Abstract
Background
Restrictive food intake in anorexia nervosa (AN) has been related to an overactive cognitive control network inhibiting intuitive motivational responses to food stimuli. However, the influence of short-term homeostatic signaling on the neural regulation of cue-induced food craving in AN is still unclear.
Methods
Twenty-five women with AN and 25 matched normal-weight women were examined on two occasions after receiving either glucose or water directly into their stomach using a nasogastric tube. Participants were blinded to the type of infusion. An event-related functional magnetic resonance imaging paradigm was used to investigate the effect of intestinal glucose load on neural processing during either simple viewing or distraction from food stimuli.
Results
Neural differences between patients with AN and normal-weight participants were found during the distraction from food stimuli, but not during the viewing condition. When compared to controls, patients with AN displayed increased activation during food distraction in the left parietal lobule/precuneus and fusiform gyrus after water infusion and decreased activation in ventromedial prefrontal and cingulate regions after intestinal glucose load.
Conclusions
Independent of the cephalic phase and the awareness of caloric intake, homeostatic influences trigger disorder-specific reactions in AN. Food distraction in patients with AN is associated with either excessive higher-order cognitive control during physiological hunger or decreased internally directed attention after intestinal glucose load. These findings suggest that food distraction plays an important role in the psychopathology of AN. This study was registered on clinicaltrials.gov with identifier: NCT03075371.
Key words: Anorexia nervosa, fMRI, food craving, food distraction, homeostatic mechanisms
Introduction
Anorexia nervosa (AN) is a severely debilitating psychosomatic disorder, most commonly observed in young women and characterized by low body weight, preoccupation with food and an intense fear of gaining weight (American Psychiatric Association, 2013). Previous studies have proposed alterations in cognitive control as well as an altered homeostatic appetite regulation as underlying pathological mechanisms of reduced food intake and starvation in AN (Friederich, Wu, Simon, & Herzog, 2013; Kaye, Wierenga, Bailer, Simmons, & Bischoff-Grethe, 2013a; Tortorella et al., 2014). However, there is still a paucity of research related to the interaction between homeostatic and non-homeostatic factors that control food intake in AN. An imbalance between neural reward and inhibition networks is considered a hallmark feature of AN and might account for the pathological feeding behavior typically observed in patients (Kaye et al., 2013b). In addition to excessive cortical top-down control over limbic-striatal brain regions during food processing, there is evidence for homeostatic disturbances in individuals with AN (Warren, 2011). An aberrant release of gastrointestinal peptides (Casper, 1996; Tanaka et al., 2003), as well as a central nervous system resistance to hormonal satiety signaling (Miljic et al., 2006), is considered to be either a direct consequence of long-term starvation, or an underlying factor in the development and maintenance of dysfunctional food-related behaviors, such as restrained eating or binging and purging (Harada et al., 2008; Lockie & Andrews, 2013; Monteleone & Maj, 2013).
Food intake is driven by non-homeostatic (e.g. food hedonics) and homeostatic (e.g. peripheral physiology) mechanisms and both interact for an adaptive regulation of energy balance (Berthoud, 2006). Previous studies investigating the effect of satiety on neural processing of food stimuli did not control for the cephalic phase of food consumption such as sight, smell, and taste. However, it has been postulated that top-down effects have a strong influence on the subjective perception of sensory properties of food stimuli and therefore on the neural processing of these stimuli (Robinson et al., 2013; Robinson, Kersbergen, & Higgs, 2014). Specifically, projections from brain areas such as the ventral prefrontal cortex (i.e. the subgenual anterior cingulate cortex), amygdala, and hippocampus to the hypothalamus play a decisive role in the suppression of metabolic signals (Berthoud, 2011; Petrovich, Ross, Holland, & Gallagher, 2007). The state of prolonged caloric deprivation is defined as homeostatic hunger, and is driven by a complex series of physiological processes such as hormonal signaling and gastric secretion (Espel-Huynh, Muratore, & Lowe, 2018). Homeostatic satiety, on the other hand, refers to post-ingestive physiological processes such as changes in physical signals of distension as well as metabolic signaling via gut peptide hormones. Therefore, in order to investigate the influence of homeostatic hunger and satiety on disorder-specific processing of food-related stimuli in AN, we employed an experimental design capable of eliminating influences associated with the cephalic phase of food consumption and with the awareness of caloric ingestion. In a sample of women with AN and a matched normal-weight control group, we administered glucose and water on two different occasions directly into the stomach by means of a nasogastric tube. The glucose and water conditions will represent homeostatic satiety and hunger respectively. Since participants were blinded to the type of infusion administered during each study session, this allowed us to examine the impact of homeostasis on neural regulation of cue-induced food craving independently from disorder-related expectations towards food consumption. Therefore, we hypothesized that by abolishing the cephalic phase of food consumption, any observed differences in neural processing will be exclusively related to post-gastric signaling. Taken together, the present study provides a promising approach to gain new and valuable insights into the psychobiological mechanisms of altered intero- and exteroceptive food processing in AN.
Methods
Participants
Twenty-six female patients with AN and 32 age- and education-matched healthy normal-weight women (CON group) were enrolled in the present study. A total of seven participants (N = 1 AN, N = 6 CON) were excluded from the final data analyses due to excessive head movement. The task employed in this study was piloted first and findings from the normal-weight control group (Stopyra et al., 2019) revealed no significant effect of intragastric glucose load on neuronal correlates during the distraction from food. The distraction from high-caloric food images recruited fronto-parietal brain regions, indicative of underlying attentional mechanisms. In the present study, the same healthy participants were included; however, in order to match both groups regarding age, education years, and handedness (one left-handed participant in the AN as well as the CON group), one healthy control participant from the previous study was excluded. Twelve of the patients with AN were of binge-purging subtype. Each participant was screened for medical and psychiatric disorders using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th edition (SCID; Wittchen, Zaudig, & Fydrich, 1997). All other Axis I and II disorders were excluded with exception of a history of (N = 17) or current (N = 12) unipolar depression. Patients with AN received no medication other than antidepressants (of SSRI type) (N = 5). The study was approved by the ethics committee of the medical faculty of the University of Heidelberg and it was in accordance with the ethical standards of the Declaration of Helsinki in 1975 as revised in 2008. Prior to participation all participants gave informed written consent. See Table 1 for demographics.
