Abstract
Background:
Contradictory evidence exists for association of hyperuricemia and kidney function. To investigate the association of hyperuricemia and kidney function decline (hyperuricemia question) and effect of urate-lowering therapies (ULTs) on kidney function (ULT question), we performed a systematic review and meta-analysis.
Methods:
MEDLINE, Embase, Cochrane Central Register of Controlled Trials, and CINAHL were searched from inception to July 2020. We selected observational studies for the hyperuricemia question and controlled trials for the ULT question. Two investigators independently assessed study eligibility and abstracted the data. Risk of bias was assessed using the Newcastle–Ottawa Scale and Cochrane risk of bias tool. Meta-analysis was done using the inverse variance method and random effect model. We estimated odds ratio (OR), hazard ratio (HR), risk ratio (RR), and the mean difference (MD). Evidence certainty was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.
Results:
Of 12,037 studies screened, 131 studies with 3,414,226 patients were included. Hyperuricemia was associated with a significant risk of rapid estimated glomerula filtration rate (eGFR) decline ⩾3 ml/min per 1.73 m2 per year (OR 1.38, 95% CI 1.20–1.59; low certainty), albuminuria (OR/HR 1.94, 95% CI 1.34–2.79; very low certainty), chronic kidney disease (OR/HR 2.13, 95% CI 1.74–2.61; very low certainty), and kidney failure (HR 1.53, 95% CI 1.18–1.99; very low certainty). Compared with control, ULT use for ⩾1 year was associated with significantly more improved eGFR (MD 1.81 ml/min per 1.73 m2, 95% CI 0.26–3.35; very low certainty), serum creatinine (MD −0.33 mg/dl, 95% CI −0.47 to −0.19; low certainty), and proteinuria (MD −5.44 mg/day, 95% CI −8.49 to −2.39; low certainty), but no difference in kidney failure.
Conclusion:
Hyperuricemia is associated with worsening eGFR, albuminuria, chronic kidney disease, and kidney failure. ULT use for ⩾1 year may improve kidney function.
Registration:
The protocol was registered at PROSPERO database, CRD42015013859.
Keywords: crystal arthropathies, gout, hyperuricemia, kidney
Introduction
Hyperuricemia, generally defined as serum urate (sUA) level above 6 mg/dl in the literature,1 has a prevalence of 21% in the United States.2 It is associated with a higher risk of hypertension, coronary artery disease, and stroke.3 Hyperuricemia is implicated in the pathophysiology of kidney disease,4,5 making clinical investigations an active research interest.
Previous meta-analyses showed that hyperuricemia is a risk factor for the development of stage 3 chronic kidney disease (CKD) and incident kidney disease [CKD, end-stage kidney disease (ESKD), albuminuria, or elevated serum creatinine composite].6,7 To the best of the authors’ knowledge, no systematic review to date has assessed whether hyperuricemia is associated with early markers of kidney function decline, that is, estimated glomerular filtration rate (eGFR) or albuminuria as a separate outcome. Most focused on a composite CKD outcome.
Systematic reviews examining the effect of urate-lowering therapy (ULT) on kidney function provided contradictory results.8–18 Systematic reviews reported variable findings: improvement in eGFR report in some9,13–15,17,18 but not in others;8,11,12 improvement in serum creatinine reported in some8,9,11,14,15,17,18 but not others;12,13 improvement in proteinuria/albuminuria reported in one16 but not in others;8,9,11,15,18 and reduction in kidney failure risk reported in some14,16,18 but not in other systematic reviews.8,15 Two recently published randomized controlled trials (RCTs) showed no significant preservation of kidney function with the use of ULT,19,20 which contradicts findings from the previous RCTs. Therefore, an updated systematic review is needed to answer these important questions. Questions also exist as to whether kidney function preservation varies by the type of ULT or the duration of ULT use.10
The increasing prevalence of hyperuricemia in the general population,2,21 the contradictory nature of the available evidence, and the availability of new data19,20 were the key motivations for conducting this systematic review. A robust evidence base can help to develop evidence-based treatment guidelines, as pointed out in the 2016 European League Against Rheumatism treatment guidelines for gout.22 Therefore, our aim was to conduct a systematic review and meta-analysis to examine the effect of hyperuricemia on kidney function and the effect of ULT use on kidney function.
Methods
Data sources and searches
We performed this systematic review and meta-analysis according to the Cochrane Handbook for Intervention Reviews and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guidelines.23,24 An expert Cochrane librarian (CMH) searched major electronic databases (PubMed, Embase, CINAHL, and Cochrane Central Register of Controlled Trials, via Cochrane Library) from inception to July 2020 (Supplemental material Appendix 1 online). We also searched the bibliography of identified reports and review articles for additional references. We considered all full published reports as potentially eligible studies irrespective of the language, since the inclusion of abstracts can provide inconsistent results.25 We registered study protocol in the PROSPERO database (http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015013859).
Study selection
To assess the effect of hyperuricemia on kidney function, we included observational studies comparing kidney function between hyperuricemic and normouricemic patients, with or without underlying kidney disease. Cohorts non-representative of the general population with infectious, autoimmune glomerulopathies, or polycystic kidney diseases were excluded. We included studies with a sample size of at least 100 subjects,26,27 hyperuricemia defined as sUA >5.5 in men and >4.5 in women. Studies with no description of uric acid levels in the patient population and unknown follow-up duration were excluded (Supplemental Appendix 2c). For assessing the effect of ULT on kidney function, we considered controlled trials evaluating the effect of ULT on kidney function in patients with or without underlying kidney disease, comparing ULT with control (placebo, no treatment, or usual care) or another ULT.
Two investigators (GS and AD) independently screened all titles/abstracts and full texts to identify relevant articles. Any disagreement was resolved by consensus between abstractors and by consulting the senior author (JAS).
Data extraction and quality assessment
Three authors (GS, AD, NN) independently abstracted data using Microsoft Excel© (Redmond, WA, USA) and assessed risk of bias and certainty of evidence. Non-English studies were translated before data abstraction. When necessary, we contacted the authors for additional information. We abstracted data on study characteristics and estimates of effects (unadjusted, age/gender and multivariable-adjusted risk ratio, odds ratio, and hazard ratios) for observational studies and study outcomes, including mean and standard deviation for outcomes at pre-specified time points for all studies, using a structured, pre-piloted, data abstraction form (Supplemental Appendix 2). We used the Newcastle–Ottawa scale28 and the Cochrane risk of bias tool29 to assess the quality of observational studies and randomized trials, respectively (Supplemental Appendix 3). We rated certainty (or quality or strength) of evidence as high, moderate, low, or very low as per the GRADE method by using the GRADE handbook and GRADEpro Guideline Development Tool© (McMaster University).
Data synthesis and analysis
The primary outcomes for examining the effect of hyperuricemia on kidney function (the hyperuricemia question) were new-onset stage 3 CKD (eGFR <60 ml/min per 1.73 m2 and albuminuria), composite renal failure (eGFR to <15 ml/min per 1.73 m2, renal replacement therapy, eGFR decline >50% or doubling of serum creatinine), new-onset albuminuria (>30 mg/day or albumin–creatinine ratio >30 mg/g creatinine), or rapid decline of eGFR (⩾3 ml/min per 1.73 m2/year) (see Supplemental Appendix 4 for definitions). For the ULT question, primary outcomes were the change in eGFR/creatinine clearance, the change in serum creatinine levels, kidney failure events (%people with reduction of eGFR to <15 ml/min per 1.73 m2 or decline in eGFR > 50% or doubling of serum creatinine or requiring dialysis), and the change in proteinuria/albuminuria [urine albumin–creatinine ratio (mg/g)]. Albumin–creatinine ratio (mg/g) was converted into 24 h urine albumin (mg/day) with a conversion factor of 1.30 We combined albuminuria with proteinuria in our main analysis for ULT question.31,32 Secondary outcomes for the ULT question were serum uric acid, serum cystatin C, serum fibrinogen, blood pressure, adverse events, and death events.
