Abstract
Very-low-carbohydrate diets or ketogenic diets are frequently used for weight loss in adults and as a therapy for epilepsy in children. The incidence and characteristics of kidney stones in patients on ketogenic diets are not well studied. Methods: A systematic literature search was performed, using MEDLINE, EMBASE, and Cochrane Database of Systematic Reviews from the databases’ inception through April 2020. Observational studies or clinical trials that provide data on the incidence and/or types of kidney stones in patients on ketogenic diets were included. We applied a random-effects model to estimate the incidence of kidney stones. Results: A total of 36 studies with 2795 patients on ketogenic diets were enrolled. The estimated pooled incidence of kidney stones was 5.9% (95% CI, 4.6–7.6%, I2 = 47%) in patients on ketogenic diets at a mean follow-up time of 3.7 +/− 2.9 years. Subgroup analyses demonstrated the estimated pooled incidence of kidney stones of 5.8% (95% CI, 4.4–7.5%, I2 = 49%) in children and 7.9% (95% CI, 2.8–20.1%, I2 = 29%) in adults, respectively. Within reported studies, 48.7% (95% CI, 33.2–64.6%) of kidney stones were uric stones, 36.5% (95% CI, 10.6–73.6%) were calcium-based (CaOx/CaP) stones, and 27.8% (95% CI, 12.1–51.9%) were mixed uric acid and calcium-based stones, respectively. Conclusions: The estimated incidence of kidney stones in patients on ketogenic diets is 5.9%. Its incidence is approximately 5.8% in children and 7.9% in adults. Uric acid stones are the most prevalent kidney stones in patients on ketogenic diets followed by calcium-based stones. These findings may impact the prevention and clinical management of kidney stones in patients on ketogenic diets.
Keywords: ketogenic diet, kidney stones, nephrolithiasis, epidemiology, meta-analysis, systematic review
1. Introduction
The ketogenic diet, initially introduced in the early nineteenth century, refers to a diet pattern that is low in carbohydrates and high in fat with a moderate proportion of protein (1.2–1.5 g/kg) [1,2]. The ketogenic diet increases the oxidation of fatty acids and ketone bodies production—creating a state of ketosis and mild acidosis [3,4]. A ketogenic diet leads to glycolysis inhibition, inhibits glutamatergic synaptic transmission, and assists in weight loss [5,6], making it popular not only for patients with obesity or metabolic syndrome, but even for athletes, both professional and amateur [7]. Ketone bodies (acetate, aceto-acetate, and beta-hydroxybutyrate) have been shown to prevent recurrent seizures [8,9,10], hence are prescribed for children with intractable seizures. However, the mechanism of the anti-seizure effects of ketone bodies is not well understood. The ketogenic diet may also have a protective effect against cognitive impairment [11] and malignancy [12]. Indications for the ketogenic diet have been extended to include glucose-1 transporter deficiency syndrome and pyruvate dehydrogenase deficiency disorders [13,14].
Multiple formulations of the ketogenic diet are currently available, including the classic keto diet, low glycemic index diet (LGID), medium-chain triglyceride diet (MCT), and modified Atkins diet. These diets differ in the proportions of lipid, carbohydrate, and protein contents [10,13,14,15]. Despite potential advantages, the ketogenic diet has multiple adverse effects. During the first four-week period, nausea, vomiting, and diarrhea are particularly common with the medium-chain triglyceride diet [16,17,18], posing a risk for acute kidney injury, hyponatremia, hypomagnesemia, hypercalciuria, hyperuricemia, and metabolic acidosis [19,20,21]. Long-term adverse effects of the ketogenic diet, including osteopenia, risk of bone fractures, alterations in vitamin D levels, are well reported [22,23,24]. Increased risk for kidney stones is well described in patients using the ketogenic diet for over a 2 year period [17,20,25,26], with complications such as obstructive uropathy, acute kidney injury, and chronic kidney disease [27,28,29].
The incidence of kidney stones among patients on the ketogenic diet ranges from 3% to 10% [20,30,31], compared to one in several thousand in the general population [17,24]. We performed a meta-analysis on the incidence and characteristics of kidney stones in patients on the ketogenic diet to better understand the kidney stones’ burden and pathophysiology in this population.
2. Materials and Methods
2.1. Search Strategies
A comprehensive search of several databases from each database’s inception to 3 May 2019 was conducted. The databases included OVID MEDLINE (1946 to April 2020), EMBASE (1988 to April 2020), and the Cochrane Database of Systematic Reviews (database inception to April 2020). The systematic literature review was conducted independently by two investigators (P.A. and C.A.), using the search strategy that consolidated the terms of (‘ketogenic diet’ OR ‘keto diet’ OR ‘atkins diet’ OR ‘low carb diet’ OR ‘low carbohydrate diet’) AND (nephrolithiasis OR ‘kidney stone’ OR ‘kidney stones’). The actual strategy listing all search terms used is available in the online Supplementary Materials. There were no restrictions on language, sample size, or study duration. This study was conducted by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (online Supplementary Materials) [32].
2.2. Study Selection
Eligible studies must be clinical trials, observational studies (cohort, case-control, or cross-sectional studies) that reported incidence and characteristics of kidney stones in patients on ketogenic diets. Retrieved articles were individually reviewed for eligibility by the two investigators (P.A. and C.A.). Discrepancies were addressed and resolved by third investigator (W.C.). Inclusion was not limited by language, age, sample size, or study duration.
2.3. Data Extraction
The following data were extracted: first author name, year of publication, number of patients, duration of follow-up, description of ketogenic diet, mean age, sex, incidence of kidney stones, type of kidney stones, and time to diagnosis of kidney stones after ketogenic diet consumption. The primary outcome included the incidence of kidney stones.
2.4. Data Synthesis and Statistical Analysis
We calculated the pooled estimated incidence of kidney stones among patients on the ketogenic diet. The pre-specified subgroup analysis based on age groups (pediatrics and adults) was performed. A random-effects model was used due to the expected clinical heterogeneity in the included populations [33]. All pooled estimates were shown with 95% confidence intervals (CIs). Heterogeneity among effect sizes estimated by individual studies was described with the I2 statistic and the chi-square test. A value of I2 of 0% to 25% represents insignificant heterogeneity, 26% to 50% low heterogeneity, 51% to 75% moderate heterogeneity and 76% to 100%, high heterogeneity [34].
Publication bias was evaluated using the Egger test [35]. A p-value of less than 0.05 indicates the presence of publication bias. The meta-analysis was performed by the Comprehensive Meta-Analysis 3.3 software (Biostat Inc, Englewood, NJ, USA). The data for this meta-analysis are publicly available through the Open Science Framework on 25 September 2020 (URL: https://osf.io/2gtk3/ (accessed on 25 September 2020)).
