Abstract
The results from previous observational studies and clinical trials about the neuroprotective benefits of statins use for the prevention of dementia are contradictory. It is unclear whether the neuroprotective benefits are experienced in a specific group with a higher risk of dementia, such as patients with concurrent diabetes and hyperlipidemia. We aimed to examine the association between adherence to statins and the risk of dementia among patients with diabetes and comorbid hyperlipidemia. This was a retrospective study with a new user design. We used data from the Taiwan National Health Insurance Research Database to identify patients with diabetes and comorbid hyperlipidemia. The occurrence of dementia was the study outcome. The adherence to statins was the exposure, which was measured by the proportion of days covered (PDC) of statins. The good adherence included patients with ≥80% PDC of statins. Cox proportional hazards regression models were used to evaluate the association between adherence to statins and dementia. Among 18,125 included individuals with diabetes and comorbid hyperlipidemia, 33.5% had good adherence to statins. Compared to poor adherence to statins, good adherence to statins was not significantly associated with a reduced risk of dementia (hazard ratio = 0.94; 95%confidence interval = 0.70–1.24) among patients with diabetes and comorbid hyperlipidemia. Good adherence to statins was not found to be associated with the risk of dementia among patients with diabetes and comorbid hyperlipidemia in Taiwan. Future studies with a more diverse study population are needed to evaluate the neuroprotective effects of statins use on dementia prevention.
Keywords: adherence, dementia, diabetes, hyperlipidemia, statins
What do we already know about this topic?
Statins have potential benefits of delaying dementia, although there is no cure for dementia currently.
How does your research contribute to the field?
Adherence to statins was not found to be associated with a reduced risk of dementia among diabetic patients with comorbid hyperlipidemia.
What are your research’s implications toward theory, practice, or policy?
Healthcare providers should have a more conservative attitude toward the effectiveness of statins on dementia before further studies with a longer follow-up period and a more precise definition of good adherence to statins.
Introduction
Dementia is a progressive neurodegenerative disease that gradually impairs memory and cognitive function among patients. There are 7.7 million new cases of dementia each year globally, and the incidence is still increasing.1 Patients with diabetes have a nearly two-fold higher risk of developing dementia than individuals without diabetes and the majority of them are type 2 diabetes due to the age of the populations involved.2 Patients with hyperlipidemia also have an increased risk of developing dementia.3 Patients with diabetes and hyperlipidemia are more likely to develop dementia than patients with diabetes alone.3 Furthermore, hyperlipidemia commonly cooccurs with diabetes.3 Compared to patients without diabetes, patients with diabetes have been shown to have a six-fold probability of developing hyperlipidemia.3 Therefore, patients with concurrent diabetes and hyperlipidemia have an increased risk of developing dementia.
Patients with hyperlipidemia often require statins as medication treatment. In addition to lowering cholesterol, statins use has been suggested to have a neuroprotective effect.4-7 Prior studies reported the potential mechanisms for neuroprotective effect of statins to reduce the risk of dementia including (1) lowering the cholesterol level, (2) decreasing cardiovascular risk factors, (3) reducing the deposition of β-amyloid plaques, (4) increasing vascular dilation through endothelial nitric oxide (NO) synthase, and then increasing cerebral blood flow, and (5) inhibiting inflammatory and oxidative stress markers that relevant to hyperlipidemia.4,6-13
However, meta-analyses of randomized controlled trials14 and meta-analyses of observational studies5,15 have reported contradictory results about the potential neuroprotective benefits of statins in the prevention of dementia. Observational studies have shown that statins use reduced the risk of dementia among patients with diabetes and patients with hyperlipidemia.5,15 In contrast, the protective effect of statins use on dementia was not observed in clinical trials.14
Previous observational studies that reported a positive association between statins use and the prevention of dementia had several limitations in not considering adherence to statins, using a prevalent user design, and often only including statins nonusers as the reference group.16,17 For example, patients with high cardiovascular risk or with previous stroke are more likely to have good adherence. Prevalent statins users are less likely to be susceptible to its side effects and more likely to have good adherence to statins than new statins users. Furthermore, studies that included statins nonusers as the reference group (ie, studies that lacked an active comparator) may have either overestimated or underestimated the neuroprotective effect. These major limitations from previous studies could lead to bias when assessing the neuroprotective effect of statins on the prevention of dementia and further limit the assessment of the association between statins use and dementia when considering adherence. Thus, it is important to know whether neuroprotective benefits from statins are experienced in a specific patient group with a high risk of developing dementia, such as patients with concurrent diabetes and hyperlipidemia. Therefore, we conducted a pharmacoepidemiologic study that aimed to examine whether good adherence to statins was associated with a reduced risk of developing dementia among individuals with diabetes and comorbid hyperlipidemia.
