Abstract
Objectives:
As the aging population grows, interest in applying the concept of frailty to older adults with cancer has increased. This study examines the prevalence of frailty in older patients with multiple myeloma using three frailty models.
Methods:
In this secondary analysis of a prospective cohort study, 40 adults aged ≥65 with myeloma completed the Cancer and Aging Research Group geriatric assessment within three months of initial diagnosis. Geriatric assessment data was used to categorize patients’ frailty status according to three indices: The International Myeloma Working Group (IMWG) Frailty Index, the Revised Myeloma Comorbidity Index (R-MCI), and the Carolina Frailty Index (CFI). Agreement between the indices was examined using Cohen’s kappa.
Results:
Twenty-eight patients were classified as frail by at least one of the models. However, only slight agreement exists on the classification of frailty among the indices, with little concordance among the models (Kappa 0.03-0.12). Only three patients were categorized as frail by all three models.
Conclusion:
In a cohort of 40 older adults with newly diagnosed multiple myeloma, three frailty indices have differing approaches to operationalizing frailty resulting, in different patients being categorized as frail. Little agreement existed between the models. Further studies are needed to explore the utility of these models in predicting treatment toxicity and prognosis.
Keywords: Multiple myeloma, elderly, geriatric assessment, frailty, survival
Introduction
Multiple Myeloma is an incurable hematological malignancy characterized by the bone marrow being invaded by plasma cells [1]. Over 60% of patients with this disease are over the age of 65 meaning that multiple myeloma mainly affects the geriatric population [2]. Older patients may have a lower likelihood of response and higher rates of treatment-related toxicity relative to younger patients. This can be explained by age-associated factors, such as frailty, and other biological and prognostic factors [3–9]. Frailty is a collective deterioration of multiple physical and physiological functions yielding a lower tolerance of stressors such as cancer and treatments [10].
The concept of frailty has been applied in patients with cancer to inform a more personalized treatment approach [11]. One of the greatest challenges in treating older patients is the ability to differentiate ‘fit’ and ‘frail’ patients, where ‘fit’ patients are more likely to tolerate more aggressive treatments, while ‘frail’ patients are at greater risk for developing toxicities when using the same aggressive treatments [12].
To date, there is no universally accepted way to identify or define frailty [11]. The phenotypic approach defines frailty as having three or more of the following factors: unintentional weight loss, weakness, poor endurance and energy, slowness and low physical activity [13]. Another approach is the concept of frailty as a deficit accumulation that increases with age, in which the cumulative presence of aging-associated deficits yields a frailty index; this approach allows the use of a broad number of objective or subjective measures, provided they are aging-associated [14]. Nearly 70 frailty measures were identified in a recent systematic review, underscoring the plethora of approaches to operationalizing frailty [15].
Identifying frail patients with cancer may allow clinicians to propose more personalized treatment plans for each individual patient rather than basing the treatment solely on a patient’s age. Multiple indices have been developed to help classify older patients with myeloma or cancer in general. Two approaches developed in older adults with myeloma are the International Myeloma Working Group (IMWG) [16] frailty index and the Revised Myeloma Comorbidity Index (R-MCI) [17], which both incorporate comorbidities, age and functional status. Another model is the Carolina Frailty Index (CFI), developed in patients with various malignancies [18], which uses an accumulation of deficits approach. The accumulation of deficits approach predicts overall survival (OS) in patients with myeloma, though the CFI specifically has not been used in patients with myeloma [12]. Each of these three separate models employs different variables, though each operationalizes the concept of frailty. There is currently no standard or uniformly accepted method to score frailty in a clinical setting [12]. Our objective was to examine the prevalence of frailty based on three approaches to operationalizing frailty and evaluate concordance between the different approaches.
Methods
Patient Population and Study Design
This study is a secondary analysis of a pilot prospective cohort study of older adults with multiple myeloma [19]. Participants were recruited from two comprehensive cancer centers, Siteman Cancer Center, St. Louis, MO and Duke University. Eligible patients for this study were 65 years of age or older, received a diagnosis of myeloma by International Myeloma Working Group criteria, and enrolled within three months of initial diagnosis; participants were allowed to enroll after initiation of treatment. If patients had an estimated life expectancy of less than six months, had smoldering myeloma or concomitant amyloidosis, or were not expecting to return to the participating treatment center for care, they were excluded from this study. Forty-seven patients who met the criteria were approached and seven declined to participate, resulting in 40 patients enrolled in the study between 2012-2015. This study was approved by the institutional review board at both institutions. All patients were registered through the Siteman Cancer Center database at Washington University and gave written informed consent.
