Abstract
Background
Sepsis typically results in enhanced coagulation system activation and microthrombus formation. Microparticle (MP) production promotes coagulation and enhances pro-coagulation. This study investigated how circulating MP levels and tissue factor-bearing MP (TF+-MP) activity caused coagulation in patients with septic disseminated intravascular coagulation (DIC).
Methods
Thirty patients with septic DIC and 30 healthy controls were studied from December 2017 to March 2019. Patient blood samples were collected at enrolment (day 1) and on days 3 and 5; DIC scores and Sequential Organ Failure Assessment (SOFA) scores were recorded. TF+-MP activity was measured using TF-dependent factor Xa generation experiments. Circulating MP concentrations were determined by MP capture assay. Clotting factor activity, antithrombin level, soluble thrombomodulin, and serum tissue factor pathway inhibitor (TFPI) concentrations were measured.
Results
Patients with septic DIC had lower circulating MP levels than healthy control patients. Circulating MP levels in patients with septic DIC were positively correlated with DIC scores and negatively correlated with coagulation factors, but TF+-MP activity did not correlate with clotting factor levels and TFPI.
Conclusions
In patients with septic DIC, circulating MP levels are important in promoting coagulation activation and increasing clotting factor consumption. TF+-MP activity may not be the main form of active TF.
Keywords: Coagulation factor, circulating microparticle, tissue factor-bearing microparticle activity, tissue factor pathway inhibitor, thrombomodulin, disseminated intravascular coagulation, sepsis
Introduction
Sepsis refers to diseases with a systemic inflammatory response syndrome and organ dysfunction that are caused by infections, and it is a common critical disease in intensive care units (ICUs). The global mortality rate for sepsis is approximately 30%, and disseminated intravascular coagulation (DIC) often occurs in patients with systemic infection.1 Overactivation of the coagulation system, damage to the anticoagulant system, and widespread formation of microcirculatory thrombosis leading to organ failure are the main clinical features of septic DIC.2 Compared with patients without septic DIC, the mortality rate for sepsis patients with DIC is twice as high.3
For sepsis, the inflammatory response and coagulation activation affect each other mutually. On the one hand, inflammatory factors, cytokines, and chemokines can activate the coagulation system and destroy the physiological anticoagulation mechanism. For example, tissue factor pathway inhibitor (TFPI) cannot effectively counteract tissue factor (TF) activity. The endothelial-related anticoagulant system, especially the protein C system, is damaged, and up-regulation of plasminogen activator inhibitor type-1 (PAI-1) hinders fibrin clearance. On the other hand, the activated coagulation system also significantly affects the inflammatory response. The severity of sepsis increases with the degree of coagulation disorders.4,5
Microparticles (MPs) are small bubble-like membrane particles (0.1–1 µm in diameter) that originate from a variety of blood cells and endothelial cells. They carry components such as proteins and lipids from the parental cell membrane and cytoplasm. Under pathological conditions, MPs have strong coagulative properties and have various functions in inflammation and coagulation.6,7 Phosphatidylserine, which is exposed on platelets, leukocytes, and endothelial cell microbubbles, contributes to the procoagulant activity in sepsis. The circulating MPs in sepsis mice had a greater coagulative effect than those in sham-operated mice. The release of MPs carrying TF into the blood can specifically activate the coagulation pathway and cause DIC.8–10 Total thrombin (TG) production was induced by platelet MPs (PMPs) that are produced in the septic model of caecal ligation. Compared with the healthy control group, MPs that were obtained from the blood of patients with meningococcal sepsis had a stronger thrombin production capacity in vitro.11,12
We measured the circulating MP levels and the TF-bearing MP (TF+-MP) activity in patients with septic DIC and evaluated the roles of both in the activation of the coagulation pathway and the consumption of coagulation factors.
Patients and methods
Patients
Patients were selected from those who were admitted to the ICU at the First Affiliated Hospital of Harbin Medical University from December 2017 to March 2019. All patients were over 18 years old and met the 2016 SEPSIS 3.0 diagnostic criteria and DIC diagnostic criteria of the International Society on Thrombosis and Haemostasis (ISTH).13,14 No patients with end-stage cardiopulmonary diseases, cancer, or end-stage cancer, chronic liver or kidney dysfunction, end-stage liver or kidney failure, or haematological system diseases were included. Healthy volunteers of the same age and sex who had not taken any drugs were designated as the control group. In preliminary experiments that were conducted before this experiment to optimize the procedure (data not shown), the circulating MP levels and TF+-MP activity levels in the non-DIC sepsis patients were between those in the septic DIC patients and healthy volunteers. Compared with the experimental results of septic DIC patients, the difference was not significant. All enrolled patients received anti-infection treatment as early as possible, adequate drainage of the infected site, early fluid resuscitation treatment, and organ support treatment.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University (Harbin, China; approval number HYYKY/WZLS 201810), and it meets the requirements of the ethics principles in the Declaration of Helsinki of the World Medical Association (WMA) and the International Ethical Guidelines for Biomedical Research Involving Human Subjects of the Council for International Organizations of Medical Sciences (CIOMS).15,16 We obtained written informed consent from the patients’ family members.