Materials and Methods
Data Source
The data for this study were collected from Longitudinal Cohort of Diabetes Patients (LHDB) in National Taiwan Insurance Research Database (NHIRD). NHIRD is an administrative claims database that contains beneficiaries’ demographic characteristics, diagnoses, inpatient and outpatient procedures, medication prescriptions, and enrollment information of the enrollees in Taiwan National Health Insurance program. The program is a single-payer compulsory insurance plan that covers 99.5% of the Taiwan population.18 The analyzed LHDB contains a random sample of 120 000 incident cases of diabetes in each calendar year from 2003 to 2006, and all of the participants were followed up to 2013 as a longitudinal cohort.19
Study Design and Participants
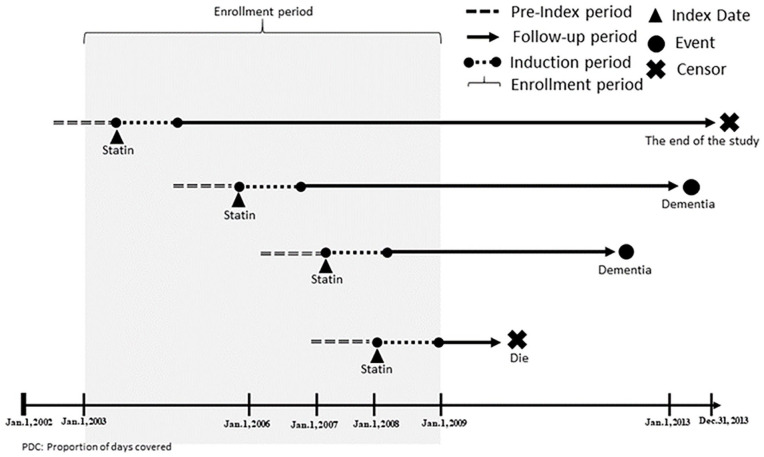
We only included type 2 diabetes individuals with comorbid hyperlipidemia and statins use. The enrollment period was from 2003 to 2007. To be included in the study cohort, individuals needed to have the diagnosis of hyperlipidemia (ICD-9-CM = 272.0–272.4) ≥1 month after the diagnosis of diabetes (ICD-9-CM code = 250.xx, except for 250.x1 and 250.x3); this ensured that hyperlipidemia was a comorbid with diabetes. We used statins prescriptions to ensure the validity of the hyperlipidemia diagnosis. The index date was the date of the first statins prescription after the diagnosis of hyperlipidemia. To verify new users of statins, individuals receiving any statins during the 1-year pre-index period were excluded. The study design is illustrated in Figure 1.
Figure 1.
Illustration of the study design.
Individuals were excluded if they had dementia (ICD-9-CM code = 290.0-290.4, 294.1, 294.2, 331.0-331.2), Huntington’s disease (ICD-9-CM code = 333.4), Creutzfeldt-Jakob disease (ICD-9-CM code = 046.11, 046.19), cerebral degeneration (ICD-9-CM code = 331.8), or Parkinson’s disease (ICD-9-CM code = 332.0, 332.1) during the 1-year pre-index period. Moreover, individuals aged under 50 were excluded.
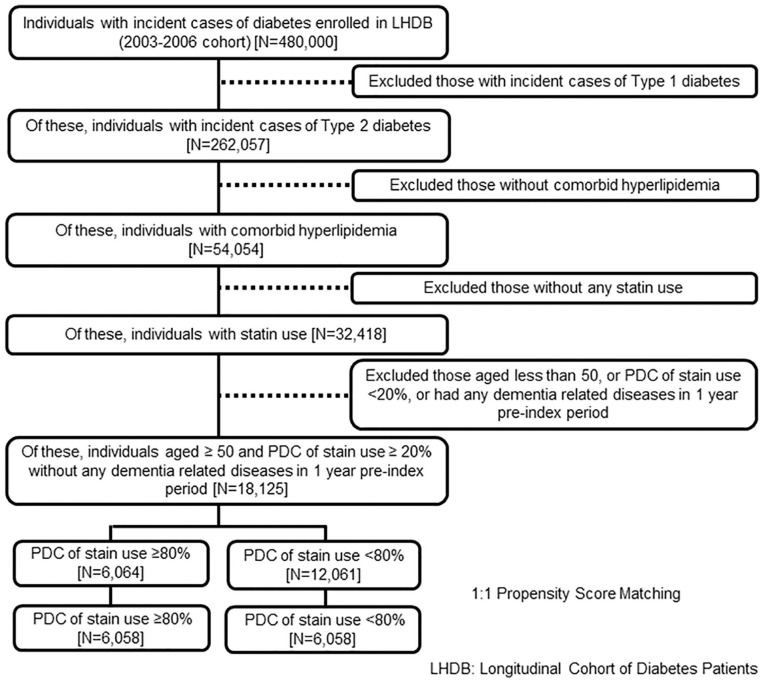
The active comparator design was used to categorize individuals into the good adherence group and the reference group. The interval-based proportion of days covered (PDC) of statins use over a 1-year period starting from the index date was used to measure adherence. The one-year period after the index date was the induction period for dementia. The induction period was designed to exclude the event of dementia caused by factors other than the level of statins use. The period also allowed us to calculate the PDC of statins use. The follow-up period was the time between the end of the induction period (the date that was 1 year later than the index date) and the date of incident dementia or the date of censoring. The maximum follow-up time was 10 years in all eligible individuals. Figure 2 shows the enrollment process for the study population.
Figure 2.
The enrollment process of the study population.
Outcomes Measures
The outcome of this study was the incidence of dementia. The definition of incident dementia was the first inpatient or outpatient diagnosis of dementia (ICD-9-CM code: 290.0-290.4, 294.1, 294.2, 331.0-331.2) during the follow-up period.