Table 1.
Demographics and clinical characteristics of the CON and AN groups
| CON group (N = 25) | AN group (N = 25) | p | |||
|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | ||
| Illness duration (years) | – | – | 6.68 | 4.54 | – |
| BMI (kg/m2)** | 21.82 | 1.47 | 15.51 | 1.41 | <0.001 |
| Education (years) | 12.88 | 0.60 | 12.40 | 1.23 | 0.087 |
| BDI-II** | 4.12 | 3.80 | 20.66 | 13.62 | <0.001 |
| EDE-Q – total score** | 11.08 | 9.10 | 78.40 | 33.05 | <0.001 |
| EDE-Q – restraint** | 2.24 | 3.91 | 18.24 | 8.18 | <0.001 |
| EDE-Q – eating concern** | 0.52 | 0.71 | 13.68 | 7.87 | <0.001 |
| EDE-Q – weight concern** | 2.52 | 2.08 | 16.36 | 8.03 | <0.001 |
| EDE-Q – shape concern** | 5.80 | 5.18 | 30.12 | 12.40 | <0.001 |
BDI, Beck depression inventory; BMI, body mass index; EDE-Q, eating disorder examination questionnaire.
**Significant differences between CON and AN groups at p < 0.001.
Self-report measures
Hunger was assessed with a 100-mm visual analogue scale ranging from 0 to 100 before and after each functional magnetic resonance imaging (fMRI) session. The German version of the Eating disorder examination questionnaire (EDE-Q; Hilbert, Tuschen-Caffier, Karwautz, Niederhofer, & Munsch, 2007) was administered in order to assess the severity of disturbed eating patterns as well as the degree of weight and shape concerns. The German version of the Beck Depression Inventory (BDI-II; Hautzinger, Keller, & Kühner, 2006) was used to assess the severity of depressive symptoms.
Procedure
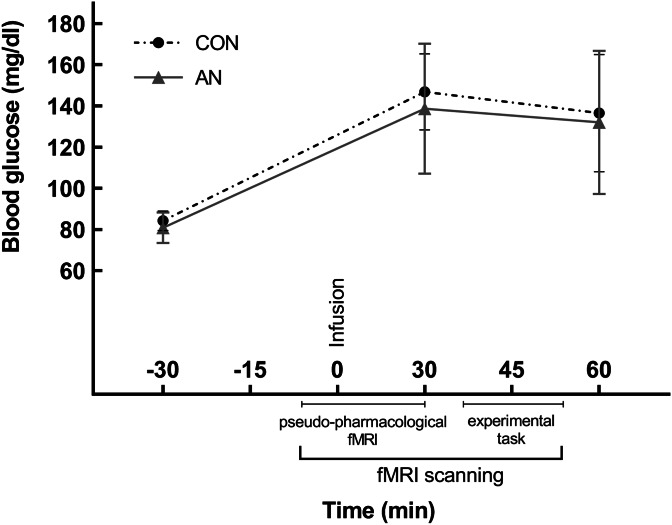
During the first study session, all participants were interviewed with the SCID, filled out questionnaires and their weight and height were measured. Before entering the MRI scanner a fine-bore nasogastric tube (Flocare Nutrisoft, Nutricia GmbH, Erlangen) was placed with its tip in the gastric ventricle ~5 cm below the xiphoid process and fixed with adhesive tape to the participants' cheek and nose. At the beginning of each fMRI session, a pseudo-pharmacological fMRI scan of 5 min was carried out, which was followed by the infusion of either 75 g glucose dissolved in 300 ml water (Accu-Chek® Dextrose O.G-T., Roche, Basel, Switzerland) or 300 ml of plain water. A total of 75 g of glucose, as administered in our study protocol, is the amount of glucose that is normally used in the Oral Glucose Tolerance Test, a standard method to assess glucose sensitivity and therefore, allows for the comparison of our results with previous studies. Furthermore, previous research has shown that 75 g of glucose is a sufficient dose in order to evoke differences in neural response (Brown & Riby, 2013; Sünram-Lea, Foster, Durlach, & Perez, 2001). The administration of water or glucose took ~3 min. The water and glucose solution were administered by the use of a disposable 50 ml syringe (Chirana® 50 ml catheter with Luer adapter, Chirana T. Injecta, A.S.) through the nasogastric tube directly into the stomach. The accurate position of the nasogastric tube was controlled by auscultating during air insufflation. After the infusion of glucose or water, participants underwent additional 30 min of pseudo-pharmacological fMRI. These results will be reported elsewhere. Subsequently, participants performed the experimental task (17 min). Each fMRI session lasted a total of 60 min. A graphical depiction of the fMRI protocol can be derived from Fig. 1. The order of glucose/water infusion was balanced across the two scanning sessions and assigned randomly across participants. Furthermore, three blood samples were taken 30 min before fMRI scanning, 30 min after intragastric infusion (before the experimental paradigm) and 60 min after intragastric infusion (after the experimental paradigm). Means and standard deviations of the behavioral and physiological parameters can be derived from online Supplementary Table S1.
Fig. 1.
Mean and standard deviation of blood glucose levels before and after intragastric glucose administration in the AN and CON groups.
