 1960s and the late 1990s5–10 (reviewed in REF.11). Advances in the technology for the detection of mRNAs and newly synthesized proteins through molecular and cell biology approaches began to emerge around 2000, resulting in several publications showing that mRNA translation not only occurs in axons but also provides a variety of functional benefits12–15 (reviewed in REF.16). For example, since a single mRNA can be used to generate many copies of a protein, the transport of mRNAs to the axon provides the neuron with the ‘locally sourced’ capacity for proteome modifications, allowing the axon to spatially and temporally regulate the introduction of new proteins. Local translation also affords a means to restrict the activity of proteins to the site at which they are needed by providing an ‘on demand’ mechanism that regulates when and exactly where a protein is generated. Thus, localized protein synthesis allows a subcellular region — such as an axon or even a subaxonal domain — to finely tune gene expression over both space and time17.
Ribosomes.
RNA–protein complexes composed of 60S and 40S subunits that are responsible for translating mRNAs into protein.
Although dendritic protein synthesis was accepted by the scientific community much earlier than axonal protein synthesis18, the number of known functions of axonally synthesized proteins may soon exceed those that we know of for dendritically synthesized proteins. The distances that axons extend from the cell body make them more amenable to physical separation from the cell body than dendrites, meaning that knowledge of axonal mRNA populations and the functions that their encoded proteins serve is now advancing at a rapid pace. RNA profiling studies have uncovered hundreds to thousands of mRNAs in distal axons and have demonstrated some differences between axonal mRNA profiles across different neuronal populations, physiological conditions and developmental growth states (reviewed in REF.19). Undoubtedly, many more functions of axonally synthesized proteins will be uncovered as more specific experimental tools are developed.
RNA profiling.
The measurement of the identities and abundances of RNAs across different cell or tissue populations or subcellular sites and in different physiological conditions or growth states.
Similarly to mRNA translation in dendrites and non-neuronal cells, where and when axonal mRNAs are translated is determined by the interactions of those mRNAs with a combination of RNA-binding proteins (RBPs) and the subsequent modifications of those interactions. mRNAs in the neuronal soma interact with RBPs to assemble into non-membrane-bound ribonucleoproteins (RNPs). Recent publications have provided insights into the nature of these RNPs and have uncovered novel mechanisms for the delivery of mRNAs into axons and the translational regulation of these localized mRNAs. In this Review, we will try to place these mRNA transport and translation mechanisms into the context of the functional outcomes that are linked to the axonally synthesized proteins. RNA regulons are cohorts of mRNAs that are co-regulated by individual RBPs and encode proteins with complementary or synergistic cellular functions20, providing a mechanism through which the neuron can orchestrate the localization and translation of hundreds of different functionally related axonal mRNAs over space and time. Here we argue that RNA regulons extend beyond the initial description of an mRNA family interacting with a single RBP to include groups of mRNAs that interact with combinations of RBPs, subcellular organelles, organelle-like structures and receptors. Intracellular signalling pathways layer onto these RNA regulons to bring further temporal and spatial specificity to axonal mRNA translation.
RNA-binding proteins.
(RBPs). Proteins that can bind RNA via RNA structures (‘motifs’) and that are involved in RNA processing, trafficking, stability and translational regulation.
Ribonucleoproteins.
(RNPs). Complexes of RNA-binding proteins and RNAs that associate, often in the form of a granule or organelle-like structures.
Sorting mRNAs for axon localization
A central theme that has emerged from studies examining subcellular RNA localization, both in neurons and in other systems, is the idea that the cell recognizes which mRNAs to localize on the basis of sequence structures within the mRNAs themselves. These cis elements or motifs, typically (but not exclusively) found in 3′ untranslated regions (UTRs) of the mRNAs21, are recognized by RBPs. Together the mRNA and RBP eventually form an RNP that is capable of localization, whether the RNP is linked directly to motor proteins or localizes to a subcellular region by binding to vesicles trafficking on motor proteins (FIG. 1).
Fig. 1 |. ribonucleoprotein (rNP)-coupled transport of mRNAs into axons.
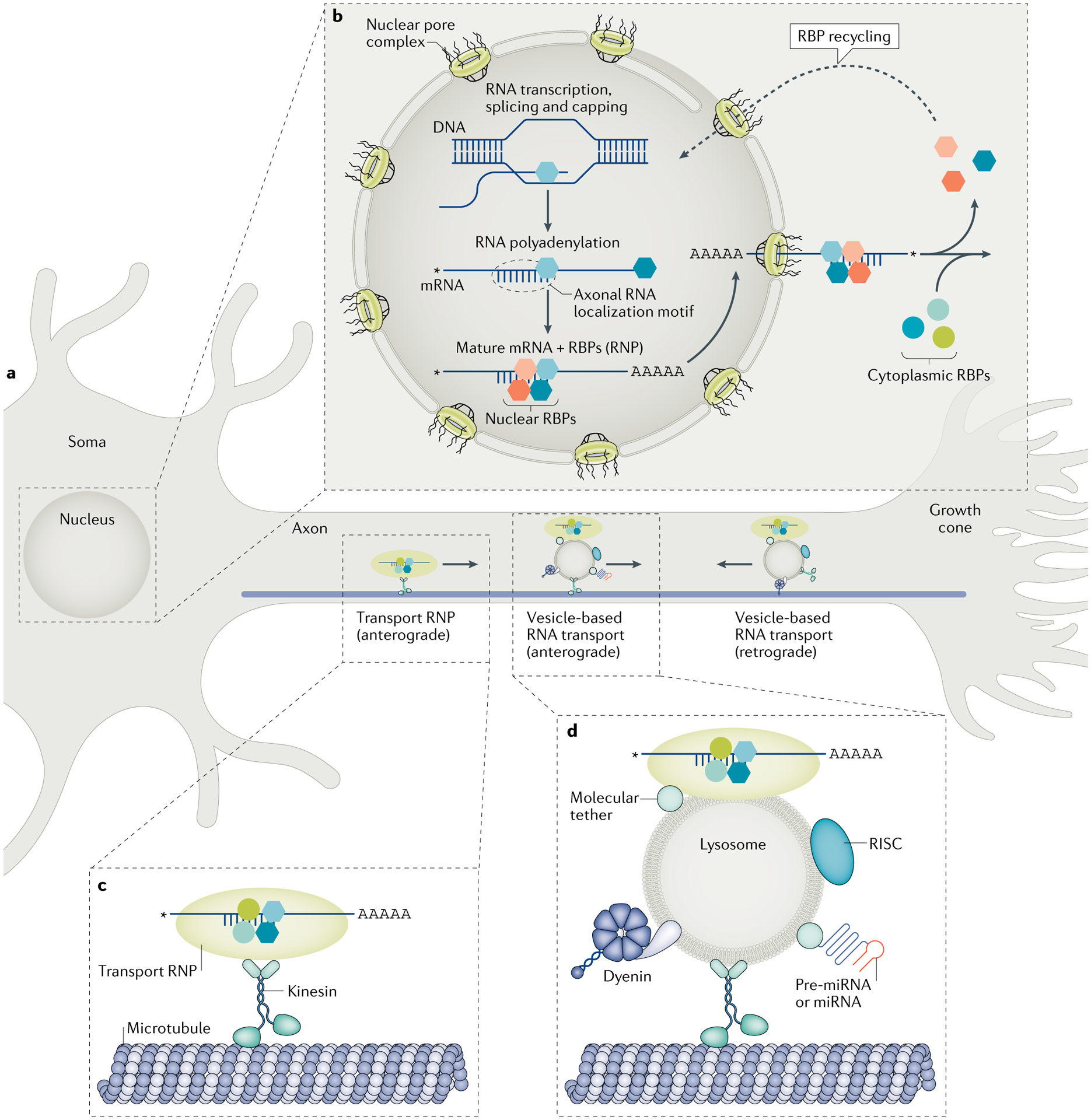
a | Schematic of a neuron showing the anterograde transport of RNPs that are engaged with motor proteins directly (known as transport RNPs) or indirectly (through binding to vesicles) for microtubule-based transport along the axon shaft. Although it is not clear whether transport RNPs also move in a retrograde direction, retrograde vesicle-based RNA transport has been reported recently77. b | Some RNA-binding proteins (RBPs) initially interact with newly transcribed RNAs in the nucleus to form an RNP. Drawing from studies in other systems, these nuclear RNPs are thought to exit the nucleus through the nuclear pore, after which they are remodelled through the addition of some new RBPs and the removal of others, with some of the released RBPs returning to the nucleus27,33. c,d | RNPs can bind kinesin motor proteins as a ‘transport RNP’ (panel c) or can bind to vesicles (endosomes or lysosomes) through molecular tether proteins (panel d). RNPs may also utilize adaptor proteins for binding to kinesin (not shown). Pre-microRNAs (pre-miRNAs) and mature miRNAs have also been shown to associate with vesicles for transport into axons84. In addition, the RNA-induced silencing complex (RISC), which is needed for miRNA processing, has been shown to associate with vesicles76,116, although it is not clear whether it functions directly on or adjacent to the vesicle for processing co-trafficked pre-miRNAs or whether it acts on different pre-miRNAs that already resided in the axon.
Motor proteins.
Proteins that bind to microtubules or microfilaments and transport cargos from one site in a cell to another.
mRNAs are generally thought to exit the nucleus through the nuclear pore complex in the form of an RNP that can include exon junction complex (EJC) components22 (FIG. 1b). EJC proteins bound to 3′ UTR exon junctions are used by the cell to identify mRNAs that will be subjected to nonsense-mediated decay. Given that nonsense-mediated decay has been demonstrated for both axonal and dendritic mRNAs, this suggests that the neuronal mRNAs that are trafficked to subcellular sites can carry along EJC-associated proteins from the nucleus to the axon23. As discussed below, several RBPs thought to be predominantly nuclear have been observed in rat axons and shown to bind to axonal localization motifs24. It should be noted that some RNPs may be too large to transit through the nuclear pore complex; indeed, an alteRNAtive mechanism of nuclear export known as nuclear envelope budding was recently suggested25.
Nuclear pore complex.
A protein complex that transects the nuclear membrane and controls the exchange of many components between the nucleus and the cytoplasm.
Nonsense-mediated decay.
A mechanism for the selective degradation of mRNAs that contain premature stop codons within, or have exon–exon junctions within, their 3′ untranslated regions.