Definitions and diagnosis of DIC
The DIC score and Sequential Organ Failure Assessment (SOFA) score were calculated for enrolled patients with septic DIC. The SOFA score calculates dysfunction of the six organ systems and dysfunction severity, including the respiratory, clotting, liver, cardiovascular, kidney, and nervous systems.17 The DIC score was calculated using the ISTH DIC score criteria, including prothrombin time (PT), platelets (PLTs), fibrinogen (FIB) level, and fibrinogen/fibrin degradation products (FDP).14
Plasma collection
Fasting venous blood samples (day 1; D1) were collected as soon as possible within 6 hours for the patients who met the diagnostic criteria for enrolment and also on the mornings of day 3 (D3) and day 5 (D5). Fresh peripheral blood samples were used for the blood cell count and coagulation indexes using routine laboratory test methods.
Platelet-free plasma (PFP) was prepared from the collected blood within 1 hour in an anticoagulant test tube containing sodium citrate for MP detection. Platelet-poor plasma (PPP) was obtained by centrifuging at 1500 ×g at room temperature for 15 minutes. The platelets were then removed by centrifugation at 13,000 × g for 2 minutes.18 PFP was separated and stored at −80°C until analysis. Blood samples from healthy volunteers were collected and preserved at the same time for comparison.
Laboratory analysis
An automatic flow cytometer (XFA6100; Nanjing Pulang Co., Nanjing, China) was used for the complete blood count analysis, which included white blood cells (WBCs), PLTs, and haemoglobin (Hb). An automatic coagulation analyser (CA7000; Sysmex, Kobe, Japan) was used to test the following coagulation indexes: PT, activated partial thromboplastin time (APTT), fibrinogen level (FIB), D-dimer level (D-D), and FDP.
The following indexes were measured at the same time in the frozen blood samples from patients and healthy controls. Serum thrombomodulin (sTM) and TFPI levels were measured using the Quantikine® Thrombomodulin and Tissue Factor Pathway Inhibitor ELISA Kit (R & D, Minneapolis, MN, USA). The activity of clotting factors and the antithrombin (AT) level were determined using a CS5100 automatic haemagglutination analyser (Sysmex; Kobe, Japan).
Analysis of microparticles
Circulating microparticle concentration
In accordance with the operating instructions (ZYMUPHEN MP Activity Assay; Hyphen Biomed, Neuville sur Oise, France),18 frozen PFP plasma samples from patients and the healthy control group were thawed at 37°C and diluted with Sample Diluent at 1:20. Then, 100 µL of the diluted sample was added to the micropores coated with streptavidin and biotinylated Annexin V and incubated for 1 hour at 37°C. The sample was then washed five times with 300 µL of diluted wash buffer, and 100 µL of R1 (Bovine FXa–FVa mixture, containing calcium) and 50 µL of R2 (purified human prothrombin) were added. The samples were incubated for 10 minutes at 37°C. Next, 50 µL of R3 (thrombin-specific chromogenic substrate) were added and incubated at 37°C for 3 minutes. Finally, 50 µL of 2% citrate were added to terminate the reaction. After stabilization for 10 minutes, the absorbance was measured at 405 nm. Using the calibrator provided in the kit at “C” nM, the standard solutions were prepared. The calibration curve was calculated based on the absorbance results of the standard solutions. The MP concentration obtained for the sample tested was deduced from the calibration curve by multiplying the measured concentration by the dilution ratio. The results were expressed as nanomolar phosphatidylserine equivalents (nM eq. PhtdSer).
