Abstract
Background
Coronavirus disease 2019 (COVID-19) is still a pandemic, with a high mortality rate in severe/critical cases. Therapies based on the Shenghuang Granule have proved helpful in viral infection and septic shock.
Hypothesis/Purpose
The objective of the current study was to compare the efficacy and safety of the traditional Chinese medicine, Shenhuang Granule, with standard care in hospitalized patients with severe/critical COVID-19.
Study Design and Methods
This was an open-label, multicenter, randomized, controlled clinical trial. At 4 medical centers, a total of 111 severe/critical patients were randomly assigned to receive Shenhuang Granule (SHG group) twice a day for 14 days, in addition to standard care, or to receive standard care alone (Control group). The maximal follow up time was 75 days. The clinical endpoint was clinical improvement and mortality.
Results
54 patients were assigned to the control group and 57 to the SHG group. The overall mortality was 75.9% (41/54) in the control group, and 38.6% (22/57) in the SHG group (p < 0.01 vs. control). The post hoc analysis showed that in the severe category, the mortality of the control group vs. the SHG group was 58.8% (10/17) vs. 5.3% (1/19) (p < 0.01); while in the critical category, it was 83.8% (31/37) vs. 55.3% (21/38) (p < 0.05). In the severe category, the mortality of patients who eventually received an invasive ventilator in the control vs. the SHG group was 58.8% (10/17) vs. 0 (0/19) (p < 0.01). Administration of SHG was associated with increased lymphocytes and decreased adverse events.
Conclusion
Shenhuang Granule is a promising integrative therapy for severe and critical COVID-19.
Keywords: COVID-19, Traditional Chinese Medicine, Shenhuang Granule, Mortality, Clinical trial
Abbreviations: ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CK, creatine kinase; CK-MB, creatine kinase muscle-brain isoform; COVID-19, Coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; IL-6, interleukin-6; IQR, interquartile range; K, potassium; LDH, lactate dehydrogenase; Na, sodium; PCT, Procalcitonin; PT, prothrombin time; SHG, Shenhuang Granule; TNF-α, tumor necrosis factor-alpha; α-HBDH, α-hydroxybutyrate dehydrogenase
Graphical abstract
Introduction
Since the first case of coronavirus disease-2019 (COVID-19) was reported in December 2019 in Wuhan, China (Zhu et al., 2020), COVID-19 has become a pandemic (Engineering and University, 2020). Currently, the number of confirmed active cases is still continuously increasing globally (Engineering and University, 2020). Its mortality rate could reach as high as 16.4 per 100 confirmed cases in some countries (Engineering and University, 2020), and up to 78% in severe/critical cases (National and Centre, 2020; Zhou et al., 2020). So far, there is no specific therapy for COVID-19, and many hospitals have used off-label or compassionate-use therapies, such as lopinavir–ritonavir, chloroquine, convalescent plasma, and remdesivir (Holshue et al., 2020). Remdesivir has been authorized for emergency use by the Food and Drug Administration (FDA) (Administration, 2020), but it has not been shown to improve mortality in severe COVID-19 patients(Beigel et al., 2020), and no published data support usage among critically ill cases. Dexamethasone has also been shown to beneficial in severe COVID-19 (Horby et al., 2020), but this is controversial(Singh et al., 2020).
In fighting the COVID-19 outbreak, more than 90% of patients have used traditional Chinese medicine (TCM) as an integrative therapy in China (The State Council, 2020). Recently, one TCM product, Lianhuaqingwen capsule, has been shown in one randomized controlled clinical trial (RCT) to ameliorate clinical symptoms of mild COVID-19 (Hu et al., 2020). However, no RCT of TCM focusing on life threatening severe/critical cases has been reported yet.
Our group has conducted a series of clinical and basic science research on a traditional Chinese medicine therapy for acute abdominal diseases (Fang et al., 2007; Liang et al., 2017), viral (including COVID-19) (Feng et al., 2020) or bacterial infections (Fang et al., 2019), and sepsis or septic shock (Chen et al., 2008; Chen et al., 2012b; Wang et al., 2019). Our methods have also been accepted into the treatment guidelines or expert consensus of the Chinese Medical Doctor Association (CMDA) for integrative management of sepsis/septic shock (PHYSICANS. and Shoch and sepsis commission, 2019), and acute upper respiratory tract infections (Fang et al., 2019). Based on our data, a formula known as Shenhuang Granule (SHG) was developed (Fang et al., 2017). With respect to the components of SHG, and according to TCM theory, rhubarb can reduce heat and promote blood circulation and resolve dampness. In the latest publication, it has been shown to possess extensive pharmacological activity, including antitumor activity, regulation of gastrointestinal flora, protection of the intestinal mucosal barrier, and anti-inflammatory activity (Xiang et al., 2020). Rhubarb may also interfere with the progression of severe infectious diseases (Xiang et al., 2020). Ginseng is believed to increase strength, increase blood volume, promote longevity and appetite. Recent studies also suggest that ginseng exhibits activity against microbes, inflammation, cytotoxicity, hemagglutination and viruses (Kim and Yang, 2018). The present study was a prospective, multicenter, randomized, controlled, and open-label clinical trial to confirm the efficacy and safety of SHG in hospitalized adult patients with severe/critical COVID-19.