In Drosophila melanogaster oocytes, the eventual subcellular localization of an mRNA can be determined while the mRNA is still in the nucleus26, and this is likely also to be the case for other organisms and cell types. It is, however, not clear whether an mRNA’s localization fate is determined in the nucleus or cytoplasm in neurons, and there is circumstantial evidence that both scenarios occur. In mice, Actb mRNA requires zipcode-binding protein 1 (ZBP1; also known as IMP1 or IGF2BP1) to bind to a motif in its 3′ UTR in the cytoplasm for localization into axons12, but also requires that the mRNA is initially bound by ZBP2 (also known as FUBP2 or KHSRP) in the nucleus27. In addition to EJC proteins, a number of heterogeneous nuclear RNPs and other RBPs with well-known roles in RNA splicing and nuclear organization have been shown to bind to axonal mRNA localization motifs, suggesting that these proteins could initiate their interaction with axonally targeted mRNAs in the nucleus24,28–30 (FIG. 1b). However, stimulation of axons with different trophic and pathfinding stimuli, including neurotropic stimuli and injury, was shown to alter axonal RNA populations of rat sensory neurons in the absence of ongoing transcription31, which suggests that co-transcriptional determination of RNA fate through nuclear RBP interactions may not be important for all axonal mRNA localization. In Xenopus oocytes, RNPs are remodelled along the way to their final location with the addition and removal of different RBPs32,33, implying that the fate of an mRNA may be continually fine-tuned by the complement of proteins bound to the transcript.
Several short RNA motifs or cis elements that are necessary and sufficient for localization of mRNAs into axons have been identified, including the ‘zip code’ motif (present in ACTB mRNA) in chick neurons and the MAIL motif (present in Kpnb1 mRNA) and the AU-rich element (ARE; present in Gap43 and Nrn1 mRNAs) in rat and mouse neurons12,34–36. However, no consensus sequences have emerged from studies of these and other axonal mRNA localization elements so far, with the exception of the ARE, which is found in many mRNAs. The question of consensus motifs for axonal localization is further complicated by the finding that RBPs can have multiple effects on the mRNAs to which they bind. For example, HU proteins bind to ARE-containing mRNAs and affect both their survival and their translation37, with the neuronal-specific HUD (also called ELAVL4) binding to Gap43 and Nrn1 AREs and stabilizing these mRNAs in rat neurons38. But it has also been shown that the AREs in Gap43 and Nrn1 mRNAs can drive localization of these mRNAs35,36. The cis elements identified to date range in size from 30–40 nucleotides to a few hundred nucleotides. The longer motifs may represent complex secondary or tertiary structures or binding sites for multiple RBPs that have not yet been defined. It has been shown that even when small motifs of 40–125 nucleotides are used for affinity purification of RBPs from rat peripheral nervous system (PNS) axons, many RBPs can specifically bind to a single RNA motif24. Similar results were observed for the MAIL motif that is responsible for the localization of Kpnb1 mRNA (encoding importin subunit-β1), although it was discovered that nucleolin was the predominant RBP bound to that motif28. The reader is referred to a recent review that outlines known axonal RNA localization motifs and, when known, the RBPs that bind to those motifs39. These RNA localization motifs are by no means unique to neurons, and mRNA localization driving cis elements have been identified in bacteria, metazoans, plants and insects40–43.
RNA-sequencing analysis of the rat PNS axon mRNAs that co-precipitate with several axonal RBPs has further shown that there are tens to hundreds of different interacting mRNAs for each RBP, suggesting that these different mRNAs may be co-transported by the same RBP24. The interactomes of the axonal heterogeneous nuclear RNPs K, H1 and F have been assessed bioinformatically, showing that these rat RBPs bind to RNA regulons linked to axon growth; however, another axonal RBP, the La (also known as SSB) protein, does not bind to growth-related regulons but binds to a different cohort of axonal mRNAs24 (FIG. 2). While binding of an RBP to axonal mRNAs could represent the formation of a transport RNP, the interaction could represent the formation of an RNP with other functions. Another bioinformatic study took a different approach and searched for established binding sites for the RBP splicing factor proline and glutamine rich (SFPQ) in mRNAs that had been shown to localize into axons; this established an RNA regulon associated with axonal survival that included Bcl2l2 and Lmnb2 mRNAs29. However, as outlined above, RNA profiling studies have shown that other RBPs bind to many different mRNAs, so it is likely that an unbiased test for axonal SFPQ targets would provide a more systematic assessment of an axonal SFPQ RNA regulon. RNA interactomes for ZBP1, FMRP, and HUD — RBPs that have been linked to axonal mRNA localization — have shown hundreds of mRNAs specifically binding to these proteins44–47. However, these analyses were performed with whole cell or tissue extracts rather than the subcellular compartment(s) relevant for their localizing activities. The RNA interactomes of other RBPs linked to axonal localization, such as nucleolin and hermes, have similarly not been tested at the level of axons. There is clearly a need for more unbiased profiling of RBP–mRNA interactions directly within axons.
Fig. 2 |. Defining axonal mRNA regulons.

An RNA-binding protein (RBP) can interact with multiple axonal mRNAs that encode proteins with complementary functions, thereby defining an axonal RNA regulon24,29. The schematic depicts three axonal RNA regulons in which related mRNAs (depicted in different shades of blue, red or purple) are bound by a single shared RBP. Additional RBPs bind to this complex, either in the nucleus or in the cytoplasm, to establish a ribonucleoprotein (RNP) for axonal localization. The regulon-defining RBP is shown here as a nuclear RBP, on the basis of the finding that heterogeneous nuclear RNPs bind to axonal mRNAs that are associated with axon growth24. This does not exclude the possibility of a regulon defined by cytoplasmic RBPs. Axonal RNA regulons for axon growth24 and mitochondrial function (axon survival)29 have been reported, while a regulon for injury signalling in the axon was inferred from work showing that nucleolin is needed for the axonal localization of both Kpnb1 and Mtor mRNAs28,30.
Interactomes.
Sets of molecular interactions; for example, an RNA-binding protein has both an ‘RNA interactome’ and a ‘protein interactome’ that define the macromolecules that it interacts with.
An RNA consensus motif that retains mRNAs in the neuronal soma, a fate that is conferred by its interaction with Pumilio 2, has recently been uncovered48. This finding supports the contention, long held by many in the field, that mRNAs do not lack subcellular localizing activity but are rather actively restricted from subcellular sites through specific RNA motifs. Such cell body retention may differ for individual mRNAs between different neuron types, since Actg mRNA localizes into axons of cultured motor neurons but is retained in the cell body of cortical and sensory neurons49. Ironically, the soma-restricting RNA motif is, thus far, better defined as a consensus than the axon-localizing motifs, which suggests that the number of different RBPs that can drive axonal mRNA localization is potentially greater than the number that can restrict mRNAs to the neuronal cell body.
A further consideration when one is attempting to understand the mechanisms that drive the axonal localization of mRNAs is the affinities of mRNAs for individual RBPs. Different motifs can clearly compete for binding to an RBP23, and it has been shown that heterologous overexpression of axonal RNA localization motifs can block the axonal localization of multiple mRNAs50.
mRNA transport into axons
RBPs and mRNAs assemble into granules for transport into axons.
According to a reductionist view, an axonally localizing mRNA is first bound by an RBP and then linked to motor proteins through adaptor proteins for transport into axons. On the basis of several lines of evidence, multiple proteins can bind to an mRNA via localization motifs as well as other regions of the mRNA (FIG. 1c). For example, ZBP1 and heterogeneous nuclear RNP R bind to the 3′ UTR of Actb mRNA51,52, and a complex containing both HUD and ZBP1 must bind to the ARE of Gap43 for axonal localization, at least in rat PNS neurons35. Proteins that do not directly bind RNA can also be part of a transport granule and have an impact on axonal mRNA transport and translation; for example, SMN helps to assemble RNPs but also localizes into axons and is needed for the proper axonal localization of Actb and Gap43 mRNAs53,54. As noted above, RNPs are transported into axons and dendrites as membraneless, organelle-like granules that range in size, 100 nm or greater in diameter55. Both in axons and dendrites there is evidence that granule populations can be distinguished on the basis of the RBPs that they contain56–59 and, although not fully characterized, multiplex in situ hybridization studies have shown that there is a limited number of mRNAs within dendritic RNA transport granules60. Finally, work in HeLa cells shows that single mRNAs are present in smaller granules containing IMP1 and YBX1 (REF.61). Although how the different RNP sizes affect function is not clear, the RNA granule size variations may reflect the existence of transport granules with different RNA–RBP compositions and/or the growing and shrinking of granules through the addition or removal of constituents for different needs.
Adaptor proteins.
Proteins containing specific protein-binding sites that facilitate interactions between protein binding partners (for example, proteins linking cargos to motor proteins).
Transport granule.
An RNA-protein complex, or ribonucleoprotein, that is needed for transport of mRNAs to subcellular sites.
RNPs form through a self-assembly process. In some (but not all) RNPs, liquid–liquid phase separation (LLPS) generates phase-separated condensates in which mRNAs and proteins are selectively concentrated62. It is therefore appealing to speculate that RNA transport granules may also be generated through LLPS, although it is not clear whether and how motor proteins can tether to phase-separated condensates for the distribution of mRNAs along the axon and it is unlikely that all RNPs self-assemble by LLPS. Several lines of evidence indicate that LLPS occurs as a result of the presence of intrinsically disordered regions or low-complexity regions (LCRs) within select RBPs, which self-oligomerize through non-specific weak interactions (reviewed in REF.63). FUS, TDP43 and TIA1, RBPs the genes of which are subject to mutations causing amyotrophic lateral sclerosis (ALS), each undergo this type of self-assembly through LCRs64. Notably, RNP granules are likely to fuse and split, allowing the constant mixing of molecules within these structures as well as their exchange with the cytoplasm65 (although the larger pathological aggregates that result from ALS mutations appear less dynamic63). Post-translational modifications can alter the LLPS tendencies of some RBPs. For FMRP, an RBP that localizes to dendrites and axons, phosphorylation increases its LLPS, while methylation decreases this property66. In contrast, phosphorylation of G3BP1 decreases its LLPS ability based on work in vitro and in cell lines67,68. LLPS by axonal G3BP1 is increased immediately following axotomy in rats, with newly synthesized casein kinase 2α phosphorylating G3BP1 and decreasing the numbers of axonal G3BP1 granules69.
Liquid–liquid phase separation.
(LLPS). A process in which solutions of macromolecules (proteins and nucleic acids) transition to form a membraneless phase-separated cytoplasmic condensate.
