Abstract
Among the secondary fungal infections in Coronavirus-19 (COVID-19) infection, Aspergillosis has been reported more often than Mucormycosis. Disseminated mucormycosis is almost always a disease of severely immunosuppressed hosts. We report a young obese Asian male who was admitted with an acute anterior cerebral artery (ACA) territory infarct and severe COVID-19 pneumonitis to the intensive care unit (ICU). He had a complicated stay with recurrent episodes of vasoplegic shock and multi-organ dysfunction. At autopsy, he was confirmed to have disseminated mucormycosis. We believe this to be the first documented case of disseminated mucormycosis in an immunocompetent host with COVID-19 infection. The lack of sensitive non-invasive modalities and biomarkers to diagnose mucormycosis, along with the extremely high mortality in untreated cases, present a unique challenge to clinicians dealing with critically ill patients with COVID-19.
Keywords: COVID-19, Secondary infections, Fungal, Mucormycosis
Introduction
Numerous factors, including lymphopenia, exposure to steroids, and a dysregulated immune response can predispose to severe opportunistic super-infections in coronavirus disease-19 (COVID-19). However, the reported incidence in the literature is low, partly due to limited microbiologic sampling, especially from the airways [1]. Also, severe COVID-19 infection can be complicated by multi-system dysfunction and thrombo-embolic phenomenon, making it difficult to differentiate progressive disease from secondary invasive fungal infections.
Mucormycosis is often challenging to detect but is a potentially treatable fungal infection. There are a handful of reports on pulmonary, gastric, rhino-orbital and rhino-orbito-cerebral mucormycosis in the COVID-19 literature [[2], [3], [4], [5], [6]]. The autopsy report of our case has been discussed in a previous post-mortem study series [7]. Interest is emerging in South Asia, especially among diabetic patients with COVID-19 infection [8].
Here, we report a case of disseminated mucormycosis identified at autopsy in a young male admitted with an acute anterior cerebral artery (ACA) territory infarct and severe COVID-19 pneumonitis.
Case presentation
In April 2020, a 22-year-old Asian-descent male with a body mass index of 44 and a known history of hypothyroidism was transferred to our ICU with severe COVID pneumonitis requiring mechanical ventilation and an acute ACA territory cerebrovascular event (CVA). He had no prior history of diabetes mellitus and his average blood glucose during his ICU stay was 8.4 mmol/l. He had presented to his local hospital with a 4-day history of fever, cough, and new onset of left-sided weakness. In addition to daily aspirin and low molecular weight heparin (LMWH), hydroxychloroquine and azithromycin were started as per our hospital protocol at the time. Chest radiography (CXR) showed extensive consolidation in the right mid-to-lower lung zones and multifocal air space opacities bilaterally, consistent with COVID-19 infection. Serial laboratory parameters are summarised in Table 1.
Table 1.
Laboratory investigation reports.
