Abstract
Liposomal spherical nucleic acids (L-SNAs) show significant promise as cancer immunotherapeutics. L-SNAs are highly modular nanoscale assemblies defined by a dense, upright radial arrangement of oligonucleotides around a liposomal core. Herein, we establish a set of L-SNA design rules by studying the biological and immunological properties of L-SNAs as a function of liposome composition. To achieve this, we synthesized liposomes where the lipid phosphatidylcholine headgroup was held constant, while the diacyl lipid tail chain length and degree of saturation were varied, using either 1,2-dioleylphosphatidylcholine (DOPC), 1,2-dimyristoyl-phosphatidylcholine (DMPC), 1,2-dipalmitoylphosphatidylcholine (DPPC), or 1,2-distearoyl-phosphatidylcholine (DSPC). These studies show that the identity of the constituent lipid dictates the DNA loading, cellular uptake, serum stability, in vitro immunostimulatory activity, and in vivo lymph node accumulation of the L-SNA. Furthermore, in the 4T1 mouse model of triple-negative breast cancer (TNBC), the subcutaneous administration of immunostimulatory L-SNAs synthesized with DPPC significantly decreases the production of lung metastases and delays tumor growth as compared to L-SNAs synthesized using DOPC, due to the enhanced stability of L-SNAs synthesized with DPPC over those synthesized with DOPC. Moreover, the inclusion of cell lysates derived from Py8119 TNBC cells as antigen sources in L-SNAs leads to a significant increase in antitumor efficacy in the Py8119 model when lysates are encapsulated in the cores of L-SNAs synthesized with DPPC rather than DOPC, presumably due to increased codelivery of adjuvant and antigen to dendritic cells in vivo. This difference is further amplified when using lysates from oxidized Py8119 cells as a more potent antigen source, revealing synergy between the lysate preparation method and liposome composition in synthesizing immunotherapeutic L-SNAs. Together, this work shows that the biological properties and immunomodulatory activity of L-SNAs can be modulated by exchanging liposome components, providing another handle for the rational design of nanoscale immunotherapeutics.
Short abstract
The molecular identity of the lipids that comprise liposomal spherical nucleic acids modulates their biological properties and provides a handle for their rational design as cancer immunotherapeutics.
Regarded as one of the more successful biomolecule packing systems to date,1 liposomes are useful in a wide variety of biomedical applications, including drug delivery,2,3 biosensing,4,5 diagnostics,6,7 gene delivery,8 and immunomodulation.9−13 The chemical composition of liposomes determines their performance in biological systems.14 Indeed, it has been well-established that the phase transition temperature (TC) of the constituent lipids dictates the phospholipid bilayer membrane fluidity, which heavily influences the liposome’s biological properties, including permeability and lipid exchange.15 The TC of a phospholipid is determined by the chemical identity and charge of the hydrophilic headgroup as well as the chain length and degree of saturation of the diacyl lipid tail.16 At temperatures at or below the TC, liposomes exist in a gel phase, where lipid exchange is limited and membrane fluidity is low. At temperatures exceeding the TC, liposomes exist in a liquid-crystalline phase, where the membrane is more fluid and the dynamics of lipid exchange are increased. In general, the TC increases as the diacyl lipid chain length increases, due to increased van der Waals forces between the acyl chains.17 However, introducing double bonds into the diacyl lipid tail decreases the TC, as the packing of the hydrophobic chains is disrupted by structural kinks introduced by the unsaturated bonds.18 Together, these subtle changes to the chemical structure of the individual lipid components dictate the supramolecular properties of the liposome and are important to consider when designing liposome-scaffolded materials.
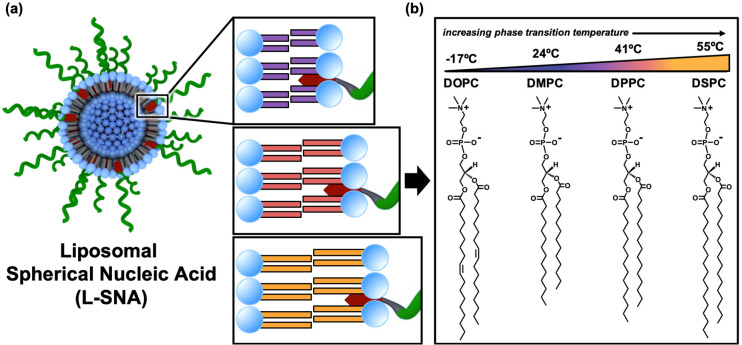
Because of their biocompatibility, modularity, and favorable physical properties,1,19−23 liposomes are attractive scaffolds for synthesizing spherical nucleic acids (SNAs). SNAs are a unique class of nucleic acid defined by the dense, highly oriented arrangement of oligonucleotides around a nanoparticle core.11,24−28 SNAs exhibit markedly different biological properties from their linear nucleic acid analogues,24 including rapid cellular uptake without the need for transfection reagents.25 Liposomal SNAs (L-SNAs, Figure 1a) are synthesized by embedding oligonucleotides that have been functionalized with lipophilic moieties (e.g., cholesterol) into the outer membrane of a liposome’s phospholipid bilayer.11,27,29−31 Diverse L-SNA constructs with tunable properties can be rapidly generated by changing the lipophilic oligonucleotide anchor11,32 or altering the sequence, backbone, and surface density of the oligonucleotide.33
Figure 1.