Several lines of evidence point to the RNA transport granule as providing a level of translational control by preventing the translation of neuronal mRNAs while they are in transit to distal neurites70 (FIG. 3a,b). For example, ZBP1 binding blocks translation of Actb mRNA in NG108-15 neuroblastoma and HEK293 cells, but phosphorylation of ZBP1 by SRC-family kinases reduces its affinity for mRNA, thereby releasing it for translation71. The FMRP-containing RNPs that undergo phase separation, as mentioned above, contain eukaryotic translation initiation factor 4E-binding protein 2 and microRNA (miRNA) miR-125b, both of which have the capacity to inhibit generalized cap-dependent and specific mRNA translation66. Biochemical purification of RNA granules through either affinity purification for motor protein interaction or differential sedimentation has shown that they contain many different translation factors, RBPs, RNA helicases and ribosome components58,72,73, indicating that a transport granule is likely to contain many different proteins with the capacity to directly or indirectly regulate translation. It is notable, however, that these approaches result in the analysis of a mixture of different RNA granules, so the published granule protein and mRNA compositions likely reflect combinations of many different granule types rather than an individual granule population. Furthermore, evidence has been provided for the transition of FMRP-containing granules from RNA transport granules to polysome-interacting units58. This is likely to reflect multifunctionality of FMRP, a common feature of RBPs, but also emphasizes the dynamic nature of RNPs and underscores the need for better temporal resolution when one is defining RNA granule composition. In addition, the transport of stalled polysomes into dendrites was recently suggested in a study showing that the RNA helicase UPF1 is needed for polysome stalling on Map1b mRNA in the cell soma but also for dendritic Map1b mRNA localization74. This raises the possibility that mRNAs that undergo translation that is initiated by multiple ribosomes can be translationally stalled and redirected to subcellular domains as a mechanism for spatial and temporal translational control in neurons.
Fig. 3 |. The fate of mRNAs on reaching their axonal destinations.

a | Schematic of a neuron showing the transport of mRNA and pre-microRNAs (pre-miRNAs) to their axonal destinations. Ribosomes and translation factors accumulate in growth cones and at spots along the axon shaft, including at branch points in cultured neurons6,15,153; these regions have been referred to as ‘hotspots’ for translation in axons. Axonal mRNAs are presumably released from a transport ribonucleoprotein (RNP) or vesicle (endosome or lysosome) at or near those hotspots. However, axonal translation is likely not limited to these regions. The released mRNAs can be translated or sequestered into axonal mRNA storage depots. b | mRNAs in the transport granule are thought to be translationally suppressed during their transport72,154 and single RNA-binding proteins (RBPs) have been shown to prevent mRNA translation when bound71. On arrival in axons and release from RBPs, mRNAs can be immediately translated or they can be stored in stress granule-like compartments as seen for G3BP1 granules in rodents and TIAR-2 granules in Caenorhabditis elegans90,91. c | Vesicle-coupled mRNAs are thought to be similarly translationally repressed by RBPs during their transport. On release of the RNP from the vesicle and subsequent release of RBPs, the mRNA can be immediately translated or stored in axonal stress granule-like compartments. RNPs bound to lysosomes include G3BP1 (REF.77), which has also been shown to store mRNAs in axons91, suggesting that the transport RBP may be remodelled on release to become a storage depot for axonal mRNAs. d | RNA-induced silencing complex (RISC) components have been shown to associate with vesicles76,116, suggesting that vesicle co-transported pre-miRNAs may provide a second means to suppress the translation of local mRNAs until needed. That is, an ‘on demand’ maturation of the pre-miRNA that happens before or after its release from the vesicle could place it and RISC components in close proximity to the released mRNAs and allow translational silencing.
Polysome.
A complex of multiple ribosomes bound to an mRNA that is typically regarded as a site of active translation.
Some axonal RNAs associate with vesicles for transport and translation.
Several recent publications have demonstrated a previously unrecognized association of some axonal mRNAs with membrane bound organelles — including endosomes, multivesicular bodies, lysosomes, and proteins involved in endoplasmic reticulum (ER)–Golgi complex trafficking — that have been linked to RNA regulation across different cellular systems (BOX 1). In neurons, recent studies have shown that endosomes, lysosomes and mitochondria contribute to axonal RNA transport and/or translational regulation75–77 (FIG. 1d). In the case of RNA transport, RNPs that bind to vesicles have been described as ‘hitchhiking’, which implies a somewhat random process in which a passing vehicle picks up a passenger in search of a ride78. This mechanism is energetically advantageous, having the potential to transport many axonal cargoes at once, and so is undoubtedly driven by specific interactions that have undergone centuries of evolution. A similar hitchhiking mechanism has been documented in numerous other organisms that show polarized subcellular organization (BOX 1), such as the filamentous fungus Ustilago maydis79.
Box 1 |. organelle-based mRNA transport and translation mechanisms.
mRNAs have been shown to ‘hitchhike’ onto components of the endocytic membrane transport pathway in a number of polarized cells types. endosomes, lysosomes, and multivesicular bodies are components of the endocytic membrane transport pathway155. These membrane-bound organelles have a characteristic luminal pH and are defined by their specific rib GtPase protein content. endosomes can originate by budding from the Golgi complex or by inteRNAlization from the plasma membrane. endocytosis of plasma membrane proteins can generate ‘signalling endosomes’ for intracellular signalling or that can be recycled back to the plasma membrane to regulate membrane components such as receptors and ion channels. Other endosomes are transported between different regions of the cell (in a mechanism known as transcytosis), such as axonal and dendritic compartments. early endosomes (expressing RAB5A) can undergo maturation to become late endosomes and multivesicular bodies (expressing RAB7), which have key roles in the sorting of endocytosed cargos and can fuse with the lysosomes that mediate protein degradation. Multivesicular bodies can also fuse with the plasma membrane to release exosomes. together, these components bring flexibility to the control of RNA localization and translation in specific subcellular regions.
The yeast Saccharomyces cerevisiae provided early evidence for differential RNA transport in specific cellular subcompartments. ASH1 and several other mRNAs were shown to localize and co-migrate with the cortical endoplasmic reticulum (er) during daughter cell budding, using the RNA-binding protein (rBP) she2 as a tether156–159. COPI proteins drive localization of mRNAs for translation at or near mitochondria in yeast, and these mRNAs mislocalize to the ER when the mRNA–COPI protein complex is disrupted160. the transition from a yeast-like cell to a polarized hyphal growth in the fungus Ustilago maydis requires the transport of CDC3 mRNA, which hitchhikes on RAB5A-positive endosomes in a complex with the RBP RRM4 and the adaptor protein uPa1 (REFS79,113). ribosomes associated with these vesicles provide an efficient means to asymmetrically deliver and establish polar growth79,161. endosomes were also recently implicated in transport of GluB mRNA to the cortical ER in endosperm cells of rice162.
Recent work in mammalian cells extends the roles of the ER in the synthesis of cytoplasmic proteins. the translation of mRNAs encoding secreted and membrane-bound proteins is well established to occur at the rough ER, but it was recently shown that translation of a broad population of mRNAs encoding cytoplasmic proteins also occurs at the ER163. another recent study found distinct populations of ribosome-bound mRNAs interacting with the ER proteins LRRC59 and SEC61B in HEK293 cells, with particular enrichment of mRNAs encoding organelle-associated proteins163. this suggests that the ER provides a compartmentalized subcellular environment for the translation of specific mRNAs.
Some initial hints that vesicle-based RNA transport is present in neurons could be inferred from work showing that COPB1, a subunit of the coatomer complex that coats vesicles that are transported between the ER and the Golgi complex, contributes to the microtubule-based transport of murine Kor (also known as Oprk1) mRNA into sensory axons80. Similarly, a protein component of the endosome sorting complex required for transport (ESCRT), ESCRTII, was shown to be an RBP for Drosophila bicoid mRNA81. ESCRT plays a role in endosome sorting and multivesicular body formation, but has many additional functions, including receptor signalling82. More recent work shows that ESCRTII colocalizes with actb mRNA, the axon guidance protein receptor Dcc and early endosomes in the growth cones of Xenopus retinal ganglion cells83. These and other studies provided links between vesicle trafficking-associated proteins and axonal mRNAs that could impact axonal mRNA transport and/or translation. Consistent with these hints, recent studies from several groups have provided direct links between axonal RNPs and the endosomal pathway, spanning from early endosomes to lysosomes, pointing to these vesicles as transport and translational control vehicles for RNAs75–77,84 (FIG. 1d).
Endosomes and lysosomes are well suited as hitchhiking platforms for axons since they can undergo long-range bidirectional transport along microtubules85. Adaptor proteins, such as pleckstrin homology domain-containing family M member 2 (PLEKHM2; also known as SKIP) — in combination with ADP-ribosylation factor-like protein 8 (ARL8) — and HOOK proteins, link the vesicles to kinesin and dynein motor proteins for anterograde and retrograde axonal transport, respectively86,87. The movement of endosomes and lysosomes is characterized by directional changes and pauses, and many of these organelles undergo retrograde and anterograde transport in relatively equal proportions88. Although we generally think of axonal mRNAs as moving anterogradely, there are instances of retrograde movements of RBPs and RNAs. For example, the axonal RBP La is anterogradely transported in its native form and retrogradely transported after sumoylation89. Other examples of the retrograde movement of RNPs are mentioned below. Even though the function of mRNAs moving retrogradely is currently not clear, having an ‘on demand’ mechanism to relocalize mRNA cohorts from one region to another within the axon could be advantageous in responding to different stimuli, provided that there is some specificity to the response with regard to the delivered mRNAs. Thus, bidirectional transport of vesicles within axons could provide the means to reposition mRNAs and translational machinery along the axon.
It was recently shown that the RBPs G3BP1, TDP43, and caprin 1 hitchhike on lysosomes in the axons of cultured rodent cortical neurons75. G3BP1 and caprin 1 are components of stress granules, organelle-like structures used to store translationally silent mRNAs during periods of cellular stress68. Notably, intra-axonal stress granule-like structure formation by G3BP1 (in rats and mice) or the stress granule protein TIAR-2 (in Caenorhabditis elegans) has been shown to decrease axonal regeneration after axotomy by decreasing axonal mRNA translation90,91. G3BP1-containing RNA granules interact with lysosomes through the adaptor protein annexin A11 (ANXA11) for transport along axons in rat, zebrafish and stem cell-derived human neurons75. Annexins are a protein family that show conserved membrane binding and contain Ca2+-interacting domains92. ANXA11’s interaction with lysosome and endosome compartments occurs via its carboxy terminus and is Ca2+ dependent. Disruption of this interaction through the introduction of protein encoded by a mutant ANXA11 gene linked to ALS blocks transport of G3BP1-containing RNPs and Actb mRNA75. G3BP1 contains three LCRs and is known to self-assemble via LLPS. Recent work indicates this self-assembly requires interactions with RNAs67,68. Furthermore, the amino-terminal region of ANXA11 also contains an LCR sequence that promotes LLPS that may enable its interaction with RNPs75.
The relationship between the ANXA11-carrying lysosome linked G3BP1 granules that are transported along axons and G3BP1’s role in stress granules is not clear. For example, it is unknown whether the G3BP1 RNA transport granule is distinct from the axonal G3BP1-containing stress granule-like structure or whether the transport granule matures into an axonal mRNA storage depot once it has docked in the axons. Further work will clearly be needed to resolve this question, but the RNP modifications that are documented to occur in transit in Xenopus oocytes33 provide an appealing mechanism for changes in granule function (FIG. 3c). Overall, these recent studies provide a provocative mechanism for the movement of large RNA granules rapidly across axons, and the proteins linking RNPs to vesicles have clear links to human diseases. Notably, neurons cultured to model ALS, spinal muscular atrophy or peripheral neuropathies have shown alterations in axonal mRNA localization93–97.
