Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic requires appropriate measures for containing infection spreading. Endoscopic procedures are considered at increased risk of infection transmission. We evaluated organizational aspects and personal behaviours in Italian Endoscopic Units during phase II of the pandemic.
Methods
A questionnaire on organizational aspects and use of personal protective equipment (PPE) were e-mailed to gastroenterologists working in Endoscopic Units. Data were analysed accordingly to the National Health Institute and Gastroenterology Societies recommendations.
Results
Data of 117 centres were collected, and different shortcomings emerged. Specific protocols for containing infection and training programs for operators were lacking in 20 and 30% of centres, respectively, and telephone triage 24–72 h before the endoscopy was not implemented in 25% of hospitals. In 30% of centres, the slot time for endoscopies and between examinations was not prolonged. PPE, masks, shirts and gloves were universally adopted, although with some differences. In 20% of centres, a FFPE-FFP3 mask was not adopted during endoscopic examinations. Postendoscopy patient tracking/contact was completed in only one-third of centres.
Conclusions
Our survey provides information on organizational and medical behaviours during COVID-19 phase II in Italy, which could be useful for adopting appropriate measures for containing COVID-19 spread during phase II.
Keywords: coronavirus disease 2019, digestive endoscopy, organization, protection
Introduction
The current coronavirus disease 2019 (COVID-19) pandemic raised relevant shortcomings in implementing immediate and adequate measures to contain the spread of infection, especially in the hospital areas [1–6]. Although virus transmission mainly relies on air with salivary droplets, biological liquids may be involved, such as saliva and faeces, where complete viral particles are present, even for long periods following infection recovery [7–11]. According to the National Health Institute recommendations, only urgent endoscopic examinations were carried out in Italy during the peak of infection outbreak (phase I) [2]. The containment measures implemented in different European countries have allowed some countries to overcome the peak phase, and in June 2020, Italy entered into phase II. As such, endoscopy activity was resumed, including outpatients needing examination within 2 months, according to official advice [12–16]. A recent survey showed that containment measures for phase I COVID-19 infection recommended by the National Health Institute and Gastroenterology Societies were, at least in part, disregarded in Italy [17]. An Italian survey showed that gastrointestinal endoscopy appears to be relatively safe for both patients and operators during phase I when using adequate protective measures [19]. Therefore, we designed this survey to evaluate the organizational aspects implemented in endoscopic units during phase II of the pandemic.
Methods
Two authors (F.M. and R.V.) prepared a specific questionnaire on behalf of AIGO (Italian Association of Hospital Gastroenterologists and Endoscopists) [20]. The questionnaire included two domains, one on the organizational aspects (17 items) and one on the use of different personal protective equipment (PPE) (six items). It was e-mailed to AIGO associates who were required to anonymously reply by accessing a specific link. Information on hospitals’ setting was also registered. Data were analysed accordingly to the National Health Institute and Gastroenterology Societies recommendations.
Results
A total of 117 Digestive Endoscopy Units, distributed across the entire country, took part in the survey, including public (70%), academic (20%) and private (10%) hospitals (Fig. 1). More than 4000, between 2000 and 4000 and less than 2000 endoscopic examinations yearly were performed in 58, 35 and 7% of involved centres, respectively. Questions and answers of the questionnaire are provided in Table 1. Specific protocols for containing infections according to recommendations were not implemented in nearly 20% of centres, and training or requalification programs for adequate containment measures (social distancing, ambient disinfection, adequate use of PPE) were missed in 3 out of every 10 endoscopy Units. For outpatients, a telephone triage 24–72 h before the procedure was performed in 75% of the centres (by nursing staffs in 40%, doctors in 17%, and administrative personnel in 26% of cases). However, all centres accomplished a triage on the day of the examination, postponing endoscopy in suspected cases. Nearly three-quarter of responders asserted performing COVID-19 diagnostic tests before endoscopic examination in all inpatients and in outpatients with positive triage, whilst in the remaining centres (26.5%) all patients were potentially considered as infected so that no preliminary diagnostic tests were carried out. The number of daily endoscopic examinations was reduced in all centres, as a result of applying the suggested preventive measures (distancing, triage, use of PPE, environments sanitization, aeration, etc.). Specifically, the duration of upper endoscopy and colonoscopy was increased by 10–30 min in 55 and 70% of centres, respectively, whilst that of endoscopic retrograde choloangiopancreatography and endoscopic ultrasound was prolonged in only 32% of hospitals. The scheduled 30 min interval between two examinations for ambient aeration was adopted in only one-quarter of centres, and dedicated endoscopic rooms for COVID-19 positive patients were organized in less than half of surveyed hospitals. Postendoscopy patient tracking/contact suggested in guidelines was completed in one-third of centres. Regarding the use of PPE, masks, shirts and gloves were universally adopted, although with some differences. In 79% of endoscopy units, working staff wear masks with filtering capacity FFPE-FFP3 with associated visor in 28.6% or with additional surgical mask (13.7%), whilst in the remaining 21% centres, only a surgical mask was used. In nearly 40% of centres, prolonged or reuse or sanitization masks was adopted.