Liposomal SNA (L-SNA) design parameters. (a) Schematic representation of the L-SNA, wherein DNA (green) is embedded into the outer phospholipid membrane of a liposome core using a hydrophobic DNA anchor (depicted in red). The modularity of the L-SNA architecture allows for the rapid generation of diverse constructs by using different phospholipids. (b) Chemical structure and phase transition temperature (TC) of the phospholipids used in the synthesis of the L-SNAs employed in these studies.
When comprised of immunostimulatory oligonucleotides (CpG-1826), L-SNAs function as potent cancer immunotherapeutics.9,10 Prior research has revealed that the identity of the lipophilic moiety used to embed DNA into the liposomal membrane changes the stability of the L-SNA, which in turn alters the biological and immunological properties of the material.11 Moreover, when incorporating tumor-associated antigens (TAAs) into the L-SNA, the nanoscale arrangement and attachment chemistry of immunomodulatory components on the scaffold can be used to modulate the kinetics of antigen presentation and costimulatory marker expression, which dictate their antitumor efficacy.10,28 For cancers without identified TAAs, L-SNAs have been used to encapsulate tumor cell lysates as potent antigen sources.9 In mouse models of triple-negative breast cancer (TNBC), it has been found that the process by which lysates are generated prior to L-SNA incorporation changes the available antigen pool, which impacts the immunotherapeutic potency of the construct.
Herein, we sought to determine whether the molecular identity of the lipids comprising L-SNAs could be used as an additional handle for controlling their biological properties and immunotherapeutic function. Toward this end, we synthesized a series of L-SNAs using liposomes comprised of lipids with varying TC. In this approach, the hydrophilic headgroup of the phospholipids remained unchanged, while the chemical identity of the hydrophobic acyl chains was systematically varied (Figure 1b). In this way, the surface chemistry remained the same, but the bilayer composition differed. We evaluated the resulting L-SNAs for their in vitro serum stability, cellular uptake, immune cell activation, and immunotherapeutic function in orthotopic syngeneic mouse models of TNBC. Through these analyses, we determined that the liposome scaffold composition regulates the biological and immunological properties of L-SNAs; namely, the more stable the L-SNA, the better the in vivo performance. Significant antitumor efficacy and inhibition of lung metastasis formation was observed in the 4T1 model of TNBC when animals were administered L-SNAs synthesized using lipids with higher TC values, indicating that liposome stability governs L-SNA efficacy in this model. Moreover, the inclusion of cell lysates from Py8119 TNBC cells into the core of L-SNAs synthesized from lipids that support higher TCs significantly increased the resultant antitumor efficacy in the Py8119 model, likely due to higher codelivery of immunotherapeutic components to dendritic cells in vivo by the more stable L-SNA scaffold. This trend became more pronounced when lysates from oxidized Py8119 cells were utilized as the antigen source, indicating that the lysate preparation method and L-SNA stability are additive contributors. Together, these results indicate that L-SNA stability can be modulated by exchanging the lipid components and that the most potent constructs are those synthesized using the most stable liposome scaffolds.
Results and Discussion
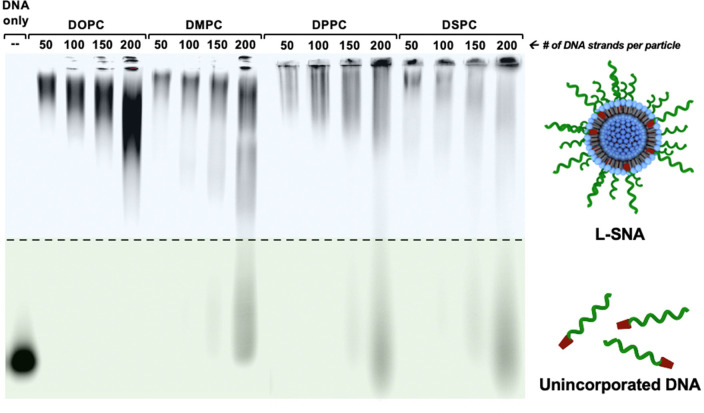
To assess the effect of liposome composition on the properties of L-SNAs, 80 nm liposomes comprised of lipids with varying TC (Figure 1b) were synthesized using 1,2-dioleylphosphatidylcholine (DOPC, TC = −17 °C), 1,2-dimyristoyl-phosphatidylcholine (DMPC, TC = 24 °C), 1,2-dipalmitoylphosphatidylcholine (DPPC, TC = 41 °C), or 1,2-distearoyl- phosphatidylcholine (DSPC, TC = 55 °C).16 Following overnight incubation with DNA that is doubly functionalized with cholesterol and Cy5 (Table S1, entry 1) at T > TC for all lipids, colloidally stable L-SNAs formed from all liposome scaffolds. The maximum DNA loading per particle was observed for L-SNAs synthesized with DOPC, as evidenced by the absence of free unincorporated DNA by gel electrophoresis at the highest liposome to DNA ratio (Figure 2). We hypothesize that this is because cholesterol is more readily intercalated into the membrane bilayer of DOPC-based liposomes than those comprised of fully saturated phospholipids, due to conformational rearrangement of lipids in the liquid-crystalline phase.34,35 Indeed, the maximum DNA loading per particle was reduced in L-SNAs comprised of lipids with higher TC values (i.e., DMPC, DPPC, and DSPC), as evidenced by the presence of free DNA at loadings exceeding 150 strands per particle (0.31 pmol/cm2, Figure 2). This is presumably due to decreased membrane fluidity of liposomes comprised of lipids with higher TC.35 Dynamic light scattering (DLS) analysis confirmed that the L-SNA hydrodynamic diameter was identical regardless of core composition (Table S2).
