Abstract
Background
Antiretrovirals, including tenofovir, can suppress human immunodeficiency virus (HIV) infection but cannot completely eradicate it. Patients with HIV infection are administered antiretroviral drugs over a long term; thus, managing consequent adverse drug reactions, such as renal dysfunction and bone mineral loss, is important. Currently, highly sensitive biomarkers that can detect adverse drug reactions early have not been well studied.
Methods
This single-center, prospective, observational study explored changes in the biomarkers of renal function, bone metabolism, and lipid profile before and after switching from tenofovir disoproxil fumarate (TDF) to tenofovir alafenamide (TAF) in patients with HIV infection.
Results
All 31 enrolled patients had been treated with antiretrovirals for more than 5 years. The rate of proteinuria decreased significantly after starting TAF-containing antiretroviral regimen. The urinary liver-type fatty acid binding protein (L-FABP)/creatinine ratio was significantly decreased at 3 and 6 months after switching to TAF compared with that before switching to TAF (− 0.5 μg/g Cr at 3 months, and − 0.8 μg/g Cr at 6 months; p < 005 for both at 3 and 6 months). The urinary N-terminal telopeptide (NTx)/creatinine ratio decreased over the study period, and the ratios were significantly different between 3 and 6 months (− 11 nmol/mmol Cr at 3 months, − 15.2 nmol/mmol Cr at 6 months; p = 0.0069 at 3 months, p < 0.0001 at 6 months). Low density lipoprotein-cholesterol level significantly increased at 3 (+ 26 mg/dL) and 6 months (+ 13 mg/dL) compared with that at the baseline (p < 0.0001).
Conclusions
Switching from TDF to TAF decreased the levels of renal and bone biomarkers, such as urinary L-FABP and NTx, but increased low density lipoprotein-cholesterol levels. Future studies should evaluate if these biomarkers, such as urinary L-FABP and NTx, truly detect serious adverse drug reactions early.
Keywords: HIV-infection, Antiretrovirals, Biomarkers, Tenofovir disoproxil fumarate, Tenofovir alafenamide
Background
Antiretrovirals (ARVs) have been successfully used to suppress human immunodeficiency virus (HIV) infection, and the life expectancy of the HIV-infected patients treated with ARVs has considerably increased, comparable to that of HIV-uninfected patients [1]. However, ARVs cannot completely eradicate HIV; therefore, lifelong therapy with ARVs is still required.
Long-term therapy with ARVs can cause adverse drug effects. Tenofovir disoproxil fumarate (TDF) was used since 2001 in the world and since 2004 in Japan. TDF has recommended as a part of initial regimens for most treatment naïve patients [2]. The adverse drug effects commonly caused by TDF include renal dysfunction and bone density loss [3]. Tenofovir alafenamide (TAF), a prodrug of tenofovir, was approved in Japan in 2017. TAF has improved permeability into the lympho-reticular cells and reduces the systemic concentration of tenofovir by 10% compared to TDF [4]. Clinical trials have shown that switching from TDF to TAF has improved the estimated glomerular filtration rate (eGFR) and levels of creatinine, urinary protein, and urinary biomarkers such as retinol-binding protein (RBP) [5, 6]. A pooled analysis of 26 clinical trials have also supported the improved renal safety of TAF compared to TDF [7].
Although switching to TAF from TDF showed renal improvement with potent viral suppression, highly sensitive biomarkers that indicate these subclinical changes after switching to TAF are not well-identified.
The urinary liver-type fatty acid binding protein (L-FABP) is expressed in the human proximal tubules, and its excretion via urine reflects tubulointerstitial damage [8]. L-FABP could be a prognostic biomarker for the development of renal dysfunction in HIV-infected patients [9].
Among the biomarkers of bone metabolism, bone-specific alkaline phosphatase (BAP) is a glycoprotein on the surface of osteoblasts that is released during bone formation. The N-terminal fragments of telopeptide (NTx) of type 1 collagen after degradation of the bone matrix is considered a resorption marker [10]. These markers have been evaluated in several studies [11–13] in patients on a TDF-containing regimen of antiretrovirals.
In this study, we aimed to explore the changing trends in biomarkers of renal function and bone metabolism, and lipid profile while switching from TDF- to TAF-containing regimen in HIV-infected patients.
