Supplemental Digital Content is available in the text.
Keywords: alanine aminotransferase, aspartate aminotransferase, COVID-19, liver injury, SARS-CoV-2
Abstract
Background/aims
The number of cases with coronavirus disease 2019 (COVID-19) has exceeded seven million worldwide. However, the data describing the global prevalence of liver injury associated with COVID-19 is lacking secondary to the novelty of this ongoing pandemic. Therefore, we conducted a meta-analysis to determine the association between COVID-19 and liver injury.
Methods
A systematic literature search of indexed databases including, PubMed, Medline, and Embase databases from inception to 14 April 2020, was used to identify studies that reported data of liver chemistry in patients diagnosed with COVID 19. The overall prevalence of abnormal liver chemistry and relevant 95% confidence interval was used to estimate the pooled results studies.
Results
Sixty-four studies with 11 245 patients with COVID-19 were included. The pattern of abnormal liver enzymes was notable for higher aspartate aminotransferase (AST) than alanine aminotransferase (ALT) levels. The overall global prevalence of elevated AST, ALT, total bilirubin, gamma-glutamyltransferase (GGT), and alkaline phosphatase was 23.2, 21.2, 9.7, 15.0, and 4.0%, respectively. The prevalence of elevated AST was substantially higher among those with severe cases (45.5%) compared to non-severe cases (15.0%). Co-existing chronic liver disease presented up to 37.6% of patients with COVID-19.
Conclusion
A fourth of COVID-19 patients had elevated liver enzymes and associated with disease severity. Our study may be used as a guide for clinicians and epidemiologists to proactively identify other sources of injury and illness in patients diagnosed with COVID-19. Intensive monitoring for liver injury may be needed in cases with severe COVID-19.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus more commonly referred to as ‘coronavirus’ and the infectious disease it causes coronavirus disease 2019 (COVID-19). The first reservoir of SARS-COV-2 and disease outbreak was identified in Wuhan, China, in December 2019. As of May 2020, the number of global cases has exceeded seven million worldwide, with the WHO declaring the outbreak of a pandemic in March 2020.
While respiratory and systemic symptoms such as fever, fatigue, and myalgia have been the primary manifestations of symptomatic COVID-19, literature has reported gastrointestinal and hepatic manifestations. Symptoms of nausea, vomiting, and abdominal pain have been reported by infected patients [1,2]. In addition, indirect and direct evidence of liver injury via abnormal liver enzymes and postmortem biopsies identifying moderate microvesicular steatosis with mild lobular and portal inflammation has been reported [3].
SARS-CoV-2 is primarily spread via droplet transmission with respiratory symptoms being the most common manifestation secondary to host seeding via angiotensin-converting enzyme 2 (ACE2) receptors primarily found in type II alveolar cells of lungs. However, ACE2 receptors are also extensively expressed within biliary and hepatic epithelial cells, posing a potential nidus of infection [4]. Gastrointestinal and hepatic manifestations of COVID-19, in addition to literature identifying viral shedding in feces, raises the question of a possible fecal-oral route of transmission or hepatic first-pass effect [2,5,6].
Recent studies suggest a significant prevalence of abnormal aminotransferase levels in patients with COVID-19. However, data describing the global prevalence of liver injury associated with COVID-19 is lacking. Currently, there is no systematic review and meta-analysis summarizing the available studies documenting liver chemistry in patients with COVID-19. The aim of our study was to investigate the association between COVID-19 and liver injury by performing a systematic review and meta-analysis on available indexed studies.