Figure 2.
DNA loading capacity of various liposome scaffolds. The DNA loading onto liposomes was assessed using native gel electrophoresis and Cy5-labeled DNA. L-SNAs can be successfully formed using liposomes comprised of all phospholipids tested (DOPC, DMPC, DPPC, and DSPC) at DNA loadings of up to 100 strands per particle, as evidenced by the reduction in DNA mobility without the presence of unincorporated DNA, following incubation of cholesterol-functionalized, Cy5-labeled DNA with liposomes at T > TC for all constructs.
To evaluate the DNA dissociation from the L-SNA scaffold in biological media, L-SNAs were synthesized using liposomes containing 1% rhodamine-labeled lipids (Figure S1) and DNA doubly functionalized with cholesterol and Cy5 (Table S1, entry 1). On the L-SNA scaffold, Cy5 and rhodamine are within the radius required for Förster resonance energy transfer (FRET), with the FRET signal indicative of the presence of intact L-SNAs. FRET-capable L-SNAs were incubated in 10% fetal bovine serum (FBS) at 37 °C, and their stability was evaluated as a function of the decrease in FRET signal over time (Figure S2). To account for any potential variations in dye incorporation per particle and to normalize the data for comparison, the apparent rate constant, k, was calculated for each L-SNA using a one-phase exponential decay equation (Figure S2) to determine the initial rate of FRET decrease. The calculated k values of the L-SNAs decrease as a function of increasing TC (Figure 3a), indicating that the DNA dissociation rate is slower in higher-TC L-SNAs, and that these L-SNAs are more stable. Indeed, the initial rate of FRET decay for L-SNAs synthesized with DSPC was determined to be approximately 60% lower than those synthesized with DOPC at 37 °C. Importantly, the trends in the rate of DNA dissociation from the SNA scaffold as a function of TC become more pronounced at increased temperatures (Figure S3). The rate of DNA dissociation from L-SNAs synthesized with DSPC is ∼95% lower than L-SNAs synthesized from DOPC when serum stability is assessed at 65 °C. Moreover, the differences in the DNA dissociation rate become negligible when L-SNAs are incubated at 20 °C (Figure S4), due to decreased membrane fluidity of all L-SNA constructs.
Figure 3.
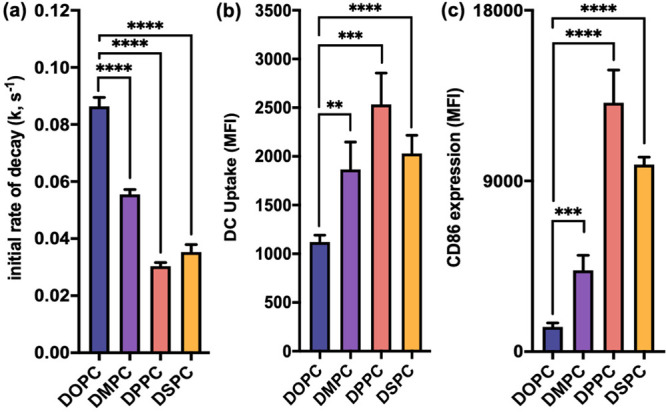
In vitro serum stability, cellular uptake, and immune activation by L-SNAs. (a) Plot of the initial rate of decay, k, as a function of decrease in FRET signal over time. Changing the liposome scaffold from DOPC to one comprising phospholipids of higher TC significantly decreases the rate of DNA dissociation from L-SNAs, thus increasing the stability of the overall construct. (b) Cellular uptake of L-SNAs by DCs as a function of liposome scaffold. Uptake is significantly increased by synthesizing L-SNAs from all higher-TC lipids. (c) DC activation as a function of L-SNA composition. Changing the liposome scaffold from DOPC to one comprising phospholipids of higher TC significantly increases the observed expression of CD86. Statistical analysis was performed using an unpaired t test, where “**” represents a p value of <0.01, “***” represents a p value of <0.001, and “****” represents a p value of <0.0001. Error bars represent standard deviations. MFI represents median fluorescence intensity.
To correlate the in vitro serum stability with biological outcomes, the cellular uptake of all L-SNAs by primary immune cells was evaluated as a function of liposome scaffold. Increased cellular uptake as a function of TC was observed in bone-marrow-derived dendritic cells (DCs), with an overall upward trend between TC and cellular uptake, showing an approximately 80% increase in uptake of L-SNAs comprised of DSPC as compared to DOPC (Figure 3b). Intriguingly, the cellular uptake by DCs was consistently highest for L-SNAs synthesized from DPPC in biological replicates (n = 3). We hypothesize that this is because the TC of DPPC (41 °C) is close to the incubation temperature used for these assays (37 °C), and it is known that liposomes become increasingly permeable at temperatures close to the TC,36 thus allowing for increased L-SNA membrane flexibility and greater association with cell surfaces.
The effect of L-SNA composition on downstream biological processes was assessed via in vitro DC activation, as these cells are functional antigen-presenting cells and have been shown to be efficiently activated by immunostimulatory L-SNAs.10,12,31,37 L-SNAs were synthesized using immunostimulatory oligonucleotides (CpG-1826)38 functionalized with cholesterol (Table S1, entry 2) and liposomes comprised of DOPC, DMPC, DPPC, or DSPC. Following incubation with L-SNAs, DC activation was evaluated as a function of CD86 expression, which is a surface protein present on mature immune cells that promotes T cell differentiation and survival.39 A dramatic increase in immune activation was observed when DCs were incubated with the more stable L-SNAs (Figure 3c), revealing a 6.5-fold increase in CD86 expression by L-SNAs synthesized with DSPC, as compared to those synthesized with DOPC. Consistent with the cellular uptake trends observed in DCs, L-SNAs comprised of DPPC induced the highest expression of CD86, resulting in a nearly 10-fold increase in CD86 expression as compared to L-SNAs comprised of DOPC (Table S3).