We used the inverse variance method, random effect as the main model. Effect estimate measures calculated pooled estimated odds ratio (OR), hazard ratio (HR), risk ratio (RR), or mean difference (MD). We calculated the 95% confidence interval (CI) and produced forest plots using the RevMan 5.3 software.33 Heterogeneity was assessed by I2 and Cochrane Q test for each pooled analysis.34 We used the IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY, USA) for Egger’s regression test. Summary of findings graphs were created using GraphPad Prism version 8.0.1 for Windows, GraphPad Software, San Diego, California, USA, www.graphpad.com.
We separately analyzed studies of hyperuricemia that provided unadjusted versus adjusted estimates. We also analyzed cross-sectional studies and longitudinal studies separately, since the confidence in findings and the interpretation of evidence is likely different by the study type. We pooled OR, HRs, and RRs, but also examined studies separately for each analysis as applicable.35 Follow-up duration-adjusted effect estimates were estimated by stratified analysis. Since the study duration differed between studies, based on the available data, we developed duration-adjusted estimates of overall effects for each outcome. To ensure robustness of inferences with respect to analysis, we developed stratified estimates to control for the variation in durations. Each stratum was constructed using studies of similar durations. We constructed four strata of study duration with 0–4, 4–8, 8–12, and >12 years. The cut points were selected so that each stratum has at least three studies and to have the maximum number of studies with close duration times, based on the distribution of study duration. To estimate the stratified duration adjusted OR, we used two methods: method 1 constructs the overall stratified estimate by combining the stratum estimates of ORs proportional to the size of each stratum. The second method is the common method of estimating log of ORs within each stratum and then exponentiating the combined estimated log ORs (combined proportional to sizes). The within strata estimates were weighted mean of ORs (method 1) and weighted mean of log ORs (method 2), where the weight of the reported OR of each study was the study precision, which is the inverse of the study reported variance (standard error-squared) of the estimated ORs or log ORs. Note that the standard error of the combined estimate in the common method (method 2) uses an approximation formula, method 1 provided a confirmatory alternative set of estimates. Note that within strata estimates are based on studies of similar durations. We considered, but after discussion decided against, performing a simple per year analysis using regression, which assumes a linear time effect on the effect, and would incorrectly penalize studies with longer durations.
For the ULT question, separate analyses based on duration and type of ULT were conducted. For all outcomes, except adverse events, mean differences were pooled together. For some outcomes, changes from baseline scores were pooled with mean differences, as recommended.36 For adverse events, we pooled the number of events from each study to obtain RRs.
Results
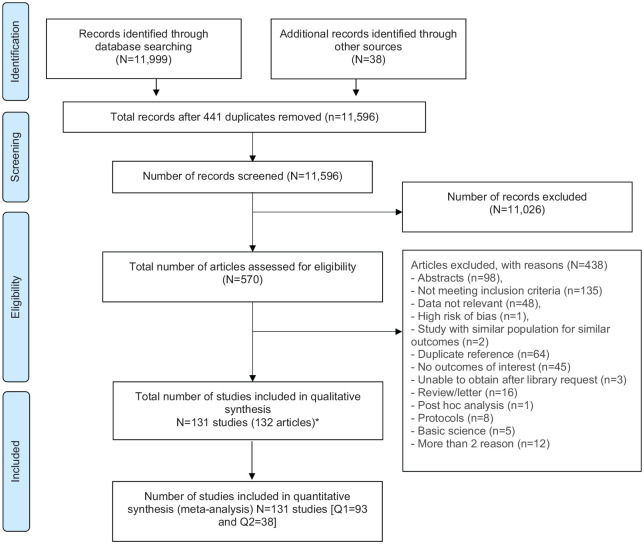
Of the 12,037 studies screened, we reviewed full texts for 570 potentially eligible studies and included 131 studies in this systematic review (Figure 1). Of these, 93 studies, with 3,408,787 patients, qualified for the hyperuricemia question and 38 studies, with 5439 patients, qualified for the ULT question. All studies except four studies of plasma/blood urate levels37–40 assessed sUA levels; 11 studies did not specify plasma versus serum.41–51 All studies were in the English language except four studies in Chinese,39,52–54 which were translated and abstracted.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart for study selection.
*Two reports from one study: Sezai 2013 and Sezai 2015.
Hyperuricemia and the risk of kidney disease
For the hyperuricemia question, study follow-up duration varied from 6 months to 31 years. The sUA cut-off threshold for hyperuricemia ranged from 5.61 to 8.50 mg/dl in men and 4.60–7.81 mg/dl in women among studies (Supplemental Appendix 2).
Fifty-nine studies assessed new-onset stage 3 or more CKD; others examined kidney failure (n = 24), albuminuria (n = 21), rapid decline of eGFR/year (n = 17), serum creatinine (n = 5), or decline in creatinine clearance (n = 1; detailed definitions in Supplemental Appendix 4). Cross-sectional studies were analyzed separately from the longitudinal studies.
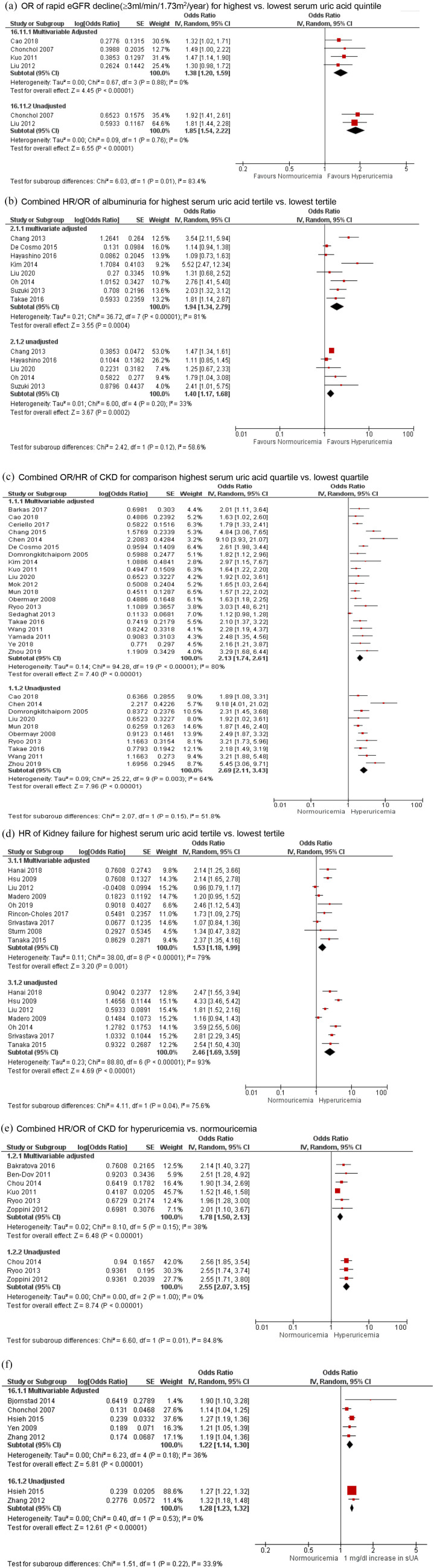
In the main analysis of the longitudinal studies (n = 68 studies; n = 3,181,286 people), compared with the lowest respective category, the risk estimates for the highest sUA tertile/quartile/quintile were as follows: rapid eGFR decline (⩾3 ml/min per 1.73 m2 per year), OR 1.38 (95% CI 1.20–1.59, p < 0.00001; low certainty evidence, I2 of 0%); albuminuria, OR/HR 1.94 (95% CI 1.34–2.79, p = 0.0004; very low certainty evidence, substantial heterogeneity, I2 of 81%); new-onset of CKD stage 3, OR/HR 2.13 (95% CI 1.74–2.61, p < 0.00001; very low certainty evidence, substantial heterogeneity, I2 of 80%); and kidney failure, HR 1.53 (95% CI 1.18–1.99, p = 0.001; very low certainty evidence, substantial heterogeneity, I2 of 79%) [Figure 2(a) to (d); Table 1]. Compared with normouricemia, hyperuricemia was associated with increased risk of development of new-onset albuminuria (low certainty evidence), CKD stage 3 (very low certainty evidence), and kidney failure (low certainty evidence) [Figure 2(e), Table 1; Supplemental Appendix 5a].