TF+-MP activity assay
TF+-MP activity was measured using a TF+-MP-dependent FXa generation assay that has been previously described.19 MPs were pelleted from 360 µL of PPP by centrifugation at 20,000 ×g for 90 minutes at 4°C, washed twice with HBSA (137 mM NaCl, 5.38 mM KCl, 5.55 mM glucose, 10 mM HEPES, and 0.1% bovine serum albumin, pH 7.5), and re-suspended in 180 µL of HBSA. The samples were incubated with either a neutralising antibody to human TF (hTF1; 10 µg/mL) (8 µL) or an isotype-matched murine monoclonal IgG antibody (Purified Mouse IgG1, κ Isotype Control; 10 µg/mL) (8 µL) (BD Biosciences, San Jose, CA, USA) for 20 minutes at 25°C. After incubation, 50-µL aliquots were added to duplicate wells of a 96-well plate. Next, 50 µL of coagulation factor mixture (including 10 µL 50 nM FVIIa [Enzyme Research Laboratories, South Bend, IN, USA] + 20 µL 750 nM FX [Enzyme Research Laboratories] + 20 µL 25 mM Ca2+) were added to each sample, and the mixture was incubated for 2 hours at 37°C. FXa generation was stopped by the addition of 25 µL of 25 mM EDTA buffer, and 25 µL of the chromogenic substrate S2765 (4 mM) (Chromogenix, Bedford, MA, USA) were added and incubated at 37°C for 15 minutes. Then, a microplate reader was used for 90 minutes of continuous reading (absorbance at 405 nm). Known amounts of purified FXa (Enzyme Research Laboratories) were used for a reference line to calculate FXa formation from the fluorescence tracings. TF-dependent FXa generation was determined by subtracting the amount of FXa that was generated in the presence of HTF1 from the amount of FXa that was generated in the presence of the control antibody.
Statistical analysis
SPSS v.20.0 software (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. The Wilcoxon sign-rank test was used for paired sample comparisons between patients and healthy controls at each time point. Spearman’s rank-correlation was used to analyse the correlations among various indexes. Additionally, p < 0.05 was considered to be statistically significant. In the correlation analysis, to reduce false-positive results, p < 0.01 was specifically defined as statistically significant given the repeated measurement data that were involved.
Results
Patients
Thirty patients with septic DIC were included in the study (median age, 56 [19–86] years), including 25 men and five women. Four of these patients died within 24 hours after the D1 blood collection, and 26 patients showed improvement of their condition. Thirty healthy controls with a median age of 55 [22–90] years were included, and no statistical difference in age was found between the two groups. The main characteristics of the patients are shown in Table 1. The sources of septic DIC were bacteraemia (n = 10), pneumonia (n = 6), abdominal infection (n = 12), intracranial infection (n = 1), and abscess of the pharynx (n = 1).
Table 1.
Patient characteristics.
| Variable | Septic DIC group, n=30 | Control group, n=30 | p-value |
|---|---|---|---|
| Sex (n) | |||
| Female | 5 (16.7%) | 5 (16.7%) | |
| Male | 25 (83.3%) | 25 (83.3%) | |
| Total | 30 | 30 | |
| Age (years) | 56 (19–86) | 55 (22–90) | 0.404 |
| SOFA score at enrolment | 14 (8–22) | ||
| DIC score at enrolment | 5 (5–7) | ||
| Pathogeny | |||
| Bacteraemia | 10 (33.3%) | ||
| Pneumonia | 6 (20%) | ||
| Abdominal infection | 12 (40%) | ||
| Intracranial infection | 1 (3.3%) | ||
| Pharynx abscess | 1 (3.3%) | ||
| Treatment | |||
| Platelet transfusion | 19 (63.3%) | ||
| Plasma infusion | 19 (63.3%) | ||
| Anticoagulants | 0 |
Data on scores and age are expressed as the median. Reference ranges are expressed as the minimum and maximum.
SOFA, Sequential Organ Failure Assessment; DIC, disseminated intravascular coagulation.
Changes in coagulation indexes in patients with septic DIC
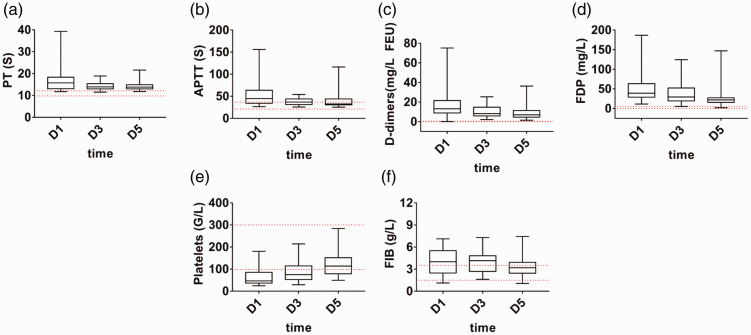
On D1 in the septic DIC group, PT and APTT were significantly prolonged (p < 0.001 and p = 0.002, respectively), and the PLT count was significantly decreased (p < 0.001). Clotting factors and platelets were consumed. D-D and FDP increased significantly (p < 0.001 and p = 0.001, respectively), and activation of the coagulation pathway and increased thrombin production occurred in the patients. With an improvement at D5, the above indexes gradually recovered, and the degree of coagulation disorder decreased. However, FIB did not change significantly in this process (Figure 1a–f).
Figure 1.