Geriatric Assessment
Participants completed a geriatric assessment of 113 questions comprising measures from the Cancer and Aging Research Group Geriatric Assessment Tool (CARG) [20]. Our assessment included previously validated measures of functional status [21], comorbidity [22], psychological state, social activity and support [21], nutrition, and cognition [23], physical symptoms [24], social and family well-being [25], emotional and functional well-being [26] and neuropathy items [27]. Other clinical parameters recorded at baseline included: disease stage using the International Staging System (ISS) [28], cytogenetic abnormalities, hemoglobin and creatinine.
Definitions of Frailty
We examined three different models of frailty using data available in the Cancer and Aging Research Group tool to define frailty in our cohort: the IMWG frailty model [16], the R-MCI [17] and the CFI [18]. In the IMWG frailty index, frailty is categorized based on the Katz Activity of Daily Living (ADL), the Lawton Instrumental Activities of Daily Living (IADL) scale, the Charlson Comorbidity Index (CCI) and age. The R-MCI was constructed using the Revised Myeloma Comorbidity website [29] which categorizes frailty using an additive scoring system comprising renal disease measured by estimated glomerular filtration rate (eGFR), lung disease, Karnofsky Performance Rating Scale (KPS), age, frailty and cytogenetics. The CFI defined frailty using items including IADLs, physical function, comorbidities, number of daily medications, vision, hearing, nutrition, mental health and social activity as well as objective measures of physical function and cognition from the measures in the CARG tool. Our data collection began prior to the publication of the IMWG and R-MCI, therefore, our dataset did not include every variable employed in the calculation of those indices. See Supplementary Table 1 for full details of the application of the available geriatric assessment data to approximate the IMWG and R-MCI models. The CFI frailty model was replicated precisely as published, as all necessary variables were included in the Cancer and Aging Research Group geriatric assessment. With all three models, patients are categorized as fit, intermediate or frail based on summary scores, as detailed in Supplementary Table 1.
Statistical Analysis
The variables and scores from all three models were calculated as previously stated by [16–18] and as adapted for our dataset (Supplemental Table 1). Patients categorized as fit/intermediate were combined in a “non-frail” category for each model. Continency tables were used to analyze the overlap of patients categorized as frail by each model; Cohen’s kappa was used to measure agreement.
Results
Forty patients were enrolled. Baseline characteristics are listed in Table 1. Of the 40 participants, 17 (42.5%) had not yet received myeloma therapy and 23 (57.5%) had initiated therapy a median of 25.0 days ± standard deviation 23.7 prior to the geriatric assessment.
Table 1.
Baseline characteristics of 40 older patients with newly diagnosed multiple myeloma
| Characteristic | N (%) or Median ± Standard Deviation |
|---|---|
| Age, years | 69.5 ± 5.07 |
| Sex | |
| Female | 15 (37.5%) |
| Male | 25 (62.5%) |
| Race | |
| White | 31 (77.5%) |
| Black | 5 (12.5%) |
| Other | 4 (10%) |
| International Staging System | |
| Stage I | 11 (27.5%) |
| Stage II | 13 (32.5%) |
| Stage III | 8 (20%) |
| Missing | 5 (12.5%) |
| Deletion 17p | 8 (20%) |
| Charlson Comorbidity Index | 0.00 ± 1.59 |
| Timed Up & Go | 12.25 ± 4.91 seconds |
| Instrumental Activities of Daily Living (IADLs) | |
| Independent in all IADLs | 15 (37.5%) |
| Dependent in one or more IADLs | 25 (62.5%) |
Frailty Categories
By applying the IMWG frailty index, three (7.5%) patients were categorized as fit, fifteen (37.5%) were intermediate-fit and 22 (55%) were frail. With the R-MCI, four (10%) patients were categorized as fit, 29 (72.5%) as intermediate and seven (17.5%) as frail. When using the CFI, seventeen (42.5%) patients were categorized as fit, eight (20%) were intermediate and fifteen (37.5%) were frail. There were no differences in the prevalence of frailty between patients who had started myeloma therapy and those who had not with any of the 3 frailty indices.