Fig. 1.
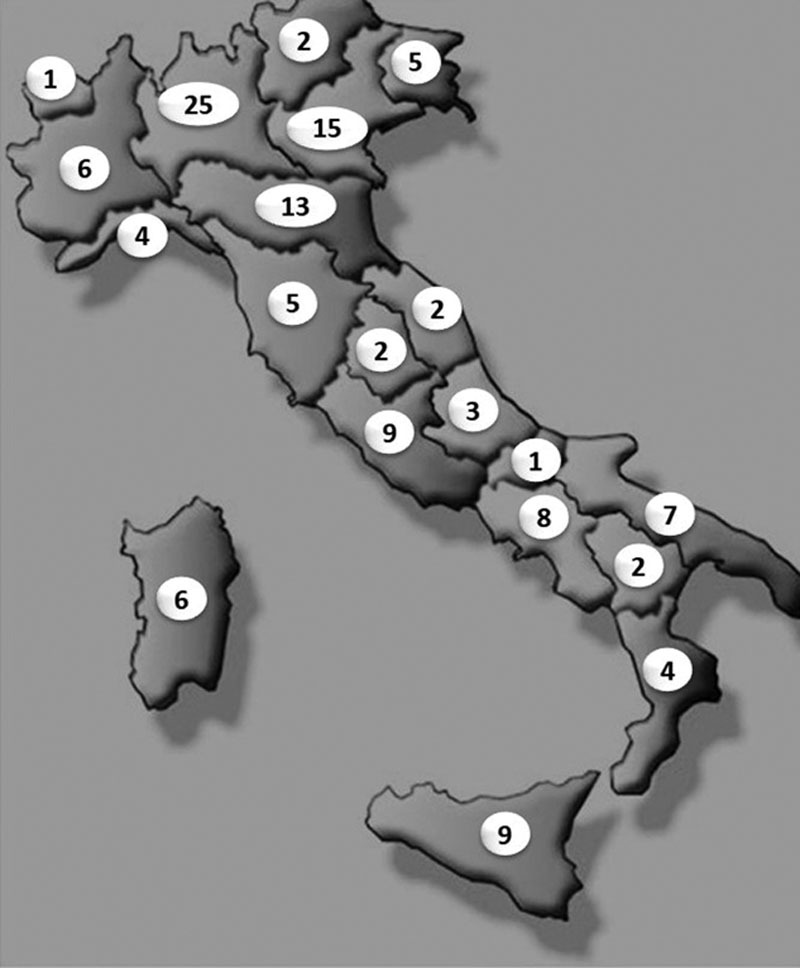
Distribution of participating centres across Italy. The number indicates centres involved in each Italian Region.
Table 1.