Stimuli and task
Before entering the MRI, participants were instructed to choose eight images out of a sample of 85 images depicting high calorie food. Participants were asked to choose those food images that they found most appetizing and for which they experienced craving in that moment. Eight non-food objects (non-food-related household objects) were matched for color and visual complexity. The experimental paradigm was a modified version of an emotion regulation task (Kanske, Heissler, Schonfelder, Bongers, & Wessa, 2011; Stopyra et al., 2019) and was presented with Presentation software version 18.3 (Neurobehavioral Systems Inc., Berkeley, CA, United States). A graphical example of a distraction trial can be seen in online Supplementary Fig. S1. The task required participants to either look attentively at the content of the presented images (viewing condition) or to solve an arithmetic equation (e.g. 7 + 8 − 5 = 14) as fast and accurately as possible (distraction condition). After the presentation of a fixation cross (jittered duration of 3000–4500 ms), the viewing and distraction condition started with an initial induction phase (1000 ms), in which either an image of food or non-food object was presented. In the viewing condition, the induction phase was followed by viewing instructions (1000 ms) which were shown as a semi-transparent overlay, followed by the presentation of the image for another 5000 ms. In the distraction condition, the induction phase was followed by the arithmetic equation, which was continuously presented for 6000 ms as a semi-transparent overlay. Participants had to indicate by a button press whether the equation was solved correctly or incorrectly. Each experimental condition (viewing and distraction of images) was followed by a rating of participants' current craving (desire) for the presented food (non-food object). Participants rated the intensity of their craving (desire) on a 9-point Likert scale ranging from very low to very high using self-assessment manikins. For each experimental condition every image was presented twice, resulting in the presentation of 64 pseudo-random trials.
Biochemical analysis of glucose
Assessment of glucose concentrations was performed at the central laboratory of the University Clinic Heidelberg on a Siemens Advia 2400 device using the hexokinase method. To assess differences between sessions and groups, we performed a repeated measures ANOVA with satiety (water v. glucose) and time point (before/after infusions of water/glucose) as within-subjects factors, and group as a between-subjects factor. Post-hoc tests were performed with two-sample t tests to assess group differences and paired t tests for differences between time points (two-tailed, p < 0.05).
Image acquisition
Functional imaging was performed on a 3-Tesla Siemens Trio MRI scanner (Siemens Medical Solutions, Erlangen, Germany) at the University Clinic Heidelberg, Germany. In total, 523 functional T2*-weighted images were acquired in one run lasting 17.30 min. An interleaved slice order with a repetition time (TR) of 2000 ms, an echo time (TE) of 30 ms, a flip angle (FA) of 80°, and a voxel size 3 × 3 × 4 mm was used. Each volume consisted of 30 axial slices with a slice thickness of 4 mm with no inter-slice gap and a field of view (FOV) of 192 × 192 × 120 mm. A T1-weighted high-resolution anatomical image with 192 slices (1 × 1 × 1 mm voxel size) was acquired. The scanning parameters for the anatomical image were: FA = 9°, TR = 1900 ms, TE = 2.52 ms, FOV = 256 × 256 × 256 mm.
Data analysis
The analysis of functional imaging data was performed with Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, London, United Kingdom) running in MATLAB version 7.13 (Mathworks, Inc.). Pre-processing of functional scans included slice-time correction, realignment, and spatial normalization to a standard stereotactic space defined by the Montreal Neurological Institute template with a voxel size of 3 × 3 × 4 mm. Spatial smoothing with a full-width at half maximum Gaussian smoothing kernel of 8 mm was applied.
On the single-subject level, significant hemodynamic changes for each condition were assessed by means of a General Linear Model. Events (starting with the presentation of the instructions) and experimental conditions (i.e. food distraction, non-food distraction, viewing food, viewing non-food objects) were defined by the stimulus onset and duration. Subsequently, experimental conditions were modeled and convolved with the hemodynamic response function which resulted in four regressors of interest. A high-pass filter with a cut-off of 128 s was applied to remove low-frequency noise. Furthermore, we entered the following regressors of no interest into the design matrix: movement parameters, ratings, and the induction phase. In order to examine changes in the blood oxygen level-dependent signal, the individual statistical parametric maps were calculated for contrasts of interest which were: (1) viewing food compared to viewing non-food objects (viewing food–viewing non-food) and (2) distraction from food compared to distraction from non-food objects (distraction food–distraction non-food).
At the second level analysis, the resulting contrast images of the fixed effects analysis were entered in a random effects analysis. At first, a 2 × 2 repeated measures ANOVA with group and homeostatic states as factors was calculated. Paired t tests were conducted to assess differences in brain activation between homeostatic satiety states. To examine group differences, independent two-sample t tests were conducted. BDI-II scores were entered as covariates in the group analyses, as depression may influence the neural response to rewarding stimuli (Admon & Pizzagalli, 2015). All results were thresholded at a whole-brain family-wise error (FWE) cluster corrected p < 0.05, and only clusters exceeding 40 voxels are reported. For the visualization of group statistics the MRIcron software (Chris Rorden, Version 4, April 2011, http://people.cas.sc.edu/rorden/mricron/index.html) was used.
Regression analysis
To investigate the relationship between brain activation and food craving during food distraction (i.e. distraction food–distraction non-food), a multiple regression analysis was carried out by entering the food craving scores as regressors of interest in our model. Only results significant at a FWE cluster corrected p < 0.05 and exceeding 40 voxels are reported.
Results
Behavioral results
As shown in Table 1, there were no significant group differences regarding age and years of education between both groups. However, significant differences were found in body mass index (BMI), BDI, and the EDE-Q scores (all Ps < 0.001, see Table 1).
In order to investigate whether participants were blinded to the nature of the liquid, a Pearson's χ2 test was conducted, revealing that the CON and AN groups guessed at the chance level for the first (χ2 = 0.37, p = 0.543 for CON and χ2 = 1.07, p = 0.302 for AN) and second session (CON: χ2 = 3.22, p = 0.07; AN: χ2 = 0.43, p = 0.51).
Hunger ratings
In both groups, paired t tests of subjective hunger ratings revealed no significant differences in hunger before and after scanning during the glucose (CON: t(24) = 1.8, p = 0.085; AN: t(22) = 0.09, p = 0.926) and water (CON: t(24) = −0.118, p = 0.907; AN: t(24) = 0.72, p = 0.477) conditions.