| Blood Tests | Day 1 07/04/20 |
Day 4 10/04/20 |
Day 9 15/04/20 |
Day 13 19/04/20 |
Day 17 23/04/20 |
Date of Death 27/04/20 |
Normal ranges |
|---|---|---|---|---|---|---|---|
| White Cell Count | 9.7 | 17.9 | 26 | 24.95 | 17 | 27.5 | 4.4−10.1 (109/L) |
| Lymphocyte Count | 1.7 | 0.4 | 1.6 | 6.25 | 0.5 | 1.7 | 1.3−3.7 (109/L) |
| Creatinine | 189 | 322.5 | 155 | 357 | 84 | 80 | 60−120 (μmol/L) |
| CRP | 189 | 357.5 | 375 | 392.5 | 308 | 378 | 0−10 (mg/L) |
| D-dimer | 3318 | 3565 | 6394 | 13645 | 6189 | – | 0−240 (ng/mL) |
| Ferritin | 254 | 7078 | 1913 | 933 | 973 | 535 | 32−284 (ug/L) |
| LDH | 2023 | 8630 | – | 2654 | 1268 | 3945 | 266−500 (IU/L) |
| hsTroponin | 298.1 | 1130 | 443.8 | – | 19.1 | 74.5 | <19.8 (ng/L) |
| Procalcitonin | – | – | 62.02 | – | 97.22 | – | <0.07 (ug/L) |
| Glucose | 6.5 | 8.4 | 8.6 | 5.6 | 10 | 6.6 | 4−7.8 (mmol/L) |
| Amylase | 92 | 670.5 | 126 | 93.5 | 25 | 27 | 28−100 (U/L) |
| Creatine Kinase | 4283 | 51287 | 5157 | – | 496 | 3407 | 25−171 (U/L) |
| Alanine transaminase | 69 | 284 | 203 | 115 | 104 | – | 8−40 (IU/L) |
| Alkaline Phosphatase | 47 | 99 | 239 | 154 | 153 | – | 30−130 (U/L) |
| Total Bilirubin | 148 | 140 | 134 | 81 | 158 | – | 0−20 (μmol/L) |
| APTT | 50.7 | 46.7 | 55.5 | 81 | 52.5 | – | 26−36 (sec) |
| Microbiology |
|---|
| SARS CoV-2 PCR |
| 05/04/20 Detected |
| 19/04/20 Not Detected |
| 23/04/20 Not detected |
| Tracheal aspirate Culture |
| 20/04/20 No growth |
| Urine Cultures |
| 05/04/20 No growth |
| 20/04/20 No growth |
| Blood Cultures |
| 08/04/20 & 09/04/20 No growth |
| 12/04/20 No growth |
| 13/04/20 Growth of Staphylococcus epidermidis in one bottle (insignificant) |
| Bronchoalveolar lavage (BAL) |
| 23/04/20 No growth (including AFB, bacterial and fungal cultures), viral PCR panel negative |
| Pericardial Fluid |
| 27/04/20 Bacterial and AFB cultures negative |
| Serum Aspergillus Galactomannan (GAL) |
| 13/04/20 Negative (Index value:0.040) |
| 26/04/20 Negative (Index value:0.040 |
| Serum 1,3- β -D Glucan (BDG) |
| 26/04/20 Negative (<30 pg/mL) |
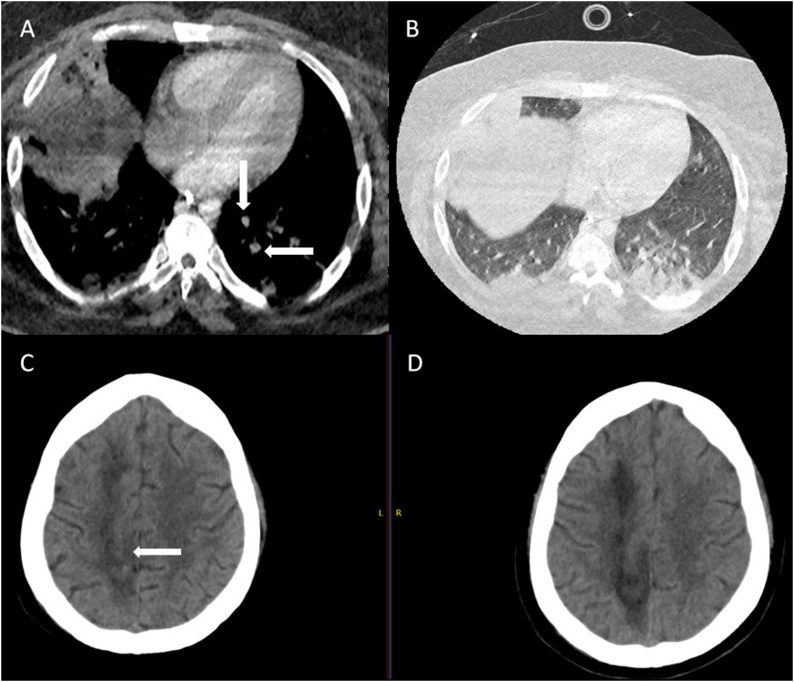
His stay was punctuated by recurrent vasoplegic episodes. The first one on day 3 was associated with low Pa02/Fi02 (P/F) ratio, poor lung mechanics, high ventilatory pressures, high vasopressor requirement (noradrenaline 0.35 mcg/min and vasopressin 0.06units/mL at peak), high amylase (peak 670 U/l) and a raised CRP (peak 393 mg/l). A combination of steroids, continuous veno-venous hemodiafiltration (CVVHDF), and escalation of empirical anti-microbial therapy to meropenem and teicoplanin was associated with a brief dramatic improvement in all the above parameters such that by day 5, in addition to improving P/F ratio and ventilatory mechanics, he was off all