Abstract
Objective
To investigate the relationship between asymmetric prominent hypointense vessels (prominent vessel sign, PVS) on susceptibility-weighted imaging (SWI) and leptomeningeal collateralization in patients with acute ischemic stroke due to large vessel occlusion.
Methods
We retrospectively enrolled patients with M1 segment occlusion of the middle cerebral artery who underwent emergency magnetic resonance imaging and digital subtraction angiography within 24 hours from stroke onset. The extent of PVS on SWI was assessed using the Alberta Stroke Program Early CT Score (ASPECTS). Leptomeningeal collateralization on digital subtraction angiography images was assessed using the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) scale. Spearman’s rank correlation test was performed to explore the correlation of ASITN/SIR scores with SWI-ASPECTS and SWI-diffusion-weighted imaging (DWI) mismatch scores.
Results
Thirty-five patients were enrolled. There was no significant correlation between SWI-ASPECTS and ASITN/SIR scores. However, SWI-DWI mismatch scores were positively correlated with ASITN/SIR scores.
Conclusion
The range of PVS on SWI did not closely reflect the collateral status, while the range of SWI-DWI mismatch was significantly correlated with the leptomeningeal collateralization. In patients with acute anterior circulation stroke due to large vessel occlusion, larger SWI-DWI mismatch was associated with better leptomeningeal collaterals.
Keywords: Acute ischemic stroke, susceptibility-weighted imaging, diffusion-weighted imaging, leptomeningeal collateralization, large vessel occlusion, prominent vessel sign
Introduction
The cerebral collateral circulation refers to the subsidiary network of vascular channels that stabilize cerebral blood flow (CBF) when principal conduits fail.1 It is the main cause of heterogeneity of stroke in terms of the severity of the ischemic penumbra, the size of the infarction, the duration and severity of cerebral ischemia, and whether stroke occurs after arterial occlusion. Evaluation of the collateral circulation is helpful for clinical decision-making and prediction of the prognosis.2,3
Susceptibility-weighted imaging (SWI) is a magnetic resonance imaging (MRI) technique that exploits the differences in magnetic susceptibility between tissues for imaging.4 In patients with acute ischemic stroke, asymmetric prominent hypointense vessels appear on SWI; this is called the prominent vessel sign (PVS).5,6 It is widely accepted that the occurrence of the PVS is caused by an increase in the oxygen extraction fraction (OEF) in ischemic brain tissue, which leads to an increase in the deoxyhemoglobin concentration in blood vessels.7,8
The clinical and imaging implications of the PVS have recently attracted increasing attention. Several studies have explored the relationship between the PVS and collateral circulation, but the results are controversial. Verma et al.9 found that extensive prominent cortical veins on SWI correlated with poor leptomeningeal collateralization, whereas Park et al.10 found that the presence of more extensive hypointense vessels on SWI was associated with better collateral flow. Therefore, the relationship between the PVS and collateral circulation remains unclear.
Digital subtraction angiography (DSA) is the gold standard technique for evaluation of the cerebral collateral circulation, and it can clearly show the structure and compensation at all levels.11 DSA findings are graded according to the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) scale.12 In this study, we used the ASITN/SIR scale to assess the collateral circulation and investigate the relationship between the PVS and leptomeningeal collateralization in patients with stroke caused by large vessel occlusion.
