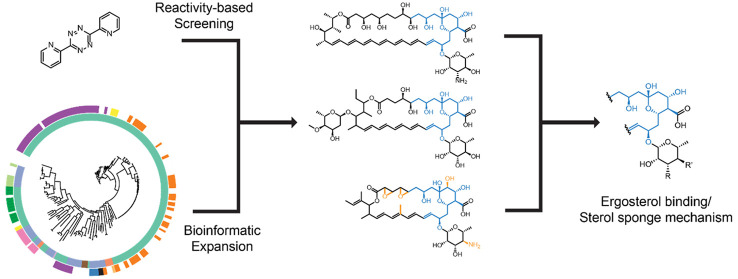
Abstract
Amphotericin-like glycosylated polyene macrolides (GPMs) are a clinically and industrially important family of natural products, but the mechanisms by which they exert their extraordinary biological activities have remained unclear for more than half a century. Amphotericin B exerts fungicidal action primarily via self-assembly into an extramembranous sponge that rapidly extracts ergosterol from fungal membranes, but it has remained unclear whether this mechanism is applicable to other GPMs. Using a highly conserved polyene–hemiketal region of GPMs that we hypothesized to represent a conserved ergosterol-binding domain, we bioinformatically mapped the entirety of the GPM sequence-function space and expanded the number of GPM biosynthetic gene clusters (BGCs) by 10-fold. We further leveraged bioinformatic predictions and tetrazine-based reactivity screening targeting the electron-rich polyene region of GPMs to discover a first-in-class methyltetraene- and diepoxide-containing GPM, kineosporicin, and to assign BGCs to many new producers of previously reported members. Leveraging a range of structurally diverse known and newly discovered GPMs, we found that the sterol sponge mechanism of fungicidal action is conserved.
Short abstract
A combination of genome-mining and chemical reactivity identified new antifungal natural products. Such glycosylated polyene macrolides share a common mode of action via ergosterol extraction.
Introduction
Glycosylated polyene macrolides (GPMs) such as amphotericin B (AmB), nystatin, and natamycin are clinically and industrially important fungicidal small molecules.1 AmB, the most prominent GPM, has been used as the last line of defense against life-threatening invasive fungal infections since the 1950s with minimal development of resistance,2,3 and AmB continues to reveal new horizons for small molecule function.4−6 However, despite extensive investigations for more than half a century, the mechanism(s) by which GPMs exert their extraordinary biological activities remains controversial, limiting extensive efforts to harness, improve, or replicate their properties.
Multiple hypotheses have been advanced for the resistance-refractory fungicidal mechanism of GPMs. These include membrane permeabilization, generation of reactive oxygen species, and, most recently, formation of an extramembranous “sterol sponge” that extracts ergosterol from lipid bilayers.7−16 The prevailing model for AmB’s fungicidal activity, and that of many other members of the GPM family, has long been ion-channel-mediated membrane permeabilization.12,13,17 This mechanism continues to appear in modern textbooks and publications, including by groups aiming to improve the pharmaceutical properties of AmB or other GPMs.18−22 However, recent studies have decoupled ion channel formation from the fungicidal activity of AmB and shown that only glycosylation-dependent ergosterol sequestration is essential.9,15,23 Specifically, a synthetic derivative of AmB lacking a C35 hydroxyl group did not form ion channels but retained ergosterol binding and fungicidal activity.8 A subsequent extensive series of solid-state NMR, transmission electron microscopy (TEM), and cell biological studies further revealed that AmB forms large extramembranous aggregates that rapidly extract ergosterol from lipid bilayers, akin to a “sterol sponge”.16 In a key experiment directly connecting the sterol sponge mechanism to the fungicidal activity of AmB, it was shown that precomplexation of the AmB sterol sponge with ergosterol, thus saturating its sterol-binding capacity, mitigated both the ergosterol extracting and fungicidal activities of AmB.16 Earlier studies linked the mode of action for the structurally related but smaller natural product natamycin to ergosterol binding and disruption of sterol-dependent membrane protein functions in an ion-channel-independent manner.9,10,14,24 Fungal ergosterol is essential for many membrane-mediated functions, including membrane protein activity and delivery, cell signaling, endocytosis, exocytosis, vacuole fusion, and cell division.25−29 It is thus logical that rapid sequestration of this vital sterol could drive multifaceted and resistance-evasive fungicidal action. We therefore hypothesized that GPM natural products may exert a common fungicidal activity via the sterol sponge mechanism. Testing this global hypothesis required a comprehensive map of the structures and functions of the GPM natural product family. Thus, we set out to bioinformatically define the entire GPM sequence-function space to enable discovery of new GPMs and to evaluate the mode of action for known and newly discovered compounds. GPMs such as AmB are biosynthesized analogously to other type I polyketides. The carbon framework is first constructed by polyketide synthases (PKSs) followed by tailoring modifications that include O-glycosylation and cytochrome-P450-mediated hydroxylations and epoxidations.30−33 The vast majority of reported GPMs fall into three major classes: tetraenes (nystatin- and natamycin-like), trans-heptaenes (AmB-like), and aromatic cis-heptaenes bearing an aminoacetophenone moiety (candicidin-like). Three minor classes of GPMs have also been reported, including pentaenes (eurocidins), diglycosylated GPMs (selvamicin, NPP, and 67-121C), and carbamide-containing GPMs (rimocidin B and CE-108, Supplemental Figure S1).34−38 Despite a wide range of sizes and tailoring modifications, all known GPMs display a highly conserved substructure comprised of a polyene that extends into a hemiketal ring (represented by carbons 13–21 on AmB). A variable hexose sugar is appended between the polyene and hemiketal moieties. We speculate that this framework represents the sterol-binding domain for the GPMs.
To rapidly screen for both known and new GPMs and to take advantage of the conserved polyene region of GPMs, we developed a hybrid strategy that combines bioinformatic prioritization of the genes encoding the conserved framework with reactivity-based screening. The latter procedure involves rapid and sensitive identification of natural products via the chemoselective targeting of an organic functional group of interest. This chemistry-based approach can be guided by genomic prioritization to only include strains capable of installing the targeted functional group, thus expediting natural product discovery. We have used reactivity-based screening to identify α/β-unsaturated carbonyl-containing natural products via 1,4-nucleophilic addition, reactive carbonyl-containing compounds via oxime ligation, and primary ureido-group-containing compounds via nucleophilic addition onto glyoxal-based probes.39−42 Other research groups have reported related approaches for elucidating the proteomic targets of natural products, chemoselective natural product enrichment, and methodology to covalently target di/trienes using a nitrosopyridine-based probe.43−46 To target the electron-rich polyene common to all GPMs (Supplemental Figure S1), we co-opted the well-known tetrazine ligation that was recently employed to detect isonitrile-containing natural products.47,48
Herein, we report the identification and classification of all GPMs predicted from genomes available in GenBank. This effort increased the number of putative GPM biosynthetic gene clusters (BGCs) by an order of magnitude. We then combined reactivity-based screening and bioinformatic prioritization to discover, isolate, and characterize kineosporicin, a first-in-class GPM with methyl substitution in the polyene region. Our data also identified new GPM producers and BGCs with previously unknown or incomplete genomic regions. With a comprehensive map of the GPM family of natural products and a diverse panel of tetraene-, pentaene-, and heptaene-containing GPMs in hand, we performed a series of biophysical and fungicidal assays that revealed ergosterol sequestration through the sterol sponge mechanism is the unifying molecular activity for this class of antifungal natural products.