Because immunostimulatory L-SNAs synthesized with DPPC (henceforth referred to as “DPPC-SNAs”) showed the highest in vitro stability, uptake, and CD86 expression, the immune uptake and responses generated by this construct were more comprehensively analyzed and compared to the standard L-SNA construct, which is synthesized with DOPC (henceforth referred to as “DOPC-SNA”). DPPC-SNAs were consistently taken up to a greater extent by DCs than DOPC-SNAs at all time points analyzed (Figure S5). In addition, DPPC-SNAs showed increased immune activation over DOPC-SNAs, as measured by the expression of surface proteins CD80 and MHC-II (Figure S6) and the secretion of the cytokine TNF-α (Figure S7) following 24 h of incubation.
To determine whether superior in vitro DC uptake correlates with higher in vivo lymph node targeting, the accumulation of DPPC-SNAs in the lymph nodes was compared to that of DOPC-SNAs as a function of time. To achieve this, healthy mice were administered fluorophore-labeled SNAs via subcutaneous injection at a dose of 2.5 nmol of DNA per construct (n = 3). The lymph node accumulation of DPPC-SNAs was 4-fold higher than DOPC-SNAs at 2 h postinjection (Figure S8), while the concentration was equivalent at 24 h postinjection (Figure S9). Together, this indicates that DPPC-SNAs are shuttled more rapidly to the lymph nodes in vivo, without a loss in long-term retention.
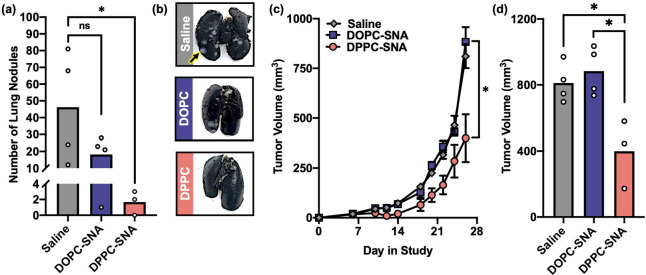
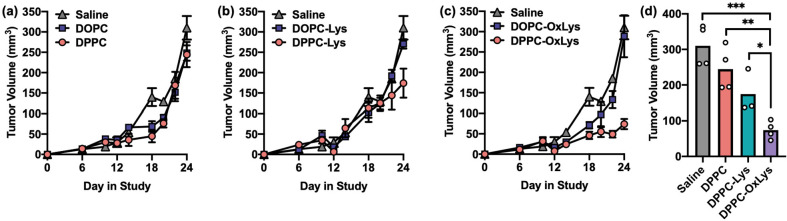
The administration of CpG-1826 in the 4T1 model of TNBC can suppress the spontaneous formation of lung metastases, as a form of “adjuvant-only” immunotherapy.40,41 Thus, to evaluate whether L-SNA stability and in vitro immunostimulatory activity correlated with in vivo outcomes, the activity of DOPC-SNAs was compared to DPPC-SNAs in the 4T1 mouse model (Figure 4). Mice bearing 4T1 tumors were peritumorally administered DOPC-SNAs and DPPC-SNAs via subcutaneous injection at a dose of 5 nmol of CpG-1826 on days 6, 10, and 15 postinoculation (n = 4 per group). As a negative control, an additional set of animals (n = 4) was administered saline. At day 28 of the study, animals were sacrificed, lungs were perfused per literature protocol,42 and the number of lung nodules was counted. DPPC-SNAs significantly inhibited the formation of lung metastases (Figure 4a,b), whereas DOPC-SNAs were ineffective. Interestingly, this trend held when evaluating primary tumor growth (Figure 4c,d), with DPPC-SNAs significantly suppressing tumor growth, while DOPC-SNAs had no effect on primary tumor growth throughout the duration of the study. Consistent with in vitro results (vide supra), L-SNA potency in the 4T1 model directly correlates with liposome stability.
Figure 4.
In vivo antimetastic and antitumor activity of L-SNAs in the 4T1 TNBC model. (a) Lung metastasis production, as measured by the number of lung nodules identified at day 28 in the study, following administration of saline (gray bar), DOPC-SNAs (blue bar), or DPPC-SNAs (pink bar). White dots represent individual animals in each group. (b) Representative photos of lungs excised from each group. (c) Primary tumor growth in the 4T1 model following administration of saline (gray diamonds), DOPC-SNAs (blue square), or DPPC-SNAs (pink circle). Error bars represent standard errors of the mean. (d) Comparison of tumor volume between treatment groups at day 28 in the study. White dots represent individual animals in each group. Statistical analysis was performed using an unpaired t test, where “*” represents a p value of <0.05, and “ns” represents a p value of >0.05.