3. Results
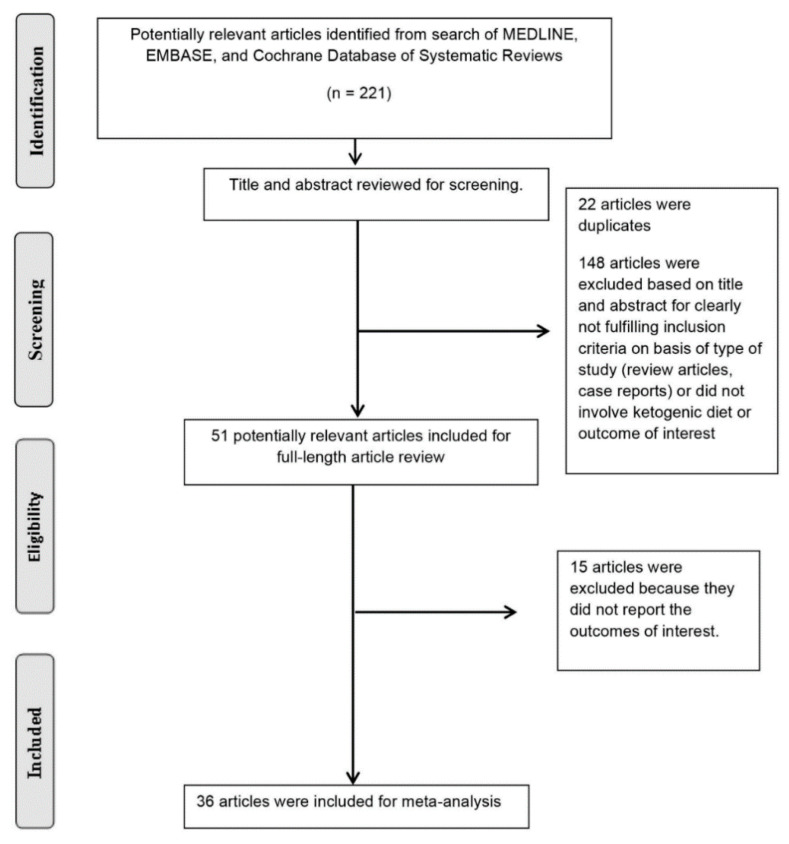
A total of 221 potentially relevant articles were identified and screened. Fifty-one articles were assessed in detail of which 36 studies with 2795 patients on ketogenic diets were enrolled in our meta-analysis (Figure 1 and Table 1). The definitions and reported adverse effects observed in ketogenic diets in different studies are shown in Table 2.
Figure 1.
The literature retrieval, review, and selection process.
Table 1.
Characteristics of studies included in this systematic review.
| Reference | Description of Ketogenic Diet |
Total Number of Patients on Ketogenic Diet |
Mean Age of Included Patients |
Mean Duration of KGD |
No. of Patients with Kidney Stones | % of Patients with Kidney Stones |
24 h Urine Study |
|---|---|---|---|---|---|---|---|
| Holler A. et al. [36] | Classical KD (16), Modified Atkins diet (MAD) (14) | 31 | Median 5.5, mean 5.5 (0.1–15.7 Y) | N/A | 0 | 0 | Hypercalciuria in 12/22 |
| Rener et al. [37] | 2.5:1 to 45/3/20201 | 15 | <3 Y | Average 13 M (4–16 M) | 1 | 6.60% | N/A |
| Attar H. et al. [38] | Modified Atkins diet | 13 | 23–72 Y | Range 1–21 M | 1 | 7.69% | N/A |
| Felix et al. [39] | Modified Atkins diet | 5 | 6–12 Y | 4 M | 0 | 0 | 1 had hypercalciuria |
| Lapp et al. [40] | KGD in 7 and Modified Atkins diet in 19 | 26 | >18 Y | N/A | 4 | 15% | N/A |
| Nerurkar et al. [41] | KGD | 17 | <3 Y | N/A | 1 | 5.88% | N/A |
| Nangia et al. [42] | KGD | 59 | 4.5 Y (range 0.2–22 Y) | Mean 2 Y (range 0–5.5 Y) | 3 | 5% | N/A |
| Gainza et al. [43] | KGD | 41 | 4.7 Y (range 1–13 Y) | Mean 5.79 Y (3–10.6) | 3 | 7.10% | Hypercalciuria in 11 (26.2%) |
| Mak et al. [44] | KGD (MCT oil diet) | 13 | 7.3 Y | N/A | 1 | 7.70% | Increase Ca/Cr ratio in 1 patient |
| Furth et al. [45] | N/A | 112 | 5 Y | N/A | 6 | 5.30% | Elevated Ca/Cr ratio |
| Rios et al. [46] | 4:1 (1.5:1–4.5:1) KGD | 22 | Range 1–19 Y (min age 18 M) | 25 M (1–54 M) | 2 | 9.09% | N/A |
| Sharma et al. [47] | 3:1 KGD in <18 M, 4:1 KGD in >18 M | 23 | (range 6 M–5 Y) | N/A | 1 | 3.70% | Elevated Ca/Cr ratio |
| Kang et al. [48] | 3:1 KGD | 40 | Median ± IQR- 15 ± 13.0 (range 6–60 M) | N/A | 2 | 5.00% | N/A |
| Wibisono et al. [49] | Classic KGD, MCT diet, Modified Atkins diet | 48 | 3.8 Y (range 2.3–7 Y) | Range 1–14 Y | 2 | 4.00% | N/A |
| Simm et al. [50] | 4:1 to 2:1 KGD | 29 | 6.4 Y (range 3.3–17.8 Y) | Mean 2.1 Y (range 0.5–6.5 Y) | 1 | N/A | N/A |
| Guzel et al. [51] | Olive oil-based KGD | 389 | Median 4.0 (2–7)Y | 12 M | 12 | 3% | N/A |
| Hassan et al. [52] | Classic 4:1 KGD (49 of 52 pts), rest with modified diet supplemented by MCT oil | 52 | 5 Y, 6 M ± 3 Y, 4 M | N/A | 1 | 1.90% | Increased calcium oxide in urine |
| Takeoka et al. [53] | 3:1 to 4.1 KGD + Topiramate | 14 | Mean age 4.7 Y | N/A | 0 | 0% | N/A |
| Kossoff et al. [54] | KGD (older children on 4:1 diet and younger on 3:1) without carbonic anhydrase inhibitors | 221 | 5.1 Y (SD 4.5, range 16.5 Y) | N/A | 15 | 6.70% | N/A |
| Kossoff et al. [55] | KGD (older children on 4:1 diet and younger on 3:1) with topiramate or zonisamide | 80 | 4.8 Y (SD 2.4, range 6.5 Y) | N/A | 5 | 6.30% | N/A |
| Kossoff et al. [56] | 4:1 KGD in 9, 3.5:1 in 1 and 3:1 in 13 | 23 | 1.1 Y (range 0.5–24 M) | N/A | 2 | 8.60% | N/A |
| Kang et al. [57] | 4:1 KGD | 129 | 64.9 (±59.3) M | 12.0 (±10.1) M | 4 | 3.10% | N/A |
| Mackay et al. [58] | N/A | 26 | Median age 6.1 Y (range 2.3–13.2) | N/A | 0 | 0% | Increased urine calcium in 8% |
| Groesbeck et al. [59] | 4:1 KGD in 19, 3:1 KGD in 9 | 28 | 3 Y 9 M (range 6 M–13 Y 6 M) | 7 Y 9 M | 7 | 25% | Increase Ca Cr ratio in 14 pts |
| Sampath et al. [60] | 3:1 (56%) or 4:1 KGD | 195 | Median 3 Y (0.5–15 Y) | Median 12 M (range 1–72 M) | 13 | 7% | N/A |
| Raimann et al. [61] | 4:1 in 16, 3.5:1 in 3 and 3:1 in 2 + Calcium and MV supplement | 21 | 6.2 Y (range 6 M–17 Y) | 15 pt completed 1 Y of KGD 2.6 Y (1–6.3 Y) | 2 | 10% | Hypercalciuria in both stone formers |
| Caraballo et al. [62] | N/A | 140 | 5 Y (range 1–18 Y | 3.5 Y (range 1–20 Y) | 6 | 4.28% | N/A |
| Dressler et al. [63] | 4:1 in 36, 3:1 in 53, 3.5:1 in 6, 2.5:1 in 17, 2:1 in 3 | 115 | 2.86 ± 3.1 (min 0.0–max 16.8) | N/A | 4 | 3.40% | N/A |
| Hallbook et al. [64] | N/A | 290 | 5.3 (0.6–18.6) | 2 Y | 7 | N/A | N/A |
| Khoo et al. [65] | 4:1 in 12, 3:1 in 11, 2:1 in 3, MAD in 4 | 30 | 6.8 Y (8 M to 17 Y) | 8 M (range 7 days to 6 Y) | 4 | 13% | N/A |
| Lim et al. [66] | N/A | 204 | 4.8 Y (range 0.3–33.9 Y) | Median 17 M (95% CI 9–24 M) | 2 | 0.98% | N/A |
| McNally et al. [67] | 3:1 or 4;1 KGD | 195 (KGD + hypercalciuria so polycitra K given) |
4.3 Y in reactive group |
15.6 (13.1) | 13 | 6.70% | N/A |
| Park et al. [68] | N/A | 16 | Age range (0.1–40 Y) | N/A | 1 | 6.25% | N/A |
| Draaisma et al. [69] | Classic KGD (67.6%), MCT diet (2.9%), MAD (19.1%) or LGIT (7.4%), other (1.5%) | 68 | 5.7 ± 4.3 Y | 25.6 ± 24.8 M | 6 | 8.80% | N/A |
| Roehhl et al. [70] | Modified KGD | 55 | Mean 38 Y (range 17–70 Y) | N/A | 0 | 0% | N/A |
| Lambrechts et al. [71] | MCT diet and Classic KGD | 26 | 7 Y | 0 | 0 | N/A | N/A |
Abbreviations: KGD—ketogenic diet, MCT—medium chain triglyceride, F/H—family history, Y—years, M—months, pts—patients, Ca—calcium, Cr—creatinine, MV—multivitamin, K citrate—potassium citrate, RR—relative risk, NR—not recorded, gp—group, LGIT—low glycemic index treatment, MAD—modified Atkin’s diet.