Materials and methods
Patients
This retrospective study was based on a prospectively maintained database at our center from August 2013 to August 2017.13 The inclusion criteria were (1) age of ≥18 years, (2) admission for acute ischemic stroke within 24 hours from symptom onset, (3) performance of emergency MRI (including SWI and diffusion-weighted imaging [DWI]) within 24 hours from symptom onset, (4) performance of DSA after MRI within 24 hours from symptom onset, and (5) DSA-confirmed M1 segment occlusion of the middle cerebral artery (including terminal T-shaped occlusion of the internal carotid artery). Patients with insufficient image quality were excluded. The study protocol was approved by the Ethics Committee of PLA Rocket Force Characteristic Medical Center (approval number: KY2013031). Informed consent was not required because of the retrospective nature of the study. We have de-identified all protected health information to ensure patient privacy. The STROBE guidelines were used for the reporting of this study.14
MRI protocol
Imaging data for the prospective database were collected using a Siemens Skyra 3.0-T MRI system (Siemens Healthineers, Erlangen, Germany), and uniform imaging parameters were used. DWI was performed with the following parameters: b-values, 0 and 1000 s/mm2; repetition time (TR), 4300 ms; echo time (TE), 98 ms; matrix, 173 × 192 pixels; and field of view (FOV), 220 × 220 mm. For SWI, the magnitude and phase images were obtained with the following parameters: TR, 27 ms; TE, 20 ms; flip angle, 15°; matrix, 138 × 256 pixels; FOV, 168 × 300 mm; slice thickness, 1.5 mm; and 80 slices. Minimum-intensity projection images were reconstructed with a thickness of 12 mm.
Data collection and analysis
Clinical data for the prospective database were collected through a standardized case report form. The following clinical variables were prospectively recorded: demographics (age and sex), medical history (hypertension, diabetes, hyperlipidemia, coronary heart disease, previous ischemic stroke, and antiplatelet therapy), and clinical characteristics (baseline National Institutes of Health Stroke Scale [NIHSS] score, mean arterial pressure, fasting glucose concentration, cardioembolism, time from onset to MRI, time from onset to DSA, length of stay in hospital, NIHSS score at discharge, and modified Rankin scale score at 3 months). All MRI and DSA images generated were saved on a compact disc read-only memory (CD-ROM).
