Abstract
Objective:
To investigate the effects of monoclonal antibodies against Aβ on cognition, function, amyloid PET and other biomarkers, as well as risk for amyloid-related imaging abnormalities (ARIA) and other adverse events, in Alzheimer’s disease (AD).
Methods:
Pubmed, Web of Science, ClinicalTrials.gov and gray literature were searched for phase III RCTs and random-effects meta-analyses were performed.
Results:
Seventeen studies (12,585 patients) were included. Antibodies statistically improved the cognitive outcomes ADAS-Cog {SMD = −0.06 [95% CI (−0.10; −0.02), I 2 = 0%]} and MMSE {SMD = 0.05 [95% CI (0.01; 0.09), I 2 = 0%]} by small effect sizes, but did not improve the cognitive/functional measure CDR-SOB {SMD = −0.03 [95% CI (−0.07; 0.01), I 2 = 18%]}. Moreover, antibodies decreased amyloid PET SUVR {SMD = −1.02 [95% CI (−1.70; −0.34), I 2 = 95%]} and CSF p181-tau {SMD = −0.87 [95% CI (−1.32; −0.43), I 2 = 89%]} by large effect sizes. They also increased risk for ARIA {RR = 4.30 [95% CI (2.39; 7.77), I 2 = 86%]} by a large effect size. Antibody effects on reducing amyloid PET SUVR were correlated with their effects on improving ADASCog (r = +0.68, p = 0.02). In subgroup analyses by individual drug, Aducanumab improved ADAS-Cog, CDRSOB, ADCS-ADL by small effect sizes and decreased amyloid PET SUVR and CSF p181-tau by large effect sizes. Solanezumab improved ADAS-Cog, MMSE by small effect sizes, and increased (improved) CSF Ab 1–40 levels by a moderate effect size. Bapineuzumab, Gantenerumab and Crenezumab did not improve any clinical outcomes. Bapineuzumab and Gantenerumab decreased CSF p181-tau by a small and large effect size, respectively. All drugs except Solanezumab increased ARIA risk.
Conclusions:
In this meta-analysis of phase III trials in AD, we found that monoclonalantibodies against Aβ induced clinical improvements of small effect size, biomarker improvements of large effect size, and increase in risk for the hallmark adverse event, ARIA, by a large effect size, when all drugs were pooled together. Among individual drugs, Aducanumab produced the most favorable effects followed by Solanezumab. These findings provide moderate support for the continuous development of anti-Aβ monoclonal antibodies as a treatment for AD.
Keywords: monoclonal antibodies, Amyloid beta, Alzheimer’s, meta-analysis
1. Introduction
The Amyloid Hypothesis for Alzheimer’s disease (AD) posits that abnormal brain accumulation of amyloid-beta (Aβ) is the key pathogenic event that triggers a complex cascade leading to tau pathology, neurodegeneration, and cognitive decline (Hardy and Selkoe, 2002; Selkoe and Hardy, 2016). This hypothesis brought focus on the balance between Aβ production and clearance in AD, informing therapeutic strategies aiming to either decrease Aβ production such as β-secretase inhibitors and γ-secretase inhibitors or increase Aβ clearance such as active and passive immunotherapy (Blennow et al., 2006; Hardy and Selkoe, 2002; Panza et al., 2019).
Of all anti-Aβ approaches, passive immunotherapy using monoclonal antibodies against Aβ has been best tolerated and given its mechanistic selectivity, it has been widely considered as the therapeutic candidate of choice (Panza et al., 2019). As has been the case with most anti-Aβ approaches, individual trials testing anti-Aβ monoclonal antibodies have largely reported lack of efficacy (Panza et al., 2019), but newer drugs of this class are still being investigated (Cummings et al., 2020). Recently, investigators of Aducanumab trials reported some relatively promising effects, therefore re-energizing the popularity of the Amyloid Hypothesis and the interest in monoclonal antibodies against Aβ (Schneider, 2020). However, there is controversy regarding Aducanumab’s efficacy (Biogen, 2020; Knopman et al., 2020; Sabbagh and Cummings, 2020) and at present, FDA is reviewing Aducanumab’s data (Biogen, 2020, 2021).
Currently, there is no consensus on the therapeutic potential of monoclonal antibodies against Aβ in AD. While research on this drug class is ongoing (Cummings et al., 2020), it is important to examine the totality of evidence on their effects based on results for antibodies that reached phase III stage of development. With this systematic review and meta-analysis, we aimed to investigate the effects of monoclonal antibodies against Aβ on clinical outcomes of interest (cognitive function/functional abilities), biomarkers related to Aβ and tau pathologies, and the risk for select adverse events including the hallmark adverse event associated with this drug class, amyloid-related imaging abnormalities (ARIA), in patients with AD.
The goal of this systematic review and meta-analysis is not to challenge assessments on the efficacy of individual anti-Aβ monoclonal antibodies that were made by the original studies’ investigators and sponsors or are being deliberated by regulatory authorities based on analyses of individual studies and individual-subject data. The evidence presented here is based on meta-analyses (with input data at the study level) and serves mainly as a comprehensive assessment of all antibodies tested in phase III trials, an objective comparison of the effects of individual drugs across individual trials, and, hopefully, an aid for further therapeutic development for this drug class. The generated evidence cannot be used to substitute or supersede the analysis of individual trials or influence the approval process for any individual drug.
2. Methods
2.1. Search Strategy and selection criteria
To conduct this systematic review and meta-analysis, we followed the PRISMA guidelines (Moher et al., 2009). We searched for literature on Medline/Pubmed, Web of Science and ClinicalTrials.gov. We additionally searched for abstracts and presentations of scientific meetings. The initial search was performed through April 9, 2020 and an additional search was performed on 9/10/2020 to include data from recently reported studies. Two reviewers (KA, DK) conducted independent searches and disagreements were resolved by consensus. We used combinations of the next keywords for the literature search: “Alzheimer’s”, “sporadic”, “mild cognitive impairment”, “monoclonal antibody”, “passive immunotherapy”, “AAB-003”, “PF-05236812”, “Aducanumab”, “BIIB037”, “BAN2401”, “mAb158”, “Bapineuzumab”, “AAB-001”, “Crenezumab”, “MABT5102A”, “RG7412”, “Donanemab”, “N3pG-Aβ Monoclonal Antibody”, “LY3002813”, “GSK933776”, “Gantenerumab”, “RO4909832”, “RG1450”, “LY2599666”, “LY3372993”, “MEDI1814”, “Ponezumab”, “PF-04360365”, “SAR228810”, “Solanezumab”, “LY2062430”.
To be included in the meta-analysis, a study had to: (1) be a phase III parallel design double-blind placebo controlled RCT; (2) be published/presented in any language (3) include participants with mild cognitive impairment (MCI) or any stage of sporadic AD.
A study was excluded if it: (1) was a narrative/systematic review, meta-analysis, phase I, II or I/II RCT, open-label trial, single-arm trial, prospective cohort study, case-control study, cross-sectional study, case-series study, case report study, opinion/editorial, post-hoc or secondary analysis of a main study; (2) involved non-human subjects; (3) involved participants diagnosed with dementia other than sporadic AD (we excluded familial AD among other dementias); (4) did not report cognitive/functional outcomes.
2.2. Data analysis
Two reviewers (KA, KD) extracted data from the identified articles independently and any disagreements were resolved by consensus. Summary measures for continuous outcomes were expressed as Standardized Mean Difference (SMD) [95% Confidence Interval (CI)] and for binary outcomes as Risk Ratio (RR) [95% CI]. Throughout the manuscript, the terms SMD, effect size and Hedges’ g are used interchangeably, as they are considered equivalent (Higgins JPT). The following outcomes were considered: (i) primary: AD Assessment Scale-Cognitive Subscale (ADAS-Cog), Mini Mental State Examination (MMSE) and Clinical Dementia Rating scale-Sum of Boxes (CDR-SOB); (ii) secondary: amyloid PET Standardized Uptake Volume Ratio (SUVR), CSF p(Thr181)-tau, and risks for the following adverse reactions: ARIA, major depression/depression, anxiety, headaches, falls, cardiac disorders and seizures/convulsions; (iii) tertiary: Neuropsychological Test Battery (NTB), AD Cooperative Study-Activities of Daily Living (ADCS-ADL), Disability Assessment for Dementia (DAD), Dependence Scale (DS), CSF Aβ1–40, CSF Aβ1–42, and volumetric MRI (vMRI).The terms “primary outcome”, “secondary outcome” and “tertiary outcome” in our meta-analyses do not correspond to the respective designated outcomes of the original studies, which were not identical across studies anyway. The outcomes of this meta-analysis were ranked as “primary”, “secondary” and “tertiary” mainly based on their importance for demonstrating efficacy, but also based on our interest in the underlying mechanism (e.g., reduction in Aβ deposition), and their availability in as many as possible of the original studies. More specifically, we defined as “primary”, those clinical outcomes that were reported by all or the majority of the original studies; as “secondary”, the biomarker and adverse event outcomes that were reported by the majority of the original studies; as “tertiary”, other outcomes of interest that were reported by few original studies only or were reported by multiple studies but were of less mechanistic importance.