Agreement in categorization of participants as frail between the three models was only slight. Of the 28 patients categorized as frail by one of the three indices, only three (11%) were categorized as frail by all three models. The IMWG and R-MCI model had two (7%) frail patients in common (Kappa 0.11, p=0.33). Six (21%) patients were categorized as frail by both the IMWG index and the CFI (Kappa 0.03, p=0.62). There were two (7%) patients classified as frail in the CFI and R-MCI (Kappa 0.12, p=0.04). Eleven were categorized as frail by only the IMWG frailty index, four by only the CFI. See Figure 1 for representation of overlap in categorization of the 28 patients categorized as frail.
Figure 1.
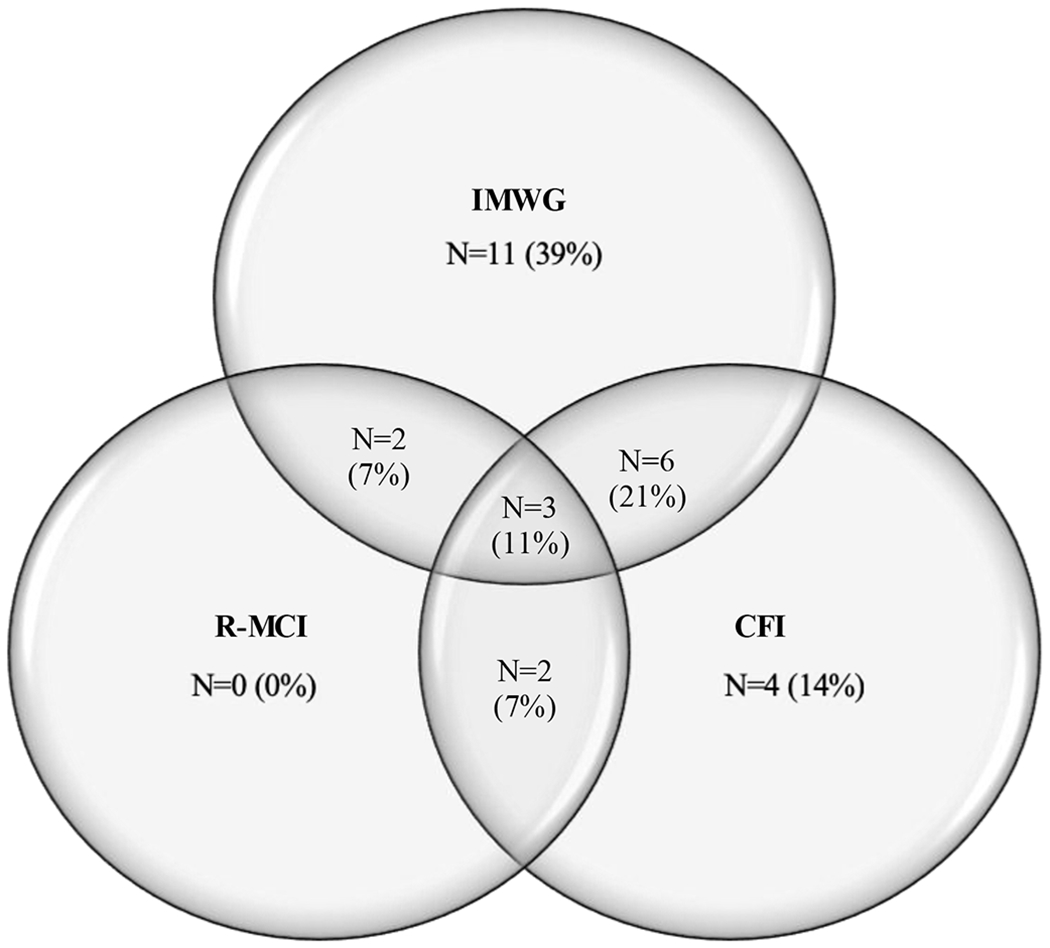
Overlap in categorization of the 28 patients categorized as frail Acronyms: IMWG, International Myeloma Working Group; CFI, Carolina Frailty Index; R-MCI, Revised Myeloma Comorbidity Index
Table 2 summarizes demographic and geriatric assessment characteristic s among the patients categorized as frail using the different frailty models.