In the retrospective analysis, the MRI scans were independently reviewed by two readers who were blinded to the clinical findings and DSA results, and decisions were reached by consensus. A PVS was defined as a local prominence of hypointense vessels on SWI with either an increased vessel number or diameter in the target area relative to the non-target area. The extent of abnormalities on SWI or DWI was assessed using the Alberta Stroke Program Early CT Score (ASPECTS).15 The area served by the middle cerebral artery was divided into 10 sections: the caudate nucleus, lentiform nucleus, internal capsule, six cortical areas overlying the middle cerebral artery area (M1, M2, M3, M4, M5, and M6), and the insular cortex. To calculate the ASPECTS, 1 point was subtracted from 10 for each area of ischemic changes, including restricted diffusion on DWI and asymmetric prominent hypointense vessels on SWI. SWI-DWI mismatch was defined by a regional increase in the vessel number or diameter on SWI extending beyond the DWI hyperintensity territory in the affected hemisphere.16,17 The SWI-DWI mismatch score was calculated using the ASPECTS scoring system (Figures 1 and 2).
Figure 1.
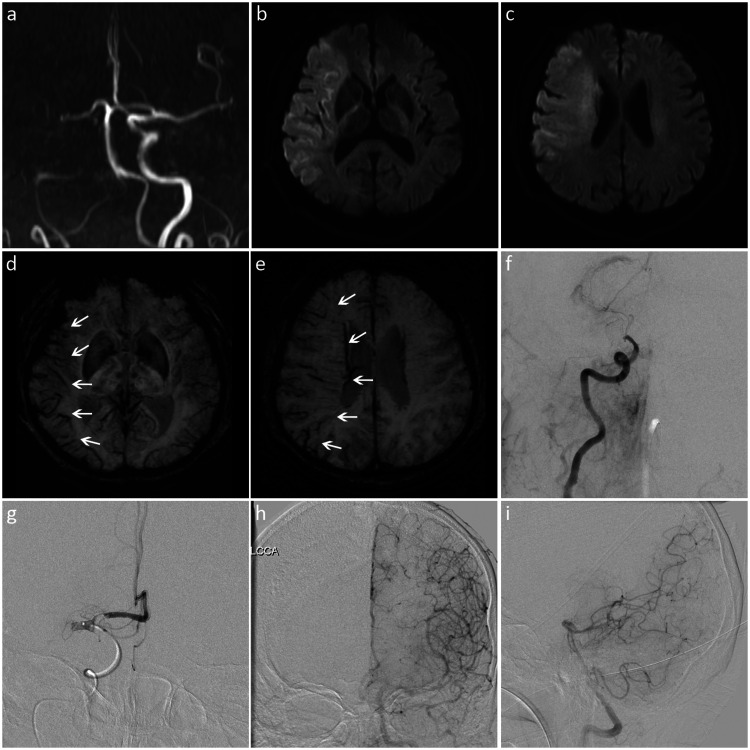
Images from a patient with a low SWI-DWI mismatch score and poor leptomeningeal collateralization. (a) MRA showed occlusion of the right internal carotid and middle cerebral arteries. (b, c) DWI revealed restricted diffusion in the internal capsule, insular cortex, and M1 to M6 zones of the right middle cerebral artery territory. (d, e) SWI showed the prominent vessel sign (white arrows) in seven regions (right insular and M1 to M6). The SWI-ASPECTS value was 3 (10 − 7 = 3), and the SWI-DWI mismatch score was 0 (7 − 7 = 0). (f) DSA showed occlusion of the right terminal internal carotid artery. (g) Microcatheterography confirmed a T-shaped occlusion at the end of the internal carotid artery (including the proximal M1 of the middle cerebral artery). (f–i) There was no collateral blood supply to the right middle cerebral artery supplying area, and the ASITN/SIR score was 0.
SWI, susceptibility-weighted imaging; DWI, diffusion-weighted imaging; MRA, magnetic resonance angiography; ASPECTS, Alberta Stroke Program Early CT Score; DSA, digital subtraction angiography; ASITN/SIR, American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology.
Figure 2.
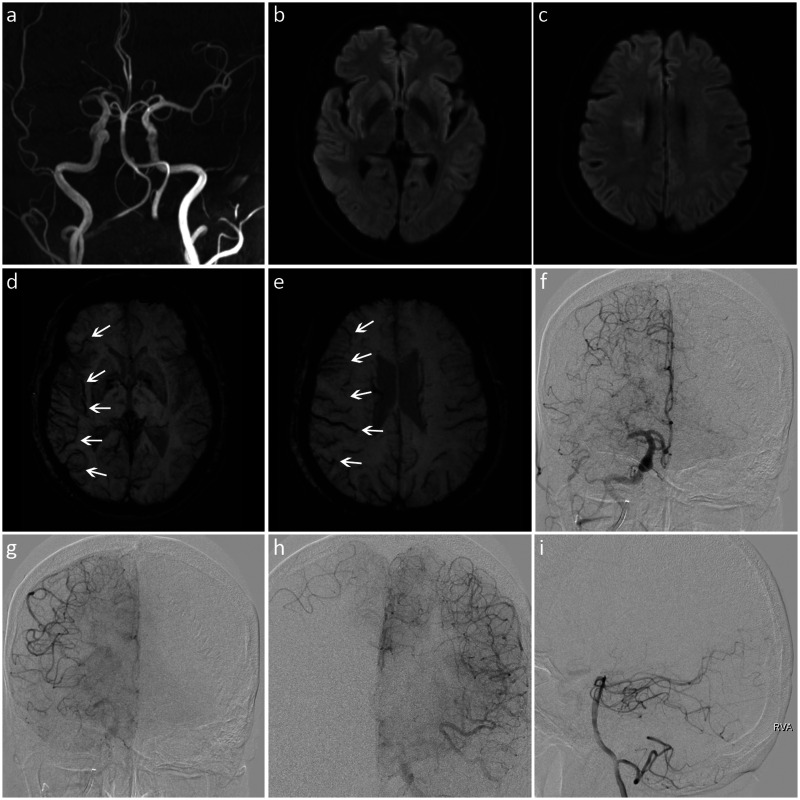
Images from a patient with a high SWI-DWI mismatch score and good leptomeningeal collateralization. (a) MRA showed M1 segment occlusion of the right middle cerebral artery. (b, c) DWI revealed restricted diffusion in the right lentiform nucleus and paraventricular region. (d, e) SWI showed the prominent vessel sign (white arrows) in seven regions (right insular and M1 to M6). The SWI-ASPECTS value was 3 (10 − 7 = 3), and the SWI-DWI mismatch score was 7 (7 − 0 = 7). (f) DSA indicated occlusion of the proximal M1 segment of the right middle cerebral artery. (f–i) The ischemic area was compensated by the leptomeningeal collaterals of the right anterior cerebral artery, and the ASITN/SIR score was 3.