Table 2.
Definitions and reported adverse effects observed in ketogenic diets in different studies.
| Author | Different Types of Ketogenic Diet |
Side Effects/Complication of Ketogenic Diet besides Renal Stones |
|---|---|---|
| Holler A. et al. [36] | Classical KGD and MAD | Constipation, increased bromine level (3.2%) |
| Rener et al. [37] | KGD 2.5:1 to 4:1 | Vomiting |
| Attar H. et al. [38] | MAD | NR |
| Felix et al. [39] | MAD | Weight loss, hyperlipidemia |
| Lapp et al. [40] | KGD, MAD | Gallstones (3.8%), hyperlipidemia (3.8%) |
| Nerurkar et al. [41] | KGD not specified | Constipation (57%) |
| Nangia et al. [42] | KGD 3:1 to 4:1 | Constipation (39%), acidosis (21%), nausea/emesis (14%), increased seizures (7%). |
| Gainza et al. [43] | KGD not specified | Osteopenia (38.1%), severe metabolic acidosis (9.5%), recurrent pneumonia (21.4%), neutropenia (0.5%), fatty liver (0.1%), easy bruising (4.8%) |
| Mak et al. [44] | KGD—Protein + carb (<19%) of caloric requirements MCT 60–70% of caloric requirements |
Weight loss (46%), diarrhea (38%), bad temper (7.6%), abdominal cramps (15%), nausea (15%), bad body smell (7.6%) |
| Furth et al. [45] | NR | |
| Rios et al. [46] | KGD 4:1 (1.5:1 to 4.5:1) | Nausea and vomiting (26.3%), hypercholesterolemia (64.7%), anorexia (31.8%), constipation (40.9%), symptomatic acidosis (9.09%), carnitine deficiency (9.09%) |
| Sharma et al. [47] | Classical KGD 3:1 or 4:1 | Vomiting (75%), asymptomatic hypocalcemia, Constipation (75%), weight loss, hypoalbuminemia |
| Kang et al. [48] | Classical KGD 4:1 | Dehydration, GI discomfort, hyperlipidemia, hyperuricemia, symptomatic hypoglycemia, lipoid aspiration pneumonia, hypoproteinemia, hypomagnesemia, repeated hyponatremia |
| Wibisono et al. [49] | Classical KGD, MCT, MAD | Constipation, hypertriglyceridemia, hypercholesterolemia, diarrhea, lethargy, iron deficiency, GERD, vomiting, hypoglycemia |
| Simm et al. [50] | KGD 2:1 to 4:1 | Osteopenia, fracture |
| Guzel et al. [51] | KGD 2.5:1 and 4:1 | Hyperlipidemia (50.8%), selenium deficiency (26.9%), constipation (26.2%), sleep disturbances (20%), hyperuricemia (3%), hepatic effects (2.6%), hypoproteinemia (2.6%), hypoglycemia(1.5%) |
| Hassan et al. [52] | Classic 4:1 KGD or MCT diet | Constipation (85%), gall bladder stone (1.9%), hyponatremia (1.9%) |
| Takeoka et al. [53] | KGD not specified | Nausea/vomiting (7%), irritability (7%), lethargy (21%), sedation (14%) |
| Kossoff et al. [54] | KGD 3:1 to 4:1 | NR |
| Kossoff et al. [55] | KGD 3:1 to 4:1 | Sedation (27%), rash, irritability |
| Kossoff et al. [56] | KGD 3:1 to 4:1 | Severe GERD (13%), hip dislocation (0.4%) |
| Kang et al. [57] | Dehydration, GI discomfort, hyperlipidemia, hyperuricemia, hypoglycemia | |
| Mackay et al. [58] | Classical KGD 3:1 to 4.2:1 | Asymptomatic hypoglycemia (24%), poor linear growth (20%), hyperlipidemia (16%), vomiting (12%), hypocarnitinemia (8%), hypercalciuria (8%), constipation (8%), osteopenia (4%), pancreatitis (4%), Diarrhea (4%) |
| Groesbeck et al. [59] | 60.7% on classical KDT 7% MAD, 32% other KGD |
Fractures (21.4%), hyperlipidemia (7%), constipation (53%) |
| Sampath et al. [60] | KGD 3:1 (56%) or 4:1 | NR |
| Raimann et al. [61] | KGD 4:1 (3 pts 3.5:1 2 pts 3:1) | Hypercholesterolemia 64% (at 12 months) 15% (at 18 months), growth retardation |
| Caraballo et al. [62] | KGD | GI side effects (30.5%), hyperlipidemia (9.7%), weight gain (2.3%), hypocarnitinemia (3.7%), hypercalciuria (6.9%), hypoglycemia (5.5%), dehydration (6.4%) |
| Dressler et al. [63] | KGD 3:1, 4:1 or 2.5:1 | Carnitine deficiency (13%), growth deficit (5.2%), weight gain (1.7%), hypertriglyceridemia (29.5%), hypercholesterolemia (10.4%) |
| Hallbook et al. [64] | KGD 3:1 or 4:1 ratio | Hyperlipidemia (6%), bone fractures (0.9%) |
| Khoo et al. [65] | Classical KGD (81.2%), MAD (18.75%) | Constipation (43%), hunger (23%), excessive weight gain or loss (20%), vomiting (10%), hyperuricemia (30%), hypocalcemia (20%) |
| Lim et al. [66] | NR | GI side effects (nausea, vomiting, and constipation), Inadequate weight gain or significant weight loss, ketoacidosis, hepatotoxicity, renal dysfunction, sinus tachycardia, osteoporosis |
| McNally et al. [67] | KGD unspecified | NR |
| Park et al. [68] | KGD 4:1 (87.5%), KGD 3:1 (12.5%) | Regurgitation, constipation, aspiration, hypertriglyceridemia, hypoproteinemia, nausea, vomiting |
| Draaisma et al. [69] | Classic KGD (67.6%), MCT diet (2.9%) MAD (19.1%), LGID (7.4%), others (1.5%) |
Decrease in BMD 0.22 Z-score/year |
| Roehhl et al. [70] | Modified ketogenic diet 15 gm carb vs. 50 gm carb diet |
Constipation (9%) |
| Lambrechts et al. [71] | KGD | GI side effects (30%) |
Abbreviations: KGD—ketogenic diet; MAD—modified Atkins diet; MCT—medium chain triglyceride diet; NR—non-report; LGID—low glycemic index diet.
