Abstract
Background:
Recent changes to the legal status of cannabis across various countries have renewed interest in exploring its use in Parkinson’s disease (PD). The use of cannabinoids for alleviation of motor symptoms has been extensively explored in pre-clinical studies.
Objective:
We aim to systematically review and meta-analyze literature on the use of medical cannabis or its derivatives (MC) in PD patients to determine its effect on motor function and its safety profile.
Methods:
We reviewed and analyzed original, full-text randomized controlled trials (RCTs) and observational studies. Primary outcomes were change in motor function and dyskinesia. Secondary outcomes included adverse events and side effects. All studies were analyzed for risk of bias.
Results:
Fifteen studies, including six RCTs, were analyzed. Of these, 12/15 (80%) mention concomitant treatment with antiparkinsonian medications, most commonly levodopa. Primary outcomes were most often measured using the Unified Parkinson Disease Rating Scale (UPDRS) among RCTs and patient self-report of symptom improvement was widely used among observational studies. Most of the observational data lacking appropriate controls had effect estimates favoring the intervention. However, the controlled studies demonstrated no significant motor symptom improvement overall. The meta-analysis of three RCTs, including a total of 83 patients, did not demonstrate a statistically significant improvement in UPDRS III score variation (MD −0.21, 95% CI −4.15 to 3.72; p = 0.92) with MC use. Only one study reported statistically significant improvement in dyskinesia (p < 0.05). The intervention was generally well tolerated. All RCTs had a high risk of bias.
Conclusion:
Although observational studies establish subjective symptom alleviation and interest in MC among PD patients, there is insufficient evidence to support its integration into clinical practice for motor symptom treatment. This is primarily due to lack of good quality data.
Keywords: adjuvant therapy, cannabidiol, cannabinoids, medical cannabis, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative condition after Alzheimer’s disease.1 Between 1990 and 2016, the age-standardized prevalence of PD in Canada increased from 16.5% to 67%, totaling 103,903 in 2016.2 Bradykinesia, rigidity, postural instability and resting tremor are the motor symptoms that clinically characterize this disease. These symptoms are the result of marked loss of dopaminergic neurons in the substantia nigra pars compacta.3
First-line treatment involves administering dopamine precursors such as levodopa to correct the dopamine deficiency. However, chronic use of the drug can result in loss of drug efficacy and the development of motor complications. These include response fluctuations with “on-off” periods and levodopa-induced dyskinesia (LID), which can affect up to 35% of patients within 2 years of use.4,5 These limitations surrounding dopaminergic treatments have produced increased interest in novel treatments targeting non-dopaminergic systems such as the endocannabinoid system (ECS).6
Endocannabinoids of the ECS act at cannabinoid receptor type 1 (CB1) receptors to modulate the activity of dopamine and other neurotransmitters in the basal ganglia, rendering the ECS a potential target for pharmacological intervention in PD.7 The ECS is in itself implicated in the pathology of PD. This is evidenced by increased levels of the endogenous endocannabinoid anandamide (AEA) in the cerebrospinal fluid of both untreated8 and treated9 PD patients, and decreased CB1 receptor mRNA expression in the brain tissue of patients who died with idiopathic PD.6 Cannabinoids offer both antiparkinsonian and neuroprotective properties as therapeutic mechanisms in PD treatment. Pre-clinical studies have demonstrated cannabinoids act to suppress excitotoxicity, glial activation, and oxidative injury that cause degeneration of dopaminergic neurons.4,10–12 Recent studies have shown CB1 receptor antagonists could prove useful in the treatment of both PD symptoms and LID, and CB1 receptor agonists could have a role in reducing LID.13,14
Cannabinoids are divided into three categories: endogenous, plant-derived phytocannabinoids and synthetic. The most studied endogenous endocannabinoids are AEA and 2-arachidonoylglycerol.15 The principal phytocannabinoids responsible for the therapeutic effects of cannabis are ∆9-tetrahydrocannabidiol (Δ9-THC) and cannabidiol (CBD). Common cannabis preparations vary in their ratio of CBD to Δ9-THC, the latter being the psychoactive component of cannabis.15 The synthetic cannabinoids approved for use by Health Canada are nabiximols and nabilone.16
Medical use of cannabis has been legal in Canada since 2001 and as such, it has been explored for a variety of medical indications. With the passage of the Cannabis Act legalizing recreational cannabis in 2018, there has been renewed interest in the scientific exploration of potential therapeutic uses of cannabis. A number of reviews have explored cannabinoid-based therapies for the treatment of chronic neurodegenerative diseases and movement disorders such as PD.3,17–22 While pre-clinical studies show promise, gaps remain in bridging these results to human trials with clinical applications.23 We embarked on this review to expand our work into real-world observational data, looking specifically at efficacy for motor symptoms in PD and safety, and the applicability of these results in a Canadian context.
Objective
The objective of this systematic review was to identify literature exploring the efficacy and safety of cannabis and its derivatives for the treatment of motor symptoms in adults with PD. The questions we sought to investigate were as follows: (1) What is the direction and magnitude of effect of medical cannabis (MC) in alleviating motor symptoms and dyskinesia in adult PD patients? (2) What side effects and adverse events are associated with the use of cannabis and its derivatives? This review focuses on data from both randomized controlled trials (RCTs) and observational studies. Given the paucity of data, we included data from studies both with and without controls or comparators in this review.
Methods
We conducted a systematic review and meta-analysis of primary research literature investigating the use of cannabis and its derivatives for the treatment of PD with improvement of the cardinal motor symptoms and dyskinesia as the primary outcomes. Our study was conducted in accordance with the PRISMA 2009 Checklist for systematic reviews and meta-analyses24 (Supplemental Table S1). RCTs and observational studies were included in our search, which excluded case reports, case series and review articles. The population of interest was adult patients 18 years of age and older, diagnosed with idiopathic PD treated with MC or its derivatives. Studies were excluded if patients had any comorbid neurodegenerative disorders.
We performed electronic searches of PsychInfo, EMBASE, MEDLINE, and Evidence-Based Medicine Reviews – Cochrane Central Register of Controlled Trials (EBM-CCRCT) from the date of their inception to 2019. The search was initially done on 2 December 2019 and subsequently updated on 24 December 2020. Searches were restricted to yield only English-language results. MeSH headings and keywords used included but were not limited to: “Parkinson’s disease,” “parkinsonism,” “cannabis,” “cannabinoids,” “nabiximols,” “medical cannabis,” and “nabilone.” A sample search strategy from the MEDLINE database is available (Supplemental Figure S1).
All electronic search results were uploaded onto the Covidence platform to enable independent two-reviewer title and abstract screening. All titles were reviewed by two reviewers, authors SJT and BR, for inclusion based on the described population, intervention, outcome and study design criteria (Supplemental Table S2).
Outcome measures and data extraction
Primary outcomes included improvement of motor function encompassing tremor, bradykinesia, rigidity, and postural instability, and improvement in LID. Secondary outcomes looked at safety data, specifically adverse events and side effects associated with the use of MC. Authors SJT and BR reviewed included studies and extracted study design, population characteristics, treatment duration and dosing, follow-up, funding sources, motor function outcome data and safety data. Data reporting concomitant treatments with standard antiparkinsonian therapy were also extracted. RCTs with outcome data presented using mean and standard deviation were included in the meta-analysis. Observational studies were considered for inclusion in the meta-analysis if mean and standard deviation data were reported for both a treatment and control or comparator group.
Bias assessment
Risk of bias was assessed at the study level. Controls were evaluated using the Cochrane Risk of Bias Tool for RCTs25 and crossover trials.26 Randomization and concealment of allocation, blinding of participants and study personnel, incomplete data, selective reporting, period and crossover effects, and other sources of bias such as sample size were assessed. Risk of bias among uncontrolled observational studies was assessed using Modified Newcastle–Ottawa Criteria for Cross-Sectional Studies.27 Studies were assessed on the basis of selection, comparability and outcome. Good studies were judged to have a minimum score of 8, satisfactory studies had a minimum score of 5 and unsatisfactory studies had scores between 0 and 4.
Statistical analysis
A mean difference and its 95% confidence interval (CI) were calculated. Standard deviations (SDs) were derived using the 95% CIs. A forest plot was created using the Cochrane Collaboration RevMan v5.3 software. A random-effects model was used to account for clinical heterogeneity of the meta-analyzed studies. Values of I2 >50% and p < 0.10 were considered to indicate significant heterogeneity.