Methods
Patients and setting
This observational study was conducted prospectively at the University of Tokyo Hospital, a 1217-bed tertiary-care teaching hospital in Tokyo, Japan. The study was approved by the research ethics committee of the Faculty of Medicine of the University of Tokyo. All the patients have provided written informed consent.
HIV-infected adult (20 years old and more) patients were enrolled in the study from July 2017 to June 2018. The enrollment criteria were as follows: (1) patients who had already received TDF-containing antiretroviral regimen for at least a year, (2) patients who could visit the outpatient clinic, and undergo blood and urine tests at least once every 3 months, and (3) if the physician had determined that the patients preferred TAF over TDF to be concerned about possible adverse drug reactions such as renal dysfunction and bone mineral loss for long treatment.
The study period of all the patients was for 6 months. Additional urine samples, and venous blood samples were collected for testing in a commercial laboratory during the study period.
Data collection and selection
The collected patient data included age, sex, body weight, height, underlying disease (diabetes mellitus, hypertension, hyperlipidemia, chronic kidney disease (CKD), chronic hepatitis C virus infection, and chronic/history of hepatitis B virus infection), a history of acquired immunodeficiency syndrome (AIDS), and the prescribed antiretroviral regimen by medical chart review. Chronic kidney disease was defined as eGFR < 60 mL/min/1.73 m2 during the study period or previous diagnosis by their respective doctors. Body mass index was calculated as weight in kilograms divided by the square of height in meters: weight (kg)/height (m)2.
The following laboratory data were reviewed using the medical charts: CD4+ lymphocyte count, plasma HIV RNA level, complete blood count, serum phosphate level, low and high density lipoprotein cholesterol levels, triglyceride (TG) level, urinalysis, and serum creatinine level. Glomerular filtration rate (GFR) was calculated with serum creatinine and cystatin C levels.
In addition, serum and urine samples were tested for the following in a commercial laboratory (LSI Medience Corporation): urinary liver-type fatty acid binding protein level (U-L-FABP; latex agglutination immunoturbidimetric assay), urinary phosphate level, urinary N-acetyl-beta-d-glucosaminidase (U-NAG), cystatin-C (latex agglutination turbidimetry), urinary N-terminal telopeptide (U-NTx; enzyme immunoassay), and serum BAP (chemiluminescent enzyme immunoassay). Using this data the following creatinine ratios were calculated: U-L-FABP/creatinine (Cr), U-NAG/Cr, and U-NTx/Cr. To assess renal proximal tubular dysfunction, the rate of tubular reabsorption of phosphate (percent tubular reabsorption of phosphate; %TRP) was calculated according to the following formula:
Proteinuria was defined by a value of ≥ 1+ by dipstick urinalysis.
Statistical analysis
The Wilcoxon signed-rank test was performed using the JMP Pro software version 14.2.0 (SAS institute Inc., Cary, NC, USA) to analyze trends of biomarkers before and after switching from TDF to TAF. The significance level of the two-tailed test was set at 0.05.
Results
Thirty-one patients were enrolled in our study. The patients’ characteristics are shown in Table 1. All the patients were men, and the median age was 45 years. The average BMI was 23.6, and 5 patients had low body weight (< 55 kg). Thirty patients were Japanese, and one was an African. All the patients had been treated with ARVs for more than 5 years. Half of the TDF-containing antiretroviral regimens also consisted of integrase inhibitors, such as dolutegravir and raltegravir. Among all the ARVs, only TDF was changed during the study period. Two patients in the study had baseline CKD. Eleven patients were diagnosed with hyperlipidemia, among which, two of them received statin treatment before switching to TAF.
Table 1.