Notably, recent studies have reported that a significant number of organelles labelled by the lysosome marker LAMP1 lack major lysosomal hydrolases98, so it remains unknown whether those lysosomes linked to RNA transport are actually active lysosomes. One can speculate that there are different vesicle types linked to axonal RNPs, in a manner similar to the mixture of different RNA granules present in the biochemical preparations outlined above. Further work will be needed to define the nature and potential variations of the vesicles associated with RNA localization. Furthermore, Rab7a-carrying late endosomes have recently been implicated as sites of mRNA translation in Xenopus laevis retinal ganglion cell axons77. These Rab7a vesicles frequently paused at axonal mitochondria and transported mRNAs that encode mitochondrial proteins, thus providing a delivery (and potentially synthesis) platform to support axonal mitochondrial function. The introduction of rab7a mutations identical to those that cause Charcot–Marie–Tooth 2B neuropathy in humans in these neurons decreased both intra-axonal protein synthesis and neuronal mitochondrial function77. It is important to mention that most of the axonal mRNAs examined in this study were transported into axons in a vesicle-independent fashion. In combination with the work in G3BP1 granules outlined above, this emphasizes the fact that vesicle-based RNA localization and translation mechanisms account for the localization of only a subset of axonal mRNAs.
Vesicle-associated transport extends to axonal miRNAs.
Work in several different neuronal systems has shown that non-coding RNAs (ncRNAs) localize into both axons and dendrites. These include small miRNAs and PIWI-interacting RNAs19,99. miRNAs modulate translation and/or survival of mRNAs in axons100–106. Although not as well studied as miRNAs, PIWI-interacting RNAs are also presumed to regulate axonal mRNA survival on the basis of what is known of their functions in other systems107. There is evidence that pre-miRNAs, the precursors that must be processed for miRNA functionality, can be transported into both axons and dendrites and then processed locally in response to different stimuli108,109. This provides a mechanism to locally modulate the axonal translatome, but it is not clear how these ncRNAs actually get into axons. There are reports of the transfer of ncRNAs as well as ribosomes to axons from glial cells110 (BOX 2), but many of the experimental systems in which axonal ncRNAs were initially observed used compartmentalized cultures in which glia are not included in the axonal compartment. This suggests that mechanisms have evolved to move ncRNAs into axons, and that these mechanisms exhibit specificity that means that not all ncRNAs make their way into axons and dendrites.
Box 2 |. Axonal ribosome dynamics.
Early electron microscopy studies showing that mature CNS axons do not contain polysomes led to the unfortunate conclusion that axons were not capable of protein synthesis164. More recent work has shown that translation in CNS axons largely occurs through single ribosomes bound to mRNAs (monosomes)165, which may help to explain why it was missed by the classical electron microscopy work6 and has implications for our understanding of how the neuron regulates axonal protein synthesis. Consistent with this, there are now several reports of mRNAs and translational machinery in CNS axons, even in the mature CNS166–169. indeed, recent work examining axonal translation in the forebrain proposed that translation in CNS axons is likely to be “more a rule than an exception”166. in addition, there are instances in which polysomes have been detected in vertebrate peripheral nervous system (PNS) axons, suggesting that translation could occur in axons in a manner similar to that in dendrites6,170.
A study in 2008 raised the intriguing possibility that ribosomes could be transferred to distal PNS axons from surrounding schwann cells110. although transfer was initially observed in distal severed axons (that is, in the segment that will go on to degenerate), it was more recently shown that transfer to injured PNS axons still connected to the soma is also possible171,172. it is not clear whether this mechanism extends to the CNS, but it is intriguing to consider it in light of the distances that axons extend. very recent work suggested that the transfer may be microtubule dependent and include glial-derived mRNAs173. At least in vitro, Schwann cell-derived exosomes can transfer miRNAs to neurons and modify axon outgrowth174. One therefore has to keep in mind that at least some of the ribosomes and RNAs identified in axons could derive from other cells. This is particularly the case in vivo, where axons extend manyfold longer distances than they do in culture conditions (where much of the work on axonal mRNA transport/translation has been performed). Other cellular systems were recently shown to transfer mRNA from one cell to another through membrane nanotubes175; the close relationship of glia to axons could facilitate such a mechanism.
Axons contain an extensive population of mRNAs, including many that encode ribosomal proteins (RPs)19. this is curious, given that biogenesis of ribosome subunits was thought to occur strictly in the nucleolus. However, there is evidence that ribosomes are not a homogeneous population, and several lines of evidence point to the existence of functionally distinct ribosome cohorts176,177. This raises the possibility that local translation of axonal RP mRNAs could alter ribosome function if the proteins could be added to the ribosome subunits locally in axons. indeed, a recent article showed RNA motif-dependent translation of axonal RP mRNA codons and the addition of the nascently translated rP to the axonal ribosome178. Other recent work showed that different RPs associate with the guidance cue receptors DCC, neuropilin 1 (NRP1) and ROBO2 through an RNA-dependent mechanism134. Proteosome-dependent degradation of some axonal ribosome constituents as axons initiate synapse formation has been shown, which emphasizes the dynamic nature of axonal ribosomes179.
Similarly to mRNAs, pre-miRNAs have been shown to be transported through interactions between RBPs and vesicles76,84 (FIG. 1d). Neuronal pre-miR-134 localizes into dendrites via the DEAH-box RNA helicase DHX36, which directly binds to pre-miR-134’s terminal loop111. So far, RBPs that are required for axonal localization of pre-miRNAs or miRNAs have not been reported. However, it is known that pre-miRNA-338’s localization into rat sympathetic axons is microtubule dependent, and mass spectrometry of proteins co-precipitating with pre-miRNA-338 identified RBPs, translational machinery constituents, components of the 40S and 60S ribosomal subunits and important motor proteins112. Pre-miR-338 associates with mitochondria and modulates translation of cellular mRNAs encoding mitochondrial proteins. This association with mitochondria is an intriguing parallel with coding RNAs, and raises the possibility of vesicle-mediated trafficking of axonal pre-miRNAs and/or miRNAs. Indeed, recent work in Xenopus provided evidence that axonal pre-miRNAs can localize into axons through association with vesicles84. This study showed that Xenopus pre-miR-181a-1 co-traffics with late endosome and/or lysosome compartments, with the pre-miRNA showing both anterograde and retrograde movements along axons to ultimately reside in the central domain of the retinal ganglion cell growth cone. Importantly, super-high-resolution microscopy showed that the pre-miRNA molecules are tethered to the outer membrane of the late endosomes and lysosomes rather than inside the vesicle84. G3BP1 RNPs were similarly seen adjacent to the outer membrane of lysosomes by electron microscopy of human U2OS cells75. Although details on how pre-miR-181a-1 docks onto the vesicular membrane are still missing, it is intriguing to speculate this role may be served by the molecular adaptor ANXA11, as is the case for the axonal G3BP1-containing RNA granules75, or the protein UPA1, as is the case for the link between the RBP RRM1 and endosomes in filamentous fungi79,113. Notably, it has been shown that both the enzyme Dicer, a ribonuclease needed for miRNA maturation that had been shown to localize into axons previously114,115, and miR-124 co-traffic along with acidic vesicles in murine motor axons76. This raises the possibility that pre-miRNAs may co-traffic with the machinery needed to cleave them into mature miRNAs, which is analogous to sending the ‘factory’ as a single unit into the axons rather than simply bringing the parts to the factory (FIG. 3c).
A critical unanswered question is whether the pre-miRNA processing occurs entirely separately from the transported granule or whether vesicle-associated pre-miRNA is processed during transport to regulate mRNAs within the granule. These possibilities are not mutually exclusive, so both may occur depending on the pre-miRNA and RNA cargos, which would bring substantial flexibility for regulating dynamics of axonal mRNAs and translation. Also, the translational activity of different mRNAs could change depending on the granule’s subcellular environment, meaning that there may be an advantage to selectively attenuating the translation of some but not all mRNAs that are adjacent to or being transported by a vesicle compartment. Notably, the RNA-induced silencing complex (RISC) that is needed for miRNA function depends on the endosomal pathway116, supporting the idea miRNA functionality is linked to transported vesicles.
Organizing translation ‘on demand’
Axonally synthesized proteins support developmental and regenerative axon growth, axon maintenance and axon survival, and play a role in neurodegeneration through their local functions in the axon or by generating retrograde signals that are sent to the soma117,118 (FIG. 4). Many of these retrograde signals modify transcriptional programmes in the nucleus, indicating that axonally synthesized proteins can broadly impact the neuronal transcriptome (reviewed in REFS119,120). There have been hints at the specificity of the axon’s translational responses to different stimuli. For example, attractive cues increase translation of actb mRNA in growth cones, whereas repulsive cues stimulate translation of cofilin mRNA in Xenopus laevis121,122. The links between axonal RNAs and vesicles as well as the association of axonal RNAs with other organelles and organelle-like structures discussed earlier raise the question of whether these structures help to organize the translational specificity of the axon’s response to different stimuli and physiological states. Indeed, there is evidence for a higher level of organization for translational regulation that is likely to help to match the local translatome to the temporal and spatial physiological needs of the axon’s domains and subdomains. RNA granules isolated from neurons and the brain of different species have, in some cases, been shown to contain translation factors and ribosome subunits in addition to RBPs and mRNAs58,59,72,73,123. This, coupled with the translational suppression or stalling of transported mRNAs outlined earlier herein74, suggests that the RNA transport granule — on its own or in association with vesicles — could provide a platform to mobilize mRNA translation once the RNP reaches the correct location in the axon (FIG. 3). It is clear that different stimuli can newly activate or increase translation of different mRNAs to provide an ‘on demand’ mechanism to change the axonal proteome. In the following sections, we suggest that the regulatory platforms described above provide a level of organization for translational control in axons that parallels the concept of RNA regulons.
Fig. 4 |. Distinct axonal translational control mechanisms have different functional outcomes.