hemodynamic support, amylase had almost normalized and CRP had dropped to 196 mg/l. On days 6 and 7, he had the second episode of septic shock leading to the addition of tigecycline and caspofungin along with termination of steroids. He had no significant positive cultures (Table 1). CT pulmonary angiography (CTPA) on day 11 revealed a segmental pulmonary embolism in the left lower lobe with peripheral consolidation in lower lobes and right upper lobe (Fig. 1A and B). He was switched from LMWH to Argatroban with APTT target range of 47–78, in view of low anti-thrombin III levels.
Fig. 1.
Computed tomography (CT) pulmonary angiogram study showing (A) segmental pulmonary emboli, and (B) basal consolidation.
CT head showing (C) petechial hemorrhagic transformation (D) ischaemic changes in the brain.
His vasopressor requirement continued to fluctuate with raised inflammatory markers, and he had another vasoplegic episode on day 13 and received noradrenaline (0.3 mcg/min), vasopressin (0.06 units/mL), and gentamicin. Fungal biomarkers (Serum 1,3-β -d glucan (BDG) and galactomannan (GM)) were negative (Table 1). He continued to be comatose despite multiple sedation weaning trials and developed a right-sided non-reactive pupil. Repeat CT head showed new petechial haemorrhages within the known right ACA infarct with no mass effect (Fig. 1C and D). Tracheostomy and bronchoalveolar lavage (BAL) were performed and samples for bacterial, fungal, mycobacterial cultures and viral PCR were negative (Table 1).
Terminally, he developed features of pericarditis and cardiac tamponade, in addition to profound intractable vasoplegia, and despite an emergency pericardiocentesis, he succumbed to the disease on day 20 of ICU stay. He was on CVVHDF throughout except for two brief periods in view of metabolic hyperlactaemic acidosis, anuria, and rhabdomyolysis.
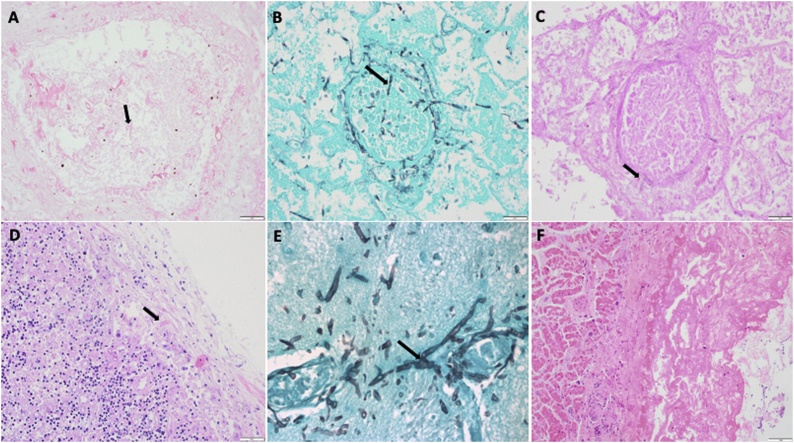
At autopsy, disseminated mucormycosis involving the lungs (a focus of necrosis of about 5 cms in the right lower lobe with evidence of invasive mycosis with broad aseptate hyphae which was Mucorales PCR positive on microscopy), pericardium, hilar nodes, and brain was identified (Fig. 2A–F). Thrombo-emboli were seen in the lungs, brain, pharynx, nasal mucosa, and trachea. There was evidence of recent haemorrhagic transformation in the previously infarcted area in the brain as well as thrombotic and congested capillaries in and around the areas of infarction with evidence of fungal hyphae in the vessels suggestive of hematogenous dissemination, necrotic vasculitis, and cerebral invasion. Besides, he had severe COVID 19 pneumonia, acute haemorrhagic pancreatitis, steatohepatitis, thyroiditis, and acute tubular injury of the kidneys with myoglobin casts.