Patients’ characteristics
| Patients’ characteristics | |||
|---|---|---|---|
| Age: median (range) | 45 (33–73) | Comorbidity | |
| Sex: male:female | 31:0 | Chronic kidney disease | 2 |
| BMI: average (range) | 23. 6 (16.5–36.1) | Diabetes mellitus | 2 |
| History of AIDS | 13 | Hypertension | 5 |
| PCP | 5 | Hyperlipidemia | 11 |
| Lymphoma | 2 | HCV infection | 3 |
| CMV retinitis | 2 | HBV infection | 20 |
| Candida esophagitis | 2 | Antiretrovirals with tenofovir | |
| Kaposi’s sarcoma | 1 | DRV/r | 10 |
| Disseminated MAC | 1 | RAL | 8 |
| Cryptococcus meningitis | 1 | DTG | 8 |
| Duration of ART | EFV | 4 | |
| Less than 5 years | 11 | FPV/r | 1 |
| 5–10 years | 19 | ||
| More than 10 years | 1 | ||
| Nadir CD4+ T-cell count, (cells/μL) | |||
| Less than 100 | 15 | ||
| 100–199 | 6 | ||
| 200–499 | 9 | ||
| Unknown | 1 | ||
BMI body mass index [calculated as weight in kilograms divided by the square of height in meters: weight (kg)/height (m2)], AIDS acquired immunodeficiency syndrome, PCP Pneumocystis pneumonia, CMV cytomegalovirus, MAC Mycobacterium avium complex, HCV hepatitis C virus, HBV hepatitis B virus, DRV/r ritonavir-boosted darunavir, RAL raltegravir, DTG dolutegravir, EFV efavirenz; FPV/r ritonavir-boosted fosamprenavir, ART antiretroviral therapy
There was no significant change in CD4+ T cell count, and a rate of successful viral suppression was maintained. The median CD4+ T cell counts were 448, 385, and 442 cells/µL at 0, 3, and 6 months, respectively, after switching from TDF to TAF. The rates of HIV RNA suppression (less than 100 copies/mL) were 93.5%, 90.3%, and 100% at 0, 3, and 6 months, respectively. All patients remained on ART during the study period. No events of bone fractures, acute coronary syndrome, and renal failure occurred.
Biomarkers of renal function
The biomarkers of renal function such as the eGFR values calculated using creatinine, and with cystatin-C did not show any statistical change during the study period (Table 2). The rate of proteinuria decreased significantly after starting TAF-containing antiretroviral regimen.
Table 2.
Analysis of serum and urine biomarkers before and after changing to tenofovir alafenamide among 31 patients
| Median | Duration after switching | p value | |||
|---|---|---|---|---|---|
| 0 months | 3 months | 6 months | 3 months | 6 months | |
| Serum creatinine (mg/dL) | 0.89 (0.68–1.16) | 0.90 (0.66–1.11) | 0.89 (0.6–1.25) | 0.179 | 0.098 |
| Estimated GFR using creatinine | 73.1 (50.1–108.4) | 72.6 (53.2–110.2) | 74.8 (47.2–123.3) | 0.238 | 0.098 |
| Serum cystatin C (mg/L) | 0.88 (0.65–1.39) | 0.87 (0.64–1.26) | 0.88 (0.62–1.26) | 0.942 | 0.807 |
| Estimated GFR using cystatin C | 91.2 (50.2–130.5) | 91.5 (56.4–131.6) | 92.3 (56.4–136.2) | 0.833 | 0.226 |
| Urine-NAG/urine-creatinine (U/g Cr) | 3.72 (0–56.9) | 5.31 (0.83–28.6) | 3.93 (0–29.4) | 0.723 | 0.739 |
| Urine-L-FABP/urine-creatinine (μg/g Cr) | 2.3 (0.6–63.9) | 1.8 (0.6–31.2) | 1.5 (0.3–15) | 0.0166* | 0.0002* |
| %TRP | 85.1 (73.7–92.7) | 87.7 (73.6–97.7) | 86.9 (74.2–94.2) | 0.026* | 0.200 |
| Serum inorganic phosphorus (mg/dL) | 3.1 (2.5–4.1) | 3.0 (2.1–4.3) | 3.1 (2.2–4.7) | 0.992 | 0.841 |
| Proteinuria (%) | 13.3 | 6.67 | 6.67 | 0.023* | 0.023* |
| Urine occult blood: positive (%) | 6.7 | 6.7 | 10 | 0.573 | 0.973 |
| Serum LDL-cholesterol (mg/dL) | 106 (67–159) | 132 (84–199) | 119 (82–207) | < 0.0001* | < 0.0001* |
| Serum triglyceride (mg/dL) | 144 (41–874) | 146 (47–700) | 175 (43–669) | 0.192 | 0.273 |
| Serum BAP (μg/L) | 10.8 (7.0–21.6) | 11.7 (6.2–21.1) | 11.0 (7.4–19.8) | 0.894 | 0.150 |
| Urine NTx/urine Cr (nmol/mmol Cr) | 48 (27.4–84.9) | 37 (20.2–83.4) | 32.8 (14.7–67.4) | 0.0034* | < 0.0001* |
GFR glomerular filtration rate, NAG N-acetyl-beta-d-glucosaminidase, L-FABP liver-type fatty acid binding protein, TRP tubular reabsorption of phosphate, LDL low density lipoprotein, BAP bone-specific alkaline phosphatase, NTx N-terminal fragments of telopeptide
*Urinalysis data were obtained from 30 of the 31 patients
U-L-FABP/Cr ratios had significantly decreased at 3 and 6 months after switching to TAF, compared to the value before switching to TAF, as shown in Fig. 1a) (− 0.5 μg/g Cr at 3 months, and − 0.8 μg/g Cr at 6 months; p = 0.0166 at 3 months, p = 0.0002 at 6 months). U-NAG/Cr ratios did not show any statistical change during the study period.
