Abstract
Posttranslational modification of a protein with glycosylphosphatidylinositol (GPI) is a conserved mechanism exists in all eukaryotes. Thus far, >150 human GPI-anchored proteins have been discovered and ~30 enzymes have been reported to be involved in the biosynthesis and maturation of mammalian GPI. Phosphatidylinositol glycan biosynthesis class A protein (PIGA) catalyzes the very first step of GPI anchor biosynthesis. Patients carrying a mutation of the PIGA gene usually suffer from inherited glycosylphosphatidylinositol deficiency (IGD) with intractable epilepsy and intellectual developmental disorder. We generated three mouse models with PIGA deficits specifically in telencephalon excitatory neurons (Ex-M-cko), inhibitory neurons (In-M-cko) or thalamic neurons (Th-H-cko), respectively. Both Ex-M-cko and In-M-cko mice showed impaired long-term fear memory and were more susceptible to kainic acid-induced seizures. In addition, In-M-cko demonstrated a severe limb-clasping phenotype. Hippocampal synapse changes were observed in Ex-M-cko mice. Our Piga conditional knockout mouse models provide powerful tools to understand the cell-type specific mechanisms underlying inherited GPI deficiency and to test different therapeutic modalities.
Introduction
The international focus on human congenital disorders of glycosylation (CDG) has grown considerably in the past 10 years along with great advances in next-generation sequencing technology. A new glycosylation disorder was reported, on average, every 17 days in 2013 (1). One important classification of CDG is glycosylphosphatidylinositol (GPI) anchor biosynthesis defects (2). GPI anchoring of proteins is a conserved posttranslational modification among all eukaryotes, and at least 150 human proteins are GPI-anchored proteins (GPI-APs), including receptors, adhesion molecules, complement regulators and enzymes (3). Biosynthesis and maturation of mammalian GPI-APs requires about 30 genes (3). Germline mutations of any of these genes could cause inherited glycosylphosphatidylinositol deficiency (IGD) with a wide spectrum of symptoms, including pronounced neurologic impairments, such as intellectual disability and intractable seizures, early-onset epileptic encephalopathies, cerebral and/or cerebellar atrophy, hypotonia, motor incoordination and ataxia, cortical visual impairment and sensorineural deafness (4).
Phosphatidylinositol glycan biosynthesis class A protein (PIGA) catalyzes the very first step of GPI anchor biosynthesis and the loss function of the gene encoding PIGA (PIGA) completely abolishes GPI anchor production (5). The group of Matsumoto and others have applied whole-exome sequencing and identified multiple PIGA germline mutations in patients with neurological symptoms from 15 unrelated families (6–15). Therefore, it would be important to study the detailed molecular, cellular and neural circuit mechanisms underlying the IGD encephalopathy by using Piga mutated animal models. Complete disruption of the Piga gene caused early embryonic lethality (16). Takeda and Kinoshita developed a conditional Pigaflox mouse line, in which exon 6 of Piga is flanked by two identically orientated loxP sites, and proved that Piga function is essential for proper skin differentiation and maintenance (17). More recently, this conditional knock out (cko) system was used in Nestin-Cre mice to delete Piga in the neuroepithelial stem cell population that differentiates into neurons, astrocytes and oligodendrocytes. These mutated mice showed deficits in cerebellar and white matter development, ataxia and tremor and did not survive past weaning. The early lethality of these mice excludes the possibility to examine their cognitive and epileptic phenotype thoroughly (18). In addition, Nestin is also expressed in a variety of tissues and progenitor cells, including pancreatic islets, skeletal muscle satellite cells, developing myotomes, testis, hair follicle, heart and the non-hematopoietic fraction of the bone marrow (19), further adding to the complexity of explaining the mutants’ phenotype.
In the current study, by using three Cre recombinase (Cre) mouse lines including Emx1-Cre, Vgat-Cre and Pkcd-Cre lines, we successfully ablated Piga in three populations of neurons: telencephalon excitatory neurons; inhibitory neurons and thalamic neurons, respectively. As Piga is localized on the X chromosome, a male mouse bearing a floxed Piga (Pigaflox/Y) has only targeted Piga locus and will completely disrupt Piga in Cre-expressing cell lineages (hemizygous cko). Whereas a female mouse bearing a loxP site (Pigaflox/+) will have Piga partially ablated in Cre-expressing cell lineages due to random X chromosome inactivation (mosaic cko). Emx1-Cre hemizygous cko mice (Ex-H-cko) and Vgat-Cre hemizygous cko mice (In-H-cko) failed to survive after birth. Emx1-Cre mosaic cko mice (Ex-M-cko), Vgat-Cre mosaic cko mice (In-M-cko) and Pkcd-Cre hemizygous cko mice (Th-H-cko) survived to adulthood. Further analysis revealed that Ex-M-cko and In-M-cko mutants performed poorly in fear memory task, and their susceptibility to kainic acid (KA)-induced seizures was significantly increased. In addition, In-M-cko mutants demonstrated a robust limb-clasping phenotype and decreased bodyweight. Our animal models with GPI deficit in different populations of neurons provided a useful tool to screen effective treatments of IGD patients.
Results
Generation of different neuronal population-specific Piga cko mice
In the Pigaflox mouse line, three loxP sites with identical orientation were inserted within the Piga gene on X chromosome, with one loxP localized in intron 5 and the other two loxP sites flanking a Neo gene localized downstream of exon 6 (Fig. 1A). Previous work using this mouse line proved that deletion of exon 6 upon Cre recombination resulted in an efficient ablation of Piga and disrupted the cell surface expression of GPI-APs (16–18,20). Our in situ hybridization on adult mouse brain showed that Piga is widely expressed in almost all the neurons and has a very strong expression in CA1 pyramidal cells and dentate gyrus (DG) granule cells of the hippocampus and piriform cortex (Fig. 1B). To investigate the functions of different neuronal populations in the encephalopathy of IGD, we crossed Pigaflox/+ female mice with the following three Cre lines of male mice: Emx1-Cre line, which induces recombination in excitatory neurons in the cerebral cortex and limbic structures (Fig. 1C) (21); Vgat-Cre line, which induces recombination in all inhibitory neurons throughout the brain (Fig. 1D) (22), and Pkcd-Cre line, which induces recombination in almost the entire thalamus (Fig. 1E) (23). As Piga is localized on the X chromosome, theoretically by this breeding strategy we could get male hemizygous cko mice with the genotype of ‘Pigaflox/Y, Cre+’, and female mosaic cko mouse with the genotype of ‘Pigaflox/+, Cre+’. The Emx1-Cre line is a knock-in mouse, we used the homozygous Emx1Cre/Cre so that all the offspring carried the Cre gene. The Vgat-Cre line and Pkcd-cre line are BAC transgenic lines. The Vgat-Cre mice we used harbor homozygous transgenic alleles so that all the offspring expressed Cre. The Pkcd-cre mice we obtained harbor heterozygous transgenic alleles thus the offspring could be either positive or negative for Cre. We performed polymerase chain reaction (PCR) analysis of tail DNA from 22-day-old offspring to genotype both the Piga alleles and Cre. We obtained double positive female mice, Pigaflox/+, Cre+, from the breeding pairs of all the three Cre lines, meaning that mice with mosaic conditional knockout of Piga in excitatory neurons (Ex-M-cko), inhibitory neurons (In-M-cko) and thalamus neurons (Th-M-cko) could survive after weaning. We never obtained Pigaflox/Y, Cre+ male mouse from the Emx1-Cre and Vgat-Cre breeding groups, meaning that complete deletion of Piga in excitatory neurons or inhibitory neurons led to early death during embryo stage or soon after birth. However, we did get Pigaflox/Y, Cre+ male mice from Pkcd-cre breeders, meaning that the mutants with Piga totally disrupted in thalamus (Th-H-cko) could survive after weaning. Unless specified, in all the experiments, Ex-M-cko and IN-M-cko mutants were compared with their female littermates (Piga+/+, Cre+); Th-H-cko mutants were compared with their male littermates (Pigaflox/Y, Cre−). Table 1 shows the genotyping results from the first-round crossing of all the three breeding groups. We further examined E13.5 embryos from two Pigaflox/+ X Emx1-Cre breeding pairs and three Pigaflox/+ X Vgat-Cre breeding pairs. From 22 embryos of Emx1-Cre breeders, there were two embryos that showed obvious microcephaly and their genotype was Pigaflox, Cre+ (Fig. 1F and G). From 32 embryos of the Vgat-Cre breeders, three embryos displayed an amorphous sphere morphology with the genotype Pigaflox, Cre+ (Fig. 1H and I).