Coagulation indexes in the septic disseminated intervascular coagulation (DIC) group (a–f). The red dotted line indicates the normal range. Data are expressed as the median (interquartile range). The ends of the whiskers represent the maximum and minimum values. a, Prothrombin time (PT). b, Activated partial thromboplastin time (APTT). c, D-dimer level (DD). d, Fibrinogen/fibrin degradation products (FDP). e, Platelets (PLTs). f, Fibrinogen level (FIB).
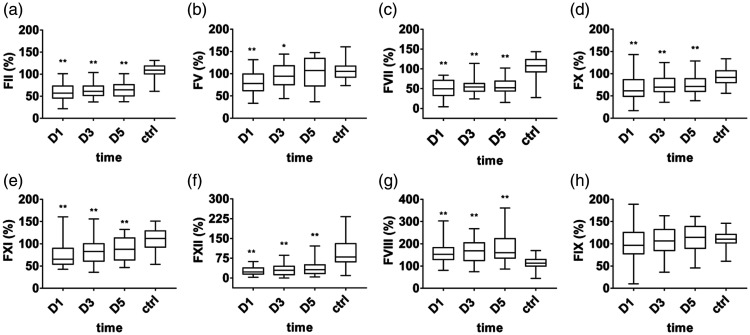
Changes in clotting factors in patients with septic DIC
During the occurrence of DIC in sepsis (D1), the clotting factors FII, FV, FVII, FX, FXI, and FXII were significantly reduced in the septic DIC group (p < 0.001). The coagulation pathway was activated, and the clotting factors were consumed. After improvement on D5, the clotting factors gradually recovered (Figure 2 a–f). Both intrinsic and extrinsic coagulation pathways were activated in septic DIC. However, in the septic DIC group on D1, clotting factor FVIII expression was increased (p = 0.001), and clotting factor FIX consumption was not significant (Figure 2 g–h).
Figure 2.
Activity of clotting factors in the septic disseminated intervascular coagulation (DIC) group. Data are expressed as the median (interquartile range). The ends of the whiskers represent the maximum and minimum values. **A significant difference was found between the patient group and the healthy control group (p < 0.01); *A difference was found between the patient group and the healthy control group (p < 0.05). a, Clotting factor FII. b, Clotting factor FV. c, Clotting factor FVII. d, Clotting factor FX. e, Clotting factor FXI. f, Clotting factor FXII. g, Clotting factor FVIII. h, Clotting factor FIX.
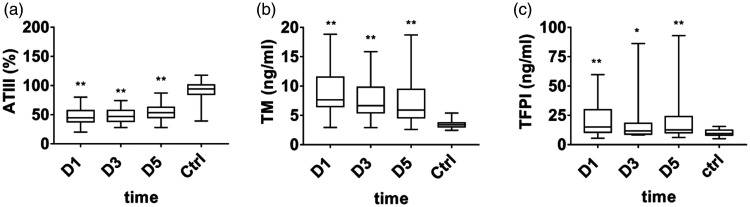
Changes in the anticoagulation system
The results showed that the AT level (median, 44.5% [37.75%–57%]) was significantly lower in patients with septic DIC at D1 than in healthy controls (median, 94% [84.75%–101.25%]; p < 0.001). Serum sTM (median, 7.66 [6.51–11.52] ng/mL) and TFPI (median, 15.13 [10.52–29.91] ng/mL) levels were significantly higher than those of healthy controls (sTM median, 3.4 [3.05–3.73] ng/mL; TFPI median, 9.45 [8.03–12.46] ng/mL; p < 0.001). At this time, the natural anticoagulants (AT, sTM, and TFPI) were seriously damaged, and the endothelial system was also damaged. After D5, the condition improved, and AT, sTM, and TFPI levels gradually recovered. The damaged anticoagulant system was restored (Figure 3).
Figure 3.
Anticoagulant indexes in septic disseminated intervascular coagulation (DIC). Data are expressed as the median (interquartile range). The ends of the whiskers represent the maximum and minimum values. **A significant difference was found between the patient group and healthy control group (p < 0.01). *A difference was found between the patient group and the healthy control group (p < 0.05). a, Antithrombin III (ATIII). b, Thrombomodulin (TM). c, Tissue factor pathway inhibitor (TFPI).