Methods
Study protocol and search strategy
A systematic literature search of indexed databases including, PubMed, Medline, and Embase databases inception to 14 April 2020, was used to identify all available studies that reported data of liver chemistry. Reported data included aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, alkaline phosphatase (ALP), and gamma-glutamyltransferase (GGT), among patients with COVID-19. Three investigators (K.W., P.P., and P.U.) independently screened and reviewed the literature using the search strategy that included the terms ‘COVID-19’, ‘novel coronavirus 2019’, ‘liver chemistry’, ‘liver function test’, ‘laboratory data’, and ‘clinical characteristics’ as described in Supplementary Methods, supplement digital content 1, http://links.lww.com/EJGH/A563. Limitations based on language, publication status, or publication dates were not applied. An additional literature review was performed using the bibliography of the included articles. The systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline provided in the Supplementary Method, supplement digital content 1, http://links.lww.com/EJGH/A563 [7].
Eligibility criteria
To be eligible for this meta-analysis, studies must meet the following inclusion criteria: (1) observational study; (2) study reports liver chemistry data including AST, ALT, total bilirubin, ALP, and GGT among patients diagnosed with SARS-CoV-2 (COVID-19). Review articles, book chapters, or expert opinions were excluded. Inclusion was not limited by study size, and retrieved articles were reviewed independently for inclusion by the same three investigators (K.W., P.U., and P.P.) with disagreements resolved by consensus.
Data abstraction
The investigators used a data collection form to extract the following data: name of the first author, publication date, study date, region where the study was conducted, number of participants, demographics of participants, methods used to determine COVID-19 diagnosis, data of liver chemistry, and concurrent chronic liver disease. Methods used to diagnose SARS-Cov-2 infection was dependent upon each study and included real-time reverse transcription PCR (RT-PCR) and next-generation sequencing, quantitative RT-PCR, clinical criteria of novel coronavirus-infected pneumonia, and viral nucleic acid identification. The degree of severity of COVID-19 (severe- vs. non-severe) was defined using the American Thoracic Society guidelines for community-acquired pneumonia [8]. To ensure accuracy, this data extraction process was independently performed by three investigators (K.W., P.P., and P.U.) and was reviewed by the senior investigator (D.K.).
Statistical analysis
The overall prevalence of abnormal liver chemistry and relevant 95% confidence interval (CI) was used to estimate the pooled results of the studies. A random-effect model was chosen because the consensus belief that a fixed-effect model that all studies should yield the same result is universally not true for observational studies. The heterogeneity test was conducted by using the I2 statistic to quantify the variability between the included studies. A value of I2 of 0–25% represents insignificant heterogeneity, 26–50% represents low heterogeneity, 51–75% represents moderate heterogeneity, and more than 75% represents high heterogeneity [9]. We used Comprehensive Meta-analysis 3.0 software (Englewood, New Jersey, USA) for statistical analysis.
Results
A total of 563 indexed articles were identified for potential inclusion using the described search strategy (86 from PubMed, 297 from MEDLINE, 174 from EMBASE, and six from other sources). After the exclusion of 240 duplicated articles, 323 unique articles were identified. Titles and abstracts of the 323 unique articles were reviewed. Two hundred fifty-nine articles were further excluded after an independent full-length review based on the aforementioned exclusion criteria. A total of 64 studies with 11 245 patients diagnosed with COVID-19 were included in this meta-analysis. The literature retrieval, review, and selection process are shown in detail in Fig. 1. The details of each study are described in Supplementary Table 1, supplement digital content 2, http://links.lww.com/EJGH/A564. The mean value of overall AST, ALT, total bilirubin, ALP, and GGT are 32.9, 31.8, 10.1, 68.1, and 35.9, respectively (Table 1). Notably, COVID-19 patients who were admitted to the ICU tended to have higher AST and ALT levels than those who were not admitted to an ICU. Compared to patients with non-severe COVID-19, those with severe disease had higher liver enzymes. Patients with gastrointestinal symptoms (nausea, vomiting, diarrhea, etc.) had higher AST and ALT levels than those without gastrointestinal symptoms. The pattern of abnormal liver enzymes among patients with COVID-19 was notable for higher AST than ALT levels, predominantly in ICU cases. As shown in Fig. 2, the overall prevalence of elevated AST was 23.2% (95% CI 19.8–27.1), and elevated ALT was 21.2% (95% CI 17.2–25.9). The prevalence of elevated total bilirubin, GGT, and ALP was 9.7% (95% CI 6.8–13.8), 15.0% (10.3–21.3), and 4.0% (2.5–6.3), respectively. The prevalence of elevated AST was substantially higher among those with severe cases (45.5%) compared to non-severe cases (15.0%). While most studies were from China (22.8% for AST; 22.3% for ALT), the prevalence of elevated AST and ALT was comparable in non-Chinese studies (42.0% for AST; 21.7% for ALT). We noted co-existing chronic liver disease up to 37.6% of patients with COVID-19. Increased prevalence and higher levels of mean liver enzymes elevation were noted in pregnant women compared to non-pregnant cases.