To evaluate whether the observed differences in L-SNA activity were tumor-model-dependent, the antitumor activity of L-SNAs in the Py8119 model of TNBC was also evaluated. Unsurprisingly, administration of DPPC-SNAs and DOPC-SNAs as “adjuvant-only” immunotherapeutics was ineffective in this model (Figure 5a), as immunostimulation alone is often insufficient at raising an antitumor immune response,41 with a few notable exceptions that include the aforementioned 4T1 model. Since no common TAAs have been identified for TNBC, lysates from Py8119 cells were generated and then utilized as antigen sources. These lysates were encapsulated into L-SNAs comprised of DOPC (DOPC-Lys-SNAs) or DPPC (DPPC-Lys-SNAs). The antitumor efficacy of both constructs was compared in vivo following L-SNA administration at days 6, 10, and 15 at a dose of 5 nmol of CpG-1826 and 10 μg of lysate. Excitingly, there was a ∼60% reduction in tumor growth when DPPC-Lys-SNAs were administered, as compared to when saline was administered to animals. Conversely, there was no difference in tumor growth when DOPC-Lys-SNAs were administered to animals (Figure 5b), again showing the dependence of antitumor efficacy on L-SNA stability.
Figure 5.
In vivo antitumor activity of L-SNAs in the Py8119 TNBC model. (a) Antitumor efficacy of “adjuvant-only” L-SNAs as a function of liposome stability, following administration of saline (gray triangle), DOPC-SNAs (blue square), or DPPC-SNAs (pink circle). (b) Antitumor efficacy of L-SNAs encapsulating Py8119 lysates as a function of liposome stability. Animals were administered saline (gray triangle), DOPC-Lys-SNAs (blue square), or DPPC-Lys-SNAs (pink circle). (c) Antitumor efficacy of L-SNAs encapsulating oxidized Py8119 lysates as a function of liposome stability. Animals were administered saline (gray triangle), DOPC-OxLys-SNAs (blue square), or DPPC-OxLys-SNAs (pink circle). (d) Comparison of tumor volume between DPPC-containing treatment groups at day 28 in the study. White dots represent individual animals in each group. Error bars represent standard errors of the mean. Statistical analysis was performed using an unpaired t test, where “*” represents a p value of <0.05, “**” represents a p value of <0.01, and “***” represents a p value of <0.001.
Oxidizing cancer cells prior to lysate generation often increases their immunogenicity,43−45 and incorporating oxidized lysates into the core of L-SNAs leads to dramatic increases in the antitumor efficacy in mouse models of TNBC.9 Therefore, to determine whether the effects of tumor cell oxidation and L-SNA stability were additive, L-SNAs containing lysates from oxidized Py8119 cells were synthesized using DOPC (DOPC-OxLys-SNAs) and DPPC (DPPC-OxLys-SNAs). Strikingly, DPPC-OxLys-SNAs significantly suppressed tumor growth over the duration of the study (Figure 5c), while DOPC-OxLys-SNAs were ineffective at these doses of DNA and lysate. Collectively, these data indicate that the effects of the lysate preparation method and L-SNA stability are synergistic, a trend which becomes clearer when the study end point for all DPPC-based L-SNAs is compared (Figure 5d). While DPPC-SNAs are ineffective at reducing tumor growth in the Py8119 model, the inclusion of lysates into the L-SNA (DPPC-Lys-SNAs) renders these materials effective, and maximum antitumor efficacy is observed when lysates from oxidized cells are used as the antigen sources (DPPC-OxLys-SNAs), revealing the importance of both the antigen processing method and liposome stability in these constructs.
Conclusions
Synthesizing L-SNAs from liposomes comprised of lipids with identical phosphatidylcholine headgroups, but a varied diacyl lipid tail, allows for single-variable analysis of biological properties. Through these studies, we have determined that the serum stability, cellular uptake, immune activation, and antitumor activity of L-SNAs can be augmented by using liposome scaffolds comprised of lipids with higher TC values while keeping the nanoparticle size and surface chemistry identical. The dynamics of lipid exchange in liposomes is a function of membrane fluidity, which decreases as TC increases. Thus, synthesizing L-SNAs from lipids with higher TC produces structures whose dynamics of lipid exchange are slower. In turn, the rate of DNA dissociation from the L-SNA scaffold is significantly decreased when SNAs are synthesized from lipids with higher TC, leading to greater interactions between the oligonucleotides and liposome core and an overall increased structural stability. Because the L-SNA architecture drives its biological properties, prolonged preservation of this structure in biological media likely leads to the observed enhancements in cellular uptake, immune cell activation, and in vivo lymph node accumulation. These results indicate that the biological interactions of nanomaterials are not solely determined by nanoscale size and surface chemistry but rather that the chemical identity of the components of noncovalent assemblies also plays a substantial role.
We have previously shown that seemingly small chemical and structural changes to the L-SNA platform can have profound impacts on resulting SNA biological properties and functions. For example, changing the chemistry used to anchor DNA into the L-SNA affects the in vitro serum stability, immune activation, and in vivo tissue distribution due to differences in L-SNA stability.11,32 Moreover, altering the chemical bond that releases peptide antigens from immunostimulatory L-SNAs enhances immune activation,31 and modifying the chemical composition and structural arrangement of antigenic cargo within immunostimulatory L-SNAs dramatically affects downstream antitumor efficacy because of differences in signaling kinetics.9,10 In line with these findings, we now show that the composition of the liposomal core can be used to modulate the biological properties of L-SNAs, providing another tunable handle in their rational design as immunotherapeutics.