Materials and methods
Study design
The study was a randomized, controlled, multicenter, open-label trial that was conducted at 4 medical centers in Hubei Province, China: Leishenshan Hospital of Wuhan, Tongji Hospital, Wuhan Mental Health Center, and Huangshi Hospital of TCM (The Infectious Disease Hospital).
Study participants or legal representatives of family members signed the informed consent, which abides by the principles of the Declaration of Helsinki and the regulations on quality management of clinical trials in China. The study protocol was approved by the Ethics Committee (approval number HSZY-PJ-2020-001-01) and registered with the Chinese Clinical Trial Registry (ChiCTR2000029777). The Institutional Review Board (Shanghai University of Traditional Chinese Medicine) approved the study prior to data collection.
Patients
Patients were diagnosed as equal to or worse than the severe case (severe or critical) according to the national interim guideline of diagnosis and treatment for coronavirus disease 2019 (COVID-19) set by the China National Health Commission (China, 2020). All patients were provided written informed consent.
Inclusion criteria
Subjects must meet all of the following requirements:
-
1
Hospitalized patients with COVID-19 were confirmed by pathogenic detection viral diagnostic techniques (nucleic acid test) (Esbin et al., 2020).
-
2
Meet any one of the criteria in the severe or critical category according to the national guideline of diagnosis and treatment (China, 2020)
-
3
Be ≥18 years of age
Exclusion criteria
-
1
Pregnant or lactating women
-
2
Allergy to herbs of Shenhuang Granule
-
3
History of a severe primary disease, including unresectable tumors, blood diseases (such as acute leukemia and purpura hemorrhagica) , HIV, severe liver dysfunction (serum bilirubin level > 205.2 µmol/l) or severe kidney dysfunction (serum creatinine > 442μmol/l or urine volumes ≤ 200 ml/day)
-
4
Presence of obstructive pneumonia, interstitial pulmonary fibrosis, alveolar proteinosis, and hypersensitivity pneumonitis caused by obstructive lung tumors
-
5
Have a severe psychiatric disorder
Suspension criteria
The criteria for suspension of participation are as follows:
-
1
Poor compliance with investigators
-
2
Incomplete data that might affect analysis of the results
-
3
Voluntary withdrawal
-
4
Using other medications which were not listed in the trial protocol that might affect analysis of the results
Interventions
The Shenhuang Granule is a formulation of the following raw herbs: 50 g of Panax ginseng C. A. Mey (Renshen) root, 40 g of Rheum palmatum L. stem (Dahuang), 30g of Sargentodoxa cuneata stem (Hongteng), 30 g of Taraxacum mongolicum whole plant (Pugongying), 50 g of Aconiti Lateralis Radix Praeparata stem (Fuzi) and 6g of Whitmania pigra Whitman (Shuizhi) whole organism, After a series of extraction and manufacturing processes, the final product is a concentrated granule which is 1:5 of the raw herbs. The granules were packaged into two sachets before serving. The SHG was provided and manufactured by Beijing Tcmages Pharmaceutical Co., Ltd. The product was approved by the National Medical Product Administration (China) (Approval number: Jing 20180032). Patients underwent a 14-day treatment, and laboratory tests (complete blood count, general urine analysis, fecal occult blood test, hepatic function and renal function) were ordered on day 1, 3, 5, 7 and 14 since enrollment in the trial. The SHG was dissolved in warm water and taken orally, with a dosing regimen of two sachets per day. For critical patients with difficulty taking medication, the full dose of the SHG solution was administered via a feeding tube. After 14 days, SHG was given twice daily until death or discharge.
Patients were allowed to remain on standard care according to the national guideline of diagnosis and treatment for novel Coronavirus pneumonia (the new term is COVID-19) (Interim Version 7) (China, 2020). One group received standard care and Shenhuang Granule (SHG group), while the other group received only standard care (control group). Detailed information about standard care is located in Table 1 .
Table 1.
Treatments received after enrolment
| Total (N=111) | Control (N=54) | SHG (N=57) | Difference (95% CI) | p value | |
| Receiving injection of interferon alfa-2b | 24 (21.6) | 12 (22.2) | 12 (21.1) | 1.07 (0.43 to 2.65) | 0.881 |
| Receiving lopinavir–ritonavir | 10 (9.0) | 9 (16.7) | 1 (1.8) | 11.2 (1.37 to 91.73) | 0.016* |
| Vasopressors | 61 (55.0) | 35 (64.8) | 26 (45.6) | 2.20 (1.02 to 4.72) | 0.042* |
| Renal replacement therapy | 10 (9.0) | 5 (9.3) | 5 (8.8) | 1.06 (0.29 to 3.89) | 1.00 |
| Highest oxygen therapy support | |||||
| Non-invasive mechanical ventilation | 37 (33.3) | 14 (25.9) | 23 (40.4) | 1.93 (0.86 to 4.33) | 0.107 |
| Invasive mechanical ventilation | 58 (52.3) | 35 (64.8) | 23 (40.4) | 0.37 (0.17 to 0.79) | 0.010# |
| Extracorporeal membrane oxygenation or mechanical ventilation | 4 (3.6) | 2 (3.7) | 2 (3.5) | 1.06 (0.14 to 7.79) | 0.956 |
| Antibiotic | 100 (90.1) | 52 (96.3) | 48 (84.2) | 4.88 (1.00 to 23.71) | 0.033* |
| Corticosteroids therapy after trial enrollment | 48 (43.2) | 19 (35.2) | 29 (50.9) | 0.52 (0.24 to 1.12) | 0.095 |
| Other oral patent TCM product | 25 (22.5) | 14 (25.9) | 11 (19.3) | 1.46 (0.60 to 3.59) | 0.403 |
Data are median (IQR) or n (%).
