Abstract
The function of neural circuits and networks can be controlled, in part, by modulating the synchrony of their components’ activities. Network hypersynchrony and altered oscillatory rhythmic activity may contribute to cognitive abnormalities in Alzheimer disease (AD). In this condition, network activities that support cognition are altered decades before clinical disease onset, and these alterations predict future pathology and brain atrophy. Although the precise causes and pathophysiological consequences of these network alterations remain to be defined, interneuron dysfunction and network abnormalities have emerged as potential mechanisms of cognitive dysfunction in AD and related disorders. Here, we explore the concept that modulating these mechanisms may help improve brain function in these conditions.
Certain activities of neurons and neuronal networks are associated with the successful encoding of memories and retention of new information, and thus may be necessary for learning and memory. In Alzheimer disease (AD), schizophrenia, epilepsy and other neurological and psychiatric diseases that cause cognitive impairment, network activities supporting cognition are altered, even during preclinical stages1–5, that is, before symptoms are noticed by the patient or can be detected by neurocognitive exams. These network alterations include activation and deactivation deficits, abnormal oscillatory rhythmic activity and network hypersynchrony. In people at high risk of developing AD, for example, abnormal activation and deactivation of specific networks during memory encoding can be detected decades before the predicted onset of clinical disease6–9. As these functional network alterations widely overlap with the brain regions that ultimately develop pathological hallmarks and atrophy in AD, they may be a harbinger and possibly even a cause of clinical disease manifestations.
Robust network alterations are associated with diverse cognitive disorders, but the mechanisms and pathophysiological consequences of the alterations are poorly understood. Do alterations in network activity contribute to cognitive impairment or are they incidental by-products of disease-induced cellular dysfunction? Do alterations in network synchrony cause the dysfunction of microcircuits, larger distributed networks, or both? Most importantly, could cognitive alterations be prevented and even reversed by improving the function of cells that promote specific network activities?
Recent findings suggest that altered network activity can indeed contribute to cognitive impairment in AD and that network activities can be experimentally or behaviorally manipulated to improve cognitive functions in patients at risk of AD and related mouse models. In this Review, we explore two main concepts — that network abnormalities and interneuron dysfunction contribute to cognitive deficits in AD and related conditions, and that blocking or counteracting these mechanisms could be of both symptomatic and disease-modifying therapeutic value.
Neuronal synchrony and brain function
Cognitive functions depend on brain states that range from maximal focus and concentration to inattentiveness, drowsiness and sleep. These states probably do not reflect a continuous functional gradient of neuronal activities but rather represent distinct operating modes of brain activity that are closely linked to — and possibly determined by — changes in neuronal synchrony, the degree to which neuronal activities are correlated. Such synchrony fluctuates considerably with behavioural and brain states10. The degree of synchrony among neuronal populations (network synchrony) can be determined by measuring and correlating neuronal activities at multiple sites, for example, through electroencephalography (EEG) or the recording of local field potentials (LFPs) with multielectrode arrays. During non-active states, such as slow-wave sleep or quiet wakefulness, cortical neuronal activities at different sites tend to be synchronized and slowly fluctuate at high amplitudes (FIG. 1a). During active behaviours, such as paying attention or learning, this highly synchronized pattern of brain activity abruptly ceases, and neuronal activities at different sites become desynchronized and fluctuate at higher frequencies and smaller amplitudes11 (FIG. 1a).