SWI, susceptibility-weighted imaging; DWI, diffusion-weighted imaging; MRA, magnetic resonance angiography; ASPECTS, Alberta Stroke Program Early CT Score; DSA, digital subtraction angiography; ASITN/SIR, American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology.
Leptomeningeal collateralization was assessed using the ASITN/SIR scale by an experienced neurointerventionalist who was unaware of the MRI data. The ASITN/SIR score was determined by DSA and was graded as follows: Grade 0, no visible collaterals to the ischemic site; Grade 1, slow collaterals to the periphery of the ischemic site with persistence of some of the defect; Grade 2, rapid collaterals to the periphery of the ischemic site with persistence of some of the defect and to only a portion of the ischemic territory; Grade 3, collaterals with slow but complete angiographic blood flow of the ischemic bed by the late venous phase; and Grade 4, complete and rapid collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion. The patients were divided into a good collateral group and a poor collateral group according to their ASITN/SIR scores. Good collaterals were defined as an ASITN/SIR score of 3 or 4, and poor collaterals were defined as an ASITN/SIR score of 0 to 2.18
Statistical analysis
Statistical analysis was performed with SAS software version 9.2 (SAS Institute, Cary, NC, USA). Categorical variables and continuous variables are expressed as number (percentage) and median (interquartile range), respectively and were compared using Fisher’s exact test and the Wilcoxon rank sum test, respectively. Spearman’s rank correlation test was used to analyze the correlation of the ASITN/SIR scores with the SWI-ASPECTS value and SWI-DWI mismatch scores. A P value of <0.05 was considered statistically significant.
Results
Thirty-five patients with M1 segment occlusion of the middle cerebral artery who underwent emergency MRI and DSA within 24 hours from stroke onset were enrolled. Fifteen patients had poor collaterals and 20 patients had good collaterals. The patients’ characteristics are shown in Table 1. Their median age was 71 years, and women accounted for 48.6%. The median onset-to-MRI time was 3.4 hours (interquartile range, 2.8–4.6 hours). Eighty percent of patients completed MRI scans within 6 hours of onset. The PVS was seen on SWI in all patients.
Table 1.
Characteristics of patients with poor and good leptomeningeal collaterals.
| Total (n = 35) | Poor collaterals (n = 15) | Good collaterals (n = 20) | P value | |
|---|---|---|---|---|
| Age, years | 71 (59–79) | 78 (59–79) | 70 (60–78) | 0.463 |
| Female | 17 (48.6) | 8 (53.3) | 9 (45.0) | 0.738 |
| Hypertension | 27 (77.1) | 13 (86.7) | 14 (70.0) | 0.419 |