The concept of RNA regulons (FIG. 2) can be extended to encompass groups of axonal mRNAs that exhibit translational regulation on the basis of the functional effects of the encoded proteins. The schematic illustrates examples of these regulatory mechanisms. a | Late endosome-coupled mRNA transport has been linked to several mRNAs involved in the support of mitochondrial function, which is needed for collateral branching of axons77,141,142. b | Some axonal mRNAs that encode injury response-associated and axon regeneration-associated proteins are translationally silenced by storage in G3BP1 granules (stress granule-like structures) in mammalian peripheral nervous system axons; phosphorylation of G3BP1 (G3BP1PS149) triggers disassembly of these granules and the release of the mRNAs for translation67,68,91. TIAR-2 granules have similarly been linked to the storage of growth-associated axonal mRNAs in the axons of Caenorhabditis elegans, with phosphorylation of TIAR-2 favouring granule disassembly90. c | Cell surface receptors can sequester mRNAs and ribosome subunits in the axoplasm, releasing these for synthesis of proteins presumably needed for axon guidance on the receptor binding to those guidance cues133,134. d | Increased levels of axoplasmic Ca2+ following injury can trigger endoplasmic reticulum (ER) stress-associated phosphorylation of eukaryotic translation initiation factor 2A (EIF2A), which can suppress generalized protein synthesis but paradoxically upregulate the synthesis of proteins needed for response to injury119,144,145,147,148. Motifs in both the 5′ untranslated region (UTR) and the 3′ UTR of axonal mRNAs have been shown to modulate both their transport into axons and their translation within axons; these post-transcriptional mechanisms are mediated by RNA-binding proteins and by translation initiation factors interacting with those motifs. Such a mechanism could therefore underlie the injury response. Notably, signals activated by trophic and tropic stimuli can converge on translation initiation factors to regulate axonal mRNA translation in addition to the receptor-mediated storage mechanism depicted in panel d. e | Although not as clearly linked to RNA regulons, the repression of translation in axons as well as potentially ‘on demand’ degradation of some axonal mRNAs can be regulated through microRNAs (miRNAs). Thus far, this regulatory mechanism has been linked to mitochondrial function100,112. f | Several of the axonally translated proteins that are regulated by these mechanisms can contribute to axon-to-soma signalling to further regulate gene expression117.
Platforms to store mRNAs within axons until needed.
The axon must make a choice of whether to translate or store an mRNA once its transport ceases. It was recently shown that axonal G3BP1 granules are used to store mRNAs in mature rat PNS axons91 (FIG. 4b). Kpnb1 and Nrn1 mRNAs are stored in these granules in mammalian axons, but Gap43 mRNA is not stored and its translation is not affected by G3BP1 overexpression or aggregate disassembly. The translation of mRNAs stored in G3BP1 granules is needed for optimal axon regrowth after axotomy, since G3BP1 granule disassembly as a result of the application of a cell-permeable peptide from G3BP1’s acidic domain in rodents or the knockdown of TIAR-2 in C. elegans increases axon growth90,91. Thus, the sequestering of axonal mRNAs in stress granule-like structures can be thought of as a general mechanism for modulating an RNA regulon for axon growth after injury, at least as tested thus far. The finding that Gap43 mRNA is not regulated by G3BP1 points to the existence of distinct cohorts of mRNAs in axons, regulated by different cellular platforms. From comparison of the published profiles of axonal mRNAs from rat and mouse neurons124–126 with those of mRNAs co-precipitating with G3BP1 from whole cell or tissue lysates of various species127–130, there are potentially many G3BP1-interacting axonal mRNAs beyond Kpnb1, Nrn1 and Actb mRNAs that have been published to date75,91. However, it is not known whether mRNA storage by G3BP1 and TIAR-2/TIA1 is something that the neuron utilizes for normal functions and thus whether the response to axotomy simply takes advantage of a normal physiological mechanism. If such non-stressed roles exist for G3BP1 and TIAR-2/TIA1 granules in neurons, then we should stop referring to these axonal structures as ‘stress granules’.
Another mechanism for storing axonal mRNAs has been uncovered that provides potential for direct modulation by extracellular ligands. Adenomatous polyposis complex (APC), a microtubule plus-end-binding protein, was shown to bind mRNAs in pseudopodia of motile fibroblasts131. APC prominently localizes to rat neuronal growth cones, and it has been shown that growth cone mRNAs and axonal microtubules are linked through their interactions with APC132. It was unclear whether APC plays a role in transport of the mRNAs, but the association of APC with microtubules and its concentration in the growth cone raise the more likely possibility that it serves as a storage depot for some localized mRNAs.
Further evidence for storage of mRNAs in growth cones came from a study showing that ribosome components and translation factors bind the cytoplasmic tail of the guidance cue receptor DCC in murine growth cones, with ligand binding to DCC activating protein synthesis adjacent to the receptor133. This provided a compelling mechanism for directly coupling translation to extracellular stimuli – essentially, DCC is ‘geared up and ready’ to rapidly generate new proteins for the growth cone once activated (FIG. 4c). This concept was recently extended to include other guidance cue receptors, including neuropilin 1 (NRP1) and ROBO2, but not EPHB2 (REF.134). This ribosome–receptor coupling is mRNA dependent, and distinct RBPs and mRNA populations co-precipitate with each receptor134. The ligand-dependent assembly of polysomes along the receptor-associated mRNAs requires endocytosis, thereby providing a link to the vesicular transport mechanisms outlined above. It will be of interest to determine whether other ligand–receptor systems are similarly geared up for translation. Nonetheless, the ribosome–receptor coupling and stress granule-like storage outlined above point to stimulus-dependent intra-axonal mechanisms that are specifically tuned to release cohorts of mRNAs for translation.
Axonal mitochondria and adjacent sites bring a mechanism for translational regulation.
Although not known as a storage depot, mitochondria are emerging as another platform for regulating translation of localized mRNA cohorts (FIG. 4a). The local translation of nuclear-encoded mitochondrial protein mRNAs was initially seen in the squid giant axon135. This was later replicated in mammalian neurons, where mRNAs encoding mitochondrial proteins are translated adjacent to or even on the mitochondrial membrane136–138. For example, the axonally synthesized products of CoxIV (also known as Cox4i1) and Lmnb2 mRNAs maintain mitochondrial function and are needed for axon survival138,139, but it is not presently known whether these mRNAs are translated at or near axonal mitochondria. These and other examples suggest that there is a mitochondrial-linked RNA regulon that includes mRNAs that encode proteins needed for mitochondrial function in axons. It has been shown that the axonally synthesized protein BCL-2L2 (also called BCL-W) supports axon survival by inhibiting inositol 3-phosphate receptor 1 (IP3R1) on the axonal ER membrane95. Since ER Ca2+ released by IP3R1 activation can precipitate the release of mitochondrial Ca2+ that ultimately causes axon degeneration140, this brings Bcl2l2 mRNA into the mitochondrial-linked RNA regulon and implicates SFPQ, the protein that cotransports axonal Bcl2l2 and Lmnb2 mRNAs29, in the regulation of this regulon.
Mitochondrial respiration has also been shown to support localized protein synthesis. For example, protein synthesis-dependent collateral branching of axons requires that transported mitochondria stall along axons to generate a localized source of ATP for translation141. Similarly, physiological stimuli that activate synaptic plasticity require a focal source of ATP (mitochondria) to support mRNA translation at the synapse, creating a compartmentalized region of localized protein synthesis adjacent to the activated synapse142. It is appealing to speculate that the filopodia that transition into an axon branch when induced by nerve growth factor (NGF)141,143 similarly represent compartmented regions of the axon that support mRNA translation. This is also a further indication that extracellular stimuli can converge on this perimitochondrial translation compartment.
Translational regulation in axons by calcium and stress signalling.
After axonal injury in the rat PNS, an influx of extracellular Ca+2 into the injured axon and subsequent Ca2+ release from the axonal ER have been shown to mobilize the translation of stored axonal mRNAs39 (FIG. 4d). This increased level of axoplasmic Ca2+ activates the translation of Kpnb1, Ranbp1, Stat3 and Vim (encoding vimentin) mRNAs within a few hours after crush injury of the sciatic nerve. The release of Ca2+ from axonal ER stores also increases translation of Calr mRNA in axons through phosphorylation of the translation initiation factor EIF2A144. It was recently shown that the axotomy-induced increase in Calr mRNA translation requires activation of PERK, thereby pointing to a contribution of localized ER stress145. Since the ER chaperone proteins needed for folding nascently synthesized proteins are Ca2+ dependent146, depletion of ER Ca2+ triggers ER stress and results in an unfolded protein response. Activation of the unfolded protein response in axons promotes translation of Calr, Grp78 (also known as Hspa5 and Bip) and Luman (also known as Creb3) mRNAs in axons144,145,147 and promotes axon regeneration after PNS nerve injury148. Together, these observations point to ER-associated signalling as a platform to regulate local synthesis of axonal injury-associated proteins. It will be of interest to determine whether this translational regulatory mechanism is used to respond to other types of axonal stress and whether this involves a shared ‘stress RNA regulon’. Axonal translation in response to the SEMA3A guidance cue was recently shown to similarly require phosphorylation of EIF2A149, suggesting that this ER-modulated RNA regulon likely has functions beyond injury. These signalling pathways likely layer onto the translational platforms outlined above to bring further temporal and spatial specificity. Additionally, ribosomes themselves may provide some specificity to axonal translation, as a result of both the mechanisms through which they get into axons and their composition (BOX 2).
Unfolded protein response.
A molecular response that occurs when levels of unfolded proteins increase in the endoplasmic reticulum; this reduces overall protein synthesis to decrease continued load of unfolded proteins and allows the cell to respond to different types of stress.
Summary and future perspectives
As we hope the reader will glean from this Review, neurons tightly regulate mRNA transport into axons and translation within axons to enable many different functions. Thankfully, the field has been able to rapidly transition from proving that translation occurs in axons to understanding the biology of the system. Mutations in genes encoding proteins needed for axonal mRNA transport, storage and translation have been linked to human neurological disorders associated with axon degeneration, including peripheral neuropathies and motor neuron degeneration, emphasizing the importance of the functions that axonally synthesized proteins serve. Indeed, expression of ALS-associated mutations in the gene encoding TDP43 in mice precipitate broad but specific changes in the axonal mRNA and miRNA populations73.
Publications emerging over the last few years point to a higher level of organization for regulating the axonal proteome through localized translation. RNPs and organelle-like RNA granules (some assembled through LLPS and some true membrane-bound organelles) have been shown to provide molecular platforms that play a role not only in the transport of mRNAs but also in their translation. By co-packaging ribosome subunits, translation factors and pre-miRNAs with some of these transport vessels, the neuron can effectively send out much of the factory for translation to sites in the axon or just deliver the mRNA templates. Storage of mRNAs once localized, in granules and associated with receptors, brings a compartmentalizing feature for translation ‘on demand’ independent of the neuronal cell body. Cohorts of mRNAs sharing these different platforms — that is, RNA regulons — bring the opportunity to functionally organize mRNA transport and translation in order to drive the axon’s response to different stimuli and physiological conditions to optimally and autonomously fill its needs.