p < 0.05,
p < 0.01 vs. the control group.
Sample size
Considering a previous study on COVID-19 using TCM, the clinically effective rate in the treatment group improved from 61.11% to 91.18%. The sample size was calculated according to the parameters α = 0.05 (two-sided test) and β = 0.1. By comparing the clinically effective rates of the two groups with the sample size estimation formula, we determined that we needed to recruit at least 60 participants in each group to achieve sufficient statistical power. Since the withdrawal rate was less than 15%, a total of 160 participants were ultimately needed in the two groups, with 80 participants in each group, including both the severe and critical category. We used the calculation tool located at https://www.cnstat.org/samplesize/.
Randomization
Patients meeting the eligibility criteria were randomized into two groups (control group or SHG group) in a ratio of 1:1. At the coordinating center (Tongji Hospital), consecutive numbers were prepared by a statistician who was not involved in the trial, and assigned to each hospital according to a center-stratified random order generated by SPSS software (version 19), then patients were randomly assigned to one of the two treatment groups: either standard care alone, or standard care with SHG. Eligible patients were allocated to receive medication in individually numbered packs.
Outcomes and measurements
The primary clinical endpoint was clinical improvement, which was defined as a one-point reduction in the patients’ admission status on a six-point ordinal scale according to the national interim guideline of diagnosis and treatment (China, 2020), or live discharge from the hospital, whichever came first. Detailed clinical categories were defined as the following:
1. Mild cases
Clinical symptoms were mild and no signs of pneumonia on imaging.
2. Moderate cases
Fever and respiratory symptoms complicated with radiological findings of pneumonia.
3. Severe cases
Adult cases meeting any of the following criteria:
3.1 Respiratory distress (≥ 30 breaths/ min);
3.2 Oxygen saturation ≤ 93% at rest;
3.3 Arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≦ 300 mmHg (l mmHg = 0.133 kPa).
In high-altitude areas (at an altitude of over 1,000 meters above sea level), PaO2/FiO2 shall be corrected by the following formula:
PaO2/ FiO2 * [Atmospheric pressure (mmHg)/760]
Cases with chest imaging that showed obvious lesion progression within 24-48 h > 50% shall be managed as severe cases.
4. Critical cases
Cases meeting any of the following criteria:
4.1 Respiratory failure that requires mechanical ventilation;
4.2 Shock;
4.3 With other organ failure that requires ICU care;
5. Discharge criteria
-
(1)
Body temperature returns to normal for more than 72 h;
-
(2)
Respiratory symptoms significantly improve (saturation of blood oxygen > 94% and respiratory rate ranges from 16/min to 24/min);
-
(3)
Pulmonary imaging shows obvious reduction of inflammation;
-
(4)
Nucleic acid tests negative twice consecutively on respiratory tract samples such as sputum and nasopharyngeal swabs (sampling interval being at least 24 h).
The six-point ordinal scale was defined as: 6 = death; 5 = critical; 4 = severe; 3 = moderate; 2 = mild; and 1 = discharge alive.
Secondary outcomes were mortality, rate of advancing to critical category or receiving an invasive ventilator in the severe category. Safety outcomes included treatment-emergent adverse events, serious adverse events, and premature discontinuations of study drug.
Statistical analysis
The statistical analysis was performed in a blinded manner using the SPSS 19.0 software. To test if the data was normally distributed, the Komogoroff-Smirnoff test was performed. Continuous variables characterizing each study group are expressed as means with standard errors or medians with interquartile ranges and analyzed by unpaired Student's t-test or Wilcoxon nonparametric statistics. Categorical variables will be reported as frequencies and proportions and analyzed with a Chi-square test or Fisher's exact test. After separating the total participants into the severe and critical category, a post hoc analysis for subgroup was conducted. A p value < 0.05 is considered as statistically significant.
Results
Patients
Hospitalized patients were recruited from January 14, 2020 to March 28, 2020. The last follow up date was April 22, 2020 with the maximal time of follow up as 75 days. No patients were enrolled after March 29. Because of the control of the outbreak in Wuhan, the data safety and monitoring board recommended that the study be terminated and data analyzed on April 22.