The RCTs included in our meta-analysis contained multiple subgroups, which differed in terms of participant characteristics (e.g., ApoE genotype) or received different doses of the drug. To appropriately handle the variation among study subgroups, we followed the recommendation to use subgroup as the unit of analysis, thereby considering each subgroup as a separate study (Borenstein M, 2009a). To account for the between-studies variation in our statistical analysis, we followed the recommendation to use a random-effects model (Borenstein M, 2009b). Two common measures of heterogeneity in meta-analyses were used, τ2 and I2. To estimate the between-study variance τ2, we used the DerSimonian-Laird method which is commonly used in meta-analyses in Medicine whenever the number of included studies is large and there is no substantial heterogeneity (both are true for the majority of the performed meta-analyses) (Harrer, 2019). Heterogeneity between studies was also expressed with the commonly used I2 statistic, which is the percentage of variability in the effect sizes not caused by sampling error. The I2 cut-offs 25%, 50% and 75% were used to characterize heterogeneity as low, moderate and substantial, respectively (Harrer, 2019).
Risk of bias was assessed with the “Revised Cochrane risk-of-bias tool for randomized trials” (RoB 2) (Sterne et al., 2019).We assessed for bias arising from the randomization process, bias due to deviations of intended interventions, bias due to missing outcome data, bias in outcome measurements and bias in selection of reported results. Individual domains of risk of bias and studies could be characterized as of: “low risk”, “some concerns”, or “high risk”. Publication bias was assessed with inspection of funnel plots, Egger’s statistic and imputation of “missing studies” with the Duval & Tweedie’s trim-and-fill procedure (Harrer, 2019).
To be as comprehensive as possible, the main analysis included data from all available sources (peer-reviewed manuscripts, conference presentations, ClinicalTrials.gov reports). To address any potential concerns for including non-peer-reviewed data extracted from conference presentations and reports on ClinicalTrials.gov, we also performed a sensitivity analysis including data from peer-reviewed manuscripts only.
We performed subgroup analyses by individual drug, shared drug characteristics [i.e., by sub-grouping human vs. humanized murine antibodies; antibodies with indiscriminate targeting of all Aβ types (monomers, oligomers, fibrils) vs. targeting monomers vs. targeting oligomers and fibrils; antibodies with high vs. low ARIA risk]. Meta-regressions by baseline MMSE, age, apoE genotype, sex, race and AD medications at baseline were performed to examine whether these variables affected efficacy. For statistically significant results, we calculated “Number Needed to Treat” (NNT) or “Number Needed to Harm” (NNH) (Kraemer and Kupfer, 2006) to assess their clinical relevance..
To assess the association between changes in Aβ deposition and changes in cognitive performance across studies, we computed the Pearson’s r correlation co-efficients between the effect sizes for change in amyloid PET and (i) ADAS-Cog, (ii) CDR-SOB, as well as between CSF p181-tau and (i) ADAS-Cog, (ii) CDR-SOB. Statistical analyses were performed using R version 3.6.3 and the packages “dmetar”, “meta”, “metafor”, and “metaSEM” (Harrer, 2019). This manuscript was cleared for publication according to NIH regulations.
3. Results
The literature search yielded 12 eponymous phase III RCTs (12 ClinicalTrials.gov registries), which contained 17 studies, considering different subgroups within certain RCTs as separate studies according to our methodology (CREAD1, 2020; CREAD2, 2020; Doody et al., 2014; EMERGE/ENGAGE_Investigators, 2019; Honig et al., 2018; Ostrowitzki et al., 2017; Salloway et al., 2014; Vandenberghe et al., 2016) (table 1). Figure 1 provides details on study selection. The meta-analysis included n = 12,585 participants and k = 17 studies. Given these parameters, the meta-analysis had 100% statistical power assuming moderate between-studies heterogeneity and any possible effect size (small, medium, large) (supplemental figure 1).
Table 1.
Baseline characteristics of included phase III clinical trials
| First author, (study’s name) | Year of study publication/data presentation | Number of participants (treatment/placebo) | AD stage (MMSE score for inclusion) | Baseline MMSE§ by Treatment vs. Placebo | Baseline ADAS-Cog§, ¥ by Treatment vs. Placebo | Baseline CDR-SOB§ by Treatment vs. Placebo | Age§ by Treatment vs. Placebo | Apoe4 carriage (treatment/placebo) | Drug, dose | Study duration (weeks) | Primary Efficacy outcome | Other cognitive, functional measures | Reason for study discontinuation, if applicable | Biomarker/neuroimaging outcomes† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salloway et al.* (Study 301) | 2014 | 314/493 | Mild/moderate (16–26) | 21.2 (3.4) 21.2 (3.2) |
22.4 (9.7) 22.2 (10.1) |
NR | 73.1 (9.3) 71.9 (10.1) |
All non-carriers | Bapineuzumab, 0.5 mg/kg | 78 | ADAS-Cog11, DAD | NTB, CDR-SOB, MMSE, DS | NA | SUVR (PIB-PET), CSF ptau, whole brain vMRI |
| Salloway et al.** (Study 301) | 2014 | 307/493 | Mild/moderate (16–26) | 21.2 (3.3) 21.2 (3.2) |
22.2 (10.0) 22.2 (10.1) |
NR | 73.5 (9.1) 71.9 (10.1) |
All non-carriers | Bapineuzumab, 1.0 mg/kg | 78 | ADAS-Cog11, DAD | NTB, CDR-SOB, MMSE, DS | NA | SUVR (PIB-PET), CSF ptau, whole brain vMRI |
| Salloway et al.*** (Study 302) | 2014 | 658/432 | Mild/moderate (16–26) | 20.8 (3.1) 20.7 (3.2) |
23.5 (9.4) 23.9 (9.5) |
NR | 72.8 (8.0) 72.3 (8.4) |
All carriers | Bapineuzumab, 0.5 mg/kg | 78 | ADAS-Cog11, DAD | NTB, CDR-SOB, MMSE, DS | NA | SUVR (PIB-PET), CSF ptau, whole brain vMRI |
| Doody et al. * (EXPEDITION 1) | 2014 | 506/506 | Mild/moderate (16–26) | 21.0 (4.0) 21.0 (3.0) |
22.0 (8.0) 22.0 (9.0) |
NR | 75.0 (7.9) 74.4 (8.0) |
57.3% carriers 61.3% carriers |
Solanezumab, 400 mg | 80 | ADAS-Cog11/14, ADCS-ADL | CDR-SOB, NPI, RUD-Lite, EQ-5D, QOL-AD, MMSE | NA | Plasma Aβ1–40/1–42, CSF Aβ1–40/1–42, CSF total tau/ptau, hippocampal and whole brain vMRI, SUVR (18F-florbetapir-PET) |
| Doody et al. ** (EXPEDITION 2) | 2014 | 521/519 | Mild/moderate (16–26) | 21.0 (3.0) 21.0 (3.0) |
24.0 (9.0) 23.0 (10.0) |
NR | 72.5 (8.0) 72.4 (7.8) |