Figure 1.

Creation of three Piga conditional knockout mouse lines and confirmation of specific deletion of Piga in different neuronal populations. (A) The construction of Pigaflox mouse. The binding sites of three primers for genotyping and detection of Piga disruption, and that of in situ hybridization probe are indicated. (B) In situ hybridization of Piga in adult mouse brain. (C–E) The X-gal staining of Rosa-NLS-LacZ reporter mice crossed with Emx1-Cre (C), Vgat-Cre (D) and Pkcd-Cre (E) lines, respectively. (F–I) E13.5 embryos with the genotype of PigaWT,Cre+ (F); Pigaflox, Cre+ (G) from the Emx-Cre breeding group and E13.5 embryos with the genotype of PigaWT, Cre+ (H); Pigaflox, Cre+ (I) from the Vagt-Cre breeding group. (J and K) PCR was performed using neuronal DNA from different brain regions with all the three primers (Primers 1–3) indicated in (A). (L and M) In situ hybridization of Piga with Pigaflox, Cre− male control (L) and Th-H-cko (M). Higher magnification of thalamus region of (L) and (M) were shown in (P) and (Q), respectively. (N and O) Immunohistochemistry of netrin-G1 with Pigaflox, Cre− male control (N) and Th-H-cko(O). Higher magnification of cortex region of (N) and (O) were shown in (R) and (S), respectively. Scale bars of (B–E), (L–Q): 1 mm; scale bar of (F–I): 5 mm; scale bar of (R and S):0.5 mm
Table 1.
The mosaic and hemizygous mutants obtained from each cre lines in the first round breeding
| Breeding strategy | Number of breeding pairs | Number of offsprings | Number of ♀offsprings | Number of ♂offsprings | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Piga flox/+, Cre+ | Piga flox/+, Cre− | Piga +/+, Cre+ | Piga +/+, Cre− | Piga flox/Y, Cre+ | Piga flox/Y, Cre− | Piga +/Y, Cre+ | Piga +/Y, Cre− | |||
| Emx-cre♂ × | 10 | 45 | 13 | 0 | 12 | 0 | 0 | 0 | 20 | 0 |
| Piga flox/+♀ | ||||||||||
| Vgat-cre♂ × | 10 | 37 | 16 | 0 | 9 | 0 | 0 | 0 | 12 | 0 |
| Piga flox/+♀ | ||||||||||
| Pkcd-cre♂ × | 10 | 51 | 5 | 12 | 5 | 8 | 5 | 4 | 7 | 5 |
| Piga flox/+♀ | ||||||||||
We are interested in possible phenotypes resembling intellectual disability and epilepsy; therefore, we focused on the cko mice that can survive after weaning. To confirm the deletion of Piga in specific targeted neuronal populations, we obtained neurons from hippocampus, striatum, thalamus, and cortex using a well-established NeuN-labeled Fluorescence-Activated Cell Sorting (FACS) method (24) and performed PCR using three primers shown in Figure 1A. The bands for ‘wild type (WT)’, ‘targeted’ and ‘disrupted’ Piga are about 250, 420 and 550 bp, respectively (Fig. 1A and J). Our results showed that in Ex-M-cko mutants, where Cre targeted Pigaflox in excitatory neurons, the ‘disrupted’ band from hippocampus was the most prominent, which is in consistent with the fact that the hippocampus GABAergic inhibitory interneurons represent only ~ 10–15% of the total neuronal population (25) (Fig. 1J). In contrast, in In-M-cko where Cre targeted Pigaflox inhibitory neurons, the ‘disrupted band’ from striatum was the most prominent, consistent with the fact that 95% of neurons in striatum are GABAergic inhibitory neurons (26) (Fig. 1J). In Th-H-cko, as expected, there was only a disrupted band from thalamic neurons but not from those from other brain regions (Fig. 1K). In addition, there was no ‘disrupted’ band from tails of all the mutants, indicating the specificity of our cko system. To further confirm the specificity and efficiency of Piga ablation, we generated a probe that covers ~790 bp of non-coding region of Piga exon 6. In situ hybridization on Th-H-cko tissue showed a dramatic decrease of signal in thalamus compared with that from a control littermate, whereas the signal in hippocampus and cortex was comparable with that from the control (Fig. 1L, M, P and Q). NetrinG1 is synaptic adhesion GPI-AP, and it is mainly distributed at synaptic sites in adults (27,28). We did immunohistochemistry for netrinG1 on Th-H-cko mutants. The signal of netrinG1 on cortex layer 4 was completely lost, whereas the signal in hippocampus CA1 stratum lacunosum moleculare, DG outer molecular layer and piriform cortex layer Ia was comparable with that from the control (Fig. 1N, O, R and S). This result demonstrated that GPI synthesis deficit in thalamus neurons disrupted the synaptic delivery of netrin-G1 through thalamocortical axons, whereas the delivery of netrinG1 from entorhinal cortex to hippocampus through temporoammonic axons and lateral perforant axons and the delivery of netrinG1 from olfactory bulb to piriform cortex through mitral cell axons were intact. In summary, by PCR of the disrupted Piga product, in situ hybridization of Piga and examination of the surface delivery of a known GPI-AP, we provide convincing evidence that the ablation of Piga was specific in different neuronal populations.
The deficit of PIGA in inhibitory neurons resulted in weight loss and limb clasping
We examined the phenotypes of Ex-M-cko, In-M-cko and Th-H-cko mutants. Ex-M-cko, In-M-cko mutants were compared with Piga+/+, Cre+ female control littermates. Th-H-cko mutants were compared with ‘Pigaflox/Y, Cre−’ male control littermates. All these mutants could survive as long as their control littermates, at least for 1 year. The most distinctive difference was that all In-M-cko mutants showed smaller body size (Fig. 2A). We compared the body weight of 7-month-old mice (n = 12/group), a one-way analysis of variance (ANOVA) showed there was a significant difference among female Ex-M-cko, In-M-cko and Piga+/+, Cre+ control (Fig. 2F), F(2,33) = 59.35, P < 0.0001. Post hoc comparisons using the Bonferroni test indicated that the mean value for In-M-cko (M = 18.28 g, Standard Error(SE) = 0.64) was significantly lower and ~60% of that of the controls (M = 28.82 g, SE = 0.71, P < 0.0001). The mean body weight for Ex-M-cko (M = 25.06 g, SE = 0.72) was slightly but statistically significantly lower than controls as well, P < 0.01. An unpaired t-test was performed to compare the weight between male Th-H-cko mutants and ‘Pigaflox/Y, Cre−’ controls (Fig. 2G). There was no significant difference in the weight of Th-H-cko (M = 34.73, SE = 0.75) and control mice (M = 33.85, SE = 0.67; t(22) = 0.099, P = 0.756).
Figure 2.