Fig. 1.
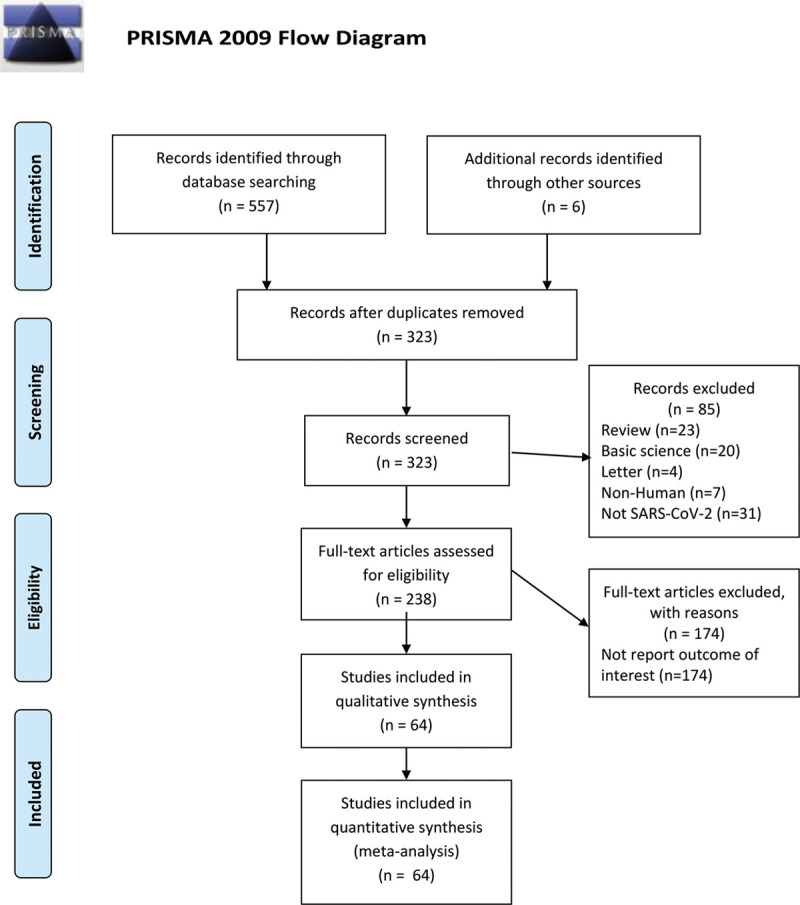
Literature review process.
Table 1.