Primary Exposure
The primary exposure was defined as whether patients had good adherence to statins. Adherence was measured by the PDC. NHIRD includes information on days’ supply, and the PDC was assessed as the cumulative number of days on statins according to the prescription records divided by the one-year period after the index date. The PDC was recommended by Pharmacy Quality Alliance as the preferred method to calculate chronic medication adherence.20 The active comparator design was used with patients categorized into the good adherence group and the reference group. Patients were in the good adherence group if the PDC ≥ 80%, and patients were in the reference group if they had a PDC between 20% and 80%, in accordance with clinical evidence providing support for a standard PDC threshold of 80%.20 To make the 2 groups more comparable, we excluded patients with a PDC < 20% because these patients could be temporary statins users. Furthermore, very poor adherence could be driven by many confounders, including social economic status, regional factors, education level, or mental disorders. Most of these confounders could not be fully assessed and ruled out in our claim-based study. To ensure validity, we further assessed whether patients switched between the use of different types of statins and other anti-hyperlipidemic drugs. If the users switched between different kinds of statins or switched to a combination of statins, the number of days on any statin treatments was counted for the PDC. In contrast, if the users switched to other anti-hyperlipidemic drugs without statins, the number of days for those treatments was excluded from the cumulative number of days covered by statin therapy.
Covariates
Covariates in the study were categorized into demographic and clinical characteristics. The demographic characteristics included age, gender and region, and these 3 covariates were assessed on the index date. Clinical characteristics included diabetes severity, hyperlipidemia severity, comorbidities and medication-related variables. All covariates were measured in the one-year pre-index period.
Diabetes severity was measured by the diabetes disease duration and the number of oral hypoglycemic agents that patients used. The diabetes disease duration was defined by the year when the individual was first diagnosed with type 2 diabetes. The number of oral hypoglycemic agents was determined according to the inpatient or outpatient records of prescriptions over the 1-year pre-index period.
Hyperlipidemia severity was measured by the mean defined daily dose of statins and the intensity of statins. The mean defined daily dose of statins was based on the first year of statin therapy. The prescriptions in any kind of statins were converted into the number of defined daily doses (DDDs). DDD is the assumed average maintenance dose per day for statins.21 The intensity of statins can be classified into high-intensity, moderate-intensity, and lower-intensity statin therapy in accordance with the American College of Cardiology/American Heart Association guidelines on the treatment of blood cholesterol.22 The intensity of statins was based on the average expected LDL-C response to a specific statin and dose.
Comorbid diseases were measured during the pre-index period, including cardiovascular diseases, cerebrovascular events, peripheral vascular diseases, chronic pulmonary disease, psychiatric disorders, nephropathy, hypertension, thyroid diseases, liver disease, malignancy, and autoimmune diseases.
Medication-related variables were divided into 8 prescription categories and defined based on the pre-index period. They were antihypertensive treatment, other anti-hyperlipidemic drugs, antithrombotic medications, antidepressants, benzodiazepines, antipsychotic medications, anticholinergic drugs, and digitalis glycosides.
Statistical Analyses
The propensity score was used to match characteristics between the good adherence and comparison groups in our study. The 1:1 propensity score matching through the greedy matching process was performed.23
Descriptive statistics were used to describe and compare the demographic and clinical characteristics between the good adherence and comparison groups. For continuous variables, an independent samples t-test was used. For categorical variables, the chi-square test was used. A Cox proportional hazards model was performed to study the time to dementia diagnosis and to compare the hazard ratio (HR) of incident dementia between the exposure group and the reference group. Individuals were censored at the time of the following situations: the end of the study period on December 31, 2013, the earliest date of insurance withdrawal, or at the time of death if they died during follow-up before dementia diagnosis. All the data analyses were conducted using SAS software, version 9.4 (SAS Institute, Cary, North Carolina, USA). In this study, a two-tailed p value <0.05 was considered statistically significant.
Sensitivity Analysis
We further conducted a time-dependent Cox proportional hazards model in the sensitivity analysis. When a key independent variable or covariates change during the follow-up period, it is appropriate to use time-varying explanatory variables. In our study, the key independent variable was good adherence to statins, which was a variable that would keep varying in the follow-up period. Therefore, the time-dependent status of statins use was incorporated into the Cox proportional hazards model. The status of statins use was identified yearly in the follow-up period. If the participants’ PDC of statins use in a year was ≥80%, they were in the good adherence group in that year. In contrast, if the participants’ PDC of statins use in a year was <80%, they were in the reference group in that year. This study was reviewed and obtained an exemption and a waiver for informed consent from the Taipei Medical University Joint Institutional Review Board (TMU-JIRB No: N201704057).
Results
Table 1 shows the characteristics of the study population before and after propensity score matching. Among 18 125 eligible individuals with type 2 diabetes and comorbid hyperlipidemia, 6064 had good adherence to statins. After 1:1 propensity score matching, 6058 individuals with type 2 diabetes and comorbid hyperlipidemia were in the good adherence group, and 6058 were in the comparison group. After 1:1 propensity score matching, most characteristics between patients with and without good adherence to statins were balanced.
Table 1.
Characteristics of Good Adherence to Statin Group and Low Adherence to Statin Group among Patients with Concurrent Diabetes and Hyperlipidemia.