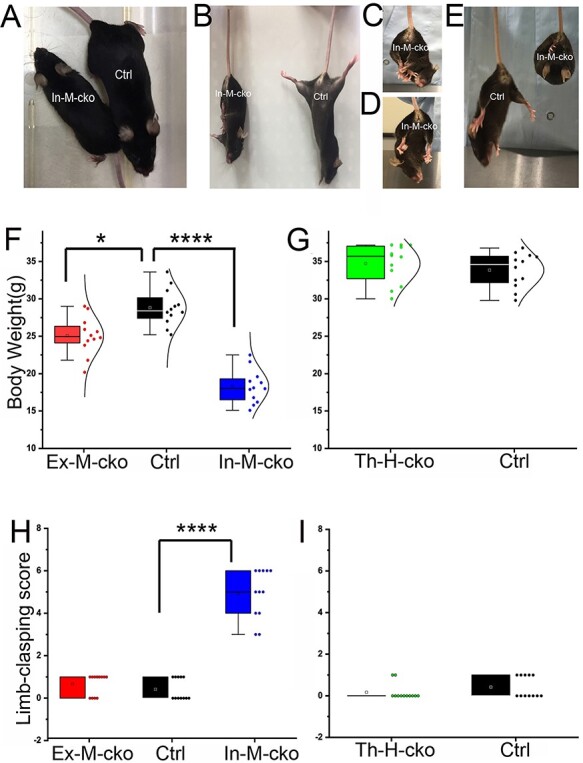
The body weight and limb-clasping phenotype in Piga mutants. (A) Body size was compared between In-M-cko mutant and its control littermate of same sex. (B–E) Limb-clasping phenotype in In-M-cko mutants. In-M-cko mutants clasped limbs (B–D) and showed a bat-like posture (E). (F and G) Body weight was compared between Piga mutants and their same sex controls. (H and I) The limb-clasping score was compared between Piga mutants and their same gender controls. In the box charts, the boxes are determined by the 25th and 75th percentiles. The whiskers are determined by the 5th and 95th percentiles. Mean and medium values are shown as the small square and line inside the boxes. Each individual value is plotted beside the box (F–I).
Another prominent phenotype of In-M-cko mutants was limb clasping (Fig. 2B–D) and a bat-like posture (Fig. 2E) when picked up by their tails. In fact, all the In-M-cko mice demonstrated obvious limb clasping. We evaluated the limb clasping of each cko group (n = 12/group). A Kruskal–Wallis H test showed that there was a statistically significant difference in limb-clasping score among female Ex-M-cko, In-M-cko and Piga+/+, Cre+ controls (Fig. 2H), (χ2(2) = 29.40, P < 0.0001), with a mean rank limb-clasping score of 16 for Ex-M-cko, 30.5 for In-M-cko and 9 for controls. A post hoc test adjusted by Bonferroni correction showed that there was a significance between In-M-cko and control, P < 0.0001, whereas the difference between Ex-M-cko and control did not reach statistical significance, P = 0.25. A Mann–Whitney U test was applied to compare the limb clasping between male Th-H-cko mutants and ‘Pigaflox/Y, Cre−’ control (Fig. 2I). There was no significant difference between the mean rank of Th-H-cko (M = 11) and that of control (M = 14; U = 54, P = 0.19). All of the mutants including In-M-cko demonstrated normal gaits, and none of these mutants showed tremor, at least by the age of 1 year old. In summary, mosaic cko from either excitatory or inhibitory neuronal populations showed reduced bodyweight, and this weight reduction was especially remarkable in In-M-cko mutant. In addition, In-M-cko mutant demonstrated a robust limb-clasping phenotype.
The deficit of PIGA in either inhibitory neurons or excitatory neurons impaired fear memory and caused an enhanced vulnerability to KA-induced seizures
Prominent clinical features of IGD patients are intellectual disability and epilepsy. We examined whether our cko mice developed similar phenotypes. We used a classical contextual and cued fear conditioning task to test learning and memory ability of mutants (n = 11/group). On Day1, a mouse learns to associate a context and an auditory cue to electric shocks. The next day they are tested to see if they remember the context associated with aversive stimuli, and the third day they are tested to see if they remember the tone paired with a shock (Fig. 3A). A mixed factorial ANOVA was performed to compare the freezing level on each day of Ex-M-cko, In-M-cko and Piga+/+, Cre+ controls (Fig. 3B). For Day1, conditioning day, Macuchly’s test indicated that the assumption of sphericity had been violated so that Greenhouse–Geisser correction was used; there was no significant interaction between genotype and time: F(5.25, 78.80) = 0.70, P = 0.63; and there was no significant difference for genotype: F(2, 30) = 1.02, P = 0.374. For Day2 contextual memory test day, Mauchly’s test indicated that the assumption of sphericity had not been violated, χ2(9) = 7.9, P = 0.54, there was no significant interaction between genotype and time: F(8,120) = 0.965, P = 0.47; however, the main effect for genotype was significant: F(2, 30) = 13.8, P < 0.0001. Bonferroni corrected post hoc test showed that the freezing level of In-M-cko mutants was significantly lower than that of control, P < 0.0001; and the freezing level of Ex-M-cko showed a tendency to be lower than that of control and was approaching statistical significance, P = 0.058. The multiple comparison of mean values (corrected by Bonferroni test) at each time block among three genotypes is shown in Table 2A and B. In-M-cko froze significantly less than control at each time block, whereas Ex-M-cko froze significantly less than control at the fourth block. For Day3 cued memory test day, there was no significant difference among three groups: F(2, 30) = 0.30, P = 0.74. A mixed factorial ANOVA was performed to compare the freezing level on each day of male Th-H-cko mutants and ‘Pigaflox/Y, Cre−’ (Fig. 3C). For Day1, there was no significant difference between the two groups, F(1,18) = 1.69, P = 0.21. For Day2, there was no significant difference between the two groups, F(1,18) = 0.49, P = 0.49. For Day3, F(1,18) = 0.01, P = 0.92. The pain sensations of all the three mutants were comparable with their control mice (data not shown). Our results revealed that deficit of PIGA in either inhibitory or excitatory neurons decreased the long-term memory of contextual fear, consistent with the important role of GPI-APs in cognitive functions.
Figure 3.

Piga mutants showed altered cognition and susceptibility to KA induced seizures. (A) A diagram of fear conditioning test. (B and C) The freezing percentage was compared between Piga mutants and their same sex controls. (D and E) The behavioral seizure score was compared between Piga mutants and their same sex controls. In the line charts, Mean ± SE are indicated for each time point.
Table 2.
The simple effects test result of the contextual fear memory among Ex-M-cko, In-M-cko and control. The Bonferroni test corrected P value of each comparison between the freezing percentage of two genotype groups at each time point in contextual fear memory task.
| Simple effects test | Measure: | freezing | |
|---|---|---|---|
| Time (min) | (I) group | (J) group | Sig. b |
| 1 | Ex-M-cko | In-M-cko | .006 |
| Ctrl | .569 | ||
| In-M-cko | Ex-M-cko | .006 | |
| Ctrl | .000 | ||
| Ctrl | Ex-M-cko | .569 | |
| In-M-cko | .000 | ||
| 2 | Ex-M-cko | In-M-cko | .033 |
| Ctrl | .486 | ||
| In-M-cko | Ex-M-cko | .033 | |
| Ctrl | .001 | ||
| Ctrl | Ex-M-cko | .486 | |
| In-M-cko | .001 | ||
| 3 | Ex-M-cko | In-M-cko | .091 |
| Ctrl | .164 | ||
| In-M-cko | Ex-M-cko | .091 | |
| Ctrl | .001 | ||
| Ctrl | Ex-M-cko | .164 | |
| In-M-cko | .001 | ||
| 4 | Ex-M-cko | In-M-cko | 1.000 |
| Ctrl | .036 | ||
| In-M-cko | Ex-M-cko | 1.000 | |
| Ctrl | .004 | ||
| Ctrl | Ex-M-cko | .036 | |
| In-M-cko | .004 | ||
| 5 | Ex-M-cko | In-M-cko | .159 |
| Ctrl | .221 | ||
| In-M-cko | Ex-M-cko | .159 | |
| Ctrl | .002 | ||
| Ctrl | Ex-M-cko | .221 | |
| In-M-cko | .002 | ||
Based on estimated marginal means
* The mean difference is significant at the .05 level.