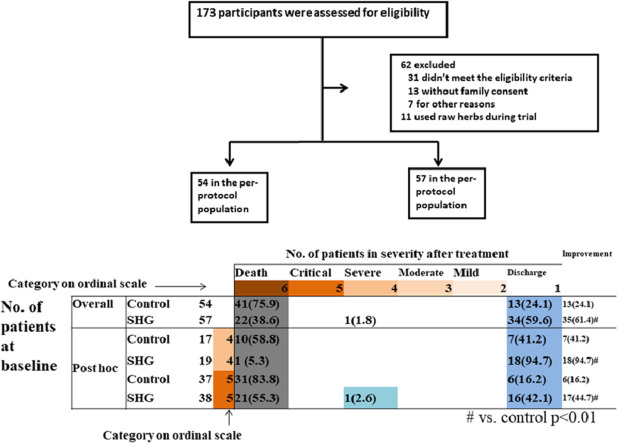
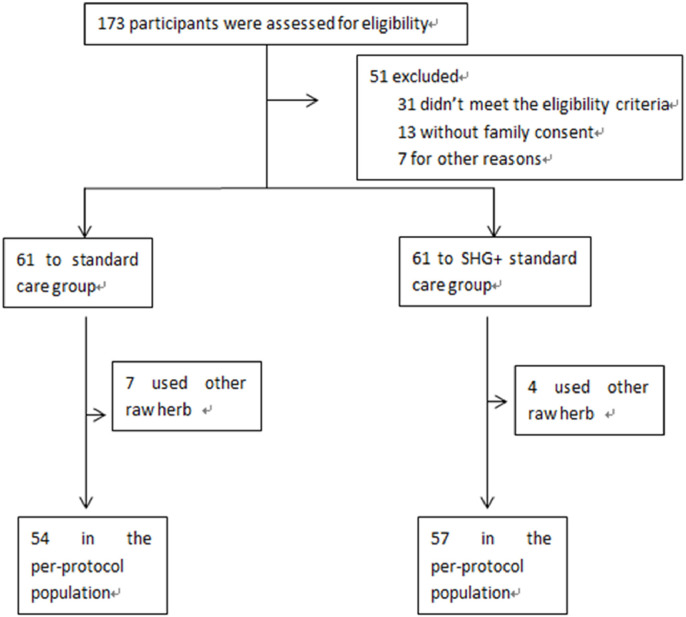
A total of 173 cases were assessed for eligibility. Some patients were willing to participate in the trial but requested to take only standard care or only Shenhuang Granule, which was defined as “other reasons” in the exclusion criteria and they were thus excluded from enrollment in the trial. A total of 122 patients eventually underwent randomization. 61 patients each were assigned to either the control group or the SHG group. 7 and 4 from each group, respectively, received other individualized raw herbs due to rapid advancement of severity and were thus excluded from final analysis (Figure 1 ).
Figure 1.
Trial profile
The median age of study participants was 66.0 years (IQR 56.0-72.0); the sex distribution was 70.4% (38/54) male vs. 29.6% (16/54) female in the control group and 57.9% male (33/57) vs. 42.1% (24/57) female in the SHG group (Suppl. Table 1). The time from symptoms onset to trial enrollment in the control group was 16 days (IQR 14-23.3) and in the SHG group it was 19 days (IQR 8.5-28.0). More patients in the control group had fever, heart rate > 100/min, respiratory rate > 24/min, or oxygen saturation < 94%. However, the rate of receiving oxygen therapy such as mechanical ventilation and noninvasive oxygen support was similar in the two groups. Total white blood cell count, neutrophil count, lymphocyte count and interleukin – 6 (IL-6) were also comparable between the control and SHG group. The percentage of patients receiving corticosteroid therapy was also comparable between the two groups. Lactate dehydrogenase (LDH), D Dimer and interleukin-10 (IL-10) were higher in the control group, and PaO2 were higher in the SHG group (Suppl. Table 1).
Primary outcome
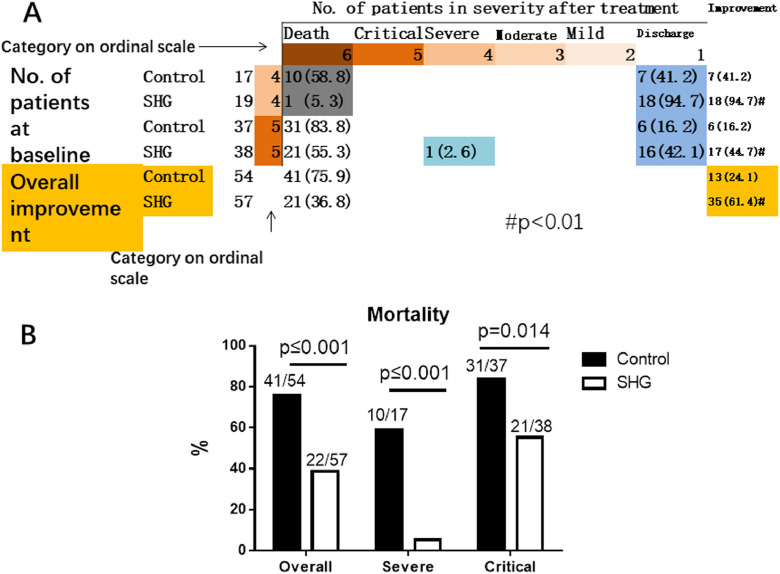
The overall improvement rate was 24.1% (13/54) in the control group, compared to 61.4% (35/57) in the SHG group (p < 0.01). The post hoc analysis showed that in the severe category, patients in the control group had an improvement rate of 41.2% (7/17), which was significantly lower than the SHG group of 94.7% (18/19) (p < 0.01). In the critical category, the control group had an improvement rate of 16.2% (6/37), which was also lower than the SHG group of 44.7% (17/38) (p < 0.05) (Fig. 2 A).