Questions and answers
| Question: Have you… | Yes | Not | NAa |
|---|---|---|---|
| Organizational aspects | |||
| Institutional specific COVID protocol? | 96 (82) | 21 (18) | – |
| Performed training programs for endoscopic personnel? | 80 (69) | 36 (31) | 1 |
| Performed pretriage call 24 h or more before examination? | 87 (75) | 29 (25) | 1 |
| Postponed the procedure if positive pretriage? | 89 (100) | – | 28 |
| Postponed procedure if positive clinical or physical triage preprocedure were found? | 111 (95) | 6 (5) | – |
| Postponed the procedure after COVID swab and/or serological test, if triage was positive? | 75 (67) | 37 (33) | 5 |
| Performed COVID swab and/or serological test, before procedure in all patients? | 86 (74) | 31 (26) | – |
| Prolonged slot time for endoscopic procedures? | 94 (80) | 23 (20) | – |
| EGD | 52 (55) | 42 (45) | 23 |
| From standard 30–40 min | 34 | ||
| From standard 30–50 min | 11 | ||
| From standard 30–60 min | 7 | ||
| Colonoscopy | 61 (70) | 26 (30) | 30 |
| From standard 50–60 min | 50 | ||
| From standard 50–70 min | 11 | ||
| ERCP/EUS (operative) | 20 (32) | 43 (68) | 54 |
| From standard 90–100 min | 11 | ||
| From standard 90–120 min | 9 | ||
| Polypectomy (standard 10 min) | 44 (65) | 24 (35) | 49 |
| From standard 10–20 min | 27 | ||
| From standard 10–30 min | 17 | ||
| Prolonged interval between procedures 30/60 min? | 29 (25) | 87 (75) | 1 |
| Performed endoscopic room sanitization between procedures? | 66 (57) | 50 (43) | 1 |
| Adopted different rooms for COVID patients? | 49 (42) | 68 (58) | – |
| Adopted safe biopsy transport (closed box) | 61 (53) | 55 (47) | 1 |
| Adopted re-call interview after 14 days | 42 (36) | 75 (64) | – |
| Personal protective equipment use | |||
| Used respirator during procedures | 117 (100) | – | – |
| FFP2/FFP3 respirator | 104 | ||
| Surgical mask | 13 | ||
| Add on face shield to surgical mask to FFP2/FFP3? | 74 (63) | 43 (37) | – |
| Adopted prolonged or reuse or sanitization mask? | 73 (62) | 44 (38) | – |
| Prolonged respirator use up to 8 h | 58 | ||
| Reuse after 7 days | 3 | ||
| Sanitization (alcool, H2O2, Heat, UV) | 12 | ||
| Adopted head-set use | 104 (89) | 12 (11) | |
| Adopted overshoes | 76 (65) | 41 (35) | – |
| Adopted double gloves | 91 (78) | 26 (22) | – |
COVID, coronavirus disease; EGD, esophagogastroduodenoscopy; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound.
Not available; N (%).
Discussion
This survey evaluated the organizational aspects for limiting COVID-19 diffusion in Endoscopic Units during phase II of pandemic. In detail, specific statements were provided by different Institutional and Scientific organizations for optimizing governance of phase II infection, namely when endoscopic examinations were also allowed for outpatients without emergency. More than 100 centres were involved, so that data are consistent. Basically, the results showed that official recommendations were not followed in a rate ranging from 20–30% [12–16]. Among other shortcomings, patient preselection by a telephone triage the days before endoscopy was lacking in more than 20% of centres, which is an impressive finding. Indeed, this procedure would limit infection outbreak in the community by preventing subjects with suspected infection to move into the hospital setting and would refer them to a general practitioner for COVID-19 appropriate screening [1–6,12–16,18]. We found that the time slot for the endoscopic procedures was not increased by over 30% of the centres. It may be argued that this affected compliance with the containment behaviours (triage, dressing/undressing PPE, environments sanitization, the interval between exams, etc.) of operators. Moreover, the suggested interval between two endoscopic examinations (30 min in case of negative pressure room and 60 min in standard room) was accomplished only in a quarter of interviewed centres, and postendoscopy patient tracking/contact at 2 weeks was completed only in one-third of centres [12,16]. All these behaviours might exert understanding harmful effects for both operators and patients. Another critical issue was that a training program for operators on the correct spacing, dirty/clean paths, PPE use (donning/doffing, fit, buddy system, safe reuse, etc.) was not implemented in 30% of centres [1–6,12–16].
As far as the PPE use is concerned, in about 20% of the centres, PPE with lower containment capacity (surgical masks, nonwaterproof gowns, single pair of gloves) were used in low-risk patients. This surely provides cost savings, but expose operators to the risk of COVID transmission in case of asymptomatic infected patients. Moreover, in near half centres, the masks were used for a time prolonged up to 8 hours, as well as head-set, overshoes and double gloves use was lacking in 20–30% of centres.
In conclusion, data of our survey showed that appropriate organizational and medical behaviours were adopted in the majority of Italian Endoscopic Units during COVID-19 phase II. However, some shortcomings emerged (i.e. preendoscopic triage, prolongation of timing between endoscopies, dedicated endoscopic room, patient’s follow-up were not appropriate in 20–50% of centres, etc.). Therefore, adhesion to preventive measures according to guidelines/recommendations should be improved, particularly when considering the risk for both operators and patients of COVID-19 infection and further pandemic diffusion. These information might be particularly useful for implementing protective behaviours in prevision of a potential pandemic recurrence in the next autumn in our country—as well as in others—where the infection is in phase II, and in adopting appropriate measures in those still in phase I. The battle against the pandemic continues!