Adjustment for multiple comparisons: Bonferroni.
We did not see spontaneous behavioral seizures in the cko mutant mice. To test if the susceptibility to KA-induced seizures was changed, we injected KA (20 mg/kg) intraperitoneally to observe acute behavioral seizure responses. The seizure score was evaluated every 20 min on the basis of a modified Racine scale (29) totally for 120 min postinjection (n = 8/group). A mixed factorial ANOVA was performed to compare the seizure scores of female Ex-M-cko, In-M-cko and PigaWT, Cre+ controls (Fig. 3D). We observed a significant main effect for genotype: F(2, 30) = 29.20, P < 0.0001. Bonferroni corrected post hoc test showed that the seizure score of In-M-cko mutants was significantly higher than that of control, P < 0.0001, and Ex-M-cko also demonstrated more seriously seizures than control, P = 0.001. The multiple comparation of mean values (corrected by Bonferroni test) at each time block among the three genotypes is shown in Table 3A and B. A mixed factorial ANOVA was also performed to compare seizure score of male Th-H-cko mutants and ‘Pigaflox/Y, Cre−’ control (Fig. 3E). There was no significant difference between the two groups, F(1,14) = 2.88, P = 0.11. Therefore, deficit of Piga in either inhibitory or excitatory neurons increased the vulnerability of cko mutants to KA-induced behavioral seizures.
Table 3.
The mean freezing percentage value of Ex-M-cko, In-M-cko and control group at each time point in contextual fear memory task.
| Measure: | freezing | ||
|---|---|---|---|
| group | time | Mean | Std. Error |
| Ex-M-cko | 1min | 49.541 | 5.028 |
| 2min | 57.568 | 5.605 | |
| 3min | 48.568 | 6.716 | |
| 4min | 46.277 | 6.946 | |
| 5min | 46.741 | 5.320 | |
| In-M-cko | 1min | 25.450 | 5.028 |
| 2min | 36.064 | 5.605 | |
| 3min | 26.973 | 6.716 | |
| 4min | 38.264 | 6.946 | |
| 5min | 31.586 | 5.320 | |
| Ctrl | 1min | 59.086 | 5.028 |
| 2min | 68.936 | 5.605 | |
| 3min | 67.573 | 6.716 | |
| 4min | 72.600 | 6.946 | |
| 5min | 60.686 | 5.320 | |
Table 4.
The simple effects test result of the seizure score among Ex-M-cko, In-M-cko and control. The Bonferroni test corrected P value of each comparison between the seizure score of two genotype groups at each time point.
| Simple effects test | Measure: | seizure | |
|---|---|---|---|
| Time | (I) group | (J) group | Sig. b |
| 1 | Ex-M-cko | In-M-cko | . |
| Ctrl | . | ||
| In-M-cko | Ex-M-cko | . | |
| Ctrl | . | ||
| Ctrl | Ex-M-cko | . | |
| In-M-cko | . | ||
| 2 | Ex-M-cko | In-M-cko | .452 |
| Ctrl | 1.000 | ||
| In-M-cko | Ex-M-cko | .452 | |
| Ctrl | .147 | ||
| Ctrl | Ex-M-cko | 1.000 | |
| In-M-cko | .147 | ||
| 3 | Ex-M-cko | In-M-cko | .045 |
| Ctrl | .600 | ||
| In-M-cko | Ex-M-cko | .045 | |
| Ctrl | .002 | ||
| Ctrl | Ex-M-cko | .600 | |
| In-M-cko | .002 | ||
| 4 | Ex-M-cko | In-M-cko | .205 |
| Ctrl | .017 | ||
| In-M-cko | Ex-M-cko | .205 | |
| Ctrl | .000 | ||
| Ctrl | Ex-M-cko | .017 | |
| In-M-cko | .000 | ||
| 5 | Ex-M-cko | In-M-cko | .120 |
| Ctrl | .003 | ||
| In-M-cko | Ex-M-cko | .120 | |
| Ctrl | .000 | ||
| Ctrl | Ex-M-cko | .003 | |
| In-M-cko | .000 | ||
| 6 | Ex-M-cko | In-M-cko | .041 |
| Ctrl | .000 | ||
| In-M-cko | Ex-M-cko | .041 | |
| Ctrl | .000 | ||
| Ctrl | Ex-M-cko | .000 | |
| In-M-cko | .000 | ||
| 7 | Ex-M-cko | In-M-cko | .035 |
| Ctrl | .059 | ||
| In-M-cko | Ex-M-cko | .035 | |
| Ctrl | .000 | ||
| Ctrl | Ex-M-cko | .059 | |
| In-M-cko | .000 | ||
*The mean difference is significant at the .05 level.
b Adjustment for multiple comparisons: Bonferroni.
Table 5.
The mean seizure score of Ex-M-cko, In-M-cko and control group at each time point.
| Measure: | seizure | ||
|---|---|---|---|
| group | factor1 | Mean | Std. Error |
| Ex-M-cko | 1 | .000 | .000 |
| 2 | 1.375 | .296 | |
| 3 | 2.875 | .200 | |
| 4 | 3.750 | .230 | |
| 5 | 4.000 | .323 | |
| 6 | 4.125 | .263 | |
| 7 | 3.500 | .385 | |
| In-M-cko | 1 | .000 | .000 |
| 2 | 2.000 | .296 | |
| 3 | 3.625 | .200 | |
| 4 | 4.375 | .230 | |
| 5 | 5.000 | .323 | |
| 6 | 5.125 | .263 | |
| 7 | 5.000 | .385 | |
| Ctrl | 1 | .000 | .000 |
| 2 | 1.125 | .296 | |
| 3 | 2.500 | .200 | |
| 4 | 2.750 | .230 | |
| 5 | 2.250 | .323 | |
| 6 | 2.375 | .263 | |
| 7 | 2.125 | .385 |
The deficit of Piga in excitatory neurons caused a decreased excitatory synaptic density and synapse size in hippocampus CA1
The brain structure of all the mutants including Ex-M-cko (Fig. 4B), In-M-cko (Fig. 4C) and Th-H-cko (Fig. 4D) is comparable with that of WT controls (male Piga+/Y, Cre−, littermates of Th-H-cko) (Fig. 4A). Furthermore, there was no obvious difference of the morphology of cerebella (Fig. 4E–H). We expected that the complete deletion of Piga might cause the abnormal barrel formation in layer IV of somatosensory cortex in Th-H-cko mutants and examined it by Nissl staining and cytochrome oxidase (CO) staining. However, we observed that barrels were normally formed in both Th-H-cko mutants (Fig. 4J and L) and ‘Pigaflox/Y, Cre−’ control (Fig. 4I and K), and there was not a noticeable difference between them.
Figure 4.

Piga mutants showed altered synaptic density and size in hippocampus CA1 (increase font for x-axis). (A–H) Nissl staining of forebrain and cerebellum of WT (A and E), Ex-M-cko (B and F), In-M-cko (C and G) and Th-H-cko (D and H) revealed normal gross brain structure of mutants.(I–L) CO and Nissl staining of tangential sections of somatosensory cortex of Pigaflox, Cre− male control (I and K) and Th-H-cko (J and L) suggested a normal barrel pattern in mutants. (M–P) PSD95 punctate number and punctate size was compared between Ex-M-cko mutants (M) and ‘PigaWT, Cre+’ control mice (N). In the box charts, the boxes are determined by the 25th and 75th percentiles. The whiskers are determined by the 5th and 95th percentiles. Mean and medium values are shown as the small square and line inside the boxes. Each individual value is plotted beside the box (O and P). Scale bar: 1 mm for (A–L); 12.5 μm for (M and N).