| Before propensity score matching |
Absolute standardized differences | After propensity score matching |
Absolute standardized differences | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Good adherence to statin (N = 6064) | Poor adherence to statin (N = 12 061) | Good adherence to statin (N = 6058) | Poor adherence to statin (N = 6058) | |||||||
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | |||
| Age, mean (SD) | 62.34 | 8.8 | 62.32 | 8.9 | 0.15 | 62.34 | 8.9 | 62.34 | 8.8 | 0.03 |
| Male | 2778 | 45.8 | 5737 | 47.6 | 0.26 | 2777 | 45.8 | 2767 | 45.7 | 0.02 |
| Region | ||||||||||
| Northern | 1984 | 32.7 | 3663 | 30.4 | 0.52 | 32.7 | 2007 | 33.1 | 0.05 | |
| Northwestern | 718 | 11.8 | 1455 | 12.1 | 718 | 11.9 | 684 | 11.3 | ||
| Central | 1093 | 18 | 2214 | 18.4 | 1093 | 18 | 1060 | 17.5 | ||
| Southwestern | 836 | 13.8 | 1816 | 15.1 | 836 | 13.8 | 864 | 14.3 | ||
| Southern | 1082 | 17.8 | 2154 | 17.9 | 1081 | 17.9 | 1103 | 18.2 | ||
| Eastern and others | 351 | 5.8 | 759 | 6.3 | 351 | 5.8 | 340 | 5.6 | ||
| Diabetes cohort | ||||||||||
| 2003 | 2066 | 34.1 | 4234 | 35.1 | 0.82 | 2065 | 17 | 2059 | 17 | 0.06 |
| 2004 | 1721 | 28.4 | 3509 | 29.1 | 1716 | 14.2 | 1732 | 14.3 | ||
| 2005 | 1393 | 23 | 2639 | 21.9 | 1393 | 11.5 | 1382 | 11.4 | ||
| 2006 | 884 | 14.6 | 1679 | 13.9 | 884 | 7.3 | 885 | 7.3 | ||
| Number of OHAs | ||||||||||
| 0 class of OHAs | 160 | 2.6 | 425 | 3.5 | 0.53 | 160 | 2.6 | 151 | 2.5 | 0.15 |
| 1 class of OHAs | 1210 | 20 | 2627 | 21.8 | 1209 | 20 | 1260 | 20.8 | ||
| 2 classes of OHAs | 2400 | 39.6 | 4943 | 41 | 2399 | 39.6 | 2371 | 39.1 | ||
| 3 classes of OHAs | 1418 | 23.4 | 2591 | 21.5 | 1416 | 23.4 | 1411 | 23.3 | ||
| 4 classes of OHAs | 621 | 10.2 | 1035 | 8.6 | 621 | 10.3 | 617 | 10.2 | ||
| 5 classes of OHAs | 200 | 3.3 | 364 | 3 | 200 | 3.3 | 204 | 3.4 | ||
| 6 classes of OHAs | 55 | 0.9 | 76 | 0.6 | 53 | 0.9 | 44 | 0.7 | ||
| Insulin | 1289 | 21.3 | 2249 | 18.7 | 0.32 | 1287 | 21.2 | 1258 | 20.8 | 0 |
| MDDD of statins | ||||||||||
| MDDD > 0.61 | 2193 | 36.2 | 4398 | 36.5 | 0.03 | 2191 | 36.2 | 2237 | 36.9 | 0.04 |
| MDDD ≤ 0.61 | 3871 | 63.8 | 7663 | 63.5 | 3867 | 63.8 | 3821 | 63.1 | ||
| Intensity of statins | ||||||||||
| High | 218 | 3.6 | 405 | 3.4 | 0.17 | 217 | 3.6 | 223 | 3.7 | 0.03 |
| Moderate and low | 5846 | 96.4 | 11 656 | 96.6 | 5841 | 96.4 | 5835 | 96.3 | ||
| Atrial fibrillation | 111 | 1.8 | 208 | 1.7 | 0 | 111 | 1.83 | 107 | 1.77 | 0.02 |
| Heart failure | 418 | 6.9 | 813 | 6.7 | 0.28 | 418 | 6.9 | 419 | 6.92 | 0.01 |
| Coronary artery disease | 1653 | 27.3 | 3113 | 25.8 | 0.21 | 1650 | 27.24 | 1663 | 27.45 | 0.06 |
| Atherosclerosis | 159 | 2.6 | 348 | 2.9 | 0.40 | 159 | 2.62 | 157 | 2.59 | 0.01 |
| Transient cerebral ischemia | 204 | 3.4 | 458 | 3.8 | 0.40 | 204 | 3.37 | 208 | 3.43 | 0.02 |
| Stroke | 485 | 8 | 869 | 7.2 | 0.62 | 485 | 8.01 | 478 | 7.89 | 0.01 |
| Diabetic PVD | 245 | 4 | 538 | 4.5 | 0.28 | 245 | 4.04 | 236 | 3.9 | 0.01 |
| Other peripheral vascular disease | 197 | 3.3 | 458 | 3.8 | 0.12 | 197 | 3.25 | 193 | 3.19 | 0.02 |
| Arteries of the extremities | 37 | 0.6 | 65 | 0.5 | 0.00 | 37 | 0.61 | 30 | 0.5 | 0.04 |
| Chronic obstructive pulmonary disease | 332 | 5.5 | 696 | 5.8 | 0.41 | 331 | 5.46 | 328 | 5.41 | 0.03 |
| Sleep apnea | 51 | 0.8 | 79 | 0.7 | 0.00 | 51 | 0.84 | 50 | 0.83 | 0.02 |
| Depression | 317 | 5.2 | 629 | 5.2 | 0.40 | 317 | 5.23 | 312 | 5.15 | 0.04 |
| Schizophrenia | 30 | 0.5 | 79 | 0.7 | 0.00 | 30 | 0.5 | 33 | 0.54 | 0.02 |
| Bipolar | 42 | 0.7 | 98 | 0.8 | 0.00 | 42 | 0.69 | 47 | 0.78 | 0.00 |