Table 2.
Characteristics of patients categorized as frail by different frailty indices
| Frail by International Myeloma Working Group criteria (N=22) | Frail by Revised Myeloma Comorbidity Index (N=7) | Frail by Carolina Frailty Index (N=15) | |
|---|---|---|---|
| Age (mean ± standard deviation) | 73.1 ± 5.7 years | 74.4 ± 6.3 years | 70.1 ± 3.8 years |
| Female Gender (number, percent) | 7 (31.8%) | 5 (71.4%) | 7 (46.7%) |
| International Staging System: Stage I/II/III (number, frequency)* | 4 (23.5%) / 5(29.4%) /8 (47.1%) | 0 / 3(42.9%)/ 4 (57.1%) | 2 (15.4%)/ 7 (53.8%) /4 (30.8%) |
| Karnofsky performance status (Mean ± standard deviation) | 75.5 ± 13.4 | 61.4 ± 9.0 | 70.67 ± 12.8 |
| Charlson Comorbidity Index Score (Mean ± standard deviation) | 1.6 ± 1.9 | 1.7 ± 1.4 | 0.8 ± 1.0 |
| Patients reporting one or more falls in the past 6 months(number, percent) | 7 (31.8%) | 3 (42.9%) | 8 (53.3%) |
| Timed Up and Go(mean ± standard deviation) | 14.2 ± 5.9 seconds | 15.2 ± 4.1 seconds | 14.3 ± 3.6 seconds |
| Orientatio n-Memory-Concentration Total(mean ± standard deviation) | 4.3 ± 5.2 | 4.1 ± 8.0 | 3.9 ± 5.0 |
| Dependence in any instrumental activities of daily living(number, percent) | 15 (68.2%) | 6 (85.7%) | 15 (100%) |
| Mental health inventory (mean ± standard deviation) | 75.3 ± 15.0 | 71.1 ± 17.7 | 66.0 ± 16.7 |
Discussion
Our analysis of older adults with myeloma showed that three frailty indices had only slight agreement. Our findings are similar to a recent report by Murillo et al. In their cohort of 98 older adults with myeloma, there was a 57% discordance rate between the IMWG and Fried model in categorizing patients as frail [30]. This is problematic as making treatment decisions based upon different methods of frailty classification could result in similar patients being assigned different treatments, depending on which model is utilized. This underscores the need for prospective validation of these methods as predictors of chemotherapy toxicity and other patient-centered outcomes. Each of these different scoring methods applies different approaches operationalizing frailty, comprising different aging-associated domains.
The IMWG frailty model was the first model published in patients with myeloma, and has been widely applied [16]. While the study in which IMWG frailty model was developed had a much larger sample size (N= 869) than our study, both studies enrolled only newly diagnosed older adults with myeloma. In that study, 70% of patients were categorized as fit/intermediate fit using the IMWG frailty model, while 30% were frail. In our sample, 45% of patients were categorized as fit/intermediate fit, while 55% were categorized as frail. Of note, our dataset only included two Activities of Daily Living (bathing and dressing), whereas the IMWG model covered all six of the Katz ADLs (bathing, dressing, toileting, transferring, continence and feeding). Thus, though the prevalence of frailty in the present study was higher than in the development study, our estimate may actually underestimate the number of patients in our cohort categorized as frail using the IMWG model. The likely reason for this difference in prevalence of frailty is that the IMWG model was developed in a cohort of clinical trial participants, who tend to be more fit, while our study enrolled a more heterogeneous cohort.
In comparing our cohort to the study in which the R-MCI was developed, we found a similarly low rate of patients categorized as frail. The R-MCI included patients with newly diagnosed myeloma, without a lower age limit: 59% of patients were under 65 years of age. In the R-MCI cohort, 13.5% of patients were categorized as frail, compared with 12.5% in our study. Engelhardt found that the R-MCI defines a higher risk population in comparison to other models, including the IMWG [31].