Results
Identification of Glycosylated Polyene Macrolide BGCs
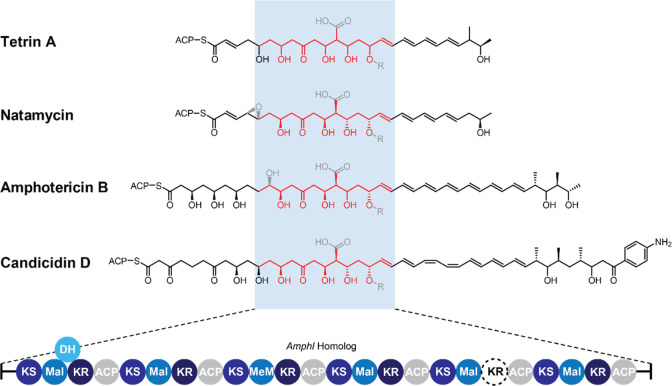
Aside from PKS genes, a core set of tailoring enzymes define the minimal GPM BGC with a few exceptions. These enzymes include mycosamine aminotransferases (AmphDII-homologues), glycosyltransferases (AmphDI-homologues), and cytochrome P450s responsible for backbone (AmphL-homologues) and hemiketal exocyclic methyl (AmphN-homologues) oxidation.31,37,49−54 However, structurally divergent GPMs may lack one or more of these modifying enzymes as in the case of perimycin (lacks AmphN) and selvamicin (lacks AmphN and AmphDII).34,38 Although no single tailoring modification is universal to all known GPMs, our PKS module analysis (see Methods) suggested that all reported GPM BGCs share a single, conserved polyketide gene highly similar to amphI, responsible for installing the carbon backbone spanning the terminal portion of the polyene through the conserved hemiketal (Figure 1 and Supplemental Figure S1).55 In the majority of closed genomes, AmphI appears as a single open-reading frame with an inactive fifth ketoreductase, enabling spontaneous formation of the conserved six-membered hemiketal. We thus chose AmphI as a BLASTp query to generate a comprehensive, nonredundant list of potential GPM BGCs.56 The local genomic contexts of the AmphI-encoding gene were retrieved and functionally annotated using RODEO (Rapid ORF Description & Evaluation Online).57 Retrieved sequences where an AmphI-homologue (Figure 1) was encoded in the genomic vicinity of homologues of known GPM modification enzymes (e.g., mycosamine aminotransferase, glycosyltransferase, or backbone-acting cytochrome P450s) were classified as candidate GPM BGCs and subjected for additional PKS module analysis using antiSMASH and PRISM.55,58 From the above analysis, a total of 178 GPM BGCs were identified. AmphI conservation as an intact, six-module PKS megasynthase was observed even when examining uncharacterized BGCs generated from this initial data set. This bioinformatic workflow was also applied to the above-mentioned GPM-modifying enzymes to account for cases where AmphI sequences (nearly 9500 amino acids in length) were incomplete owing to partial genome assembly. This process yielded an additional 74 GPM-encoding BGCs. In total, these efforts provided a list of 252 high-probability GPM BGCs (104 complete and 148 partial BGCs) that encompassed all previously reported GPMs (Supplemental File 1 and Figure S2).
Figure 1.
AmphI is highly conserved in GPM BGCs. All known GPM BGCs encode an AmphI homologue, responsible for installing the conserved carbon framework (red). All homologues feature inactivation of the fifth ketoreductase (KR5, dashed circle). Macrolide-tailoring modifications are shown in gray. Mal, malonyl-CoA loading. MeM, methylmalonyl-CoA loading. ACP, acyl-carrier protein. KS, ketosynthase. DH, dehydratase. Final structures for representative GPMs are in Supplemental Figure S1.
Analysis of GPM-Producing BGCs
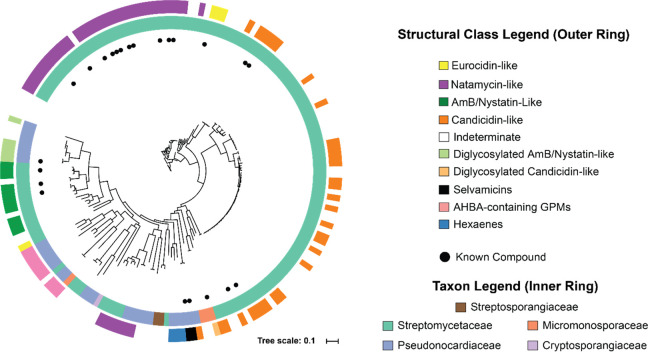
A maximum-likelihood phylogenetic tree was next constructed using the amino acid sequences of the AmphI homologues (Figure 2). The sequence relatedness of AmphI homologues and the various GPM-tailoring enzymes correlated well with known GPM structural diversity (Supplemental Figure S2). This visualization of the sequence-structure space allowed us to prioritize efforts on GPMs with predicted structural novelty. Of the 178 full-length AmphI proteins represented on the phylogenetic tree, 155 have no reported association with any isolated natural product. The consolidated set of GPM BGCs allowed the construction of highly precise, custom profile hidden Markov models (pHMMs) from characterized macrolide-tailoring enzymes (Supplemental File 2) to aid in recognition and differentiation of the plethora of cytochrome P450s and sugar aminotransferases.
Figure 2.
AmphI phylogenetic analysis. Shown is a maximum-likelihood tree of full-length AmphI homologues (n = 178) with colored annotations representing the GPM type and taxonomic family. Sequences associated with known GPM structures are denoted with black dots (structures provided in Supplemental Figure S1). Distance of each branch from the center of the tree is correlated to sequence diversity. Leaves marked “indeterminate” are AmphI homologues from incomplete BGCs where the GPM type could not be confidently assigned. AHBA, 3-amino-5-hydroxybenzoic acid.
All GPM BGCs are from the phylum Actinobacteria with the following taxonomic family distribution: Streptomycetaceae (n = 140, 79% of the total), Pseudonocardiaceae (n = 31, 17%), Micromonosporaceae (n = 4, 2%), Streptosporangiaceae (n = 2, 1%), and Cryptosporangiaceae (n = 1, Figure 2). Natamycin-like BGCs represent the structural plurality with 22% of the total GPM sequence-function space, followed by candicidin-like cis-heptaenes (18%), AmB- and nystatin-like GPMs (9%), and pentaenes (3%). Likely owing to sequencing bias, Streptomycetaceae harbor the largest fraction of the identified GPM BGCs (2053 genomes available from NCBI as of mid-2020, <7% encode a GPM BGC). Streptomycetaceae also contain the only two instances of strains harboring two GPM BGCs (Streptomyces sp. 769 and Streptomyces noursei ATCC 11455, with nystatin and natamycin BGCs). Comparatively fewer Pseudonocardiaceae have sequenced genomes and thus encode GPM BGCs with a much greater frequency (356 genomes in NCBI, ∼9% contain a GPM BGC). Further, the GPMs derived from Pseudonocardiaceae, such as selvamicin, are predicted to be more structurally distinct owing to sequence divergence of the AmphI-homologue and other post-PKS-modifying enzymes found within the BGC (Figure 2 and Supplemental Figure S2).34 While soil-dwelling Actinobacteria harbor the majority of GPM BGCs, selvamicin and multiple other sequence-divergent BGCs are derived from animal-associated bacteria and may play important ecological roles.
Sequence-Divergent Pseudonocardiaceae GPM BGCs
A total of 19 uncharacterized Pseudonocardiaceae BGCs were predicted to produce structurally distinct GPMs. Two clades formed upon phylogenetic analysis of thioesterases from linear and macrolide-forming polyene natural products with all new GPM thioesterases part of the macrolide-forming clade (Supplemental Figure S3).59 Only 14 BGCs could be confidently classified, while the other 5 could not be assigned owing to draft/incomplete genomes (entirety of PKSs not contained on a single sequencing contig). Three of these GPM BGCs encode a predicted tetraene-containing GPM that would feature an unprecedented methyl-substituted polyene region. The methyl substitution would be found at the 4 position of the tetraene, arising from the predicted usage of methylmalonyl- instead of malonyl-CoA by the PKS (Supplemental Figure S4).60 Our pHMM models also predicted the presence of a perosamine (4,6-dideoxy-4-aminomannose) instead of a mycosamine (3,6-dideoxy-3-aminomannose) sugar. Another eight BGCs encode a predicted macrolide framework built upon a 3-amino-5-hydroxybenzoic acid (AHBA) starter unit. Two of these BGCs are previously predicted AHBA-containing pentaene GPMs from ant-associated symbionts,35 while the remaining six are predicted all-trans, AHBA–heptaene GPMs (Supplemental Figure S5). Two of these AHBA–heptaene GPMs are also predicted to display 6-deoxymannose, owing to the genome-wide omission of the requisite aminotransferase. A separate set of three BGCs are predicted to produce an unprecedented hexaene-containing GPM (Supplemental Figure S6). Each of these three new types of GPM BGCs found in Pseudonocardiaceae encode cytochrome P450 enzymes that do not match our custom set of pHMMs (see Methods).