Average value of liver chemistry and prevalence of elevated liver chemistry among patients with confirmed COVID-19
| AST | ALT | Total bilirubin | ALP | GGT | |
|---|---|---|---|---|---|
| Overall | |||||
| Overall | 32.9 (n = 4876) | 31.8 (n = 5098) | 10.1 (n = 2656) | 68.1 (n = 1274) | 35.9 (n = 1228) |
| Adult | 29.7 (n = 4683) | 27.6 (n = 4818) | 10.5 (n = 2575) | 68.1 (n = 1274) | 35.9 (n = 1228) |
| Children | 29.0 (n = 12) | 16.3 (n = 37) | NA | NA | NA |
| Pregnancy | 171.0 (n = 9) | 253.8 (n = 9) | NA | NA | NA |
| ICU | |||||
| Overall | 102.5 (n = 77) | 56.0 (n = 77) | 13.2 (n = 77) | 83.6 (n = 7) | 47.4 (n = 7) |
| Adult | 102.5 (n = 77) | 56.0 (n = 77) | 13.2 (n = 77) | 83.6 (n = 7) | 47.4 (n = 7) |
| Non-ICU | |||||
| Overall | 31.5 (n = 130) | 25.0 (n = 130) | 10.1 (n = 130) | NA | NA |
| Adult | 31.5 (n = 130) | 25.0 (n = 130) | 10.1 (n = 130) | NA | NA |
| GI symptoms | |||||
| Overall | 41.8 (n = 426) | 49.5 (n = 426) | 11.9 (n = 177) | NA | NA |
| Adult | 41.8 (n = 426) | 49.5 (n = 426) | 11.9 (n = 177) | NA | NA |
| Non-GI symptoms | |||||
| Overall | 33.0 (n = 866) | 37.4 (n = 866) | 11.6 (n = 678) | NA | NA |
| Adult | 33.0 (n = 866) | 37.4 (n = 866) | 11.6 (n = 678) | NA | NA |
| Non-severe cases | |||||
| Overall | 25.4 (n = 1825) | 22.4 (n = 1819) | 9.0 (n = 626) | 64.3 (n = 382) | 26.3 (n = 382) |
| Adult | 26.6 (n = 1737) | 22.9 (n = 1737) | 9.0 (n = 626) | 64.3 (n = 382) | 26.3 (n = 382) |
| Severe cases | |||||
| Overall | 37.2 (n = 928) | 31.5 (n = 924) | 11.1 (n = 196) | 69.3 (n = 57) | 42.5 (n = 57) |
| Adult | 39.5 (n = 915) | 32.6 (n = 915) | 11.1 (n = 196) | 69.3 (n = 57) | 42.5 (n = 57) |
| China | |||||
| Overall | 32.1 (n = 4796) | 32.2 (n = 5018) | 10.4(n = 2581) | 68.3 (n = 1209) | 35.9 (n = 1228) |
| Adult | 29.1 (n = 4618) | 27.5 (n = 4748) | 10.7 (n = 2510) | 68.3 (n = 1209) | 35.9 (n = 1228) |
| Children | 29.0 (n = 12) | 16.3 (n = 37) | NA | NA | NA |
| Pregnancy | 171.0 (n = 9) | 253.8 (n = 9) | NA | NA | NA |
| Outside China | |||||
| Overall | 32.0 (n = 80) | 27.5 (n = 80) | 6.4 (n = 75) | 67.0 (n = 65) | NA |
| Adult | 38.0 (n = 70) | 29.3 (n = 70) | 6.8 (n = 65) | 67.0 (n = 65) | NA |
| Children | NA | NA | NA | NA | NA |
| Pregnancy | NA | NA | NA | NA | NA |
The degree of severity of COVID-19 (severe vs. non-severe) was defined using the American Thoracic Society guidelines for community-acquired pneumonia.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase.
Fig. 2.