Fig. 2.
A – Angio-invasive fungus in the lung infarct showing broad irregular aseptate hyphae (mucor).
B – Angio-invasive mucor in the lung - Grocott stain.
C – Angio-invasive mucor in the lung - PAS stain.
D – Mucor in the hilar node.
E – Cerebral mucor- Grocott stain.
F – Mucor fibrinous pericarditis.
The final cause of death following autopsy was multi-organ failure secondary to disseminated mucormycosis due to SARS-CoV-2 infection. Hypothyroidism, steatohepatitis, thrombo-embolic disease and an elevated body mass index were noted as other contributory factors.
Discussion
This is the first documented case of disseminated mucormycosis in the absence of typical risk factors, in a patient who was also positive for SARS-CoV-2 infection. In a recent review of 80 cases with COVID-19 associated mucormycosis (CAM), rhino-orbital cerebral mucormycosis was the most common presentation. Uncontrolled diabetes mellitus and systemic steroid use were the major co-morbid predisposing features [9].
The pathogenesis of invasive fungal infections (IFIs) in COVID-19 is intriguing. Fungi can establish infection in a heightened inflammatory state as readily as in an immunosuppressed state due to modification in host signalling pathways [10]. Persistent lymphopenia, as noted in our patient, with attenuated circulating levels of CD3, CD4, and CD8 cells and the exaggerated immune response with pro-inflammatory cytokines IL-1 β and IL-6 leading to tissue damage set the perfect stage for fungal infection and dissemination [11,12]. Steroids and poor glycaemic control can add further insult to the injury.
In its disseminated form, mucormycosis is often a disease of the severely immunosuppressed host including solid organ and stem cell transplant patients, graft versus host disease, patients with haematologic malignancies, severe neutropenia, and patients on steroids and voriconazole prophylaxis [13,14]. From previous case reports and our case, it seems feasible that the degree of immune dysfunction during SARS-CoV-2 infection is profound enough to predispose to mucormycosis in the absence of traditional risk factors [3,4].
Though our patient’s clinical and laboratory parameters demonstrated persistent sepsis with multi-organ dysfunction, he had negative bacterial and fungal workup with no response to empirical broad-spectrum anti-microbial therapy. His clinical course was attributed to progressively severe COVID-19 disease. The diagnosis of mucormycosis was not considered ante-mortem as he had no characteristic radiological findings of mucormycosis, and thrombo-embolic manifestations were thought to be consistent with severe SARS-CoV-2 infection. In retrospect, the right sided non-reactive pupil may have been due to a third nerve palsy secondary to cerebral mucormycosis as the bleed was too minor to manifest as a mass effect. We suggest that in the index case, the primary site of mucormycosis infection was the lung, as has been often reported in the literature, with local migration to the mediastinum and haematogenous spread to the brain. Cerebral involvement is the commonest secondary site, manifesting as focal neurologic deficits, though viscera and skin may also be involved [15,16]. The reported mortality in disseminated mucormycosis is between 68–96 % and is influenced by various factors, including host immunosuppression, timely diagnosis, initiation of therapy with amphotericin-B, and early surgical debridement [14,16,17].