While we imply that these axonal RNA regulons are specific for different axonal responses and functions, there are undoubtedly some overlapping mRNAs between these regulons. It is intriguing to speculate that different mechanisms are used for transport or translation of these overlapping mRNAs depending on the functions they serve. There is perhaps some evidence for this already with Calr mRNA having distinct 3′ UTR motifs for ligand-dependent versus constitutive transport into axons150. It is also conceivable that the axon makes use of combinations of just a few RNA regulons to mount specific responses to different stimuli and physiological states. To fully understand the functional organization of axonal RNA regulons implied here, we need to better systematically define the different mRNAs that support different functions of the axon at both the transport level and the translational level. As we learn more about these mechanisms, there will undoubtedly be connections between these and additional regulatory layers for axonal functions and responses. There are some hints of connections in the literature now. For example, transport of G3BP1 granules through ANX11A was suggested to require Ca2+ release from lysosomes75, and G3BP1 is deacetylated by HDAC6, with acetylated G3BP1 blocking its interaction with RNAs151. Axonal HDAC6 is activated by Ca2+ and deacetylates MIRO1 to decrease mitochondrial transport in axons152. It will be of interest to determine how this may affect the compartmentalized synthesis of mitochondrial proteins outlined above and how the axon balances this and other post-translational modifications to fine-tune axonal protein synthesis.
Acknowledgements
The authors are supported by the following funding sources for work related to the topic of this Review: US National Institutes of Health (R01-NS089633 and R01-NS041596 to J.L.T.; K01-NS105879 to T.P.S.), US National Science Foundation (MCB-1020970 to J.L.T.), Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (to J.L.T), South Carolina Spinal Cord Injury Research Fund (2019-PD-02 to P.K.S.), South Carolina EPSCoR Stimulus Research Program (18-SR04 to J.L.T.) and the University of South Carolina Research Office ASPIRE programme (to J.L.T. and A.N.K.). J.L.T. is the incumbent SmartState Chair of Childhood Neurotherapeutics at the University of South Carolina.
Footnotes
Competing interests
J,L.T. and P.K.S. have a US Patent for G3BP1 as a target for accelerating axon regeneration (US Patent 10,668,128). A.N.K., P.K.S. and J.L.T. have applied for a US patent for G3BP1 as a target for preventing neurodegeneration.
References
- 1.Steward O & Levy WB Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J. Neurosci 2, 284–291 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steward O & Schuman EM Protein synthesis at synaptic sites on dendrites. Annu. Rev. Neurosci 24, 299–325 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Kosik KS Life at low copy number: how dendrites manage with so few mRNAs. Neuron 92, 1168–1180 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Ohashi R & Shiina N Cataloguing and selection of mRNAs localized to dendrites in neurons and regulated by RNA-binding proteins in RNA granules. Biomolecules 10, 167 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobias G & Koenig E Axonal protein synthesizing activity during the early outgrowth period following neurotomy. Exp. Neurol 49, 221–234 (1975). [DOI] [PubMed] [Google Scholar]
- 6.Koenig E & Martin R Cortical plaque-like structures indentify ribosome-containing domains in the Mauthner cell axon. J. Neurosci 16, 1400–1411 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koenig E Synthetic mechanisms in the axon—II: RNA in myelin-free axons of the cat. J. Neurochem 12, 357–361 (1965). [DOI] [PubMed] [Google Scholar]
- 8.Tennyson VM The fine structure of the axon and growth cone of the dorsal root neuroblast of the rabbit embryo. J. Cell Biol 44, 62–79 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guiditta A, Menichini E, Capano C & Langella M Active polysomes in the axoplasm of the squid giant axon. J. Neurosci. Res 28, 18–28 (1991). [DOI] [PubMed] [Google Scholar]
- 10.Giuditta A, Metafora S, Flesani A & Rio AD Factors for protein synthesis in the axoplasm of giant squid axon. J. Neurochem 28, 1393–1395 (1977). [DOI] [PubMed] [Google Scholar]
- 11.Holt CE, Martin KC & Schuman EM Local translation in neurons: visualization and function. Nat. Struct. Mol. Biol 26, 557–566 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Zhang HL et al. Neurotrophin-induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron 31, 261–275 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Hanz S et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 40, 1095–1104 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Brittis PA, Lu Q & Flanagan JG Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell 110, 223–235 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Zheng JQ et al. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J. Neurosci 21, 9291–9303 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung H, Yoon BC & Holt CE Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat. Rev. Neurosci 13, 308–324 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das S, Singer RH & Yoon YJ The travels of mRNAs in neurons: do they know where they are going? Curr. Opin. Neurobiol 57, 110–116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steward O mRNA localization in neurons: a multipurpose mechanism? Neuron 18, 9–12 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Kar A, Lee S & Twiss J Expanding axonal transcriptome brings new functions for axonally synthesized proteins in health and disease. Neuroscientist 24, 111–129 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keene JD RNA regulons: coordination of post-transcriptional events. Nat. Rev. Genet 8, 533–543 (2007). [DOI] [PubMed] [Google Scholar]; This review summarizes the evidence for mRNA cohorts encoding functionally linked proteins, termed ‘RNA regulons’, being co-regulated by shared RNPs. These regulons provide functional organization for post-transcriptional regulation.
- 21.Andreassi C, Crerar H & Riccio A Post-transcriptional processing of mRNA in neurons: the vestiges of the RNA world drive transcriptome diversity. Front. Mol. Neurosci 11, 304 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Hir H, Gatfield D, Izaurralde E & Moore MJ The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J. 20, 4987–4997 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colak D, Ji SJ, Porse BT & Jaffrey SR Regulation of axon guidance by compartmentalized nonsense-mediated mRNA decay. Cell 153, 1252–1265 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S et al. hnRNPs binding to the axonal localization motifs of Nrn1 and HMGB1 mRNAs define growth-associated RNA regulons. Mol. Cell Proteom 17, 2091–2106 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parchure A, Munson M & Budnik V Getting mRNA-containing ribonucleoprotein granules out of a nuclear back door. Neuron 96, 604–615 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Palacios IM RNA processing: splicing and the cytoplasmic localisation of mRNA. Curr. Biol 12, R50–R52 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Pan F, Huttelmaier S, Singer RH & Gu W ZBP2 facilitates binding of ZBP1 to beta-actin mRNA during transcription. Mol. Cell Biol 27, 8340–8351 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perry RB et al. Nucleolin-mediated RNA localization regulates neuron growth and cycling cell size. Cell Rep. 16, 1664–1676 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cosker KE, Fenstermacher SJ, Pazyra-Murphy MF, Elliott HL & Segal RA The RNA-binding protein SFPQ orchestrates an RNA regulon to promote axon viability. Nat. Neurosci 19, 690–696 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terenzio M et al. Locally translated mTOR controls axonal local translation in nerve injury. Science 359, 1416–1421 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willis DE et al. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J. Cell Biol 178, 965–980 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis RA, Gagnon JA & Mowry KL PTB/hnRNP I is required for RNP remodeling during RNA localization in Xenopus oocytes. Mol. Cell Biol 28, 678–686 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis RA & Mowry KL Ribonucleoprotein remodeling during RNA localization. Differentiation 75, 507–518 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Perry RB et al. Subcellular knockout of importin beta1 perturbs axonal retrograde signaling. Neuron 75, 294–305 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoo S et al. A HuD-ZBP1 ribonucleoprotein complex localizes GAP-43 mRNA into axons through its 3′ untranslated region AU-rich regulatory element. J. Neurochem 126, 792–804 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akten B et al. Interaction of survival of motor neuron (SMN) and HuD proteins with mRNA cpg15 rescues motor neuron axonal deficits. Proc. Natl Acad. Sci. USA 108, 10337–10342 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otsuka H, Fukao A, Funakami Y, Duncan KE & Fujiwara T Emerging evidence of translational control by AU-rich element-binding proteins. Front. Genet 10, 332 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomes C et al. Axonal localization of neuritin/CPG15 mRNA is limited by competition for HuD binding. J. Cell Sci 130, 3650–3662 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith TP, Sahoo PK, Kar AN & Twiss JL Intra-axonal mechanisms driving axon regeneration. Brain Res. 1740, 146864 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes SC & Simmonds AJ Drosophila mRNA localization during later development: past, present, and future. Front. Genet 10, 135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kannaiah S & Amster-Choder O Protein targeting via mRNA in bacteria. Biochim. Biophys. Acta 1843, 1457–1465 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Lazzaretti D & Bono F mRNA localization in metazoans: a structural perspective. RNA Biol. 14, 1473–1484 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian L, Chou HL, Fukuda M, Kumamaru T & Okita TW mRNA localization in plant cells. Plant Physiol. 182, 97–109 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolognani F, Contente-Cuomo T & Perrone-Bizzozero NI Novel recognition motifs and biological functions of the RNA-binding protein HuD revealed by genome-wide identification of its targets. Nucleic Acids Res. 38, 117–130 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conway AE et al. Enhanced CLIP uncovers IMP protein-RNA targets in human pluripotent stem cells important for cell adhesion and survival. Cell Rep. 15, 666–679 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darnell JC et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maurin T et al. HITS-CLIP in various brain areas reveals new targets and new modalities of RNA binding by fragile X mental retardation protein. Nucleic Acids Res. 46, 6344–6355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez JC et al. Pum2 shapes the transcriptome in developing axons through retention of target mRNAs in the cell body. Neuron 104, 931–946 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moradi M et al. Differential roles of alpha-, beta-, and gamma-actin in axon growth and collateral branch formation in motoneurons. J. Cell Biol 216, 793–814 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donnelly CJ et al. Limited availability of ZBP1 restricts axonal mRNA localization and nerve regeneration capacity. EMBO J. 30, 4665–4677 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossoll W et al. Specific interaction of Smn, the spinal muscular atrophy determining gene product, with hnRNP-R and gry-rbp/hnRNP-Q: a role for Smn in RNA processing in motor axons? Hum. Mol. Genet 11, 93–105 (2002). [DOI] [PubMed] [Google Scholar]
- 52.Glinka M et al. The heterogeneous nuclear ribonucleoprotein-R is necessary for axonal beta-actin mRNA translocation in spinal motor neurons. Hum. Mol. Genet 19, 1951–1966 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Fallini C, Donlin-Asp PG, Rouanet JP, Bassell GJ & Rossoll W Deficiency of the survival of motor neuron protein impairs mRNA localization and local translation in the growth cone of motor neurons. J. Neurosci 36, 3811–3820 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donlin-Asp PG et al. The survival of motor neuron protein acts as a molecular chaperone for mRNP assembly. Cell Rep. 18, 1660–1673 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moujaber O & Stochaj U Cytoplasmic RNA granules in somatic maintenance. Gerontology 64, 485–494 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Miller LC et al. Combinations of DEAD box proteins distinguish distinct types of RNA: protein complexes in neurons. Mol. Cell Neurosci 40, 485–495 (2009). [DOI] [PubMed] [Google Scholar]
- 57.Christie SB, Akins MR, Schwob JE & Fallon JR The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits. J. Neurosci 29, 1514–1524 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El Fatimy R et al. Tracking the fragile X mental retardation protein in a highly ordered neuronal ribonucleoparticles population: a link between stalled polyribosomes and RNA granules. PLoS Genet. 12, e1006192 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akins MR et al. Axonal ribosomes and mRNAs associate with fragile X granules in adult rodent and human brains. Hum. Mol. Genet 26, 192–209 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Batish M, van den Bogaard P, Kramer FR & Tyagi S Neuronal mRNAs travel singly into dendrites. Proc. Natl Acad. Sci. USA 109, 4645–4650 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mateu-Regue A et al. Single mRNP analysis reveals that small cytoplasmic mRNP granules represent mRNA singletons. Cell Rep. 29, 736–748 e734 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Langdon EM & Gladfelter AS A new lens for RNA localization: liquid-liquid phase separation. Annu. Rev. Microbiol 72, 255–271 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Guo L & Shorter J It’s raining liquids: RNA tunes viscoelasticity and dynamics of membraneless organelles. Mol. Cell 60, 189–192 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xue YC et al. Dysregulation of RNA-binding proteins in amyotrophic lateral sclerosis. Front. Mol. Neurosci 13, 78 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gopal PP, Nirschl JJ, Klinman E & Holzbaur EL Amyotrophic lateral sclerosis-linked mutations increase the viscosity of liquid-like TDP-43 RNP granules in neurons. Proc. Natl Acad. Sci. USA 114, E2466–E2475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsang B et al. Phosphoregulated FMRP phase separation models activity-dependent translation through bidirectional control of mRNA granule formation. Proc. Natl Acad. Sci. USA 116, 4218–4227 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang P et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 181, 325–345 e328 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guillen-Boixet J et al. RNA-induced conformational switching and clustering of G3BP drive stress granule assembly by condensation. Cell 181, 346–361 e317 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sahoo P et al. A translational switch drives axonal stress granule disassembly through casein kinase 2α. Curr. Biol 10.1016/j.cub.2020.09.043 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Yang at al. (2020) and Guillen-Boixet et al. (2020), this study shows the role of RNA interactions in LLPS by stress granule protein G3BP1, with Sahoo et al. (2020) focusing on axonal G3BP1.