| Anxiety | 1347 | 22.2 | 2757 | 22.9 | 0.33 | 1347 | 22.24 | 1324 | 21.86 | 0.03 |
| Chronic kidney disease | 286 | 4.7 | 565 | 4.7 | 0.60 | 286 | 4.72 | 264 | 4.36 | 0.02 |
| Hypertension | 4360 | 71.9 | 8364 | 69.4 | 0.29 | 4356 | 71.9 | 4317 | 71.26 | 0.07 |
| Liver disease | 1724 | 28.4 | 3892 | 32.3 | 0.07 | 1722 | 28.43 | 1686 | 27.83 | 0.01 |
| Autoimmune diseases | 2 | 0.03 | 2 | 0.02 | 0.00 | 2 | 0.03 | 2 | 0.03 | 0.00 |
| Malignancy | 392 | 6.5 | 689 | 5.7 | 0.28 | 392 | 6.47 | 380 | 6.27 | 0.02 |
| Thyroid diseases | 355 | 5.9 | 659 | 5.4 | 0.40 | 355 | 5.86 | 334 | 5.51 | 0.04 |
| Beta-Blockers | 3087 | 50.91 | 5843 | 48.45 | 0.03 | 3085 | 50.92 | 3129 | 51.65 | 0.03 |
| ACEI | 2667 | 43.98 | 5022 | 41.64 | 0.19 | 2664 | 43.97 | 2699 | 44.55 | 0.03 |
| CCB | 3503 | 57.77 | 6641 | 55.06 | 0.42 | 3501 | 57.79 | 3525 | 58.19 | 0.02 |
| Diuretics | 2695 | 44.4 | 4856 | 40.3 | 0.27 | 2694 | 44.5 | 2655 | 43.8 | 0.01 |
| ARB | 2145 | 35.4 | 3508 | 29.1 | 0.36 | 2141 | 35.3 | 1986 | 32.8 | 0.08 |
| Fibrate | 872 | 14.4 | 2357 | 19.5 | 0.59 | 871 | 14.4 | 901 | 14.9 | 0.01 |
| Bile acid sequestrants | 13 | 0.2 | 16 | 0.1 | 0.00 | 13 | 0.2 | 6 | 0.1 | 0.02 |
| lipid modifying agents | 107 | 1.8 | 191 | 1.6 | 0.00 | 107 | 1.8 | 80 | 1.3 | 0.05 |
| Nicotinic acid | 10 | 0.16 | 31 | 0.3 | 0.00 | 10 | 0.2 | 12 | 0.2 | 0.02 |
| Vitamin K antagonists | 106 | 1.8 | 143 | 1.2 | 0.28 | 106 | 1.8 | 75 | 1.2 | 0.05 |
| Aspirin | 2397 | 39.5 | 4638 | 38.5 | 0.35 | 2394 | 39.5 | 2409 | 39.8 | 0.02 |
| Clopidogrel | 377 | 6.2 | 488 | 4.05 | 0.28 | 375 | 6.2 | 287 | 4.7 | 0.07 |
| Cilostazol | 120 | 2 | 201 | 1.7 | 0.28 | 120 | 2 | 112 | 1.9 | 0.02 |
| Ticlopidine | 122 | 2 | 216 | 1.8 | 0.00 | 121 | 2 | 121 | 2 | 0.04 |
| Dipyridamole | 1214 | 20 | 2457 | 20.4 | 0.11 | 1212 | 20 | 1282 | 21.2 | 0.00 |
| Antidepressants | 1063 | 17.5 | 2062 | 17.1 | 0.35 | 1063 | 17.6 | 1070 | 17.7 | 0.02 |
| Benzodiazepines | 3773 | 62.2 | 7468 | 61.9 | 0.12 | 3772 | 62.3 | 3750 | 61.9 | 0.05 |
| Typical antipsychotics | 128 | 2.1 | 261 | 2.2 | 0.01 | 128 | 2.1 | 111 | 1.8 | 0.01 |
| Atypical antipsychotics | 301 | 5 | 697 | 5.8 | 0.01 | 301 | 5 | 308 | 5.1 | 0.01 |
| Nonselective COX inhibitors | 5611 | 92.53 | 11 125 | 92.2 | 0.50 | 5607 | 92.6 | 5601 | 92.5 | 0.04 |
| Selective COX-2 inhibitors | 828 | 13.65 | 1526 | 12.7 | 0.21 | 828 | 13.7 | 848 | 14 | 0.04 |
| Anticholinergic drugs | 5706 | 94.1 | 11 308 | 93.8 | 0.28 | 5703 | 94.1 | 5696 | 94 | 0.03 |
| Digitoxin | 294 | 4.85 | 532 | 4.4 | 0.28 | 294 | 4.85 | 281 | 4.64 | 0.02 |
| Metildigoxin | 6 | 0.1 | 18 | 0.2 | 0.00 | 6 | 0.1 | 6 | 0.1 | 0.01 |
T2DM = type 2 diabetes mellitus; SD = standard deviation; OHA = oral hypoglycemic agents; MDDD = mean defined daily dose; PVD = peripheral vascular diseases; ACEI = angiotensin-converting-enzyme; CCB = calcium channel blocker; ARB = angiotensin receptor blockers.
Table 2 indicates the incident rates and results from the Cox proportional hazard model after propensity score matching. Of 6058 good adherence statins users, 272 people had diagnoses of dementia over a mean follow-up time of 6.57 years. The hazard of incident dementia among individuals with type 2 diabetes and comorbid hyperlipidemia was not statistically different between the good adherence group and the comparison group (HR = 0.94; 95% CI = 0.80-1.11). Similar results were observed in the sensitivity analysis, which considered statins use as a time-varying exposure (HR = 0.93; 95% CI = 0.70-1.23).