In the 4T1 TNBC mouse model, administration of immunostimulatory L-SNAs synthesized with DPPC significantly decreased tumor growth and lung metastasis formation, as compared to L-SNAs synthesized with DOPC, indicating that more stable L-SNA constructs are required for maximal immunostimulation in vivo. Moreover, in TNBC models where “adjuvant-only” therapy is ineffective, the inclusion of tumor cell lysates in the core of the L-SNAs reveals a significant dependence on L-SNA composition with respect to antitumor efficacy, where structural stability determines potency. In the Py8119 mouse model, lysates encapsulated in L-SNAs synthesized with DPPC were more effective than their DOPC analogues at stalling tumor growth; a trend that became more pronounced when incorporating lysates from oxidized Py8119 cells into the L-SNA scaffold, revealing synergy between the lysate incorporation method and L-SNA stability.
Taken together, this work convincingly shows that seemingly subtle changes to the chemical structure of nanoscale immunotherapeutics can profoundly impact their performance and that liposome stability can be used as a variable to control the biological properties of L-SNAs. These results have important implications for the development of therapeutic L-SNAs that extend beyond cancer immunotherapy. For example, in applications where SNAs are systemically administered (e.g., gene regulation), L-SNAs synthesized using high-TC lipids may maximize the serum stability and blood circulation. Furthermore, in applications where high stability is not advantageous, such as those where encapsulated cargo needs to be released from the core of the L-SNA following cellular uptake,9,46 L-SNAs with intermediate stability may be the most useful. Therefore, it is critical to carefully consider the liposome scaffold when designing both L-SNAs, as well as other liposome-based nanoparticle systems, for biomedical applications.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health Award U54CA199091. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The project was also supported by the Prostate Cancer Foundation and the Movember Foundation award 17CHAL08, the Polsky Urologic Cancer Institute of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University at Northwestern Memorial Hospital, and the Air Force Research Laboratory agreement FA8650-15-2-5518. The U.S. Government is authorized to reproduce and distribute reprints for Governmental purposes notwithstanding any copyright notation thereon. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of Air Force Research Laboratory or the U.S. Government. C.E.C. was supported by a Postdoctoral Fellowship, PF-20-046-01 - LIB, from the American Cancer Society, as well as the Eden and Steven Romick Postdoctoral Fellowship through the American Committee for the Weizmann Institute of Science. J.W.D. acknowledges support by the Chemistry of Life Processes Predoctoral Training Program at Northwestern University. L.B. acknowledges support from Northwestern URG award 923ACADYR1916683. The content is solely the responsibility of the authors and does not necessarily represent the official views of Northwestern University.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.1c00181.
Materials and methods; Scheme S1. Synthetic scheme of 1,2-distearoyl-sn-glycero-3-phosphorylethanolamine (DSPE)-Rho; Table S1. DNA sequences; Table S2. DLS analysis of L-SNAs; Table S3. Calculated increase (%) in CD86 expression; Figure S1. Structure of rhodamine-labeled lipids; Figure S2. FRET decay curves; Figure S3. In vitro serum stability at 65 °C; Figure S4. In vitro serum stability at 20 °C; Figure S5. Time-course analysis of L-SNA uptake by DCs in vitro; Figure S6. Expression of CD80 and MHC-II in vitro; Figure S7. Secretion of TNF-α in vitro; Figure S8. Accumulation in lymph nodes at 2 h; Figure S9. Accumulation in lymph nodes at 24 h (PDF)
Author Contributions
C.E.C. designed the research; C.E.C., C.D.K., J.W.D., and L.B. performed the research; C.E.C., C.D.K., J.W.D., L.B., and C.A.M. analyzed the data; C.E.C. and C.A.M. wrote the manuscript.
The authors declare the following competing financial interest(s): C.A.M. has financial interests in Exicure, Inc., which could potentially benefit from the outcomes of this research.
Supplementary Material
References
- Bozzuto G.; Molinari A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T. M.; Cullis P. R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Delivery Rev. 2013, 65 (1), 36–48. 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Sercombe L.; Veerati T.; Moheimani F.; Wu S. Y.; Sood A. K.; Hua S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally M.; Bailey K.; Sugihara K.; Grieshaber D.; Vörös J.; Städler B. Liposome and Lipid Bilayer Arrays Towards Biosensing Applications. Small 2010, 6 (22), 2481–2497. 10.1002/smll.201000644. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Boyd B. J. Liposomes in biosensors. Analyst 2013, 138 (2), 391–409. 10.1039/C2AN36140J. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G.; Florence A. T. Liposomes in Drug Delivery. Drugs 1993, 45 (1), 15–28. 10.2165/00003495-199345010-00003. [DOI] [PubMed] [Google Scholar]