56.8% carriers 59.5% carriers |
Solanezumab, 400 mg | 80 | ADAS-Cog11, ADCS-ADL | CDR-SOB, NPI, RUD-Lite, EQ-5D, QOL-AD, MMSE | NA | Plasma Aβ1–40/1–42, CSF Aβ1–40/1–42, CSF total tau/ptau, hippocampal and whole brain vMRI, SUVR (18F-florbetapir-PET) |
| Honig et al. (EXPEDITION 3) | 2018 | 1057/1072 | Mild (20–26) | 22.8 (2.8) 22.6 (2.9) |
28.9 (8.3) 29.7 (8.5) |
3.9 (1.9) 3.9 (2.0) |
72.7 (7.8) 73.3 (8.0) |
69.3% carriers 66.3% carriers |
Solanezumab, 400 mg | 76 | ADAS-Cog14 | MMSE, ADCS-ADL, ADCS-iADL, CDR-SOB, FAQ, iADRS, | NA | Plasma Aβ1–40/1–42, CSF Aβ1–40/1–42, CSF total tau/ptau, whole brain vMRI, SUVR (18F-florbetapir-PET and flortaucipir-PET) |
| Ostrowitzki et al.* | 2017 | 271/266 | Prodromal (≥24) | 25.7 (2.3) 25.7 (2.1) |
23.1 (6.9) 23.5 (7.2) |
2.2 (1.0) 2.1 (1.0) |
70.3 (7.0) 69.5 (7.5) |
79.0% carriers 70.3% carriers Apoe4 was tested in a subgroup of pts |
Gentenerumab, 105 mg | 104 | CDR-SOB | ADAS-Cog13, MMSE, CANTAB, FCSRT, NPI, FAQ | Futility based on interim analysis | SUVR (18F-florbetapir-PET), hippocampal and whole brain vMRI, CSF Aβ1–42, CSF total tau/ptau |
| Ostrowitzki et al.** | 2017 | 260/266 | Prodromal (≥24) | 25.7 (2.2) 25.7 (2.1) |
23.0 (6.2) 23.5 (7.2) |
2.0 (0.9) 2.1 (1.0) |
71.3 (7.1) 69.5 (7.5) |
61.5% carriers 70.3% carriers Apoe4 was tested in a subgroup of pts | Gentenerumab, 225 mg | 104 | CDR-SOB | ADAS-Cog13, MMSE, CANTAB, FCSRT, NPI, FAQ | Futility based on interim analysis | SUVR (18F-florbetapir-PET), vMRI, CSF Aβ1–42, CSF total tau/ptau |
| Vandenberghe et al.* (Study 3000) | 2016 | 255/328 | Mild/moderate (16–26) | 20.8 (3.2) 20.8 (3.1) |
23.2 (10.0) 22.9 (10.2) |
NR | 71.1 (NR) 69.7 (NR) |
All non-carriers | Bapineuzumab, 0.5 mg/kg | 78 | ADAS-Cog11, DAD | CDR-SOB, NTB, DS | NA | SUVR (PIB-PET), plasma Aβ, CSF ptau, whole brain vMRI |
| Vandenberghe et al.** (Study 3000) | 2016 | 253/328 | Mild/moderate (16–26) | 20.8 (3.1) 20.8 (3.1) |
23.5 (9.3) 22.9 (10.2) |
NR | 70.7 (NR) 69.7 (NR) |
All non-carriers | Bapineuzumab, 1.0 mg/kg | 78 | ADAS-Cog11, DAD | CDR-SOB, NTB, DS | NA | SUVR (PIB-PET), plasma Aβ, CSF ptau, whole brain vMRI |
| Vandenberghe et al.*** (Study 3001) | 2016 | 650/431 | Mild/moderate (16–26) | 20.9 (3.1) 21.0 (3.0) |
23.2 (8.9) 22.6 (8.9) |
NR | 70.9 (NR) 70.2 (NR) |
All carriers | Bapineuzumab, 0.5 mg/kg | 78 | ADAS-Cog11, DAD | CDR-SOB, NTB, DS | NA | SUVR (PIB-PET), plasma Aβ, CSF ptau, whole brain vMRI |
| EMERGE study* | 2019 | 543/548 | Early (MCI and Mild AD) (24–30) | 26.3 (1.72) 26.4 (1.78) |
22.5 (6.76) 21.9 (6.73) |
2.46 (1.01) 2.47 (1.00) |
70.6 (7.45) 70.8 (7.4) |
66.7% carriers 67.0% carriers |
Aducanumab, 1–6 mg/kg | 78 | CDR-SOB | MMSE, ADAS-Cog, ADCS-ADL-MCI | NA | SUVR (18F-florbetapir-PET), CSF total tau/ptau, SUVR (tau-PET) |
| EMERGE study** | 2019 | 547/548 | Early (MCI and Mild AD) (24–30) | 26.3 (1.68) 26.4 (1.78) |
22.2 (7.08) 21.9 (6.73) |
2.51 (1.05) 2.47 (1.00) |
70.6 (7.47) 70.8 (7.4) |
66.7% carriers 67.0% carriers |
Aducanumab, 1–10 mg/kg | 78 | CDR-SOB | MMSE, ADAS-Cog, ADCS-ADL-MCI | NA | SUVR (18F-florbetapir-PET), CSF total tau/ptau, SUVR (tau-PET) |
| ENGAGE study* | 2019 | 547/545 | Early (MCI and Mild AD) (24–30) | 26.4 (1.78) 26.4 (1.73) |
22.5 (6.30) 22.5 (6.56) |
2.43 (1.01) 2.40 (1.01) |
70.4 (6.96) 69.8 (7.72) |
71.5% carriers 69.0% carriers |
Aducanumab, 1–6 mg/kg | 78 | CDR-SOB | MMSE, ADAS-Cog, ADCS-ADL-MCI | NA | SUVR (18F-florbetapir-PET), CSF total tau/ptau, SUVR (tau-PET) |
| ENGAGE study** | 2019 | 555/545 | Early (MCI and Mild AD) (24–30) | 26.4 (1.77) 26.4 (1.73) |
22.4 (6.54) 22.5 (6.56) |
2.40 (1.01) 2.40 (1.01) |
70.0 (7.65) 69.8 (7.72) |
68.1% carriers 69.0% carriers |
Aducanumab, 1–10 mg/kg | 78 | CDR-SOB | MMSE, ADAS-Cog, ADCS-ADL-MCI | NA | SUVR (18F-florbetapir-PET), CSF total tau/ptau, SUVR (tau-PET) |
| CREAD1 study | 2020 | 404/409 | Prodromal to mild (≥22) | NR | NR | NR | 71.0 (7.9) 70.3 (8.4) |
NR | Crenezumab 60mg/kg | 100 | CDR-SOB | ADAS-Cog, CDR-GS, MMSE, ADCS-ADL, ADCS-iADL, Dependence measure derived from ADCS-ADL, NPI, QoL-AD, ZCI-AD Scale Score, EQ-5D Questionnaire Domain Score for Participants, EQ-5D Questionnaire Domain Score for Caregivers | Futility based on interim analysis | Plasma Aβ1–40/1–42, whole brain vMRI, ventricle/hippocampal volume on MRI |
| CREAD2 study | 2020 | 407/399 | Prodromal to mild (≥22) | NR | NR | NR | 71.1 (7.5) 70.7 (7.9) |
NR | Crenezumab 60mg/kg | 100 | CDR-SOB | ADAS-Cog, CDR-GS, MMSE, ADCS-ADL, ADCS-iADL, Dependence measure derived from ADCS-ADL, NPI, QoL-AD, ZCI-AD Scale Score, EQ-5D Questionnaire Domain Score for Participants, EQ-5D Questionnaire Domain Score for Caregivers, FAQ | Futility based on interim analysis | Plasma Aβ1–40/1–42, whole brain vMRI, ventricle/hippocampal volume on MRI |
Mean (SD)
ADAS-Cog version 11 for Salloway/Doody/Vandenberghe studies, version 13 for Ostrowitzki/CREAD studies and version 14 for Honig/EMERGE/ENGAGE studies
Data on biomarker and neuroimaging outcomes were available only for sub-populations of the initial sample sizes of individual studies; NR: Not Reported; NA: Not Applicable; ADAS-Cog: Alzheimer's Disease Assessment Scale-Cognitive Subscale; DAD: Disability Assessment for Dementia; NTB: Neuropsychological Test Battery; CDR-SOB: Clinical Dementia Rating-Sum of Boxes; MMSE: Mini Mental State Examination; DS: Dependence Scale; SUVR: standardized uptake value ratio; ADCS-ADL: Alzheimer’s Disease Cooperative Study-Activities of Daily Living; NPI: Neuropsychiatric Inventory; RUD-Lite: Resource Utilization in Dementia Lite; EQ-5D: European Quality of Life-5 Dimensions; QOL-AD: Quality of Life in Alzheimer’s Disease; ADCS-iADL: ADCS instrumental subscale; FAQ: Functional Activities Questionnaire; iADRS: Integrated Alzheimer’s Disease Rating Scale; CANTAB: Cambridge Neuropsychological Test Automated Battery; FCSRT: Free and Cued Selective Reminding Test; CDR-GS: Clinical Dementia Rate-Global Score; QoL-AD: Quality of Life - Alzheimer's Disease; ZCI-AD Scale Score: Zarit Caregiver Interview for Alzheimer's Disease Scale Score; FAQ: Functional Activities Questionnaire Note: Based on results of an interim analysis, CREAD 1 and 2 were discontinued because Crenezumab was unlikely to meet the primary endpoint. Outcome data was available only for few participants that completed the study and not the initial CREAD populations assigned to the study groups.