The Carolina Frailty Index differs from the IMWG and R-MCI indices in its use of the accumulation of deficits approach to frailty; to our knowledge, this is the first application of the CFI in patients with multiple myeloma. In its development, the CFI only included patients over the age of 65 but enrolled patients with multiple types of cancer and included patients either during or after treatment. Twelve percent of the CFI cohort had hematologic malignancies [18]. In the study in which the CFI was developed, 82% of patients were categorized as fit or intermediate while 18% were categorized as frail. In the subgroup of patients with hematologic malignancies, 23% were categorized as frail. In our study, 37% were categorized as frail. This difference in prevalence may reflect the differences in inclusion, where patients in the CFI cohort, including those with hematologic malignancies, may have undergone treatment, which may have mitigated some symptoms and resulted in improved functional status. Our cohort enrolled patients with recently diagnosed myeloma who may have had symptoms of active disease, such as pain or fatigue, potentially impacting their geriatric assessment.
Consideration of the variables comprising each frailty model (Supplementary Table) aids in understanding the differences in patients categorized as frail by each model (Table 2). Patients categorized as frail by the IMWG or R-MCI tended to be older than those categorized as frail by CFI, reflecting the fact that the IMWG and R-MCI both include age as a component of frailty, while the CFI does not. While all 3 indices incorporate comorbidities into assessment of frailty, comorbidities in the CFI are those in the original Cancer and Aging Research Group geriatric assessment, explaining the lower Charlson Comorbidity Score in the patients categorized as frail by the CFI. Falls and depression are incorporated as components of the CFI, reflected in the higher proportion of patients reporting a prior fall and more symptoms of depression in the group categorized as frail by the CFI model than in the IMWG or R-MCI. In the CFI, each of the individual instrumental activities of daily living is a component of the model, rather than the summary score, as in the IMWG and R-MCI, resulting in all patients categorized as frail using the CFI being dependent in one or more IADLs.
Of the three models of frailty studied, only the IMWG has been assessed as a predictor of toxicity of therapy. The next step in improving the clinical utility of these scores will be to examine the ability of these indices to discriminate risk of toxicity in order to optimize treatment, and help patients live as long as possible with the best quality of life. We are unable to assess the utility of these models in predicting toxicity in our cohort, as toxicity was not prospectively ascertained during the study period. The variations between these three models need to be reviewed since interest in the concept of frailty is increasing and it is being applied in clinical trials. A strategy examining outcomes myeloma treatment stratified based on frailty is being tested in the Myeloma Research Council Myeloma XIV (FiTNEss trial) [NCT03720041], which will employ the IMWG frailty model. Measures of frailty should be valid, reliable, and associated with outcomes of importance to older adults, including not only toxicity of therapy, but maintenance of independence and cognitive function [33].
Clinicians should also be aware that the accuracy of prognostic scores and their relevance in clinical practice reflect nuances of the methods used to develop the measure and the populations in which the indices were developed. Components of prognostic scores should be readily available in real-world practice. For example, the original R-MCI study methods utilized the Fried frailty variable, which requires grip strength to categorize weakness; when disseminated with an online tool at www.myelomacomorbidityindex.org, this component has been approximated with a frailty variable that includes Karnofsky Performances Status, Timed Up and Go, Instrumental Activities of Daily Living, and a subjective assessment of fitness. Each of the indices included slightly different patient populations: the R-MCI included patients across the age spectrum, the IMWG development cohort included only clinical trial participants, and the CFI included patients at different places in their cancer journey. Each of these factors may have influenced the model development, underscoring the need for external validation of each.
Geriatric assessment spans domains including functional status, comorbidities, medications, depression, cognition, falls and objective measures of physical function. The IMWG and R-MCI encompass function and comorbidities, while the CFI includes other domains of geriatric assessment, including sensory impairment (hearing/vision), mental health, medication, cognition and an objective measure of physical function. The IMWG and R-MCI models retain age as one of their components, raising the question whether age serves as a surrogate for other aging-associated vulnerabilities. Cognition and physical performance are two potential factors of frailty that are not included in the IMWG or R-MCI models. In a geriatric assessment used to evaluate patients with chronic lymphocytic leukemia (CLL), the Timed Up & Go and the DEMTECT (a test of cognition) are objective scales that predict hospitalizations and OS [34]. In acute myelogenous leukemia, cognitive impairment and poorer performance on the Short Physical Performance Battery predict OS [35]. The use of additional aging-associated vulnerabilities, including cognition and objective measures of function in frailty measures in myeloma may add to existing models and allow further discrimination of older adults with myeloma at risk for poorer outcomes. While most of these prognostic scores were tested for overall survival, outcomes unrelated to survival that older adults value, such as quality of life and disabilities, await examination [12].