Craving ratings
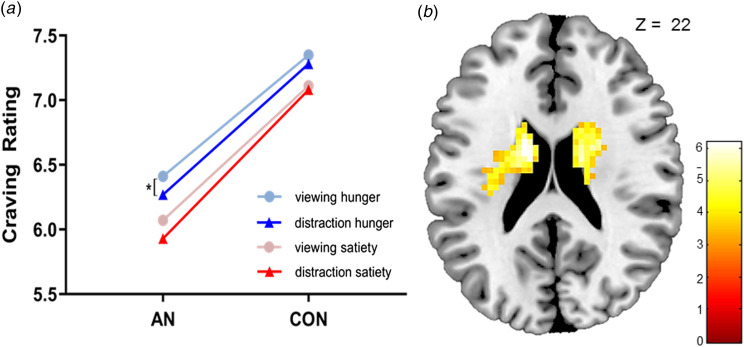
A 2 × 2 repeated-measures ANOVA with satiety state as a within, and group as a between factor, revealed a significant effect of the group on the satiety state, F(2,44) = 4.62, p = 0.015. Paired t tests revealed no differences in craving levels between homeostatic satiety states in the AN and CON group for both the viewing and distraction condition (all Ps > 0.05). The CON group did not exhibit significant differences in cue-induced craving levels during fMRI scanning between the distraction and viewing condition in the glucose (t(24) = 0.32, p = 0.754) and water condition (t(24) = 1.6, p = 0.122). During the water condition, individuals with AN demonstrated significant differences in subjective craving levels during the distraction compared to the viewing condition (t(24) = 2.56, p = 0.017); craving was higher during viewing (M = 6.1, s.d. = 1.94) than during distraction (M = 5.9, s.d. = 1.99). However, during the glucose conditions, patients with AN showed no significant differences in craving between the distraction and viewing condition (Ps > 0.05). Furthermore, there were no significant differences between the restrictive (N = 13) and bulimic (N = 12) AN subtype in craving levels during the water and glucose condition (all Ps < 0.05). Regardless of the homeostatic state (water v. glucose) and experimental condition (viewing v. distraction), the CON group exhibited higher food craving compared to the AN group (all Ps < 0.05, see Fig. 2a).
Fig. 2.
(a) Interaction between craving intensity and task instruction (distraction v. viewing). Under homeostatic hunger, patients with AN significantly decreased their craving when distracting themselves compared to viewing food images (p = 0.017). (b) In patients with AN, craving rating was negatively associated with dorsal striatal activation during the distraction from food craving in the homeostatic hunger condition. This association was neither found in the CON group nor in the satiety condition.
Blood parameter analyses
A 2 × 2 × 3 repeated-measures ANOVA with group, homeostatic satiety state, and time point of blood sampling demonstrated a significant interaction of the satiety state and time point, F(2, 84) = 121.03, p < 0.001. Paired t tests revealed that both groups had a significant increase in blood glucose compared to baseline blood glucose levels (AN group: t(20) = 9.68, p < 0.001; CON group: t(24) = 16.73; p < 0.001). Moreover, there were no significant group differences in blood glucose levels before participants underwent the experimental paradigm (t(45) = 1.06, p = 0.295) in the glucose condition. In both groups and during the execution of the experimental paradigm, blood glucose levels were significantly higher during glucose load compared to the water condition (homeostatic hunger) (AN group: t(20) = 8.8, p < 0.001; CON group: t(24) = 18.61, p < 0.001). See Fig. 1 and online Supplementary Table S1 for the temporal course of blood glucose levels for the AN and CON groups.
fMRI results
Comparison of satiety states in AN group
Paired t tests comparing differences in BOLD activity in response to the distraction food–distraction non-food contrast revealed stronger activation in the precuneus during the water compared to the glucose condition (peak of activation at x = −6, y = −49, z = 14, t = 5.45, p = 0.02, no. of voxels: 66; see online Supplementary Fig. S2). Comparing the glucose condition to the water condition, there were no differences for the distraction food–distraction non-food contrast. Furthermore, no significant differences between glucose and water were observed in the viewing food–viewing non-food contrast.
Comparison of satiety states in CON group
The control group did not exhibit significant differences in brain activation between glucose and water administration in the mentioned contrasts of interest.
Group comparison
The 2 × 2 repeated measures ANOVA with group and homeostatic satiety states as factors revealed a significant interaction effect during distraction food–distraction non-food in the left precuneus (peak of activation at x = −6, y = −55, z = 10, no. of voxels: 44, F(1, 93) = 15.66). No significant interaction effect was found in the viewing food–viewing non-food contrast.
AN v. CON group
An independent two-sample t test showed significant differences between the AN and CON group in the distraction food–distraction non-food contrast during the water condition. Patients with AN compared to CON exhibited significantly higher BOLD activity in the left inferior parietal lobule (IPL)/precuneus, the fusiform gyrus, and middle occipital areas during the water condition (see Table 2, Fig. 3a). There were no further group differences neither for the glucose condition nor the viewing food–viewing non-food contrast.
Table 2.
Between group results obtained from whole brain second level analysis
| Group: metabolic state Contrast |
Hemisphere | BA | x | y | z | No. of voxels | T-value | p value |
|---|---|---|---|---|---|---|---|---|
| AN>CON: Homeostatic hunger | ||||||||
| Distraction food–distraction non-food | ||||||||
| Middle occipital gyrus | L | 18/19 | −36 | −82 | −2 | 321 | 5.16 | <0.001 |
| Inferior parietal lobule | L | 40 | −33 | −43 | 38 | 72 | 4.44 | 0.04 |
| Precuneus | L | 7 | −27 | −49 | 50 | 3.72 | ||
| Fusiform gyrus | L | 37 | −33 | −49 | −18 | 94 | 4.32 | 0.01 |
| CON>AN: Homeostatic satiety | ||||||||
| Distraction food–distraction non-food | ||||||||
| Posterior cingulate cortex | L | 31 | −3 | −58 | 22 | 377 | 5.62 | <0.001 |
| Parahippocampal gyrus | L | 30 | −9 | −40 | −2 | 4.05 | ||
| Superior frontal gyrus | L | 10 | −18 | 56 | 10 | 233 | 4.64 | <0.001 |
| Medial orbitofrontal | L | 11 | −6 | 35 | −18 | 4.42 | ||
| Anterior cingulate cortex | L | 32 | −15 | 44 | 6 | 4.17 | ||
BA, Brodmann area; L, left; R, right.
Results were uncorrected (voxel p < 0.001; cluster >40 voxels, p < 0.05).
Fig. 3.