Figure 2.
Hyperuricemia and multivariable-adjusted and unadjusted risk of chronic kidney disease, end stage kidney disease, albuminuria and estimated glomerular filtration rate decline in longitudinal analysis. (a) OR of rapid eGFR decline (⩾3 ml/min/per 1.73 m2/year) for highest versus lowest serum uric acid quintile. (b) Combined HR/OR of albuminuria for highest serum uric acid tertile versus lowest tertile. (c) Combined OR/HR of CKD for comparison of highest serum uric acid quartile versus lowest quartile. (d) HR of kidney failure for highest serum uric acid tertile versus lowest tertile. (e) Combined HR/OR of CKD for hyperuricemia versus normouricemia. (f) Combined HR/OR of rapid eGFR decline (⩾3 ml/min/per 1.73 m2/year) for every 1 mg/dl increase in serum urate.
CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; IV, inverse variance; OR, odds ratio; sUA, serum urate
Table 1.
Association of hyperuricemia with kidney outcomes (the hyperuricemia question).
| Comparisons | New onset stage 3 or more CKD | New onset albuminuria | Kidney failure | New onset rapid decline of eGFR (⩾3 ml/min per 1.73 m2 per year) |
|---|---|---|---|---|
| Highest versus lowest sUA tertile/quartile/quintile | sUA quartiles: OR/HR 2.13, 95% CI 1.74–2.61, p < 0.00001, I2 of 80%*§; very low certainty evidence (n = 20) | sUA tertiles: OR/HR 1.94, 95% CI 1.34–2.79, p = 0.0004, I2 of 81%; multivariable adjusted¶; very low certainty evidence (n = 8) | sUA tertiles: HR 1.53, 95% CI 1.18–1.99, p = 0.001, I2 of 79%; multivariable adjusted§; very low certainty evidence (n = 9) | sUA quintiles: OR 1.38, 95% CI 1.20– 1.59, p < 0.00001, I2 of 0%; multivariable adjusted§; low certainty evidence (n = 4) |
| Hyperuricemia versus normouricemia | OR/HR 1.78, 95% CI 1.50– 2.13, p < 0.00001, I2 of 38%; multivariable adjusted†¶; very low certainty evidence (n = 6) | OR 3.05, 95% CI 1.06–8.77, p = 0.04, I2 of 83%; multivariable adjusted¶; low certainty evidence (n = 2) | HR 2.08, 95% CI 1.23–3.51, p = 006, I2 of 91%; multivariable adjusted§; low certainty evidence (n = 6) | No data |
| Every 1 mg/dl increase of sUA | OR/HR 1.15, 95% CI 1.09–1.22, p < 0.00001, I2 of 68%; multivariable adjusted§; very low certainty evidence (n = 11) | OR/HR 1.30, 95% CI 1.10–1.53, p = 0.002, I2 of 63%‡§; very low certainty evidence (n = 5) | HR 1.07, 95% CI 1.01–1.12, p = 0.01, I2 of 81%; multivariable adjusted§; very low certainty evidence (n = 10) | OR 1.22, 95% CI 1.14–1.30, p < 0.00001, I2 of 36%; multivariable adjusted§; very low certainty evidence (n = 5) |
Separate analyses for OR and HR were significant for CKD stage 3 or higher.
Corresponding separate analyses for OR and HR were significant for CKD.
Separate analyses of OR and HR, which were significant for albuminuria.
Regardless of baseline kidney function.
Normal baseline kidney function.
CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; OR, odds ratio; sUA, serum urate.
Every 1 mg/dl increase of sUA was associated with an increased risk of the following outcomes: new-onset albuminuria, OR/HR 1.30 (95% CI 1.10–1.53, p = 0.002; very low certainty evidence, substantial heterogeneity, I2 of 63%); rapid eGFR decline per year, OR 1.22 (95% CI 1.14–1.30, p < 0.00001; very low certainty evidence, moderate heterogeneity, I2 of 36%); CKD stage 3, OR/HR 1.15 (95% CI 1.09–1.22, p < 0.00001; very low certainty evidence, substantial heterogeneity, I2 of 68%); and kidney failure, HR 1.07 (95% CI 1.01–1.12, p = 0.01; very low certainty evidence, substantial heterogeneity, I2 of 81%) (Supplemental Appendix 5b–e). Two retrospective studies found association of gout with new onset stage 3 CKD, HR 2.15 (95% CI 1.49–3.10, I2 of 100%).
After adjusting the analyses for follow-up duration, we noted minimal to no differences from original estimates (Table 1) and no change in interpretation of the results, further supporting the robustness of these analyses. The results of these sensitivity analyses are shown in Table 2.
Table 2.
Adjusted effect estimates of the association of hyperuricemia with kidney outcomes based on follow-up duration of studies for the hyperuricemia question.
| Comparisons | Outcomes | Original non stratified estimates* | Method 1: weighted stratified estimates of the original OR/HR†§ | Method 2: exponentiating the weighted stratified estimates of the log OR/HR‡§ |
|---|---|---|---|---|
| Highest versus lowest sUA tertile/quartile/quintile | New onset stage 3 or more CKD | OR/HR 2.13, 95% CI 1.74–2.61 | OR/HR 1.92, 95% CI 1.83–2.00 | OR/HR 2.70, 95% CI 2.60–2.81 |
| New-onset albuminuria | OR/HR 1.94, 95% CI 1.34–2.79 | OR/HR 1.69, 95% CI 1.53–1.85 | OR/HR 2.38, 95% CI 2.18–2.61 | |
| Kidney failure | HR 1.53, 95% CI 1.18–1.99 | HR 1.39, 95% CI 1.27–1.51 | HR 1.57, 95% CI 1.44–1.71 | |
| New-onset rapid decline of eGFR (⩾3 ml/min per 1.73 m2 per year) | OR1.38, 95% CI 1.20–1.59 | OR 1.38, 95% CI 1.23–1.52 | OR 1.38, 95% CI 1.24–1.53 | |
| Hyperuricemia versus normouricemia | New-onset stage 3 or more CKD | OR/HR 1.78, 95% CI 1.50–2.13 | OR/HR 1.99, 95% CI 1.80–2.17 | OR/HR 1.97, 95% CI 1.81–2.15 |
| New onset albuminuria | OR 3.05, 95% CI 1.06–8.77 | OR 3.54, 95% CI 3.10–3.98 | OR 3.08, 95% CI 2.59–3.67 | |
| Kidney failure | HR 2.08, 95% CI 1.23–3.51 | HR 3.47 95% CI 3.19–3.75 | HR 2.51, 95% CI 2.24–2.80 | |
| Every 1 mg/dl increase in sUA | New-onset stage 3 or more CKD | HR/OR 1.15, 95% CI 1.09–1.22 | OR/HR 1.15, 95% CI 1.11–1.18 | OR/HR 1.16, 95% CI 1.12–1.19 |
| New onset albuminuria | HR/OR 1.30, 95% CI, 1.10–1.53 | HR/OR 1.21, 95% CI 1.08–1.33 | HR/OR 1.24, 95% CI 1.11–1.38 | |
| Kidney failure | HR 1.07, 95% CI 1.01–1.12 | HR 1.05, 95% CI 1.02–1.07 | HR 1.06, 95% CI 1.03–1.08 | |
| New-onset rapid decline of eGFR (⩾3 ml/min/1.73 m2 per year) | OR 1.22, 95% CI 1.14–1.30 | OR 1.22, 95% CI 1.16–1.27 | OR 1.22, 95% CI 1.17–1.27 |
Effect estimates obtained after combining individual study estimates using random effect (generic inverse variance) method of meta-analysis.
Obtained after combining the stratum estimates of odds ratios proportional to the size of each stratum.
Obtained after calculating log of odds ratios within each stratum and then exponentiating the combined estimated log odds ratios (combined proportional to sizes).
Each stratum is based on follow-up duration of included studies in original non-stratified estimates (0–4, 4–8, 8–12, >12 years).
CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; OR, odds ratio; sUA, serum urate.