Reactivity-Based Detection of Electron-Rich Alkenes
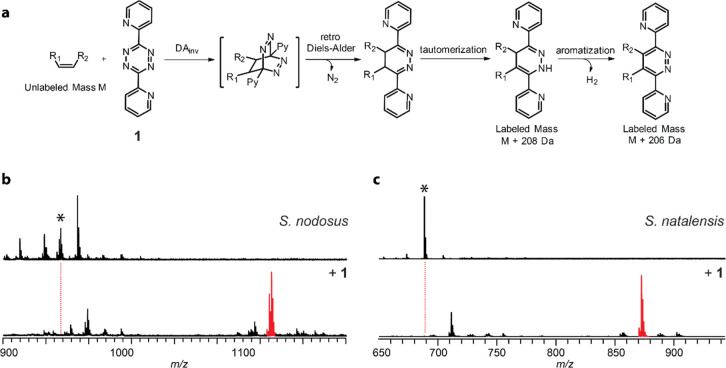
The above data set provides a roadmap for the isolation of new GPMs and specifically highlights Pseudonocardiaceae as a taxonomic group worthy of further pursuit. However, using bioinformatics to guide the discovery of new natural products that may or may not be produced at isolable quantities is time-consuming and often unsuccessful.61 Thus, to accelerate the discovery of new GPMs, we sought a reactivity-based strategy to detect natural products containing electron-rich olefins. We rationalized that the well-known tetrazine ligation could be co-opted for this purpose.47 While strained alkene substrates greatly enhance the rate of the tetrazine ligation,62 reaction specificity is more important for our purposes, and incomplete labeling is often preferable, as the presence of both labeled and unlabeled target molecule in the same sample facilitates hit identification by mass spectrometric analysis. We chose 3,6-di-2-pyridyl-1,2,4,5-tetrazine (1) as the reactivity-based probe given its commercial availability, solution stability, and the ability to introduce readily ionizable dihydropyridazine and/or pyridazine adducts for sensitive detection by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS, Figure 3a). When dihydropyridazine and pyridazine adducts are both present, +206/208 Da diagnostic masses are observed, providing an unambiguous labeling pattern when working with cellular extracts. We further selected 1 based on the hypothesis that the bis-pyridyl chromophore would intensify the peaks observed by MALDI-TOF-MS though a “label-assisted” absorption of UV laser energy.63,64
Figure 3.
Validation of 1 for reactivity-based screening of GPMs. (a) Reaction scheme of tetrazine ligation. Unique labeling pattern offset by 2 Da is expected from dihydropyridazine (+208 Da) and pyridazine (+206 Da) adducts. (b) MALDI-TOF mass spectra of S. nodosus control extract (unreacted, top) and extract reacted with probe 1 (bottom). (c) Identical to panel b but using S. natalensis extract. Mass of the parental GPM (unlabeled) is denoted with an asterisk, while the labeled GPM is indicated in red.
Given that the tetrazine ligation is commonly used in bioconjugation reactions with intentionally strained alkene substrates, we considered two potential pitfalls to the strategy: (i) slow, off-target reactivity with electron-deficient alkenes and (ii) steric factors that may prevent labeling of electronically viable olefins. To evaluate the scope of reactivity of 1, reactions were conducted on a panel of 10 structurally diverse, olefin-containing compounds. After reaction with 1, samples were subjected to MALDI-TOF-MS analysis along with an unreacted negative control (Supplemental Figures S7 and S8). Sterically hindered and/or electron-deficient olefins, such as those found in himbacine, colubrine, bryostatin I, and streptovaricin C, resulted in no observed labeling under the reaction conditions employed. The methyl-flanked diene of streptovaricin C apparently occluded labeling by 1, while the less sterically encumbered and more electron-rich, methyl-flanked triene on everolimus yielded a modest extent of labeling.
We next sought to confirm or refute if 1 was suitable for screening in the context of bacterial extracts. Therefore, we cultivated the AmB and natamycin producers (Streptomyces nodosus ATCC 14899 and Streptomyces natalensis ISP-5314, respectively) and extracted the exported metabolites from stationary growth phase cultures with MeOH prior to reaction with 1. Efficient labeling of amphotericin A/B and natamycin were observed by MALDI-TOF-MS (Figure 3).
Phylogeny-Driven Prioritization of Novel Tetraenes from Pseudonocardiaceae
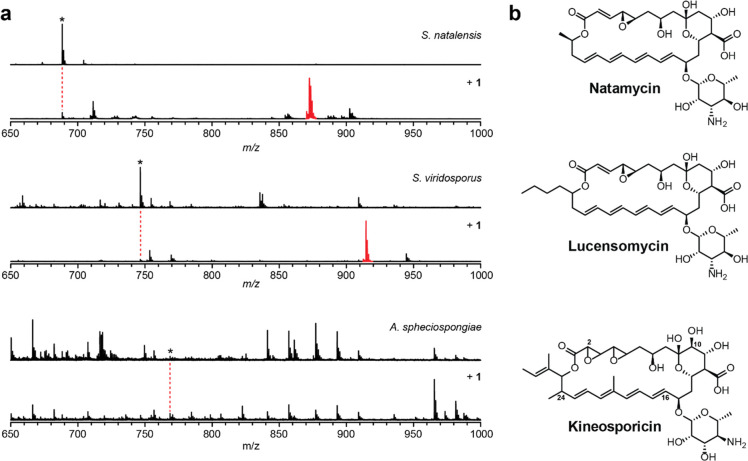
The polyene region of GPMs has also been implicated in sterol-binding.16,65 We therefore focused our discovery effort toward the newly identified GPM BGCs with predicted methyl-substituted polyene regions. This putative framework is structurally unprecedented for GPMs and could provide insight into the structure–activity relationships of GPM-mediated sterol binding and potentially challenge the hypothesis that the sterol sponge mechanism is conserved. As this divergent clade of tetraene BGCs encodes PKS enzymes predicted to utilize both malonyl and methylmalonyl units for the polyene region, we tested strains from both BGC types. Actinokineospora spheciospongiae DSM 45935 (predicted methyltetraene) and Streptomyces viridosporus (predicted tetraene) were cultivated using a variety of growth media, which was followed by reacting the methanolic extracts with 1. A single tetrazine adduct was observed in the S. viridosporus extract, while no labeling was observed for A. spheciospongiae (Figure 4). We reasoned that a lack of reactivity toward 1 could arise for methyltetraene-containing metabolites owing to steric occlusion, not an necessarily absence of GPM production. Indeed, UV–vis spectrophotometric analysis of the A. spheciospongiae extract revealed a spectrum characteristic of tetraene-containing macrolides (Supplemental Figure S9).66 High-resolution and tandem MS (HR-MS/MS) was subsequently performed on both compounds, which provided theoretical molecular formulas. The data acquired for the A. spheciospongiae compound (hereafter kineosporicin) best matched C38H55NO15 (m/z 765.3572 Da theoretical, 765.3568 Da observed, 0.5 ppm error). The S. viridosporus-derived mass was a perfect match to lucensomycin (synonymous with etruscomycin and NSC-143257) a known GPM from Streptomyces lucensis, (C36H53NO13, 707.3517 Da theoretical, 707.3523 Da observed, 0.8 ppm error).67 MS/MS analysis confirmed the generation of daughter ions consistent with the loss of a single amino-sugar (e.g., mycosamine or the isobaric perosamine) and multiple losses of water consistent with other GPMs tested in this study.
Figure 4.
Reactivity-based screen of putative tetraene-containing GPMs. (a) Methanol-extracted metabolites from S. natalensis (natamycin producer) and S. viridosporus show robust labeling after reaction with probe 1, while no labeling was observed for A. spheciospongiae. Mass of the parental GPM (unlabeled, top) is denoted with an asterisk, while the labeled GPM is indicated in red (bottom). (b) Structures of tetraene-containing GPMs with key carbons of kineosporicin numbered.