| Diabetes | 7 (20.0) | 3 (20.0) | 4 (20.0) | 1.000 |
| Hyperlipidemia | 12 (34.3) | 4 (26.7) | 8 (40.0) | 0.489 |
| Coronary heart disease | 9 (25.7) | 2 (13.3) | 7 (35.0) | 0.244 |
| Previous ischemic stroke | 8 (22.9) | 3 (20.0) | 5 (25.0) | 1.000 |
| Antiplatelet therapy | 8 (22.9) | 1 (6.7) | 7 (35.0) | 0.101 |
| NIHSS score on admission | 9 (5–14) | 10 (9–16) | 8 (5–12) | 0.124 |
| Mean arterial pressure, mmHg | 107 (101–118) | 110 (99–122) | 106 (102–109) | 0.351 |
| Fasting glucose, mmol/L | 6.8 (5.9–8.2) | 6.5 (5.9–7.8) | 6.9 (5.8–8.3) | 0.641 |
| Cardioembolism | 16 (45.7) | 8 (53.3) | 8 (40.0) | 0.506 |
| Time from onset to MRI, minutes | 205 (166–276) | 222 (169–600) | 202 (160–266) | 0.368 |
| SWI-ASPECTS | 3 (3–4) | 3 (3–4) | 3 (3–3) | 0.297 |
| DWI-ASPECTS | 7 (6–9) | 5 (3–8) | 8 (7–9) | <0.001 |
| SWI-DWI mismatch score | 5 (3–7) | 2 (2–3) | 7 (6–7) | <0.001 |
| Time from onset to DSA, minutes | 280 (238–355) | 280 (238–741) | 273 (237–341) | 0.537 |
| Length of stay in hospital, days | 15 (9–20) | 20 (14–26) | 13 (9–16) | 0.011 |
| NIHSS score at discharge | 3 (0–13) | 9 (1–23) | 1 (0–6) | 0.026 |
| 3-month mRS score | 1 (0–4) | 4 (0–5) | 1 (0–2) | 0.030 |
Data are presented as n (%) or median (interquartile range).
ASPECTS, Alberta Stroke Program Early CT Score; DSA, digital subtraction angiography; DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale; SWI, susceptibility-weighted imaging.
There were no significant differences in age, sex, NIHSS score on admission, time from onset to MRI, time from onset to DSA, or other factors between patients with good and poor collaterals. The scores of DWI-ASPECTS (8 vs. 5, P < 0.001) and SWI-DWI mismatch (7 vs. 2, P < 0.001) were significantly higher in patients with good than poor collaterals. Patients with good collaterals had a shorter hospital stay (13 vs. 20 days, P = 0.011), lower NIHSS scores at discharge (1 vs. 9, P = 0.026), and better clinical outcomes (modified Rankin scale score of 1 vs. 4, P = 0.030).
There was no significant correlation between SWI-ASPECTS and ASITN/SIR scores (r = −0.099). However, ASITN/SIR scores were positively correlated with DWI-ASPECTS (r = 0.714, P < 0.001) and SWI-DWI mismatch scores (r = 0.818, P < 0.001) (Figure 3).
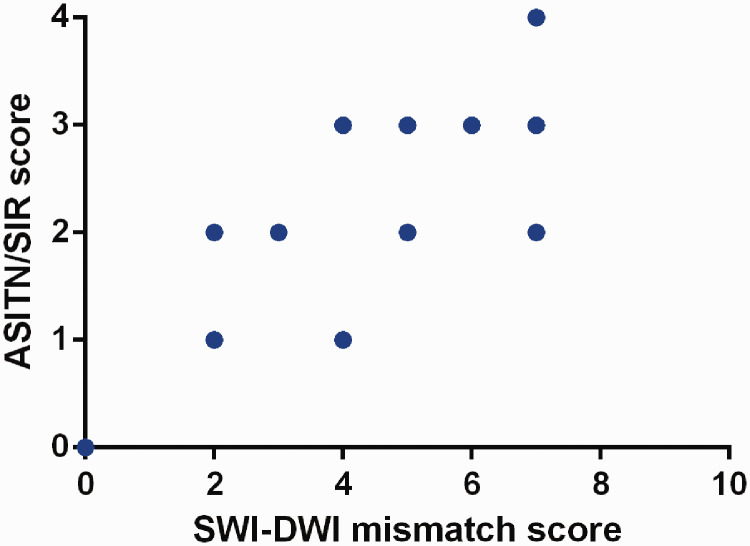
Figure 3.
Scatter plot showing the correlation between the SWI-DWI mismatch score and the ASITN/SIR score. The SWI-DWI mismatch score was positively correlated with the ASITN/SIR score (Spearman test, r = 0.818, P < 0.001).
SWI, susceptibility-weighted imaging; DWI, diffusion-weighted imaging; ASITN/SIR, American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology.