Figure 2.
Severity Status at Baseline and after Treatment, and final mortality. A. Severity Status at Baseline and after Treatment. For each severity category, percentages were calculated with the number of patients at baseline as the denominator. Improvement (light blue cells), no change (beige) and worsening (gray) in severity status are shown. B. Mortality of patients in the control group or the SHG group.
P value is shown in the figure.
Secondary outcome
The mortality was 75.9% (41/54) in the control group compared to 38.6% (22/57) in the SHG group (p < 0.01). After post hoc analysis, we found that in the severe category, the mortality of the control group was 58.8% (10/17) compared to 5.3% (1/19) for the SHG group (p < 0.01). The trend was similar in the critical category, where the control group had a mortality of 83.8% (31/37) compared to 55.3% (21/38) for the SHG group (p < 0.05) (Figure 2B).
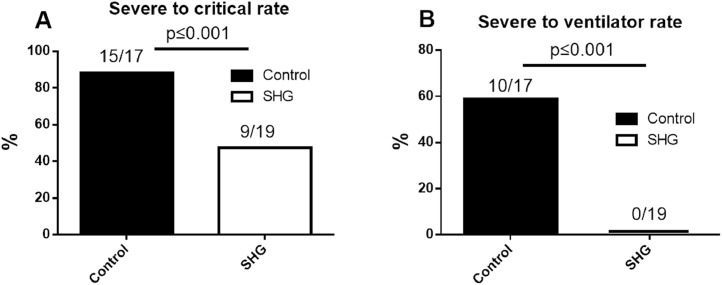
88.2% (15/17) of severe patients in the control group advanced to critical status, while only 47.4% (9/19) (p < 0.01) of the SGH group advanced to critical status (Figure 3 A). Meanwhile, 58.8% (10/17) of the control group patients in the severe category eventually received an invasive ventilator, but to our surprise, 0% (0/19) of patients in the SHG group received an invasive ventilator (p < 0.01) (Figure 3 B).
Figure 3.
The rate of advancement to critical status (A) or using an invasive ventilator (B) of the control group or the SHG group in patients enrolled as severe status.
P value is shown in the figure.
After administration of SHG, an increased lymphocyte count and a decreased total white blood cell and neutrophil count were observed (Suppl. Fig. 1).
Adverse events
Compared to the control group, we did not observe a significant increase in adverse events from SHG. On the contrary, some adverse events such as increased blood sugar level, increased blood total bilirubin level, thrombocytopenia, respiratory failure or acute respiratory distress syndrome, cardiopulmonary failure and multiorgan dysfunction syndrome were decreased after administration of SHG (Table 2 ). No premature discontinuations of study drug were observed in this trial (Table 2).
Table 2.
Summary of adverse events.
| Total (N=111) | Control (N=54) | SHG (N=57) | Difference (95% CI) | P value | |
|---|---|---|---|---|---|
| Adverse events (%) | |||||
| Any | 110 (99.1) | 54 (100) | 56 (98.2) | 0.98 (0.95 to 1.02) | 1.00 |
| Hypoalbuminemia | 59 (53.2) | 38 (70.4) | 21 (36.8) | 4.07 (1.84 to 9.01) | <0.001# |
| Hypokalemia | 13 (11.7) | 9 (16.7) | 4 (7.0) | 2.65 (0.77 to 9.19) | 0.114 |
| Increased blood glucose | 73 (65.8) | 43 (79.6) | 30 (52.6) | 3.52 (1.52 to 8.17) | 0.003# |
| Anemia | 58 (52.3) | 29 (53.7) | 29 (50.9) | 1.12 (0.53 to 2.36) | 0.766 |
| Rash | 1 (0.9) | 0 | 1 (1.8) | 0.98 (0.95 to 1.02) | 1.00 |
| Thrombocytopenia | 57 (51.4) | 36 (66.7) | 21 (36.8) | 3.43 (1.57 to 7.49) | 0.002# |
| Increased total bilirubin | 26 (23.4) | 19 (35.2) | 7 (12.3) | 3.88 (1.47 to 10.21) | 0.004# |
| Increased blood lipids | 43 (38.7) | 24 (44.4) | 19 (33.3) | 1.6 (0.74 to 3.45) | 0.23 |
| Increased white blood cell count | 66 (59.5) | 43 (79.6) | 23 (40.4) | 5.78 (2.48 to 13.49) | <0.001# |
| Increased blood urea nitrogen | 49 (44.1) | 31 (57.4) | 18 (31.6) | 2.92 (1.34 to 6.35) | 0.006 |
| Increased neutrophil | 79 (71.2) | 49 (90.7) | 30 (52.6) | 8.82 (3.07 to 25.38) | < 0.001# |
| Aspartate aminotransferase increased | 58 (52.3) | 35 (64.8) | 23 (40.4) | 2.72 (1.26 to 5.88) | 0.01# |