Fig. 1.
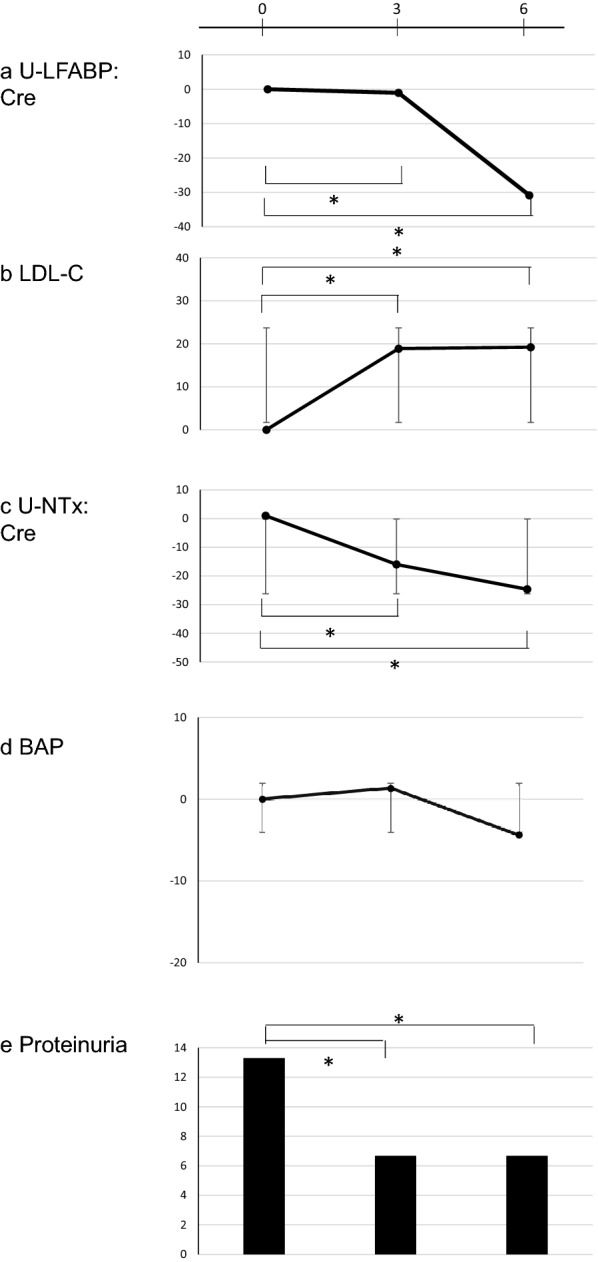
Rate of change in biomarkers before and after switching from tenofovir disoproxil to tenofovir alafenamide. Vertical axis represents the rate of change (%) of each biomarker compared to the baseline (before switching antiretrovirals). a Rate of change in urine-liver-type fatty acid binding protein/creatinine (L-FABP/Cr) levels. b Rate of change in low-density lipoprotein cholesterol (LDL-C) levels. c Rate of change in urine-N-terminal telopeptide/creatinine (U-NTx/Cr) levels. d Rate of change in bone-specific alkaline phosphatase (BAP) levels. e Percentage of detection of proteinuria
The value of %TRP had significantly increased at 3 months (p = 0.026), but the rate of change in each patient was low.