Acknowledgements
None.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gralnek IM, Hassan C, Beilenhoff U, Antonelli G, Ebigbo A, Pellisè M, et al. ESGE and ESGENA position statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy. 2020; 52:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19: Consigli FISMAD per l’assistenza ai pazienti con malattie dell’apparato digerente e per gli operatori sanitari in Gastroenterologia. https://fismad.it/wp-content/uploads/2020/04/FISMAD_COVID19_REV01_ita.pdf. [Accessed 29 July 2020]
- 3.Repici A, Maselli R, Colombo M, Gabbiadini R, Spadaccini M, Anderloni A, et al. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020; 92:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soetikno R, Teoh AYB, Kaltenbach T, Lau JYW, Asokkumar R, Cabral-Prodigalidad P, Shergill A. Considerations in performing endoscopy during the COVID-19 pandemic. Gastrointest Endosc. 2020; 92:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Razai MS, Doerholt K, Ladhani S, Oakeshott P. Coronavirus disease 2019 (COVID-19): a guide for UK GPs. BMJ. 2020; 368:m989. [DOI] [PubMed] [Google Scholar]
- 6.Sultan S, Lim JK, Altayar O, Davitkov P, Feuerstein JD, Siddique SM, et al. AGA Institute rapid recommendations for gastrointestinal procedures during the COVID-19 pandemic. Gastroenterology. 2020; 159:739–758.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C, Gao G, Xu Y, Pu L, Wang Q, Wang L, et al. SARS-Cov-2–positive sputum and feces after conversion of pharyngeal samples in patients with COVID-19. Ann Int Med. 2020; 172:832–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020; 51:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin AWH, Chu J, Perera M, Hui KPY, Yen HL, Chan MCW, et al. Stability of SARS-COV-2 in different environmental conditions. Lancet Microbe. 2020; 1:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong Cohort: systematic review and meta-analysis. Gastroenterology. 2020; 159:81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020; 382:1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gastroenterologia ed Endoscopia Digestiva (fase II Emergenza COVID) Documento FISMAD – Parte 1: Attività di endoscopia digestive. https://fismad.it/wpcontent/uploads/2020/04/FISMADCovid19Fase2_Parte1ENDO.pdf. [Accessed 29 July 2020]
- 13.Digestive Health Physicians Association. Joint AGA/DHPA guidance: recommendations for resumption of elective endoscopy during the COVID-19 pandemic. https://www.dhpassociation.org/2020/04/27/aga-dhpa-resume-endoscopy-covid19. [Accessed 29 July 2020]
- 14.Chiu PWY, Ng SC, Inoue H, Reddy DN, Ling Hu E, Cho JY, et al. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements). Gut. 2020; 69:991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayee B, Thoufeeq M, Rees CJ, Penman I, East J. Safely restarting GI endoscopy in the era of COVID-19. Gut. 2020; 69:2063–2070. [DOI] [PubMed] [Google Scholar]
- 16.Gralnek IM, Hassan C, Beilenhoff U, Antonelli G, Ebigbo A, Pellisè M, et al. ESGE and ESGENA position statement on gastrointestinal endoscopy and COVID-19: an update on guidance during the post lockdown phase and selected results from a membership survey. Endoscopy. 2020; 52:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Repici A, Pace F, Gabbiadini R, Colombo M, Hassan C, Dinelli M; ITALIAN GI-COVID19 Working Group. Endoscopy units and the coronavirus disease 2019 outbreak: a multicenter experience from Italy. Gastroenterology. 2020; 159:363–366.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rapporto ISS_COVID-19 n° 12-2020. Telemedicina. https://www.iss.it/documents/20126/0/Rapporto+ISS+COVID-19+n.+12+telemedicina.pdf/37b4b856-603a-76c1-1b85-5ff9c662bbbb?t=1586860608120. [Accessed 29 July 2020]
- 19.Repici A, Aragona G, Cengia G, Cantù P, Spadaccini M, Maselli R, et al. ; ITALIAN GI-COVID19 Working Group. Low risk of COVID-19 transmission in GI endoscopy. Gut. 2020; 69:1925–1927. [DOI] [PubMed] [Google Scholar]
- 20.Kohn A, Zullo A, Monica F, Soncini M, Cannizzaro R, Milazzo G, et al. AIGO research output: a potential matter for postgraduate non-academic hospital specialist training in gastroenterology. Dig Liver Dis. 2020; 52:1210–1212. [DOI] [PubMed] [Google Scholar]