Limitations of this study include the fact that over half of the participants had received over 3 weeks of treatment prior to completing the geriatric assessment. This allowance was made in the eligibility criteria to allow participants who needed urgent initiation of therapy to participate. We found no difference in the prevalence of frailty between those who had started therapy and those who had not. Similarly, there was difference in treatment discontinuation due to toxicity between those who had started therapy and those who had not, reassuring us that the geriatric assessment was reflective of baseline function rather than toxicity; however, we cannot evaluate whether lower grade toxicity was present, impacting geriatric assessment findings. Another limitation was that follow-up was immature; only 9 deaths had occurred at the time of the analysis. This, coupled with small sample size, precludes accurate characterization of survival by frailty status.
In summary, in a prospective cohort of 40 older adults with newly diagnosed multiple myeloma, we found that different approaches to operationalizing the concept of frailty will result in a different classification for an individual patient. Our findings highlight the differences in currently available approaches to applying the concept of frailty to older adults with cancer. This problem is not unique to oncology, as there is a continued lack of consensus on defining the concept of frailty in the general geriatric population [11]. Further studies are needed to establish the role of frailty indices in predicting toxicity of therapy and other outcomes of importance in older adults with multiple myeloma.
Supplementary Material
Acknowledgements:
This publication was made possible by Grant Number K12CA167540 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH), Grant Number R03 AG042374 through the National Institute of Aging and Grant Number UL1 TR000448 through the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCI, NCATS or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Abramson N, Shattil SJ. M-Components. JAMA J Am Med Assoc. 1973; 223: 156–159. [PubMed] [Google Scholar]
- 2.Palumbo A, Anderson K. Medical Progress Multiple Myeloma. N Engl J Med. 2011; 364: 1046–1060. [DOI] [PubMed] [Google Scholar]
- 3.Bringhen S, Mateos MV, Zweegman S, Larocca A, Falcone AP, Oriol A, et al. Age and organ damage correlate with poor survival in myeloma patients: Meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica. 2013; 98: 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenhoff S, Hjorth M, Westin J, Brinch L, Bäckström B, Carlson K, et al. Impact of age on survival after intensive therapy for multiple myeloma: A population-based study by the Nordic Myeloma Study Group. Br J Haematol. 2006; 133: 389–396. [DOI] [PubMed] [Google Scholar]
- 5.Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, et al. Continuous Lenalidomide Treatment for Newly Diagnosed Multiple Myeloma. N Engl J Med. 2012; 366: 1759–1769. [DOI] [PubMed] [Google Scholar]
- 6.Palumbo A, Waage A, Hulin C, Beksac M, Zweegman S, Gay F, et al. Safety of thalidomide in newly diagnosed elderly myeloma patients: A meta-analysis of data from individual patients in six randomized trials. Haematologica. 2013; 98: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus Melphalan and Prednisone for Initial Treatment of Multiple Myeloma. N Engl J Med. 2008; 359: 906–917. [DOI] [PubMed] [Google Scholar]
- 8.Miguel JFS, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma. J Clin Oncol. 2013; 31: 448–455. [DOI] [PubMed] [Google Scholar]
- 9.Schaapveld M, Visser O, Siesling S, Schaar CG, Zweegman S, Vellenga E. Improved survival among younger but not among older patients with Multiple Myeloma in the Netherlands, a population-based study since 1989. Eur J Cancer. 2010; 46: 160–169. [DOI] [PubMed] [Google Scholar]
- 10.Mccarthy AL, Peel NM, Gillespie KM, Berry R, Walpole E, Yates P, et al. Validation of a frailty index in older cancer patients with solid tumours. BMC Cancer. 2018; 18: 892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, et al. Searching for an operational definition of frailty: A delphi method based consensus statement. the frailty operative definition-consensus conference project. Journals Gerontol - Ser A Biol Sci Med Sci. 2013; 68: 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mian HS, Wildes TM, Fiala MA. Development of a Medicare Health Outcomes Survey Deficit-Accumulation Frailty Index and Its Application to Older Patients With Newly Diagnosed Multiple Myeloma. JCO Clin Cancer Informatics. 2018; 2: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in Older Adults: Evidence for a Phenotype. Journals Gerontol Ser A Biol Sci Med Sci. 2001; 56: M146–156. [DOI] [PubMed] [Google Scholar]