3.1. Incidence of Kidney Stones among Patients on Ketogenic Diets
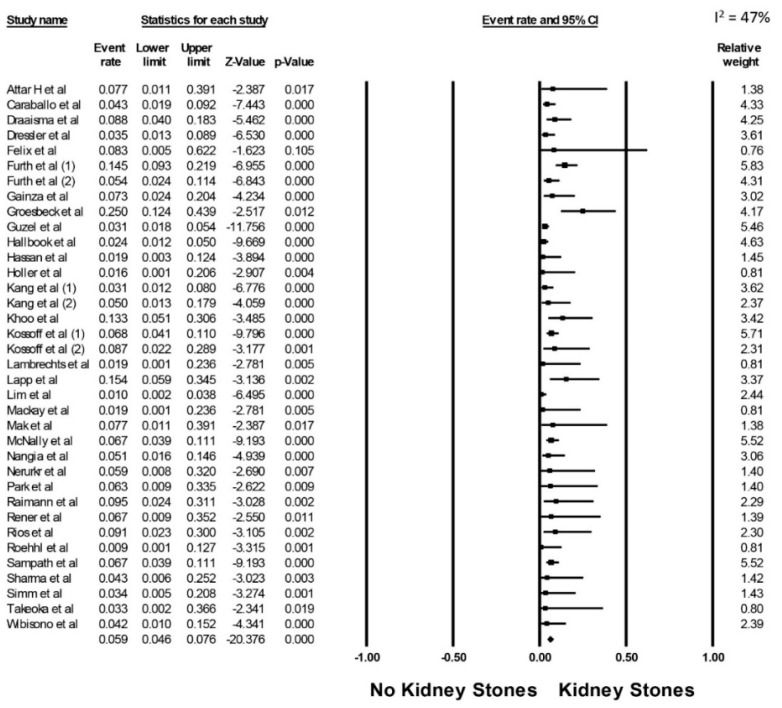
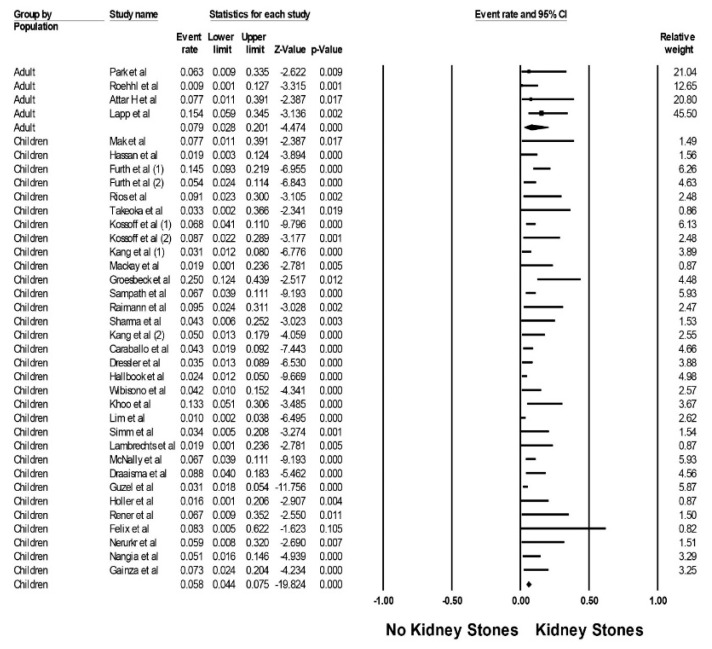
The estimated pooled incidence of kidney stones was 5.9% (95% CI, 4.6–7.6%, I2 = 47%, Figure 2) in patients on ketogenic diets at a mean follow-up time of 3.7 +/− 2.9 years. Subgroup analyses demonstrated the estimated pooled incidence of kidney stones of 5.8% (95% CI, 4.4–7.5%, I2 = 49%) in children and 7.9% (95% CI, 2.8–20.1%, I2 = 29%) in adults (Figure 3), respectively.
Figure 2.
Pooled estimated incidence of kidney stones.
Figure 3.
Pooled estimated incidence of kidney stones by patient population.
3.2. Type of Kidney Stones among Patients on Ketogenic Diet
Within reported studies, 48.7% (95% CI, 33.2–64.6%) of kidney stones were uric stones, 36.5% (95% CI, 10.6–73.6%) were calcium-based (CaOx/CaP) stones, and 27.8% (95% CI, 12.1–51.9%) were mixed uric acid and calcium-based stones, respectively.
3.3. Evaluation for Publication Bias
Using Egger’s regression asymmetry tests, there was no significant publication bias found in this meta-analysis. The Egger’s regression test demonstrated no significant publication bias in all analyses (p > 0.05).
4. Discussion
Our analysis reports a pooled incidence of kidney stones at 5.6% in patients treated with a ketogenic diet after four years. The incidence of nephrolithiasis in the general population is reported at 0.3% per year in men and 0.25% per year in women [72]. In our study, the incidence of kidney stones is identical in children and adults. This finding contradicts the hypothesis that children are more susceptible to kidney stone formation due to extended treatment duration with the ketogenic diet, small renal tubular lumen, and relatively less renal reserve. However, studies included in this analysis did not report the recurrence of kidney stones; it is possible children may be predisposed to recurrent kidney stones due to prolonged exposure to the ketogenic diet. More studies are required to understand the risk of recurrent kidney stones with the ketogenic diet.
Uric acid stones are the most common stones in patients receiving the ketogenic diet, followed by calcium-based stones and uric acid–calcium mixed stones. In contrast, calcium oxalate stones are the most common stones in the general population [72]. The exact mechanism of nephrolithiasis following the ketogenic diet is unclear. However, it is likely related to hypocitraturia and acidosis, common in people consuming a high-protein and low-alkali diet [20]. Acidosis contributes to significant reabsorption of citrate in the proximal tubule, further contributing to hypocitraturia [25,73,74,75,76,77,78]. A more generous amount of free calcium is available for stone formation in a low-citrate environment [73,79]. Chronic acidosis also leads to demineralization of the bone and increased calcium excretion [17,20]. Hypercalciuria, immobilization, anti-epileptic drugs, and fat malabsorption further precipitate urinary calcium. Moreover, the low urine pH seen in patients with a low-alkali diet contributes to uric acid crystals [73]. Obesity, insulin resistance, and an animal-protein diet are associated with low urine pH [80]. The uric acid stone may act as a nidus for calcium-based nephrolithiasis formation [73]. Furthermore, fluid intake restriction is traditionally applied to children receiving the ketogenic diet, making them susceptible to stone formation [73].