(a) Brain activation during the distraction from food in the AN group compared to the CON group during homeostatic hunger. The SPM t-map was rendered on a T1-weighted template image supplied with MRIcron. (b) Brain activation during the distraction from food in the CON group compared to the AN group during homeostatic satiety. The SPM t-map was rendered on a T1-weighted template image supplied with MRIcron.
CON v. AN group
During the glucose condition, distraction food–distraction non-food activated the posterior cingulate gyrus, ventral prefrontal regions (medial and superior frontal), and the perigenual anterior cingulate cortex in the CON group compared to the AN group (see Table 2, Fig. 3b). During the water condition, no further group differences were observed.
Regression analyses
Furthermore, we conducted a multiple regression analysis with subjective craving levels during the distraction food–distraction non-food contrast during the water condition. BOLD activation in the bilateral dorsal striatum was negatively associated with craving levels in the AN group (see Fig. 2b and online Supplementary Table S2). No association between craving levels and brain activation was observed in the CON group. Furthermore, no significant results were found during the glucose condition for the AN and CON group.
Discussion
In this study, bypassing the cephalic phase during glucose ingestion allowed the sole investigation of bottom-up, homeostatic influences on the neural regulation of cue-induced food craving in patients with AN. Our findings indicate that prolonged caloric deprivation facilitates the ability of patients to reduce their craving for food and as opposed to healthy controls, influences neural processing during the distraction from food stimuli. We observed increased activation in higher-order cognitive control regions (inferior parietal lobule, precuneus) in the hunger condition and decreased activation in fronto-cingular brain regions after glucose load, reflecting decreased internally directed attention in the AN group.
Distraction from food increased activity in the left precuneus in patients with AN when comparing homeostatic hunger with homeostatic satiety. Interestingly, previous studies on AN found increased activation in the precuneus during viewing of food pictures (Rothemund et al., 2011), but decreased activation when instructed to think about how much they want to eat the displayed food (Scaife, Godier, Reinecke, Harmer, & Park, 2016). Furthermore, research investigating neural processing during exposure to drug-cues in addictive disorders has consistently observed increased activation in this region (Holsen et al., 2012) and found a mediating role of the precuneus during craving resistance in addiction (Courtney, Ghahremani, London, & Ray, 2014; Santel, Baving, Krauel, Münte, & Rotte, 2006; Wierenga et al., 2015). Taken together with the long held assumption of similarities between compulsive traits in AN and addiction (Barbarich-Marsteller, Foltin, & Walsh, 2011; Godier & Park, 2015; Kaye et al., 2013b), our results point to a homeostatically-dependent increased higher-order cognitive control mechanism in response to cue-induced food craving among patients with AN. The observed decrease of subjective craving levels during distraction from food stimuli supports this interpretation. However, since we employed food pictures chosen by patients, our data might be biased regarding the subjective perception of valence. Future studies should investigate the validity of regulation paradigms regarding their ability to tap into typical disorder-related processing.
When compared to normal-weight controls, the AN group exhibited increased activation in the IPL including the precuneus and the fusiform gyrus during the distraction from food stimuli during homeostatic hunger. These regions have been observed during the processing of a number of eating disorder-related stimuli in AN, such as body image (Miyake et al., 2010; Vocks et al., 2010; Wagner, Ruf, Braus, & Schmidt, 2003), food pictures (Santel et al., 2006; Scaife et al., 2016), emotional processing (Fonville, Giampietro, Surguladze, Williams, & Tchanturia, 2014; Suda et al., 2014) as well as negative social interactions (McAdams, Lohrenz, & Montague, 2015). In addition to the above described cognitive control functions of the precuneus, the IPL has been strongly related to visuo-spatial and attentional processes (Behrmann, Geng, & Shomstein, 2004) and might point towards an increased attentional engagement in AN related to disorder-specific stimuli. This finding is supported by eye tracking studies, which suggest that patients with AN exhibit at least an initial attentional engagement to food stimuli (Giel et al., 2011). In light of these findings, the observed results can be seen as a sign of enhanced attentional engagement to food stimuli in patients with AN, necessary to successfully resist food craving, especially during a negative energy balance.
The negative association between increased activation in the dorsal striatum and decreased craving levels in AN is in line with previous observations of striatal alterations during food reward processing and executive control in AN (Holsen et al., 2012; Wierenga et al., 2017; Zastrow et al., 2009). Kaye and colleagues (Kaye, Fudge, & Paulus, 2009) postulated that dopaminergic alterations in striatal circuits may contribute to altered reward and executive functioning, and represent the difficulty of patients with AN to appropriately evaluate and respond to salient stimuli. Furthermore, when asked to choose between different visually depicted food stimuli, patients with AN show increased connectivity between the dorsal striatum and dorsolateral prefrontal cortex, an association found to be related to subsequent food intake (Foerde, Steinglass, Shohamy, & Walsh, 2015). The authors conclude that fronto-striatal networks, that are crucial in the development of habitual behaviors (Tricomi, Balleine, & O'Doherty, 2009), may subserve maladaptive eating behavior in AN. Therefore, given that dorsal-striatal activity was negatively associated with craving ratings during homeostatic hunger, our findings might reflect an automatic, learned strategic response to food stimuli in AN, facilitating avoidance of food and dietary restriction. Noteworthy, the opposite pattern, namely a positive relation between appetite and dorsal striatal activity has been reported in participants of normal-weight as well as participants with obesity (Contreras-Rodriguez, Martin-Perez, Vilar-Lopez, & Verdejo-Garcia, 2017; Volkow et al., 2002), further corroborating our findings of altered neural regulation of food craving in AN. In order to increase the understanding of the development and maintenance of AN, future research should examine the effect of homeostatic satiety states on the neuronal processing of disorder-related stimuli in weight-recovered individuals. Studying weight-recovered or short-time ill adolescents might provide insight with regard to state and trait variables underlying behavioral and neuronal alterations of AN.