Figure 1.
Flow diagram of study selection process.
Six studies tested Bapineuzumab (Salloway et al., 2014; Vandenberghe et al., 2016), 3 tested Solanezumab (Doody et al., 2014; Honig et al., 2018), 2 tested Gantenerumab (Ostrowitzki et al., 2017), 4 tested Aducanumab (EMERGE/ENGAGE_Investigators, 2019) and 2 tested Crenezumab (CREAD1, 2020; CREAD2, 2020). Baseline participant characteristics are provided in table 1. Regarding risk of bias, all studies were deemed as either “low risk” or as raising “some concerns” (supplemental figure 2).
The terms “improved”, “improvement”, “increased risk” and similar expressions, which are being used to describe effects of antibodies on clinical and biomarker outcomes and risks of adverse events in this meta-analysis, refer to the direction of statistically significant differences and are not assessments of their clinical importance. For statistically significant results, we characterize the size of an effect as small, moderate, or large based on widely accepted criteria (i.e., effect sizes ~0.2 may be considered small, ~0.5 moderate, and ~0.8 large). To provide yet another metric to assess clinical significance, we calculated the “Number Needed to Treat” or “Number Needed to Harm”. It is out of the scope of this study to determine which statistically significant results should be considered as sufficient evidence for a drug to be considered as clinically efficacious for purposes of regulatory approval. Ultimately, investigators and sponsors of the original trials, as well as regulatory authorities are best positioned to determine this based on individual-subject data.
3.1. Primary outcomes (main clinical outcomes)
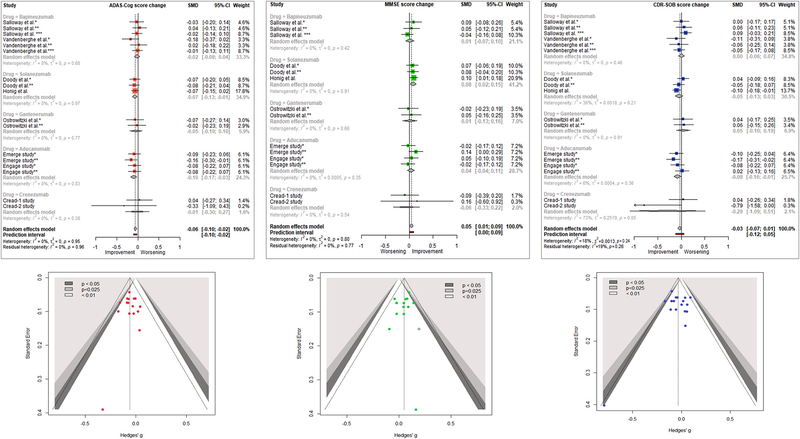
Compared with placebo, treatment with monoclonal antibodies statistically improved performance on the cognitive measures ADAS-Cog {SMD = −0.06 [95% CI (−0.10; −0.02), I2 = 0%]} (negative change indicates improvement) and MMSE {SMD = 0.05 [95% CI (0.01; 0.09), I2 = 0%]} (positive change indicates improvement). These changes were of small effect size. No improvement on the cognitive/functional measure CDR-SOB {SMD = −0.03 [95% CI (−0.07; 0.01), I2 = 18%]} (negative change indicates improvement) was found. Funnel plots were symmetric for all three primary outcomes and Egger’s test p-values were 0.92, 0.35 and 0.83, respectively; thus, there was no evidence for publication bias. Imputation of potentially “missing studies” did not shift results (figure 2).
Figure 2. Forest plots and funnel plots of meta-analyses of primary outcomes.
ADAS-Cog: Alzheimer disease assessment scale - Cognitive subscale; MMSE: Mini Mental State Examination; CDR-SOB: Clinical dementia rating scale – Sum of boxes; SMD: Standardized Mean Difference; Hedges’ g = SMD. Negative values for ADAS-Cog and CDR-SOB and positive values for MMSE indicate improvement.
Subgroup analysis by drug revealed that ADAS-Cog was statistically improved by Aducanumab and Solanezumab separately; MMSE was statistically improved only by Solanezumab; and CDR-SOB was statistically improved only by Aducanumab (figure 2). All these changes were of small effect size. Interestingly, the effect sizes of Aducanumab for ADAS-Cog from its original studies were larger than the corresponding effect sizes for MMSE, and the pooled effect for all Aducanumab studies reached significance for ADAS-Cog, but not MMSE. For Solanezumab, effects on ADAS-Cog and MMSE were of similar size in the original studies. The pooled effect for all Solanezumab studies reached significance for both ADAS-Cog and MMSE and corresponding effect sizes were similar to each other.
Additional subgroup analyses revealed no clear impact of antibody type, preferential Aβ target and ARIA risk. However, there was some evidence that antibodies binding specifically to one (Solanezumab) or two Aβ conformations (pooled Gantenerumab and Aducanumab) had some favorable effects on cognitive outcomes, whereas antibodies binding non-specifically to any Aβ conformation (pooled Bapinezumab and Crenezumab) did not produce any effects on cognitive outcomes (supplemental tables 1, 2, 3).
Meta-regressions of ADAS-Cog, MMSE and CDR-SOB outcomes by baseline participant MMSE, age, apoE genotype, percentage of females, percentage of whites, and percentage of participants on symptomatic medication for AD did not reach significance, suggesting that these factors did not affect drug responses (supplemental figures 8, 9, 10).
3.2. Secondary outcomes (main biomarker and adverse event outcomes)
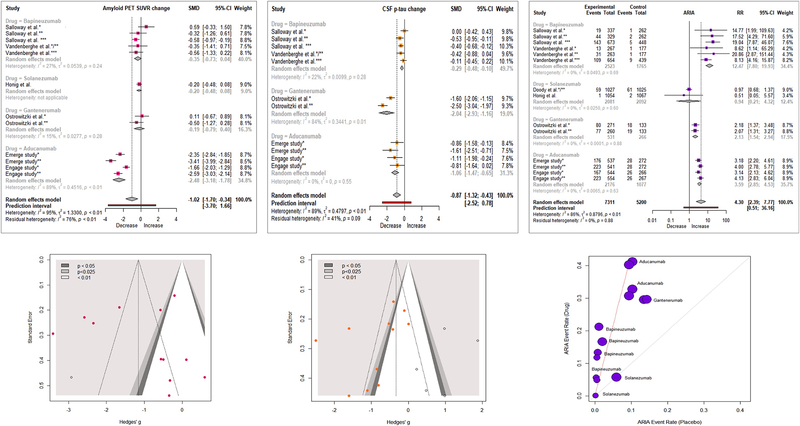
Taking note that biomarker and neuroimaging outcomes were available for subsets of study participants (table 1), we found that, compared with placebo, monoclonal antibodies substantially reduced amyloid PET SUVR {SMD = −1.02 [95% CI [(−1.70; −0.34), I2 = 95%]}, and CSF p181-tau levels {SMD = −0.87 [95% CI (−1.32; −0.43), I2 = 89%]} (figure 3). No publication bias was observed for amyloid PET SUVR and CSF p181-tau. Imputation of potentially “missing studies” did not shift results (figure 3)
Figure 3. Forest and funnel/L’ Abbe plots of meta-analyses of secondary outcomes.
Amyloid PET SUVR: Amyloid PET Standardized uptake value ratio; CSF p-tau: Tyr181-phosphorylated tau protein concentration in CSF; ARIA: amyloid related imaging abnormalities. SMD: Standardized Mean Difference; Hedges’ g = SMD
Subgroup analysis by drug showed that amyloid PET SUVR was decreased only by Aducanumab, with a very strong effect. All drugs with data for CSF p181-tau (Aducanumab, Gantenerumab and Bapineuzumab) decreased it individually and also when combined in a meta-analysis; individual effects were very strong for Gantenerumab and Aducanumab (figure 3). Solanezumab studies did not report data on CSF p181-tau.