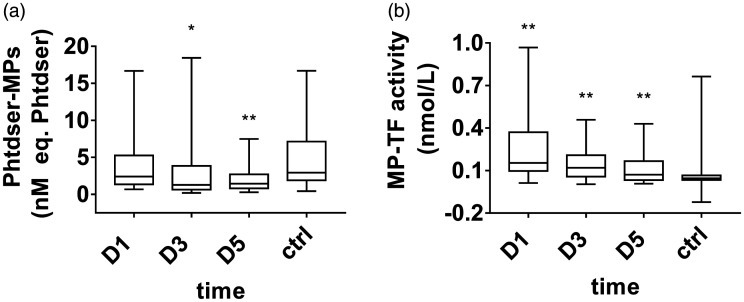
Changes in microparticles in patients with septic DIC
The circulating MP levels (median, 2.41 [1.35–5.25] nM eq. PhtdSer) decreased at D1 in septic DIC compared with the control group (median, 2.93 [1.89–7.13] nM eq. PhtdSer). With the improvement of the disease, the circulating MP levels on D3 and D5 further decreased. A significant difference was found between D5 (median, 1.45 [0.81–2.70] nM eq. PhtdSer) and the healthy control patients (median, 2.93 [1.89–7.13] nM eq. PhtdSer; p = 0.006; Figure 4a).
Figure 4.
Circulating microparticle (MP) levels and tissue factor-bearing microparticle (TF+-MP) activity in septic disseminated intervascular coagulation (DIC). Data are expressed as the median (interquartile range). The ends of the whiskers represent the maximum and minimum values. **A significant difference was found (p<0.01); *a difference was found (p<0.05). a, Phosphatidylserine (PhtdSer)-MPs in the septic DIC group and control group. b, TF+-MP activity in the septic DIC group and control group.
The TF+-MP activity level (median 0.15 [0.09–0.37] nmol/L) at D1 in the septic DIC group was higher than that of the healthy control group (median 0.04 [0.03–0.07] nmol/L), and the difference was significant (p < 0.001). After the D3 to D5 conditions improved, TF+-MP activity gradually decreased (Figure 4b).
Microparticle correlation analysis
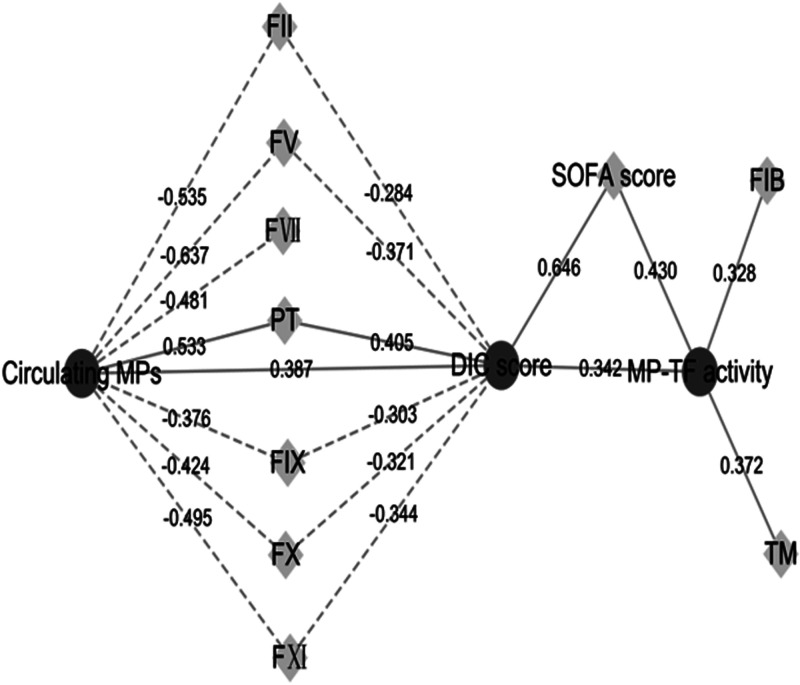
MP correlation analysis results showed that circulating MP levels and clotting factors (FII, FV, FVII, FX, FIX, and FXI) were negatively correlated in the septic DIC group (p < 0.001). A strong positive correlation (p < 0.001) was observed for PT, which is a coagulation index reflecting the consumption of exogenous clotting factors. Circulating MP levels were correlated with the DIC score (p < 0.001) and are closely related to the degree of coagulation disorder and the consumption of clotting factors.
No correlation was found between the TF+-MP activity and clotting factor levels (FII [ρ = −0.245], FV [ρ = −0.049], FVII [ρ = −0.085], FIX [ρ = −0.104], FX [ρ = −0.229], and FXI [ρ = −0.123]). TF+-MP activity was not associated with TFPI (ρ = 0.19). The TF+-MP activity was correlated with the DIC score (p = 0.002) and SOFA score (p < 0.001); when the DIC score and SOFA score were higher, TF+-MP activity was stronger. sTM is a marker of endothelial damage, and TF+-MP activity was correlated with the level of soluble TM (ρ = 0.372, p = 0.001; Figure 5).
Figure 5.
Correlation analysis results of microparticles (MPs). The dotted lines represent a negative correlation, and the solid lines represent a positive correlation. The value is the correlation coefficient. All p values <0.01.