These results are in contrast to food distraction during the glucose condition, where normal-weight women, compared to patients with AN, exhibited increased activation in brain regions involved in self-referential processing including incentive salience and emotion regulation (Raichle et al., 2000). These brain areas constitute the default mode network, which is activated during internally directed attention as, for example, during self-reflection and deactivated when attention is externally directed, as, for example, during attentionally demanding tasks (Hayden, Smith, & Platt, 2010). Against this background, the present findings indicate that patients with AN when compared to controls show less internally directed attention due to attentional deployment for the food distraction task. Thus, the findings during homeostatic satiety point towards excessive higher-order cognitive control and decreased internally cued attention to food craving, which may explain the tolerance to starvation in patients with AN. Given that group differences have exclusively been found for the distraction but not the viewing condition, these findings further support the importance of a food distraction mechanism in the psychopathology of AN.
Limitations
There are several limitations to our study. Since we only employed glucose to evoke homeostatic satiety, this cannot precisely mimic the effects of a nutritious meal, as macronutrients are essential for a satiating effect (Chambers, McCrickerd, & Yeomans, 2015). Furthermore, since we only examined distraction from food stimuli, investigating other regulation strategies, such as cognitive reappraisal or avoidance using the current experimental design would be of interest. Therefore, the employed paradigm might be unable to tap into the naturalistic restraining eating behavior observed in AN. Moreover, the success of different regulation strategies has been found to depend on inter-individual preferences (Giuliani, Calcott, & Berkman, 2013) and might differ between the two subtypes of AN. Given the dysfunctional self-regulatory capacities in AN (Fischer & Munsch, 2012), future studies need to examine different self-regulation strategies in AN. Patients with AN exhibit high rates of comorbid anxiety disorders (Kaye, Bulik, Thornton, Barbarich, & Masters, 2004) and therefore, the present study might have only studied a subsample of AN. The exclusion of co-morbid anxiety disorders might impede inferences to the general population of patients with AN. Additionally, the distraction from visually presented food stimuli may not be suitable for triggering reactions related to the psychopathology of AN. According to Murray and Strigo (Murray & Strigo, 2018), neuroimaging food cue paradigms should be situated in the context most salient to the psychopathology of AN, which refers mainly to food consumption and the intention to eat, rather than passive exposure to food stimuli. Given that patients with AN often display paradoxical approach towards as well as avoidance behavior away from food, future studies should aim at designing a more naturalistic approach by examining patients' intention to eat. For example, patients having to choose food that they are required to eat after fMRI data acquisition could hold more validity than passive viewing paradigms. Furthermore, the two subtypes of AN might exhibit food craving for different foods, which should be accounted for when studying appetitive behavior in patients with AN.
Conclusions
The behavioral and neurobiological findings indicate that food distraction plays an important role in the psychopathology of AN. In patients with AN, food distraction during homeostatic hunger was associated with increased activation in brain regions involved in higher-order control, possibly reflecting a mechanism to tolerate food restriction despite emaciation. Furthermore, during homeostatic satiety, food distraction in patients with AN was associated with decreased self-related processing and internally-cued cognition, which may facilitate increased cognitive control for the food distraction task. These mechanisms might promote avoidance of food ingestion via an automatic response to food stimuli, thereby facilitating the resistance to food cravings among patients with AN.
Acknowledgements
The study was funded by a grant of the Deutsche Forschungsgemeinschaft (SI 2087/2-1).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291719003970.
click here to view supplementary material
Conflict of interests
The authors report no financial relationships with commercial interests.
References
- Admon, R., & Pizzagalli, D. A. (2015). Dysfunctional reward processing in depression. Current Opinion in Psychology, 4, 114–118. doi: 10.1016/j.copsyc.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington: American Psychiatric Publishing. [Google Scholar]
- Barbarich-Marsteller, N. C., Foltin, R. W., & Walsh, B. T. (2011). Does anorexia nervosa resemble an addition? Current Drug Abuse Reviews, 4(3), 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann, M., Geng, J. J., & Shomstein, S. (2004). Parietal cortex and attention. Current Opinion in Neurobiology, 14(2), 212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Berthoud, H. R. (2006). Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity, 14(Suppl 8), 197S–200S. doi: 10.1038/oby.2006.308. [DOI] [PubMed] [Google Scholar]
- Berthoud, H. R. (2011). Metabolic and hedonic drives in the neural control of appetite: Who is the boss? Current Opinion in Neurobiology, 21(6), 888–896. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, L. A., & Riby, L. M. (2013). Glucose enhancement of event-related potentials associated with episodic memory and attention. Food & Function, 4(5), 770–776. doi: 10.1039/c3fo30243a. [DOI] [PubMed] [Google Scholar]
- Casper, R. (1996). Carbohydrate metabolism and its regulatory hormones in anorexia nervosa. Psychiatry Research, 62(1), 85–96. doi: 10.1016/0165-1781(96)02984-8. [DOI] [PubMed] [Google Scholar]
- Chambers, L., McCrickerd, K., & Yeomans, M. R. (2015). Optimising foods for satiety. Trends in Food Science and Technology, 41, 149–160. doi: 10.1016/j.tifs.2014.10.007. [DOI] [Google Scholar]
