Abstract
Purpose
To evaluate the post- coronavirus disease-19 (COVID-19) outcome of thyroid function in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related thyrotoxicosis.
Methods
This was a single-center prospective study involving 29 patients (11 females, 18 males; median age 64 years, range: 43–85) with thyrotoxicosis diagnosed after hospitalization for COVID-19 and then followed-up for a median period of 90 days (range: 30–120) after hospital discharge. At follow-up, patients were evaluated for serum thyrotropin (TSH), free-thyroxine (FT4), free-triiodiothyronine (FT3), TSH receptor antibodies (TRAb), thyroglobulin antibodies (TgAb), thyroperoxidase antibodies (TPOAb) and ultrasonographic thyroid structure.
Results
After recovery of COVID-19, serum TSH values significantly increased (P < 0.001) and FT4 values significantly decreased (P = 0.001), without significant change in serum FT3 (P = 0.572). At follow-up, 28 subjects (96.6%) became euthyroid whereas overt hypothyroidism developed in one case. At the ultrasound evaluation of thyroid gland, hypoecogenicity was found in 10 patients (34.5%) and in these cases serum TSH values tended to be higher than those without thyroid hypoecogenity (P = 0.066). All subjects resulted to be negative for TgAb, TPOAb and TRAb.
Conclusion
In a short-term follow-up, thyroid function spontaneously normalized in most subjects with SARS-CoV-2-related thyrotoxicosis. However, thyroid hypoecogenicity was found in a remarkable number of them and future longer-term studies are needed to clarify whether this ultrasonographic alteration may predispose to develop late-onset thyroid dysfunction.
Keywords: Covid-19, SARS-CoV2, Thyroid, Thyrotoxicosis, Hyperthyroidism, Thyroiditis
Introduction
The coronavirus disease-19 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to affect the global community and in our country more than 2 million of individuals have been infected by SARS-CoV-2.
Although the pulmonary system is the main target of SARS-CoV2 infection [1], extrapulmonary organs can be affected with potential negative impact on clinical outcome of COVID-19 [2]. Interestingly, alterations of thyroid hormones resembling a subacute thyroiditis (SAT) have been consistently reported in a substantial number of subjects with COVID-19 [3–11], suggesting that thyroid gland may be a frequent target of SARS-CoV2 infection [12, 13]. However, the clinical relevance of thyrotoxicosis associated with SARS-CoV2 infection is still unclear [3, 4, 14, 15] and data on the outcome of thyroid function after recovery of COVID-19 are scanty [14]. Specifically, it is still unknown whether thyroid gland may be persistently damaged by SARS-CoV2, such as demonstrated for other tissues and organs in individuals with COVID-19 [16].
In this prospective study, we evaluated the outcome of thyroid function and ultrasonographic structure after recovery of COVID-19 in individuals who had developed thyrotoxicosis during hospital stay for SARS-CoV2 infection.
Materials and methods
This is a prospective single-center study performed on consecutive patients who had been hospitalized for COVID-19 at the IRCCS, Humanitas Research Hospital, Rozzano-Milan, Italy in the period between March 1st and April 1st 2020 [3]. The inclusion criteria were: 1) hospitalization for COVID-19 diagnosed by real-time reverse-transcriptase–polymerase-chain-reaction assay of nasal and pharyngeal swab specimens and/or bronco-alveolar lavage fluid associated with clinical and radiological signs of pneumonia [17]; 2) primary thyrotoxicosis diagnosed during hospital stay [3]; 3) duration of follow-up ≥30 days after hospital discharge. Exclusion criteria were: 1) treatment with levo-thyroxine or anti-thyroid drugs before and at the time of first thyrotropin (TSH) evaluation; 2) treatment with drugs interfering with thyroid function after recovery of COVID-19.
The diagnosis of clinical and subclinical thyrotoxicosis was based on suppressed serum TSH values associated with high or normal serum free-thyroxine (FT4), respectively. Among 58 patients who developed a SARS-CoV2-related thyrotoxicosis [3], 23 patients (39.7%) died, whereas 6 patients (10.3%) were lost at follow-up. Therefore, 29 patients (11 females, 18 males; median age 64 years, range: 43–85) were enrolled in this prospective study. Nobody of these patients received corticosteroids during hospital stay.
The first end-point was the evaluation of serum TSH values after at least 30 days of follow-up. As secondary end-points, we also evaluated serum FT4 (24 cases), free-triiodiothyronine (FT3) (14 cases), TSH receptor antibodies (TRAb) (29 cases), thyroglobulin antibodies (TgAb) (29 cases) and thyroperoxidase antibodies (TPOAb) (29 cases) and ultrasonographic thyroid structure (29 cases) at the follow-up.