Lipid profile and biomarkers of bone metabolism
Low density lipoprotein-cholesterol (LDL-C) levels significantly increased at 3 months and 6 months compared to the baseline [+ 26 mg/dL at 3 months, and + 13 mg/dL at 6 months; p < 0.0001) (Fig. 1b)]. The significance was maintained regardless of co-administration with other ARVs, or hyperlipidemia at baseline.
BAP levels had not changed during the study period. The U-NTx/Cr ratios decreased during the study period, and there were statistical significances between all the points [− 11 nmol/mmol Cr at 3 months, − 15.2 nmol/mmol Cr at 6 months; p = 0.0069 at 3 months, p < 0.0001 at 6 months) (Fig. 1c)].
Discussion
In this study, we reported the changes in biomarkers of renal function and bone metabolism, and lipid profile when switching from TDF to TAF in HIV-infected patients. During the study, all the patients continued the TAF-containing regimen of ARVs that showed potent viral suppression. There were no serious adverse effects in the first 6 months after switching to TAF-containing regimen.
There were no noticeable changes in the eGFR calculated using creatinine levels, and cystatin-C levels during the study period. The L-FABP levels and the rate of proteinuria were significantly decreased. These results might be subclinical for majority of the participants without having CKD. However, it has been reported that the renal function in Japanese HIV-infected patients decreased depending on the duration of treatment with TDF, especially in patients with low body weight [14]. Another observational study of a 12-year period also showed that decreased eGFR occurred after 3 months of TDF-containing regimen of ARVs, which was also related to low body weight [15].
Proteinuria is commonly observed in HIV-infected patients [16]. Its risk factors are exposure to TDF, older age, low CD4+ T-cell counts, anti-hepatitis C virus antibodies, and race [16–18]. Because the patients in our study were not older age and have already acquired high CD4+ T-cell counts by treatment including TDF on enrollment, major reason of the improvement of proteinuria might be discontinuation of TDF exposure. As Proteinuria itself is one of risk factors for CKD [19] and fragility fracture [17] in HIV-infected patients, early detection of proteinuria could motivate switching ARVs.
Because L-FABP was reported as a potential marker of CKD for patients without albuminuria [20], the detection of subclinical changes using urinary L-FABP prior to the progression to CKD is important in patients taking TAF, especially in those with low body weight.
%TRP change was observed at 3 months but not at 6 months. %TRP was a marker of renal proximal tubular dysfunction and calculated as value of one minus fractional excretion of phosphate. The fractional excretion of phosphate was reported as a possible marker of end-stage renal diseases such as dialysis in moderate to advanced CKD patients [21]. However, %TRP did not showed continuous change at 6 months after switching to TAF in our study. This result might because the difference of the patients’ background.
The elevation of biomarkers of lipids such as LDL-C was observed to have significance. Previous studies have also shown the elevation in the LDL-C and TG levels, but the range of elevation depended on the observational period, and the characteristics of the participants [22–24]. One of the major risks for hyperlipidemia that was identified after switching to TAF was the elevation at beginning in TG or LDL-C levels [25] although opposite results also reported [26]. In our study, LDL-C levels increased regardless of the hyperlipidemia at baseline. This risk population, that needs lipid-lowering drugs after switching TAF from TDF, must be extensively studied in the future.
Among the biomarkers of bone metabolism, U-NTx significantly decreased in our study. Previous study showed TDF-sparing regimen decreased the level of biomarkers of bone metabolism such as osteocalcin and bone alkaline phosphatase [13], and switching from TDF to TAF also improved bone mineral density [27]. Although it is unclear whether tenofovir directly influences osteoblasts, a change in gene expression was observed with tenofovir exposure in vitro [28]. Because U-NTx levels related bone mineral density levels in older men and women [29], U-NTx could also be used to predict current osteoporosis in TDF-exposure patients.
Here, the renal function and bone metabolism had improved within 6 months after switching to TAF. However, attention must be drawn to whether the lipid metabolism could be elevated up to the level that needs statin treatment.
According to patients’ genetic background [30, 31] the presence of various comorbidities, and body weight [14], clinicians might be required to determine whether to continue TDF or switch to TAF to prevent the progression to renal tubular dysfunction or metabolic disorders, using biomarkers such as urinary L-FABP and NTx. Its tailor-made design would help lives without disabilities of HIV-infected patients on ARVs.