| Constipation | 2 (1.8) | 0 | 2 (3.5) | 0.97 (0.92 to 1.01) | 0.496 |
| Nausea | 5 (4.5) | 4 (7.4) | 1 (1.8) | 4.48 (0.49 to 41.42) | 0.198 |
| Diarrhea | 11 (9.9) | 9 (16.7) | 2 (3.5) | 5.5 (1.13 to 26.76) | 0.02* |
| Vomiting | 5 (4.5) | 4 (7.4) | 1 (1.8) | 4.48 (0.49 to 41.42) | 0.198 |
| Abnormal serum sodium | 36 (32.4) | 29 (53.7) | 7 (12.3) | 8.29 (3.19 to 21.53) | <0.001# |
| Increased serum potassium | 37 (33.3) | 27 (50) | 10 (17.5) | 4.7 (1.98 to 11.18) | <0.001# |
| Serious adverse events (%) | |||||
| Any | 98 (88.3) | 53 (98.1) | 45 (78.9) | 14.13 (1.77 to 112.94) | 0.002# |
| Respiratory failure or acute respiratory distress syndrome | 66 (59.5) | 43 (79.6) | 23 (40.4) | 5.78 (2.48 to 13.49) | <0.001# |
| Cardiopulmonary failure | 39 (35.1) | 31 (57.4) | 8 (14.0) | 8.26 (3.28 to 20.75) | <0.001# |
| Pulmonary embolism | 2 (1.8) | 2 (3.7) | 0 | 1.04 (0.99 to 1.09) | 0.234 |
| Cardiac arrest | 46 (41.4) | 30 (55.6) | 16 (28.1) | 3.20 (1.46 to 7.05) | 0.003# |
| Acute coronary syndrome | 2 (1.8) | 1 (1.9) | 1 (1.8) | 1.06 (0.06 to 17.33) | 1.00 |
| Tachycardia | 12 (10.8) | 6 (11.1) | 6 (10.5) | 1.06 (0.32 to 3.52) | 0.92 |
| Septic shock | 11 (9.9) | 5 (9.3) | 6 (10.5) | 0.87 (0.25 to 3.03) | 0.823 |
| Sepsis | 11 (9.9) | 4 (7.4) | 7 (12.3) | 0.57 (0.16 to 2.08) | 0.39 |
| Bronchitis | 5 (4.5) | 1 (1.9) | 4 (7.0) | 0.25 (0.03 to 2.31) | 0.364 |
| Thrombocytopenia | 49 (44.1) | 34 (63) | 15 (26.3) | 4.76 (2.12 to 10.68) | <0.001# |
| Increased D-dimer | 94 (84.7) | 51 (94.4) | 43 (75.4) | 5.54 (1.49 to 20.54) | 0.005# |
| Hemorrhage of lower digestive tract | 4 (3.6) | 0 | 4 (7.0) | 0.93 (0.87 to 1.00) | 0.119 |
| Acute kidney injury | 19 (17.1) | 12 (22.2) | 7 (12.3) | 2.04 (0.74 to 5.65) | 0.165 |
| Multiple organ dysfunction syndrome | 37 (33.3) | 28 (51.9) | 9 (15.8) | 5.74 (2.36 to 13.98) | <0.001# |
Data are n (%) and include all events reported after enrollment in research. Some patients had more than one adverse event. No patients discontinued the drug unless hospital discharge or early death. COVID-19 = coronavirus disease 2019.
p < 0.05,
p < 0.01 vs. the control group.
Discussion
To the best of our knowledge, this is one of the very first clinical trials to be conducted in real time during the actual start of the future world-wide pandemic at the very center of the outbreak. Our clinical trial results showed that Shenhuang Granule (SHG) improved recovery from COVID-19 in both severe and critically ill patients. After administration of SHG, the overall mortality was reduced by 37.3%, and was reduced by 50.5% in the severe category, compared to that of the control group. The chance of survival increased 2.8-fold compared to that of the control group in the critical category. For severe patients, SHG also appears to reduce disease progression to the critical category and to reduce the need for invasive ventilation therapy.
Our past basic and clinical research has also demonstrated that SGH was associated with a reduction of inflammatory mediators (Chen et al., 2012a; Chen et al., 2012b) and the regulation of intestinal flora (Liang et al., 2017; Wang et al., 2019). Our previous study showed that Rheum palmatum L. stem can block the progression and reverse the prognosis of severe inflammation (Liang et al., 2017). SHG, as a complex herbal mixture that contains numerous bioactive constituents, most likely acts at multiple levels and on multiple targets to improve COVID-19 outcomes.