Discussion
In this study, we investigated the relationship between the PVS and leptomeningeal collateralization in patients with M1 segment occlusion of the middle cerebral artery. We found no significant correlation between the range of the PVS and the leptomeningeal collateral circulation. However, the SWI-DWI mismatch scores were positively correlated with the ASITN/SIR scores, suggesting that larger SWI-DWI mismatch is associated with better leptomeningeal collaterals.
When CBF decreases because of occlusion of the cerebral artery, the OEF increases to maintain the stability of the cerebral metabolic rate of oxygen (OEF = CMRO2 / (CaO2 × CBF)).19 The increase in the OEF leads to an increase in the concentration of deoxyhemoglobin in the blood vessels, which results in the occurrence of the PVS. As the CBF decreases, the mean transit time (MTT) on perfusion-weighted imaging (PWI) also increases (MTT = CBV / CBF).19 Several studies have shown that in patients with acute ischemic stroke, the range of the PVS on SWI was closely related to the range of MTT prolongation on PWI.20–22 The PVS provides perfusion information comparable with the MTT and reflects the range of hypoperfusion.
Studies have shown that SWI-DWI mismatch provides similar information to MTT-DWI mismatch. Luo et al.23 found that SWI-DWI mismatch was not significantly different from MTT-DWI mismatch in evaluating the ischemic penumbra and that SWI-DWI mismatch was positively correlated with MTT-DWI mismatch (r = 0.76, P < 0.001). Another study also indicated that the difference in SWI-DWI versus MTT-DWI scores did not reach statistical significance.24 Therefore, like MTT-DWI mismatch, SWI-DWI mismatch represents brain tissue that is hypoperfused but not yet irreversibly damaged.
Verma et al.9 reported that an extensive PVS on SWI was correlated with poor leptomeningeal collateralization, whereas a less pronounced PVS was correlated with good leptomeningeal collateralization. These results are inconsistent with ours. A previous study analyzed the collateral status of patients with total SWI-DWI mismatch.25 Total SWI-DWI mismatch refers to the phenomenon in which some patients show negative DWI but exhibit an extensive PVS on SWI. All patients enrolled in that study exhibited an extensive PVS on SWI and good collateral flow. Total SWI-DWI mismatch was associated with good collaterals. These extreme cases refute the opinion that an extensive PVS on SWI is associated with poor leptomeningeal collateralization, and the findings of that study support our result that larger SWI-DWI mismatch is associated with better leptomeningeal collaterals. In the study by Verma et al.,9 the mean SWI-ASPECTS and DWI-ASPECTS in the good collateral group were 4.10 and 6.35, respectively, while those in the poor collateral group were 2.69 and 3.31, respectively. If the SWI-DWI mismatch score had been calculated, the score in the group with good collaterals would be expected to be higher than that in the group with poor collaterals.
Park et al.10 revealed that a more extensive PVS on SWI in acute ischemic stroke was associated with better collateral flow. They suggested that the PVS on SWI may be a useful surrogate marker for predicting an increased OEF and PWI-DWI mismatch in the acute ischemic hemisphere. The main difference between the study by Park et al.10 and ours is the time from stroke onset to MRI. In the study by Park et al.,10 more than half of the patients underwent MRI at least 6 hours after onset, some as long as 63 hours. However, all patients included in our study underwent MRI within 24 hours of symptom onset, and 80% of them completed MRI scans within 6 hours. We found that in the hyperacute stage of ischemic stroke, the PVS appeared not only in the zone of PWI-DWI mismatch but also in the diffusion-restricted area on DWI. Theoretically, in the hyperacute stage, the PVS may occur in the area of core infarction because of the increase in the OEF. When most of the cells in the infarction core area are necrotic, the OEF is reduced and the PVS becomes less obvious. A recent study showed that in acute large vessel occlusion stroke with poor collateral circulation, the OEF in the core infarction area may be high or low and the PVS may be present or absent.26 Thus, the PVS in the area of the core infarction does not truly reflect the collateral circulation. Previous studies have shown that the range of SWI-DWI mismatch rather than the range of the PVS is consistent with the zone of PWI-DWI mismatch.23,24 Inspired by this, we analyzed the relationship between SWI-DWI mismatch and leptomeningeal collateralization. We found that the SWI-DWI mismatch scores were significantly correlated with the ASITN/SIR scores.