Compared with placebo, monoclonal antibodies substantially increased the risk for the hallmark adverse event of these drugs, ARIA {RR = 4.30 [95% CI (2.39; 7.77), I2 = 86%]} (figure 3). Subgroup analysis by drug showed that all drugs, except Solanezumab, were responsible for this increase (figure 3). ARIA risk was not reported in Crenezumab studies.
Meta-analyses of the effects of all drugs on risk ratios for other adverse events showed that when compared with placebo, all antibodies combined did not increase the risk for major depression/depression {RR = 1.14 [95% CI (0.92; 1.42), I2 = 0%]}, anxiety {RR = 1.02 [95% CI (0.83; 1.25), I2 = 0%]}, headaches {RR = 1.08 [95% CI (0.97; 1.19), I2 = 0%]}, falls {RR = 1.03 [95% CI (0.92; 1.15), I2 = 0%]}, cardiac disorders {RR = 0.95 [95% CI (0.68; 1.33), I2 = 35%]} and seizures/convulsions {RR = 1.28 [95% CI (0.32; 5.15), I2 = 19%]}. In subgroup analyses by individual drugs, we found that Aducanumab increased the risk for headaches {RR = 1.28 [95% CI (1.19; 1.38), I2 = 0%]}.
3.3. Tertiary outcomes (other clinical and biomarker outcomes)
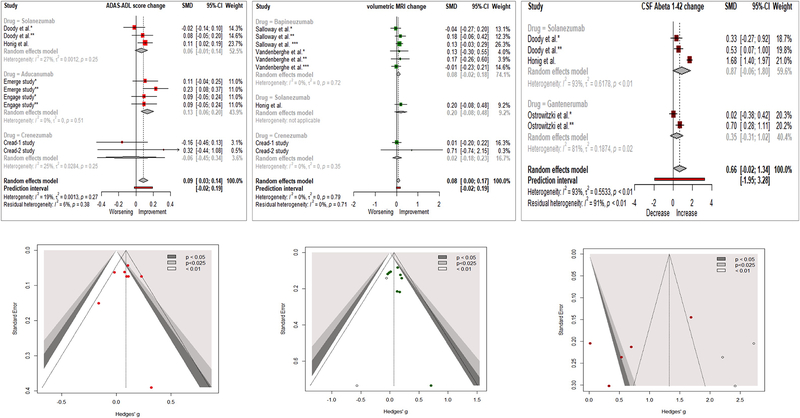
A meta-analysis of “AD Cooperative Study-Activities of Daily Living” included data from nine studies testing Solanezumab, Aducanumab or Crenezumab and showed statistical improvement with monoclonal antibodies {SMD = 0.09 [95% CI (0.03; 0.14), I2 = 19%]}. Subgroup analyses showed that only Aducanumab produced a statistically significant effect, although Solanezumab showed a trend towards improvement (figure 4). Meta-analyses of “Neuropsychological Test Battery”, “Disability Assessment for Dementia” and “Dependence Scale” were based exclusively on data from six Bapineuzumab studies and did not show any drug effects (supplementary figure 3).
Figure 4. Forest and funnel plots of meta-analyses of tertiary outcomes.
SMD: Standardized Mean Difference; Hedges’ g = SMD
A meta-analysis of CSF Aβ1–42 (from 3 Solanezumab and 2 Gantenerumab studies) showed no difference between treatment and placebo {SMD: 0.66 [95% CI (−0.02; 1.34), I2 = 93%]} (figure 4). A subgroup analysis showed that Solanezumab was mainly responsible for that trend. A meta-analysis of CSF Aβ1–40 (from 3 Solanezumab studies) also showed a statistical increase (improvement) for the antibody group {SMD: 0.51 [95% CI (0.14; 0.87), I2 = 57%]} (supplementary figure 4).
A meta-analysis of vMRI included six Bapineuzumab studies, one Solanezumab study and two Crenezumab studies, showing that monoclonal antibodies (statistically) preserved whole brain volume more than placebo {SMD: 0.08 [95% CI (0.00; 0.19) I2 = 0%]}. Interestingly, subgroup analyses showed no statistical improvement per individual drug, although the effect for all drugs combined reached statistical significance (figure 4). No publication bias was found for tertiary outcomes.
3.4. Number Needed to Treat/Harm
The calculated Number Needed to Treat was >20 for all primary (ADAS-Cog, MMSE, CDR-SOB) outcomes (table 2). Conversely, Number Needed to Treat ranged between 1–6 for amyloid PET SUVR and CSF p181-tau (table 2). Finally, Number Needed to Harm for ARIA ranged between 4–8 (table 2).
Table 2.
Clinical interpretation of effect sizes
| Outcomes for which statistical significance was reached | Drug(s) included in quantitative synthesis | Effect size | NNT or NNH | Point-change approximation on original scale | Clinically important change | |
|---|---|---|---|---|---|---|
| Clinical | ADAS-Cog | Bapineuzumab, Solanezumab, Gantenerumab, Aducanumab, Crenezumab | SMD = −0.06 | 30 | < 2 | 2–3 |
| Aducanumab | SMD = −0.10 | 18 | < 2 | 2–3 | ||
| Solanezumab | SMD = −0.07 | 25 | < 2 | 2–3 | ||
| MMSE | Bapineuzumab, Solanezumab, Gantenerumab, Aducanumab, Crenezumab | SMD = 0.05 | 35 | < 1 | 1–3 | |
| Solanezumab | SMD = 0.08 | 22 | < 1 | 1–3 | ||
| CDR-SOB | Aducanumab | SMD = −0.08 | 22 | < 1 | 1–2 | |
| Biomarker | Amyloid PET SUVR | Bapineuzumab, Solanezumab, Gantenerumab, Aducanumab | SMD = −1.02 | 2 | N/A | N/A |
| Aducanumab | SMD = −2.48 | 1 | N/A | N/A | ||
| CSF p-tau | Bapineuzumab, Gantenerumab, Aducanumab | SMD = −0.87 | 2 | N/A | NA | |
| Bapineuzumab | SMD = −0.29 | 6 | N/A | N/A | ||
| Gantenerumab | SMD = −2.04 | 1 | N/A | N/A | ||
| Aducanumab | SMD = −1.06 | 2 | N/A | N/A | ||
| Adverse event | ARIA | Bapineuzumab, Solanezumab, Gantenerumab, Aducanumab | RR = 4.30 | 7 | N/A | N/A |
| Bapineuzumab | RR = 12.47 | 8 | N/A | N/A | ||
| Gantenerumab | RR = 2.13 | 6 | N/A | N/A | ||
| Aducanumab | RR = 3.59 | 4 | N/A | N/A | ||
ADAS-Cog: Alzheimer's Disease Assessment Scale-Cognitive Subscale; CDR-SOB: Clinical Dementia Rating-Sum of Boxes; MMSE: Mini Mental State Examination; SUVR: standardized uptake value ratio; ARIA: Amyloid related imaging abnormalities; NNT: Number Needed to Treat (for clinical/biomarker outcomes); NNH: Number Needed to Harm (for ARIA); SMD: Standardized Mean Difference; RR: Risk Ratio; N/A: Not Applicable
3.5. Correlation between amyloid/p-tau pathology and clinical outcomes
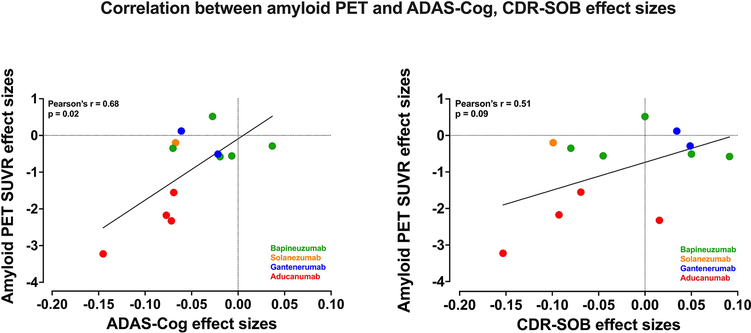
Antibody effects on reducing amyloid PET SUVR were correlated with their effects on decreasing (improving) ADAS-Cog (Pearson’s r = +0.68, p = 0.02) (figure 5). No correlation between amyloid PET SUVR and CDR-SOB effect sizes was found (r = +0.51, p = 0.09). These analyses included effect sizes for Bapineuzumab, Solanezumab, Gantenerumab and Aducanumab.