Table 2.
Association between Adherence to Statin and Risk of Dementia: Results from Cox Proportional Hazard Model after Propensity Score Matching.
| Group | Number of dementia events | Follow-up (years) |
Cox proportional hazard model |
||
|---|---|---|---|---|---|
| Total | Mean | Hazard ratio | 95% CI | ||
| Good adherence group [PDC ≥ 80%] (N = 6058) | 272 (4.5%) | 39 773 | 6.57 | 0.94 | (0.80-1.11) |
| Reference group [80% < PDC] (N = 6058) | 300 (5.0%) | 40 282 | 6.65 | Reference | |
CI = confidence interval; PDC = proportion of days covered.
Discussion
In this large cohort study with an active comparator design, good adherence to statins were not found to be associated with a lower risk of dementia than poor adherence to statins among individuals with type 2 diabetes and comorbid hyperlipidemia. The results were consistent in the sensitivity analysis. Our findings provided evidence that good adherence to statins did not lower the risk of dementia among individuals with diabetes and comorbid hyperlipidemia.
The prevalence of good adherence to statins in our study (33.5%) was lower than that (nearly 50%) in previous studies.24,25 This could be explained by 2 reasons. First, our study population included patients with diabetes and comorbid hyperlipidemia. Unlike patients with hyperlipidemia who may only need to use statins,25 patients in our study needed to take statins and diabetic drugs, which could lower the rate of good adherence because previous studies showed that polypharmacy increased the complexity of the medication regimen and decreased adherence.26,27 Second, the length of the PDC measurement period was shorter than that in previous studies.24 A longer PDC measurement period tends to lead to a higher rate of good medication adherence. When compared with a prior study using the same PDC measurement period as we did (ie, 1 year) among patients with diabetes, a similar prevalence of good adherence, ranging from 34.9% to 37.6%, was found between the study and our study.28
In our study, good adherence to statins was not associated with a lower risk of dementia among individuals with concurrent diabetes and hyperlipidemia. Our findings were different from several previous observational studies that indicated the protective effect of statins on dementia.5,16,29 The difference could result from 2 reasons. First, the active comparator design used in our study decreased the bias from confounding by indication. Instead, previous studies often compared the effect of statins on the risk of dementia between statins users and nonusers.16,29 Selecting nonusers as the reference group could induce bias due to the indication effect or the sick-stopper effect, leading to the overestimation or underestimation of the neuroprotective effect of statins on the prevention of dementia. For example, the indication bias could lead to an underestimation of the protective effect, but the sick-stopper effect from older and frail patients could lead to an overestimation of the effect. Unlike previous studies, our current study had 2 groups with similar treatment indications, which led to more robust evidence.
Second, the definition of statins without considering medication adherence in most previous observational studies could not thoroughly reflect the effectiveness of statins in real-world settings.5,16,29,30 Previous studies defined statins use often with the assumption of good adherence but did not actually measure adherence.17 However, adherence to statins could substantially decline over time in the real world.31 The current study measured adherence and considered the effectiveness of statins, which could provide better evidence for real-world experiences. Therefore, the protective effect of statins on the risk of dementia observed in previous observational studies could be biased due to not precisely measuring statins adherence.32,33
From a clinical perspective, healthcare providers should have a more conservative attitude toward the effectiveness of statins on dementia. The protective effect of statins on dementia reported in previous studies was a concern. Adherence to statins was not associated with a reduced risk of dementia in our study, so healthcare providers still need to closely monitor high-risk patients with good statins adherence. From a research perspective, we consider our findings to reflect more real-world situations. In our study, we closed the gap of the inconsistent association between statins and dementia and provided a more robust result with a rigorous pharmacoepidemiologic study design that assessed statins adherence with an active comparator.
Our study had several strengths. First, the active comparator design could largely reduce bias due to the confounding by indication effect. Second, the assessment of new drug users could eliminate the prevalent user bias. Third, we created an induction period to ensure that the outcome occurred after the exposure, thus reducing exposure misclassification and preventing protopathic biases. Finally, we captured the time-varying status of statins use in a sensitivity analysis to confirm the consistent association between good adherence to statins and risk of dementia.
Regardless of the strengths, the study still had several limitations. First, the follow-up time was relatively short for the disease progression. However, compared to previous studies with only a 3 to 4 year follow-up period,34,35 this study provided a relatively longer follow-up period. Second, propensity score matching could only reduce measurable confounders but not unmeasurable confounders (eg, apolipoprotein E [ApoE] genotype). Prior studies showed that the proportion of subjects with the ApoE genotype was significantly higher among patients with dementia than among patients without dementia (64.3% vs 35.8%; P < .01).36 Mutations in this gene result in an accumulation of β-amyloid plaques.37 Third, the measurement of adherence in this study was based on the prescription records rather than the patients’ real compliance. We used the PDC to measure adherence, which is a reliable and valid method recommended by the Pharmacy Quality Alliance for measuring adherence in the claims data.20 The PDC threshold of 80% is the level at which the medication has a reasonable likelihood of achieving the most clinical benefit. Fourth, in the outcome measurement, we defined the incident dementia using the first inpatient or outpatient diagnosis. Considering dementia as a progressive neurodegenerative disease, it is less likely to misclassify the individuals with dementia into individuals without dementia when using the first inpatient or outpatient diagnosis. However, a single diagnosis of dementia might still not indicate a true diagnosis because the diagnosis code might be used for other insurance claims purposes. In addition, previous studies found statin use could be protective for patients with mild cognitive impairment (MCI).38,39 However, patients with MCI were less likely to be captured in claim-based data. Thus, a misclassification bias could still exist. Fifth, we use adherence as the exposure, which could lead to a healthy user bias and could not completely separate the neuroprotective effect of statins use from health behaviors such as good statins adherence. Sixth, the diagnosis of dementia can be more precise with neuropsychological functioning and testing scores in additional to the diagnosis code from inpatient or outpatient records. Due to the nature of the data, we were unable to obtain those scores. However, in Taiwan National Health Insurance regulation, the specificity of dementia is relatively high because it is required to pass several evaluations (eg, Clinical Dementia Rating, Comprehensive Neuropsychological Test, brain computed tomography and blood test) assessed by psychiatrists. Therefore, it is less likely to misclassify individuals without dementia as with dementia, and then less likely to bias our results. Finally, the generalizability in this study may be limited to individuals with type 2 diabetes and comorbid hyperlipidemia in Taiwan.