Discussion
Flow cytometry mainly detects particles that are larger than 500 nm, and a few improved flow cytometers can detect particles at 200 nm with certain limitations. However, the exposure of membrane phosphatidylserine is characteristic of MP formation. A capture assay measures all clotting activities that are related to MPs by thrombin production, and it is more specific to MP-related clotting activities. Thus, we used the functional test method to measure the MPs.20,21
Our study found that compared with the healthy control group, circulating MP levels in patients with sepsis DIC at D1 to D5 were decreased. This result is consistent with previous research conclusions from other scholars. For example, compared with the healthy control group, the number of circulating MPs in patients with multiple organ dysfunction syndrome (MODS) and sepsis that was measured by flow cytometry was decreased.22 Although different from our quantitative method of analysis for MPs, the same results were achieved; in sepsis, the level of circulating MPs decreased. In addition, some experiments have noted that the circulating MP level in young infected patients is lower than that in young uninfected patients.23 However, some experimental results from other studies were contrary to our results. For example, studies have shown that total circulating MPs in patients with sepsis could be greater than in patients without sepsis. Patients with septic shock had higher levels of PhtdSer+-MPs with a phenotypic change in DIC. In DIC patients, endothelial-derived CD105+-MPs are increased as well as leucocyte-derived CD11a+-MPs when platelet-derived GPIb+-MPs were very low, reflecting thrombocytopenia.24–26 This finding may be related to patient selection, inclusion time, different sepsis stages, different detection methods, and unknown cellular modulation mechanism. There are many explanations for the decrease in circulating MP levels in sepsis DIC. For example, in sepsis DIC, circulating MPs are isolated in tissue organs through adhesion factors and other mechanisms, acting as an immune thrombus, whereas fewer circulating MPs are released.18 After monocyte activation, phagocytosis enhancement can increase the ability to clear MPs.23 In addition, decreased MP subtypes were noted in sepsis. For example, compared with the healthy control group, the level of monocyte-derived MPs in sepsis decreased, and MPs from leukocytes (CD45+) in septic shock patients were also decreased.27,28 All of the above factors may lead to a reduction in circulating MP levels.
There was a positive correlation between circulating MP levels and the DIC score in septic DIC, suggesting that circulating MPs may play a role in coagulation disorders. The coagulation cascade reaction shows the extent of clotting factor consumption, which can reflect the degree of coagulation activation. Some studies have noted that the surface of the MPs can enlarge the haemostasis reaction by exposing the binding sites of coagulation factors.29 A correlation was found between circulating MPs and coagulation factor consumption. Our experiments showed a significant negative correlation between circulating MP levels and coagulation factors (FII, FV, FVII, FIX, FX, and FXI) in septic DIC. A strong positive correlation with the PT index was noted, reflecting the consumption of coagulation factors in the external coagulation pathway. This finding further suggests that circulating MPs play an important role in activation of the coagulation pathway and consumption of coagulation factors. Combined with the results of the correlation analysis, the relatively high circulating MP levels may have a stronger ability to activate the coagulation pathway, thereby increasing the consumption of coagulation factors and the degree of coagulation disorders. Some studies have shown that the sepsis shock group has a higher number of circulating MPs and more significant clot formation compared with the sepsis group.30 This supports our hypothesis. Close correlations were found between the circulating MP levels in septic DIC and the endogenous pathway clotting factors FIX and FXI and the exogenous pathway clotting factor FVII. Circulating MPs play a major role through simultaneous activation of intrinsic and extrinsic coagulation pathways.
Previous experiments have investigated the role of TF+-MP activity or MPs from different cell sources in septic DIC.30–32 We evaluated the role of TF+-MP activity in septic DIC from the perspective of coagulation factor consumption and coagulation pathway activation. TF+-MP activity was not correlated with the clotting factors in the coagulation pathway. In septic DIC patients, TF+-MP activity is not closely related to clotting factor consumption. Therefore, although TF+-MP activity is enhanced in septic DIC and TF+-MP activity is also correlated with the DIC score and SOFA score, TF+-MPs activity does not play a major role in activation of the coagulation pathway and consumption of clotting factors. TF+-MP activity may play a role in predicting the severity of coagulation disorders and organ dysfunction in patients with septic DIC to a certain extent. The lack of correlation between TF+-MP activity and TFPI also suggests a weak relationship between TF+-MP activity and circulating active TF. In sepsis, active TF expression varies. For example, studies have shown that monocytes, non-haematopoietic cells, and endothelial cells can express TF and may play an important role in activating coagulation in a sepsis model. Selective inhibition of TF activity during treatment may reduce haemorrhagic complications.33 Our study suggests that TF+-MPs may not be the main form of active TF in septic DIC. It suggests that the strong procoagulant effect of TF in septic DIC may not be realised by TF+-MPs. This finding may have some significance to guide the direction of clinical research. Our experiments also show that circulating MP levels play an important role in the consumption of coagulation factors. The main mechanism of the pro-coagulant properties of circulating MPs may be related to other mechanisms, such as phospholipid and coagulation factor site exposure, rather than TF+-MP activity. In addition, the experimental results showed that TF+-MP activity was significantly correlated with TM. Endothelial injury can expose more TF and release endothelial MPs (EMPs) containing TF expression.34 This finding suggests that TF+-MP activity may result from endothelial injury in septic DIC.