Results
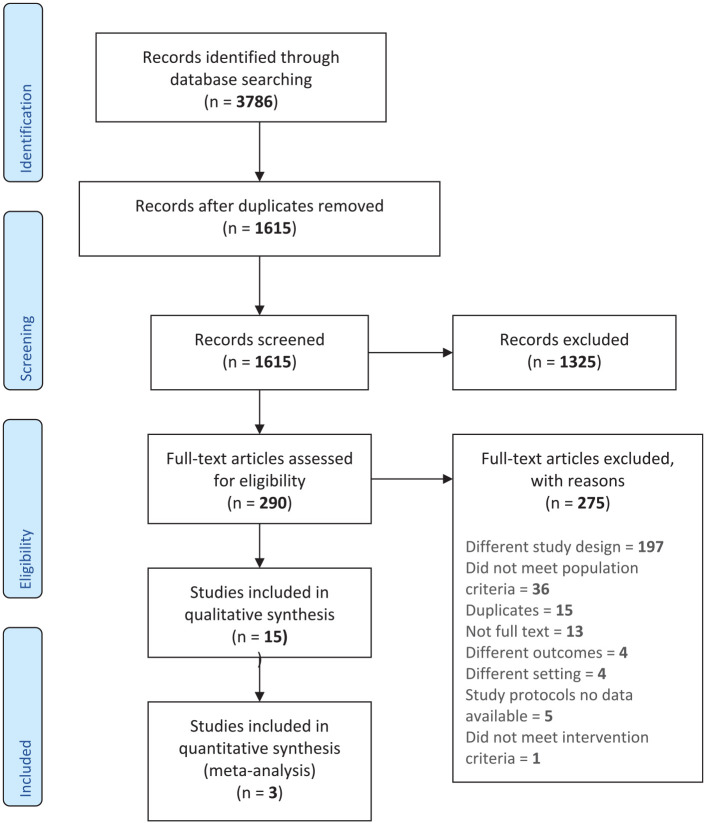
The search strategy yielded 1615 studies eligible for inclusion after duplicates were removed between the inception of the databases and December 2020 (Figure 1). All titles and abstracts were reviewed independently by two authors (SJT and BR) per inclusion criteria outlined above. In total, 28 abstracts were selected for full-text review. Of these, 13 observational studies were identified as having only abstract publications, with no associated full text to review. Ultimately, 15 primary studies met full inclusion criteria, including six RCTs and nine observational studies (Table 1). Of the 15 included papers, 13 studies reported statistical analysis of motor function data. Two of the observational studies28,29 only included the number of participants who self-reported motor symptom improvement. Most of the papers were published since 2010: five studies were published in the 2000s and 10 studies were published between 2010 and 2020. A total of 3079 patients were included in studies evaluating the primary outcome of efficacy; of these, 133 were enrolled in RCTs while the remaining 2946 were enrolled in observational studies. Data from 2266 patients (12 studies) were used to examine the secondary outcome of safety.
Figure 1.
PRISMA flow schema of literature search.
Table 1.
Included study treatment and duration.
| Study | Study design | Study setting | Treatment | Concomitant treatment | Duration | Outcomes |
|---|---|---|---|---|---|---|
| Controlled trials | ||||||
| Sieradzan et al.30 | RCT double-blind, placebo-crossover study | England | Nabilone or placebo (0.03 mg/kg) in 2 split doses 12 h and 1 h before levodopa administration | No antiparkinsonian meds from 9 pm the previous evening Levodopa, mean 582 mg Pergolide (n = 6), mean 2.9 mg Apomorphine 25 mg (n = 1) |
Two levodopa challenges performed 2 weeks apart | Effect on LID |
| Carroll et al.31 | RCT double-blind, placebo-crossover study | England | Cannador (ethanolic extract of Cannabis sativa standardized to 3.5 mg Δ9-THC and 1.25 mg cannabidiol per capsule) dosed based on body weight: mean dose 0.146 mg/kg/day Placebo capsules contained synthetic oil |
No antiparkinsonian meds from 9 pm the previous evening *Normal anti-PD meds in supplementary table, unavailable |
Two treatment phases each 4 weeks long separated by a 2-week washout period | • Effect on severity and duration dyskinesia • Effect on function, quality of life, sleep, pain and overall parkinsonism |
| Mesnage et al.32 | Randomized control trial | France | Anandamide, SR141716 20 mg Placebo Neurotensin SR 48692 180 mg Neurokinin B SR 142801 200 mg |
Levodopa treatment + single suprathreshold dose of levodopa (50 mg higher than the usual effective dose) before and at the end of treatments/placebo | 16 days | • Effect on motor symptoms • Effect on LID |
| Chagas et al.33 | Randomized control trial | Brazil | Placebo and CBD in identical capsules CBD 75 mg and 300 mg (powdered form 99.9% purity, dissolved in corn oil, placed in gelatin capsules) |
Stable doses of anti-Parkinson medication for at least 30 days before the trial | 6 weeks | • Effect on motor symptoms, dyskinesia and overall parkinsonism • Effect on well-being and quality of life • Possible neuroprotective effects |
| de Faria et al.34 | Randomized controlled trial crossover | Brazil | Placebo and CBD in identical capsules 300 mg (powdered form 99.9% purity, dissolved in corn oil, placed in gelatin capsules) | Levodopa (n = 19) Pramipexole (n = 11 Amantadine (n = 8) Selegiline (n = 3) Biperiden (n = 2) Entacapone (n = 1) |
Two 3-h sessions separated by a 15-day interval | • Effect on anxiety measures • Effect on tremor induced by a Simulated Public Speaking Test |
| Peball et al.35 | Randomized control trial | Austria | Nabilone dose titrated in phase I Placebo corn starch (identical to nabilone) |
Steady medication for >30 days prior to screening | 4 weeks | • Effect on mentation, behavior and mood aspects of parkinsonism • Effect on motor symptoms and overall parkinsonism • Effect on quality of life, sleep and pain |
| Observational studies | ||||||
| Venderova et al.36 | Observational retrospective questionnaire-based study | Prague | ~0.5 tsp of fresh/dried leaves orally (n = 1 inhaled) Frequency once a day 52.9% |
Continued anti-parkinsonian therapy | 32% (n = 27) used for <3 months 64% (n = 54) used for ⩾3 months |
• Descriptors of use • Self-reported effect on motor symptoms, overall parkinsonism, dyskinesia and pain |
| Zuardi et al.37 | Observational open-label pilot study | Brazil | 150 mg CBD tablet (CBD in powder, approximately 99.9% pure dissolved in corn oil) Dose increased weekly by 150 mg depending on the clinical response |
Stable doses of anti-PD medication for at least 7 days Median levodopa 1050 mg/day |
4 weeks | • Effect on psychosis • Effect on motor symptoms, dyskinesia and overall parkinsonism |
| Lotan et al.38 | Observational open-label study | Israel | Smoked their regular dose of cannabis (0.5 g inhaled) | Baseline assessment done w/o taking regular meds Levodopa (n = 19) Rasagiline (n = 7) Amantadine (n = 6) Anticholinergics (n = 6) Pramipexole (n = 4) Stalevo (n = 1) |
30 mins | • Effect on motor symptoms • Effect on non-motor symptoms |
| Finseth et al.29 | Observational retrospective questionnaire-based study | USA | Unknown | PD medications, not described | Unknown | • Descriptors of use • Self-reported effect on quality of life, mood, sleep, energy and motor symptoms |
| Shohet et al.39 | Observational open-label study | Israel | 1 g prescribed cannabis RoA: - Smoking (n = 18) - Vaporizer(n = 2) |
Levodopa (n = 14), mean 608 ± 410 mg (R 250–1000) Anticholinergic agents (n = 5) Dopamine agonists (n = 6) Amantadine (n = 3) Rasagiline (n = 5) |
30 min | • Effect on pain • Effect on motor symptoms |
| Balash et al.40 | Observational retrospective questionnaire-based study | Israel |
Cannabis sativa flowers/leaves or oil Mean daily dose 0.9 ± 0.5 g 80.9% (n = 38) smoked |
Unknown | Duration of MC use 19.1 ± 17.0 months | • Descriptors of use • Self-reported effect on motor and non-motor symptoms |
| Kindred et al.41 | Observational retrospective questionnaire-based study | USA | Formulation unspecified RoA: - Smoke only 40.9% - Edibles only 6.3% - Smoke + edibles 19.5% |
Unknown | Use lasted longer than 12 mo: 69.8% 4.6 (2.4) days/week | • Descriptors of use • Self-reported effect on motor and non-motor symptoms |
| Micheli et al.28 | Observational retrospective questionnaire-based study | Argentina | Cannabis oil 96.7% (115/121) | Concomitant PD treatment 91.7% (111/121) | 66.54 ± 84.24 days | • Descriptors of use • Self-reported effect on motor and non-motor symptoms |
| Yenilmez et al.42 | Observational retrospective questionnaire-based study | Germany | Doses unknown THC as gum extract or leaves (inhaled primarily) CBD oil/liquid drops or capsules |
Unknown | Unknown | • Descriptors of use • Self-reported effect on motor and non-motor symptoms |
LID, levodopa-induced dyskinesia; RCT, randomized controlled trial; THC, tetrahydrocannabinol; PD, Parkinson’s disease; CBD, cannabidiol; RoA, route of administration.
Study characteristics
We identified six RCTs examining the use of MC for the treatment of PD motor symptoms.30–35 Sieradzan et al.,30 Carroll et al.31 and de Faria et al.34 used randomized crossover trial designs with 14 to 15-day washout periods in between treatment phases. Nine observational studies were included in the analysis, of which six were questionnaire-based survey studies. All studies included were done in an outpatient setting. Six studies (40%) were conducted in Europe, three (20%) in the Middle East, four (27%) in South America and two (13%) in the United States (Table 1).
Population characteristics
The total sample size of patients enrolled in RCTs (n = 6) was 133 patients, of whom 87 individuals were allocated to receive treatment with cannabis derivatives, while 88 patients were allocated to comparators (Table 2). Non-controlled open-label studies (n = 3) enrolled 48 patients who received treatment with cannabis.
Table 2.