Conclusion and Relevance
In conclusion, good adherence to statins was not found to be associated with a reduced risk of dementia among patients with diabetes and comorbid hyperlipidemia. Healthcare providers should be aware that the neuroprotective effect of statins among patients with concurrent diabetes and hyperlipidemia may not be as strong as reported in previous studies. Future studies with a more diverse study population are needed to further evaluate the neuroprotective effect of statins on dementia prevention.
Acknowledgments
This study is based in part on data from the National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the National Health Insurance Administration, Ministry of Health and Welfare, or National Health Research Institutes.
Footnotes
Authors’ Note: Jin-Liern Hong is currently an employee of Takeda. The results were presented in part at the 2018 International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Conference, May 19 to 23, 2018, Baltimore, MD, USA.
Author Contributions: Chung-Hsuen Wu oversaw the implementation and quality assurance of the study, contributed to the interpretation of these results, and critically reviewed the manuscript. Ching-Yuan Chang conceived of the study, conducted the analyses, and drafted the manuscript. Fang-Ju Lin and Jin-Liern Hong contributed to the interpretation of these results and critically reviewed the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported, in part, by a research grant from the Ministry of Science and Technology, Taiwan (MOST 105-2320-B-038-018 and MOST 106-2320-B-038-018 to Dr. Wu) and an investigator grant from Taipei Medical University and Taipei Medical University Hospital (TMU 105TMU-TMUH-20, to Dr. Wu). The funders had no role in the study design, data collection and analysis, result interpretation, publication decision, or manuscript preparation.
ORCID iD: Chung-Hsuen Wu  https://orcid.org/0000-0003-0495-1922
https://orcid.org/0000-0003-0495-1922
References
- 1. Wortmann M. Dementia: a global health priority-highlights from an ADI and World Health Organization report. Alzheimers Res Ther. 2012;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64-74. doi: 10.1016/s1474-4422(05)70284-2 [DOI] [PubMed] [Google Scholar]
- 3. Kuo SC, Lai SW, Hung HC, et al. Association between comorbidities and dementia in diabetes mellitus patients: population-based retrospective cohort study. J Diabetes Complications. 2015;29:1071-1076. doi: 10.1016/j.jdiacomp.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 4. Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627-1631. [DOI] [PubMed] [Google Scholar]
- 5. Wong WB, Lin VW, Boudreau D, Devine EB. Statins in the prevention of dementia and Alzheimer’s disease: a meta-analysis of observational studies and an assessment of confounding. Pharmacoepidemiol Drug Saf. 2013;22:345-358. doi: 10.1002/pds.3381 [DOI] [PubMed] [Google Scholar]
- 6. Hyman BT, Strickland D, Rebeck GW. Role of the low-density lipoprotein receptor-related protein in beta-amyloid metabolism and Alzheimer disease. Arch Neurol. 2000;57:646-650. doi: 10.1001/archneur.57.5.646 [DOI] [PubMed] [Google Scholar]
- 7. Zamrini E, McGwin G, Roseman JM. Association between statin use and Alzheimer’s disease. Neuroepidemiology. 2004;23:94-98. doi: 10.1159/000073981 [DOI] [PubMed] [Google Scholar]
- 8. Shoji M, Hirai S, Yamaguchi H, Harigaya Y, Kawarabayashi T. Amyloid beta-protein precursor accumulates in dystrophic neurites of senile plaques in Alzheimer-type dementia. Brain Res. 1990;512:164-168. [DOI] [PubMed] [Google Scholar]
- 9. Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230-235. doi: 10.1161/01.cir.100.3.230 [DOI] [PubMed] [Google Scholar]
- 10. Das UN. Statins and the prevention of dementia. CMAJ. 2001;165:908-909. [PMC free article] [PubMed] [Google Scholar]
- 11. Bodovitz S, Klein WL. Cholesterol modulates alpha-secretase cleavage of amyloid precursor protein. J Biol Chem. 1996;271:4436-4440. doi: 10.1074/jbc.271.8.4436 [DOI] [PubMed] [Google Scholar]
- 12. Sparks DL, Hunsaker JC, III, Scheff SW, Kryscio RJ, Henson JL, Markesbery WR. Cortical senile plaques in coronary artery disease, aging and Alzheimer’s disease. Neurobiol Aging. 1990;11:601-607. doi: 10.1016/0197-4580(90)90024-t [DOI] [PubMed] [Google Scholar]
- 13. Notkola IL, Sulkava R, Pekkanen J, et al. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology. 1998;17:14-20. doi: 10.1159/000026149 [DOI] [PubMed] [Google Scholar]