In this experiment, no specific trends and significant differences in the changes in FIB in septic DIC were found. Although a positive correlation between TF+-MP activity and FIB was found, further studies are needed. The above results also suggest that the potential role of MPs may be complex and diverse. For example, treatment with recombinant human APC (rhAPC) significantly increased circulating endothelial protein C receptor-expressing MPs (EPCR+-MPs) in sepsis patients, and these MPs show anticoagulant activity and play a beneficial physiological role.35 Furthermore, different markers of the same MP may have different evaluation results. Therefore, more comprehensive consideration is required for the research and design of MP functions.
Limitations
Our experiment had some limitations. We did not thoroughly analyse the different cell sources of the MPs by flow cytometry. If the interval between the blood sample collection time points can be appropriately extended in septic patients, we may be able to better evaluate the sepsis recovery process. In addition, the sample size of patients who died was relatively small. The sample size should be increased, and further comparisons should be conducted.
Conclusion
The circulating MP levels were lower in patients with septic DIC than in healthy controls. The circulating MP levels play an important role in promoting coagulation activation and increasing clotting factor consumption. TF+-MPs activity may not be the main form of active TF. This suggests that we need a new understanding of the mechanism of circulating MPs that promote coagulation in patients with septic DIC.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This work was supported by the Research and Innovation Fund for Graduate Students of Harbin Medical University (project no: yjskycx2018-46hyd).
Authors’ contributions: Shishuai Meng designed and completed the experiment index test and wrote the article. Kai Kang, Dongshen Fei, and Songlin Yang collected and preserved the blood samples. Quankuan Gu coordinated the experiment. Mingyan Zhao and ShangHa Pan reviewed and verified the experimental progress.
ORCID iD: Mingyan Zhao https://orcid.org/0000-0001-5484-9817
References
- 1.Davis RP, Miller-Dorey S, Jenne CN. Platelets and coagulation in infection. Clin Transl Immunology 2016; 5: e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikezoe T. Thrombomodulin/activated protein C system in septic disseminated intravascular coagulation. J Intensive Care 2015; 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gando S, Shiraishi A, Yamakawa K, et al. Role of disseminated intravascular coagulation in severe sepsis . Thromb Res 2019; 178: 182–188. [DOI] [PubMed]
- 4.Levi M, Van Der Poll T. Coagulation and sepsis. Thromb Res 2017; 149: 38–44. [DOI] [PubMed] [Google Scholar]
- 5.Iba T, Yamada A, Hashiguchi N, et al. New therapeutic options for patients with sepsis and disseminated intravascular coagulation. Pol Arch Med Wewn 2014; 124: 321–328. [DOI] [PubMed] [Google Scholar]
- 6.Johnson BL, III, Kuethe JW, Caldwell CC. Neutrophil derived microvesicles: emerging role of a key mediator to the immune response. Endocr Metab Immune Disord Drug Targets 2014; 14: 210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Słomka A, Urban SK, Lukacs-Kornek V, et al. Large extracellular vesicles: have we found the holy grail of inflammation. Front Immunol 2018. 9: 2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang JG, Manly D, Kirchhofer D, et al. Levels of microparticle tissue factor activity correlate with coagulation activation in endotoxemic mice. J Thromb Haemost 2009; 7: 1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delabranche X, Berger A, Boisramé-Helms J, et al. Microparticles and infectious diseases. Med Mal Infect 2012; 42: 335–343. [DOI] [PubMed] [Google Scholar]
- 10.Meziani F, Delabranche X, Asfar P, et al. Bench-to-bedside review: circulating microparticles–a new player in sepsis. Crit Care 2010; 14: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Zhang S, Luo L, et al. Platelet-derived microparticles regulates thrombin generation via phophatidylserine in abdominal sepsis. J Cell Physiol 2018; 233: 1051–1060. [DOI] [PubMed] [Google Scholar]
- 12.Nieuwland R, Berckmans RJ, McGregor S, et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood 2000; 95: 930–935. [PubMed] [Google Scholar]
- 13.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor FB, Jr, Toh CH, Hoots WK, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost 2001; 86: 1327–1330. [PubMed] [Google Scholar]