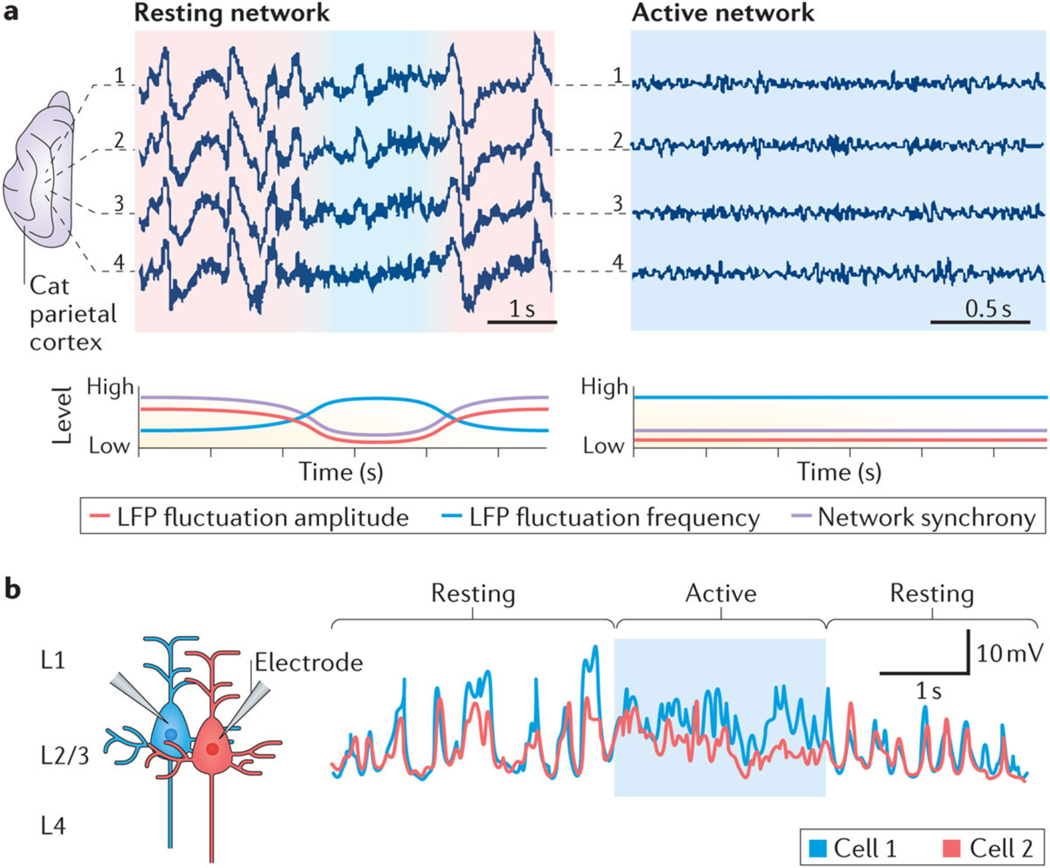
Figure 1 |. Synchrony and functional states of networks.
The degree of correlated neuronal activity (synchrony) reflects the functional state of networks and circuits. a | To illustrate this relationship at the network level, the top panels show local field potentials (LFPs) recorded from four electrodes (1–4) inserted 1 mm apart into the cat parietal cortex (suprasylvian gyrus) during sleep (left) and wakefulness (right). Network activity during resting and non-active states is predominantly characterized by synchronized slow-frequency and high-amplitude fluctuations (red shading). By contrast, network activity during active states is characterized by desynchronized fast-frequency and low-amplitude fluctuations (blue shading). The bottom panels show hypothetical representations of the amplitude (red line) and frequency (blue line) of LFP fluctuations and of the associated network synchrony (pink line). b | Membrane potential recordings of two layer 2/3 (L2/3) pyramidal neurons from mouse parietal cortex (whisker barrel cortex) during resting and active (whisker use) periods illustrate that neurons desynchronize during active periods. Part a is republished with permission of Society for Neuroscience, from Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states, Destexhe, A., Contreras, D. & Steriade, M., 19, 11, 1999; permission conveyed through Copyright Clearance Center Inc. Part b is from REF.12, Nature Publishing Group.
The fundamental relationship between the synchrony of neuronal activities and functional states is also evident at the circuit level (FIG. 1b). When mice are resting, adjacent layer 2 pyramidal cells in the somatosensory cortex show synchronous high-amplitude fluctuations in membrane potentials, which result in relatively low signal-to-noise ratios for evoking action potentials relative to ongoing fluctuations; however, with active whisker use, this high-amplitude synchrony of membrane potentials ceases abruptly, and signal-to-noise ratios increase12,13. Thus, increasing synchrony among neurons and recruiting them into slow-frequency and high-amplitude fluctuations seem to be basic mechanisms for ‘switching off’ specific brain regions. By contrast, the suppression of high-amplitude fluctuations and desynchronization of neuronal activities increases the ability of neurons to respond to external information-rich inputs12. Synchrony and functional states are closely related in many neocortical and hippocampal regions, suggesting that synchrony reflects a fundamental principle of nervous system design. Here, we propose that deficits in synchrony are an important pathogenic mechanism of AD-related cognitive dysfunction.