The study was approved by the Ethics Committee of IRCCS Humanitas Research Hospital, and the patients gave their consent to use the clinical and biochemical data for research purposes.
Biochemical assays
Serum TSH, FT4, FT3 were measured at 8.00 a.m. using chemiluminescent methods on the Beckman Coulter DxI 800 Access® immunoassay system. In our laboratory, the reference ranges of TSH, FT4 and FT3 were 0.34–4.80 mU/L, 7.82–17.29 pmol/L and 3.38–6.45 pmol/L, respectively. TRAb were determined using the TRACE (Time-Resoved Amplified Cryptate Emission) on the Kryptor analyzer and reference range in our laboratory was <1.8 IU/L. Overt thyrotoxicosis was defined by low TSH values and serum FT3 and/or FT4 above the reference ranges. Overt hypothyroidism was defined by high TSH values and serum FT4 and/or FT3 below the reference ranges. Subclinical thyroid dysfunction was defined when TSH was either low or high accompanied by FT4 and FT3 in the reference ranges.
Ultrasound evaluation of thyroid gland
Ultrasonographic examination of the thyroid was performed with a linear transducer 5–14 (CANON APLIO A). The echogenicity was evaluated by a standardized comparison of thyroid parenchyma with the adjacent sternohyoideus, sternothyroideus and sternocleidomastoideus muscles, in a longitudinal scan of the thyroid lobes. Thyroid volume was calculated measuring the three axes of the thyroid lobes; for all measurements, the transducer kept perpendicular the skin surface. The length was measured from the most cranial to the most caudal part of the lobe on a screen picture following the longitudinal axis of the lobe; the maximal width and depth of the lobe were measured horizontally on a screen picture cross-sectional to the longitudinal axis of the lobe, taken from the middle half of the lobe in the lateral plane. Thyroid volume was calculated for each lobe separately using the formula for a rotation ellipsoid (length × width × depth × π/6) [18].
Statistical analyses
Data were presented as median and range, unless otherwise stated. The un-paired comparisons were performed by Mann–Whitney’s and Kruskal–Wallis’ tests, whereas paired data were compared by Wilcoxon’s and Friedman’s tests. Frequencies were compared by the Chi-Square’s test, with Fisher correction when appropriate. Association between TSH values and thyroid volume was sought by calculating the Spearman’s rank correlation coefficient. A p value <0.05 was considered as significant.
Results
Table 1 reports the individual data of 29 subjects with SARS-CoV2-related thyrotoxicosis who were included in the prospective evaluation of thyroid function and structure. As compared to patients with SARS-CoV2-related thyrotoxicosis who died during hospital stay, the enrolled subjects were significantly younger (64 years, range: 43–85 vs. 79 years, range: 67–90; P < 0.001) and had higher serum FT3 values (4.50 pmol/L, range: 3.28–7.33 vs. 4.05 pmol/L, range: 3.00–5.06; P = 0.016), without significant differences in sex (P = 0.608), serum FT4 (P = 0.934) and TSH (P = 0.233) values.
Table 1.
Individual clinical data of patients with SARS-CoV-2-related thyrotoxicosis prospectively evaluated after recovery of COVID-19
| BASELINE | FOLLOW-UP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age (years) | TSH (mU/L) | FT4 (pmol/L) | FT3 (pmol/L) | Duration (days) | TSH (mU/L) | FT4 (pmol/L) | FT3 (pmol/L) | Thyroid volume (mL) | Thyroid hypoecogenicity |
| M | 66 | 0.33 | 14.36 | 4.78 | 30 | 1.90 | 15.00 | NA | 12 | NO |