Baseline characteristics of included studies.
| Study | Population (male/female) | Mean age (years) | Mean PD duration (years) | Hoehn and Yahr stage | Intervention | Comparator | Funding and conflicts |
|---|---|---|---|---|---|---|---|
| Controlled trials | |||||||
| Sieradzan et al.30 | 7 (3/4) | 59 (49–69) | 8 (3–12) | Mean: 3 (3–4) | Nabilone (0.03 mg/kg) capsule | Placebo | Ø |
| Carroll et al.31 | 19 (12/7) | 67 (51–78) | 14 (4–32) | Mean: 3 (2.5–4) | 3.5 mg Δ9-THC and 1.25 mg cannabidiol per capsule Mean dose 0.146 mg/kg/day |
Placebo capsules contained synthetic oil | Ø |
| Mesnage et al.32 | 24 | Anandamide: 56.8 (8) Placebo: 65 (7) Neurotensin: 56 (8) Neurokinin: 60.8 |
Anandamide: 13.2 (4) Placebo: 12 (3) Neurotensin: 11.6 (2) Neurokinin: 16.5 |
Ø | Anandamide: 20 mg | Placebo Neurotensin: 180 mg Neurokinin B: 200 mg |
Ø |
| Chagas et al.33 | 21 (15/6) | CBD 75 mg: 65.86 ± 10.59 (51–82) CBD 300 mg: 63.43 ± 6.48 (53–71) Placebo: 67.29 ± 7.23 (57–75) |
CBD 75 mg: 8.14 ± 5.64 (2–15) CBD 300 mg: 6.86 ± 3.72 (3–12) Placebo: 9.86 ± 4.71 (5–17) |
Ø | CBD (powdered form 99.9% purity dissolved in corn oil placed in gelatin capsules) 75 mg 300 mg |
Placebo in identical capsules | Ø |
| de Faria et al.34 | 24 (22/2) | 64.13 ± 9.72 | 6.5 ±5.03 | Range 1–2.5 | CBD (powdered form 99.9% purity dissolved in corn oil placed in gelatin capsules) 300 mg | Placebo in identical capsules | State of São Paulo Research Assistance Foundation National Council for Scientific and Technological Development |
| Peball et al.35 | 38 (24/14) | Nabilone: 65.38 ± 7.94 (66.83) Placebo: 63.95 ± 8.04 (65.92) |
Nabilone: 7.83 ± 5.47 (7.25) Placebo: 7.39 ± 5.14 (5.75) |
Nabilone: 1.84 ± 0.50 (2.00) (95% CI 1.60; 2.08) Placebo: 1.95 ± 0.41 (2.00) (95% CI 1.75; 2.14) 12.26 |
Nabilone (median dose = 0.75 mg) dose determined in open-label nabilone titration | Placebo | AOP Orphan Pharmaceuticals AG provided investigational medicinal product and placebo; compensation of in-person study visits Funding from Medical University of Innsbruck |
| Observational studies | |||||||
| Venderova et al.36 | 339 (195/139) | 65.7 (36–92) years | 8.5 (<1–30) | Ø | ~0.5 tsp of fresh/dried leaves orally (n = 1 inhaled), frequency once a day 52.9% | Ø | Supported by Czech Ministry of Education |
| Zuardi et al.37 | 6 (4/2) | 58.8 ± 14.9 | 10.6 ± 3.7 | Ø | Initial dose 150 mg CBD tablet, increased q weekly by 150 mg (~99.9% pure powder dissolved in corn oil) | Ø | Funding: national and state science grants in Brazil Sponsored by THC-Pharm (Frankfurt, Germany) and STI-Pharm |
| Lotan et al.38 | 22 (13/9) | 65 (10.2) | 7.3 (4.8) | Median: 1.5 (1–3) | Smoked cannabis (amt inhaled 0.5 g) | Ø | Ø |
| Finseth et al.29 | 207 (125/82) | 68.9 (10.9) Cannabis (49–75) |
8.15 (6.9) Cannabis (2–11) |
Ø | Cannabis users n = 9 (4%) | Ø | Ø |
| Shohet et al.39 | 20 | 62.4 ± 9 (43–78) | 6.8 ± 3.5 (2–14) | Median: 2.2 ± 0.8 (1–4) | 1 g prescribed cannabis Smoking n = 18 Vaporizer n = 2 |
Ø | Ø |
| Balash et al.40 | 47 (40/7) | 64.2 (10.8) | 10.8 (8.3) | Median: 3 | Mean daily dose 0.9 ± 0.5 g 80.9% (n = 38) smoking |
Ø | Conflicts: L.B.S.a, YB.b |
| Kindred et al.41 | 454 (263/191) | 61.1 (9.5) Users: 60.0 (9.2) Non-users: 61.7 (9.5) |
Ø | Ø | Medicinal use: 72.3% Current use: 36.6% Past use: 66.3% Has medical card: 38.4% |
Ø | Conflicts: W.R.S.c |
| Micheli et al.28 | 503 (272/231) | Overall: R 27 – 93 Users (n = 121): 68.56 ± 9.78 |
Users (n = 121): 7.3 ± 5.3 | Ø | Cannabis oil 96.7% (115/121) | Ø | Ø |
| Yenilmez et al.42 | 1348 (737/609) | Overall: 71.6 ± 8.9 Users (n = 113): 66.4 ± 10.7 |
Overall: 11.6 ± 7.2 Users: 11.6 ± 6.5 |
Ø | THC 9.7% (11/113) CBD 39.8% (45/113) THC + CBD 20.4% (23/113) |
Ø | Conflicts: O.F.d and C.B.e |
Employee of Tikun Olam Co (Israeli pharmaceutical company developing cannabis-based medicinal extracts).
Previous head of the Israeli Ministry of Health program for Medical Use of Cannabis in 2003 to 2012; CSO of One World Cannabis Israel (company dedicated to the research of cannabis and cannabinoids and their medical properties) at the time of the study.
Consultant/speaker for EMD Serono, Acorda, TEVA, Genzyme, and Mallinckrodt.
Congress attendance fees: AbbVie, Abbott/St. Jude; Lecture fee: Daiichi-Sankyo.
Fees for advisory board participation: UCB Pharma, Zambon; Lecture fees: AbbVie Pharma, BIAL Pharma, Desitin, GE Health-care, Grunenthal Pharma, Licher GmbH, Medtronic, Novartis, TAD Pharma, UCB Pharma, Zambon Pharma. THC, tetrahydrocannabinol; CBD, cannabidiol.
The mean age of participants among studies investigating the primary outcome, excluding Micheli et al.28 as they reported a range instead of a mean, was 68.1 years. The mean age of participants enrolled in studies exploring the secondary outcome was 70.4 years. All studies except Sieradzan et al.30 and Zuardi et al.37 had mean ages above 60 years. The mean PD duration of participants enrolled in studies investigating the primary outcome, excluding Kindred et al.41 and Micheli et al.28 as they did not report mean duration, was 10.5 years, while that of those investigating the secondary outcome was 10.9 years. Only seven studies reported the Hoehn and Yahr (H&Y) stage of their PD patients, of which three reported means, three reported medians and one reported a range. The mean H&Y stage was 1.78 among the three studies, while the median among the other three studies was 2.2. Baseline characteristics are summarized in Table 2.
Interventions
Of the nine studies that were intervention based, three used the phytocannabinoid CBD (33%), two used the synthetic cannabinoid nabilone (22%), two used the participants’ own home cannabis (22%), one used the endocannabinoid anandamide (11%) and one used an ethanolic extract of Cannabis sativa standardized to 3.5 mg Δ9-THC and 1.25 mg CBD per capsule (11%) (Table 2). Comparators included placebo in identical capsules, neurotensin, neurokinin B or no control group. Doses of the treatment were widely variable between studies, employing standard,32–34,38,39 weight-based30,31 and escalating doses.35,37 The route of administration was most commonly oral capsules (78% of intervention-based studies, n = 7), although 22% (n = 2) were primarily smoked.38,39 Treatment duration ranged from single-dose administration to 6 weeks.
Among the questionnaire-based observational studies (n = 6), 575 of 2898 study participants reported cannabis use. Formulations were largely undescribed, with the majority of studies characterizing route of administration instead as smoked/inhaled, vaporized, edible or sublingual oil. Mean duration of use ranged from approximately 2 months to 1.5 years.
Concomitant treatment
Patient use of standard antiparkinsonian medications was mentioned in 12/15 (80%) of the included studies (Table 1). Only five studies (33%), two RCTs and three observational, describe specific agents used by enrolled patients for standard antiparkinsonian therapy. One study did provide these data in a supplemental table, but it was unavailable at the time of data extraction.31 Levodopa was the most frequently used agent, although other common ones included pramipexole, rasagiline, amantadine, dopamine agonists such as pergolide and unspecified anticholinergics. Of the five studies, one study provided doses of antiparkinsonian agents used by enrolled patients30, one study39 provided the mean levodopa dose, and one study37 provided median levodopa dose. These latter two studies did not provide doses for agents other than levodopa. A single study32 evaluated MC use after the administration of a suprathreshold dose of levodopa—that is, higher than the usual effective dose. This is notable as it may have blunted the effects of the intervention on PD motor symptoms and dyskinesia. Some 20%40–42 of the included studies did not comment on concomitant treatment. Additionally, 20%30,31,38 intentionally withheld levodopa the day before or the day of treatment to examine motor function during the functional “off” period.