The clinical outcome of the SHG group is also likely associated with the herb dosing and combination. The regular dose of Panax ginseng is 5-10 grams, but 50 grams were used in SHG, which is 5-10 times higher than the regular dose. Similarly, the dose of (40 g) of Rheum palmatum L. stem is 3-13 times higher than the regular dose (3-12 g). Intriguingly, Panax ginseng is not commonly used to manage acute inflammation or infection in textbooks of TCM as it would be considered to aggravate these acute disorders. Nevertheless, our previous basic and clinical research showed a beneficial role of Panax ginseng, especially if a high dose was used (Fang et al., 2017). High dose of Panax ginseng and Aconiti Lateralis Radix Praeparata stem (Fuzi) have been reported to improve cardiac function in heart failure conditions (Yan et al., 2018). Meanwhile, Whitmania pigra Whitman (Shuizhi) whole organism can be beneficial for micro-thrombosis (Ren et al., 2019), a critical factor for prognosis of COVID-19.
The mortality rate in the critical control group in this trial was as high as 83.8%. We believe this was at least partly associated with the care provided by our medical centers in supporting treatment for COVID-19 in China. The tertiary medical centers in this clinical trial were designated as referral centers to receive severe/critical cases only. The high mortality rate might also be associated with our long follow up time. In a report examining outcomes of COVID-19 patients in New York, with a median follow-up time of 4.5 days, the mortality of patients receiving mechanical ventilation was initially 88.1% (Richardson et al., 2020). We consider the trend observed in New York hospitals for severe/critical COVID-19 patients is likely similar to what we observed in Hubei Province, China, if the follow-up time was long enough, for example 75 days.
We did observe that some baseline parameters such as fever, heart rate, respiratory rate, oxygen saturation, LDH, IL-6 and D Dimer were different between the control and SHG group. We think this is probably due to the diagnostic criteria: any one of three criteria met would be diagnosed as a severe case. Although the disease severity was comparable between the two groups; and the rate of receiving mechanical ventilation was similar in the two groups, we still consider this baseline difference undesirable.
Our study had some limitations: First, the sample size of the present study is relatively small. Second, initiation of SHG treatment was quite late in some patients. Third, there was no viral load data. Fourth, the study population only included patients within the Hubei Province of China. Fifth, because of the emergency nature of the trial, placebos were not prepared. A larger and more rigorous international multicenter RCT with enough participants may ultimately confirm the safety, tolerability and efficacy of SHG.
In summary, we integrated Shenhuang Granule into standard care and showed improved clinical outcomes in both severe and critical COVID-19 patients. Administration of SHG in the severe category also reduced the rate of advancement to critical status and the percentage of patients that went on to receive invasive ventilator therapy.
Conclusion
SHG is a promising integrative therapy for severe/critical COVID-19.
Trial protocol
The full trial protocol can be accessed by contacting the corresponding author.
Authors’ contributions
J.F., S.Z. and B.F. contributed to the study design, data analysis and interpretation. J.C., D.P. and T.F. wrote the original draft. Q.X., Y.F. and T.H. contributed to the statistical analysis and edited the manuscript. X.X., G.Y. and D.Z. contributed to the data collection and edited the manuscript. D.Z., W.Z. and S.S. contributed to performing search and data collection. D.Z. and J.W. supervised the study. Z.Y., X.L. and X.W. interpreted the data, reviewed the manuscript, and prepared the final version of the article. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Funding source
Emergency Committee of the World Federation of Chinese Medicine Societies and Shanghai Society of Traditional Chinese Medicine, Novel Coronavirus Pneumonia Emergency Tackling Key Project (SJZLJZ.N01); National Key Research and Development Program of China (2018YFC1705900).
Declaration of Competing Interest
The Shenhuang granule is manufactured by Beijing Tcmages Pharmaceutical Co., LTD (Number: Jing 20180032). Beijing Tcmages Pharmaceutical Co played no part in study design; collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
The authors declare that they have no competing interests.
Acknowledgements
We thank all the patients who agreed to participate in our clinical trial and the staff who have taken part in our trial. We also thank Dr. Arthur Yin Fan at McLean Center for Complementary and Alternative Medicine, PLC; Dr. Yongming Li at the American TCM Society, Dr. Canhui Li at Toronto University, Dr. Iman Majd at University of Washingon, Dr Sherman Gu at the Federation of Chinese Medicine & Acupuncture Societies of Australia (FCMA), and Dr. Paul S. Amieux at Bastyr University for their critical reviewing and constructive suggestions for this manuscript. We also specially thank Dr. Paul S. Amieux for his valuable time and efforts in helping with technical details and grammar editing.
Footnotes
Clinical Trial Registration: [Chinese Clinical Trial Register number, ChiCTR2000029777].
References
- Administration, F.a.D., 2020. remdesivir EUA Letter of Authorization.