Nevertheless, there were some limitations to our study. First, the information on the changes in lifestyle, such as new exercise habits and dietary patterns, were not collected using a questionnaire, although the treating physicians had recorded the changes in lifestyle and adherence to ARVs during each visit and no other drugs was changed. Second, the study did not have long observational period. Finally, this study was a single-centered observational study, and all except one was Japanese.
Conclusions
The TAF-containing regimen of ARVs could be safe with potent viral suppression for 6 months after switching from a TDF-containing regimen of ARVs. Switching from TDF to TAF decreased the levels of biomarkers such as urinary L-FABP and NTx but increased LDL-C levels. Future studies should evaluate if these biomarkers truly detect serious adverse drug reactions early.
Acknowledgements
Not applicable.
Abbreviations
- HIV
Human immunodeficiency virus
- ARV
Antiretrovirals
- TDF
Tenofovir disoproxil fumarate
- TAF
Tenofovir alafenamide
- eGFR
Estimated glomerular filtration rate
- RBP
Retinol-binding protein
- L-FABP
Liver-type fatty acid binding protein
- BAP
Bone-specific alkaline phosphatase
- NTx
N-terminal fragments of telopeptide
- CKD
Chronic kidney disease
- AIDS
Acquired immunodeficiency syndrome
- TG
Triglyceride
- CrCl
Creatinine clearance
- NAG
N-Acetyl-beta-d-glucosaminidase
- TRP
Tubular reabsorption of phosphate
- BMI
Body mass index
- LDL-C
Low density lipoprotein-cholesterol
- PCP
Pneumocystis pneumonia
- CMV
Cytomegalovirus
- MAC
Mycobacterium avium complex
- HCV
Hepatitis C virus
- HBV
Hepatitis B virus
- DRV/r
Ritonavir-boosted darunavir
- RAL
Raltegravir
- DTG
Dolutegravir
- EFV
Efavirenz
- FPV/r
Ritonavir-boosted fosamprenavir
- ART
Antiretroviral therapy
Authors’ contributions
MI designed this study, acquired and analyzed the patients’ data and samples, and was a major contributor in writing the manuscript. YW and SO enrolled the patients in this study and collected the samples. KO, SY, SO, and KM revised the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the research ethics committee of the Faculty of Medicine of the University of Tokyo. All the patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mahoko Ikeda, Email: mhk-ikeda@umin.ac.jp.
Yoshitaka Wakabayashi, Email: wakabayashi-tky@umin.ac.jp.
Koh Okamoto, Email: kokamoto-tky@umin.ac.jp.
Shintaro Yanagimoto, Email: yanagimoto@hc.u-tokyo.ac.jp.
Shu Okugawa, Email: okugawa-tky@umin.ac.jp.
Kyoji Moriya, Email: moriya-tky@umin.org.
References
- 1.Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS. 2016;11(5):492–500. doi: 10.1097/COH.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. https://clinicalinfo.hiv.gov/sites/default/files/inline-files/AdultandAdolescentGL.pdf. Accessed 1 Dec 2020.