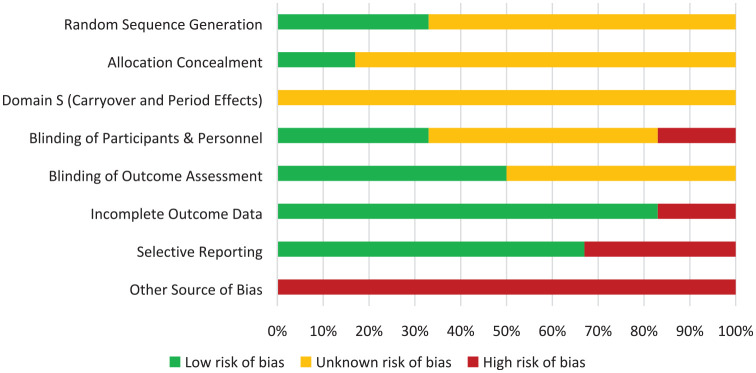
Risk of bias
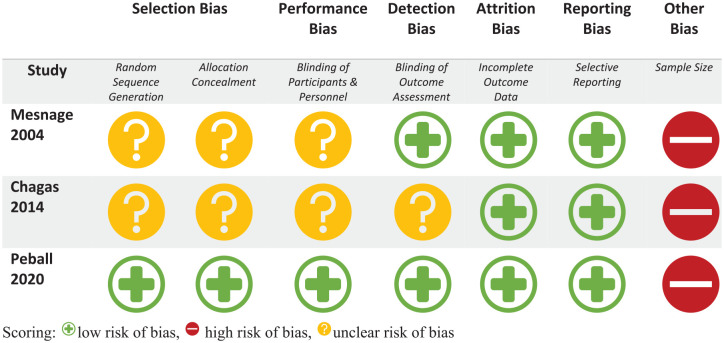
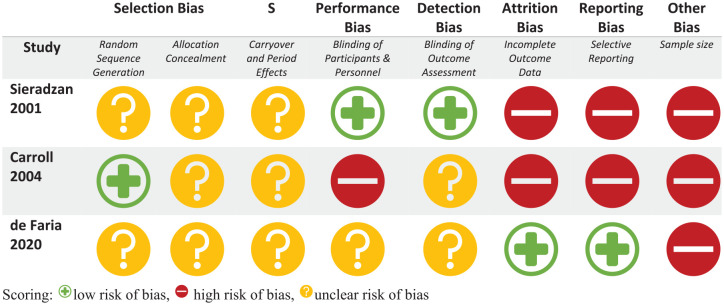
The risk of bias of the RCTs is represented in risk-of-bias graphs (Figures 2 and 3), Table 3, and a risk of bias summary (Figure 4).
Figure 2.
Bias assessment for randomized controlled trials using the Cochrane risk of bias tool criteria.
Scoring: low risk of bias, high risk of bias, unclear risk of bias.
Figure 3.
Bias assessment for crossover trials using the Cochrane risk of bias tool criteria.
Scoring: low risk of bias, high risk of bias, unclear risk of bias.
Table 3.
Cochrane risk of bias table for included randomized controlled trials and crossover trials.
| Study | Cochrane risk of bias tool criteria | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Selection bias | S | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias | |||
| Random sequence generation | Allocation concealment | Carryover and period effects | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Sample size | ||
| Sieradzan et al.30 | 1 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 7 |
| Carroll et al.31 | 2 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 5 |
| Mesnage et al.32 | 1 | 1 | 1 | 2 | 2 | 2 | 0 | 9 | |
| Chagas et al.33 | 1 | 1 | 1 | 1 | 2 | 2 | 0 | 8 | |
| de Faria et al.34 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 0 | 9 |
| Peball et al.35 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 12 | |
Figure 4.
Risk of bias summary across all included randomized controlled trials and crossover trials.
Random sequence generation: All included studies were randomized but only two of the six studies adequately described the method used to generate the random sequence.
Concealment of allocation: Only one study described how the sequence was concealed; the remaining five did not report any such information.
Domain S (carryover and period effects): While adequate washout periods in all three crossover trials were in place to mitigate carryover effects, none of the studies reported on-period effects.
Blinding of participant and personnel: Only two of the six studies provided adequate explanations of how blinding was achieved. There was high risk of bias in one study wherein the majority of participants correctly identified their treatment allocation at the end of the study period despite the double-blind nature of the trial.31
Blinding of outcome assessment: Three studies adequately described how outcome assessors were blinded to the intervention, while the remaining three were judged to have unclear risk of bias.
Incomplete outcome data: Two studies were at high risk of bias as two randomized patients in both studies were excluded from the analysis. The remaining five studies had low risk of bias with outcome data available for all randomized patients.
Selective reporting: Four studies were noted to be at low risk of bias with all trials analyzed appropriately in accordance with their pre-specified plan. Two studies were judged to have high risk of bias for presenting results selected from multiple eligible analyses of the data.
Other potential bias: All six studies had less than 50 patients in each treatment arm, representing high risk of bias.
Overall, all RCTs included in the analysis were judged to be at high risk of bias (Figures 2 and 3).
Among the nine observational studies, three were judged to be unsatisfactory with respect to risk of bias and the remaining six studies were judged to be satisfactory (Table 4). None of the observational studies achieved the minimum score to be considered good with respect to risk of bias. Deficits were noted most markedly across studies in terms of sample size selection, ascertainment of exposure, descriptions of non-respondents and outcome assessment method.
Table 4.
Modified Newcastle–Ottawa criteria for cross-sectional studies.
| Study | Selection | Comparability | Outcome | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of sample | Sample size | Ascertainment of exposure | Non-respondents | Subjects in different outcome groups are comparable | Assessment method | Statistical test | ||
| Venderova et al.36 | * | * | ** | * | 5 | |||
| Zuardi et al.37 | * | ** | ** | * | 6 | |||
| Lotan et al.38 | * | ** | ** | * | 6 | |||
| Finseth et al.29 | * | ** | 3 | |||||
| Shohet et al.39 | * | ** | ** | * | 6 | |||
| Balash et al.40 | * | ** | * | 4 | ||||
| Kindred et al.41 | * | * | ** | * | 5 | |||
| Micheli et al.28 | * | * | ** | 4 | ||||
| Yenilmez et al.42 | * | * | ** | * | 5 | |||
Scoring: Very Good Studies 9–10 points; Good Studies 7–8 points; Satisfactory Studies 5–6 points; Unsatisfactory Studies 0–4 points.
Primary outcome: Motor function
Effectiveness with respect to motor function improvement was most widely measured across studies using part 3 of the Unified Parkinson Disease Rating Scale (UPDRS), which has been revised for clinical use by specialists from the Movement Disorder Society.43 The UPDRS III sub-scale is examination based and is a sum of scores from 27 clinical observations with the total score ranging from 0 to 108.
Motor function outcome data are summarized in Table 5. Thirteen studies examined motor function, of which four were RCTs, three were observational open-label studies and six were retrospective questionnaire-based studies. These studies were highly heterogeneous with respect to outcome measurement. They used the Unified Parkinson Disease Rating Scale (UPDRS), measurements of tremor amplitude, subjective motor disability scales, or patient self-reports of motor symptom improvement as outcome metrics. Only one of the four RCTs, which enrolled 24 patients who were given a single dose of oral CBD, showed a significant improvement in terms of tremor amplitude (p < 0.05), but not frequency (p = 0.899), using a single-dose administration of MC.34 The results of this study were not amenable to meta-analysis owing to significant heterogeneity in the constructs of the outcome measurement tool and in the statistics used to report the data despite using a random-effects model.
Table 5.
Motor function and dyskinesia data of included studies.