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.-d., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the Treatment of Covid-19 — Final Report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Wang H., Fang B., Chen H., Guo Q., Lu J., Wang B. Study on anti-pyretic and anti-inflammator effect of Shufeng Jiebiao decoction. International Journal of Traditional Chinese Medicine. 2012;34:613–616. [Google Scholar]
- Chen H., wANG Y., Zou C., Fang B., Guo Q., Tian Y. Clinical Study on“Quyu J iedu Yiqi Decoction”in Tr eating Sever e Sepsis. ACTA UNIVERSITATIS TRADITIONIS MEDICALIS SINENSIS PHARMACOLOGIAEQUE SHANGHAI. 2008;22:30–31. [Google Scholar]
- Chen M., Fei A., Lu W., Tang F., Wang F., Xu W., Yu Y., Fang B. Clinical research on the therapeutic effect of Xuebijin injection on sepsis and its influence on PECAM − 1 and coagulation function. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2012;21:1156–1158. [Google Scholar]
- China, N.H.C.o.t.P.s.R.o., 2020. Guideline of diagnosis and treatment for New Coronavirus Pneumonia (Interim version 7).
- Engineering, T.C.f.S.S.a., University, C.a.J.H., 2020. Coronavirus COVID-19 global cases.
- Esbin M.N., Whitney O.N., Chong S., Maurer A., Darzacq X., Tjian R. Overcoming the bottleneck to widespread testing: a rapid review of nucleic acid testing approaches for COVID-19 detection. RNA. 2020;26:771–783. doi: 10.1261/rna.076232.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang B., Cui Y., Li Z., Li Y., Yu X., Hu S., Wang G., Rui Q. Expert consensus on application of Chinese patent medicine for acute upper respiratory tract infection. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care. 2019;26:1–10. [Google Scholar]
- Fang B., Gao P., He S., Hao C., Shen P., Zhang Y., Zhang J., Fang B., Gao P., He S., Chen H., Shen P., Zhang Y., Zhang J. Clinical early intervent ion of Tongxia Huayu Decoct ion on pancreatic microcirculatory disturbance in severe acute pancreatitis. Journal of Chinese In tegrative Medicine. 2007;5:134–136. doi: 10.3736/jcim20070206. [DOI] [PubMed] [Google Scholar]
- Fang B., Sun L., Bo J., Chen M., Song J., Wu Q., Huang J. The Discussion of Acute Deficiency Syndrome Theory and its Clinical Application in Emergency Medicine. Journal of Emergency in Traditional Chinese Medicine. 2017;26:1724–1726. [Google Scholar]
- Feng Q., Li X., Zhe Z., Zhao F., Li W., Li H., Deng J., Cao M., Qing C., Guo Q., Fang B., Liu L., yang A., Yan S. Study on method of “three protections” in damp pestilence and treatment of COVID¯19 based on harmonic method. Shanghai J. Taditional Chin. Med. 2020;54:41–45. [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K., Washington State -nCo, V.C.I.T. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Effect of Dexamethasone in Hospitalized Patients with COVID-19 – Preliminary Report. medRxiv. 2020 2020.2006.2022.20137273. [Google Scholar]
- Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B., Liu Q., Song Y., Li X., Duan Z., Zheng Q., Yang Z., Liang J., Han M., Ruan L., Wu C., Zhang Y., Jia Z.H., Zhong N.S. Efficacy and Safety of Lianhuaqingwen Capsules, a repurposed Chinese Herb, in Patients with Coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2020 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.R., Yang C.S. Protective roles of ginseng against bacterial infection. Microb Cell. 2018;5:472–481. doi: 10.15698/mic2018.11.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Ni X., Wang Y., Xie J., Zhang X., Gu H., Zhang J. Jinhong Tablet Reduces Damage of Intestinal Mucosal Barrier in Rats with Acute Biliary Infection via Bcl-2/Bax mRNA and Protein Regulation. Evidence-based complementary and alternative medicine: eCAM. 2017;2017 doi: 10.1155/2017/4985926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National, I.C., Centre, A.R., 2020. COVID-19 Report.
- PHYSICANS C.C.O.E., Shoch and sepsis commission C.r.h. China guidelines of emergency treatment of sepsis/sepsis shock. Infection Inflammation Repariment. 2019;20:3–22. [Google Scholar]
- Ren S.H., Liu Z.J., Cao Y., Hua Y., Chen C., Guo W., Kong Y. A novel protease-activated receptor 1 inhibitor from the leech Whitmania pigra. Chin. J. Natural Med. 2019;17:591–599. doi: 10.1016/S1875-5364(19)30061-5. [DOI] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Consortium, a.t.N.C.-R. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Majumdar S., Singh R., Misra A. Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician's perspective. Diabetes Metabolic Syndrome. 2020;14:971–978. doi: 10.1016/j.dsx.2020.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The State Council, T.P.s.R.o.C., 2020. Latest developments in epidemic control on March 23.
- Wang B., Zhang W., fANG B. Intestinal flora, intestinal mucosal immunity and sepsis. Chem. Life. 2019;39:1153–1158. [Google Scholar]
- Xiang H., Zuo J., Guo F., Dong D. What we already know about rhubarb: a comprehensive review. Chin. Med. 2020;15 doi: 10.1186/s13020-020-00370-6. 88-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Wu H., Ren J., Liu Y., Wang S., Yang J., Qin S., Wu D. Shenfu Formula reduces cardiomyocyte apoptosis in heart failure rats by regulating microRNAs. J. Ethnopharmacol. 2018;227:105–112. doi: 10.1016/j.jep.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]