- 3.Casado JL. Renal and bone toxicity with the use of tenofovir: understanding at the end. AIDS Rev. 2016;18(2):59–68. [PubMed] [Google Scholar]
- 4.Ruane PJ, DeJesus E, Berger D, Markowitz M, Bredeek UF, Callebaut C, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr. 2013;63(4):449–455. doi: 10.1097/QAI.0b013e3182965d45. [DOI] [PubMed] [Google Scholar]
- 5.Gallant JE, Daar ES, Raffi F, Brinson C, Ruane P, DeJesus E, et al. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: a randomised, double-blind, active-controlled phase 3 trial. Lancet HIV. 2016;3(4):e158–e165. doi: 10.1016/S2352-3018(16)00024-2. [DOI] [PubMed] [Google Scholar]
- 6.Mills A, Arribas JR, Andrade-Villanueva J, DiPerri G, Van Lunzen J, Koenig E, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16(1):43–52. doi: 10.1016/S1473-3099(15)00348-5. [DOI] [PubMed] [Google Scholar]
- 7.Gupta SK, Post FA, Arribas JR, Eron JJ, Jr, Wohl DA, Clarke AE, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33(9):1455–1465. doi: 10.1097/QAD.0000000000002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoyama T, Kamijo-Ikemori A, Sugaya T, Hoshino S, Yasuda T, Kimura K. Urinary excretion of liver type fatty acid binding protein accurately reflects the degree of tubulointerstitial damage. Am J Pathol. 2009;174(6):2096–2106. doi: 10.2353/ajpath.2009.080780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hikasa S, Shimabukuro S, Hideta K, Higasa S, Sawada A, Tokugawa T, et al. Urinary liver-type fatty acid-binding protein levels as a potential risk factor for renal dysfunction in male HIV-infected Japanese patients receiving antiretroviral therapy: a pilot study. Int J STD AIDS. 2018;29(14):1424–1431. doi: 10.1177/0956462418788432. [DOI] [PubMed] [Google Scholar]
- 10.Bieglmayer C, Dimai HP, Gasser RW, Kudlacek S, Obermayer-Pietsch B, Woloszczuk W, et al. Biomarkers of bone turnover in diagnosis and therapy of osteoporosis: a consensus advice from an Austrian working group. Wien Med Wochenschr. 2012;162(21–22):464–477. doi: 10.1007/s10354-012-0133-9. [DOI] [PubMed] [Google Scholar]
- 11.Stellbrink HJ, Orkin C, Arribas JR, Compston J, Gerstoft J, Van Wijngaerden E, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51(8):963–972. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 12.Calza L, Magistrelli E, Colangeli V, Borderi M, Conti M, Mancini R, et al. Improvement in renal function and bone mineral density after a switch from tenofovir/emtricitabine plus ritonavir-boosted protease inhibitor to raltegravir plus nevirapine: a pilot study. Antivir Ther. 2016;21(3):217–224. doi: 10.3851/IMP2995. [DOI] [PubMed] [Google Scholar]
- 13.Bloch M, Tong WW, Hoy J, Baker D, Lee FJ, Richardson R, et al. Switch from tenofovir to raltegravir increases low bone mineral density and decreases markers of bone turnover over 48 weeks. HIV Med. 2014;15(6):373–380. doi: 10.1111/hiv.12123. [DOI] [PubMed] [Google Scholar]
- 14.Nishijima T, Kawasaki Y, Tanaka N, Mizushima D, Aoki T, Watanabe K, et al. Long-term exposure to tenofovir continuously decrease renal function in HIV-1-infected patients with low body weight: results from 10 years of follow-up. AIDS. 2014;28(13):1903–1910. doi: 10.1097/QAD.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki S, Nishijima T, Kawasaki Y, Kurosawa T, Mutoh Y, Kikuchi Y, et al. Effect of tenofovir disoproxil fumarate on incidence of chronic kidney disease and rate of estimated glomerular filtration rate decrement in HIV-1-infected treatment-naive Asian patients: results from 12-year observational cohort. AIDS Patient Care STDS. 2017;31(3):105–112. doi: 10.1089/apc.2016.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeder AJ, Hilge R, Schrader S, Bogner JR, Seybold U. Medium-grade tubular proteinuria is common in HIV-positive patients and specifically associated with exposure to tenofovir disoproxil fumarate. Infection. 2016;44(5):641–649. doi: 10.1007/s15010-016-0911-1. [DOI] [PubMed] [Google Scholar]