Potential benefits of urine alkalization with oral potassium citrate in children with a urine calcium to creatinine ratio of >0.20 mg/mg to prevent kidney stone formation is well reported [20,25,75,78]. Genetic polymorphisms in transporters, such as renal sodium citrate cotransporter, is a known risk factor in recurrent stone formers [81,82]. McNally et al. reported that the empiric use of oral citrate in children treated with a classic ketogenic diet led to a reduction in the incidence of kidney stones from 6.75% to 0.9% without an increase in adverse effects [67]. The international ketogenic diet study group agreed that oral citrates appear to prevent kidney stones; however, there was mixed opinion on its empiric use (class III) [9]. The consensus statement is unchanged since 2009 due to the lack of new evidence. Hence, we need a well-designed study to analyze the empiric use of urine alkalization therapy. A ketogenic diet is generally prescribed for weight loss in adults, who require a shorter duration of therapy; the empiric use of oral citrates may not be necessary. However, this remains to be elucidated by future studies.
Purine-rich foods (red meat, fish, poultry, beer, and legumes) increase the uric acid load [80]. The digestion of animal protein produces a transient acidic environment, which results in a lower urine pH, promoting the precipitation of uric stones [80]. Since uric stones are the most common stones in patients receiving a ketogenic diet, switching from animal proteins to plant-based proteins results in lower uric acid excretion, but, sequentially, lower uric acid stone formation is unclear. Siener et al. reported that patients consuming a balanced diet of vegetables and animal proteins had higher urine pH and urine uric acid concentration than those on a typical western diet [83]. It is also recommended that patients with symptomatic hypercalcemia, hyperuricosuric calcium urolithiasis, and urate nephropathy should be prescribed a urate-lowering agent [80]. However, empiric use of xanthine oxidase inhibitors in patients on a ketogenic diet requires further investigation.
Other measures to mitigate the risk of renal stones include liberalizing fluid intake and avoiding the initial fasting phase at the start of ketogenic diets [84,85]. Modification of the diet regimen to allow small, frequent meals might help decrease gastrointestinal side effects and avoid volume depletion [86]. Screening for underlying metabolic disorders should be considered before initiation of a ketogenic diet to help avoid substantial acidosis [87]. Considering the long-term risk of bone fractures and osteopenia, the 2018 international ketogenic diet group recommended periodic DEXA scan screening for evaluation of bone mineral density [9]. Epidemiological studies have shown a temporal relationship between idiopathic osteoporosis and kidney stones. In addition, changing dietary patterns, including the ketogenic diet, could possibly be an important environmental trigger in the association, as well [88]. Bone health should be monitored closely in patients on the ketogenic diet and more clinical trials are needed to further define the negative impacts on bone health [89]. Although prophylactic calcium and vitamin D is recommended in all people on the ketogenic diet for bone health [23,24], athletes with dermal calcium loss during exercise/sweating and obese subjects restricting dairy are at further risk of worsening bone health if not on adequate calcium supplements [7,89]. However, given the reported risk of nephrolithiasis from hypercalciuria, supplementation remains a challenge [20,25]. Periodic urine chemistry analyses to measure the calcium to creatinine ratio, calcium, citrate, and oxalate can help identify patients at risk for kidney stone formation, and timely referral to a nephrologist should be considered. Patients with a family history of nephrolithiasis should be screened before starting a ketogenic diet due to their higher risk for stone formation.
In the era of precision medicine, further studies are needed to understand the use of genetic variants to further personalize management, even in the dietary therapy field [90]. Our study has the following limitations. First, the observational studies included in the analysis are susceptible to shortcomings inherent to the design. In addition, sources of heterogeneity could be due to differences in patient population and definitions of ketogenic diet as described in Table 2. Second, important risk factors, such as family history of nephrolithiasis, physical activity, exposure to sunlight, presence of ketone bodies in the blood or urine and environmental exposure, were not mentioned in the included studies. Third, recurrent kidney stones are not differentiated from the first episodes; recurrent kidney stones might be more common in children. Fourth, the glomerular filtration rate decline was not the primary outcome in most of the included studies. Fifth, the pooled sample size for adults is smaller than children. Lastly, data on the estimated incidence of kidney stones in elderly and CKD patients on ketogenic diets were limited.
5. Conclusions
In conclusion, the estimated incidence of kidney stones in patients on ketogenic diets is 5.6%, which is comparable among adults and children. Uric acid stones are the most prevalent kidney stones in patients treated with ketogenic diets, followed by calcium-based stones. These findings may impact the prevention and management of kidney stones in patients treated with ketogenic diets.
Supplementary Materials
The following are available on https://www.mdpi.com/article/10.3390/diseases9020039/s1, the actual strategy listing all search terms; the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.
Author Contributions
Conceptualization, P.A., C.A., C.T., P.H., K.K., J.M., P.V., D.F.G.A., P.M., P.P., T.B. and W.C.; data curation, P.A. and C.A.; formal analysis, W.C.; investigation, P.A., C.A. and W.C.; methodology, P.A., C.T., S.R.K. and W.C.; project administration, C.A., P.M. and T.B.; resources, T.B.; software, W.C.; supervision, C.T., P.H., S.R.K., K.K., J.M., P.V., D.F.G.A., P.P. and W.C.; validation, P.A., C.A., P.H., J.M., P.M. and W.C.; visualization, C.T., T.B. and W.C.; writing—original draft, P.A.; writing—review and editing, P.A., C.A., C.T., P.H., S.R.K., K.K., J.M., P.V., D.F.G.A., P.P., T.B. and W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors have no commercial associations that might be a conflict of interest for this article.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paoli A., Cenci L., Grimaldi K. Effect of ketogenic mediterranean diet with phytoextracts and low carbohydrates/high-protein meals on weight, cardiovascular risk factors, body composition and diet compliance in Italian council employees. Nutr. J. 2011;10:112. doi: 10.1186/1475-2891-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawkes C.P., Roy S.M., Dekelbab B., Frazier B., Grover M., Haidet J., Listman J., Madsen S., Roan M., Rodd C., et al. Hypercalcemia in Children Using the Ketogenic Diet: A Multicenter Study. J. Clin. Endocrinol. Metab. 2021;106:e485–e495. doi: 10.1210/clinem/dgaa759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha S.R., Kossoff E.H. The Ketogenic Diet. Neurology. 2005;11:161–170. doi: 10.1097/01.nrl.0000160818.58821.d2. [DOI] [PubMed] [Google Scholar]