- 14.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005; 173: 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buta BJ, Walston JD, Godino JG, Park M, Kalyani RR, Xue QL, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Research Reviews. 2016; 26: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palumbo A, Bringhen S, Mateos MV, Larocca A, Facon T, Kumar SK, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood. 2015; 125: 2068–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelhardt M, Domm AS, Dold SM, Ihorst G, Reinhardt H, Zober A, et al. A concise revised myeloma comorbidity index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica. 2017; 102: 910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerard EJ, Deal AM, Chang Y, Williams GR, Nyrop KA, Pergolotti M, et al. Frailty index developed from a cancer-specific geriatric assessment and the association with mortality among older adults with cancer. JNCCN J Natl Compr Cancer Netw. 2017; 15: 894–902. [DOI] [PubMed] [Google Scholar]
- 19.Wildes TM, Tuchman SA, Klepin HD, Mikhael J, Trinkaus K, Stockerl-Goldstein K, et al. (2018). Geriatric Assessment in Older Adults with Multiple Myeloma. Journal of the American Geriatrics Society. 10.1111/jgs.15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. In: Journal of Clinical Oncology. 2011; 29: 3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart A, Sherbourne CD, Hays RD, Wells KB, Nelson EC, Kamberg C, et al. Summary and Discussion of MOS Measures. In: Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. 1992; 345–371. [Google Scholar]
- 22.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. Journals Gerontol. 1981; 37: 428–434. [DOI] [PubMed] [Google Scholar]
- 23.Kawas C, Karagiozis H, Resau L, Corrada M, Brookmeyer R. Reliability of the Blessed Telephone Information-Memory-Concentration Test. J Geriatr Psychiatry Neurol. 1995; 8: 238–242. [DOI] [PubMed] [Google Scholar]
- 24.Podsiadlo D The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J Am Geriatr Soc. 1991; 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 25.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991; 32: 705–714. [DOI] [PubMed] [Google Scholar]
- 26.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 27.Calhoun EA, Welshman EE, Chang CH, Lurain JR, Fishman DA, Hunt TL, et al. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group - Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer. 2003; 13: 741–748. [DOI] [PubMed] [Google Scholar]
- 28.Greipp PR, Miguel JS, Dune BGM, Crowley JJ, Barlogje B, Bladé J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005; 23: 3412–3420. [DOI] [PubMed] [Google Scholar]
- 29.http://www.myelomacomorbidityindex.org/ Engelhardt M, Dold SM, Ihorst G, Knaus J, Schumacher M. R-MCI webpage [Internet]. 2015. [accessed 4 September 2018]
- 30.Murillo A, Cronin AM, Laubach JP, Hshieh TT, Tanasijevic AM, Richardson PG, et al. Performance of the International Myeloma Working Group myeloma frailty score among patients 75 and older (2018). Journal of Geriatric Oncology. 10.1016/jgo.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Engelhardt M, Dold SM, Ihorst G, Zober A, Möller M, Reinhardt H, et al. Geriatric assessment in multiple myeloma patients: Validation of the international Myeloma Working Group (IMWG) score and comparison with other common comorbidity scores. Haematologica. 2016; 101: 1110–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bataille R, Annweiler C, Beauchet O. Multiple myeloma international staging system: “staging” or simply “aging” system? Clinical Lymphoma, Myeloma and Leukemia. 2013; 13: 635–637. [DOI] [PubMed] [Google Scholar]
- 33.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the Treatment Preferences of Seriously Ill Patients. N Engl J Med. 2002; 346: 1061–1066. [DOI] [PubMed] [Google Scholar]
- 34.Goede V, Bahlo J, Chataline V, Eichhorst B, Dürig J, Stilgenbauer S, et al. Evaluation of geriatric assessment in patients with chronic lymphocytic leukemia: Results of the CLL9 trial of the German CLL study group. Leuk Lymphoma. 2016; 57: 789–796. [DOI] [PubMed] [Google Scholar]
- 35.Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Pardee TS, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121:4287–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