Figure 5. Correlations between amyloid PET SUVR and ADAS-Cog, CDR-SOB effect sizes.
Limitation: data for amyloid PET SUVR were reported only for sub-populations of the original studies. Each correlation relies on the assumption that effect sizes for PET in these sub-populations are representative of those for the entire populations.
On the other hand, antibody effects on reducing CSF p181-tau were not correlated with their effects on decreasing (improving) ADAS-Cog (Pearson’s r = +0.32, p = 0.33) or their effects on decreasing (improving) CDR-SOB (Pearson’s r = +0.02, p = 0.96). These analyses included effect sizes for Bapineuzumab, Gantenerumab and Aducanumab, which reported CSF p181-tau.
3.6. Sensitivity analyses
Sensitivity analyses (i.e., excluding Aducanumab and Crenezumab data, which have not been published as peer-reviewed manuscripts) did not yield different results for the major outcomes of interest (ADAS-Cog, MMSE, CDR-SOB, amyloid PET SUVR, CSF p181-tau and ARIA) compared to the main analyses (supplemental figures 5, 6). The effect size for amyloid PET SUVR decreased in the sensitivity analysis {SMD: −0.29 [95% CI (−0.50; −0.07), I2 = 10%]}. This difference can be attributed primarily to the exclusion of Aducanumab’s effect in the sensitivity analysis. As with the main analysis, the pooled effect on CDR-SOB did not reach significance in the sensitivity analysis. The risk for the hallmark adverse event ARIA was similar in both analyses. For “AD Cooperative Study-Activities of Daily Living”, there was a small but disadvantageous change in the sensitivity analysis. For vMRI, there was a small but advantageous change (supplemental figure 7). Finally, the correlation between amyloid PET SUVR and ADAS-Cog lost its significance in the sensitivity analysis (Pearson’s r = −0.31, p = 0.45). This finding can be attributed primarily to the absence of the effects of Aducanumab studies.
4. Discussion
This meta-analysis included data from all reported phase III RCTs of anti-Aβ monoclonal antibodies in sporadic AD. Robust data syntheses of all included studies (12,585 participants) showed statistical improvements for monoclonal antibodies on cognitive outcomes such as ADAS-Cog and MMSE, and a trend towards improvement on CDR-SOB, a measure that assesses both cognition and function. The statistically significant cognitive benefits of monoclonal antibodies revealed in this meta-analysis were particularly noteworthy considering that the majority of original studies did not reach significance on ADAS-Cog and MMSE. Additional meta-analyses also showed that monoclonal antibodies statistically improved a functional measure (AD Cooperative Study-Activities of Daily Living), reduced amyloid burden (amyloid PET SUVR) and a tau biomarker (CSF p181-tau), preserved brain volume (vMRI), and also increased risk of the hallmark adverse event of this drug class, ARIA.
We updated and expanded upon the results of earlier meta-analyses (Foroutan et al., 2019; Mo et al., 2017; Penninkilampi et al., 2017), which allowed us to reach novel conclusions. Critically, we included the recently reported Aducanumab phase III results (EMERGE/ENGAGE_Investigators, 2019; Schneider, 2020), which may have contributed to showing some statistically significant effects, as well as the most recently reported Crenezumab phase III results. Aducanumab data included in this meta-analysis were extracted from the presentation given at “Clinical Trials on AD” conference that took place in December 5, 2019 (EMERGE/ENGAGE_Investigators, 2019). From this presentation, we only included data from the intention-to-treat analysis. Data from post-hoc analyses were excluded. To be as comprehensive as possible, we performed two analyses: one including all available data (from peer reviewed publications, published presentations at scientific meetings, and data posted on ClinicalTrials.gov) and one including data from peer-reviewed manuscripts only. To gain pharmacodynamic insights, we synthesized biochemical and neuroimaging biomarker outcomes and examined whether clinical effects relate to effects on biomarkers.
Overall, monoclonal antibodies demonstrated strong target engagement and biomarker responses. The effect sizes for amyloid PET SUVR and CSF p181-tau reductions were large (~1.00 and 0.9 respectively) (figure 3). However, the clinical benefits of monoclonal antibodies had rather small effect sizes (0.06 for ADAS-Cog, 0.05 for MMSE), despite their statistical significance (figures 2, 5). Such effect sizes correspond to point-changes on these scales that may be of small and questionable clinical significance (table 2) (Andrews et al., 2019; Birks, 2006; Cohen, 1992; Hensel et al., 2007; Schrag et al., 2012). Moreover, monoclonal antibodies produced only a trend towards improving function/cognition on CDR-SOB (figure 2), an important measure for proof of efficacy in AD trials (FDA, 2018). The small effect sizes for clinical improvements observed, may allow some skeptics to claim that an inherent low ceiling exists for the therapeutic efficacy of monoclonal antibodies or, perhaps, any Aβ-reducing strategy. Interestingly, we found that reduction of amyloid deposition measured by PET was moderately correlated with cognitive improvements on ADAS-Cog. Although it is tempting to speculate that agents producing even greater Aβ reductions than already achieved may be able to produce more robust cognitive and perhaps functional improvements, extrapolating the correlation curve beyond our current data may be misleading. Regarding individual drugs, Aducanumab produced statistically favorable results for multiple clinical and biomarker outcomes. Aducanumab statistically improved ADAS-Cog, and it was the only drug that statistically improved CDR-SOB and “AD Cooperative Study-Activities of Daily Living” (figures 2, 4), therefore potentially benefiting both cognition and function. The effect sizes for these improvements were small (effect sizes < 0.2 correspond to clinically minor score changes on the ADAS-Cog, CDR-SOB and “AD Cooperative Study-Activities of Daily Living” scales) (table 2) (Andrews et al., 2019; Birks, 2006). In addition, Aducanumab decreased brain Aβ burden and CSF p-tau by large effect sizes (ideal Number Needed to Treat) (table 2). The combination of statistical improvements in multiple clinical outcomes and strong target engagement/disease-modifying properties identify Aducanumab as the most promising candidate among this drug class (figures 2–4). Aducanumab is currently being considered for potential approval by the FDA (Biogen, 2021; Sabbagh and Cummings, 2020), but there is no consensus among experts on whether Aducanumab has demonstrated efficacy or not (Knopman et al., 2020; Sabbagh and Cummings, 2020). It is interesting to note that the high dose EMERGE study was associated with both greater amyloid PET SUVR reduction and a more favorable effect on ADAS-Cog and CDR-SOB compared to the low dose EMERGE study (see the two red points towards the left that correspond to the EMERGE studies in both graphs of figure 5). Disappointingly, the high dose ENGAGE study was not associated with a more favorable effect on ADAS-Cog and CDR-SOB despite the greater reduction on amyloid deposition when compared to the low dose ENGAGE study (see the two red points towards the right that correspond to the ENGAGE studies in both graphs of figure 5). It is also paradoxical that the high dose Aducanumab in ENGAGE trial produced a similar effect on ADAS-Cog with the low dose Aducanumab, but a worse effect on MMSE and CDR-SOB (figure 2). These conflicting findings naturally raise concerns about the robustness of Aducanumab effects.
Solanezumab also showed promise by statistically improving two cognitive measures (ADAS-Cog and MMSE) and showing trends towards improving statistically a functional (“AD Cooperative Study-Activities of Daily Living”) outcome (figures 2 and 4). Even though Solanezumab did not decrease PET amyloid burden or CSF p181-tau, it increased CSF Aβ1–40 and, at trend, CSF Aβ1–42, suggesting favorable effects on Aβ dynamics. All statistical improvements by Solanezumab had small effect sizes (< 0.2), except for Aβ1–40 which was of moderate effect size (~ 0.5). Bapinezumab, Gantenerumab and Crenezumab did not improve clinical and amyloid pathology outcomes. However, Bapineuzumab and Gantenerumab decreased CSF p181-tau (figure 3).