- 4.Stafstrom C.E., Spencer S. The ketogenic diet: A therapy in search of an explanation. Neurology. 2000;54:282. doi: 10.1212/WNL.54.2.282. [DOI] [PubMed] [Google Scholar]
- 5.Hartman A.L., Stafstrom C.E. Harnessing the power of metabolism for seizure prevention: Focus on dietary treatments. Epilepsy Behav. 2013;26:266–272. doi: 10.1016/j.yebeh.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nylen K., Likhodii S., Burnham W.M. The ketogenic diet: Proposed mechanisms of action. Neurotherapeutics. 2009;6:402–405. doi: 10.1016/j.nurt.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klesges R.C., Ward K.D., Shelton M.L., Applegate W.B., Cantler E.D., Palmieri G.M.A., Harmon K., Davis J. Changes in Bone Mineral Content in Male Athletes. JAMA. 1996;276:226–230. doi: 10.1001/jama.1996.03540030060033. [DOI] [PubMed] [Google Scholar]
- 8.Wheless J.W. History of the ketogenic diet. Epilepsia. 2008;49:3–5. doi: 10.1111/j.1528-1167.2008.01821.x. [DOI] [PubMed] [Google Scholar]
- 9.Kossoff E.H., Zupec-Kania B.A., Amark P.E., Ballaban-Gil K.R., Bergqvist A.G.C., Blackford R., Buchhalter J.R., Caraballo R.H., Cross J.H., Dahlin M.G., et al. Optimal clinical management of children receiving the ketogenic diet: Recommendations of the International Ketogenic Diet Study Group. Epilepsia. 2009;50:304–317. doi: 10.1111/j.1528-1167.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- 10.Kossoff E.H., Zupec-Kania B.A., Rho J.M. Ketogenic Diets: An Update for Child Neurologists. J. Child Neurol. 2009;24:979–988. doi: 10.1177/0883073809337162. [DOI] [PubMed] [Google Scholar]
- 11.Davidson T., Hargrave S., Swithers S., Sample C., Fu X., Kinzig K., Zheng W. Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience. 2013;253:110–122. doi: 10.1016/j.neuroscience.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedland S.J., Mavropoulos J., Wang A., Darshan M., Demark-Wahnefried W., Aronson W.J., Cohen P., Hwang D., Peterson B., Fields T., et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate. 2008;68:11–19. doi: 10.1002/pros.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klepper J., Leiendecker B. GLUT1 deficiency syndrome—2007 update. Dev. Med. Child Neurol. 2007;49:707–716. doi: 10.1111/j.1469-8749.2007.00707.x. [DOI] [PubMed] [Google Scholar]
- 14.Wexler I.D., Hemalatha S.G., McConnell J., Buist N., Dahl H.-H.M., Berry S.A., Cederbaum S.D., Patel M.S., Kerr D.S. Outcome of pyruvate dehydrogenase deficiency treated with ketogenic diets: Studies in patients with identical mutations. Neurology. 1997;49:1655–1661. doi: 10.1212/WNL.49.6.1655. [DOI] [PubMed] [Google Scholar]
- 15.Meira I.D., Romão T.T., Prado H.J.P.D., Krüger L.T., Pires M.E.P., Da Conceição P.O. Ketogenic Diet and Epilepsy: What We Know So Far. Front. Neurosci. 2019;13:5. doi: 10.3389/fnins.2019.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman J.M., Kelly M.T., Freeman J.B. The Epilepsy Diet Treatment: An Introduction to the Ketogenic Diet. Demos Vermande; New York, NY, USA: 1996. [Google Scholar]
- 17.Liu Y.M., Wang H.S. Medium-chain triglyceride ketogenic diet, an effective treatment for drug-resistant epilepsy and a comparison with other ketogenic diets. Biomed. J. 2013;36:9–15. doi: 10.4103/2319-4170.107154. [DOI] [PubMed] [Google Scholar]
- 18.Tanchoco C.C., Cruz A.J., Rogaccion J.M., Casem R.S., Rodriguez M.P., Orense C.L., Hermosura L.C. Diet supplemented with MCT oil in the management of childhood diarrhea. Asia Pac. J. Clin. Nutr. 2007;16:286–292. [PubMed] [Google Scholar]
- 19.Ballaban-Gil K., Callahan C., O’Dell C., Pappo M., Moshe S., Shinnar S. Complications of the Ketogenic Diet. Epilepsia. 1998;39:744–748. doi: 10.1111/j.1528-1157.1998.tb01160.x. [DOI] [PubMed] [Google Scholar]
- 20.Bach A.C., Babayan V.K. Medium-chain triglycerides: An update. Am. J. Clin. Nutr. 1982;36:950–962. doi: 10.1093/ajcn/36.5.950. [DOI] [PubMed] [Google Scholar]
- 21.Chesney D., Brouhard B.H., Wyllie E., Powaski K. Biochemical abnormalities of the ketogenic diet in children. Clin. Pediatr. 1999;38:107–109. doi: 10.1177/000992289903800207. [DOI] [PubMed] [Google Scholar]
- 22.Bertoli S., Trentani C., Ferraris C., De Giorgis V., Veggiotti P., Tagliabue A. Long-term effects of a ketogenic diet on body composition and bone mineralization in GLUT-1 deficiency syndrome: A case series. Nutrition. 2014;30:726–728. doi: 10.1016/j.nut.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Bergqvist A.C., Schall J.I., Stallings V.A. Vitamin D Status in Children with Intractable Epilepsy, and Impact of the Ketogenic Diet. Epilepsia. 2007;48:66–71. doi: 10.1111/j.1528-1167.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 24.Bergqvist A.G.C., Schall J., Stallings V., Zemel B.S. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am. J. Clin. Nutr. 2008;88:1678–1684. doi: 10.3945/ajcn.2008.26099. [DOI] [PubMed] [Google Scholar]
- 25.Choi J.N., Song J.E., Shin J.I., Kim H.D., Kim M.J., Lee J.S. Renal stone associated with the ketogenic diet in a 5-year old girl with intractable epilepsy. Yonsei Med. J. 2010;51:457–459. doi: 10.3349/ymj.2010.51.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kielb S., Koo H.P., Bloom D.A., Faerber G.J. Nephrolithiasis associated with the ketogenic diet. J. Urol. 2000;164:464–466. doi: 10.1016/S0022-5347(05)67400-9. [DOI] [PubMed] [Google Scholar]
- 27.Rule A.D., Bergstralh E.J., Melton L.J., Li X., Weaver A.L., Lieske J.C. Kidney Stones and the Risk for Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2009;4:804–811. doi: 10.2215/CJN.05811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Zoghby Z.M., Lieske J.C., Foley R.N., Bergstralh E.J., Li X., Melton L.J., Krambeck A.E., Rule A.D. Urolithiasis and the Risk of ESRD. Clin. J. Am. Soc. Nephrol. 2012;7:1409–1415. doi: 10.2215/CJN.03210312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thongprayoon C., Hansrivijit P., Kovvuru K., Kanduri S.R., Torres-Ortiz A., Acharya P., Gonzalez-Suarez M.L., Kaewput W., Bathini T., Cheungpasitporn W. Diagnostics, Risk Factors, Treatment and Outcomes of Acute Kidney Injury in a New Paradigm. J. Clin. Med. 2020;9:1104. doi: 10.3390/jcm9041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul E., Conant K.D., Dunne I.E., Pfeifer H.H., Lyczkowski D.A., Linshaw M.A., Thiele E.A. Urolithiasis on the ketogenic diet with concurrent topiramate or zonisamide therapy. Epilepsy Res. 2010;90:151–156. doi: 10.1016/j.eplepsyres.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang H.C., Kim Y.J., Kim N.W., Kim H.D. Efficacy and Safety of the Ketogenic Diet for Intractable Childhood Epilepsy: Korean Multicentric Experience. Epilepsia. 2005;46:272–279. doi: 10.1111/j.0013-9580.2005.48504.x. [DOI] [PubMed] [Google Scholar]