Clinical suspicion of mucormycosis is generally host factor-driven in the context of angio-invasive disease [17]. While early identification and subsequent treatment are crucial to reducing morbidity and mortality, the lack of validated serological tests, biomarkers, and molecular diagnostics significantly reduces the chances of arriving at a diagnosis [17,18]. In most cases, the diagnosis requires histopathological confirmation aided by fungal cultures [18,19]. Lack of adequate sampling due to the risk of aerosolization associated with bronchoscopy and autopsy and the bleeding risk (secondary to a tissue biopsy) in those who are anti-coagulated add to the diagnostic difficulties in COVID patients. Although a variety of molecular techniques to identify mucor in blood, BAL, or body fluid samples have been reported, they suffer from lack of validation, risk of false positives in the event of contamination, and very poor sensitivity when compared to tissue samples [17,18,20]. The role of mucorales specific T cells as a diagnostic marker is yet to be established [3]. The anti-fungal drug repertoire for mucormycosis is limited, with liposomal amphotericin B being the preferred first-line agent. The newer azoles, isavuconazole, and posaconazole can be used as salvage or adjunctive therapy and stepdown therapy after initial treatment with amphotericin B. Early surgical intervention where feasible is mandatory for better outcomes [17,21].
Severe COVID-19 infection carries a significant mortality risk, hence identifying treatable co-infections and earlier intervention may improve survival. In critically ill SARS-CoV-2 infected patients with systemic infection, either unresponsive or partially responsive to conventional anti-microbial agents and with features of immune dysregulation and thrombosis, there should be a high index of suspicion for invasive mycoses, including mucormycosis especially when BDG and GM are negative. Moreover, further research into discovering novel robust diagnostic biomarkers enabling earlier detection of mucormycosis is urgently needed to prevent poor patient outcomes.
All named authors
-
•
Declare that this is an original article, that is not under consideration by another journal and has not been previously published in the current format Histopathology of the case has been discussed in a previous publication listed below.
-
•
Have seen and agreed to the submitted version of the manuscript.
-
•
Have no conflicts of interest to declare.
Sources of funding
None.
Ethical approval
Not required.
Consent
No identifying data or personal details have been used in the manuscript.
Consent has been obtained from father of the patient for publishing scientific data.
Author contribution
Vidya Krishna: Writing- Visualisation, Original draft preparation, Jaymin Morjaria- Writing: Supervision and Editing, Rona Jalandari- Data curation, Fatima Omar- Data Curation, Sundeep Kaul- Conceptualisation, Writing: Supervision and Editing.
Author agreement
This is to certify that all authors have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors' original work, hasn't received prior publication and isn't under consideration for publication elsewhere.
Declaration of previous publication
The histopathology of this case has been previously discussed in the following publication:-
Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R, Al-Sarraj S, Abdolrasouli A, Swann OC, Baillon L, Penn R, Barclay WS, Viola P, Osborn M. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020 Oct;1(6):e245-e253. doi: https://doi.org/10.1016/S2666-5247(20)30115-4. Epub 2020 Aug 20. PMID: 32844161; PMCID: PMC7440861.