Stratified analyses by effect estimate (OR versus HR) (data available on request) did not show a significant difference from combined main analyses of pooled effect estimates (as above). Analyses of the cross-sectional data (n = 25 studies, n = 185,314 persons) (Supplemental Appendix 6) were significant for the association of hyperuricemia and CKD/albuminuria. The GRADE profiles including the certainty of evidence are described in Supplemental Appendix 7.
Effect of use of ULT on kidney disease
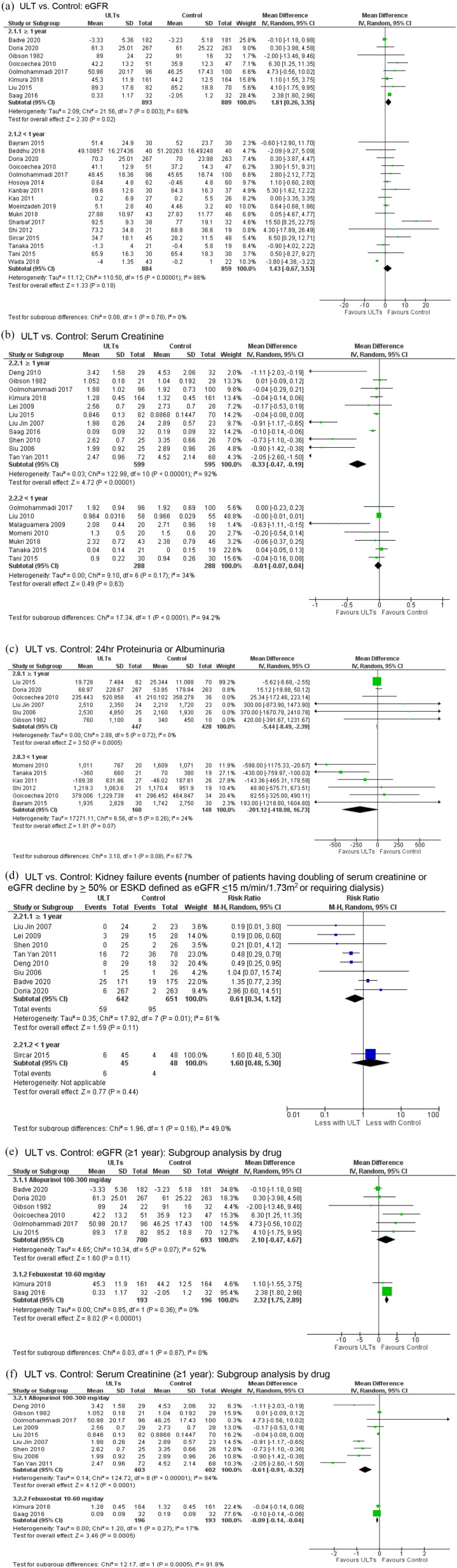
The combined main analysis for this question was based on 38 studies (n = 5439). The mean follow-up duration was 11.6 months. The sUA threshold for the definition of hyperuricemia being treated with ULT ranged from 7 mg/dl to 8 mg/dl. The intervention and the comparators are described in Table 3. Studies compared ULT with controls, where control arm included placebo, no active treatment, or usual care. Compared to control, ULT use for ⩾1 year was associated with significantly more improvement in three renal function estimands, including the eGFR (MD 1.81 ml/min per 1.73 m2, 95% CI 0.26–3.35, p = 0.02; very low certainty evidence, substantial heterogeneity, I2 of 68%) [Figure 3(a)], serum creatinine (MD −0.33 mg/dl, 95% CI −0.47 to −0.19, p < 0.00001; low certainty evidence, considerable heterogeneity, I2 of 92%) [Figure 3(b)], and proteinuria/albuminuria (MD −5.44 mg/day, 95% CI −8.49 to −2.39, p = 0.0005; low certainty evidence, I2 of 0%) [Figure 3(c)]. We found no significant reduction in the risk of kidney failure events (RR 0.61, 95% CI 0.34–1.12, p = 0.11; very low certainty evidence, substantial heterogeneity, I2 of 61%) [Figure 3(d)]. We found no significant difference in eGFR, serum creatinine, proteinuria/albuminuria, and kidney failure for ULT use <1 year versus control [Figure 3(a) to (d)].
Table 3.
Association of the ULT use with the kidney function (the ULT question).
| Comparisons¶ | Change in eGFR | Change in serum creatinine | Change in proteinuria or albuminuria | Kidney failure (patient numbers) |
|---|---|---|---|---|
| ULT versus control (⩾1 year of follow-up) | MD 1.81 ml/min per 1.73 m2, 95% CI 0.26–3.35, p = 0.02, I2 = 68%*; very low certainty evidence (n = 8) | MD −0.33 mg/dl, 95% CI −0.47 to −0.19, p < 0.00001, I2 of 92%*; low certainty evidence (n = 11) | MD −5.44 mg/day, 95% CI −8.49 to −2.39, p = 0.0005, I2 of 0%*; low certainty evidence (n = 6) | RR 0.61, 95% CI 0.34–1.12, p = 0.11, I2 of 61%‡; very low certainty evidence (n = 8) |
| ULT versus control (<1 year of follow-up) | MD 1.43 ml/min per 1.73 m2, 95% CI −0.67 to 3.53, p = 0.18, I2 of 86%*; very low certainty evidence (n = 16) | MD −0.01 mg/dl, 95% CI −0.07 to 0.04, p = 0.63, I2 of 34%*; low certainty evidence (n = 7) | MD −201.12 mg/day, 95% CI −418.98 to 16.73, p = 0.07, I2 of 24%‡; low certainty evidence (n = 6) | RR 1.60, 95% CI 0.48– 5.30, p = 0.44, I2 not applicable‡; low certainty evidence (n = 1) |
| Allopurinol 100–300 mg/day versus control (⩾1 year) | MD 2.10 ml/min per 1.73 m2, 95% CI −0.47 to 4.67, p = 0.11, I2 of 52%*; very low certainty evidence (n = 6) | MD −0.61 mg/dl, 95% CI −0.91 to −0.32, p < 0.0001, I2 = 94%*; low certainty evidence (n = 9) | MD −5.44 mg/day, 95% CI −8.49 to −2.39, p = 0.0005, I2 of 0%*; low certainty evidence (n = 6) | RR 0.61, 95% CI 0.34–1.12, p = 0.11, I2 of 61‡; low certainty evidence (n = 8) |
| Allopurinol 100–300 mg/day versus control (<1 year) | MD 2.68 ml/min per 1.73 m2, 95% CI 0.17 to 5.20, p = 0.04, I2 of 58%*; very low certainty evidence (n = 9) | MD −0.06 mg/dl, 95% CI −0.25 to 0.13, p = 0.52, I2 0%*; low certainty evidence (n = 2) | MD −113.32 mg/day, 95% CI −331.34 to 104.38, p = 0.31, I2 of 2%‡; low certainty evidence (n = 5) | No data |
| Febuxostat 10–60 mg/day versus control (⩾1 year) | MD 2.32 ml/min per 1.73 m2, 95% CI 1.75 to 2.89, p < 0.00001, I2 = 0%‡; moderate certainty evidence (n = 2) | MD −0.09 mg/dl, 95% CI −0.14 to −0.04, p = 0.0005, I2 = 17%‡; moderate certainty evidence (n = 2) | No data | No data |
| Febuxostat 10–60 mg/day versus control (<1 year) | MD 0.40 ml/min per 1.73 m2, 95% CI −2.18 to 2.97, p = 0.50, I2 of 17 %*; low certainty evidence (n = 5) | MD 0.01 mg/dl, 95% CI −0.06 to 0.09, p = 0.82, I2 of 0%*; low certainty evidence (n = 3) | MD −430.00 mg/day, 95% CI −759.97 to −100.03, I2 not applicable, p = 0.01‡; low certainty evidence (n = 1) | RR 1.60, 95% CI 0.48 to 5.30, p = 0.44, I2 not applicable‡; low certainty evidence (n = 1) |
| Allopurinol versus febuxostat (⩾1 year) | MD −0.20 ml/min per 1.73 m2, 95% CI −6.92 to 6.52, p = 0.95, I2 not applicable‡; low certainty evidence (n = 1) | No data | MD 65.40 mg/day, 95% CI −12.15 to 142.95, p = 0.10, I2 not applicable‡; low certainty evidence (n = 1) | RR 1.26, 95% CI 0.98–1.63, p = 0.07, n = 1, low certainty evidence |
| Allopurinol versus febuxostat (<1 year) | MD −0.89 ml/min per 1.73 m2, 95% CI −3.83 to 2.05, p = 0.55, I2 not applicable*; low certainty evidence (n = 1) | MD 0.12 mg/dl, 95% CI 0.00–0.24, p = 0.04, I2 not applicable; low certainty evidence (n = 1) | MD 100.70 mg/day, 95% CI 37.66–163.74, p = 0.002, I2 not applicable‡; low certainty evidence (n = 1) | No data |
| Febuxostat 10 mg/day versus topiroxostat 40 mg/day (<1 year) | MD 0.60 ml/min per 1.73 m2, 95 % CI −4.58 to 5.78, p = 0.82, I2 not applicable, very low certainty of evidence (n = 1) | No data | No data | No data |
| Topiroxostat (up to 160 mg/day) versus control (<1 year) | MD −1.42 ml/min per 1.73 m2, 95% CI −6.22 to 3.38, p = 0.56, I2 = 96%‡; low certainty evidence (n = 2) | No data | No data | No data |
| Benzbromarone 50 mg/day versus control (<1 year) | No data | MD 0.00 mg/dl, 95% CI −0.01 to 0.01, p = 0.73, I2 not applicable†; low certainty evidence (n = 1) | MD −4.20 mg/l, 95% CI −4.50 to −3.90, p < 0.00001, I2 not applicable†; low certainty evidence (n = 1)§ | No data |
| Rasburicase 4.5 mg single dose versus control (<1 year) | No data | MD −0.63 mg/dl, 95% CI −1.11 to −0.15, p = 0.01, I2 not applicable; low certainty evidence (n = 1) | No data | No data |
Regardless of baseline kidney function.