| Study | Scale | Motor function and dyskinesia data | Effect estimates |
|---|---|---|---|
| Controlled trials | |||
| Sieradzan et al.30 | Rush dyskinesia disability scale On-period duration % on-period dyskinesia |
Median total dyskinesia score
levodopa + nabilone: 17 (R 11–25) levodopa + placebo: 22 (R 16–26) Mean on-period duration (SEM) Levodopa + nabilone: 169.6 (24.1) minutes Levodopa + placebo: 156.7 (16.2) minutes % on-period dyskinesia (SEM) Levodopa + nabilone: 98.2% (0.1%) Levodopa + placebo: 96.1% (1.7%) |
p < 0.05: nabilone significantly reduced total levodopa-induced dyskinesia compared with placebo p > 0.5 no difference in on-period duration p > 0.5 no difference in % on-period dyskinesia |
| Carroll et al.31 | UPDRS IV dyskinesia score Rush dyskinesia scale Bain dyskinesia scale |
Size of treatment effect UPDRS IV score
0.52 (95% CI −0.1 to 1.1) Size of treatment effect Rush dyskinesia score −1.5 (95% CI −5.5 to 2.5) Size of treatment effect Bain dyskinesia score −0.7 (95% CI −11.9 to 10.6) |
p = 0.09, not significant worsening in UPDRS dyskinesia score with cannabis p = 0.44, not significant improvement in Rush dyskinesia score with cannabis p = 0.90, not significant improvement in Bain dyskinesia score with cannabis |
| Mesnage et al.32 | UPDRS III motor score variation (baseline-final) severity of dyskinesia |
Mean UPDRS III motor score (baseline – final)
CBD: −3 (5) Placebo: −0.5 (8.51) Severity of dyskinesia, score/min CBD Baseline: 2.6 (1.6) → Final: 2.8 (1.9) Placebo Baseline: 3.1 (1.3) → Final: 3 (1.4) |
No difference in percentage of parkinsonian motor improvement and severity of levodopa-induced dyskinesias between cannabinoid antagonists and placebo |
| Chagas et al.33 | UPDRS III motor score variation (baseline-final) UPDRS IV score variation (baseline-final) |
CBD 75 mg
UPDRS III 3.85 (5.37) UPDRS IV −0.43 (1.99) CBD 300 mg UPDRS III 3.00 (5.16) UPDRS IV 0.43 (2.64) Placebo UPDRS III 2.17 (8.23) UPDRS IV −1.00 (2.19) |
Kruskal–Wallis test: p = 0.675 No significant difference between mean score variations among the three groups for UPDRS III ANOVA: F = 0.644, Kruskal–Wallis: p = 0.538 No significant difference between mean score variations among the three groups for UPDRS IV |
| de Faria et al.34 | Simulated public speaking test accelerometer data Tapping test |
Power spectrum peak (PSP)
F(1, 20) = 6.19 Power spectrum entropy (PSE) F(1, 20) = 1.63 Power spectrum peak frequency (PSPF) F(1, 20) = 0.02 TT (bradykinesia) F(1, 21) = 0.15 |
ANOVA showed significant differences for the drug only in the PSP variable (amplitude of fundamental frequency of movement), p = 0.022, not the PSE (p = 0.216) or PSPF (p = 0.899) variables; amplitude of tremor reduced significantly, but not frequency In the tapping test no effect was observed from the drug, p = 0.701 |
| Peball et al.35 | UPDRS III (baseline – final) UPDRS motor score (baseline – final) |
Nabilone
UPDRS III −0.53 (95% CI −2.24 to 3.29) UPDRS motor −1.00 (95% CI −2.16 to 4.16) Placebo UPDRS III −2.63 (95% CI 0.25–5.02) UPDRS motor −3.53 (95% CI 0.78–6.28) |
Nabilone UPDRS III p = 1.000, UPDRS motor p = 0.790 both scores worsened in the nabilone arm, not significant Placebo UPDRS III p = 0.034, UPDRS motor p = 0.018 both scores significantly worsened in the placebo arm |
| Observational studies | |||
| Venderova et al.36 | Muscle rigidity, bradykinesia, tremor, dyskinesia subjective changes rated |
Bradykinesia alleviation: 38 (44.7%) Muscle rigidity Alleviation: 32 (37.7%) Rest tremor alleviation: 26 (30.6%) L-dopa-induced dyskinesia alleviation: 12 (14.1%) |
With ⩾3 months use reported significantly more often improvement in: • Bradykinesia (p < 0.01, X2 test) • Muscle rigidity (p < 0.01, X2 test) • Resting tremor, (p < 0.01, X2 test) • No relationship between length of cannabis use and dyskinesia patients using cannabis ⩾once/day reported improvement in dyskinesia significantly more often than those using ⩽once/day, p < 0.05, X2 test |
| Zuardi et al.37 | UPDRS motor score |
UPDRS III total motor score
Baseline: 44.5 (20.5–62) → Final: 36 (31–64) UPDRS IV score Baseline: 3 (1–7) → Final: 2.5 (0–7) |
Improvement in UPDRS III motor score, not statistically significant, Z = 1.2, Wilcoxon signed rank test improvement in UPDRS IV score, not statistically significant, Z = 0.4, Wilcoxon signed rank test |
| Lotan et al.38 | UPDRS III motor score |
UPDRS III total motor score
Baseline: 33.1 (13.8) → Final: 23.3 (10.5) UPDRS III tremor sub-score Baseline: 7.55 (4.79) → Final: 3.64 (2.8) UPDRS III rigidity sub-score Baseline: 7.55 (3.79) → Final: 6.48 (3.56) UPDRS III bradykinesia sub-score Baseline: 13.12 (6.88) → Final: 8.62 (5.5) UPDRS III posture sub-score Baseline: 1.90 (1.58) → Final: 1.55 (1.1) |
Significant improvement in mean total UPDRS motor score, t = 5.8, p < 0.001 Significant improvement in mean UPDRS tremor sub-score, p = 0.000 Improvement in mean UPDRS rigidity sub-score, p = 0.004 Significant improvement in mean UPDRS bradykinesia sub-score, p = 0.000 Improvement in mean UPDRS posture sub-score, p = 0.056 |
| Finseth et al.29 | Subjective motor symptom improvement | N = 2 (22%) reported benefits in motor symptoms | Ø |
| Shohet et al.39 | UPDRS III motor score |
UPDRS III total motor score
Baseline: 38.1 (18) → Final: 30.4 (15.6) |
Significant improvement in mean UPRDS motor score, p < 0.0001 Findings consistent between 2 raters, intra-class correlation coefficient 0.91 |
| Balash et al.40 | Falls, muscle stiffness, tremor (subjective 5-point clinical global impressions scale) | Fall complaints Prior to MC use: n = 22/47 (46.8%) With MC use: n = 6/18 (33.3%) Reported reduction in muscle stiffness n = 32/44 (72.7%) Reported reduction in tremor n = 30/41 (73.2%) |
Significant reduction in complaints of falling, p < 0.05, r2 = 0.89 Significant reduction in reports of general muscle stiffness, p < 0.001, r2 = 0.62 Significant reduction in reports of tremor p < 0.001, r2 = 0.64 |
| Kindred et al.41 | Subjective overall effectiveness (0–7 Likert scale) Guy’s Neurological Disability Scale (subjective) |
Effectiveness
6.2 (1.8) GNDS arm/hand sub-scale Use: 9.8 (1.1) Non: 9.9 (1.2) GNDS mobility sub-scale Use: 2.1 (1.4) Non: 2.2 (1.3) |
Effectiveness Likert scale not specific to motor symptoms No significant difference between users and non-users in terms of GNDS motor sub-scales |
| Micheli et al.28 | Overall symptom improvement Improvement in: stiffness, gait, tremor, motor slowness, other motor symptoms, falls, dyskinesias |
Motor symptom Improvement: 42 (34.7%) Stiffness: 15 (12.4%) Gait: 11 (9.1%) Tremor: 8 (6.6%) Motor slowness: 6 (5%) Other motor symptoms: 5 (4.1%) Falls: 1 (0.8%) Dyskinesias: 1 (0.8%) |
Ø |
| Yenilmez et al.42 | PD motor symptom improvement |
Stiffness: 21 (18.6%) Freezing: 13 (11.5%) Tremor: 17 (15.0%) Postural instability: 6 (5.3%) Dyskinesia: 2 (1.8%) Falls: 1 (0.88%) Nighttime involuntary Movements: 0 (0%) Restless legs: 6 (5.3%) |
Efficacy on stiffness/immobility/akinesia was more frequently reported in the THC group [8/16 (50%) versus 4/26 (15.4%), p = 0.03] Better efficacy compared with dopaminergic agents reported more frequently in THC group, non-Significant [12/15 (80.0%) versus 7/20 (35.0%), p = 0.06] |
SEM, standard error of the mean; UPDRS, Unified Parkinson’s Disease Rating Scale; CBD, cannabidiol; THC, tetrahydrocannabinol; PD, Parkinson’s disease; GNDS, Guy’s Neurological Disability Scale; ANOVA, analysis of variance.
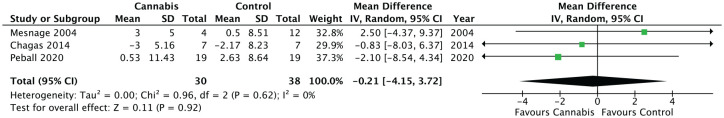
The remaining three RCTs all measured improvement in motor outcomes using the change in UPDRS III motor scores from the baseline assessment to the final one after the intervention was administered. Figure 5 summarizes these results using the calculated mean difference between the intervention (cannabis and its derivatives) and control groups. The data from these three RCTs32,33,35 analyzed results from 83 patients. There was no significant improvement in UPDRS motor scores over the duration of use in one33 of the three studies (p = 0.675). The other two RCTs32,35 reported a worsening of scores in the MC arm over the duration of use, but the change was worse in the placebo arm compared with the MC arm. As such, although meta-analysis of the variation in UPDRS III scores favored the use of cannabis with a mean difference of −0.21, this was not statistically significant (95% CI −4.15 to 3.72; p = 0.92). There was no statistical heterogeneity noted when a random-effects model was used.
Figure 5.
Change in Unified Parkinson Disease Rating Scale III motor score from baseline to final measurement between MC and control groups.
Nine of the 13 studies examining motor outcomes were observational, as outlined above. The three open-label studies demonstrated improvement in mean UPDRS III motor scores, with statistically significant (p < 0.001) improvements in two38,39 of the three studies. Notably, these two studies involved single-dose administration. Only the one study that demonstrated no significant improvement37 had a 4-week duration of use. Of the six retrospective questionnaire-based studies, two studies28,29 did not statistically analyze their results, opting to instead report the number of participants who reported subjective improvement in motor symptoms with MC use. Three of the six questionnaire-based studies (50%) demonstrated statistically significant improvement in motor symptom burden using participant self-reports. Only one41 of the questionnaire-based studies reported no significant difference in motor outcomes between MC users and non-users in terms of the motor sub-scale of a subjective neurological scale completed by participants.