Structural Elucidation of Lucensomycin and Kineosporicin
We next purified larger quantities of both tetraene GPMs for structure elucidation using a suite of NMR-based experiments. NMR data from the S. viridosporus tetraene (Supplemental Table S1 and Figure S10) corroborated the previous HR-MS/MS results and matched precisely those reported for lucensomycin.68 Structure elucidation of kineosporicin from A. spheciospongiae was expedited by a complete PKS-module-guided prediction of the carbon framework (Supplemental Figure S11). The macrolide exhibited six distinct COSY/TOCSY spin systems (Supplemental Table S2 and Figures S12–S13). HMBC correlations readily connected fragments C3–8 to C10–19 via the C9 hemiketal quaternary carbon. A singlet proton (3.45 ppm) with a downfield 13C shift (79.4 ppm) was observed at C10 as opposed to the expected geminal protons, indicating a C10 hydroxylation within the hemiketal ring, a new tailoring location likely installed by one of two uncharacterized cytochrome P450 proteins in the kineosporicin BGC (Supplemental Figure S11). HMBC correlations connected fragments C10–19 and C21–25 via an alkene carbon C20 (137.4 ppm). C20 also showed an HMBC correlation to C30 (methyl), confirming the bioinformatically predicted methyl-substituted polyene region. Fragment C27–28 was connected to C21–25 and methyl C32 via carbon C26 (134.3 ppm) to reveal a unique 2,3-unsaturated sec-butenyl substituent. Fragment C2–4 was also connected to C5–8 and C21–25 to complete the macrolide. While the C4–5 chemical shift values matched those expected for an epoxide (55.9 and 54.4 ppm, Supplemental Table S2), the chemical shift values of C2–3 were further upfield compared to the expected values for a predicted alkene (51.7 and 58.0 ppm) and indicated that kineosporicin might display two contiguous epoxides. Natural product diepoxidation is known but rare, with the C2–3 chemical shift matching known values for epoxides alpha to carbonyls (Supplemental Figure S14).69−71 As predicted, instead of a mycosamine sugar, the C3′ (3.96 ppm) and C4′ (3.36 ppm) proton chemical shifts closely match those of 4-amino-4,6-dideoxymannose, or perosamine (Supplemental Table S2 and Figure S15). HMBC correlations between the macrolide and the anomeric proton of the perosamine places glycosylation at C15. NOESY correlations allowed assignment of relative stereochemistry at C10 and the perosamine (Supplemental Figure S13).
Assignment of BGCs to Known GPMs
To better understand GPM biosynthesis and evolution, we leveraged the collection of GPM genomic data to identify the probable biosynthetic origins of all orphan GPM families. Although the first pentaene GPMs, the eurocidins, were discovered in the 1990s, no BGC has been reported for this compound.72 Upon sequencing the genome of eurocidin producers Streptomyces eurocidicus NRRL ISP-5604 and B-1677, we located and verified by reactivity-based screening and NMR the BGC predicted to produce this pentaene-containing GPM (Supplemental Figures S16–17 and Supplemental Table S3). In addition, only a partial BGC from earlier sequencing projects was available for rimocidin (NCBI identifiers: AY442225.1).54 We identified a complete rimocidin BGC within the producer strain Streptomyces rimosus NRRL WC-3558 (NCBI identifier: ASM72060v1). Although a BGC was recently reported for lucensomycin, we identified a partial BGC from a new producer S. viridosporus (NCBI identifier: NZ_MSGP01000218).68
Bioactivity Assessment
Having leveraged a suite of bioinformatic and reactivity-based natural product discovery tools to define the extent of diversity in the GPM family of natural products, we next characterized the functional properties of representative family members to test whether the sterol sponge mechanism of action is conserved. We first screened kineosporicin against a brief panel of bacteria to evaluate any growth-suppressive activity. As expected, kineosporicin was inactive toward all tested strains (Supplemental Figure S18). Antifungal testing of kineosporicin then commenced alongside four additional, structurally distinct GPMs: AmB, eurocidin D, natamycin, and lucensomycin (Table 1). Kineosporicin was active against all tested Candida and Aspergillus strains. Lucensomycin also inhibited fungal growth for all strains tested and displayed submicromolar activity nearly equivalent to AmB.
Table 1. GPM Antifungal Activitya.
| fungal strain | AmB | eurocidin D | natamycin | lucensomycin | kineosporicin |
|---|---|---|---|---|---|
| C. albicans SN250 | 0.125 | 2 | 4 | 0.25 | 2 |
| C. albicans | 0.125 | 1.5 | 4 | 0.25 | 2 |
| C. glabrata | 0.0625 | 2 | 4 | 0.125 | 2 |
| C. krusei | 0.5 | 4 | 4 | 0.25 | 4 |
| C. tropicalis | 0.25 | 2 | 4 | 0.25 | 4 |
| A. fumigatus 91 | 1.5 | 4 | 8 | 1 | 8 |
| A. fumigatus 1100 | 0.5 | 4 | 4 | 0.5 | 8 |
| A. fumigatus 1163 | 0.5 | 4 | 4 | 0.5 | 8 |
Minimum inhibitory concentrations (MICs, in μM) against Candida and Aspergillus strains were determined by microbroth dilution assay (n = 2).
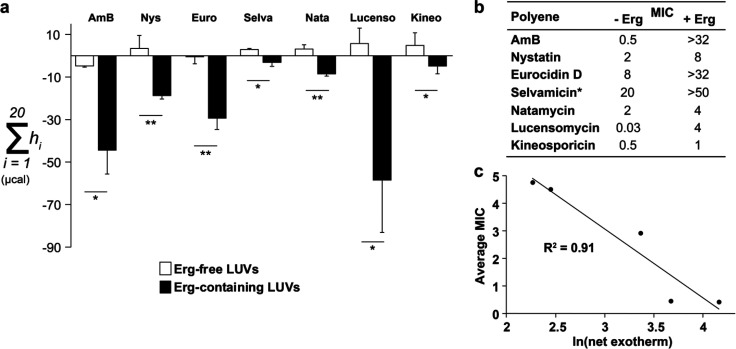
Having established shared antifungal activity, we next evaluated the ergosterol-binding activity of the five GPMs in our panel.72 Nystatin and selvamicin were included as additional comparators with nystatin being a known ergosterol binder and selvamicin reported to not bind ergosterol.34,73 Isothermal titration calorimetry was used to evaluate binding with 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) large unilamellar vesicles (LUVs) that contained or omitted ergosterol (Figure 5).9,10,23 All seven structurally distinct GPMs, including selvamicin, displayed a statistically significant binding interaction to ergosterol-embedded POPCs relative to the POPC-only negative control.
Figure 5.
GPM antifungal activity is ergosterol (erg)-dependent. (a) Isothermal titration calorimetry of a panel of GPMs against vesicles containing or omitting erg (n = 3). *p < 0.05; **p < 0.01. Abbreviations as follows: AmB (amphotericin B), Nys (nystatin), Euro (eurocidin D), Selva (selvamicin), Nata (natamycin), Lucenso (lucensomycin), Kineo (kineosporicin). (b) Minimum inhibitory concentrations (MICs, in μM, n = 3) against Saccharomyces cerevisiae were determined via microbroth dilution with and without ergosterol precomplexation. All precomplexation MICs were measured at a 1:5 molar ratio of GPM/erg except for selvamicin, which was done at a ratio of 1:1. (c) Plot of the net exotherm against average MIC for the five GPMs evaluated in Table 1.
With the generality of ergosterol binding confirmed, we performed a key experiment to test the generality of the sterol sponge mechanism. As described above, an extensive series of solid-state NMR, TEM, and cell biological studies recently revealed that AmB primarily kills fungi by forming large extramembranous aggregates that rapidly extract ergosterol from lipid bilayers. A key experiment that confirmed this mechanism involved forming of a noncovalent complex between AmB and ergosterol, in the form of a presaturated sterol sponge, and testing the biological activity of this preformed complex. We previously showed that such precomplexation reduces the capacity for AmB to extract sterols from yeast membranes and thereby increases the minimum inhibitory concentration (MIC) of AmB in antifungal assays.4,5,16 If other GPMs operate via the sterol sponge mechanism, ergosterol precomplexation should similarly mitigate the fungicidal action. Accordingly, we applied the same approach and measured the MIC against S. cerevisiae for each GPM with and without ergosterol precomplexation. All seven GPMs in the panel displayed reproducible MIC increases of at least 2-fold. We further note a strong correlation (R2 = 0.91) between the net exotherm measured by isothermal titration calorimetry and the observed average MIC against Candida and Aspergillus across the tested GPMs (Figure 5). Lastly, to evaluate potential ion channel formation, GPM-dependent potassium ion release was measured using C. albicans SN250 cultures for AmB, natamycin, kineosporicin, lucensomycin, and eurocidin D (Supplemental Figure S19). From the panel, only AmB, a known ion channel former, demonstrated release of potassium ions. Collectively, these experiments strongly support the sterol sponge mechanism of action.