Normal baseline kidney function.
Abnormal kidney function.
Benzbromarone albuminuria data units in mg/l, could not be included in all ULT combined versus control (<1 year) proteinuria/albuminuria analysis.
¶The control group consisted of placebo (n=20), usual care (n=9), no active treatment (n=3), or a direct comparison of two ULTs (n=6).
CI, confidence interval; MD, mean difference; RR, risk ratio; ULT, urate-lowering therapy.
Figure 3.
The use of urate-lowering therapies (ULTs) and their association with kidney function (eGFR, serum creatinine, proteinuria/albuminuria and kidney failure events). (a) ULT versus control: eGFR. (b) ULT versus control: serum creatinine. (c) ULT versus control: 24 h proteinuria or albuminuria. (d) ULT versus control: kidney failure events (number of patients having doubling of serum creatinine or eGFR decline by ⩾50% or ESKD defined as eGFR ⩽ 15 m/min per 1.73 m2 or requiring dialysis). (e) ULT versus control: eGFR (⩾1 year): subgroup analysis by each urate-lowering drug. (f) ULT versus control: serum creatinine (⩾1 year): subgroup analysis by each urate-lowering drug.
CI, confidence interval; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; IV, inverse variance; ULT, urate-lowering therapy.
By type of ULT
The improvement in serum creatinine and proteinuria/albuminuria were significant for ⩾1 year of treatment with allopurinol. We found no evidence of significantly greater improvement in eGFR and a lower rate of kidney failure events with allopurinol ⩾1 year (Table 3). Significant greater improvement in eGFR and serum creatinine was noted with febuxostat use for ⩾1 year (albuminuria and kidney failure data were not available from any study). No significant greater improvement in serum creatinine was found for <1 year of allopurinol or febuxostat use as compared with control, except for the improvements in eGFR with allopurinol (MD 2.68 ml/min per 1.73 m2, 95% CI 0.17–5.20), and proteinuria/albuminuria with febuxostat (MD −430 mg/day, 95% CI −760 to −100) (Table 3). Most of the evidence was of low or very low certainty, except moderate low certainty evidence for febuxostat use for ⩾1 year versus control.Head-to-head comparison of allopurinol and febuxostat use for ⩾1 year in one study (61 patients) showed no significant difference in eGFR (MD −0.20 ml/min/ per 1.72 m2, 95% CI −6.92 to 6.52) or albuminuria (MD 65.40 mg/day, 95% CI −12.15 to 143) (Supplemental Appendix 8). Similarly, no difference in eGFR at 6 months was found in head-to-head comparison between febuxostat and topiroxostat (Table 3). Most of the evidence was low or very low certainty evidence.
Secondary outcomes
Compared with control, ULT use was associated with improved sUA, cystatin C, and fibrinogen levels (Supplemental Appendix 9). Improvement in systolic blood pressure was significant in ULT group compared with control, whereas no significant improvement in diastolic blood pressure was found regardless of duration. Total adverse events were not different between ULT and the control group. The result of detailed analyses are further summarized in Supplemental Appendix 9. The GRADE profiles or the quality of evidence is described in Supplemental Appendix 10.
Summary of findings
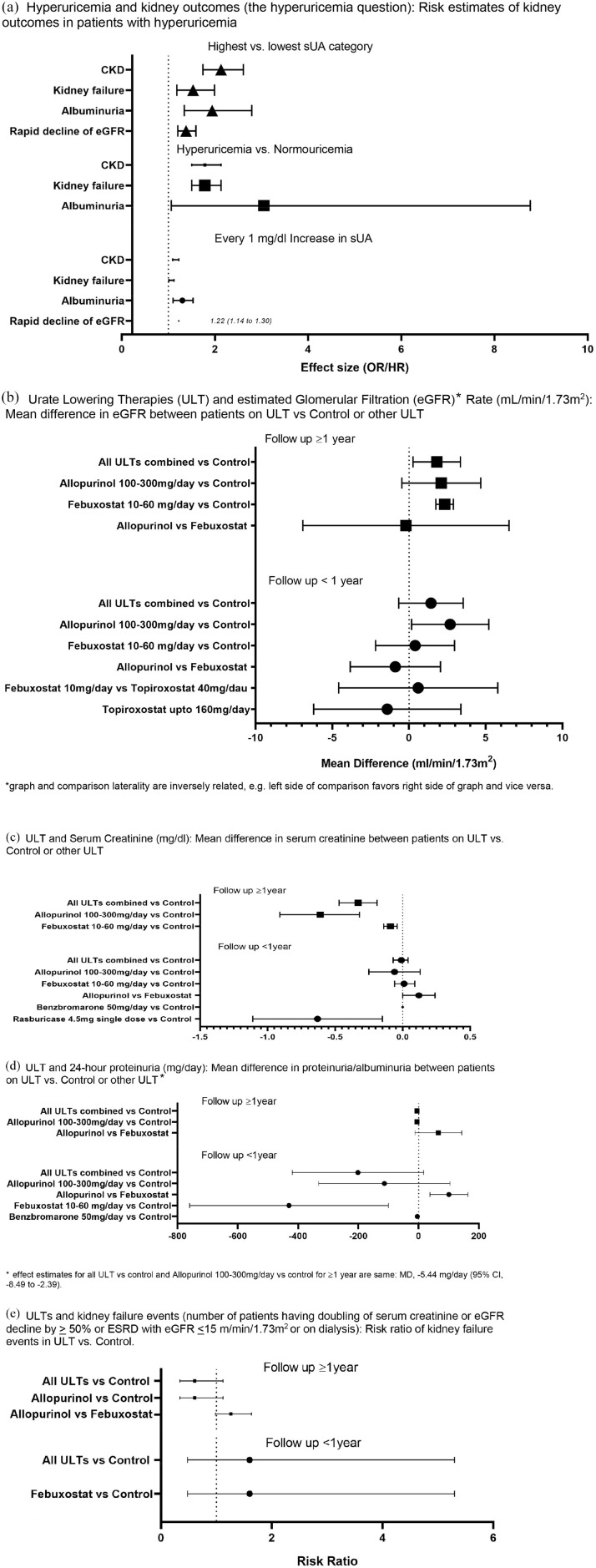
A summary of all findings is shown in Figure 4 and Supplemental Appendix 7.