- 15.Rosmini F. [ Points of view in comparison on the 5th revision of the Declaration of Helsinki]. Ann Ist Super Sanita 2002; 38: 169–174. [PubMed] [Google Scholar]
- 16.Council for International Organizations of Medical Sciences . International ethical guidelines for biomedical research involving human subjects. Bull Med Ethics 2002; 182: 17–23. [PubMed] [Google Scholar]
- 17.Khwannimit B, Bhurayanontachai R, Vattanavanit V. Comparison of the performance of SOFA, qSOFA and SIRS for predicting mortality and organ failure among sepsis patients admitted to the intensive care unit in a middle-income country. J Crit Care 2018; 44: 156–160. [DOI] [PubMed] [Google Scholar]
- 18.Shaver CM, Woods J, Clune JK, et al. Circulating microparticle levels are reduced in patients with ARDS. Crit Care 2017; 21: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khorana AA, Francis CW, Menzies KE, et al. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost 2008; 6: 1983–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szatanek R, Baj-Krzyworzeka M, Zimoch J, et al. The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci 2017; 18: 1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amiral J, Seghatchian J. The diagnostic usefulness of capture assays for measuring global/specific extracellular micro-particles in plasma. Transfus Apher Sci 2015; 53: 127–136. [DOI] [PubMed] [Google Scholar]
- 22.Joop K, Berckmans RJ, Nieuwland R, et al. Microparticles from patients with multiple organ dysfunction syndrome and sepsis support coagulation through multiple mechanisms. Thromb Haemost 2001; 85: 810–820. [PubMed] [Google Scholar]
- 23.Forest A, Pautas E, Ray P, et al. Circulating microparticles and procoagulant activity in elderly patients. J Gerontol A Biol Sci Med Sci 2010; 65: 414–420. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Meng H, Ma R, et al. Circulating microparticles, blood cells, and endothelium induce procoagulant activity in sepsis through phosphatidylserine exposure. Shock 2016; 45: 299–307. [DOI] [PubMed] [Google Scholar]
- 25.Delabranche X, Boisramé-Helms J, Asfar P, et al. Microparticles are new biomarkers of septic shock-induced disseminated intravascular coagulopathy. Intensive Care Med 2013; 39: 1695–1703. [DOI] [PubMed] [Google Scholar]
- 26.Boscolo A, Campello E, Bertini D, et al. Levels of circulating microparticles in septic shock and sepsis-related complications: a case-control study. Minerva Anestesiol 2019; 85: 625–634. [DOI] [PubMed] [Google Scholar]
- 27.Sumi YI, Ogura H, Tanaka H, et al. Paradoxical cytoskeleton and microparticle formation changes in monocytes and polymorphonuclear leukocytes in severe systemic inflammatory response syndrome patients. J Trauma 2003; 55: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 28.Mostefai HA, Meziani F, Mastronardi ML, et al. Circulating microparticles from patients with septic shock exert protective role in vascular function. Am J Respir Crit Care Med 2008; 178: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 29.Sierko E, Sokół M, Wojtukiewicz MZ. [ Endothelial microparticles (EMP) in physiology and pathology]. Postepy Hig Med Dosw (Online) 2015; 69: 925–932. [DOI] [PubMed] [Google Scholar]
- 30.Hellum M, Øvstebø R, Brusletto BS, et al. Microparticle-associated tissue factor activity correlates with plasma levels of bacterial lipopolysaccharides in meningococcal septic shock. Thromb Res 2014; 133: 507–514. [DOI] [PubMed] [Google Scholar]
- 31.Woei-A-Jin FJ, Van Der Starre WE, Tesselaar ME, et al. Procoagulant tissue factor activity on microparticles is associated with disease severity and bacteremia in febrile urinary tract infections. Thromb Res 2014; 133: 799–803. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto H, Yamakawa K, Ogura H, et al. Clinical significance of tissue factor and CD13 double-positive microparticles in Sirs patients with trauma and severe sepsis. Shock 2017; 47: 409–415. [DOI] [PubMed] [Google Scholar]
- 33.Pawlinski R, Mackman N. Cellular sources of tissue factor in endotoxemia and sepsis. Thromb Res 2010; 125: S70–S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto H, Yamakawa K, Ogura H, et al. Enhanced expression of cell-specific surface antigens on endothelial microparticles in sepsis-induced disseminated intravascular coagulation. Shock 2015; 43: 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pérez-Casal M, Thompson V, Downey C, et al. The clinical and functional relevance of microparticles induced by activated protein C treatment in sepsis. Crit Care 2011; 15: R195. [DOI] [PMC free article] [PubMed] [Google Scholar]