Although most of the included observational studies, which lack appropriate controls, demonstrate a favorable effect with the intervention, the overall evidence from controlled studies does not support a significant improvement in PD motor symptoms with MC use. Notably, all included studies had a high risk of bias.
Primary outcome: dyskinesia
Eight of the included studies reported primary outcome data pertaining to the alleviation of dyskinesia and LID (Table 5). Four of these studies were RCTs, one was an observational open-label study, and the remaining three were retrospective questionnaire-based studies. The UPDRS part IV sub-score which captures complications of therapy43 was most commonly used to measure LID data across studies. The Rush and Bain dyskinesia scales and patient self-reports of symptom improvement were also used to assess for dyskinesia in a minority of studies. Dyskinesia data were not amenable to meta-analysis owing to significant heterogeneity in measurement tools and non-combinable descriptive statistics used to report the data. Only 1/8 (12%) of the included studies reported statistically significant improvement in dyskinesia (p < 0.05) with the use of cannabis. This study was a RCT that used nabilone to assess LID alleviation.30 Although 4/8 (50%) of studies show effect estimates consistently favoring the intervention, these were not statistically significant. Of these, objective scores were used to measure dyskinesia by two RCTs and one open-label trial, while patient self-reports of improvement were used by one questionnaire-based study. Two28,42 of the questionnaire-based studies only reported the number of participants who reported subjective improvement in dyskinesia without any statistical analysis. Overall, there was insufficient evidence to support the use of MC to alleviate dyskinesia.
Secondary outcome: safety data
Safety data are summarized in Table 6. There were no severe adverse events reported in any of the 12 studies examining the secondary outcome of safety—study drugs were generally well tolerated. Only two studies33,38 used scales or adverse event surveys to capture safety data. Most studies reported the number of participants who described specific side effects, and a sub-set31,40 of these descriptively classified side effects as either physical or psychotropic in nature. Balash et al.40 provide safety data over the longest period of use, 19.1 months. They report psychotropic effects including confusion, anxiety, hallucinations, short-term amnesia and psychosis in 38% (n = 18) of participants and physical adverse effects including unsteadiness, dizziness, dyspnea and cough in 45% (n = 21) of participants.40
Table 6.
Adverse event profile of included studies.
| Study | Scale | Safety data | Effect estimates |
|---|---|---|---|
| Controlled trials | |||
| Sieradzan et al.30 | Physical adverse effects | All patients had postural fall in SBP in both off and on states n = 2 patients withdrawn after nabilone treatment, one due to vertigo and one due to symptomatic postural hypotension; n = 5 patients experienced other adverse effects including mild sedation, “floating sensation,” dizziness, hyperacusis, partial disorientation, visual hallucinations | No significant difference between placebo and nabilone groups in terms of postural fall in SBP |
| Carroll et al.31 | Physical and psychological adverse events |
Physical (UTI, dry mouth, altered taste, MSK pain, diarrhea, constipation, nausea, dizzy) cannador: n = 18 placebo: n = 9 Psychological (drowsy, detached, paranoia, nightmares, confusion, forgetful) cannador: n = 20 placebo: n = 6 |
All mild adverse events were ameliorated by dose reduction, no serious adverse events |
| Mesnage et al.32 | Ø | Ø | Anandamide well tolerated without marked adverse events |
| Chagas et al.33 | Udvalg for Kliniske Undersogelser (UKU) side effect rating scale | Ø | No significant side effects recorded in any of the groups assessed with the UKU or through verbal reports |
| de Faria et al.34 | Ø | Ø | No side effects reported during or after sessions |
| Peball et al.35 | Ø | Insomnia n = 2 in both arms; URTI n = 3 in placebo arms; only pain n = 1 in nabilone arm, n = 2 in placebo arm; Fall n = 1 in both arms; Syncope n = 2 in placebo arm only |
Overall incidences of all-cause AEs were similar between groups No severe AE occurred in any patient during the study and follow-up period |
| Observational studies | |||
| Venderova et al.36 | Ø | n = 3 discontinued using cannabis because of unspecified side effects | Ø |
| Zuardi et al.37 | Ø | Ø | No adverse event observed during treatment with CBD |
| Lotan et al.38 | NDARC medical cannabis survey adverse effects | Hypoglycemia resolved with glucose intake n = 1 dizziness n = 1 | No significant adverse effects during study Long-term adverse effects reported: somnolence, drowsiness, palpitations, bad taste |
| Finseth et al.29 | Ø | Ø | No one reported worsening of symptoms or side effects |
| Shohet et al.39 | Ø | Ø | Ø |
| Balash et al.40 | Physical and psychotropic adverse effects |
Any adverse effects
n = 28 (59.6%) Any psychotropic effects n = 18 (38.3%) Confusion n = 8 (17%) Anxiety n = 8 (17%) Hallucinations n = 8 (17%) Short-term amnesia n = 3 (6.5%) Psychosis n = 1 (2.1%) Any physical adverse effects n = 21 (44.7%) Cough n = 15 (4.7%) Dyspnea n = 2 (12.8%) Dizziness n = 6 (12.8%) Unsteadiness n = 7 (15.6%) n = 12/61 patients (7/14 excluded and 5/47 included individuals, 19.7%) stopped using MC because of ineffectiveness or intolerable adverse effects |
No hospitalizations or severe adverse effects were reported |
| Kindred et al.41 | Ø | Ø | Ø |
| Micheli et al.28 | Ø |
Any adverse effects
n = 18 (14.9%) Drowsiness n = 6 (4.95%) Motor worsening n = 4 (3.3%) Hallucinations n = 2 (1.65%) palpitations abdominal pain weight loss |
No serious adverse effects reported |
| Yenilmez et al.42 | Ø |
Any side effects
n = 41 (36.3%) Fatigue n = 22 (54%) Tachycardia n = 2 (5%) Nausea/vomiting n = 2 (5%) Ravenous appetite n = 9 (22%) Hallucinations n = 4 (10%) Visual disorder n = 5 (12%) Headache n = 4 (10%) Other n = 4 (10%) |
Overall tolerance not significantly different between THC and CBD, p = 0.06 Significantly different occurrence of side effects between THC and CBD [12/22 (54.5%) versus 7/37 (18.9%), p = 0.01] |
SBP, systolic blood pressure; UTI, urinary tract infection; MSK, musculoskeletal; AE, adverse effect; NDARC, national drug and alcohol research centre; MC, medical cannabis; CBD, cannabidiol; THC, tetrahydrocannabinol.
The most commonly reported side effects across all studies were fatigue, unsteadiness and dizziness. Only 15 (0.66%) of the 2266 participants enrolled in studies examining safety data reported stopping MC during the study period due to intolerable side effects or ineffectiveness. These culprit side effects were unspecified. Among the RCTs, only two participants were withdrawn from the interventional arm of the study—one for vertigo, and the other for symptomatic postural hypotension.30 In summary, cannabis and its derivatives were largely well tolerated across studies, although these studies generally relied on patient self-report and assessed side effects over relatively short (⩽4 weeks) durations of use.
Funding sources
Some 53% of the 15 included studies had no funding support or conflicts of interest to declare. Four studies disclosed funding support from government, education or research institutions.34–37 Two studies received support in the form of cannabis products from pharmaceutical companies—the studies reported no significant improvement37 and worsening35 of motor symptoms associated with cannabis use. Three questionnaire-based observational studies had authors who were consultants, received conference or lecture fees from pharmaceutical companies, or were employees of the pharmaceutical industry.40–42 Although a minority of studies (n = 7, 47%) do report conflicts of interest, overall, the data included in this systematic review do not appear to be significantly influenced by these.
Discussion
The primary objective of this systematic review and meta-analysis was to identify the currently available evidence for the efficacy and safety of cannabis and its derivatives for the treatment of motor symptoms and dyskinesia in adults with PD. To our knowledge this is the first systematic review and meta-analysis to include and assess RCTs, observational non-randomized interventional studies and observational questionnaire-based studies, specifically for motor symptom efficacy and safety. Previous systematic reviews either covered cannabinoids for PD among other neurodegenerative and movement disorders18,44,45 or evaluated efficacy across more broad domains without combining outcome data.46 As such, this review enhances our knowledge by evaluating and collating particular efficacy and safety endpoints among all available RCTs and real-world observational studies in the PD population. This systematic review establishes that the current evidence for the use of cannabis and its derivatives for the alleviation of motor symptoms in PD is heterogeneous and that there is a dearth of robust placebo-controlled studies.
Variable MC use duration, outcome measures, MC formulations, doses and concomitant treatments
Treatment durations among interventional studies were widely variable, ranging from single-dose administration to a maximum of 6 weeks. Additionally, no studies evaluated motor function in follow-up after cessation of the intervention. Consequently, despite improvement in motor outcomes among non-randomized observational studies, we are unable to comment on persistent benefit and long-term effectiveness in any of the included studies. Further, four of the studies that demonstrated favorable improvement in PD motor symptoms using MC without achieving statistical significance, and three of the studies that demonstrated statistically significant improvement, reported these in the context of single or one-time split-dose administration. As such, the utility of all these results in the evaluation of cannabinoids as adjunctive treatment options in PD is limited.