However, we wish to highlight in our case report the salient clinical features of the case to disseminate knowledge among clinicians on mucormycosis in COVID.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Rawson T.M., Wilson R.C., Holmes A. Understanding the role of bacterial and fungal infection in COVID-19. Clin Microbiol Infect. 2021;27(1):9–11. doi: 10.1016/j.cmi.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zurl C., Hoenigl M., Schulz E., Hatzl S., Gorkiewicz G., Krause R. Autopsy proven pulmonary mucormycosis due to rhizopus microsporus in a critically ill COVID-19 patient with underlying hematological malignancy. J Fungi (Basel) 2021;7(Jan. (2)):88. doi: 10.3390/jof7020088. PMID: 33513875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasero D., Sanna S., Liperi C., Piredda D., Branca G.P., Casadio L. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2020;(December):1–6. doi: 10.1007/s15010-020-01561-x. Epub ahead of print. PMID: 33331988; PMCID: PMC7745708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monte Junior E.S.D., Santos M.E.L.D., Ribeiro I.B., Luz G.O., Baba E.R., Hirsch B.S. Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: a case report. Clin Endosc. 2020;53(Nov. (6)):746–749. doi: 10.5946/ce.2020.180. Epub 2020 Nov 19. PMID: 33207116; PMCID: PMC7719411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta S., Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12(Sep. (9)) doi: 10.7759/cureus.10726. PMID: 33145132; PMCID: PMC7599039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2020;(September) doi: 10.1016/j.ajem.2020.09.032. S0735-6757(20)30826-3, Epub ahead of print. PMID: 32972795; PMCID: PMC7493738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanley B., Naresh K.N., Roufosse C., Nicholson A.G., Weir J., Cooke G.S. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1(Oct. (6)):e245–e253. doi: 10.1016/S2666-5247(20)30115-4. Epub 2020 Aug 20. PMID: 32844161; PMCID: PMC7440861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debroy Sumithra. Mumbai edition. Times of India; 2020. Fungal Infection Proves Deadly for Some Patients Post-Covid.https://timesofindia.indiatimes.com/city/mumbai/fungal-infection-proves-deadly-for-some-patients-post-covid/articleshow/79808382.cms December 19, Available from: [Google Scholar]
- 9.Hoenigl M., Seidel D., Carvalho A., Rudramurthy S.M., Arastefar A., Gangneux J.P. 2021. The Emergence of COVID-19 Associated Mucormycosis: Analysis of Cases From 18 Countries. Available from: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3844587. [Last accessed 25th May 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunha C., Carvalho A., Esposito A., Bistoni F., Romani L. DAMP signaling in fungal infections and diseases. Front Immunol. 2012;3:286. doi: 10.3389/fimmu.2012.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakous N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6) doi: 10.1016/j.chom.2020.04.009. 992-1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song C.Y., Xu J., He J.Q., Lu Y.Q. Immune dysfunction following COVID-19, especially in severe patients. Sci Rep. 2020;10(Sep. (1)):15838. doi: 10.1038/s41598-020-72718-9. PMID: 32985562; PMCID: PMC7522270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spellberg B., Edwards J., Jr., Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong W., Keighley C., Wolfe R., Lee W.L., Slavin M.A., Kong D.C.M. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25(Jan. (1)):26–34. doi: 10.1016/j.cmi.2018.07.011. Epub 2018 Jul 21. PMID: 30036666. [DOI] [PubMed] [Google Scholar]
- 15.Prakash H., Chakrabarti A. Global epidemiology of mucormycosis. J Fungi (Basel) 2019;5(1):26. doi: 10.3390/jof5010026. Published 2019 Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrikkos G., Skiada A., Lortholary O., Roilides E., Walsh T.J., Kontoyiannis D.P. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(Feb. (Suppl 1)):S23–34. doi: 10.1093/cid/cir866. PMID: 22247442. [DOI] [PubMed] [Google Scholar]
- 17.Cornely O.A., Alastruey-Izquierdo A., Arenz D., Chen S.C.A., Dannaoui E., Hochhegger B. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(Dec. (12)):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. Epub 2019 Nov 5. PMID: 31699664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skiada A., Pavleas I., Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi (Basel) 2020;6(4):265. doi: 10.3390/jof6040265. Published 2020 Nov 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozel T.R., Wickes B. Fungal diagnostics. Cold Spring Harb Perspect Med. 2014;4(4) doi: 10.1101/cshperspect.a019299. Published 2014 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millon L., Scherer E., Rocchi S., Bellanger A.P. Molecular strategies to diagnose mucormycosis. J Fungi (Basel) 2019;5(1):24. doi: 10.3390/jof5010024. Published 2019 Mar 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong W., Keighley C., Wolfe R., Lee W.L., Slavin M.A., Chen S.C. Contemporary management and clinical outcomes of mucormycosis: a systematic review and meta-analysis of case reports. Int J Antimicrob Agents. 2019;53(May (5)):589–597. doi: 10.1016/j.ijantimicag.2019.01.002. Epub 2019 Jan 10. PMID: 30639526. [DOI] [PubMed] [Google Scholar]