- Xia Y.; Xu C.; Zhang X.; Ning P.; Wang Z.; Tian J.; Chen X. Liposome-based probes for molecular imaging: from basic research to the bedside. Nanoscale 2019, 11 (13), 5822–5838. 10.1039/C9NR00207C. [DOI] [PubMed] [Google Scholar]
- Balazs D. A.; Godbey W. Liposomes for use in gene delivery. J. Drug Delivery 2011, 2011, 326497. 10.1155/2011/326497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callmann C. E.; Cole L. E.; Kusmierz C. D.; Huang Z.; Horiuchi D.; Mirkin C. A. Tumor cell lysate-loaded immunostimulatory spherical nucleic acids as therapeutics for triple-negative breast cancer. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 17543. 10.1073/pnas.2005794117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Qin L.; Yamankurt G.; Skakuj K.; Huang Z.; Chen P.-C.; Dominguez D.; Lee A.; Zhang B.; Mirkin C. A. Rational vaccinology with spherical nucleic acids. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (21), 10473–10481. 10.1073/pnas.1902805116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckes B.; Banga R. J.; Nguyen S. T.; Mirkin C. A. Enhancing the Stability and Immunomodulatory Activity of Liposomal Spherical Nucleic Acids through Lipid-Tail DNA Modifications. Small 2018, 14 (5), 1702909. 10.1002/smll.201702909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovic-Moreno A. F.; Chernyak N.; Mader C. C.; Nallagatla S.; Kang R. S.; Hao L.; Walker D. A.; Halo T. L.; Merkel T. J.; Rische C. H.; Anantatmula S.; Burkhart M.; Mirkin C. A.; Gryaznov S. M. Immunomodulatory spherical nucleic acids. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (13), 3892–3897. 10.1073/pnas.1502850112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangasseri D. P.; Cui Z.; Chen W.; Hokey D. A.; Falo L. D. Jr; Huang L. Immunostimulation of dendritic cells by cationic liposomes. Mol. Membr. Biol. 2006, 23 (5), 385–95. 10.1080/09687860600790537. [DOI] [PubMed] [Google Scholar]
- Lian T.; Ho R. J. Trends and developments in liposome drug delivery systems. J. Pharm. Sci. 2001, 90 (6), 667–80. 10.1002/jps.1023. [DOI] [PubMed] [Google Scholar]
- Kučerka N.; Nieh M.-P.; Katsaras J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim. Biophys. Acta, Biomembr. 2011, 1808 (11), 2761–2771. 10.1016/j.bbamem.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Beltrán-Gracia E.; López-Camacho A.; Higuera-Ciapara I.; Velázquez-Fernández J. B.; Vallejo-Cardona A. A. Nanomedicine review: clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10 (1), 11. 10.1186/s12645-019-0055-y. [DOI] [Google Scholar]
- Kheyfets B.; Galimzyanov T.; Mukhin S. Microscopic Description of the Thermodynamics of a Lipid Membrane at a Liquid–Gel Phase Transition. JETP Lett. 2018, 107 (11), 718–724. 10.1134/S0021364018110036. [DOI] [Google Scholar]
- Alberts B.; Johnson A.; Lewis J.; Raff M.; Roberts K.; Walter P.. The Lipid Bilayer. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, 2002. [Google Scholar]
- Hong J. S.; Vreeland W. N.; DePaoli Lacerda S. H.; Locascio L. E.; Gaitan M.; Raghavan S. R. Liposome-Templated Supramolecular Assembly of Responsive Alginate Nanogels. Langmuir 2008, 24 (8), 4092–4096. 10.1021/la7031219. [DOI] [PubMed] [Google Scholar]
- Lockhart J. N.; Beezer D. B.; Stevens D. M.; Spears B. R.; Harth E. One-pot polyglycidol nanogels via liposome master templates for dual drug delivery. J. Controlled Release 2016, 244, 366–374. 10.1016/j.jconrel.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Tan G.; Xu P.; He J.; Lawson L.; McPherson G. L.; John V. T. Highly aspherical silica nanoshells by templating tubular liposomes. Soft Matter 2009, 5, 3006–3009. 10.1039/b908779f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestini B. J.; Sagnella S. M.; Xu Z.; Shive M. S.; Richter N. J.; Jayaseharan J.; Case A. J.; Kottke-Marchant K.; Anderson J. M.; Marchant R. E. Surface modification of liposomes for selective cell targeting in cardiovascular drug delivery. J. Controlled Release 2002, 78 (1–3), 235–47. 10.1016/S0168-3659(01)00505-3. [DOI] [PubMed] [Google Scholar]
- Turánek J.; Mašek J.; Raška M.; Ledvina M.; Paulovičová E.; Hubatka F.; Kotouček J. Modification of liposomal surface by polysaccharides: Preparation, characterization, and application for drug targeting. Functional Polysaccharides for Biomedical Applications 2019, 433–467. 10.1016/B978-0-08-102555-0.00013-3. [DOI] [Google Scholar]
- Mirkin C. A.; Letsinger R. L.; Mucic R. C.; Storhoff J. J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996, 382, 607. 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- Cutler J. I.; Auyeung E.; Mirkin C. A. Spherical nucleic acids. J. Am. Chem. Soc. 2012, 134 (3), 1376–91. 10.1021/ja209351u. [DOI] [PubMed] [Google Scholar]