Furthermore, the lack of data on the development of tolerance with chronic MC use in the context of PD is not surprising given the relatively short durations of use among included studies. Tolerance, with its gradual dose escalation to maintain effect, remains an area of concern in the context of chronic MC for neurological diseases.47 However, pre-clinical studies have demonstrated that chronic treatment with certain cannabinoids such as CBD does not produce tolerance.48,49 Indeed, tolerance was not observed in the setting of nabiximols, which contains a 1:1 mixture of CBD and Δ9-THC, in clinical studies in the multiple sclerosis patient population.50,51 Long-term studies examining MC use for PD would be helpful to elucidate the extent of tolerance development with various cannabinoids.
Although Balash et al.40 do demonstrate statistically significant benefit over the longest duration of MC use identified in this study, their method of outcome measurement relied entirely on patient self-reports as opposed to observational scores.40 In fact, although the UPDRS was most commonly used to measure motor function and dyskinesia, the overall heterogeneity of outcome measures across studies was notable, with eight of the 15 studies using other scores or subjective self-reports of improvement as metrics. Given these inconsistencies within the available body of evidence, results reported across studies must be interpreted with caution. The results are further muddled by highly heterogeneous doses and formulations of MC across studies that also render clinical decision-making based on the current body of evidence difficult. Moreover, none of the studies were conducted in a Canadian setting—unsurprisingly, the formulations studied, with the exception of nabilone, are not eligible for prescription drug coverage in Canada, limiting the application of these studies in a Canadian context.
Moreover, while a majority (80%) of studies mention participants’ use of concomitant standard PD therapies, a minority (33%) of these describe the drugs used in detail. Given cannabinoid therapies are being explored as adjuvant therapies in these studies, more descriptive data around concomitant treatments are necessary to elucidate the benefit derived from their use. Additionally, the included studies do not specifically comment on concomitant therapies when drawing conclusions either in support of, or against, the use of cannabinoids for PD motor symptoms. Our initial search did identify a single open-label pilot study abstract (not included given lack of full text) that demonstrated significant improvement in a PD quality-of-life score, which encompasses functional mobility as a domain, specifically when CBD was added to usual PD treatment. However, the usual PD treatment was again not described in any further detail.52 We suggest that variation in baseline PD treatment may be a factor contributing to the conflicting results to support cannabis and its derivatives in PD. Future studies should examine baseline concomitant PD treatment in more detail to determine whether adjuvant benefit, or lack thereof, can be persistently demonstrated among the variety of standard treatments currently available.
Evaluation of MC early in the PD course is understudied
Cannabis and its derivatives were consistently evaluated in patients who had PD for at least 6 years prior to their enrollment in the studies (mean 10.5 years). This may in part be due to patient recruitment largely occurring in movement disorder clinics or through PD patient societies wherein patients are well differentiated. This merits special consideration in the evaluation of evidence for the use of MC in PD because the timing of administration did appear to influence the effectiveness of cannabinoids for neuroprotection in pre-clinical studies.12 In a rat model, although CBD did have an effect when treatment was initiated immediately after the lesion to model PD was induced, it did not reverse dopaminergic injury when initiated 1 week after lesion induction.12 This suggests cannabinoids may play a more prominent role in preventing disease progression in earlier stages of moderate disease or in individuals at risk of developing PD, with more limited potential to alleviate disease progression in advanced stages of PD.12 As such, future trials for MC evaluation should specifically recruit patients earlier in their PD disease course and include newly diagnosed patients to more holistically evaluate the potential efficacy of cannabinoids.
Although well tolerated overall, the side effects of MC use remain concerning given the demographic profile of PD patients
The most commonly reported side effects were fatigue, unsteadiness and dizziness, and the average age of participants enrolled in studies examining the secondary outcome was 70.4 years. Although these were reported in a minority of the total patient population included in the 12 studies examining safety data, these particular side effects are concerning given the advanced average age of the PD population. MC use in geriatric populations warrants further consideration of factors such as frailty and pill burden. Further studies should include metrics for frailty and concomitant overall pill burden that extends beyond PD medications. Additionally, psychotropic side effects are also of concern in a geriatric population when evaluating MC. Formulations vary widely with respect to dose and their proportion of Δ9-THC and CBD. Many of the included observational studies did not describe the intervention’s Δ9-THC proportion. Future evaluation of MC in PD warrants explicit measurements of Δ9-THC:CBD ratios in the interventions being tested.
No RCTs with low risk of bias
The quality of all available RCTs evaluating cannabinoids for motor symptoms in PD was poor with respect to risk of bias. While a dearth of high-quality evidence does not exclude the possibility of benefit, changes in clinical practice cannot be recommended based on the current available literature.
Strengths and limitations
This review is the first systematic review and meta-analysis of the current literature on cannabinoids for the treatment of PD motor symptoms that closely examines RCT, non-randomized intervention study and retrospective observational data. Further, previous systematic reviews only provided narrative summaries of individual studies examining the therapeutic potential of MC in PD. Ours is the first to synthesize results across studies both quantitatively and descriptively.
We acknowledge several limitations in this study. Limiting the scope of the review to efficacy with respect to motor symptoms meant that potentially important safety data from studies that examined non-motor symptoms may not have been captured in this review. We elected to focus on motor symptoms of PD, given the heterogeneity of previous work. Additionally, also due to the heterogeneity of the included studies, the data were unable to be meta-analyzed for the outcomes of dyskinesia and safety. Finally, as is the nature of review publications, the validity of the results is limited by publication bias.
Conclusion
Our review found insufficient evidence to support integration of MC into PD clinical practice for the treatment of motor symptoms, validating the results of previously published reviews. Most of the available evidence was assessed to have high risk of bias. We have sufficient evidence from retrospective questionnaire-based studies to establish subjective symptom alleviation and interest among PD patients in using MC. However, before cannabinoids can be readily integrated into PD treatment frameworks, more robust evidence in the form of high-quality RCTs with objective symptom assessment is required. Placebo-controlled investigations should be conducted with larger sample sizes, over longer durations of intervention, with consistent use of standardized tools such as the UPDRS as opposed to self-reports for outcome measurement. These studies should look at tolerance and the role of MC as an adjuvant treatment to standard parkinsonian therapies among patients with more variable disease course to elucidate the effectiveness of various formulations of MC for the treatment of motor PD symptoms.
Supplemental Material
Supplemental material, sj-jpg-1-tan-10.1177_17562864211018561 for Cannabis and its derivatives for the use of motor symptoms in Parkinson’s disease: a systematic review and meta-analysis by Susan J. Thanabalasingam, Brandan Ranjith, Robyn Jackson and Don Thiwanka Wijeratne in Therapeutic Advances in Neurological Disorders
Acknowledgments
None.
Footnotes
Author contributions: All authors were responsible for conceptualization, methodology, and revising the manuscript for critical intellectual content.
SJT had a substantial lead role in writing the original manuscript and consolidating edits for future drafts.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Susan J. Thanabalasingam  https://orcid.org/0000-0002-8948-1730
https://orcid.org/0000-0002-8948-1730
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Susan J. Thanabalasingam, Kingston Health Sciences Centre, Kingston, ON, Canada Faculty of Health Sciences, Queen’s University, Kingston, ON, Canada; Department of Medicine, Queen’s University, Kingston, ON, Canada.
Brandan Ranjith, Faculty of Health Sciences, Queen’s University, Kingston, ON, Canada.
Robyn Jackson, Kingston Health Sciences Centre, Kingston, ON, Canada; Faculty of Health Sciences, Queen’s University, Kingston, ON, Canada; Department of Medicine, Queen’s University, Kingston, ON, Canada.
Don Thiwanka Wijeratne, Division of General Internal Medicine, Queen’s University, Etherington Hall, Room 1018, 94 Stuart St., Kingston, ON K7L 3N6, Canada, 613 533-2056; Kingston Health Sciences Centre, Kingston, ON, Canada; Faculty of Health Sciences, Queen’s University, Kingston, ON, Canada; Department of Medicine, Queen’s University, Kingston, ON, Canada.