Our in situ hybridization (ISH) result revealed that Piga is expressed most strongly in hippocampus excitatory neurons (Fig. 1B). On the basis of that and the fact that Ex-M-cko showed a deficit in long-term contextual fear memory, we investigated possible changes in excitatory synapses in hippocampus CA1 stratum radiatum by immunostaining with the postsynaptic marker PSD95. The results from Ex-M-cko and control groups are shown in Figure 4M and N, respectively. An unpaired t-test was conducted to compare the punctate number between Ex-M-cko and control groups (Fig. 4O, n = 24/group). Ex-M-cko (M = 488.33, SE = 8.7) showed a significant lower number than that of control (M = 517.42, SE = 8.9); t(46) = 2.33, P = 0.024. Due to the large sample size, a Z-test was performed to compare the puncta size of two groups (Fig. 4P) (n = 11 720 puncta for Ex-M-cko; n = 12 418 puncta for control group), Z(critical two tail) = 1.96, P < 0.0001. These results suggested that the deficit of PIGA in excitatory neurons resulted in a decreased density and size of the excitatory synapses in CA1 stratum radiatum.
Discussion
As the loss function of PIGA completely abolishes GPI anchor production, Pigaflox mouse line provided a powerful genetic tool to thoroughly explore the mechanisms underlying IGD encephalopathy in animal models. Using a hCMV-Cre mouse line, which expresses Cre before the preimplantation stage, Nozaki et al. (16) revealed that complete deletion of Piga totally perturbed organ formation in the early embryo stage of hemizygous cko mice (E9). Furthermore, partial knockout of Piga impaired development of neural tube and the face in the late embryo stage of mosaic cko from E12.5 to E17.5. Lukacs et al. (20) utilized a Wnt1-Cre mouse line to target Piga around E9.5 in neural crest cells and demonstrated that the deficit of Piga led to cleft lip/cleft and craniofacial hypoplasia, with hemizygous cko showing more severe phenotype than mosaic ones on E16.5. In both cases the cko mice could not survive after birth. They also used the Nestin-Cre line to drive ablation of Piga in the central nervous system on E11.5, when the neural tube was already closed. None of the mutants survived past weaning, with 50% hemizygous cko not surviving 2 days after birth and 50% of mosaic cko not surviving 19 days after birth. The brain size of mutants was smaller than that of WT (18). In the current study, we used Emx1-Cre, Vgat-Cre and Pkcd-Cre mouse lines to target ablation to telencephalon excitatory neurons, inhibitory neurons and thalamic neurons, respectively. We observed embryos of Ex-H-cko mutants with either microencephaly or normal appearance on E13.5; the reason of this discrepancy is unclear. Some In-H-cko embryos failed to develop any organs and appeared as an armorphous sphere on E13.5, whereas we also got In-H-cko embryos bearing similar appearance with the control embryos. Previous work reported that Vgat was expressed in a few sperm (30,31); therefore, the Vgat-Cre line may express Cre in a subpopulation of sperm. Neither the In-H-cko and Ex-H-cko mutants could survive after birth. All of the mosaic mutants and thalamus-specific hemizygous mutants (Th-H-cko) could survive as long as control mice, at least for 1 year. These data suggested that the short life span in Nestin-Cre-induced Piga mosaic mutants (Nestin-M-cko) (18) was more likely due to the deficits of GPI anchors in non-neuronal cells including astrocytes and oligodendrocytes.
There was a dramatic body weight loss in In-M-cko mice and a mild body weight loss in Ex-M-cko mutants. Previous work showed that Nestin-M-cko mutants were approximately half the weight of WT (18). Another study recently showed that a mouse model carrying Pigv hypomorphic mutation (Pigv341E) weighed ~60% of the WT control (32), which was similar to our In-M-cko mutants. Thus GPI-APs in inhibitory neurons may play an important role in regulating appetite and/or energy homeostasis. It has been reported that inhibitory neurons in the lateral hypothalamic area, ventral tegmental area and central nucleus of amygdala are involved in food intake behaviors (33). Future work examining the physiological changes of the inhibitory neurons in these brain regions of IN-M-cko mutants will help to identify the mechanisms underlying the reduced body weight. Limb clasping is often seen in ataxia mouse models and is regarded as a marker of ataxia. It is intriguing that limb clasping was the most prominent motor phenotype in Nestin-M-cko (18), Pigv341E (32) and our IN-M-cko mutants. Nestin-M-cko demonstrated a reduced motor coordination in rotarod test and surface righting reflex test (18). Pigv341E performed poorly in beam walking test, rope grip test and rotarod test, and Pigv341E also showed abnormal walking pattern in footprint test as well (32). Future work will examine our mutants with all these paradigms to characterize the potential motor phenotypes. Besides, Nestin-M-cko mutants also developed a severe tremor (18), whereas Pigv341E and IN-M-cko mutants did not. Limb clasping could be related to dysfunction of the cerebellum and, indeed, Nestin-M-cko showed cerebellum atrophy and impaired Purkinje cell dendritic arborization (18). Pigv341E showed normal-sized cerebellum and unchanged Purkinje cell dendrites. The cerebellum gross structure of our IN-M-cko mutants was unchanged as well. Future work will examine the fine structures of cerebellum including dendrites and spines of Purkinje cells in our mutants.
The most debilitating symptoms of IGD patients are epilepsy and intellectual disability. Our mutants had a normal life span, allowing us to investigate cognitive and seizure-related phenotypes. Fear conditioning has been widely used to test the ability of animal to learn and memorize an association between environmental/audial cues and an electrical shock. We tested our mutant mice with this paradigm and observed a significant decrease of fear response in the contextual memory test in IN-M-cko mutants and a decrease tendency in Ex-M-cko mutants. There was also a tendency that the cued memory of IN-M-cko was reduced. Contextual fear memory requires the coordination of hippocampus and amygdala. Although the gross structure of hippocampus and amygdala in the cko mutants was normal, a significantly smaller number and size of PSD95 puncta in hippocampus CA1 of Ex-M-cko mutant was detected. This suggests that the excitatory synapse number and size was altered in Ex-M-cko due to the deficit of GPI anchor synthesis. Spatial long-term memory impairment was reported in Pigv341E hypomorphic mutant as well. In addition, there was a synaptic transmission defect in stratum radiatum of Pigv341E, including decreased EPSP amplitude, increased paired pulse facilitation and post-tetanic potentiation (32). Our ISH results revealed an enrichment of Piga in hippocampus excitatory neurons, and we previously observed facilitated PTP in stratum radiatum and impaired spatial memory in mutant mice deficient in synaptic adhesion GPI-AP, netrin-G2 (28,34). All of these data implicate an important role of GPI-APs in the function of hippocampus pyramidal cells. In future work, it will be necessary to thoroughly examine the possible changes of both excitatory and inhibitory synapses on acute hippocampal slices of our mutants by electrophysiology recordings.
KA animal models of epilepsy have been extensively used over the past decades because of their high level of similarity with human epilepsy. By systematic administration of KA, we observed an increased vulnerability to KA-induced behavioral seizures in both Ex-M-cko and IN-M-cko mutants. To our knowledge, two GPI-APs including netrin-G2 and contactin-2 have been reported to be related with epilepsy (35,36). Specifically, contactin-2 null mice displayed spontaneous episodes of seizures (37) and netrin-G1/netrin-G2 double knockout mice were more susceptible to KA-induced seizures (unpublished data). It has been commonly assumed that an imbalance between the excitatory and inhibitory synaptic transmission in the brain initiates seizure activity. Future studies will thoroughly examine the morphological, molecular and physiological changes of both excitatory and inhibitory neurons in our mutants.