Our study has several limitations. First, only 35 patients were included in the analysis; this number was very limited. Second, the PVS was determined by visual inspection and comparison rather than by objective measurement, which may have given rise to investigator bias. Third, although the ASPECTS is a rapid screening tool to assess the extent of the PVS, it is only a semiquantitative grading system. Further research using a quantitative susceptibility mapping technique would allow for quantitative analysis of the OEF and accurate determination of the extent of the PVS.27,28 If volumetric analysis is feasible, the SWI-DWI mismatch ratio could be calculated and the relationship between the PVS and collateral circulation could be more accurately delineated.
In conclusion, our study showed that the range of the PVS on SWI did not closely reflect the collateral status, whereas the range of SWI-DWI mismatch was significantly correlated with the leptomeningeal collateralization. In patients with acute ischemic stroke due to large vessel occlusion, larger SWI-DWI mismatch is associated with better leptomeningeal collaterals. SWI can be added to the routine neuroimaging protocol for patients with large vessel occlusion stroke.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work was funded by the National Key Basic Research Program of China (973 program, 2013CB733800) and the National Natural Science Foundation of China (General program, 61671440).
Author contributions: HJ, YZ, W-JJ: conceived the study. YZ, A-FL, CL, MJ, FM: collected the data. HJ, JP, CS: conducted the data analysis. HJ: drafted the manuscript. W-JJ, YZ: revised the article.
ORCID iD: Haifei Jiang https://orcid.org/0000-0002-9751-4467
References
- 1.Liebeskind DS. Collateral circulation. Stroke 2003; 34: 2279–2284. [DOI] [PubMed] [Google Scholar]
- 2.Bang OY, Goyal M, Liebeskind DS. Collateral circulation in ischemic stroke: assessment tools and therapeutic strategies. Stroke 2015; 46: 3302–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginsberg MD. The cerebral collateral circulation: relevance to pathophysiology and treatment of stroke. Neuropharmacology 2018; 134: 280–292. [DOI] [PubMed] [Google Scholar]
- 4.Haacke EM, Xu Y, Cheng YC, et al. Susceptibility weighted imaging (SWI). Magn Reson Med 2004; 52: 612–618. [DOI] [PubMed] [Google Scholar]
- 5.Chen CY, Chen CI, Tsai FY, et al. Prominent vessel sign on susceptibility-weighted imaging in acute stroke: prediction of infarct growth and clinical outcome. PLoS One 2015; 10: e0131118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang J, Gao P, Lin Y, et al. Susceptibility-weighted imaging in post-treatment evaluation in the early stage in patients with acute ischemic stroke. J Int Med Res 2019; 47: 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santhosh K, Kesavadas C, Thomas B, et al. Susceptibility weighted imaging: a new tool in magnetic resonance imaging of stroke. Clin Radiol 2009; 64: 74–83. [DOI] [PubMed] [Google Scholar]
- 8.Kesavadas C, Santhosh K, Thomas B. Susceptibility weighted imaging in cerebral hypoperfusion-can we predict increased oxygen extraction fraction? Neuroradiology 2010; 52: 1047–1054. [DOI] [PubMed] [Google Scholar]
- 9.Verma RK, Hsieh K, Gratz PP, et al. Leptomeningeal collateralization in acute ischemic stroke: impact on prominent cortical veins in susceptibility-weighted imaging. Eur J Radiol 2014; 83: 1448–1454. [DOI] [PubMed] [Google Scholar]
- 10.Park MG, Yang TI, Oh SJ, et al. Multiple hypointense vessels on susceptibility-weighted imaging in acute ischemic stroke: surrogate marker of oxygen extraction fraction in penumbra? Cerebrovasc Dis 2014; 38: 254–261. [DOI] [PubMed] [Google Scholar]