We hoped that contrasting and comparing features of antibodies may inform future therapeutic development. It is not apparent why Solanezumab and Aducanumab produced the most statistical improvements. Solanezumab is a humanized murine antibody, whereas Aducanumab is a human monoclonal antibody (Kwon et al., 2020; van Dyck, 2018). Solanezumab mainly targets Aβ monomers, whereas Aducanumab mainly targets oligomers and fibrils (Kwon et al., 2020; van Dyck, 2018). Moreover, their targeted Aβ epitopes are also different (16–26 vs 3–7) (Kwon et al., 2020; van Dyck, 2018). Gantenerumab, which, similarly to Aducanumab, has preferential affinity for Aβ oligomers and fibril, did not produce any significant effects. Interestingly, antibodies that target multiple Aβ conformations such as Bapineuzumab and Crenezumab (Kwon et al., 2020; van Dyck, 2018), did not produce any clinical or biomarker benefits. These observations suggest that antibodies with widely different properties may be effective in AD, but complete lack of Aβ target specificity may not be a desirable feature of an “ideal” antibody. This observation was also shown in subgroup analyses of ADAS-Cog and MMSE by targeted Aβ species (supplemental tables 1 and 2).
Whereas treatment as early as possible is favored by many (Aisen et al., 2020; DIAN-TU_Clinical_Trial, 2020; van Dyck, 2018), our meta-regressions did not reveal any associations between baseline MMSE and primary outcome effect sizes. Moreover, although Solanezumab trials enrolled patients with more advanced AD than Aducanumab trials (baseline MMSE 21–22.8 vs. 26.4), both drugs resulted in statistically significant improvements of clinical outcomes. Conversely, Gantenerumab, an antibody with similar preference for Aβ oligomers and an overlapping epitope target with Aducanumab (3–11/18–27 vs 3–7) (Kwon et al., 2020; van Dyck, 2018), failed to show any benefits in patients with early disease (mean MMSE: 25.7). Furthermore, in recently announced topline results from the First Dominantly Inherited Alzheimer Network Clinical Trial (DIAN-TU-001), Solanezumab and Gantenerumab failed to meet the primary cognitive endpoint in asymptomatic or mildly symptomatic mutation carriers (DIAN-TU_Clinical_Trial, 2020). Thus, although patients with early clinical disease may be desirable for AD trials (e.g., to avoid floor effects for cognitive outcomes), the available evidence suggests that administration in early disease does not guarantee drug success.
ARIA risk was high for all drugs, except for Solanezumab. Aducanumab caused both the greatest brain amyloid reduction and the greatest risk for ARIA and headaches. This finding is in accordance with the “ARIA paradox”, which posits that Aβ mobilization achieved by immunotherapies may be causally linked to both efficacy and ARIA risk (DiFrancesco et al., 2015). An optimal balance between efficacy and ARIA risk has yet to determined (Piazza and Winblad, 2016). This meta-analysis contributes to this ongoing discussion by showing that an anti-Aβ drug, Solanezumab, can produce some statistically significant effects on clinical outcomes (ADAS-Cog, MMSE) without increasing ARIA risk. The low ARIA risk with Solanezumab may be attributable to its preferential targeting of monomeric Aβ, which may be removed without affecting Aβ deposited in plaques (Honig et al., 2018; Racke et al., 2005; Sperling et al., 2011), whereas immunotherapies targeting plaques may mobilize Aβ species more likely to induce vascular damage and ARIA (Racke et al., 2005; Sperling et al., 2011).
Although Aβ brain accumulation is a pathologic hallmark of AD, the relationship between Aβ pathology and cognitive decline is indirect (Hanseeuw et al., 2019), a fact that has been used as an argument against anti-Aβ therapeutic strategies. To investigate whether cognitive benefits by monoclonal antibodies were associated with their ability to reduce Aβ brain deposition, as previously suggested (Geerts et al., 2018), we examined the correlation between effects on amyloid PET SUVR and clinical outcomes. We found that reductions in Aβ brain deposition were associated with improvements of cognition (figure 5). This finding supports the view that Aβ is a rational target and that evidence for target engagement and strong pharmacodynamic effects may be required for any anti-Aβ treatment to improve clinical outcomes. However, reduction on amyloid PET SUVR was not significantly correlated with improvement on CDR-SOB, an outcome which has lately been used as the primary outcome in AD trials since it incorporates functional assessment in addition to cognition. Although this finding was not encouraging, the correlation was to the correct direction (r = +0.51) and the p-value showed a trend towards significance (p = 0.09), a relatively promising finding.
Our sensitivity analyses (excluding non-peer reviewed data) generated results that were largely consistent with those of the main analysis. For most outcomes (ADAS-Cog, MMSE, CDR-SOB, CSF p181-tau, ARIA risk), the sensitivity analyses yielded almost identical results to those of the main analyses. Interestingly, the sensitivity analysis for amyloid PET SUVR showed a reduced effect size compared to that of the main analysis. This difference may be attributable to the exclusion of Aducanumab data from the sensitivity analysis, since Aducanumab induced the most robust amyloid reduction on PET. Excluding Aducanumab and Crenezumab (sensitivity analysis) also worsened the effect for “AD Cooperative Study-Activities of Daily Living” but improved the effect for vMRI. Finally, the positive correlation between amyloid PET reduction and cognitive improvement was rendered non-significant in the sensitivity analysis, something that may also be attributed to the exclusion of the sizable effects of Aducanumab.
Between-study heterogeneity was small (I2 < 20%) in the meta-analyses of clinical outcomes (ADAS-Cog, MMSE, CDR-SOB, ADCS-ADL), but substantial (I2 > 85%) in the meta-analyses of major biomarker outcomes (amyloid PET SUVR, CSF p181-tau) and the adverse event ARIA. Aducanumab introduced heterogeneity in the meta-analysis for amyloid PET SUVR (figure 3), because it induced robust reductions of amyloid compared to other antibodies. Similarly, different antibodies had different effects on CSF p181-tau resulting in high heterogeneity in the respective meta-analysis. In the meta-analysis for ARIA risk, Solanezumab introduced heterogeneity as it was the only drug that did not increase ARIA risk.
5. Conclusions
This meta-analysis of phase III RCTs showed that monoclonal antibodies against Aβ as a class statistically improved cognition by a small effect size and robustly decreased brain amyloid burden and CSF p181-tau suggesting some degree of disease modification, at the expense of increasing ARIA risk. Aducanumab, which is currently being reviewed by the FDA, produced the most promising clinical and biomarker results, followed by Solanezumab. Our results partly address some hypotheses discussed within the field of passive immunization trials: we couldn’t find which specific Aβ targets are the most appropriate, although, non-specific targeting of multiple species seemed to have no effect at all. In addition, this study showed that enrolling participants with mild clinical AD is not sufficient to guarantee efficacy. We also showed that cognitive effects are moderately correlated with and, therefore, may be predicted by amyloid reduction on PET (Kramer, 2020). We hope that these results will help the field better assess the merits of passive immunotherapy against Aβ, as approached to this date, and determine the next steps of therapeutic development in AD.
Supplementary Material
Highlights.
The increased power of this meta-analysis allowed us to detect statistical improvements of small effect size for clinical outcomes and of large effect size for biomarker outcomes, induced by monoclonal antibodies against Aβ in Alzheimer’s disease.
Antibody effects on reducing amyloid PET deposition were correlated with their effects on improving cognition.
Among individual drugs, Aducanumab produced the most consistent effects (statistically improved ADAS-Cog, CDR-SOB, ADCS-ADL, amyloid PET SUVR and CSF p181-tau) followed by Solanezumab (statistically improved ADAS-Cog, MMSE and CSF Aβ1–40).
Acknowledgement:
This research was supported entirely by the Intramural Research Program of the National Institute on Aging.
Footnotes
Declaration of Competing Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisen PS, Cummings J, Doody R, Kramer L, Salloway S, Selkoe DJ, Sims J, Sperling RA, Vellas B, 2020. The Future of Anti-Amyloid Trials. J Prev Alzheimers Dis 7, 146–151. [DOI] [PubMed] [Google Scholar]
- Andrews JS, Desai U, Kirson NY, Zichlin ML, Ball DE, Matthews BR, 2019. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer’s disease clinical trials. Alzheimer’s & dementia 5, 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biogen, 2020. UPDATE ON FDA ADVISORY COMMITTEE’S MEETING ON ADUCANUMAB IN ALZHEIMER’S DISEASE.