Figure 4.
Hyperuricemia, urate-lowering therapies, and kidney outcomes: summary of findings.
(a) Hyperuricemia and kidney outcomes (the hyperuricemia question): risk estimates of kidney outcomes in patients with hyperuricemia. (b) Urate-lowering therapies (ULTs) and estimated glomerular filtration rate (eGFR) (ml/min per 1.73 m2): mean difference in eGFR between patients on ULT versus control or other ULT. *Graph and comparison laterality are inversely related, that is, left side of comparison favors right side of graph and vice versa. (c) ULT and serum creatinine (mg/dl): mean difference in serum creatinine between patients on ULT versus control or other ULT. (d) ULT and 24-h proteinuria (mg/day): mean difference in proteinuria/albuminuria between patients on ULT versus control or other ULT. *Effect estimates for all ULT versus control and allopurinol 100–300 mg/day versus control for ⩾1 year are same: MD −5.44 mg/day (95% CI, −8.49 to −2.39). (e) ULTs and kidney failure events (number of patients having the doubling of serum creatinine or eGFR decline by ⩾50% or ESRD with eGFR ⩽15 m/min per 1.73 m2 or on dialysis): risk ratio of kidney failure events in ULT versus control.
CI, confidence interval; CKD, chronic kidney disease; ESRD, end-stage renal disease; HR, hazard ratio; MD, mean difference; OR, odds ratio; sUA, serum urate.
Publication bias
We assessed publication bias with funnel plots (Supplemental Appendix 11). Analyses by sUA quartiles for the development of CKD and ULT versus control for serum creatinine showed funnel plot asymmetry, indicating a publication bias. Confirmation with Egger’s regression test, publication bias was present in studies comparing sUA quartiles for the development of CKD (p = 0.002) and studies comparing ULT versus control for the change in serum creatinine (p = 0.043; Supplemental Appendix 11b).
Discussion
Based on several systematic reviews and recent Mendelian randomization studies,55,56 most clinicians interpret that there is a non-causal relationship between hyperuricemia and kidney function (hyperuricemia question). However, whether ULT use leads to improved kidney function is not known (ULT question). An interesting literature review with meta-analysis of randomized trials based on assumption that ULT provides different benefits based on the rapidity of progression of underlying kidney disease found that there was a significant improvement of eGFR/creatinine clearance with ULT use in patients with rapidly declining kidney function.57 Although results from that analysis and analysis from correspondence to that systematic review are insightful,58 there are a few limitations to the interpretation of data from the correspondence, namely, the absence of a published protocol and the non-systematic nature of the review described in the correspondence. Therefore, while interesting points were raised, further systematic research, such as our study, is needed to address these issues. In our review, based on low to very low certainty evidence, we found that hyperuricemia was associated with an increased risk of kidney outcomes including rapid eGFR decline, albuminuria, stage 3 CKD, and kidney failure. We also found that ULT use for ⩾1 year was associated with significantly more improvement in serum creatinine and proteinuria/albuminuria based on low certainty evidence and eGFR based on very low certainty evidence. The kidney function benefit was evident with both febuxostat and allopurinol use for ⩾1 year.
Key limitations from previous studies were the use of composite outcome and the lack of assessment of early markers of kidney dysfunction, which our study overcomes. Our systematic review and meta-analysis included 131 studies addressing two key clinical questions. Among the two published meta-analyses assessing the hyperuricemia question, Li et al.7 analyzed 21 cohort studies and reported an association between hyperuricemia and incident kidney disease (composite outcome defined as ESKD, CKD, albuminuria or elevated serum creatinine; RR 1.49, 95% CI, 1.27–1.75) and Li et al.6 included 13 cohort studies reporting OR/HR of 2.59 (95% CI, 2.14–3.13) for new-onset stage 3 CKD with hyperuricemia. An outdated database search upto September 2013, the use of composite outcomes,
and language restriction to English and Chinese were important limitations of previous systematic reviews.6,7 To address these shortcomings, we updated the search to 2020 with data from 90 additional studies, did not restrict to English or Chinese language, analyzed kidney outcomes separately rather than a composite outcome, and included clinical trials of ULT to examine whether lowering of sUA with ULT can improve kidney function.
For the ULT question, 12 previous meta-analyses of clinical trials evaluated the effect of ULT use on kidney function.8,9,11–15,17,18,59 Results from these meta-analyses, comparing ULT versus control (placebo, no treatment, or usual care), were contradictory, showing improvement in kidney function versus an absence of an effect.8–18 No previous meta-analyses performed head-to-head comparison of allopurinol versus febuxostat, except one systematic review of cohort studies with no meta-analysis.10 Additionally, they restricted language in the search to studies published in English and Chinese languages only7,8,11,15 and had a small number of studies (<5 total) for meta-analysis.8,15 Our sample size exceeds the previously published meta-analyses and had no language restriction. We performed head-to-head comparison of allopurinol and febuxostat.
A novel finding from our meta-analysis was that the use of ULT for 1 year or more was associated with an improvement of eGFR (very low certainty), serum creatinine (low certainty), and proteinuria/albuminuria (low certainty), but no reduction in risk of kidney failure events (very low certainty). Our results of the effect of ULTs as a category complement, rather than contradict, the findings from the recently published RCTs of allopurinol, which were done in people with Stage 3-4 CKD or diabetes, respectively.19,20 Our systematic and critical evaluation of the evidence to-date, including these two recent RCTs, provides an updated literature synthesis that can inform clinical care. We noted no significant differences between the effect of allopurinol, febuxostat, and topiroxostat on kidney function, based on a small evidence base. This indicates that more, well-powered, studies in different populations with hyperuricemia and gout, including head-to-head studies, are needed to answer these important questions.
Our findings must be interpreted considering study limitations. Data for the hyperuricemia question were derived from observational studies (as expected). Therefore, confounding bias is likely in these estimates. A major potential confounder was difference in follow-up duration between studies. We calcuated follow-up duration-adjusted effects estimated to address this issue and no difference was found, compared to the unadjusted estiamtes. Baseline kidney function was variable in observational studies, which likely led to study heterogeneity reflected in overall statistical heterogeneity (I2). Significant heterogeneity was also noted in some analyses, including that for hyperuricemia and kidney failure, which may be due to the differences in definitions for kidney failure (ICD-9 code versus eGFR <15 mg/min per 1.73 m2 versus patient on dialysis) and the difference in baseline kidney function between studies. For the ULT question, significant heterogeneity was noted, which can be explained by different baseline characteristics (low versus normal kidney function), differences in the trial design, and inclusion/exclusion criteria. Despite the inclusion of many more studies a previous meta-analyses, head-to-head data from clinical trials of allopurinol versus febuxostat were limited. Therefore, some results were inconclusive because of possible type 1 error, that is, low power.60 One recent observational study showed the superiority of allopurinol in comparison with febuxostat for reducing the risk of incident kidney disease.61 This indicates that more head-to-head trials between febuxostat and allopurinol are needed. We were unable to examine the secondary effects of other commonly prescribed medications in analyzed/enrolled studies that can only modestly lower sUA levels (atorvastatin, fenofibrate, angiotensin receptor blockers, including losartan, empagliflozin, etc.) or medications that can increase sUA levels (angiotensin converting enzyme inhibitors, diuretics, theophylline, beta and alpha-1-adrenergic antagonists, etc.). Lastly, we could not perform subgroup analysis based on race and sex due to the lack of these data in the included studies.