Discussion
Multiple antifungal modes of action have been proposed for GPMs with the majority of previous work conducted on AmB and natamycin.10,16 To permit a more holistic evaluation of GPM bioactivity, we report the expansion of high-confidence GPM BGCs by 10-fold (n = 252). We leveraged this roadmap for new GPM discovery, enhanced by a tetrazine reactivity-based strategy to link previously reported GPMs to their respective BGCs and to discover kineosporicin, a structurally unique methyltetraene-containing GPM. We obtained and tested a structurally diverse panel of GPMs, and results obtained from ergosterol-GPM isothermal titration calorimetry and MIC evaluation upon ergosterol precomplexation independently confirmed that each tested GPM directly interacts with ergosterol and that precomplexation with ergosterol reduces antifungal activity. While this result was expected for AmB, nystatin, and natamycin, it was unexpected for the GPM selvamicin, which was previously reported not to interact with ergosterol.9,10,34 Our data show selvamicin binds ergosterol-embedded LUVs in a statistically significant albeit weak manner, and this discrepancy may be due to usage of a more sensitive calorimeter (Methods). These findings confirm that GPMs primarily function via small molecule–small molecule interactions through GPM-mediated ergosterol sequestration.74,75
While alternative modes of action such as ion channel formation, reactive oxygen species generation, and membrane transporter disfunction have been proposed, these observations likely result from downstream effects of GPM-mediated binding and sequestration of ergosterol.10,11,13,14 Membrane permeabilization has been only observed in larger GPMs like AmB, and AmB-mediated channel formation has been shown to occur in an ergosterol-dependent manner. A synthesized derivative of AmB lacking a hydroxyl group at C35 retains ergosterol-binding activity but cannot form ion channels and yet retains fungicidal activity.9 It was further shown that fungicidal activity required a stoichiometric excess of AmB relative to ergosterol in the yeast.9,15,23 An extensive series of additional experiments demonstrated that ergosterol precomplexation blocks fungicidal activity and revealed that AmB primarily exists as a large extramembranous aggregate that kills fungi by rapidly extracting ergosterol from yeast membranes.16 As shown herein, this same precomplexation experiment mitigates the antifungal action of all the GPMs that were studied. Furthermore, like natamycin, kineosporicin, lucensomycin, and eurocidin do not form ion channels. Taken together, these findings show that ion channel formation is a secondary mode of action likely available only to larger GPMs and is not the primary driver of GPM fungicidal activity.22 Such secondary GPM fungicidal mechanisms are reminiscent of antibiotics like nisin or telavancin, both of which have dual modes of action involving inhibition of peptidoglycan biosynthesis and membrane depolarization.76,77
The AmB aglycone, which binds readily to LUVs and yeast cells, retains the putatively redox-active polyene motif but cannot bind ergosterol completely and lacks fungicidal activity.23 The aglycone of natamycin also does not bind ergosterol, retains its polyene motif, and completely lacks antifungal activity.8 Polyene-based oxidation is therefore unlikely to be a major contributor for the antifungal activity of GPMs.
With a unifying mechanism of action, we hoped to gain greater insight into the structural driver of sterol binding by leveraging our expanded data set of GPMs. Previous studies have shown that glycosylation and the conformational rigidity imparted by the polyene–hemiketal region are essential for ergosterol binding.9,23,65 We have shown that the carbon framework installed by AmphI homologues provides the structural foundation for both of these ergosterol-binding prerequisites. We identify a polyene–glycoside–hemiketal motif as the minimal common scaffold for all GPMs and that this motif implies direct sterol binding. Further, we demonstrate this gene-to-structure-to-function relationship with tetraene-, pentaene-, and heptaene-containing GPMs, including structurally divergent members like kineosporicin and selvamicin. In addition to our data demonstrating the selvamicin–ergosterol interaction, recent work showed that meijiemycin, a linear perosamine- and hexaene-containing polyene, exerts its antifungal activity via ergosterol binding, further supporting this hypothesis.78 The meijiemycin BGC, which also contains an amphI-homologue, encodes a linear structure with the requisite perosamine glycosylation, polyene region, and hemiketal ring as other GPMs. This suggests that the structural rigidity imparted by a polyene and AmphI-derived cyclic hemiketal along with glycosylation may be sufficient as a minimal scaffold for sterol binding. Confirming this hypothesis would enable the emergence of nonmacrolide sterol-binding compounds that may be simpler synthetic targets.
In addition to insights regarding the GPM mode of action, our panel of structurally distinct GPMs also enabled a direct comparison of antifungal activity. The presence of aliphatic groups adjacent to the macrocyclic ester appears to greatly affect antifungal activity. Lucensomycin is 8- to 16-fold more potent than natamycin, with the sole structural difference being an n-butyl (rather than methyl) substituent at C25. This trend is corroborated by previous reports where n-propyl-substituted rimocidins are more potent than methyl-substituted counterparts.79 This significant increase in antifungal activity might in part be attributable to increased binding to ergosterol as shown by isothermal titration calorimetry but may also be due to improved membrane localization and access to ergosterol due to the presence of a hydrophobic anchor. Similar membrane localization effects have been reported for the lipid tail in lipoglycopeptides and depsipeptides.77,80 Thus, aliphatic substitutions near the macrolide ester on GPMs may prove to be an effective strategy to increase antifungal potency. Additionally, C10 hydroxylation and polyene methyl substitution as found on kineosporicin did not dramatically alter antifungal activity, as kineosporicin was approximately 2-fold more effective against Candida but 2-fold less effective against Aspergillus compared to natamycin. Enzymatic C10 hydroxylation by one of two cytochrome P450 proteins encoded by the kineosporicin BGC is of special interest for future investigation due to the ubiquity of the highly conserved hemiketal ring among GPM scaffolds and may be engineered onto other GPMs to further increase aqueous solubility.
The results of this study have several important impacts. First, we mapped the entire GPM biosynthetic landscape and used our genomics and reactivity-based strategy to discover, isolate, and test new and known GPMs in a generalized strategy applicable to many other alkene-containing natural products. Our diverse panel of GPMs allowed us to confirm that the sterol sponge mechanism of antifungal action is conserved in this family of natural products. This work reinforces the proposal that binding of a functionally vital sterol leads to a resistance-refractory antifungal mode of action across the GPM family. We also demonstrated through our bioinformatic analysis that GPMs remain underexplored with the majority of diverse GPM structures, such as kineosporicin, coming from Pseudonocardiaceae. In addition, both structural and genetic conservation within this family of natural products points to an ergosterol-binding polyene–glycoside–hemiketal motif, which may inform the production of alternative sterol-binding antimicrobials. Lastly, this study sets the stage for future work, whereby the structure activity relationships dictating ergosterol and cholesterol binding may be probed with naturally derived, structurally diverse GPMs toward potentially less toxic analogues.
Acknowledgments
We thank Lingyang Zhu and Adam DiCaprio for consultation with NMR data acquisition, Graham Hudson for mass spectrometry assistance, and Ian Dailey for helpful discussions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.1c00148.
This work was supported in part by the National Institutes of Health (GM123998 to D.A.M., GM118185 and AI135812 to M.D.B., and GM112845 to C.M.R.), the David and Lucile Packard Fellowship for Science and Engineering (to D.A.M.), and Sfunga Therapeutics.
The authors declare no competing financial interest.