| F | 58 | 0.31 | 19.00 | 3.70 | 41 | 2.25 | 13.00 | 4.90 | 9.5 | NO |
| F | 85 | 0.20 | 19.00 | 7.33 | 43 | 0.70 | 10.48 | 6.00 | 9 | NO |
| F | 55 | 0.21 | 17.48 | 4.95 | 44 | 0.55 | 13.97 | 5.73 | 15 | NO |
| F | 64 | 0.30 | 22.23 | 5.28 | 60 | 3.19 | 12.50 | 3.00 | 10 | YES |
| M | 43 | 0.32 | 12.53 | 4.77 | 60 | 1.81 | 11.00 | NA | 12 | NO |
| M | 61 | 0.17 | 19.93 | 5.05 | 60 | 1.31 | 14.00 | NA | 10 | YES |
| M | 53 | 0.12 | 16.86 | 3.86 | 60 | 0.57 | 13.00 | NA | 14 | YES |
| M | 61 | 0.29 | 12.00 | 4.00 | 62 | 1.11 | 9.93 | 5.16 | 5 | YES |
| F | 60 | 0.32 | 18.50 | 4.82 | 66 | 0.70 | 16.00 | NA | 11 | NO |
| M | 48 | 0.24 | 15.68 | 4.92 | 70 | 0.45 | 11.00 | NA | 19 | NO |
| M | 49 | 0.12 | 13.65 | 4.34 | 90 | 6.78 | 4.11 | 1.80 | 6.5 | YES |
| M | 81 | 0.19 | 27.66 | 3.49 | 90 | 3.77 | 12.60 | NA | 21 | YES |
| F | 73 | 0.13 | 22.44 | 4.09 | 90 | 1.84 | 12.23 | 2.68 | 11.3 | NO |
| M | 76 | 0.20 | 15.56 | 4.53 | 90 | 1.64 | 12.87 | 4.73 | 14 | YES |
| F | 64 | 0.33 | 20.75 | 4.10 | 90 | 1.60 | 14.30 | 5.00 | 3.0 | NO |
| M | 74 | 0.33 | 16.38 | 5.20 | 90 | 1.36 | 10.62 | 6.58 | 50 | NO |
| F | 80 | 0.14 | 13.59 | 3.86 | 90 | 1.21 | 15.10 | 5.00 | 10.2 | YES |
| F | 67 | 0.29 | 22.40 | 4.50 | 90 | 0.92 | 17.00 | NA | 11 | NO |
| F | 74 | 0.30 | 18.68 | 4.50 | 90 | 0.86 | 15.00 | 4.30 | 3.2 | NO |
| M | 77 | 0.33 | 15.82 | 3.28 | 90 | 0.66 | 13.00 | NA | 18 | NO |
| M | 62 | 0.30 | 16.60 | 4.69 | 92 | 2.11 | NA | NA | 22 | YES |
| M | 57 | 0.14 | 24.05 | 4.00 | 93 | 1.50 | 12.28 | 4.04 | 9.5 | YES |
| M | 70 | 0.05 | 30.49 | 3.50 | 96 | 3.44 | NA | NA | 24 | NO |
| M | 68 | 0.08 | 18.26 | 4.74 | 96 | 0.93 | NA | NA | 12 | NO |
| F | 77 | 0.12 | 26.30 | 4.30 | 97 | 1.49 | 13.66 | 2.77 | 12 | NO |
| M | 57 | 0.20 | 16.90 | 4.50 | 100 | 0.63 | NA | NA | 16.5 | NO |
| M | 65 | 0.09 | 17.59 | 4.75 | 120 | 1.03 | NA | NA | 16 | NO |
| M | 63 | 0.33 | 19.00 | 5.00 | 120 | 0.93 | 16.00 | NA | 13 | NO |
F females, FT3 free-iodiothyronine, FT4 free-thyroxine, I increased, M males, NA not available, TSH thyrotropin
At study entry, overt and subclinical thyrotoxicosis were found in 17 patients (58.62%) and 12 (41.38%) patients, respectively (Table 1). After a median period of 90 days (range 30–120), median serum TSH values increased (29 cases; from 0.21 mU/L, range: 0.05–0.33 to 1.49 mU/L, range: 0.45–6.78; P < 0.001; Fig. 1) and FT4 values decreased (24 cases from 18.59 pmol/L, range: 12.00–27.66 to 13.00 pmol/L, range: 4.11–17.00; P = 0.001), without significant change in serum FT3 (14 cases; P = 0.572). At follow-up, 28 subjects (96.6%) became euthyroid whereas overt hypothyroidism developed in one case (TSH 6.78 mU/L, FT4 4.11 pmol/L, FT3 1.8 pmol/L) (Fig. 1).
Fig. 1.
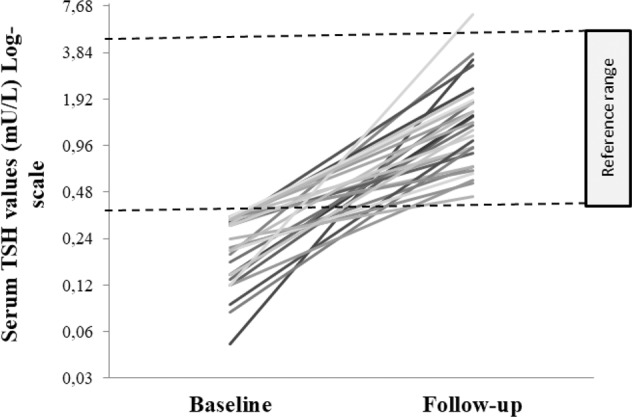
Individual outcome of serum thyrotropin (TSH) values, expressed in logarithmic scale, in 29 subjects with SARS-CoV2-related thyrotoxicosis followed up for a median period of 90 days (range 30–120)
At follow-up, all subjects resulted to be negative for TgAb, TPOAb and TRAb. At the ultrasound evaluation of thyroid gland, hypoecogenicity was found in 10 patients (34.5%) (Table 1 and Fig. 2). Patients with thyroid hypoecogenicity tended to have higher serum TSH values as compared to those without thyroid hypoecogenicity (1.57 mU/L, range: 0.57–6.78 vs. 0.93 mU/L, range: 0.45–3.44; P = 0.066). No significant association was found between TSH values and thyroid volume at follow-up (rho −0.10; P = 0.637) (Table 1).