- Contreras-Rodriguez, O., Martin-Perez, C., Vilar-Lopez, R., & Verdejo-Garcia, A. (2017). Ventral and dorsal striatum networks in obesity: Link to food craving and weight gain. Biological Psychiatry, 81(9), 789–796. doi: 10.1016/j.biopsych.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Courtney, K. E., Ghahremani, D. G., London, E. D., & Ray, L. A. (2014). The association between cue-reactivity in the precuneus and level of dependence on nicotine and alcohol. Drug and Alcohol Dependence, 141, 21–26. doi: 10.1016/j.drugalcdep.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espel-Huynh, H. M., Muratore, A. F., & Lowe, M. R. (2018). A narrative review of the construct of hedonic hunger and its measurement by the Power of Food Scale. Obesity Science & Practice, 4(3), 238–249. doi: 10.1002/osp4.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, S., & Munsch, S. (2012). Self-regulation in eating disorders and obesity – implications for treatment. Verhaltenstherapie, 22, 158–164. doi: 10.1159/000341540. [DOI] [Google Scholar]
- Foerde, K., Steinglass, J. E., Shohamy, D., & Walsh, B. T. (2015). Neural mechanisms supporting maladaptive food choices in anorexia nervosa. Nature Neuroscience, 18(11), 1571–1573. doi: 10.1038/nn.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonville, L., Giampietro, V., Surguladze, S., Williams, S., & Tchanturia, K. (2014). Increased BOLD signal in the fusiform gyrus during implicit emotion processing in anorexia nervosa. NeuroImage: Clinical, 4, 266–273. doi: 10.1016/j.nicl.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich, H.-C., Wu, M., Simon, J. J., & Herzog, W. (2013). Neurocircuit function in eating disorders. International Journal of Eating Disorders, 46(5), 425–432. doi: 10.1002/eat.22099. [DOI] [PubMed] [Google Scholar]
- Giel, K. E., Teufel, M., Friederich, H.-C., Hautzinger, M., Enck, P., & Zipfel, S. (2011). Processing of pictorial food stimuli in patients with eating disorders – a systematic review. International Journal of Eating Disorders, 44(2), 105–117. doi: 10.1002/eat.20785. [DOI] [PubMed] [Google Scholar]
- Giuliani, N. R., Calcott, R. D., & Berkman, E. T. (2013). Piece of cake. Cognitive reappraisal of food craving. Appetite, 64, 56–61. doi: 10.1016/j.appet.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godier, L. R., & Park, R. J. (2015). Does compulsive behavior in anorexia nervosa resemble an addiction? A qualitative investigation. Frontiers in Psychology, 6, 1608. doi: 10.3389/fpsyg.2015.01608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada, T., Nakahara, T., Yasuhara, D., Kojima, S., Sagiyama, K.-i., Amitani, H., … Inui, A. (2008). Obestatin, acyl ghrelin, and des-acyl ghrelin responses to an oral glucose tolerance test in the restricting type of anorexia nervosa. Biological Psychiatry, 63(2), 245–247. doi: 10.1016/j.biopsych.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Hautzinger, M., Keller, F., & Kühner, C. (2006). Beck depressions-inventar: BDI II. Revision. Frankfurt/Main: Harcourt Test Services. [Google Scholar]
- Hayden, B. Y., Smith, D. V., & Platt, M. L. (2010). Cognitive control signals in posterior cingulate cortex. Frontiers in Human Neuroscience, 4, 223. doi: 10.3389/fnhum.2010.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert, A., Tuschen-Caffier, B., Karwautz, A., Niederhofer, H., & Munsch, S. (2007). Eating disorder examination-questionnaire. Diagnostica, 53(3), 144–154. doi: 10.1026/0012-1924.53.3.144. [DOI] [Google Scholar]
- Holsen, L. M., Lawson, E. A., Blum, J., Ko, E., Makris, N., Fazeli, P. K., … Goldstein, J. M. (2012). Food motivation circuitry hypoactivation related to hedonic and nonhedonic aspects of hunger and satiety in women with active anorexia nervosa and weight-restored women with anorexia nervosa. Journal of Psychiatry & Neuroscience, 37(5), 322–332. doi: 10.1503/jpn.110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske, P., Heissler, J., Schonfelder, S., Bongers, A., & Wessa, M. (2011). How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex, 21(6), 1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kaye, W. H., Bulik, C. M., Thornton, L. M., Barbarich, N., & Masters, K. (2004). Comorbidity of anxiety disorders with anorexia and bulimia nervosa. American Journal of Psychiatry, 161(12), 2215–2251. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- Kaye, W. H., Fudge, J. L., & Paulus, M. (2009). New insights into symptoms and neurocircuit function of anorexia nervosa. Nature Reviews Neuroscience, 10(8), 573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- Kaye, W. H., Wierenga, C. E., Bailer, U. F., Simmons, A. N., & Bischoff-Grethe, A. (2013a). Nothing tastes as good as skinny feels: The neurobiology of anorexia nervosa. Trends in Neurosciences, 36(2), 110–120. doi: 10.1016/j.tins.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye, W. H., Wierenga, C. E., Bailer, U. F., Simmons, A. N., Wagner, A., & Bischoff-Grethe, A. (2013b). Does a shared neurobiology for foods and drugs of abuse contribute to extremes of food ingestion in anorexia and bulimia nervosa? Biological Psychiatry, 73(9), 836–842. doi: 10.1016/j.biopsych.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockie, S. H., & Andrews, Z. B. (2013). The hormonal signature of energy deficit: Increasing the value of food reward. Molecular Metabolism, 2(4), 329–336. doi: 10.1016/j.molmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams, C. J., Lohrenz, T., & Montague, P. R. (2015). Neural responses to kindness and malevolence differ in illness and recovery in women with anorexia nervosa. Human Brain Mapping, 36(12), 5207–5219. doi: 10.1002/hbm.23005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljic, D., Djurovic, M., Doknic, M., Pekic, S., Popovic, V., Milic, N., … Ghatei, M. (2006). Ghrelin has partial or no effect on appetite, growth hormone, prolactin, and cortisol release in patients with anorexia nervosa. The Journal of Clinical Endocrinology & Metabolism, 91(4), 1491–1495. doi: 10.1210/jc.2005-2304. [DOI] [PubMed] [Google Scholar]
- Miyake, Y., Okamoto, Y., Onoda, K., Shirao, N., Okamoto, Y., Otagaki, Y., & Yamawaki, S. (2010). Neural processing of negative word stimuli concerning body image in patients with eating disorders: An fMRI study. NeuroImage, 50(3), 1333–1339. doi: 10.1016/j.neuroimage.2009.12.095. [DOI] [PubMed] [Google Scholar]