Supplementary Material
References
- Nishimura S.; Matsumori N. Chemical Diversity and Mode of Action of Natural Products Targeting Lipids in the Eukaryotic Cell Membrane. Nat. Prod. Rep. 2020, 37 (5), 677–702. 10.1039/C9NP00059C. [DOI] [PubMed] [Google Scholar]
- Jambor W. P.; Steinberg B. A.; Suydam L. O. Amphotericins A and B: Two New Antifungal Antibiotics Possessing High Activity against Deep-Seated and Superficial Mycoses. Antibiot. Annu. 1955, 3, 574–578. [PubMed] [Google Scholar]
- Sanglard D.; Odds F. C. Resistance of Candida Species to Antifungal Agents: Molecular Mechanisms and Clinical Consequences. Lancet Infect. Dis. 2002, 2 (2), 73–85. 10.1016/S1473-3099(02)00181-0. [DOI] [PubMed] [Google Scholar]
- Muraglia K. A.; Chorghade R. S.; Kim B. R.; Tang X. X.; Shah V. S.; Grillo A. S.; Daniels P. N.; Cioffi A. G.; Karp P. H.; Zhu L.; Welsh M. J.; Burke M. D. Small-Molecule Ion Channels Increase Host Defences in Cystic Fibrosis Airway Epithelia. Nature 2019, 567 (7748), 405–408. 10.1038/s41586-019-1018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi A. G.; Hou J.; Grillo A. S.; Diaz K. A.; Burke M. D. Restored Physiology in Protein-Deficient Yeast by a Small Molecule Channel. J. Am. Chem. Soc. 2015, 137 (32), 10096–10099. 10.1021/jacs.5b05765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorghade R. S.; Kim B. R.; Launspach J. L.; Karp P. H.; Welsh M. J.; Burke M. D.. Amphotericin B Induces Epithelial Voltage Responses in People with Cystic Fibrosis. J. Cystic Fibrosis 2020. 10.1016/j.jcf.2020.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Chemistry and Biology of the Polyene Macrolide Antibiotics. Bacteriol. Rev. 1973, 37 (3), 166–196. 10.1128/BR.37.3.166-196.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski F.; Griffin D. C.; Loraine J.; Rittig M.; Delves-Broughton J.; Bonev B. B. Recognition of Membrane Sterols by Polyene Antifungals Amphotericin B and Natamycin, A (13)C MAS NMR Study. Front. Cell Dev. Biol. 2016, 4, 57. 10.3389/fcell.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray K. C.; Palacios D. S.; Dailey I.; Endo M. M.; Uno B. E.; Wilcock B. C.; Burke M. D. Amphotericin Primarily Kills Yeast by Simply Binding Ergosterol. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (7), 2234–2239. 10.1073/pnas.1117280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welscher Y. M. te; Napel H. H. t.; Balagué M. M.; Souza C. M.; Riezman H.; de Kruijff B.; Breukink E. Natamycin Blocks Fungal Growth by Binding Specifically to Ergosterol without Permeabilizing the Membrane. J. Biol. Chem. 2008, 283 (10), 6393–6401. 10.1074/jbc.M707821200. [DOI] [PubMed] [Google Scholar]
- Mesa-Arango A. C.; Trevijano-Contador N.; Román E.; Sánchez-Fresneda R.; Casas C.; Herrero E.; Argüelles J. C.; Pla J.; Cuenca-Estrella M.; Zaragoza O. The Production of Reactive Oxygen Species Is a Universal Action Mechanism of Amphotericin B against Pathogenic Yeasts and Contributes to the Fungicidal Effect of This Drug. Antimicrob. Agents Chemother. 2014, 58 (11), 6627–6638. 10.1128/AAC.03570-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A.; Holz R. Aqueous Pores Created in Thin Lipid Membranes by the Polyene Antibiotics Nystatin and Amphotericin B. Membranes 1973, 2, 377–408. [PubMed] [Google Scholar]
- Fujii G.; Chang J.-E.; Coley T.; Steere B. The Formation of Amphotericin B Ion Channels in Lipid Bilayers. Biochemistry 1997, 36 (16), 4959–4968. 10.1021/bi962894z. [DOI] [PubMed] [Google Scholar]
- te Welscher Y. M.; van Leeuwen M. R.; de Kruijff B.; Dijksterhuis J.; Breukink E. Polyene Antibiotic That Inhibits Membrane Transport Proteins. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (28), 11156–11159. 10.1073/pnas.1203375109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios D. S.; Anderson T. M.; Burke M. D. A Post-PKS Oxidation of the Amphotericin B Skeleton Predicted to Be Critical for Channel Formation Is Not Required for Potent Antifungal Activity. J. Am. Chem. Soc. 2007, 129 (45), 13804–13805. 10.1021/ja075739o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T. M.; Clay M. C.; Cioffi A. G.; Diaz K. A.; Hisao G. S.; Tuttle M. D.; Nieuwkoop A. J.; Comellas G.; Maryum N.; Wang S.; Uno B. E.; Wildeman E. L.; Gonen T.; Rienstra C. M.; Burke M. D. Amphotericin Forms an Extramembranous and Fungicidal Sterol Sponge. Nat. Chem. Biol. 2014, 10 (5), 400–406. 10.1038/nchembio.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brajtburg J.; Powderly W. G.; Kobayashi G. S.; Medoff G. Amphotericin B: Current Understanding of Mechanisms of Action. Antimicrob. Agents Chemother. 1990, 34 (2), 183–188. 10.1128/AAC.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova S. S.; Tevyashova A. N.; Olsufyeva E. N.; Bykov E. E.; Ostroumova O. S. Pore-Forming Activity of New Conjugate Antibiotics Based on Amphotericin B. PLoS One 2017, 12 (11), e0188573 10.1371/journal.pone.0188573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y.; Umegawa Y.; Takano T.; Tsuchikawa H.; Matsumori N.; Murata M. Effect of Sterol Side Chain on Ion Channel Formation by Amphotericin B in Lipid Bilayers. Biochemistry 2014, 53 (19), 3088–3094. 10.1021/bi500122c. [DOI] [PubMed] [Google Scholar]
- Antillón A.; de Vries A. H.; Espinosa-Caballero M.; Falcón-González J. M.; Flores Romero D.; González-Damián J.; Jiménez-Montejo F. E.; León-Buitimea A.; López-Ortiz M.; Magaña R.; Marrink S. J.; Morales-Nava R.; Periole X.; Reyes-Esparza J.; Rodríguez Lozada J.; Santiago-Angelino T. M.; Vargas González M. C.; Regla I.; Carrillo-Tripp M.; Fernández-Zertuche M.; Rodríguez-Fragoso L.; Ortega-Blake I. An Amphotericin B Derivative Equally Potent to Amphotericin B and with Increased Safety. PLoS One 2016, 11 (9), e0162171 10.1371/journal.pone.0162171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzyk J.; Gruszecki M.; Tutaj K.; Luchowski R.; Szlazak R.; Wasko P.; Grudzinski W.; Czub J.; Gruszecki W. I. Self-Association of Amphotericin B: Spontaneous Formation of Molecular Structures Responsible for the Toxic Side Effects of the Antibiotic. J. Phys. Chem. B 2014, 118 (48), 13821–13832. 10.1021/jp510245n. [DOI] [PubMed] [Google Scholar]
- Yamamoto T.; Umegawa Y.; Tsuchikawa H.; Hanashima S.; Matsumori N.; Funahashi K.; Seo S.; Shinoda W.; Murata M. The Amphotericin B-Ergosterol Complex Spans a Lipid Bilayer as a Single-Length Assembly. Biochemistry 2019, 58 (51), 5188–5196. 10.1021/acs.biochem.9b00835. [DOI] [PubMed] [Google Scholar]