Fig. 2.
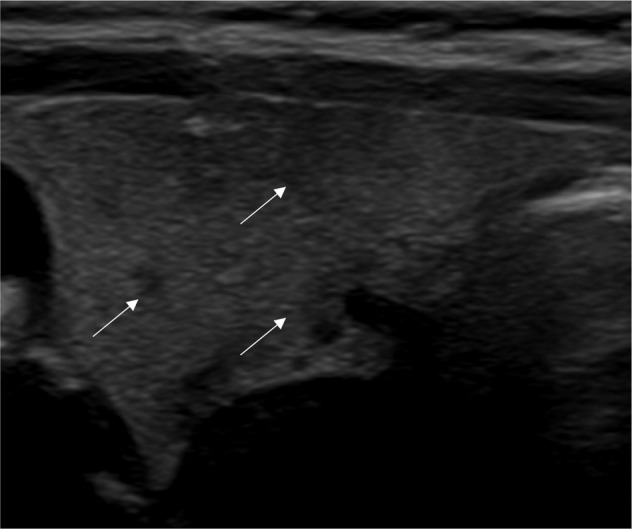
Thyroid hypoecogenicity (indicated by the arrows) in a patient with recent SARS-CoV-2-related thyrotoxicosis
Discussion
In this prospective study, all but one subjects with SARS-CoV-2-related thyrotoxicosis became euthyroid after recovery of COVID-19. However, ultrasound evaluation of thyroid structure revealed hypoecogenicity in one-third of patients in relationship with higher serum TSH values at follow-up.
Since the first months of COVID-19 outbreak, thyroid dysfunction has been reported in a remarkable number of subjects with SARS-CoV-2 infection. Specifically, thyrotoxicosis resembling SAT was consistently described in hospitalized patients with COVID-19 [3, 4, 6–11]. Thyroid cells might be potential target for SARS-CoV-2 entry as they have been found to express ACE-2 mRNA [13] and thyroid follicular and parafollicular epithelium might be directly damaged by the virus with consequent alteration of thyroid structure and function. The clinical relevance of thyroid dysfunction in the setting of COVID-19 is still uncertain. In our previous report [3], patients with overt thyrotoxicosis showed higher incidence of atrial fibrillation and thromboembolic events. In other experiences, thyroid dysfunction was associated with a unfavorable outcome of COVID-19, in terms of higher risk of acute respiratory distress, cardiac injury, fatal events and duration of hospitalization [19]. In our cohort, about 40% of patients with SARS-CoV2-related thyrotoxicosis died during hospital stay and noteworthy these subjects showed significantly lower serum FT3 values as compared to the survivors, likely reflecting the stronger inhibitory effects of severe systemic illness on peripheral conversion of T4 in T3 [14].
Infections may represent an environmental trigger of subsequent autoimmune thyroid diseases and thyroid dysfunction [20]. It is known that in the months and years following subacute viral thyroiditis there is a higher incidence of thyroid autoimmunity [21] and persistent hypothyroidism was found to develop in up to 15–20% of subjects with SAT [22–25], in relationship with persistent alterations of thyroid structure [22, 25]. Whether this is also applicable to patients after thyroiditis induced by SARS-CoV-2 is still unknown [3, 4, 14] and data on thyroid echographic parameters in this setting are scanty [3, 4]. In our prospective study, the majority of subjects (96%) achieved euthyroidism in few weeks after recovery of COVID-19, while only one subject became hypothyroidism. Such a low prevalence of overt hypothyroidism could be possibly related to a mild parenchymal damage induced by SARS-CoV-2 as compared to the typical SAT. However, a relevant number of subjects had serum TSH in the highest part of reference range in close relationship with hypoecogenicity of parenchyma at ultrasound evaluation of thyroid gland. All patients were found to be negative for thyroid autoantibodies suggesting that at least in a short-term SARS-CoV-2 infection may have not induced an autoimmune process against thyroid gland [26].
This study has limitations. The short-term follow-up and the small size of study group restricted to only survivors with subclinical or overt thyrotoxicosis likely did not allow to capture all cases developing thyroid dysfunction after resolution of SARS-CoV-2 infection [22]. However, the finding of hypoecogenicity in a remarkable number of subjects with recent SARS-CoV-2-related thyrotoxicosis provided a rationale for planning a strict follow-up in these cases for identifying possible late-onset thyroid dysfunction [27].