- 70.Pimentel J & Boccaccio GL Translation and silencing in RNA granules: a tale of sand grains. Front. Mol. Neurosci 7, 68 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huttelmaier S et al. Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature 438, 512–515 (2005). [DOI] [PubMed] [Google Scholar]
- 72.Krichevsky AM & Kosik KS Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron 32, 683–696 (2001). [DOI] [PubMed] [Google Scholar]
- 73.Kanai Y, Dohmae N & Hirokawa N Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron 43, 513–525 (2004). [DOI] [PubMed] [Google Scholar]
- 74.Langille JJ, Ginzberg K & Sossin WS Polysomes identified by live imaging of nascent peptides are stalled in hippocampal and cortical neurites. Learn. Mem 26, 351–362 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liao YC et al. RNA granules hitchhike on lysosomes for long-distance transport, using annexin A11 as a molecular tether. Cell 179, 147–164 e120 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Gershoni-Emek et al. (2018) and Cioni et al. (2019), this study links axonal mRNA transport and/or translational regulation to cellular organelles.
- 76.Gershoni-Emek N et al. Localization of RNAi machinery to axonal branch points and growth cones is facilitated by mitochondria and is disrupted in ALS. Front. Mol. Neurosci 11, 311 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cioni JM et al. Late endosomes act as mRNA translation platforms and sustain mitochondria in axons. Cell 176, 56–72 e15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salogiannis J & Reck-Peterson SL Hitchhiking: a non-canonical mode of microtubule-based transport. Trends Cell Biol. 27, 141–150 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baumann S, Konig J, Koepke J & Feldbrugge M Endosomal transport of septin mRNA and protein indicates local translation on endosomes and is required for correct septin filamentation. EMBO Rep. 15, 94–102 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bi J, Tsai NP, Lu HY, Loh HH & Wei LN Copb1-facilitated axonal transport and translation of kappa opioid-receptor mRNA. Proc. Natl Acad. Sci. USA 104, 13810–13815 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Irion U & St Johnston D bicoid RNA localization requires specific binding of an endosomal sorting complex. Nature 445, 554–558 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wollert T et al. The ESCRT machinery at a glance. J. Cell Sci 122, 2163–2166 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Konopacki FA et al. ESCRT-II controls retinal axon growth by regulating DCC receptor levels and local protein synthesis. Open Biol. 6, 150218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Corradi E et al. Axonal precursor miRNAs hitchhike on endosomes and locally regulate the development of neural circuits. EMBO J. 39, e102513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scott CC, Vacca F & Gruenberg J Endosome maturation, transport and functions. Semin. Cell Dev. Biol 31, 2–10 (2014). [DOI] [PubMed] [Google Scholar]
- 86.Rosa-Ferreira C & Munro S Arl8 and SKIP act together to link lysosomes to kinesin-1. Dev. Cell 21, 1171–1178 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reck-Peterson SL, Redwine WB, Vale RD & Carter AP The cytoplasmic dynein transport machinery and its many cargoes. Nat. Rev. Mol. Cell Biol 19, 382–398 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maday S, Twelvetrees AE, Moughamian AJ & Holzbaur EL Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron 84, 292–309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Niekerk EA et al. Sumoylation in axons triggers retrograde transport of the RNA-binding protein La. Proc. Natl Acad. Sci. USA 104, 12913–12918 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Andrusiak MG et al. Inhibition of axon regeneration by liquid-like TIAR-2 granules. Neuron 104, 290–304 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Sahoo et al. (Nat. Commun., 2018), this study focuses on functional roles of stress granule proteins and LLPSs for attenuation of axon regeneration.
- 91.Sahoo PK et al. Axonal G3BP1 stress granule protein limits axonal mRNA translation and nerve regeneration. Nat. Commun 9, 3358 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rescher U & Gerke V Annexins–unique membrane binding proteins with diverse functions. J. Cell Sci 117, 2631–2639 (2004). [DOI] [PubMed] [Google Scholar]
- 93.Rotem N et al. ALS along the axons - expression of coding and noncoding RNA differs in axons of ALS models. Sci. Rep 7, 44500 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alami NH et al. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron 81, 536–543 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pease-Raissi SE et al. Paclitaxel reduces axonal Bclw to initiate IP3R1-dependent axon degeneration. Neuron 96, 373–386 e376 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bobylev I et al. Paclitaxel inhibits mRNA transport in axons. Neurobiol. Dis 82, 321–331 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Saal L, Briese M, Kneitz S, Glinka M & Sendtner M Subcellular transcriptome alterations in a cell culture model of spinal muscular atrophy point to widespread defects in axonal growth and presynaptic differentiation. RNA 20, 1789–1802 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheng XT et al. Revisiting LAMP1 as a marker for degradative autophagy-lysosomal organelles in the nervous system. Autophagy 14, 1472–1474 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Phay M, Kim HH & Yoo S Analysis of piRNA-like small non-coding RNAs present in axons of adult sensory neurons. Mol. Neurobiol 55, 483–494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aschrafi A et al. MicroRNA-338 regulates the axonal expression of multiple nuclear-encoded mitochondrial mRNAs encoding subunits of the oxidative phosphorylation machinery. Cell Mol. Life Sci 69, 4017–4027 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kar AN, Macgibeny MA, Gervasi NM, Gioio AE & Kaplan BB Intra-axonal synthesis of eukaryotic translation initiation factors regulates local protein synthesis and axon growth in rat sympathetic neurons. J. Neurosci 33, 7165–7174 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bellon A et al. miR-182 regulates Slit2-mediated axon guidance by modulating the local translation of a specific mRNA. Cell Rep. 18, 1171–1186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang B et al. FMRP-mediated axonal delivery of miR-181d regulates axon elongation by locally targeting Map1b and Calm1. Cell Rep. 13, 2794–2807 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Dajas-Bailador F et al. microRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nat. Neurosci 15, 697–699 (2012). [DOI] [PubMed] [Google Scholar]
- 105.Hancock ML, Preitner N, Quan J & Flanagan JG MicroRNA-132 is enriched in developing axons, locally regulates Rasa1 mRNA, and promotes axon extension. J. Neurosci 34, 66–78 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y et al. The microRNA-17–92 cluster enhances axonal outgrowth in embryonic cortical neurons. J. Neurosci 33, 6885–6894 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ozata DM, Gainetdinov I, Zoch A, O’Carroll D & Zamore PD PIWI-interacting RNAs: small RNAs with big functions. Nat. Rev. Genet 20, 89–108 (2019). [DOI] [PubMed] [Google Scholar]
- 108.Kim HH, Kim P, Phay M & Yoo S Identification of precursor microRNAs within distal axons of sensory neuron. J. Neurochem 134, 193–199 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sambandan S et al. Activity-dependent spatially localized miRNA maturation in neuronal dendrites. Science 355, 634–637 (2017). [DOI] [PubMed] [Google Scholar]
- 110.Court FA, Hendriks WTJ, Mac Gillavry HD, Alvarez J & van Minnen J Schwann cell to axon transfer of ribosomes: toward a novel understanding of the role of glia in the nervous system. J. Neurosci 28, 11024–11029 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bicker S et al. The DEAH-box helicase DHX36 mediates dendritic localization of the neuronal precursor-microRNA-134. Genes Dev. 27, 991–996 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vargas JN et al. Axonal localization and mitochondrial association of precursor microRNA 338. Cell Mol. Life Sci 73, 4327–4340 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pohlmann T, Baumann S, Haag C, Albrecht M & Feldbrugge M A FYVE zinc finger domain protein specifically links mRNA transport to endosome trafficking. eLife 4, e06041 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hengst U, Cox LJ, Macosko EZ & Jaffrey SR Functional and selective RNA interference in developing axons and growth cones. J. Neurosci 26, 5727–5732 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murashov AK et al. RNAi pathway is functional in peripheral nerve axons. FASEB J. 21, 656–670 (2007). [DOI] [PubMed] [Google Scholar]
- 116.Gibbings D & Voinnet O Control of RNA silencing and localization by endolysosomes. Trends Cell Biol. 20, 491–501 (2010). [DOI] [PubMed] [Google Scholar]
- 117.Sahoo PK, Smith DS, Perrone-Bizzozero N & Twiss JL Axonal mRNA transport and translation at a glance. J. Cell Sci 131, jcs196808 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cioni JM, Koppers M & Holt CE Molecular control of local translation in axon development and maintenance. Curr. Opin. Neurobiol 51, 86–94 (2018). [DOI] [PubMed] [Google Scholar]
- 119.Terenzio M, Schiavo G & Fainzilber M Compartmentalized signaling in neurons: from cell biology to neuroscience. Neuron 96, 667–679 (2017). [DOI] [PubMed] [Google Scholar]
- 120.Tasdemir-Yilmaz OE & Segal RA There and back again: coordinated transcription, translation and transport in axonal survival and regeneration. Curr. Opin. Neurobiol 39, 62–68 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Piper M et al. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron 49, 215–228 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Strohl F et al. Single molecule translation imaging visualizes the dynamics of local beta-actin synthesis in retinal axons. Sci. Rep 7, 709 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elvira G et al. Characterization of an RNA granule from developing brain. Mol. Cell Proteom 5, 635–651 (2006). [DOI] [PubMed] [Google Scholar]
- 124.Gumy LF et al. Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA 17, 85–98 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Briese M et al. Whole transcriptome profiling reveals the RNA content of motor axons. Nucleic Acids Res. 44, e33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Minis A et al. Subcellular transcriptomics-dissection of the mRNA composition in the axonal compartment of sensory neurons. Dev. Neurobiol 74, 365–381 (2014). [DOI] [PubMed] [Google Scholar]
- 127.Edupuganti RR et al. N6-methyladenosine (m6A) recruits and repels proteins to regulate mRNA homeostasis. Nat. Struct. Mol. Biol 24, 870–878 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khong A et al. The stress granule transcriptome reveals principles of mRNA accumulation in stress granules. Mol. Cell 68, 808–820 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang K et al. Stress granule assembly disrupts nucleocytoplasmic transport. Cell 173, 958–971 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martin S et al. Preferential binding of a stable G3BP ribonucleoprotein complex to intron-retaining transcripts in mouse brain and modulation of their expression in the cerebellum. J. Neurochem 139, 349–368 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Mili S, Moissoglu K & Macara IG Genome-wide screen reveals APC-associated RNAs enriched in cell protrusions. Nature 453, 115–119 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Preitner N et al. APC is an RNA-binding protein, and its interactome provides a link to neural development and microtubule assembly. Cell 158, 368–382 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tcherkezian J, Brittis PA, Thomas F, Roux PP & Flanagan JG Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell 141, 632–644 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Koppers et al. (2019), this study provides evidence for axon guidance cue receptors storing axonal mRNAs, with ligand binding by the receptor providing an ‘on demand’ cue for translation of the stored mRNAs.