- 32.Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Easterbrook P., Gopalan R., Berlin J., Matthews D. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-Y. [DOI] [PubMed] [Google Scholar]
- 36.Holler A., Albrecht U., Baumgartner Sigl S., Zoggeler T., Ramoser G., Bernar B., Karall D., Scholl-Burgi S. Successful implementation of classical ketogenic dietary therapy in a patient with Niemann-Pick disease type C. Mol. Genet. Metab. Rep. 2021;27:100723. doi: 10.1016/j.ymgmr.2021.100723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rener-Primec Z., Trojar A., Rogač M., Neubauer D. P15.2 Ketogenic diet in infants and toddlers: Efficacy and tolerability in 15 consecutive children. Eur. J. Paediatr. Neurol. 2011;15:S92. doi: 10.1016/S1090-3798(11)70316-6. [DOI] [Google Scholar]
- 38.Attar H., Rolfe S., Marsey M., Rogers L. Results of the modified atkins diet in patients with recurrent glioma: Retrospective review. Neurology. 2016;86:16S. [Google Scholar]
- 39.Felix G., Kossoff E., Baron B., Getzoff E., Krekel C., Scheimann A. Preliminary experience of the modified atkins diet for children with prader-willi syndrome. J. Pediatr. Gastroenterol. Nutr. 2017;65:S191–S192. doi: 10.1097/MPG.0000000000001805. [DOI] [Google Scholar]
- 40.Lapp K., Sandok E., Timmler T. Modified atkins/ketogenic diet in adults with epilepsy in a rural community-based clinical practice. Epilepsy Curr. 2012:12. [Google Scholar]
- 41.Nerurkar L., Buttle J., Symonds J., Zuberi S.M., Brunklaus A. The ketogenic diet in drug-resistant epilepsy of early childhood (<3 years): Indications, tolerability and outcomes. Dev. Med. Child Neurol. 2019;61:45. doi: 10.1111/dmcn.14120. [DOI] [Google Scholar]
- 42.Nangia S., Blackford R., Nordli D.R., Berg A.T. Ketogenic diet: Extending the boundaries of its use. Epilepsy Curr. 2012;12 [Google Scholar]
- 43.Gainza M., Michoulas A., Connolly M.B., Zanotta F., Simonson C., Huh L., Selby K., Farrell K. Complications of prolonged treatment with the ketogenic diet in treatment resistant epilepsy. Epilepsia. 2013;54:348–349. doi: 10.1111/epi.12248. [DOI] [Google Scholar]
- 44.Mak S.C., Chi C.S., Wan C.J. Clinical experience of ketogenic diet on children with refractory epilepsy. Acta Paediatr. Sin. 1999;40:97–100. [PubMed] [Google Scholar]
- 45.Furth S.L., Casey J.C., Pyzik P.L., Neu A.M., Docimo S.G., Vining E.P.G., Freeman J.M., Fivush B.A. Risk factors for urolithiasis in children on the ketogenic diet. Pediatr. Nephrol. 2000;15:125–128. doi: 10.1007/s004670000443. [DOI] [PubMed] [Google Scholar]
- 46.Ríos V.G., Panico L.R., Demartini M.G., Carniello M.A. Complications of treatment of epilepsy by a ketogenic diet. Rev. Neurol. 2001;33:909–915. [PubMed] [Google Scholar]
- 47.Sharma S., Gulati S., Kalra V., Agarwala A., Kabra M. Seizure control and biochemical profile on the ketogenic diet in young children with refractory epilepsy—Indian experience. Seizure. 2009;18:446–449. doi: 10.1016/j.seizure.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Kang H.C., Chung D.E., Kim D.W., Kim H.D. Early- and Late-onset Complications of the Ketogenic Diet for Intractable Epilepsy. Epilepsia. 2004;45:1116–1123. doi: 10.1111/j.0013-9580.2004.10004.x. [DOI] [PubMed] [Google Scholar]
- 49.Wibisono C., Rowe N., Beavis E., Kepreotes H., Mackie F.E., Lawson J.A., Cardamone M. Ten-Year Single-Center Experience of the Ketogenic Diet: Factors Influencing Efficacy, Tolerability, and Compliance. J. Pediatr. 2015;166:1030–1036.e1. doi: 10.1016/j.jpeds.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 50.Simm P.J., Bicknell-Royle J., Lawrie J., Nation J., Draffin K., Stewart K.G., Cameron F.J., Scheffer I.E., Mackay M.T. The effect of the ketogenic diet on the developing skeleton. Epilepsy Res. 2017;136:62–66. doi: 10.1016/j.eplepsyres.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 51.Guzel O., Uysal U., Arslan N. Efficacy and tolerability of olive oil-based ketogenic diet in children with drug-resistant epilepsy: A single center experience from Turkey. Eur. J. Paediatr. Neurol. 2019;23:143–151. doi: 10.1016/j.ejpn.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Hassan A.M., Keene D.L., Whiting S., Jacob P.J., Champagne J.R., Humphreys P. Ketogenic diet in the treatment of refractory epilepsy in childhood. Pediatr. Neurol. 1999;21:548–552. doi: 10.1016/S0887-8994(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 53.Takeoka M., Riviello J.J., Pfeifer H., Thiele E.A. Concomitant treatment with topiramate and ketogenic diet in pediatric epilepsy. Epilepsia. 2002;43:1072–1075. doi: 10.1046/j.1528-1157.2002.00602.x. [DOI] [PubMed] [Google Scholar]
- 54.Kossoff E.H., Pyzik P.L., Furth S.L., Hladky H.D., Freeman J.M., Vining E.P.G. Kidney Stones, Carbonic Anhydrase Inhibitors, and the Ketogenic Diet. Epilepsia. 2002;43:1168–1171. doi: 10.1046/j.1528-1157.2002.11302.x. [DOI] [PubMed] [Google Scholar]
- 55.Kossoff E.H., Pyzik P.L., McGrogan J.R., Rubenstein J.E. The impact of early versus late anticonvulsant reduction after ketogenic diet initiation. Epilepsy Behav. 2004;5:499–502. doi: 10.1016/j.yebeh.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 56.Kossoff E.H., Pyzik P.L., McGrogan J.R., Vining E.P.G., Freeman J.M. Efficacy of the Ketogenic Diet for Infantile Spasms. Pediatrics. 2002;109:780–783. doi: 10.1542/peds.109.5.780. [DOI] [PubMed] [Google Scholar]
- 57.Kang H.-C., Kim H.D. Diet therapy in refractory pediatric epilepsy: Increased efficacy and tolerability. Epileptic Disord. 2006;8:309–316. [PubMed] [Google Scholar]
- 58.Mackay M.T., Nation J., Humphrey M., Harvey A.S., Bicknell-Royle J. The ketogenic diet in refractory childhood epilepsy. J. Paediatr. Child Health. 2005;41:353–357. doi: 10.1111/j.1440-1754.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- 59.Groesbeck D.K., Bluml R.M., Kossoff E.H. Long-term use of the ketogenic diet in the treatment of epilepsy. Dev. Med. Child Neurol. 2006;48:978–981. doi: 10.1017/S0012162206002143. [DOI] [PubMed] [Google Scholar]
- 60.Sampath A., Kossoff E.H., Furth S.L., Pyzik P.L., Vining E.P.G. Kidney Stones and the Ketogenic Diet: Risk Factors and Prevention. J. Child Neurol. 2007;22:375–378. doi: 10.1177/0883073807301926. [DOI] [PubMed] [Google Scholar]
- 61.Raimann T.X., Marín B.V., Burón K.V., Devilat B.M., Ugalde F.A. Ketogenic diet in refractory epilepsy: Efficacy, evolution and long-term complications. Rev. Chil. Pediatr. 2007;78:477–481. [Google Scholar]