In conclusion, this prospective and monocenter study shows that most of subjects with SARS-CoV-2-related thyrotoxicosis normalize thyroid function few weeks after resolution of COVID-19. However, thyroid hypoecogenicity was found in a remarkable number of them and future high-quality prospective and longer-term studies will clarify whether this ultrasonographic alteration may predispose to develop late-onset thyroid dysfunction.
Acknowledgments
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Humanitas COVID-19 Task force
Stefano Accornero6, Alessio Aghemo6, Ludovico Alfarone6, Hussam Ali6, Monia Aloise6, Claudio Angelini6, Ivan Arcari6, Paola Arosio6, Elena Azzolini6, Alessandra Baccarin6, Salvatore Badalamenti6, Sara Baggio6, Luca Balzarini6, Caterina Barberi6, Franca Barbic6, Viviana Barbieri6, Alessandro Barbone6, Alessio Basciu6, Chiara Benvenuti6, Ilaria Bianchi6, Monica Bocciolone6, Cristiana Bonifacio6, Federica Borea6, Mario Borroni6, Gianluigi Bresciani6, Enrico Brunetta6, Cinzia Bulletti6, Cristina Cadonati6, Lorenzo Calabro’6, Marta Calatroni6, Giuseppe Caltagirone6, Albania Antonietta Calvetta6, Francesco Cannata6, Lorenzo Canziani6, Antonio Capogreco6, Giovanni Luigi Capretti6, Elisa Carlani6, Flaminia Carrone6, Maddalena Casana6, Alice Castelli6, Elena Castelnuovo6, Angela Ceribelli6, Carlo Ceriotti6, Manuel Chiarito6, Michele Ciccarelli6, Matteo Cimino6, Gianluigi Citterio6, Leonardo Ciuffini6, Chiara Colaizzi6, Francesca Colapietro6, Guido Costa6, Ottavia Cozzi6, Vincenzo Craviotto6, Chiara Crespi6, Massimo Crippa6, Federica D’Antonio6, Felice D’Antuono6, Federico D’Orazio6, Sara Dal Farra6, Leonardo Da Rio6, Guido De Ambroggi6, Massimo De Donato6, Francesca De Lucia6, Pasquale De Nittis6, Giacomo Delle Rose6, Antonio Desai6, Maria De Santis6, Marina Di Pilla6, Franca Dipaola6, Andrea Dipasquale6, Angelo Dipasquale6, Ginevra Droandi6, Roberta Fazio6, Giuseppe Favacchio6, Carlo Fedeli6, Giuseppe Ferrante6, Elisa Chiara Ferrara6, Matteo Carlo Ferrari6, Sebastian Ferri6, Marco Folci6, Sara Foresti6, Eloisa Franchi6, Elia Fraolini6, Federica Furfaro6, Paola Galimberti6, Alessia Galtieri6, Maria Gardini6, Francesca Gavazzi6, Elena Generali6, Caterina Giannitto6, Massimo Giovanni Giorgino6, Benedetta Goletti6, Elisabetta Guarino6, Jacopo Guerrini6, Giacomo Guidelli6, Flavia Jacobs6, Hayato Kurihara6, Michele Lagioia6, Andrea Lania6, Ezio Lanza6, Elisabetta Lavezzi6, Luca Libre’6, Ana Lleo6, Ferdinando Loiacono6, Laura Loy6, Giovanni Lughezzani6, Fabio Lutman6, Marta Maccallini6, Paola Magnoni6, Alfonso Francesco Maiorino6, Alberto Malesci6, Riccardo Mantovani6, Davide Marchettini6, Arianna Marinello6, Nikolaos Markopoulos6, Enrico Marrano6, Chiara Masetti6, Gherardo Mazziotti6, Angelo Milani6, Marco Mirani6, Paola Morelli6, Francesca Motta6, Federica Mrakic Sposta6, Valeria Mundula6, Irene Nasone6, Mattia Nigro6, Paolo Omodei6, Monica Ormas6, Arianna Pagliaro6, Silvia Paiardi6, Roberta Paliotti6, Alessia Pavesi6, Rosa Pedale6, Vittorio Pedicini6, Francesco Pegoraro6, Gaia Pellegatta6, Marta Pellegrino6, Alessandra Pestalozza6, Gennaro Petriello6, Sara Piccini6, Giorgio Pivato6, Daria Pocaterra6, Laura Poliani6, Dario Poretti6, Paoletta Preatoni6, Fabio Procopio6, Manuel Profili6, Francesca Puggioni6, Luca Pugliese6, Nicola Pugliese6, Francesca Racca6, Michele Randazzo6, Damiano Regazzoli Lancini6, Francesco Reggiani6, Marta Ripoll Pons6, Stefano Rodolfi6, Giulia Ronzoni6, Lidia Ruongo6, Clara Sacco6, Michele Sagasta6, Maria Teresa Sandri6, Giuseppe Sarra6, Marzia Savi6, Iside Scarfo’6, Dana Shiffer6, Federico Sicoli6, Simone Solano6, Virginia Solitano6, Anna Stainer6, Matteo Carlo Stella6, Giuseppe Strangio6, Antonio Taormina6, Francesca Ilaria Teofilo6, Lucia Testoni6, Federica Tordato6, Chiara Torrisi6, Angela Trabucco6, Luisa Ulian6, Rossella Valentino6, Chiara Valeriano6, Walter Vena6, Simona Verlingieri6, Edoardo Vespa6, Antonio Voza6, Giuseppe Voza6, Valentina Zanuso6, Alessandra Zilli6, Aurora Zumbo6
Data availability
Data are stored in the Institutional repository Zenodo.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study was approved by the Ethical Committee of IRCCS Humanitas Research Hospital.