- 134.Koppers M et al. Receptor-specific interactome as a hub for rapid cue-induced selective translation in axons. eLife 8, e48718 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gioio AE et al. Local synthesis of nuclear-encoded mitochondrial proteins in the presynaptic nerve terminal. J. Neurosci. Res 64, 447–453 (2001). [DOI] [PubMed] [Google Scholar]
- 136.Aschrafi A, Natera-Naranjo O, Gioio AE & Kaplan BB Regulation of axonal trafficking of cytochrome c oxidase IV mRNA. Mol. Cell Neurosci 43, 422–430 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hillefors M, Gioio A, Mameza M & Kaplan B Axon viability and mitochondrial function are dependent on local protein synthesis in sympathetic neurons. Cell Mol. Neurobiol 27, 701–716 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kar AN et al. Dysregulation of the axonal trafficking of nuclear-encoded mitochondrial mRNA alters neuronal mitochondrial activity and mouse behavior. Dev. Neurobiol 74, 333–350 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yoon BC et al. Local translation of extranuclear lamin B promotes axon maintenance. Cell 148, 752–764 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Villegas R et al. Calcium release from intra-axonal endoplasmic reticulum leads to axon degeneration through mitochondrial dysfunction. J. Neurosci 34, 7179–7189 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Spillane M, Ketschek A, Merianda TT, Twiss JL & Gallo G Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Rep. 5, 1564–1575 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Rangaraju et al. (2019) and Spillane et al. (2012), this study shows that mitochondria provide a platform for localized translation, with mitochondrial function and intra-axonal translation required for neurotrophin-dependent axon branching.
- 142.Rangaraju V, Lauterbach M & Schuman EM Spatially stable mitochondrial compartments fuel local translation during plasticity. Cell 176, 73–84 e15 (2019). [DOI] [PubMed] [Google Scholar]
- 143.Spillane M et al. Nerve growth factor-induced formation of axonal filopodia and collateral branches involves the intra-axonal synthesis of regulators of the actin-nucleating Arp2/3 complex. J. Neurosci 32, 17671–17689 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vuppalanchi D et al. Lysophosphatidic acid differentially regulates axonal mRNA translation through 5′UTR elements. Mol. Cell Neurosci 50, 136–146 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Pacheco et al. (2020), Ying et al. (2015), Onate et al. (2016) and Cagnetta et al. (2019), this study provides evidence for a role of ER stress-associated signalling pathways in regulating specificity of intra-axonal mRNA translation.
- 145.Pacheco A, Merianda TT, Twiss JL & Gallo G Mechanism and role of the intra-axonal calreticulin translation in response to axonal injury. Exp. Neurol 323, 113072 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Malhotra JD & Kaufman RJ The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol 18, 716–731 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ying Z et al. The unfolded protein response and cholesterol biosynthesis link Luman/CREB3 to regenerative axon growth in sensory neurons. J. Neurosci 35, 14557–14570 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Onate M et al. Activation of the unfolded protein response promotes axonal regeneration after peripheral nerve injury. Sci. Rep 6, 21709 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cagnetta R et al. Noncanonical modulation of the eIF2 pathway controls an increase in local translation during neural wiring. Mol. Cell 73, 474–489 e475 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vuppalanchi D et al. Conserved 3′-untranslated region sequences direct subcellular localization of chaperone protein mRNAs in neurons. J. Biol. Chem 285, 18025–18038 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gal J et al. The acetylation of Lysine-376 of G3BP1 regulates RNA binding and stress granule dynamics. Mol. Cell Biol 39, e00052–00019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kalinski AL et al. Deacetylation of Miro1 by HDAC6 blocks mitochondrial transport and mediates axon growth inhibition. J. Cell Biol 218, 1871–1890 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bassell GJ et al. Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J. Neurosci 18, 251–265 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wells DG RNA-binding proteins: a lesson in repression. J. Neurosci 26, 7135–7138 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Elkin SR, Lakoduk AM & Schmid SL Endocytic pathways and endosomal trafficking: a primer. Wien. Med. Wochenschr 166, 196–204 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schmid M, Jaedicke A, Du TG & Jansen RP Coordination of endoplasmic reticulum and mRNA localization to the yeast bud. Curr. Biol 16, 1538–1543 (2006). [DOI] [PubMed] [Google Scholar]
- 157.Aronov S et al. mRNAs encoding polarity and exocytosis factors are cotransported with the cortical endoplasmic reticulum to the incipient bud in Saccharomyces cerevisiae. Mol. Cell Biol 27, 3441–3455 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Genz C, Fundakowski J, Hermesh O, Schmid M & Jansen RP Association of the yeast RNA-binding protein She2p with the tubular endoplasmic reticulum depends on membrane curvature. J. Biol. Chem 288, 32384–32393 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Darzacq X, Powrie E, Gu W, Singer RH & Zenklusen D RNA asymmetric distribution and daughter/mother differentiation in yeast. Curr. Opin. Microbiol 6, 614–620 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Trautwein M, Dengjel J, Schirle M & Spang A Arf1p provides an unexpected link between COPI vesicles and mRNA in Saccharomyces cerevisiae. Mol. Biol. Cell 15, 5021–5037 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zander S, Baumann S, Weidtkamp-Peters S & Feldbrugge M Endosomal assembly and transport of heteromeric septin complexes promote septin cytoskeleton formation. J. Cell Sci 129, 2778–2792 (2016). [DOI] [PubMed] [Google Scholar]
- 162.Tian L et al. Zipcode RNA-binding proteins and membrane trafficking proteins cooperate to transport glutelin mRNAs in rice endosperm. Plant Cell 32, 2566–2581 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hoffman AM, Chen Q, Zheng T & Nicchitta CV Heterogeneous translational landscape of the endoplasmic reticulum revealed by ribosome proximity labeling and transcriptome analysis. J. Biol. Chem 294, 8942–8958 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Steward O Alterations in polysomes associated with dendritic spines during the reinnervation of the dentate gyrus of the adult rat. J. Neurosci 3, 177–188 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Biever A et al. Monosomes actively translate synaptic mRNAs in neuronal processes. Science 367, eaay4991 (2020). [DOI] [PubMed] [Google Scholar]
- 166.Ostroff LE et al. Axon TRAP reveals learning-associated alterations in cortical axonal mRNAs in the lateral amygdala. eLife 8, e51607 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hafner AS, Donlin-Asp PG, Leitch B, Herzog E & Schuman EM Local protein synthesis is a ubiquitous feature of neuronal pre- and postsynaptic compartments. Science 364, eaau3644 (2019). [DOI] [PubMed] [Google Scholar]
- 168.Poulopoulos A et al. Subcellular transcriptomes and proteomes of developing axon projections in the cerebral cortex. Nature 565, 356–360 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shigeoka T et al. Dynamic axonal translation in developing and mature visual circuits. Cell 166, 181–192 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pannese E & Ledda M Ribosomes in myelinated axons of the rabbit spinal ganglion neurons. J. Submicrosc. Cytol. Pathol 23, 33–38 (1991). [PubMed] [Google Scholar]
- 171.Court FA et al. Morphological evidence for a transport of ribosomes from Schwann cells to regenerating axons. Glia 59, 1529–1539 (2011). [DOI] [PubMed] [Google Scholar]
- 172.Muller K et al. A predominantly glial origin of axonal ribosomes after nerve injury. Glia 66, 1591–1610 (2018). [DOI] [PubMed] [Google Scholar]
- 173.Canclini L et al. Association of microtubules and axonal RNA transferred from myelinating Schwann cells in rat sciatic nerve. PLoS ONE 15, e0233651 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lopez-Leal R et al. Schwann cell reprogramming into repair cells increases exosome-loaded miRNA-21 promoting axonal growth. J. Cell Sci 133, jcs239004 (2020). [DOI] [PubMed] [Google Scholar]
- 175.Haimovich G et al. Intercellular mRNA trafficking via membrane nanotube-like extensions in mammalian cells. Proc. Natl Acad. Sci. USA 114, E9873–E9882 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dinman JD The eukaryotic ribosome: current status and challenges. J. Biol. Chem 284, 11761–11765 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Segev N & Gerst JE Specialized ribosomes and specific ribosomal protein paralogs control translation of mitochondrial proteins. J. Cell Biol 217, 117–126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shigeoka T et al. On-site ribosome remodeling by locally synthesized ribosomal proteins in axons. Cell Rep. 29, 3605–3619 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Costa RO et al. Synaptogenesis stimulates a proteasome-mediated ribosome reduction in axons. Cell Rep. 28, 864–876 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