- 11.Piedade GS, Schirmer CM, Goren O, et al. Cerebral collateral circulation: a review in the context of ischemic stroke and mechanical thrombectomy. World Neurosurg 2019; 122: 33–42. [DOI] [PubMed] [Google Scholar]
- 12.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109–e137. [DOI] [PubMed] [Google Scholar]
- 13.Jiang HF, Zhang YQ, Pang JX, et al. Factors associated with prominent vessel sign on susceptibility-weighted imaging in acute ischemic stroke. Sci Rep 2021; 11: 5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 15.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet 2000; 355: 1670–1674. [DOI] [PubMed] [Google Scholar]
- 16.Payabvash S, Taleb S, Benson JC, et al. Susceptibility-diffusion mismatch in middle cerebral artery territory acute ischemic stroke: clinical and imaging implications. Acta Radiologica 2017; 58: 876–882. [DOI] [PubMed] [Google Scholar]
- 17.Jiang H, Zhang Y, Pang J, et al. Interactive effect of susceptibility-diffusion mismatch and recanalization status on clinical outcome in large vessel occlusion stroke. J Stroke Cerebrovasc Dis 2020; 29: 105072. [DOI] [PubMed] [Google Scholar]
- 18.Guenego A, Fahed R, Albers GW, et al. Hypoperfusion intensity ratio correlates with angiographic collaterals in acute ischaemic stroke with M1 occlusion. Eur J Neurol 2020; 27: 864–870. [DOI] [PubMed] [Google Scholar]
- 19.Leigh R, Knutsson L, Zhou J, et al. Imaging the physiological evolution of the ischemic penumbra in acute ischemic stroke. J Cereb Blood Flow Metab 2018; 38: 1500–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dejobert M, Cazals X, Annan M, et al. Susceptibility-diffusion mismatch in hyperacute stroke: correlation with perfusion-diffusion mismatch and clinical outcome. J Stroke Cerebrovasc Dis 2016; 25: 1760–1766. [DOI] [PubMed] [Google Scholar]
- 21.Luo S, Yang L, Luo Y. Susceptibility-weighted imaging predicts infarct size and early-stage clinical prognosis in acute ischemic stroke. Neurol Sci 2018; 39: 1049–1055. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Luo S. Clinical application of susceptibility-weighted imaging in the evaluation of leptomeningeal collateralization. Medicine (Baltimore) 2018; 97: e13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo S, Yang L, Wang L. Comparison of susceptibility-weighted and perfusion-weighted magnetic resonance imaging in the detection of penumbra in acute ischemic stroke. J Neuroradiol 2015; 42: 255–260. [DOI] [PubMed] [Google Scholar]
- 24.Wang T, Zhu L, Hu C, et al. The diagnostic value of susceptibility-weighted imaging for ischemic penumbra in patients with acute ischemic stroke. Technol Health Care 2017; 25: 449–457. [DOI] [PubMed] [Google Scholar]
- 25.Park MG, Yeom JA, Baik SK, et al. Total mismatch of diffusion-weighted imaging and susceptibility-weighted imaging in patients with acute cerebral ischemia. J Neuroradiol 2017; 44: 308–312. [DOI] [PubMed] [Google Scholar]
- 26.Guenego A, Leipzig M, Fahed R, et al. Effect of oxygen extraction (brush-sign) on baseline core infarct depends on collaterals (HIR). Front Neurol 2021; 11: 618765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y, Sun H, Cho J, et al. Cerebral OEF quantification: a comparison study between quantitative susceptibility mapping and dual-gas calibrated BOLD imaging. Magn Reson Med 2020; 83: 68–82. [DOI] [PubMed] [Google Scholar]
- 28.Vinayagamani S, Sheelakumari R, Sabarish S, et al. Quantitative susceptibility mapping: technical considerations and clinical applications in neuroimaging. J Magn Reson Imaging 2021; 53: 23–37. [DOI] [PubMed] [Google Scholar]