Consent to participate
The patients gave their consent to use the clinical and biochemical data for research purposes.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the Humanitas COVID-19 Task force are listed below Funding.
Contributor Information
Gherardo Mazziotti, Email: gherardo.mazziotti@hunimed.eu.
On behalf of Humanitas COVID-19 Task force:
Stefano Accornero, Alessio Aghemo, Ludovico Alfarone, Hussam Ali, Monia Aloise, Claudio Angelini, Ivan Arcari, Paola Arosio, Elena Azzolini, Alessandra Baccarin, Salvatore Badalamenti, Sara Baggio, Luca Balzarini, Caterina Barberi, Franca Barbic, Viviana Barbieri, Alessandro Barbone, Alessio Basciu, Chiara Benvenuti, Ilaria Bianchi, Monica Bocciolone, Cristiana Bonifacio, Federica Borea, Mario Borroni, Gianluigi Bresciani, Enrico Brunetta, Cinzia Bulletti, Cristina Cadonati, Lorenzo Calabro’, Marta Calatroni, Giuseppe Caltagirone, Albania Antonietta Calvetta, Francesco Cannata, Lorenzo Canziani, Antonio Capogreco, Giovanni Luigi Capretti, Elisa Carlani, Flaminia Carrone, Maddalena Casana, Alice Castelli, Elena Castelnuovo, Angela Ceribelli, Carlo Ceriotti, Manuel Chiarito, Michele Ciccarelli, Matteo Cimino, Gianluigi Citterio, Leonardo Ciuffini, Chiara Colaizzi, Francesca Colapietro, Guido Costa, Ottavia Cozzi, Vincenzo Craviotto, Chiara Crespi, Massimo Crippa, Federica D’Antonio, Felice D’Antuono, Federico D’Orazio, Sara Dal Farra, Leonardo Da Rio, Guido De Ambroggi, Massimo De Donato, Francesca De Lucia, Pasquale De Nittis, Giacomo Delle Rose, Antonio Desai, Maria De Santis, Marina Di Pilla, Franca Dipaola, Andrea Dipasquale, Angelo Dipasquale, Ginevra Droandi, Roberta Fazio, Giuseppe Favacchio, Carlo Fedeli, Giuseppe Ferrante, Elisa Chiara Ferrara, Matteo Carlo Ferrari, Sebastian Ferri, Marco Folci, Sara Foresti, Eloisa Franchi, Elia Fraolini, Federica Furfaro, Paola Galimberti, Alessia Galtieri, Maria Gardini, Francesca Gavazzi, Elena Generali, Caterina Giannitto, Massimo Giovanni Giorgino, Benedetta Goletti, Elisabetta Guarino, Jacopo Guerrini, Giacomo Guidelli, Flavia Jacobs, Hayato Kurihara, Michele Lagioia, Andrea Lania, Ezio Lanza, Elisabetta Lavezzi, Luca Libre’, Ana Lleo, Ferdinando Loiacono, Laura Loy, Giovanni Lughezzani, Fabio Lutman, Marta Maccallini, Paola Magnoni, Alfonso Francesco Maiorino, Alberto Malesci, Riccardo Mantovani, Davide Marchettini, Arianna Marinello, Nikolaos Markopoulos, Enrico Marrano, Chiara Masetti, Gherardo Mazziotti, Angelo Milani, Marco Mirani, Paola Morelli, Francesca Motta, Federica Mrakic Sposta, Valeria Mundula, Irene Nasone, Mattia Nigro, Paolo Omodei, Monica Ormas, Arianna Pagliaro, Silvia Paiardi, Roberta Paliotti, Alessia Pavesi, Rosa Pedale, Vittorio Pedicini, Francesco Pegoraro, Gaia Pellegatta, Marta Pellegrino, Alessandra Pestalozza, Gennaro Petriello, Sara Piccini, Giorgio Pivato, Daria Pocaterra, Laura Poliani, Dario Poretti, Paoletta Preatoni, Fabio Procopio, Manuel Profili, Francesca Puggioni, Luca Pugliese, Nicola Pugliese, Francesca Racca, Michele Randazzo, Damiano Regazzoli Lancini, Francesco Reggiani, Marta Ripoll Pons, Stefano Rodolfi, Giulia Ronzoni, Lidia Ruongo, Clara Sacco, Michele Sagasta, Maria Teresa Sandri, Giuseppe Sarra, Marzia Savi, Iside Scarfo’, Dana Shiffer, Federico Sicoli, Simone Solano, Virginia Solitano, Anna Stainer, Matteo Carlo Stella, Giuseppe Strangio, Antonio Taormina, Francesca Ilaria Teofilo, Lucia Testoni, Federica Tordato, Chiara Torrisi, Angela Trabucco, Luisa Ulian, Rossella Valentino, Chiara Valeriano, Walter Vena, Simona Verlingieri, Edoardo Vespa, Antonio Voza, Giuseppe Voza, Valentina Zanuso, Alessandra Zilli, and Aurora Zumbo