- Monteleone, P., & Maj, M. (2013). Dysfunctions of leptin, ghrelin, BDNF and endocannabinoids in eating disorders: Beyond the homeostatic control of food intake. Psychoneuroendocrinology, 38(3), 312–330. doi: 10.1016/j.psyneuen.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Murray, S. B., & Strigo, I. A. (2018). Anorexia nervosa, neuroimaging research, and the contextual salience of food cues: The food approach-avoidance conundrum. International Journal of Eating Disorders, 51(8), 822–825. doi: 10.1002/eat.22883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich, G. D., Ross, C. A., Holland, P. C., & Gallagher, M. (2007). Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. Journal of Neuroscience, 27(24), 6436–6441. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2000). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, E., Aveyard, P., Daley, A., Jolly, K., Lewis, A., Lycett, D., & Higgs, S. (2013). Eating attentively: A systematic review and meta-analysis of the effect of food intake memory and awareness on eating. American Journal of Clinical Nutrition, 97(4), 728–742. doi: 10.3945/ajcn.112.045245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, E., Kersbergen, I., & Higgs, S. (2014). Eating ‘attentively’ reduces later energy consumption in overweight and obese females. British Journal of Nutrition, 112(4), 657–661. doi: 10.1017/S000711451400141X. [DOI] [PubMed] [Google Scholar]
- Rothemund, Y., Buchwald, C., Georgiewa, P., Bohner, G., Bauknecht, H. C., Ballmaier, M., … Klingebiel, R. (2011). Compulsivity predicts fronto striatal activation in severely anorectic individuals. Neuroscience, 197, 242–250. doi: 10.1016/j.neuroscience.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Santel, S., Baving, L., Krauel, K., Münte, T. F., & Rotte, M. (2006). Hunger and satiety in anorexia nervosa: fMRI during cognitive processing of food pictures. Brain Research, 1114(1), 138–148. doi: 10.1016/j.brainres.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Scaife, J. C., Godier, L. R., Reinecke, A., Harmer, C. J., & Park, R. J. (2016). Differential activation of the frontal pole to high vs low calorie foods: The neural basis of food preference in anorexia nervosa? Psychiatry Research: Neuroimaging, 258, 44–53. doi: 10.1016/j.pscychresns.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopyra, M. A., Friederich, H.-C., Sailer, S., Pauen, S., Bendszus, M., Herzog, W., & Simon, J. J. (2019). The effect of intestinal glucose load on neural regulation of food craving. Nutritional Neuroscience. doi: 10.1080/1028415X.2019.1600275. [DOI] [PubMed] [Google Scholar]
- Suda, M., Brooks, S. J., Giampietro, V., Uher, R., Mataix-Cols, D., Brammer, M. J., … Campbell, I. C. (2014). Provocation of symmetry/ordering symptoms in anorexia nervosa: A functional neuroimaging study. PLoS One, 9(5), e97998. doi: 10.1371/journal.pone.0097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sünram-Lea, S., Foster, J., Durlach, P., & Perez, C. (2001). Glucose facilitation of cognitive performance in healthy young adults: Examination of the influence of fast-duration, time of day and pre-consumption plasma glucose levels. Psychopharmacology (Berl), 157(1), 46–54. doi: 10.1007/s002130100771. [DOI] [PubMed] [Google Scholar]
- Tanaka, M., Tatebe, Y., Nakahara, T., Yasuhara, D., Sagiyama, K., Muranaga, T., … Naruo, T. (2003). Eating pattern and the effect of oral glucose on ghrelin and insulin secretion in patients with anorexia nervosa. Clinical Endocrinology, 59(5), 574–579. [DOI] [PubMed] [Google Scholar]
- Tortorella, A., Brambilla, F., Fabrazzo, M., Volpe, U., Monteleone, A. M., Mastromo, D., & Monteleone, P. (2014). Central and peripheral peptides regulating eating behaviour and energy homeostasis in anorexia nervosa and bulimia nervosa: A literature review. European Eating Disorders Review, 22(5), 307–320. doi: 10.1002/erv.2303. [DOI] [PubMed] [Google Scholar]
- Tricomi, E., Balleine, B. W., & O'Doherty, J. P. (2009). A specific role for posterior dorsolateral striatum in human habit learning. European Journal of Neuroscience, 29(11), 2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocks, S., Busch, M., Grönemeyer, D., Schulte, D., Herpertz, S. C., & Suchan, B. (2010). Neural correlates of viewing photographs of one's own body and another woman's body in anorexia and bulimia nervosa: An fMRI study. Journal of Psychiatry & Neuroscience, 35(3), 163–176. doi: 10.1503/jpn.090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow, N. D., Wang, G. J., Fowler, J. S., Logan, J., Jayne, M., Franceschi, D., … Pappas, N. (2002). ‘Nonhedonic’ food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse, 44(3), 175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- Wagner, A., Ruf, M., Braus, D. F., & Schmidt, M. H. (2003). Neuronal activity changes and body image distortion in anorexia nervosa. Neuroreport, 14(17), 2193–2197. doi: 10.1097/01.wnr.0000089567.45990.d9. [DOI] [PubMed] [Google Scholar]
- Warren, M. P. (2011). Endocrine manifestations of eating disorders. Journal of Clinical Endocrinology & Metabolism, 96(2), 333–343. doi: 10.1210/jc.2009-2304. [DOI] [PubMed] [Google Scholar]
- Wierenga, C. E., Bischoff-Grethe, A., Melrose, A. J., Irvine, Z., Torres, L., Bailer, U. F., … Kaye, W. H. (2015). Hunger does not motivate reward in women remitted from anorexia nervosa. Biological Psychiatry, 77(7), 642–652. doi: 10.1016/j.biopsych.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga, C. E., Bischoff-Grethe, A., Rasmusson, G., Bailer, U. F., Berner, L. A., Liu, T. T., & Kaye, W. H. (2017). Aberrant cerebral blood flow in response to hunger and satiety in women remitted from anorexia nervosa. Frontiers in Nutrition, 4, 32. doi: 10.3389/fnut.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen, H., Zaudig, M., & Fydrich, T. (1997). SKID. Strukturiertes Klinisches Interview für DSM-IV: Achse I und II (SCID. Structured Clinical Interview for DSM-IV: Axis I and II.).
- Zastrow, A., Kaiser, S., Stippich, C., Walther, S., Herzog, W., Tchanturia, K., … Friederich, H.-C. (2009). Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. American Journal of Psychiatry, 166(5), 608–616. doi: 10.1176/appi.ajp.2008.08050775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291719003970.
click here to view supplementary material