- Banga R. J.; Chernyak N.; Narayan S. P.; Nguyen S. T.; Mirkin C. A. Liposomal Spherical Nucleic Acids. J. Am. Chem. Soc. 2014, 136 (28), 9866–9869. 10.1021/ja504845f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovic-Moreno A. F.; Chernyak N.; Mader C. C.; Nallagatla S.; Kang R. S.; Hao L.; Walker D. A.; Halo T. L.; Merkel T. J.; Rische C. H.; Anantatmula S.; Burkhart M.; Mirkin C. A.; Gryaznov S. M. Immunomodulatory Spherical Nucleic Acids. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (13), 3892–3897. 10.1073/pnas.1502850112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakuj K.; Wang S.; Qin L.; Lee A.; Zhang B.; Mirkin C. A. Conjugation Chemistry-Dependent T-Cell Activation with Spherical Nucleic Acids. J. Am. Chem. Soc. 2018, 140 (4), 1227–30. 10.1021/jacs.7b12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga R. J.; Chernyak N.; Narayan S. P.; Nguyen S. T.; Mirkin C. A. Liposomal Spherical Nucleic Acids. J. Am. Chem. Soc. 2014, 136 (28), 9866–9869. 10.1021/ja504845f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprangers A. J.; Hao L.; Banga R. J.; Mirkin C. A. Liposomal Spherical Nucleic Acids for Regulating Long Noncoding RNAs in the Nucleus. Small 2017, 13 (10), 1602753. 10.1002/smll.201602753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakuj K.; Wang S.; Qin L.; Lee A.; Zhang B.; Mirkin C. A. Conjugation Chemistry-Dependent T-Cell Activation with Spherical Nucleic Acids. J. Am. Chem. Soc. 2018, 140 (4), 1227–30. 10.1021/jacs.7b12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer J. R.; Sinegra A. J.; Ivancic D.; Yeap X. Y.; Qiu L.; Wang J.-J.; Zhang Z. J.; Wertheim J. A.; Mirkin C. A. Structure-Dependent Biodistribution of Liposomal Spherical Nucleic Acids. ACS Nano 2020, 14 (2), 1682–1693. 10.1021/acsnano.9b07254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamankurt G.; Berns E. J.; Xue A.; Lee A.; Bagheri N.; Mrksich M.; Mirkin C. A. Exploration of the nanomedicine-design space with high-throughput screening and machine learning. Nat. Biomed. Eng. 2019, 3 (4), 318–327. 10.1038/s41551-019-0351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaby J. M.; Momsen M. M.; Brockman H. L.; Brown R. E. Phosphatidylcholine acyl unsaturation modulates the decrease in interfacial elasticity induced by cholesterol. Biophys. J. 1997, 73 (3), 1492–1505. 10.1016/S0006-3495(97)78181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen T. P. W.; Lewis R. N. A. H.; McElhaney R. N. Differential scanning calorimetric study of the effect of cholesterol on the thermotropic phase behavior of a homologous series of linear saturated phosphatidylcholines. Biochemistry 1993, 32 (2), 516–522. 10.1021/bi00053a016. [DOI] [PubMed] [Google Scholar]
- Chen W.; Duša F.; Witos J.; Ruokonen S.-K.; Wiedmer S. K. Determination of the Main Phase Transition Temperature of Phospholipids by Nanoplasmonic Sensing. Sci. Rep. 2018, 8 (1), 14815. 10.1038/s41598-018-33107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z. N.; Cole L. E.; Callmann C. E.; Wang S.; Mirkin C. A. Sequence Multiplicity within Spherical Nucleic Acids. ACS Nano 2020, 14 (1), 1084–1092. 10.1021/acsnano.9b08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ortiz Z. G.; Specht C. A.; Wang J. P.; Lee C. K.; Bartholomeu D. C.; Gazzinelli R. T.; Levitz S. M. Toll-Like Receptor 9-Dependent Immune Activation by Unmethylated CpG Motifs in Aspergillus fumigatus DNA. Infect. Immun. 2008, 76 (5), 2123. 10.1128/IAI.00047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom D. M.; Manzotti C. N.; Zheng Y. What’s the difference between CD80 and CD86?. Trends Immunol. 2003, 24 (6), 313–318. 10.1016/S1471-4906(03)00111-X. [DOI] [PubMed] [Google Scholar]
- Hu J.; Xu J.; Li M.; Zhang Y.; Yi H.; Chen J.; Dong L.; Zhang J.; Huang Z. Targeting Lymph Node Sinus Macrophages to Inhibit Lymph Node Metastasis. Mol. Ther.--Nucleic Acids 2019, 16, 650–662. 10.1016/j.omtn.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodogai M.; Lee Chang C.; Wejksza K.; Lai J.; Merino M.; Wersto R. P.; Gress R. E.; Chan A. C.; Hesdorffer C.; Biragyn A. Anti-CD20 Antibody Promotes Cancer Escape via Enrichment of Tumor-Evoked Regulatory B Cells Expressing Low Levels of CD20 and CD137L. Cancer Res. 2013, 73 (7), 2127–2138. 10.1158/0008-5472.CAN-12-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler H. Accurate Identification of Experimental Pulmonary Metastases. JNCI: Journal of the National Cancer Institute 1966, 36 (4), 641–645. 10.1093/jnci/36.4.641. [DOI] [PubMed] [Google Scholar]
- Grant M. L.; Shields N.; Neumann S.; Kramer K.; Bonato A.; Jackson C.; Baird M. A.; Young S. L. Combining Dendritic Cells and B Cells for Presentation of Oxidised Tumour Antigens to CD8(+) T Cells. Clin. Transl. Immunol. 2017, 6 (7), e149–e149. 10.1038/cti.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C. L.; Kandalaft L. E.; Tanyi J.; Hagemann A. R.; Motz G. T.; Svoronos N.; Montone K.; Mantia-Smaldone G. M.; Smith L.; Nisenbaum H. L.; Levine B. L.; Kalos M.; Czerniecki B. J.; Torigian D. A.; Powell D. J. Jr; Mick R.; Coukos G. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin. Cancer Res. 2013, 19 (17), 4801–15. 10.1158/1078-0432.CCR-13-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C. L.-L.; Benencia F.; Coukos G. Whole tumor antigen vaccines. Semin. Immunol. 2010, 22 (3), 132–143. 10.1016/j.smim.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer J. R.; Wertheim J. A.; Mirkin C. A. Dual Toll-Like Receptor Targeting Liposomal Spherical Nucleic Acids. Bioconjugate Chem. 2019, 30 (3), 944–951. 10.1021/acs.bioconjchem.9b00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.