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson KD, Harris C, Cain JK, Hummer C, Goyal H, Perisetti A. Pulmonary and extra-pulmonary clinical manifestations of COVID-19. Front. Med. 2020;7:526. doi: 10.3389/fmed.2020.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur. J. Endocrinol. 2020;183(4):381–387. doi: 10.1530/eje-20-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A, Ferrante E, Orsi E, Resi V, Longari V, Cuzzocrea M, Bandera A, Lazzaroni E, Dolci A, Ceriotti F, Re TE, Gori A, Arosio M, Salvi M. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8(9):739–741. doi: 10.1016/s2213-8587(20)30266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D.T.W. Lui, C.H. Lee, W.S. Chow, A.C.H. Lee, A.R. Tam, C.H.Y. Fong, C.Y. Law, E.K.H. Leung, K.K.W. To, K.C.B. Tan, Y.C. Woo, C.W. Lam, I.F.N. Hung, K.S.L. Lam, Thyroid dysfunction in relation to immune profile, disease status and outcome in 191 patients with COVID-19. J. Clin. Endocrinol. Metabol. 106(2), e926−e935 (2021). 10.1210/clinem/dgaa813 [DOI] [PMC free article] [PubMed]
- 6.Ippolito S, Dentali F, Tanda ML. SARS-CoV-2: a potential trigger for subacute thyroiditis? Insights from a case report. J. Endocrinol. Investig. 2020;43(8):1171–1172. doi: 10.1007/s40618-020-01312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.S.A.M. Mattar, S.J.Q. Koh, S. Rama Chandran, B.P.Z. Cherng, Subacute thyroiditis associated with COVID-19. BMJ Case Reports 13(8) (2020). 10.1136/bcr-2020-237336 [DOI] [PMC free article] [PubMed]
- 8.R.M. Ruggeri, A. Campennì, M. Siracusa, G. Frazzetto, D. Gullo, Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones (Athens, Greece), 20(7), 219−221 (2021). 10.1007/s42000-020-00230-w [DOI] [PMC free article] [PubMed]
- 9.A. Brancatella, D. Ricci, D. Cappellani, N. Viola, D. Sgrò, F. Santini, F. Latrofa F, Is subacute thyroiditis an underestimated manifestation of SARS-CoV-2 infection? Insights from a case series. J. Clin. Endocrinol. Metabol. 105(10), e3742−e3746 (2020). 10.1210/clinem/dgaa537 [DOI] [PMC free article] [PubMed]
- 10.Campos-Barrera E, Alvarez-Cisneros T, Davalos-Fuentes M. Subacute thyroiditis associated with COVID-19. Case Rep. Endocrinol. 2020;2020:8891539. doi: 10.1155/2020/8891539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U. Chakraborty, S. Ghosh, A. Chandra, A.K. Ray, Subacute thyroiditis as a presenting manifestation of COVID-19: a report of an exceedingly rare clinical entity. BMJ Case Rep. 13(12) (2020). 10.1136/bcr-2020-239953 [DOI] [PMC free article] [PubMed]
- 12.Wei L, Sun S, Xu CH, Zhang J, Xu Y, Zhu H, Peh SC, Korteweg C, McNutt MA, Gu J. Pathology of the thyroid in severe acute respiratory syndrome. Hum. Pathol. 2007;38(1):95–102. doi: 10.1016/j.humpath.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.M. Rotondi, F. Coperchini, G. Ricci, M. Denegri, L. Croce, S.T. Ngnitejeu, L. Villani, F. Magri, F. Latrofa, L. Chiovato, Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. Journal of endocrinological investigation. 44(5), 1085−1090 (2021). 10.1007/s40618-020-01436-w [DOI] [PMC free article] [PubMed]