- Palacios D. S.; Dailey I.; Siebert D. M.; Wilcock B. C.; Burke M. D. Synthesis-Enabled Functional Group Deletions Reveal Key Underpinnings of Amphotericin B Ion Channel and Antifungal Activities. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (17), 6733–6738. 10.1073/pnas.1015023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Welscher Y. M.; Jones L.; van Leeuwen M. R.; Dijksterhuis J.; de Kruijff B.; Eitzen G.; Breukink E. Natamycin Inhibits Vacuole Fusion at the Priming Phase via a Specific Interaction with Ergosterol. Antimicrob. Agents Chemother. 2010, 54 (6), 2618–2625. 10.1128/AAC.01794-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm R. W.; Ejsing C. S.; Surma M. A.; Kaiser H.-J.; Gerl M. J.; Sampaio J. L.; de Robillard Q.; Ferguson C.; Proszynski T. J.; Shevchenko A.; Simons K. Segregation of Sphingolipids and Sterols during Formation of Secretory Vesicles at the Trans-Golgi Network. J. Cell Biol. 2009, 185 (4), 601–612. 10.1083/jcb.200901145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H.; McCaffery J. M.; Grote E. Ergosterol Promotes Pheromone Signaling and Plasma Membrane Fusion in Mating Yeast. J. Cell Biol. 2008, 180 (4), 813–826. 10.1083/jcb.200705076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M.; Wickner W. Ergosterol Is Required for the Sec18/ATP-Dependent Priming Step of Homotypic Vacuole Fusion. EMBO J. 2001, 20 (15), 4035–4040. 10.1093/emboj/20.15.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heese-Peck A.; Pichler H.; Zanolari B.; Watanabe R.; Daum G.; Riezman H. Multiple Functions of Sterols in Yeast Endocytosis. Mol. Biol. Cell 2002, 13 (8), 2664–2680. 10.1091/mbc.e02-04-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannich J. T.; Umebayashi K.; Riezman H. Distribution and Functions of Sterols and Sphingolipids. Cold Spring Harbor Perspect. Biol. 2011, 3 (5), a004762. 10.1101/cshperspect.a004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton J.; Weissman K. J. Polyketide Biosynthesis: A Millennium Review. Nat. Prod. Rep. 2001, 18 (4), 380–416. 10.1039/a909079g. [DOI] [PubMed] [Google Scholar]
- Caffrey P.; Lynch S.; Flood E.; Finnan S.; Oliynyk M. Amphotericin Biosynthesis in Streptomyces Nodosus: Deductions from Analysis of Polyketide Synthase and Late Genes. Chem. Biol. 2001, 8 (7), 713–723. 10.1016/S1074-5521(01)00046-1. [DOI] [PubMed] [Google Scholar]
- Carmody M.; Murphy B.; Byrne B.; Power P.; Rai D.; Rawlings B.; Caffrey P. Biosynthesis of Amphotericin Derivatives Lacking Exocyclic Carboxyl Groups. J. Biol. Chem. 2005, 280 (41), 34420–34426. 10.1074/jbc.M506689200. [DOI] [PubMed] [Google Scholar]
- Aparicio J. F.; Barreales E. G.; Payero T. D.; Vicente C. M.; de Pedro A.; Santos-Aberturas J. Biotechnological Production and Application of the Antibiotic Pimaricin: Biosynthesis and Its Regulation. Appl. Microbiol. Biotechnol. 2016, 100, 61–78. 10.1007/s00253-015-7077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Arnam E. B.; Ruzzini A. C.; Sit C. S.; Horn H.; Pinto-Tomás A. A.; Currie C. R.; Clardy J. Selvamicin, an Atypical Antifungal Polyene from Two Alternative Genomic Contexts. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 (46), 12940–12945. 10.1073/pnas.1613285113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes N. A.; Innocent T. M.; Heine D.; Bassam M. A.; Worsley S. F.; Trottmann F.; Patrick E. H.; Yu D. W.; Murrell J. C.; Schiøtt M.; Wilkinson B.; Boomsma J. J.; Hutchings M. I. Genome Analysis of Two Pseudonocardia Phylotypes Associated with Acromyrmex Leafcutter Ants Reveals Their Biosynthetic Potential. Front. Microbiol. 2016, 7, 2073. 10.3389/fmicb.2016.02073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero L.; Al-Refai M.; Nieto C.; Laatsch H.; Malpartida F.; Seco E. M. New Rimocidin/CE-108 Derivatives Obtained by a Crotonyl-CoA Carboxylase/Reductase Gene Disruption in Streptomyces Diastaticus Var. 108: Substrates for the Polyene Carboxamide Synthase PcsA. PLoS One 2015, 10 (8), e0135891 10.1371/journal.pone.0135891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.-J.; Kong D.; Han K.; Sherman D. H.; Bai L.; Deng Z.; Lin S.; Kim E.-S. Structural Analysis and Biosynthetic Engineering of a Solubility-Improved and Less-Hemolytic Nystatin-like Polyene in Pseudonocardia Autotrophica. Appl. Microbiol. Biotechnol. 2012, 95 (1), 157–168. 10.1007/s00253-012-3955-x. [DOI] [PubMed] [Google Scholar]
- Pawlak J.; Sowinski P.; Borowski E.; Gariboldi P. Stereostructure of Perimycin A. J. Antibiot. 1995, 48 (9), 1034–1038. 10.7164/antibiotics.48.1034. [DOI] [PubMed] [Google Scholar]
- Cox C. L.; Tietz J. I.; Sokolowski K.; Melby J. O.; Doroghazi J. R.; Mitchell D. A. Nucleophilic 1,4-Additions for Natural Product Discovery. ACS Chem. Biol. 2014, 9 (9), 2014–2022. 10.1021/cb500324n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxson T.; Tietz J. I.; Hudson G. A.; Guo X. R.; Tai H.-C.; Mitchell D. A. Targeting Reactive Carbonyls for Identifying Natural Products and Their Biosynthetic Origins. J. Am. Chem. Soc. 2016, 138 (46), 15157–15166. 10.1021/jacs.6b06848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy E. M.; Tietz J. I.; Blair P. M.; Mitchell D. A. Biological Characterization of the Hygrobafilomycin Antibiotic JBIR-100 and Bioinformatic Insights into the Hygrolide Family of Natural Products. Bioorg. Med. Chem. 2016, 24 (24), 6276–6290. 10.1016/j.bmc.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L. A.; Saint-Vincent P. M. B.; Guo X.; Hudson G. A.; Mitchell D. A.. Reactivity-Based Screening for Citrulline-Containing Natural Products Reveals a Family of Bacterial Peptidyl Arginine Deiminases. bioRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersch M.; Kreuzer J.; Sieber S. A. Electrophilic Natural Products and Their Biological Targets. Nat. Prod. Rep. 2012, 29 (6), 659–682. 10.1039/c2np20012k. [DOI] [PubMed] [Google Scholar]
- Odendaal A. Y.; Trader D. J.; Carlson E. E. Chemoselective Enrichment for Natural Products Discovery. Chem. Sci. 2011, 2 (4), 760–764. 10.1039/c0sc00620c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capehart S. L.; Carlson E. E. Mass Spectrometry-Based Assay for the Rapid Detection of Thiol-Containing Natural Products. Chem. Commun. 2016, 52 (90), 13229–13232. 10.1039/C6CC07111B. [DOI] [PubMed] [Google Scholar]
- Castro-Falcón G.; Millán-Aguiñaga N.; Roullier C.; Jensen P. R.; Hughes C. C. Nitrosopyridine Probe To Detect Polyketide Natural Products with Conjugated Alkenes: Discovery of Novodaryamide and Nocarditriene. ACS Chem. Biol. 2018, 13 (11), 3097–3106. 10.1021/acschembio.8b00598. [DOI] [PubMed] [Google Scholar]
- Blackman M. L.; Royzen M.; Fox J. M. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels-Alder Reactivity. J. Am. Chem. Soc. 2008, 130 (41), 13518–13519. 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-B.; Cai W.; Del Rio Flores A.; Twigg F. F.; Zhang W. Facile Discovery and Quantification of Isonitrile Natural Products via Tetrazine-Based Click Reactions. Anal. Chem. 2020, 92 (1), 599–602. 10.1021/acs.analchem.9b05147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan J.; Murphy C. D.; Caffrey P. New Insights into Polyene Macrolide Biosynthesis in Couchioplanes Caeruleus. Mol. BioSyst. 2017, 13 (5), 866–873. 10.1039/C7MB00112F. [DOI] [PubMed] [Google Scholar]
- Chen S.; Huang X.; Zhou X.; Bai L.; He J.; Jeong K. J.; Lee S. Y.; Deng Z. Organizational and Mutational Analysis of a Complete FR-008/Candicidin Gene Cluster Encoding a Structurally Related Polyene Complex. Chem. Biol. 2003, 10 (11), 1065–1076. 10.1016/j.chembiol.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Brautaset T.; Sekurova O. N.; Sletta H.; Ellingsen T. E.; Strøm A. R.; Valla S.; Zotchev S. B. Biosynthesis of the Polyene Antifungal Antibiotic Nystatin in Streptomyces Noursei ATCC 11455: Analysis of the Gene Cluster and Deduction of the Biosynthetic Pathway. Chem. Biol. 2000, 7 (6), 395–403. 10.1016/S1074-5521(00)00120-4. [DOI] [PubMed] [Google Scholar]
- Cao B.; Yao F.; Zheng X.; Cui D.; Shao Y.; Zhu C.; Deng Z.; You D. Genome Mining of the Biosynthetic Gene Cluster of the Polyene Macrolide Antibiotic Tetramycin and Characterization of a P450 Monooxygenase Involved in the Hydroxylation of the Tetramycin B Polyol Segment. ChemBioChem 2012, 13 (15), 2234–2242. 10.1002/cbic.201200402. [DOI] [PubMed] [Google Scholar]