- 62.Caraballo R., Vaccarezza M., Cersósimo R., Rios V., Soraru A., Arroyo H., Agosta G., Escobal N., Demartini M., Maxit C., et al. Long-term follow-up of the ketogenic diet for refractory epilepsy: Multicenter Argentinean experience in 216 pediatric patients. Seizure. 2011;20:640–645. doi: 10.1016/j.seizure.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Dressler A., Trimmel-Schwahofer P., Reithofer E., Gröppel G., Mühlebner A., Samueli S., Grabner V., Abraham K., Benninger F., Feucht M. The ketogenic diet in infants—Advantages of early use. Epilepsy Res. 2015;116:53–58. doi: 10.1016/j.eplepsyres.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 64.Hallböök T., Sjölander A., Åmark P., Miranda M., Bjurulf B., Dahlin M. Effectiveness of the ketogenic diet used to treat resistant childhood epilepsy in Scandinavia. Eur. J. Paediatr. Neurol. 2015;19:29–36. doi: 10.1016/j.ejpn.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Khoo T.B., Tukimin S.M.B., Syed Zainal Abidin S.M.B., Lai J.J., Yusoof N.A.B. Long-term outcome and tolerability of ketogenic diet treatment for refractory epilepsies in children—A tertiary centre Malaysian experience. Neurol. Asia. 2016;21:17–21. [Google Scholar]
- 66.Lim Z., Wong K., Olson H.E., Bergin A.M., Downs J., Leonard H. Use of the ketogenic diet to manage refractory epilepsy in CDKL5 disorder: Experience of >100 patients. Epilepsia. 2017;58:1415–1422. doi: 10.1111/epi.13813. [DOI] [PubMed] [Google Scholar]
- 67.McNally M.A., Pyzik P.L., Rubenstein J.E., Hamdy R.F., Kossoff E.H. Empiric use of potassium citrate reduces kidney-stone incidence with the ketogenic diet. Pediatrics. 2009;124:e300–e304. doi: 10.1542/peds.2009-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park E.G., Lee J., Lee J. The ketogenic diet for super-refractory status epilepticus patients in intensive care units. Brain Dev. 2019;41:420–427. doi: 10.1016/j.braindev.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Draaisma J.M., Hampsink B.M., Janssen M., Van Houdt N.B., Linders E.T., Willemsen M.A. The Ketogenic Diet and Its Effect on Bone Mineral Density: A Retrospective Observational Cohort Study. Neuropediatrics. 2019;50:353–358. doi: 10.1055/s-0039-1693059. [DOI] [PubMed] [Google Scholar]
- 70.Roehl K., Falco-Walter J., Ouyang B., Balabanov A. Modified ketogenic diets in adults with refractory epilepsy: Efficacious improvements in seizure frequency, seizure severity, and quality of life. Epilepsy Behav. 2019;93:113–118. doi: 10.1016/j.yebeh.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 71.Lambrechts D.A.J.E., De Kinderen R.J.A., Vles J.S.H., De Louw A.J.A., Aldenkamp A.P., Majoie M. A randomized controlled trial of the ketogenic diet in refractory childhood epilepsy. Acta Neurol. Scand. 2016;135:231–239. doi: 10.1111/ane.12592. [DOI] [PubMed] [Google Scholar]
- 72.Curhan G.C. Epidemiology of Stone Disease. Urol. Clin. N. Am. 2007;34:287–293. doi: 10.1016/j.ucl.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bushinsky D.A., Coe F.L., Moe O.W. Nephrolithiasis. Brenner Rector’s Kidney. 2012;9:1455–1507. doi: 10.1016/b978-1-4160-6193-9.10039-9. [DOI] [Google Scholar]
- 74.Dunn-Geier J., Ho H.H., Auersperg E., Doyle D., Eaves L., Orrbine E., Whiting S. Effect of secretin on children with autism: A randomized controlled trial. Dev. Med. Child Neurol. 2007;42:796–802. doi: 10.1111/j.1469-8749.2000.tb00692.x. [DOI] [PubMed] [Google Scholar]
- 75.Chow K., Dixon J., Gilpin S., Kavanagh J.P., Rao P.N. Citrate inhibits growth of residual fragments in an in vitro model of calcium oxalate renal stones. Kidney Int. 2004;65:1724–1730. doi: 10.1111/j.1523-1755.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 76.Rudman D., Kunter M.H., Redd S.C., Waters W.C., Gerron G.G., Bleier J. Hypocitraturia in Calcium Nephrolithiasis. J. Clin. Endocrinol. Metab. 1982;55:1052–1057. doi: 10.1210/jcem-55-6-1052. [DOI] [PubMed] [Google Scholar]
- 77.Pak C.Y. Etiology and Treatment of Urolithiasis. Am. J. Kidney Dis. 1991;18:624–637. doi: 10.1016/S0272-6386(12)80602-0. [DOI] [PubMed] [Google Scholar]
- 78.Pak C.Y., Poindexter J.R., Adams-Huet B., Pearle M.S. Predictive value of kidney stone composition in the detection of metabolic abnormalities. Am. J. Med. 2003;115:26–32. doi: 10.1016/S0002-9343(03)00201-8. [DOI] [PubMed] [Google Scholar]
- 79.Curhan G., Taylor E. 24-h uric acid excretion and the risk of kidney stones. Kidney Int. 2008;73:489–496. doi: 10.1038/sj.ki.5002708. [DOI] [PubMed] [Google Scholar]
- 80.Ngo T.C., Assimos D.G. Uric Acid Nephrolithiasis: Recent Progress and Future Directions. Rev. Urol. 2007;9:17–27. [PMC free article] [PubMed] [Google Scholar]
- 81.Okamoto N., Aruga S., Matsuzaki S., Takahashi S., Matsushita K., Kitamura T. Associations between renal sodium-citrate cotransporter (hNaDC-1) gene polymorphism and urinary citrate excretion in recurrent renal calcium stone formers and normal controls. Int. J. Urol. 2007;14:344–349. doi: 10.1111/j.1442-2042.2007.01554.x. [DOI] [PubMed] [Google Scholar]
- 82.Nicar M.J., Skurla C., Sakhaee K., Pak C.Y. Low urinary citrate excretion in nephrolithiasis. Urology. 1983;21:8–14. doi: 10.1016/0090-4295(83)90113-9. [DOI] [PubMed] [Google Scholar]
- 83.Siener R., Hesse A. The effect of a vegetarian and different omnivorous diets on urinary risk factors for uric acid stone formation. Eur. J. Nutr. 2003;42:332–337. doi: 10.1007/s00394-003-0428-0. [DOI] [PubMed] [Google Scholar]
- 84.Agarwal N., Arkilo D., Farooq O., Gillogly C., Kavak K.S., Weinstock A. Ketogenic diet: Predictors of seizure control. SAGE Open Med. 2017;5 doi: 10.1177/2050312117712887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wirrell E.C., Darwish H.Z., Williams-Dyjur C., Blackman M., Lange V. Is a Fast Necessary When Initiating the Ketogenic Diet? J. Child Neurol. 2002;17:179–182. doi: 10.1177/088307380201700305. [DOI] [PubMed] [Google Scholar]
- 86.Zupec-Kania B.A., Spellman E. An Overview of the Ketogenic Diet for Pediatric Epilepsy. Nutr. Clin. Pract. 2008;23:589–596. doi: 10.1177/0884533608326138. [DOI] [PubMed] [Google Scholar]
- 87.DeVivo D.C., Haymond M.W., Leckie M.P., Bussmann Y.L., McDougal J.D.B., Pagliara A.S. The Clinical and Biochemical Implications of Pyruvate Carboxylase Deficiency. J. Clin. Endocrinol. Metab. 1977;45:1281–1296. doi: 10.1210/jcem-45-6-1281. [DOI] [PubMed] [Google Scholar]
- 88.Rendina D., De Filippo G., Iannuzzo G., Abate V., Strazzullo P., Falchetti A. Idiopathic Osteoporosis and Nephrolithiasis: Two Sides of the Same Coin? Int. J. Mol. Sci. 2020;21:8183. doi: 10.3390/ijms21218183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Merlotti D., Cosso R., Eller-Vainicher C., Vescini F., Chiodini I., Gennari L., Falchetti A. Energy Metabolism and Ketogenic Diets: What about the Skeletal Health? A Narrative Review and a Prospective Vision for Planning Clinical Trials on This Issue. Int. J. Mol. Sci. 2021;22:435. doi: 10.3390/ijms22010435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aronica L., Volek J., Poff A., D’Agostino D.P. Genetic variants for personalised management of very low carbohydrate ketogenic diets. BMJ Nutr. Prev. Health. 2020;3:363–373. doi: 10.1136/bmjnph-2020-000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in this article.