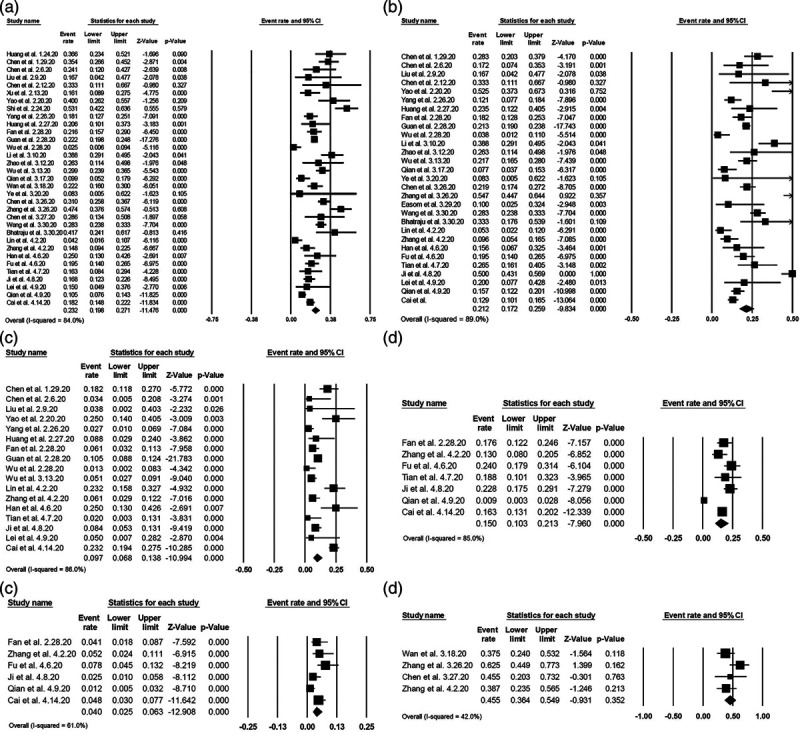
Forest plots of the included studies evaluating abnormal liver chemistries among patients with COVID-19. (a) Prevalence of elevated aspartate aminotransferase. (b) Prevalence of elevated alanine aminotransferase. (c) Prevalence of elevated total bilirubin. (d) Prevalence of elevated gamma-glutamyltransferase. (e) Prevalence of elevated alkaline phosphatase. (f) Prevalence of elevated aspartate aminotransferase in patients with severe COVID-19.
Discussions
In this meta-analysis of liver injury among patients with confirmed COVID-19, we found that AST and ALT elevations were a substantial burden in patients with COVID-19. The overall global prevalence of elevated AST, ALT, total bilirubin, GGT, and ALP was 23.2, 21.2, 9.7, 15.0, and 4.0%, respectively. Approximately 25% of patients with COVID-19 had elevated liver enzymes. In addition, our analysis aligns with previous studies reflecting a direct relationship between liver enzyme elevation and disease activity [10]. The prevalence of elevated AST was noted to be higher than ALT levels and substantially higher among those with severe cases (45.5%) compared to non-severe cases (15.0%). Lei et al. [11] reported an association of liver injury based on markers of hepatic injury and inpatient mortality in patients diagnosed with COVID-19, specifically an association with AST abnormality and risk of mortality compared to other indicators of liver injury during hospitalization. However, other studies confirming such findings are still limited [11]. Currently, the mechanism of SARS-CoV-2-associated liver injury remains unknown; previous SARS-CoV strains have been shown to injure liver parenchyma leading to lobular inflammation and apoptosis of hepatocytes [12]. It is known that the SARS-CoV-2 functional receptor, ACE2, is not only found in pulmonary parenchyma but also biliary and hepatic epithelia [12]. Detection of SARS-CoV-2 in stool and blood samples may implicate the possibility of viral exposure within the liver, which may suggest universal hepatic involvement via a first-pass effect irrespective of elevation of liver or biliary enzymes [4]. Histology from liver biopsies has shown moderate microvesicular steatosis with mild lobular and portal inflammation indicative of either direct viral injury or drug-induced liver injury [3]. It is hypothesized that a direct-virus induced cytopathic effect or a provoked immunological response from inflammatory cytokines may be a source of liver injury [13,14]. In addition, medical management of COVID-19 and patient co-morbidities with antivirals, antibiotics, and steroids, may also contribute to liver injury in patients with COVID-19. Drug-induced liver injury has been reported with the use of antiviral, remdesivir, and IL-6 inhibitor (tocilizumab). Our meta-analysis determined that patients with gastrointestinal symptoms had higher AST and ALT levels than those without [15], supporting previous literature reflecting ACE2 receptor expression within the gastrointestinal tract. Although ACE2 receptor is mainly expressed within the biliary tree, our study showed a predominance of parenchymal liver injury based on the prevalence of elevated AST (23.2%) and elevated ALT (21.2%) rather than damage to bile ducts, as reflected by GGT (9.7%) and ALP 4.0% in patients with COVID-19. Previous SARS-CoV strains have been shown to injure liver parenchyma leading to lobular inflammation and apoptosis of hepatocytes [12]. While we hypothesize that patients with chronic liver disease may have elevated liver enzymes at baseline, the novelty of this virus and ongoing pandemic limits comparative studies at this time. Because of the direct association between the number of accompanying comorbidities and mortality in patients diagnosed with COVID-19, identification of both primary and secondary presentations of disease manifestation should be identified as quickly as possible and proactively managed. Furthermore, patients with COVID-19 and co-existing end-stage liver disease may be susceptible to more pronounced liver injury compared with those without. Future studies are needed for this special sub-population to better understand medical and pharmacological management of patients with end-stage liver disease. As we learn more about the evolution of the COVID-19 pandemic, clinicians may continue to check and monitor for liver injury in patients with COVID-19. Notably, more intensive monitoring for liver injury may be needed in cases with severe COVID-19 or liver comorbidities