It is noteworthy that although we obtained Th-H-cko mutants and achieved essentially complete deletion of Piga in thalamic neurons, we did not observe any obvious abnormality of these mice. Their barrel cortex formed normally. The Pkcd-Cre induced recombination postnatally and then it took a couple of weeks to reach the maximal level (unpublished data). This timing of recombination might explain the minor effects resulted from Pkcd-Cre-induced Piga deletion. Netrin-G1 is a GPI-AP, which is highly enriched in thalamus. Netrin-G1 knockout mice showed normal barrel cortex (27), and thalamic-specific cko of netrin-G1 performed similarly to control littermates in all the behavioral paradigms we tested ((23) and unpublished data). In addition, a previous study revealed that T cells deficient in GPI-APs were functionally competent to respond to T cell receptor stimulation both in vitro and in vivo (38). Therefore GPI-APs are dispensable for some functions in certain cell populations. Future research will be needed to examine more detailed and delicate features of the thalamus related functions of Th-H-cko mutants.
Different cell populations in the nervous system have different expression profiles of GPI-APs. To date, many GPI-APs have been reported to be expressed in neurons, including Thy-1, TAG-1, NCAM120, Contactin, T-cadherin, LAMP, OBCAM, Neurotrimin, Kilon, ephrin-A, semaphoring-7A, glypicans, netrin-Gs, NgRs, MDGAs, GFRα1, PrPC, IgLON, CPG15/Neuritin. These molecules perform diverse functions in axonal fasciculation, growth and guidance, neuronal migration and synapse formation and plasticity (39–44). A few GPI-APs have been discovered to be expressed in glial cells. For examples, Glipican4 and 6 are expressed by astrocytes, with Glipican4 expression enriched in the hippocampus and Glipican6 in the cerebellum, and they play roles in synapse formation and synaptic plasticity (45). Contactin, TAG-1 and OMgp are expressed in oligodendrocytes and regulate neurite growth, myelination, nodal/paranodal domain organization and synaptic plasticity (39,40,42,44). All the IGD patients reported so far carried mutations of a molecule involved in GPI anchor synthesis or maturation. The symptoms were variable and were resulted from the sum effect of GPI-APs deficits in affected cell populations. We hypothesize that the expression of a GPI synthesizing molecule was regulated by different elements in different cell types or even subtypes. It remains to be tested explicitly whether specific or common transcription factors regulate the expression of a certain GPI synthesizing molecule in different cell populations. Another intriguing point is that in human beings, all PIGA-IGD patients are male. Their mothers, who are heterozygous carriers of PIGA mutation, were phenotypically mosaic at the cell level but do not show clear symptoms as previously described (3). In mice, the female heterozygous carriers of Piga mutations demonstrated clear deficits. It has been reported that the regulation of X-linked gene expression is quite different between human and mouse (46). Tarailo-Graovac et al. (10) showed that the pattern of X-inactivation was 94.5% skewed in the clinically unaffected carrier mother of a male IGD patient carrying a missense PIGA mutation, which might explain the mechanisms underlying the species difference of phenotypes we observed here. The phenotype severity of IGD patients can range from ‘mild’ to ‘severe’ for each patient, depending on the genotype and the residual functional activity of the protein that the mutated gene encodes. Nestin-M-cko mouse model represents part of the ‘severe’ phenotypes of IGD including delayed myelination, cerebellum atrophy and premature death. While the In-M-cko mutants we created here most authentically represent the ‘moderate’ IGD human phenotype including less severe intellectual disability and seizures, mild motor impairment, decreased bodyweight and longer life span. Nonetheless, Nestin-M-cko, Pigv341E, IN-M-cko, Ex-M-cko mutants are the only GPI-anchor deficient mouse models that can survive after birth and display the neurological phenotypes of IGD patients. Together with the coming oligodendrocyte-specific Piga cko, they will be valuable animal models to help elucidate the molecular mechanisms of IGD and test the novel therapeutic approaches for the intractable symptoms of IGD patients.
Materials and Methods
Animals
All of the mice were housed in a temperature and humidity controlled, pathogen-free environment maintained on a 12:12 h light/dark cycle, with food and water ad libitum. Pigaflox mice (17) (RBRC06211) were obtained from Dr Junji Takeda, Dr Taroh Kinoshita and RIKEN BRC. Emx-Cre, Vgat-Cre and Pkcd-Cre mice were described previously (21–23). The hemizygous and mosaic cko mice were obtained by breeding female Pigaflox/+ mice with male Cre mice and were housed in groups with mixed genotype. Unless specified, Ex-M-cko and IN-M-cko mutants aged between 16 to 32 weeks were compared with their female littermates (Piga+/+, Cre+), Th-H-cko mutants aged between 16 and 32 weeks were compared with their male littermates (Pigaflox/Y, Cre−). All assays were performed by experimenters blind to the animals’ genotype.
Genotyping and PCR analysis of neuronal specific disruption of Piga
For genotyping by using tail DNA, the PCR primer pairs were as follows:
Cre1: 5′-TCGACCAGGTTCGTTCACTC-3′and Cre2: 5′-TGACCCGGCAAAACAGGTA-3′ amplified bands of 304 bp for Cre recombinase. Primer1: 5′-ACCTCCAAAGACTGAGCTGTTG-3′ and Primer2: 5′-CCTGCCTTAGTCTTCCCAGTAC-3′ amplified bands of 420 bp for Piga floxed allele and 250 bp for WT allele.
For assessing the deletion efficiency of Piga in neuronal populations, we followed a protocol as previously described (24) with some modifications. Briefly, hippocampus, stratum, thalamus and cortex were dissected from eight brains/genotype. Single-cell suspension was obtained by trypsin digestion and cell strainer filtering and then briefly fixed in 50% ethanol. The cells were stained by first antibody anti-NeuN (Millipore) and second antibody Alexa Fluor 488 donkey anti-mouse IgG (Molecular Probes) and then sorted by BD FACSAria II. And the three PCR primers were as follows: Primer1: 5′- ACCTCCAAAGACTGAGCTGTTG-3′; Primer2: 5′-CCTGCCTTAGTCTTCCCAGTAC-3′ and Primer3: 5′-TGTGGGTTTCAGTTCATTTCAGA-3′ amplified bands of 420 bp for floxed allele, 250 bp for WT allele and 550 bp for disrupted configuration.
In situ hybridization
The probe sequence was 790 bp from the noncoding region of Piga exon 6. It was obtained by PCR from mouse hippocampus cDNA library, the following primers were used: Forward: GGGATAATGGTTTAGCCACTCA and Reverse: GAAGAGCAGCTGGTTTTGAGAT. Preparation of digoxigenin-labeled antisense riboprobes, and hybridization of free-floating sections were performed according to previously described procedures (23).
Immunohistochemistry
The netrin-G1 and PSD95 antibodies (State sources), and the immunohistochemistry procedure on free-floating sections were described in previous work (27,34).
Histology
The procedure of Nissl staining and CO staining were described previously (21).
Limb-clasping scoring
Mice were held by the tail for 5–10 s. During tail suspension, limb-clasping behavior was manually evaluated with following standards: 0—no limb clasping, normal escape extension; 1—either hind limbs or forelimbs incomplete splay and loss of mobility; 2—either hind limbs or forelimbs clasping together and loss of mobility; 3—single side hind limb and forelimb clasping together and loss of mobility; 4—three limbs clasping together and loss of mobility; 5—four limbs clasping together and loss of mobility; 6—four limbs clasping together, body forms a ball shape and loss of mobility.
Fear conditioning test
Contextual and cued fear conditioning test were performed as previously described (23).
Behavioral seizure scoring
KA-induced behavioral seizures were scored as previously described (47).
Punctate number and size quantification
Four mice from each group were analyzed. Three continuous sections around the level of Bregma 2 mm were obtained from each mouse, totally 24 images from the similar positions of stratum radiatum of each genotype were caught by Olympus FV3000 under the same condition. The punctate number and punctate size from each frame (2700 μm2) were measured with Metamorph Software.