References
- 1. Kaplin AI, Williams M. How common are the ‘common’ neurologic disorders? Neurology 2007; 68: 326–337. [DOI] [PubMed] [Google Scholar]
- 2. Ray Dorsey E, Elbaz A, Nichols E, et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018; 17: 939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aymerich MS, Aso E, Abellanas MA, et al. Cannabinoid pharmacology/therapeutics in chronic degenerative disorders affecting the central nervous system. Biochem Pharmacol 2018; 157: 67–84. [DOI] [PubMed] [Google Scholar]
- 4. More SV, Choi D-K. Promising cannabinoid-based therapies for Parkinson’s disease: motor symptoms to neuroprotection. Mol Neurodegener 2015; 10: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bastide MF, Meissner WG, Picconi B, et al. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog Neurobiol 2015; 132: 96–168. [DOI] [PubMed] [Google Scholar]
- 6. Hurley MJ, Mash DC, Jenner P. Expression of cannabinoid CB1 receptor mRNA in basal ganglia of normal and parkinsonian human brain. J Neural Transm 2003; 110: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 7. Lastres-Becker I, Fernandez-Ruiz J. An overview of Parkinson’s disease and the cannabinoid system and possible benefits of cannabinoid-based treatments. Curr Med Chem 2006; 13: 3705–3718. [DOI] [PubMed] [Google Scholar]
- 8. Pisani A, Fezza F, Galati S, et al. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson’s disease patients. Ann Neurol 2005; 57: 777–779. [DOI] [PubMed] [Google Scholar]
- 9. Marchioni C, Santos-Lobato BL, Queiroz MEC, et al. Endocannabinoid levels in patients with Parkinson’s disease with and without levodopa-induced dyskinesias. J Neural Transm 2020; 127: 1359–1367. [DOI] [PubMed] [Google Scholar]
- 10. Fernandez-Ruiz J. The endocannabinoid system as a target for the treatment of motor dysfunction. Br J Pharmacol 2009; 156: 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lastres-Becker I, Molina-Holgado F, Ramos JA, et al. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson’s disease. Neurobiol Dis 2005; 19: 96–107. [DOI] [PubMed] [Google Scholar]
- 12. García-Arencibia M, González S, de Lago E, et al. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res 2007; 1134: 162–170. [DOI] [PubMed] [Google Scholar]
- 13. Brotchie JM. CB1 cannabinoid receptor signalling in Parkinson’s disease. Curr Opin Pharmacol 2003; 3: 54–61. [DOI] [PubMed] [Google Scholar]
- 14. Junior NCF, dos- Santos-Pereira M, Guimarães FS, et al. Cannabidiol and cannabinoid compounds as potential strategies for treating Parkinson’s disease and l-DOPA-induced dyskinesia. Neurotox Res 2020; 37: 12–29. [DOI] [PubMed] [Google Scholar]
- 15. Berlekamp D. Medical cannabis: pharmacy focus on treatment options for neurologic conditions. US Pharm 2015; 41: 45–49. [Google Scholar]
- 16. Allan GM, Jamil C, Danielle R, et al. Simplified guideline for prescribing medical cannabinoids in primary care. Can Fam Physician 2018; 64: 111–120. [PMC free article] [PubMed] [Google Scholar]
- 17. Catlow B, Sanchez-Ramos J. Cannabinoids for the treatment of movement disorders. Curr Treat Options Neurol 2015; 17: 1–18. [DOI] [PubMed] [Google Scholar]
- 18. Lim K, See YM, Lee J. A systematic review of the effectiveness of medical cannabis for psychiatric, movement and neurodegenerative disorders. Clin Psychopharmacol Neurosci 2017; 15: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marudkar M, Hands O, Baillon S, et al. Therapeutic potential of cannabinoids in neurodegenerative disorders: a selective review. Curr Pharm Des 2014; 20: 2218–2230. [DOI] [PubMed] [Google Scholar]
- 20. Iuvone T, Esposito G, De Filippis D, et al. Cannabidiol: a promising drug for neurodegenerative disorders? CNS Neurosci Ther 2009; 15: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corey S. Recent developments in the therapeutic potential of cannabinoids. P R Health Sci J 2005; 24: 19–26. [PubMed] [Google Scholar]
- 22. Nuthi M, Jagnarine DA, Suryadevara U, et al. Pros and cons of medical cannabis use by people with chronic brain disorders. Curr Neuropharmacol 2017; 15: 800–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Turner H, Chueh D, Ortiz T, et al. Cannabinoid therapeutics in Parkinson’s disease: promise and paradox. J Herbs Spices Med Plants 2017; 23: 231–248. [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JPT, Savović E, Page MJ, et al. Revised cochrane risk of bias tool for randomized trials (RoB 2.0): additional considerations for cluster-randomized trials. Cochrane Methods 2020; 1–6. [Google Scholar]
- 27. Modesti PA, Reboldi G, Cappuccio FP, et al. Cross sectional study Newcastle - Ottawa quality assessment scale. PLoS One 2016; 11: 1–2. [Google Scholar]
- 28. Micheli FE, Groppo J, Contartese ML, et al. Cannabis in patients with Parkinson’s disease in Argentina. A cross sectional study. Park Relat Disord 2020; 78: 66–67. [DOI] [PubMed] [Google Scholar]
- 29. Finseth TA, Hedeman JL, Brown RP, et al. Self-reported efficacy of cannabis and other complementary medicine modalities by Parkinson’s disease patients in Colorado. Evid Based Complement Alternat Med. Epub ahead of print 2 March 2015. DOI: 10.1155/2015/874849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sieradzan KA, Fox SH, Hill M, et al. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: a pilot study. Neurology 2001; 57: 2108–2111. [DOI] [PubMed] [Google Scholar]
- 31. Carroll CB, Bain PG, Teare L, et al. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology 2004; 63: 1245–1250. [DOI] [PubMed] [Google Scholar]
- 32. Mesnage V, Houeto JL, Bonnet AM, et al. Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin Neuropharmacol 2004; 27: 108–110. [DOI] [PubMed] [Google Scholar]
- 33. Chagas MHN, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol 2014; 28: 1088–1098. [DOI] [PubMed] [Google Scholar]
- 34. de Faria SM, de Morais Fabrício D, Tumas V, et al. Effects of acute cannabidiol administration on anxiety and tremors induced by a simulated public speaking test in patients with Parkinson’s disease. J Psychopharmacol 2020; 34: 189–196. [DOI] [PubMed] [Google Scholar]
- 35. Peball M, Krismer F, Knaus HG, et al. Non-motor symptoms in Parkinson’s disease are reduced by nabilone. Ann Neurol 2020; 88: 712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Venderova K, Ruzicka E, Vorisek V, et al. Survey on cannabis use in Parkinson’s disease: subjective improvement of motor symptoms. Mov Disord 2004; 19: 1102–1106. [DOI] [PubMed] [Google Scholar]
- 37. Zuardi AW, Crippa JAS, Hallak JEC, et al. Cannabidiol for the treatment of psychosis in Parkinson’s disease. Eur Neuropsychopharmacol 2008; 18: S417–S418. [Google Scholar]
- 38. Lotan I, Treves TA, Roditi Y, et al. Cannabis (Medical Marijuana) treatment for motor and non – motor symptoms of Parkinson disease: an open-label observational study. Clin Neuropharmacol 2014; 37: 41–44. [DOI] [PubMed] [Google Scholar]
- 39. Shohet A, Roizen N, Roditi Y, et al. Effect of medical cannabis on thermal quantitative measurements of pain in patients with Parkinson’s disease. Eur J Pain. Epub ahead of print 10 October 2016. DOI: 10.1002/ejp.942. [DOI] [PubMed] [Google Scholar]
- 40. Balash Y, Bar-Lev Schleider L, Korczyn AD, et al. Medical cannabis in Parkinson disease: real-life patients’ experience. Clin Neuropharmacol 2017; 40: 268–272. [DOI] [PubMed] [Google Scholar]
- 41. Kindred JH, Li K, Ketelhut NB, et al. Cannabis use in people with Parkinson’s disease and multiple sclerosis: a web-based investigation. Complement Ther Med 2017; 33: 99–104. [DOI] [PubMed] [Google Scholar]
- 42. Yenilmez F, Fründt O, Hidding U, et al. Cannabis in Parkinson’s disease: the patients’ view. J Parkinsons Dis 2021; 11: 1–13. [DOI] [PubMed] [Google Scholar]
- 43. Perlmutter JS. Assessment of Parkinson disease manifestations. Curr Protoc Neurosci. October 2009. DOI: 10.1002/0471142301.ns1001s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Andrzejewski K, Barbano R, Mink J. Cannabinoids in the treatment of movement disorders: a systematic review of case series and clinical trials. Basal Ganglia 2016; 6: 173–181. [Google Scholar]
- 45. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014; 82: 1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bougea A, Koros C, Simitsi AM, et al. Medical cannabis as an alternative therapeutics for Parkinsons’ disease: systematic review. Complement Ther Clin Pract 2020; 39: 101154. [DOI] [PubMed] [Google Scholar]
- 47. Benbadis SR, Sanchez-Ramos J, Bozorg A, et al. Medical Marijuana in neurology. Expert Rev Neurother 2014; 14: 1453–1465. [DOI] [PubMed] [Google Scholar]
- 48. Hayakawa K, Mishima K, Nozako M, et al. Repeated treatment with cannabidiol but not Δ9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance. Neuropharmacology 2007; 52: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 49. González S, Cebeira M, Fernández-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav 2005; 81: 300–318. [DOI] [PubMed] [Google Scholar]
- 50. Barnes MP. Sativex®: Clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. Expert Opin Pharmacother 2006; 7: 607–615. [DOI] [PubMed] [Google Scholar]
- 51. Wade D. Evaluation of the safety and tolerability profile of Sativex®: is it reassuring enough? Expert Rev Neurother 2012; 12: 9–14. [DOI] [PubMed] [Google Scholar]
- 52. Chagas MHN, Penna-Pereira M, Sobreira- Neto M, et al. Cannabidiol add-on usual treatment improves the outcome of patients with Parkinson’s disease. Eur Neuropsychopharm 2013; 23: S546–S547. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-tan-10.1177_17562864211018561 for Cannabis and its derivatives for the use of motor symptoms in Parkinson’s disease: a systematic review and meta-analysis by Susan J. Thanabalasingam, Brandan Ranjith, Robyn Jackson and Don Thiwanka Wijeratne in Therapeutic Advances in Neurological Disorders