- 14.B. Khoo, T. Tan, S.A. Clarke, E.G. Mills, B. Patel, M. Modi, M. Phylactou, P.C. Eng, L. Thurston, E.C. Alexander, K. Meeran, A.N., Comninos, A. Abbara, W.S. Dhillo, Thyroid function before, during and after COVID-19. J. Clin. Endocrinol. Metabol. 106(2), e803−e811 (2021). 10.1210/clinem/dgaa830 [DOI] [PMC free article] [PubMed]
- 15.M. Chen, W. Zhou, W. Xu, Thyroid function analysis in 50 patients with COVID-19: a retrospective study. Thyroid. 31(1), 8−11 (2021). 10.1089/thy.2020.0363 [DOI] [PubMed]
- 16.Leung TYM, Chan AYL, Chan EW, Chan VKY, Chui CSL, Cowling BJ, Gao L, Ge MQ, Hung IFN, Ip MSM, Ip P, Lau KK, Lau CS, Lau LKW, Leung WK, Li X, Luo H, Man KKC, Ng VWS, Siu CW, Wan EYF, Wing YK, Wong CSM, Wong KHT, Wong ICK. Short- and potential long-term adverse health outcomes of COVID-19: a rapid review. Emerg. Microbes Infect. 2020;9(1):2190–2199. doi: 10.1080/22221751.2020.1825914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knudsen N, Bols B, Bülow I, Jørgensen T, Perrild H, Ovesen L, Laurberg P. Validation of ultrasonography of the thyroid gland for epidemiological purposes. Thyroid. 1999;9(11):1069–1074. doi: 10.1089/thy.1999.9.1069. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Lin F, Tu W, Zhang J, Choudhry AA, Ahmed O, Cheng J, Cui Y, Liu B, Dai M, Chen L, Han D, Fan Y, Zeng Y, Li W, Li S, Chen X, Shen M, Pan P. Thyroid dysfunction may be associated with poor outcomes in patients with COVID-19. Mol. Cell. Endocrinol. 2021;521:111097. doi: 10.1016/j.mce.2020.111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomer Y, Huber A. The etiology of autoimmune thyroid disease: a story of genes and environment. J. Autoimmun. 2009;32(3-4):231–239. doi: 10.1016/j.jaut.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol. J. 2009;6:5. doi: 10.1186/1743-422x-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao N, Wang S, Cui XJ, Huang MS, Wang SW, Li YG, Zhao L, Wan WN, Li YS, Shan ZY, Teng WP. Two-years prospective follow-up study of subacute thyroiditis. Front. Endocrinol. 2020;11:47. doi: 10.3389/fendo.2020.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bogazzi F, Dell’Unto E, Tanda ML, Tomisti L, Cosci C, Aghini-Lombardi F, Sardella C, Pinchera A, Bartalena L, Martino E. Long-term outcome of thyroid function after amiodarone-induced thyrotoxicosis, as compared to subacute thyroiditis. J. Endocrinol. Investig. 2006;29(8):694–699. doi: 10.1007/bf03344178. [DOI] [PubMed] [Google Scholar]
- 24.Alfadda AA, Sallam RM, Elawad GE, Aldhukair H, Alyahya MM. Subacute thyroiditis: clinical presentation and long term outcome. Int. J. Endocrinol. 2014;2014:794943. doi: 10.1155/2014/794943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishihara E, Amino N, Ohye H, Ota H, Ito M, Kubota S, Fukata S, Miyauchi A. Extent of hypoechogenic area in the thyroid is related with thyroid dysfunction after subacute thyroiditis. J. Endocrinol. Investig. 2009;32(1):33–36. doi: 10.1007/bf03345675. [DOI] [PubMed] [Google Scholar]
- 26.Cuan-Baltazar Y, Soto-Vega E. Microorganisms associated to thyroid autoimmunity. Autoimmun. Rev. 2020;19(9):102614. doi: 10.1016/j.autrev.2020.102614. [DOI] [PubMed] [Google Scholar]
- 27.Omori N, Omori K, Takano K. Association of the ultrasonographic findings of subacute thyroiditis with thyroid pain and laboratory findings. Endocr. J. 2008;55(3):583–588. doi: 10.1507/endocrj.k07e-163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are stored in the Institutional repository Zenodo.