Statistics
All analyses were conducted using SPSS Statistics21. For body weight comparation among three female groups, ANOVA was used, following by Bonferroni corrected post hoc testing. For body weight comparation between two male groups, unpaired t-test was used. For limb-clasping score comparation among three groups, Kruskal–Wallis test was used followed by Bonferroni corrected post hoc testing. For limb-clasping score comparation between two male groups, Mann–Whitney U test was used. For all of the time series data of behavioral analysis, mixed factorial ANOVA was used to compare among groups, followed by post hoc testing via the Bonferroni correction. In addition, simple effects test at each time point via the Bonferroni correction was applied when group effects existed and the data were summarized in Tables 2 and 3. For the comparation between two groups with sample size >30, two-tailed Z-Test was applied.
Acknowledgements
Authors thank Dr Taroh Kinoshita for very informative and inspiring discussion.
Conflict of Interest statement. None declared.
Funding
RIKEN Incentive Research Project (100226201701100443) to Q.Z; the Brain Science Project, Center for novel science initiatives, National Institutes Of Natural Sciences (BS291003) to Q.Z; the RIKEN Aging Project (10026-201701100263-340120) to Q.Z; the JSPS Kakenhi Grant-in-Aid for Young Scientists (17K18362) to Q.Z; the JSPS Kakenhi Grant-in-Aid for Challenging Research (19K21807) to Q.Z; the International Education and Research Laboratory Program of University of Tsukuba to L.J.Y.
Authors’ Contributions
Q.Z. conceived and designed the research; L.C.K., M.T., V.B., S.T., Y.N. and Q.Z. conducted the experiments and analyzed the data. M.K. and N.M. provided all the necessary information of clinical patients carrying PIGA mutations. J.T. provided Pigaflox mouse. S.I., S.O. and L.J.Y. provided the experimental facility and resources. Q.Z., N.M., and L.J.Y. interpreted data. Q.Z. drafted the manuscript. N.M., S.I and L.J.Y. commented on and edited the manuscript.
References
- 1. Freeze, H.H., Chong, J.X., Bamshad, M.J. and Ng, B.G. (2014) Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am. J. Hum. Genet., 94, 161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang, I.J., He, M. and Lam, C.T. (2018) Congenital disorders of glycosylation. Ann. Transl. Med., 6, 477–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kinoshita, T. (2020) Biosynthesis and biology of mammalian GPI-anchored proteins. Open Biol., 10, 190290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bellai-Dussault, K., Nguyen, T.T.M., Baratang, N.V., Jimenez-Cruz, D.A. and Campeau, P.M. (2019) Clinical variability in inherited glycosylphosphatidylinositol deficiency disorders. Clin. Genet., 95, 112–121. [DOI] [PubMed] [Google Scholar]
- 5. Kinoshita, T. (2014) Biosynthesis and deficiencies of glycosylphosphatidylinositol. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci., 90, 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnston, J.J., Gropman, A.L., Sapp, J.C., Teer, J.K., Martin, J.M., Liu, C.F., Yuan, X., Ye, Z., Cheng, L., Brodsky, R.A. and Biesecker, L.G. (2012) The phenotype of a germline mutation in PIGA: the gene somatically mutated in paroxysmal nocturnal hemoglobinuria. Am. J. Hum. Genet., 90, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belet, S., Fieremans, N., Yuan, X., Van Esch, H., Verbeeck, J., Ye, Z., Cheng, L., Brodsky, B.R., Hu, H., Kalscheueret, V.M. et al. (2014) Early frameshift mutation in PIGA identified in a large XLID family without neonatal lethality. Hum. Mutat., 35, 350–355. [DOI] [PubMed] [Google Scholar]
- 8. Kato, M., Saitsu, H., Murakami, Y., Kikuchi, K., Watanabe, S., Iai, M., Miya, K., Matsuura, R., Takayama, R., Ohba, C. et al. (2014) PIGA mutations cause early-onset epileptic encephalopathies and distinctive features. Neurology, 82, 1587–1596. [DOI] [PubMed] [Google Scholar]
- 9. Swoboda, K.J., Margraf, R.L., Carey, J.C., Zhou, H., Newcomb, T.M., Coonrod, E., Durtschi, J., Mallempati, K., Kumanovics, A., Katz, B.E. et al. (2014) A novel germline PIGA mutation in Ferro-Cerebro-cutaneous syndrome: a neurodegenerative X-linked epileptic encephalopathy with systemic iron-overload. Am. J. Med. Genet. A, 164a, 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tarailo-Graovac, M., Sinclair, G., Stockler-Ipsiroglu, S., Van Allen, M., Rozmus, J., Shyr, C., Biancheri, R., Oh, T., Sayson, B., Lafek, M. et al. (2015) The genotypic and phenotypic spectrum of PIGA deficiency. Orphanet. J. Rare. Dis., 10, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fauth, C., Steindl, K., Toutain, A., Farrell, S., Witsch-Baumgartner, M., Karall, D., Joset, P., Böhm, S., Baumer, A., Maier, O. et al. (2016) A recurrent germline mutation in the PIGA gene causes Simpson-Golabi-Behmel syndrome type 2. Am. J. Med. Genet. A, 170a, 392–402. [DOI] [PubMed] [Google Scholar]
- 12. Kim, Y.O., Yang, J.H., Park, C., Kim, S.K., Kim, M.K., Shin, M.G. and Woo, Y.J. (2016) A novel PIGA mutation in a family with X-linked, early-onset epileptic encephalopathy. Brain Dev., 38, 750–754. [DOI] [PubMed] [Google Scholar]
- 13. Lin, W.-D., Chou, I.C., Tsai, F.-J. and Hong, S.-Y. (2018) A novel PIGA mutation in a Taiwanese family with early-onset epileptic encephalopathy. Seizure, 58, 52–54. [DOI] [PubMed] [Google Scholar]
- 14. Yang, J., Wang, Q., Zhuo, Q., Tian, H., Li, W., Luo, F., Zhang, J., Bi, D., Peng, J., Zhou, D. and Xin, H. (2018) A likely pathogenic variant putatively affecting splicing of PIGA identified in a multiple congenital anomalies hypotonia-seizures syndrome 2 (MCAHS2) family pedigree via whole-exome sequencing. Mol. Genet. Genomic. Med., 6, 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neuhofer, C.M., Funke, R., Wilken, B., Knaus, A., Altmüller, J., Nürnberg, P., Li, Y., Wollnik, B., Burfeind, P. and Pauli, S. (2020) A novel mutation in PIGA associated with multiple congenital anomalies-hypotonia-seizure syndrome 2 (MCAHS2) in a boy with a combination of severe epilepsy and gingival hyperplasia. Mol. Syndromol., 11, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nozaki, M., Ohishi, K., Yamada, N., Kinoshita, T., Nagy, A. and Takeda, J. (1999) Developmental abnormalities of glycosylphosphatidylinositol-anchor-deficient embryos revealed by Cre/loxP system. Lab. Invest., 79, 293–299. [PubMed] [Google Scholar]