- Biogen, 2021. BIOGEN AND EISAI ANNOUNCE FDA’S 3-MONTH EXTENSION OF REVIEW PERIOD FOR THE BIOLOGICS LICENSE APPLICATION FOR ADUCANUMAB.
- Birks J, 2006. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev, CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, de Leon MJ, Zetterberg H, 2006. Alzheimer’s disease. Lancet 368, 387–403. [DOI] [PubMed] [Google Scholar]
- Borenstein M HL, Higgins JPT, Rothstein HR, 2009a. Independent Subgroups within a Study, Introduction to Meta‐Analysis. 2009 John Wiley & Sons, Ltd. [Google Scholar]
- Borenstein M HL, Higgins JPT, Rothstein HR, 2009b. Random-Effects Modeel, Introduction to Meta‐Analysis. 2009 John Wiley & Sons, Ltd. [Google Scholar]
- Cohen J, 1992. A power primer. Psychological bulletin 112, 155–159. [DOI] [PubMed] [Google Scholar]
- CREAD1, 2020. A Study Evaluating the Efficacy and Safety of Crenezumab Versus Placebo in Participants With Prodromal to Mild Alzheimer’s Disease (AD). (CREAD).
- CREAD2, 2020. A Study of Crenezumab Versus Placebo to Evaluate the Efficacy and Safety in Participants With Prodromal to Mild Alzheimer’s Disease (AD) (CREAD 2), ClnicalTrials.gov.
- Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K, 2020. Alzheimer’s disease drug development pipeline: 2020. Alzheimer’s & dementia 6, e12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAN-TU_Clinical_Trial, 2020. Topline Result for First DIAN-TU Clinical Trial: Negative on Primary.
- DiFrancesco JC, Longoni M, Piazza F, 2015. Anti-Abeta Autoantibodies in Amyloid Related Imaging Abnormalities (ARIA): Candidate Biomarker for Immunotherapy in Alzheimer’s Disease and Cerebral Amyloid Angiopathy. Frontiers in neurology 6, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, Siemers E, Liu-Seifert H, Mohs R, Alzheimer’s Disease Cooperative Study Steering, C., Solanezumab Study, G., 2014. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. The New England journal of medicine 370, 311–321. [DOI] [PubMed] [Google Scholar]
- EMERGE/ENGAGE_Investigators, 2019. EMERGE and ENGAGE Topline Results: Two Phase 3 Studies to Evaluate Aducanumab in Patients With Early Alzheimer’s Disease Online PP Presentation
- FDA, 2018. Early Alzheimer’s Disease: Developing Drugs for Treatment Guidance for Industry. [DOI] [PMC free article] [PubMed]
- Foroutan N, Hopkins RB, Tarride JE, Florez ID, Levine M, 2019. Safety and efficacy of active and passive immunotherapy in mild-to-moderate Alzheimer’s disease: A systematic review and network meta-analysis. Clin Invest Med 42, E53–E65. [DOI] [PubMed] [Google Scholar]
- Geerts H, Spiros A, Roberts P, 2018. Impact of amyloid-beta changes on cognitive outcomes in Alzheimer’s disease: analysis of clinical trials using a quantitative systems pharmacology model. Alzheimer’s research & therapy 10, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, Cosio DMO, Farrell M, Quiroz YT, Mormino EC, Buckley RF, Papp KV, Amariglio RA, Dewachter I, Ivanoiu A, Huijbers W, Hedden T, Marshall GA, Chhatwal JP, Rentz DM, Sperling RA, Johnson K, 2019. Association of Amyloid and Tau With Cognition in Preclinical Alzheimer Disease: A Longitudinal Study. JAMA Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ, 2002. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356. [DOI] [PubMed] [Google Scholar]
- Harrer M, Cuijpers P, Furukawa TA, & Ebert DD, 2019. Doing Meta-Analysis in R: A Hands-on Guide.
- Hensel A, Angermeyer MC, Riedel-Heller SG, 2007. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. Journal of neurology, neurosurgery, and psychiatry 78, 1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, D.J.a.A.D., How to include multiple groups from one study (Chapter 16.5.4.), in: Higgins JPT, G.S.e. (Ed.), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011).. The Cochrane Collaboration, 2011.. [Google Scholar]
- Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, Hager K, Andreasen N, Scarpini E, Liu-Seifert H, Case M, Dean RA, Hake A, Sundell K, Poole Hoffmann V, Carlson C, Khanna R, Mintun M, DeMattos R, Selzler KJ, Siemers E, 2018. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. The New England journal of medicine 378, 321–330. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Jones DT, Greicius MD, 2020. Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Kupfer DJ, 2006. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry 59, 990–996. [DOI] [PubMed] [Google Scholar]
- Kramer LD, 2020. Editorial: An Industry Perspective: Future of Anti-Amyloid Trials. J Prev Alzheimers Dis 7, 142–143. [DOI] [PubMed] [Google Scholar]
- Kwon S, Iba M, Kim C, Masliah E, 2020. Immunotherapies for Aging-Related Neurodegenerative Diseases-Emerging Perspectives and New Targets. Neurotherapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo JJ, Li JY, Yang Z, Liu Z, Feng JS, 2017. Efficacy and safety of anti-amyloid-beta immunotherapy for Alzheimer’s disease: a systematic review and network meta-analysis. Ann Clin Transl Neurol 4, 931–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowitzki S, Lasser RA, Dorflinger E, Scheltens P, Barkhof F, Nikolcheva T, Ashford E, Retout S, Hofmann C, Delmar P, Klein G, Andjelkovic M, Dubois B, Boada M, Blennow K, Santarelli L, Fontoura P, Investigators SCR, 2017. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimer’s research & therapy 9, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, Lozupone M, Logroscino G, Imbimbo BP, 2019. A critical appraisal of amyloid-beta-targeting therapies for Alzheimer disease. Nature reviews. Neurology 15, 73–88. [DOI] [PubMed] [Google Scholar]
- Penninkilampi R, Brothers HM, Eslick GD, 2017. Safety and Efficacy of Anti-Amyloid-beta Immunotherapy in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J Neuroimmune Pharmacol 12, 194–203. [DOI] [PubMed] [Google Scholar]
- Piazza F, Winblad B, 2016. Amyloid-Related Imaging Abnormalities (ARIA) in Immunotherapy Trials for Alzheimer’s Disease: Need for Prognostic Biomarkers? Journal of Alzheimer’s disease : JAD 52, 417–420. [DOI] [PubMed] [Google Scholar]
- Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, Holtzman DM, Bales KR, Gitter BD, May PC, Paul SM, DeMattos RB, 2005. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid beta. The Journal of neuroscience : the official journal of the Society for Neuroscience 25, 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh MN, Cummings J, 2020. Open Peer Commentary to “Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE Trials as reported by Biogen December 2019”. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR, Bapineuzumab, Clinical Trial, I., 2014. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. The New England journal of medicine 370, 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L, 2020. A resurrection of aducanumab for Alzheimer’s disease. The Lancet. Neurology 19, 111–112. [DOI] [PubMed] [Google Scholar]
- Schrag A, Schott JM, Alzheimer’s Disease Neuroimaging, I., 2012. What is the clinically relevant change on the ADAS-Cog? Journal of neurology, neurosurgery, and psychiatry 83, 171–173. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J, 2016. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8, 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Jack CR Jr., Black SE, Frosch MP, Greenberg SM, Hyman BT, Scheltens P, Carrillo MC, Thies W, Bednar MM, Black RS, Brashear HR, Grundman M, Siemers ER, Feldman HH, Schindler RJ, 2011. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 7, 367–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernan MA, Hopewell S, Hrobjartsson A, Junqueira DR, Juni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT, 2019. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj 366, l4898. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, 2018. Anti-Amyloid-beta Monoclonal Antibodies for Alzheimer’s Disease: Pitfalls and Promise. Biol Psychiatry 83, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Rinne JO, Boada M, Katayama S, Scheltens P, Vellas B, Tuchman M, Gass A, Fiebach JB, Hill D, Lobello K, Li D, McRae T, Lucas P, Evans I, Booth K, Luscan G, Wyman BT, Hua L, Yang L, Brashear HR, Black RS, Bapineuzumab, Clinical Study, I., 2016. Bapineuzumab for mild to moderate Alzheimer’s disease in two global, randomized, phase 3 trials. Alzheimer’s research & therapy 8, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.