In conclusion, hyperuricemia is associated with an increased risk of developing rapid eGFR decline, albuminuria, stage 3 or more CKD, and kidney failure, regardless of the baseline kidney function, based on low to very low certainty evidence. Longer ULT use (⩾1 year) improves kidney function based on low to very low certainty evidence, regardless of the baseline kidney function. This new knowledge, when balanced against the potential burden and perceived harm of starting ULTs early in the course of illness, should help patients and physicians to make the best possible evidence-based decision with consideration of cost, preferences, values, and tolerance to the risk of adverse events. However, as the certainty of available evidence is low to very low, more well designed adequately powered RCTs, including head-to-head trials between febuxostat and allopurinol and other new ULTs in patients with gout, hyperuricemia and crystalluria (urinary uric acid crystals), are required to confirm these findings. To further elucidate the pathways of renoprotection with ULTs and find efficacy differences, we also need placebo-controlled comparator studies of various drugs or different types of ULTs with kidney outcomes as primary study outcomes (xanthine oxidase inhibitors, uricosuric agents, and ULTs with novel mechanisms of action). Future studies systematically estimating harms of ULT are also needed.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X211016661 for Hyperuricemia, urate-lowering therapy, and kidney outcomes: a systematic review and meta-analysis by Gaurav Sharma, Abhishek Dubey, Nilesh Nolkha and Jasvinder A. Singh in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
We thank Dr. Siamak Noorbaloochi, a PhD biostatistician from the University of Minnesota at Minneapolis, for assisting and advising us on the sensitivity analyses by study duration and reviewing the results of these analyses for accuracy. We thank Carolyn M. Holmes at the University of Alabama at Birmingham (UAB) for doing literature searches. We also thank Shaohua Yu, formerly at UAB, a native Chinese-speaking bilingual scientist, for abstracting data from the Chinese language studies.
Footnotes
Author contributions: GS conceived the study design, performed data extraction and risk of bias assessment, data analysis, and drafted the report. AD and NN abstracted the data and performed the risk of bias assessment and reviewed the report. JAS reviewed and edited the study design, drafted the report, and made major revisions to the report. All authors have read and approved the final version of the report. GS, NN, and JAS had full access to the data and made the decision to submit for publication.
Conflict of interest statement: AD, GS, and NN have no conflicts of interest. JAS has received consultant fees from Crealta/Horizon, Medisys, Fidia, PK Med, Two Labs Inc., UBM LLC, Trio health, Medscape, WebMD, Clinical Care options, Clearview healthcare partners, Putnam associates, Focus forward, Navigant consulting, Spherix, MEdIQ, Practice Point communications, the National Institutes of Health and the American College of Rheumatology. JAS owns stock options in Amarin pharmaceuticals and Viking therapeutics. JAS owns stock options in TPT Global Tech, Vaxart pharmaceuticals and Charlotte’s Web Holdings, Inc. JAS previously owned stock options in Amarin, Viking and Moderna pharmaceuticals. JAS is on the speaker’s bureau of Simply Speaking. JAS is a member of the executive of OMERACT, an organization that develops outcome measures in rheumatology and receives arms-length funding from 12 companies. JAS serves on the FDA Arthritis Advisory Committee. JAS is the chair of the Veterans Affairs Rheumatology Field Advisory Committee. JAS is the editor and the Director of the UAB Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis. JAS previously served as a member of the following committees: member, the American College of Rheumatology’s (ACR) Annual Meeting Planning Committee (AMPC) and Quality of Care Committees, the Chair of the ACR Meet-the-Professor, Workshop and Study Group Subcommittee, and the co-chair of the ACR Criteria and Response Criteria subcommittee.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Jasvinder A. Singh  https://orcid.org/0000-0003-3485-0006
https://orcid.org/0000-0003-3485-0006
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Gaurav Sharma, Department of Internal Medicine, University of South Alabama, Mobile, AL, USA; Division of Clinical Immunology and Rheumatology, University of Alabama at Birmingham, AL, USA; Department of Internal Medicine, Seth GS Medical College and KEM Hospital, Mumbai, MH, India.
Abhishek Dubey, Department of Internal Medicine, Seth GS Medical College and KEM Hospital, Mumbai, MH, India.
Nilesh Nolkha, Department of Rheumatology, Cannock Chase Hospital, Cannock, UK.
Jasvinder A. Singh, Division of Clinical Immunology and Rheumatology, Department of Medicine at the School of Medicine and the Department of Epidemiology at the School of Public Health, University of Alabama at Birmingham, Faculty Office Tower 805B, 510 20th Street S., Birmingham, AL 35294-0022, USA; Medicine Service, VA Medical Center, Birmingham, AL 35233, USA.
References
- 1. Bardin T, Richette P. Definition of hyperuricemia and gouty conditions. Curr Opin Rheumatol 2014; 26: 186–191. [DOI] [PubMed] [Google Scholar]
- 2. Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med 2012; 125: 679–687.e1. [DOI] [PubMed] [Google Scholar]
- 3. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 2011; 63: 3136–3141. [DOI] [PubMed] [Google Scholar]
- 4. Sanchez-Lozada LG, Tapia E, Avila-Casado C, et al. Mild hyperuricemia induces glomerular hypertension in normal rats. Am J Physiol Renal Physiol 2002; 283: F1105–F1110. [DOI] [PubMed] [Google Scholar]
- 5. Sanchez-Lozada LG, Tapia E, Soto V, et al. Treatment with the xanthine oxidase inhibitor febuxostat lowers uric acid and alleviates systemic and glomerular hypertension in experimental hyperuricaemia. Nephrol Dial Transplant 2008; 23: 1179–1185. [DOI] [PubMed] [Google Scholar]
- 6. Li L, Yang C, Zhao Y, et al. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease? A systematic review and meta-analysis based on observational cohort studies. BMC Nephrol 2014; 15: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li YL, Wang L, Li J, et al. [The correlation between uric acid and the incidence and prognosis of kidney diseases: a systematic review and meta-analysis of cohort studies]. Zhonghua nei ke za zhi 2011; 50: 555–561. [PubMed] [Google Scholar]
- 8. Bose B, Badve SV, Hiremath SS, et al. Effects of uric acid-lowering therapy on renal outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant 2014; 29: 406–413. [DOI] [PubMed] [Google Scholar]
- 9. Chewcharat A, Chen Y, Thongprayoon C, et al. Febuxostat as a renoprotective agent for treatment of hyperuricemia: a meta-analysis of randomized controlled trials. Intern Med J. Epub ahead of print 9 March 2020. DOI: 10.1111/imj.14814. [DOI] [PubMed] [Google Scholar]
- 10. Hu AM, Brown JN. Comparative effect of allopurinol and febuxostat on long-term renal outcomes in patients with hyperuricemia and chronic kidney disease: a systematic review. Clin Rheumatol 2020; 39: 3287–3294. [DOI] [PubMed] [Google Scholar]
- 11. Kanji T, Gandhi M, Clase CM, et al. Urate lowering therapy to improve renal outcomes in patients with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol 2015; 16: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin T-C, Hung LY, Chen Y-C, et al. Effects of febuxostat on renal function in patients with chronic kidney disease: a systematic review and meta-analysis. Medicine (Baltimore) 2019; 98: e16311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu X, Liu K, Sun Q, et al. Efficacy and safety of febuxostat for treating hyperuricemia in patients with chronic kidney disease and in renal transplant recipients: a systematic review and meta-analysis. Exp Ther Med 2018; 16: 1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu X, Zhai T, Ma R, et al. Effects of uric acid-lowering therapy on the progression of chronic kidney disease: a systematic review and meta-analysis. Ren Fail 2018; 40: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sampson AL, Singer RF, Walters GD. Uric acid lowering therapies for preventing or delaying the progression of chronic kidney disease. Cochrane Database Syst Rev 2017; 10: CD009460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Su X, Xu B, Yan B, et al. Effects of uric acid-lowering therapy in patients with chronic kidney disease: a meta-analysis. PLoS One 2017; 12: e0187550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang H, Wei Y, Kong X, et al. Effects of urate-lowering therapy in hyperuricemia on slowing the progression of renal function: a meta-analysis. J Ren Nutr 2013; 23: 389–396. [DOI] [PubMed] [Google Scholar]
- 18. Zhang YF, He F, Ding HH, et al. Effect of uric-acid-lowering therapy on progression of chronic kidney disease: a meta-analysis. J Huazhong Univ Sci Technolog Med Sci 2014; 34: 476–481. [DOI] [PubMed] [Google Scholar]
- 19. Badve SV, Pascoe EM, Tiku A, et al. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med 2020; 382: 2504–2513. [DOI] [PubMed] [Google Scholar]
- 20. Doria A, Galecki AT, Spino C, et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med 2020; 382: 2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wallace KL, Riedel AA, Joseph-Ridge N, et al. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol 2004; 31: 1582–1587. [PubMed] [Google Scholar]
- 22. Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 2017; 76: 29–42. [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions, version 6.0. Cochrane, http://www.training.cochrane.org/handbook (2019, accessed 9 May 2021).