- 17. Tarutani, M., Itami, S., Okabe, M., Ikawa, M., Tezuka, T., Yoshikawa, K., Kinoshita, T. and Takeda, J. (1997) Tissue-specific knockout of the mouse Pig-a gene reveals important roles for GPI-anchored proteins in skin development. Proc. Natl. Acad. Sci. U. S. A., 94, 7400–7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lukacs, M., Blizzard, L.E. and Stottmann, R.W. (2020) CNS glycosylphosphatidylinositol deficiency results in delayed white matter development, ataxia and premature death in a novel mouse model. Hum. Mol. Genet., 29, 1205–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bernal, A. and Arranz, L. (2018) Nestin-expressing progenitor cells: function, identity and therapeutic implications. Cell. Mol. Life Sci., 75, 2177–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lukacs, M., Roberts, T., Chatuverdi, P. and Stottmann, R.W. (2019) Glycosylphosphatidylinositol biosynthesis and remodeling are required for neural tube closure, heart development, and cranial neural crest cell survival. Elife, 8, e45248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iwasato, T., Datwani, A., Wolf, A.M., Nishiyama, H., Taguchi, Y., Tonegawa, S., Knöpfel, T., Erzurumlu, R.S. and Itohara, S. (2000) Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature, 406, 726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ogiwara, I., Iwasato, T., Miyamoto, H., Iwata, R., Yamagata, T., Mazaki, E., Yanagawa, Y., Tamamaki, N., Hensch, T.K., Itohara, S. and Yamakawa, K. (2013) Nav1.1 haploinsufficiency in excitatory neurons ameliorates seizure-associated sudden death in a mouse model of Dravet syndrome. Hum. Mol. Genet., 22, 4784–4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang, Q., Sano, C., Masuda, A., Ando, R., Tanaka, M. and Itohara, S. (2016) Netrin-G1 regulates fear-like and anxiety-like behaviors in dissociable neural circuits. Sci. Rep., 6, 28750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guez-Barber, D., Fanous, S., Harvey, B.K., Zhang, Y., Lehrmann, E., Becker, K.G., Picciotto, M.R. and Hope, B.T. (2012) FACS purification of immunolabeled cell types from adult rat brain. J. Neurosci. Methods, 203, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pelkey, K.A., Chittajallu, R., Craig, M.T., Tricoire, L., Wester, J.C. and McBain, C.J. (2017) Hippocampal GABAergic inhibitory interneurons. Physiol. Rev., 97, 1619–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gerfen, C.R. (2004) In Paxinos, G. (ed), The Rat Nervous System, 3rd edn. Academic Press, Burlington, pp. 455–508. [Google Scholar]
- 27. Nishimura-Akiyoshi, S., Niimi, K., Nakashiba, T. and Itohara, S. (2007) Axonal netrin-Gs transneuronally determine lamina-specific subdendritic segments. Proc. Natl. Acad. Sci. U. S. A., 104, 14801–14806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsukawa, H., Akiyoshi-Nishimura, S., Zhang, Q., Luján, R., Yamaguchi, K., Goto, H., Yaguchi, K., Hashikawa, T., Sano, C., Shigemoto, R. et al. (2014) Netrin-G/NGL complexes encode functional synaptic diversification. J.Neurosci., 34, 15779–15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Racine, R.J. (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol., 32, 281–294. [DOI] [PubMed] [Google Scholar]
- 30. Aanesen, A., Andersson, E., Meister, B. and Fried, G. (2001) The vesicular GABA transporter vGAT, is present in sperm and testis. Fertil. Steril., 76, S40. [Google Scholar]
- 31. Tomoiaga, D., Aguiar-Pulido, V., Shrestha, S., Feinstein, P., Levy, S.E., Mason, C.E. and Rosenfeld, J.A. (2020) Single-cell sperm transcriptomes and variants from fathers of children with and without autism spectrum disorder. NPJ Genom. Med., 5, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de los Santos, M.R., Rivalan, M., David, F.S., Knaus, A., Stumpf, A., Velasquez, L.M., Voigt, A., Mattei, D., Long, M., Vogt, G. et al. (2020) A CRISPR-Cas9-engineered mouse model for GPI anchor deficiency mirrors human phenotype and shows hippocampal synaptic dysfunctions. bioRxiv in press, 2020.2004.2020.050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rossi, M.A. and Stuber, G.D. (2018) Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab., 27, 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang, Q., Goto, H., Akiyoshi-Nishimura, S., Prosselkov, P., Sano, C., Matsukawa, H., Yaguchi, K., Nakashiba, T. and Itohara, S. (2016) Diversification of behavior and postsynaptic properties by netrin-G presynaptic adhesion family proteins. Mol. Brain, 9, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stogmann, E., Reinthaler, E., Eltawil, S., El Etribi, M.A., Hemeda, M., El Nahhas, N., Gaber, A.M., Fouad, A., Edris, S., Benet-Pages, A. et al. (2013) Autosomal recessive cortical myoclonic tremor and epilepsy: association with a mutation in the potassium channel associated gene CNTN2. Brain, 136, 1155–1160. [DOI] [PubMed] [Google Scholar]
- 36. Dias, C.M., Punetha, J., Zheng, C., Mazaheri, N., Rad, A., Efthymiou, S., Petersen, A., Dehghani, M., Pehlivan, D., Partlow, J.N. et al. (2019) Homozygous missense variants in NTNG2, encoding a presynaptic Netrin-G2 adhesion protein, lead to a distinct neurodevelopmental disorder. Am. J. Hum. Genet., 105, 1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fukamauchi, F., Aihara, O., Wang, Y.-J., Akasaka, K., Takeda, Y., Horie, M., Kawano, H., Sudo, K., Asano, M., Watanabe, K. and Iwakura, Y. (2001) TAG-1-deficient mice have marked elevation of adenosine A1 receptors in the hippocampus. Biochem. Biophys. Res. Commun., 281, 220–226. [DOI] [PubMed] [Google Scholar]
- 38. Takahama, Y., Ohishi, K., Tokoro, Y., Sugawara, T., Yoshimura, Y., Okabe, M., Kinoshita, T. and Takeda, J. (1998) Functional competence of T cells in the absence of glycosylphosphatidylinositol-anchored proteins caused by T cell-specific disruption of the Pig-a gene. Eur. J. Immunol., 28, 2159–2166. [DOI] [PubMed] [Google Scholar]
- 39. Walsh, F.S. and Doherty, P. (1991) Glycosylphosphatidylinositol anchored recognition molecules that function in axonal fasciculation, growth and guidance in the nervous system. Cell Biol. Int., 15, 1151–1166. [DOI] [PubMed] [Google Scholar]
- 40. Karagogeos, D. (2003) Neural GPI-anchored cell adhesion molecules. Front. Biosci., 8, s1304–s1320. [DOI] [PubMed] [Google Scholar]
- 41. Javaherian, A. and Cline, H.T. (2005) Coordinated motor neuron axon growth and neuromuscular synaptogenesis are promoted by CPG15 in vivo. Neuron, 45, 505–512. [DOI] [PubMed] [Google Scholar]
- 42. Tan, R.P.A., Leshchyns’ka, I. and Sytnyk, V. (2017) Glycosylphosphatidylinositol-anchored immunoglobulin superfamily cell adhesion molecules and their role in neuronal development and synapse regulation. Front. Mol. Neurosci., 10, 378–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Um, J.W. and Ko, J. (2017) Neural Glycosylphosphatidylinositol-anchored proteins in synaptic specification. Trends Cell Biol., 27, 931–945. [DOI] [PubMed] [Google Scholar]
- 44. Gennarini, G., Bizzoca, A., Picocci, S., Puzzo, D., Corsi, P. and Furley, A.J.W. (2017) The role of GPI-anchored axonal glycoproteins in neural development and neurological disorders. Mol. Cell. Neurosci., 81, 49–63. [DOI] [PubMed] [Google Scholar]
- 45. Allen, N.J., Bennett, M.L., Foo, L.C., Wang, G.X., Chakraborty, C., Smith, S.J. and Barres, B.A. (2012) Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature, 486, 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carrel, L. and Willard, H.F. (2005) X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature, 434, 400–404. [DOI] [PubMed] [Google Scholar]
- 47. Yu, L.M.Y., Polygalov, D., Wintzer, M.E., Chiang, M.-C. and McHugh, T.J. (2016) CA3 synaptic silencing attenuates kainic acid-induced seizures and hippocampal network oscillations. eNeuro, 3, ENEURO.0003-0016.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