There are several strengths of this current study. This is the first systematic review and meta-analysis that summarizes the rapidly emerging literature identifying the prevalence of abnormal liver chemistry in COVID-19 globally. This comprehensive review of 64 indexed studies allows for a more accurate and valid estimate of the prevalence of abnormal liver chemistry in COVID-19 patients worldwide. Our study highlights that the severity of COVID-19 disease is directly associated with the elevation of AST from the subgroup analysis. However, the limitations of the study should be noted. This systematic review and meta-analysis analyzed data primarily from China and homogenous; systematic data collection was limited in most of the studies. Currently, large studies outside of China are lacking, likely due to the delay in spread to other parts of the world. Although this limits identification and prevalence of liver injury globally and in other racial groups, our study may be used as a guide for clinicians and epidemiologists to proactively identify other sources of injury and illness in patients diagnosed with COVID-19.
In summary, this study reported the overall global prevalence of elevated AST was 23.2%, and elevated ALT was 21.2% in patients diagnosed with COVID-19. Approximately 25% of COVID-19 patients had elevated liver enzymes directly related to the severity of COVID-19 disease. More intensive monitoring for liver injury may be needed in cases with severe COVID-19.
Acknowledgements
K.W. was involved in study concept and design, acquisition of data, analysis, and interpretation of data, and drafting of the manuscript. P.U. was involved in study concept and design, acquisition of data, analysis, and interpretation of data, and critical revision of the manuscript. P.P. and H.B.Z. were involved in acquisition of the data, interpretation of data, and drafting the manuscript. D.M.H. and A.A. were involved in study concept, critical revision of the manuscript, and study supervision. D.K. was involved in study concept and design, interpretation of data, drafting of the manuscript, critical revision of the manuscript, and study supervision.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.eurojgh.com.
References
- 1.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020; 5:529–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Jama. 2020; 323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020; 8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020:2020.02.03.931766. [Google Scholar]
- 5.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020. doi: 10.1111/all.14238. [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009; 62:e1–e34. [DOI] [PubMed] [Google Scholar]
- 8.Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. 2019; 200:e45–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020; 395:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020:10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, et al. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004; 39: 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Z, Chen L, Li J, Yang J, Tian C, Zhang Y, et al. Clinical Features of COVID-19 Related Liver Damage. medRxiv. 2020:2020.02.26.20026971. [Google Scholar]
- 14.Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020; 158:1518–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of gastrointestinal symptoms on patients infected with COVID-19. Gastroenterology. 2020; 158:2294–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]