- 14. McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2016;1:CD003160. doi: 10.1002/14651858.CD003160.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Macedo AF, Taylor FC, Casas JP, Adler A, Prieto-Merino D, Ebrahim S. Unintended effects of statins from observational studies in the general population: systematic review and meta-analysis. BMC Med. 2014;12:51. doi: 10.1186/1741-7015-12-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cramer C, Haan M, Galea S, Langa K, Kalbfleisch J. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology. 2008;71:344-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haag MD, Hofman A, Koudstaal PJ, Stricker BH, Breteler MM. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2009;80:13-17. doi: 10.1136/jnnp.2008.150433 [DOI] [PubMed] [Google Scholar]
- 18. Hsing AW, Ioannidis JP. Nationwide population science: lessons From the Taiwan national health insurance research database. JAMA Intern Med. 2015;175:1527-1529. doi: 10.1001/jamainternmed.2015.3540 [DOI] [PubMed] [Google Scholar]
- 19. Hou W-H, Chang K-C, Li C-Y, Ou H-T. Dipeptidyl peptidase-4 inhibitor use is not associated with elevated risk of severe joint pain in patients with type 2 diabetes: a population-based cohort study. Pain. 2016;157:1954-1959. [DOI] [PubMed] [Google Scholar]
- 20. Nau D. Pharmacy quality alliance adherence measures. https://www.pqaalliance.org/adherence-measures
- 21. World Health Organization. Use of ATC/DDD. Collaborating Centre for Drug Statistics Methodology. Accessed December 17, 2020. https://www.whocc.no/atc_ddd_index/ [Google Scholar]
- 22. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889-2934. doi: 10.1016/j.jacc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 23. Haukoos JS, Lewis RJ. The propensity score. JAMA. 2015;314:1637-1638. doi: 10.1001/jama.2015.13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Korhonen MJ, Ruokoniemi P, Ilomäki J, Meretoja A, Helin-Salmivaara A, Huupponen R. Adherence to statin therapy and the incidence of ischemic stroke in patients with diabetes. Pharmacoepidemiol Drug Saf. 2016;25:161-169. doi: 10.1002/pds.3936 [DOI] [PubMed] [Google Scholar]
- 25. McGinnis B, Olson KL, Magid D, et al. Factors related to adherence to statin therapy. Ann Pharmacother. 2007;41:1805-1811. doi: 10.1345/aph.1K209 [DOI] [PubMed] [Google Scholar]
- 26. Curkendall SM, Thomas N, Bell KF, Juneau PL, Weiss AJ. Predictors of medication adherence in patients with type 2 diabetes mellitus. Curr Med Res Opin. 2013;29:1275-1286. [DOI] [PubMed] [Google Scholar]
- 27. Sreenivasan MC, Razak R, Athira Balakrishnan C. Impact of severity of poly pharmacy on medication adherence in patients with type II diabetes mellitus. Int J Pharmacol. 2015; 4:94-97. [Google Scholar]
- 28. Colantonio LD, Rosenson RS, Deng L, et al. Adherence to statin therapy among US adults between 2007 and 2014. J Am Heart Assoc. 2019;8:e010376. doi: 10.1161/jaha.118.010376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol. 2009;67:99-109. doi: 10.1111/j.1365-2125.2008.03308.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Group HPSC. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645-654.1. [DOI] [PubMed] [Google Scholar]
- 31. Lemaitre RN, Furberg CD, Newman AB, et al. Time trends in the use of cholesterol-lowering agents in older adults: the Cardiovascular Health Study. Arch Intern Med. 1998;158:1761-1768. [DOI] [PubMed] [Google Scholar]
- 32. Corrao G, Conti V, Merlino L, Catapano AL, Mancia G. Results of a retrospective database analysis of adherence to statin therapy and risk of nonfatal ischemic heart disease in daily clinical practice in Italy. Clin Ther. 2010;32:300-310. doi: 10.1016/j.clinthera.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 33. Hartz A, He T. Why is greater medication adherence associated with better outcomes. Emerg Themes Epidemiol. 2013;10:1. doi: 10.1186/1742-7622-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zandi PP, Sparks DL, Khachaturian AS, et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry. 2005;62:217-224. doi: 10.1001/archpsyc.62.2.217 [DOI] [PubMed] [Google Scholar]
- 35. Szwast SJ, Hendrie HC, Lane KA, et al. Association of statin use with cognitive decline in elderly African Americans. Neurology. 2007;69:1873-1880. doi: 10.1212/01.wnl.0000279333.77404.d7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsiung GY, Sadovnick AD, Feldman H. Apolipoprotein E epsilon4 genotype as a risk factor for cognitive decline and dementia: data from the Canadian Study of Health and Aging. CMAJ. 2004; 171: 863-867. doi: 10.1503/cmaj.1031789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8:1-21. [DOI] [PubMed] [Google Scholar]
- 38. Smith KB, Kang P, Sabbagh MN; Alzheimer’s Disease Neuroimaging Initiative. The effect of statins on rate of cognitive decline in mild cognitive impairment. Alzheimers Dement. 2017;3:149-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kemp EC, Ebner MK, Ramanan S, et al. Statin use and risk of cognitive decline in the ADNI cohort. Am J Geriatr Psychiatry. 2020;28:507-517. doi: 10.1016/j.jagp.2019.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]