- 25. Martin JLR, Pérez V, Sacristán M, et al. Is grey literature essential for a better control of publication bias in psychiatry? An example from three meta-analyses of schizophrenia. Eur Psychiatry 2005; 20: 550–553. [DOI] [PubMed] [Google Scholar]
- 26. Long JS. Regression models for categorical and limited dependent variables. Advanced quantitative techniques in the social sciences series 7. Thousand Oaks, CA: SAGE Publications, 1997. [Google Scholar]
- 27. Nemes S, Jonasson JM, Genell A, et al. Bias in odds ratios by logistic regression modelling and sample size. BMC Med Res Methodol 2009; 9: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ontario: University of Ottawa, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2001, accessed 16 December 2009). [Google Scholar]
- 29. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moody WE, Chue CD, Inston NG, et al. Understanding the effects of chronic kidney disease on cardiovascular risk: are there lessons to be learnt from healthy kidney donors? J Hum Hypertens 2012; 26: 141–148. [DOI] [PubMed] [Google Scholar]
- 31. Atkins RC, Briganti EM, Zimmet PZ, et al. Association between albuminuria and proteinuria in the general population: the AusDiab study. Nephrol Dial Transplant 2003; 18: 2170–2174. [DOI] [PubMed] [Google Scholar]
- 32. Wu M-T, Lam K-K, Lee W-C, et al. Albuminuria, proteinuria, and urinary albumin to protein ratio in chronic kidney disease. J Clin Lab Anal 2012; 26: 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, 2014. [Google Scholar]
- 34. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deeks JJ, Higgins JP, Altman DG, et al. Analysing data and undertaking meta-analyses. In: Thomas J, Chandler J, Cumpston M, et al. (eds) Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester: John Wiley & Sons, 2019, pp.241–284. [Google Scholar]
- 37. Gibson T, Rodgers V, Potter C, et al. Allopurinol treatment and its effect on renal function in gout: a controlled study. Ann Rheum Dis 1982; 41: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kao MP, Ang DS, Gandy SJ, et al. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol 2011; 22: 1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Z, Lin Z, Lan Y, et al. Clinical efficacy of domestic benzbromarone in the treatment of hyperuricemia in the youth. China Pharm 2010; 21: 1117–1118. [Google Scholar]
- 40. Beddhu S, Filipowicz R, Wang B, et al. A randomized controlled trial of the effects of febuxostat therapy on adipokines and markers of kidney fibrosis in asymptomatic hyperuricemic patients with diabetic nephropathy. Can J Kidney Health Dis 2016; 3: 2054358116675343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Al-Rubeaan K, Siddiqui K, Alghonaim M, et al. The Saudi Diabetic Kidney Disease study (Saudi-DKD): clinical characteristics and biochemical parameters. Ann Saudi Med 2018; 38: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barkas F, Elisaf M, Liberopoulos E, et al. Uric acid and incident chronic kidney disease in dyslipidemic individuals. Curr Med Res Opin 2018; 34: 1193–1199. [DOI] [PubMed] [Google Scholar]
- 43. Rincon-Choles H, Jolly SE, Arrigain S, et al. Impact of uric acid levels on kidney disease progression. Am J Nephrol 2017; 46: 315–322. [DOI] [PubMed] [Google Scholar]
- 44. Shu D, Xu F, Su Z, et al. Risk factors of progressive IgA nephropathy which progress to end stage renal disease within ten years: a case-control study. BMC Nephrol 2017; 18: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ingsathit A, Thakkinstian A, Chaiprasert A, et al. Prevalence and risk factors of chronic kidney disease in the Thai adult population: Thai SEEK study. Nephrol Dial Transplant 2010; 25:1567–1575. [DOI] [PubMed] [Google Scholar]
- 46. Kawashima M, Wada K, Ohta H, et al. Association between asymptomatic hyperuricemia and new-onset chronic kidney disease in Japanese male workers: a long-term retrospective cohort study. BMC Nephrol 2011; 12: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liao LN, Liu CS, Li CI, et al. Three-year incidence of elevated albuminuria and associated factors in a population-based cohort: the Taichung Community Health Study. Eur J Prev Cardiol. Epub ahead of print 5 June 2014. DOI: 10.1177/2047487314537918. [DOI] [PubMed] [Google Scholar]
- 48. Lin MY, Chiu YW, Lee CH, et al. Factors associated with CKD in the elderly and nonelderly population. Clin J Am Soc Nephrol 2013; 8: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu WC, Hung CC, Chen SC, et al. Association of hyperuricemia with renal outcomes, cardiovascular disease, and mortality. Clin J Am Soc Nephrol 2012; 7: 541–548. [DOI] [PubMed] [Google Scholar]
- 50. Obermayr RP, Temml C, Knechtelsdorfer M, et al. Predictors of new-onset decline in kidney function in a general middle-European population. Nephrol Dial Transplant 2008; 23: 1265–1273. [DOI] [PubMed] [Google Scholar]
- 51. Sturm G, Kollerits B, Neyer U, et al. Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) study. Exp Gerontol 2008; 43: 347–352. [DOI] [PubMed] [Google Scholar]
- 52. Deng Y, Zhang P, Liu H, et al. Observation on allopurinol in lowering blood uric acid for slowing the progression of chronic renal failure. J Pract Med 2010; 26: 982–984. [Google Scholar]
- 53. Lei J, Li S. Clinical research on allopurinol lowering of uric acid level of chronic renal disease for the delay of the progression of renal disease. Shaanxi Med J 2009; 38: 1191–1212. [Google Scholar]
- 54. Zhou Y, Qi H, Zhao GM, et al. [Relationship between hyperuricemia and chronic kidney disease in Pudong new area of Shanghai]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 2012; 33: 351–355. [PubMed] [Google Scholar]
- 55. Ahola AJ, Sandholm N, Forsblom C, et al. The serum uric acid concentration is not causally linked to diabetic nephropathy in type 1 diabetes. Kidney Int 2017; 91: 1178–1185. [DOI] [PubMed] [Google Scholar]
- 56. Jordan DM, Choi HK, Verbanck M, et al. No causal effects of serum urate levels on the risk of chronic kidney disease: a Mendelian randomization study. PLoS Med 2019; 16: e1002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sato Y, Feig DI, Stack AG, et al. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat Rev Nephrol 2019; 15: 767–775. [DOI] [PubMed] [Google Scholar]
- 58. Steiger S, Ma Q, Anders HJ. The case for evidence-based medicine for the association between hyperuricaemia and CKD. Nat Rev Nephrol 2020; 16: 422. [DOI] [PubMed] [Google Scholar]
- 59. Su SL, Lin C, Kao S, et al. Risk factors and their interaction on chronic kidney disease: a multi-centre case control study in Taiwan. BMC Nephrol 2015; 16: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Greco T, Zangrillo A, Biondi-Zoccai G, et al. Meta-analysis: pitfalls and hints. Heart Lung Vessel 2013; 5: 219–225. [PMC free article] [PubMed] [Google Scholar]
- 61. Singh JA, Cleveland JD. Comparative effectiveness of allopurinol and febuxostat for the risk of atrial fibrillation in the elderly: a propensity-matched analysis of Medicare claims data. Eur Heart J 2019; 40: 3046–3054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X211016661 for Hyperuricemia, urate-lowering therapy, and kidney outcomes: a systematic review and meta-analysis by Gaurav Sharma, Abhishek Dubey, Nilesh Nolkha and Jasvinder A. Singh in Therapeutic Advances in Musculoskeletal Disease