- Aparicio J. F.; Fouces R.; Mendes M. V.; Olivera N.; Martín J. F. A Complex Multienzyme System Encoded by Five Polyketide Synthase Genes Is Involved in the Biosynthesis of the 26-Membered Polyene Macrolide Pimaricin in Streptomyces Natalensis. Chem. Biol. 2000, 7 (11), 895–905. 10.1016/S1074-5521(00)00038-7. [DOI] [PubMed] [Google Scholar]
- Seco E. M.; Pérez-Zúñiga F. J.; Rolón M. S.; Malpartida F. Starter Unit Choice Determines the Production of Two Tetraene Macrolides, Rimocidin and CE-108, in Streptomyces Diastaticus Var. 108. Chem. Biol. 2004, 11 (3), 357–366. 10.1016/j.chembiol.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Blin K.; Shaw S.; Steinke K.; Villebro R.; Ziemert N.; Lee S. Y.; Medema M. H.; Weber T. AntiSMASH 5.0: Updates to the Secondary Metabolite Genome Mining Pipeline. Nucleic Acids Res. 2019, 47 (W1), W81–W87. 10.1093/nar/gkz310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F.; Madden T. L.; Schäffer A. A.; Zhang J.; Zhang Z.; Miller W.; Lipman D. J. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 25 (17), 3389–3402. 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz J. I.; Schwalen C. J.; Patel P. S.; Maxson T.; Blair P. M.; Tai H.-C.; Zakai U. I.; Mitchell D. A. A New Genome-Mining Tool Redefines the Lasso Peptide Biosynthetic Landscape. Nat. Chem. Biol. 2017, 13 (5), 470–478. 10.1038/nchembio.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinnider M. A.; Merwin N. J.; Johnston C. W.; Magarvey N. A. PRISM 3: Expanded Prediction of Natural Product Chemical Structures from Microbial Genomes. Nucleic Acids Res. 2017, 45, W49–W54. 10.1093/nar/gkx320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsman M. E.; Hari T. P. A.; Boddy C. N. Polyketide Synthase and Non-Ribosomal Peptide Synthetase Thioesterase Selectivity: Logic Gate or a Victim of Fate?. Nat. Prod. Rep. 2016, 33 (2), 183–202. 10.1039/C4NP00148F. [DOI] [PubMed] [Google Scholar]
- Del Vecchio F.; Petkovic H.; Kendrew S. G.; Low L.; Wilkinson B.; Lill R.; Cortés J.; Rudd B. A. M.; Staunton J.; Leadlay P. F. Active-Site Residue, Domain and Module Swaps in Modular Polyketide Synthases. J. Ind. Microbiol. Biotechnol. 2003, 30 (8), 489–494. 10.1007/s10295-003-0062-0. [DOI] [PubMed] [Google Scholar]
- Katz L.; Baltz R. H. Natural Product Discovery: Past, Present, and Future. J. Ind. Microbiol. Biotechnol. 2016, 43 (2–3), 155–176. 10.1007/s10295-015-1723-5. [DOI] [PubMed] [Google Scholar]
- Rieder U.; Luedtke N. W. Alkene-Tetrazine Ligation for Imaging Cellular DNA. Angew. Chem., Int. Ed. 2014, 53 (35), 9168–9172. 10.1002/anie.201403580. [DOI] [PubMed] [Google Scholar]
- Mandal A.; Das A. K.; Basak A. Label-Assisted Laser Desorption/Ionization Mass Spectrometry (LA-LDI-MS): Use of Pyrene Aldehyde for Detection of Biogenic Amines, Amino Acids and Peptides. RSC Adv. 2015, 5 (129), 106912–106917. 10.1039/C5RA20678B. [DOI] [Google Scholar]
- Cabrera-Pardo J. R.; Chai D. I.; Liu S.; Mrksich M.; Kozmin S. A. Label-Assisted Mass Spectrometry for the Acceleration of Reaction Discovery and Optimization. Nat. Chem. 2013, 5 (5), 423–427. 10.1038/nchem.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpon L.; Lancelin J.-M. Solution NMR Structure of Five Representative Glycosylated Polyene Macrolide Antibiotics with a Sterol-Dependent Antifungal Activity. Eur. J. Biochem. 2002, 269 (18), 4533–4541. 10.1046/j.1432-1033.2002.03147.x. [DOI] [PubMed] [Google Scholar]
- Pandey R. C.; Rinehart K. L. Jr. POLYENE ANTIBIOTICS. VII CARBON-13 NUCLEAR MAGNETIC RESONANCE EVIDENCE FOR CYCLIC HEMIKETALS IN THE POLYENE ANTIBIOTICS AMPHOTERICIN B, NYSTATIN A1, TETRIN A, TETRIN B, LUCENSOMYCIN, AND PIMARiCIN. J. Antibiot. 1976, 29 (10), 1035–1042. 10.7164/antibiotics.29.1035. [DOI] [PubMed] [Google Scholar]
- Arcamone F.; Perego M. Isolation and characteristics of a new antibiotic: etruscomycin. Ann. Chim. 1959, 49, 345. [Google Scholar]
- Dejong C. A.; Chen G. M.; Li H.; Johnston C. W.; Edwards M. R.; Rees P. N.; Skinnider M. A.; Webster A. L. H.; Magarvey N. A. Polyketide and Nonribosomal Peptide Retro-Biosynthesis and Global Gene Cluster Matching. Nat. Chem. Biol. 2016, 12 (12), 1007–1014. 10.1038/nchembio.2188. [DOI] [PubMed] [Google Scholar]
- Ueoka R.; Bortfeld-Miller M.; Morinaka B. I.; Vorholt J. A.; Piel J. Toblerols: Cyclopropanol-Containing Polyketide Modulators of Antibiosis in Methylobacteria. Angew. Chem., Int. Ed. 2018, 57 (4), 977–981. 10.1002/anie.201709056. [DOI] [PubMed] [Google Scholar]
- Du L.; Risinger A. L.; King J. B.; Powell D. R.; Cichewicz R. H. A Potent HDAC Inhibitor, 1-Alaninechlamydocin, from a Tolypocladium Sp. Induces G2/M Cell Cycle Arrest and Apoptosis in MIA PaCa-2 Cells. J. Nat. Prod. 2014, 77 (7), 1753–1757. 10.1021/np500387h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bur D.; Nikles M.; Séquin U.; Neuburger M.; Zehnder M. Conformational Analysis of Open-Chain 1,2:3,4-Diepoxides: Comparison of Crystal Structures, NMR Data, and Molecular-Orbital Calculations. Helv. Chim. Acta 1993, 76 (5), 1863–1875. 10.1002/hlca.19930760506. [DOI] [Google Scholar]
- Nakagomi K.; Sakai S.; Tanaka H.; Tomizuka N.; Kawakami Y.; Nakajima T. STUDIES ON INHIBITORS OF RAT MAST CELL DEGRANULATION PRODUCED BY MICROORGANISMS. J. Antibiot. 1990, 43 (5), 470–476. 10.7164/antibiotics.43.470. [DOI] [PubMed] [Google Scholar]
- Zygmunt W. A.; Tavormina P. A. Steroid Interference with Antifungal Activity of Polyene Antibiotics. Appl. Microbiol. 1966, 14 (6), 865–869. 10.1128/AM.14.6.865-869.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S.; Clipstone N.; Timmermann L.; Northrop J.; Graef I.; Fiorentino D.; Nourse J.; Crabtree G. R. The Mechanism of Action of Cyclosporin A and FK506. Clin. Immunol. Immunopathol. 1996, 80 (3), S40–S45. 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- Li J.; Kim S. G.; Blenis J. Rapamycin: One Drug, Many Effects. Cell Metab. 2014, 19 (3), 373–379. 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasper H. E.; Kramer N. E.; Smith J. L.; Hillman J. D.; Zachariah C.; Kuipers O. P.; de Kruijff B.; Breukink E. An Alternative Bactericidal Mechanism of Action for Lantibiotic Peptides That Target Lipid II. Science 2006, 313 (5793), 1636–1637. 10.1126/science.1129818. [DOI] [PubMed] [Google Scholar]
- Song Y.; Lunde C. S.; Benton B. M.; Wilkinson B. J. Further Insights into the Mode of Action of the Lipoglycopeptide Telavancin through Global Gene Expression Studies. Antimicrob. Agents Chemother. 2012, 56 (6), 3157–3164. 10.1128/AAC.05403-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low Z. J.; Xiong J.; Xie Y.; Ma G.-L.; Saw H.; Thi Tran H.; Wong S. L.; Pang L. M.; Fong J.; Lu P.; Hu J.-F.; Liang Y.; Miao Y.; Liang Z.-X. Discovery, Biosynthesis and Antifungal Mechanism of the Polyene-Polyol Meijiemycin. Chem. Commun. 2020, 56 (5), 822–825. 10.1039/C9CC08908J. [DOI] [PubMed] [Google Scholar]
- Seco E. M.; Cuesta T.; Fotso S.; Laatsch H.; Malpartida F. Two Polyene Amides Produced by Genetically Modified Streptomyces Diastaticus Var. 108. Chem. Biol. 2005, 12 (5), 535–543. 10.1016/j.chembiol.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Baltz R. H.; Miao V.; Wrigley S. K. Natural Products to Drugs: Daptomycin and Related Lipopeptide Antibiotics. Nat. Prod. Rep. 2005, 22 (6), 717. 10.1039/b416648p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.