- 17.Gonciulea A, Wang R, Althoff KN, Estrella MM, Sellmeyer DE, Palella FJ, et al. Proteinuria is associated with increased risk of fragility fracture in men with or at risk of HIV infection. J Acquir Immune Defic Syndr. 2019;81(3):e85–e91. doi: 10.1097/QAI.0000000000002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee T, Scherzer R, Powe NR, Steffick D, Shahinian V, Saran R, et al. Race and other risk factors for incident proteinuria in a national cohort of HIV-infected veterans. J Acquir Immune Defic Syndr. 2014;67(2):145–152. doi: 10.1097/QAI.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schrader SY, Zeder AJ, Hilge R, Bogner JR, Seybold U. Medium-grade proteinuria is a risk factor for incident markers of chronic kidney disease. HIV Med. 2020;21(8):481–491. doi: 10.1111/hiv.12881. [DOI] [PubMed] [Google Scholar]
- 20.Khatir DS, Bendtsen MD, Birn H, Norregaard R, Ivarsen P, Jespersen B, et al. Urine liver fatty acid binding protein and chronic kidney disease progression. Scand J Clin Lab Invest. 2017;77(7):549–554. doi: 10.1080/00365513.2017.1355561. [DOI] [PubMed] [Google Scholar]
- 21.Bellasi A, Di Micco L, Russo D, De Simone E, Di Iorio M, Vigilante R, et al. Fractional excretion of phosphate (FeP) is associated with end-stage renal disease patients with CKD 3b and 5. J Clin Med. 2019;8(7):1026. doi: 10.3390/jcm8071026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kauppinen KJ, Kivela P, Sutinen J. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide significantly worsens the lipid profile in a real-world setting. AIDS Patient Care STDS. 2019;33(12):500–506. doi: 10.1089/apc.2019.0236. [DOI] [PubMed] [Google Scholar]
- 23.Schwarze-Zander C, Piduhn H, Boesecke C, Schlabe S, Stoffel-Wagner B, Wasmuth JC, et al. Switching tenofovir disoproxil fumarate to tenofovir alafenamide in a real life setting: what are the implications? HIV Med. 2020;21(6):378–385. doi: 10.1111/hiv.12840. [DOI] [PubMed] [Google Scholar]
- 24.Huhn GD, Shamblaw DJ, Baril JG, Hsue PY, Mills BL, Nguyen-Cleary T, et al. Atherosclerotic cardiovascular disease risk profile of tenofovir alafenamide versus tenofovir disoproxil fumarate. Open Forum Infect Dis. 2020;7(1):ofz472. doi: 10.1093/ofid/ofz472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacey A, Savinelli S, Barco EA, Macken A, Cotter AG, Sheehan G, et al. Investigating the effect of antiretroviral switch to tenofovir alafenamide on lipid profiles in people living with HIV. AIDS. 2020;34(8):1161–1170. doi: 10.1097/QAD.0000000000002541. [DOI] [PubMed] [Google Scholar]
- 26.Taramasso L, Di Biagio A, Riccardi N, Briano F, Di Filippo E, Comi L, et al. Lipid profile changings after switching from rilpivirine/tenofovir disoproxil fumarate/emtricitabine to rilpivirine/tenofovir alafenamide/emtricitabine: different effects in patients with or without baseline hypercholesterolemia. PLoS ONE. 2019;14(10):e0223181. doi: 10.1371/journal.pone.0223181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagins D, Orkin C, Daar ES, Mills A, Brinson C, DeJesus E, et al. Switching to coformulated rilpivirine (RPV), emtricitabine (FTC) and tenofovir alafenamide from either RPV, FTC and tenofovir disoproxil fumarate (TDF) or efavirenz, FTC and TDF: 96-week results from two randomized clinical trials. HIV Med. 2018;19(10):724–733. doi: 10.1111/hiv.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grigsby IF, Pham L, Mansky LM, Gopalakrishnan R, Carlson AE, Mansky KC. Tenofovir treatment of primary osteoblasts alters gene expression profiles: implications for bone mineral density loss. Biochem Biophys Res Commun. 2010;394(1):48–53. doi: 10.1016/j.bbrc.2010.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider DL, Barrett-Connor EL. Urinary N-telopeptide levels discriminate normal, osteopenic, and osteoporotic bone mineral density. Arch Intern Med. 1997;157(11):1241–1245. doi: 10.1001/archinte.1997.00440320149014. [DOI] [PubMed] [Google Scholar]
- 30.Danjuma MI, Egan D, Abubeker IY, Post F, Khoo S. Polymorphisms of tenofovir disoproxil fumarate transporters and risk of kidney tubular dysfunction in HIV-positive patients: genetics of tenofovir transporters. Int J STD AIDS. 2018;29(14):1384–1389. doi: 10.1177/0956462418786562. [DOI] [PubMed] [Google Scholar]
- 31.Cusato J, Calcagno A, Marinaro L, Avataneo V, D'Avolio A, Di Perri G, et al. Pharmacogenetic determinants of kidney-associated urinary and serum abnormalities in antiretroviral-treated HIV-positive patients. Pharmacogenomics J. 2020;20(2):202–212. doi: 10.1038/s41397-019-0109-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.