Amyloid-β and AD pathogenesis
The hypothesis that amyloid-β has a causal role in AD pathogenesis is supported by diverse lines of evidence, including the early accumulation of amyloid-β in the brain in people who go on to develop AD and the ability of mutations in the genes encoding the amyloid precursor protein (APP) or presenilins (PSENs), which alter amyloid-β production, to cause autosomal dominant, early-onset familial AD (FAD)14. Amyloid-β peptides are proteolytically released from APP by β-site APP-cleaving enzyme 1 (BACE1; also known as β-secretase) and γ-secretase, and exist in diverse assembly states, including monomers, oligomers, fibrils and amyloid plaques15. FAD is caused by APP duplications, mutations in APP located around the BACE 1 and γ-secretase cleavage sites or within the amyloid-β coding sequence, or by mutations in the genes that encode PSEN1 and PSEN2 (alternative catalytic subunits of the γ-secretase complex). Over 250 genetic alterations have been linked to FAD. Those alterations that have been tested for effects on APP metabolism increase the overall production of amyloid-β or the amyloid-β1–42/amyloid-β1–40 ratio16–19, or promote the accumulation of pathogenic amyloid-β assemblies20–22. Conversely, an APP mutation (A673T) that reduces the β-secretase-mediated cleavage of APP and, consequently, amyloid-β levels decreases the risk of late-onset sporadic AD23,24.
Amyloid-β oligomers elicit abnormalities in synaptic and cognitive functions in vivo and in vitro15,25–30. Neuronal expression of FAD-mutant human or humanized APP (with or without co-expression of FAD-mutant PSEN1 or PSEN2) in transgenic mice simulates key aspects of AD, including elevated levels of human amyloid-β, amyloid plaques, neuritic dystrophy, synaptodendritic impairments, astrocytosis, microgliosis, vasculopathy, network dysfunction, cognitive deficits and behavioural alterations31. We refer to these models collectively as FAD mice (Supplementary information S1 (table)). An example of such models is highlighted in Supplementary information S2 (table), which lists the AD-like alterations observed in the hAPP-J20 mouse model, which was generated in our laboratory. In combination, the above findings strongly support the amyloid-β hypothesis, which includes the notion that amyloid-β is a critical cause of cognitive decline in AD. Notably, several other factors, including tau accumulation, apolipoprotein E4 (APOE4) and inflammatory mediators, contribute to the complex pathogenesis of AD and can be introduced into FAD models through additional genetic modifications32–35.
A major unresolved question in the field is which forms of amyloid-β, tau and APOE4 are the most neurotoxic. Lead suspects include soluble amyloid-β1–42 oligomers15,30, abnormal assemblies or post-translational modifications of non-fibrillar tau35–38 and APOE4 fragments39. The importance of answering this question is illustrated by the following observations. Approximately 20% of healthy aged individuals, 60% of patients with mild cognitive impairment (MCI) and almost all patients with AD exhibit evidence of amyloid deposition in the cortex, as shown by positron emission tomography (PET) involving radiopharmaceutical ligands that bind to amyloid plaques such as Pittsburgh compound B (PiB)40. In healthy aged individuals and patients with MCI, cortical amyloid load correlated inversely with episodic memory performance40; this correlation weakened with amyloid accumulation and age and does not exist in AD40,41. Discrepancies between amyloid deposition and cognitive dysfunction may be explained, at least in part, by the early plateau of amyloid burden during disease progression42 and by dissociations between amyloid burden and the accumulation of soluble amyloid-β oligomers43. The latter may be more bioactive than amyloid-β monomers, fibrils and amyloid plaques, and readily elicit neuronal and network dysfunction26,28,30,43–50. Notably, patients with FAD who carry a mutation in the amyloid-β sequence that increases amyloid-β oligomerization49 have prominent cognitive impairments without PiB-positive (PiB+) amyloid deposition22, suggesting that AD can be caused by pathogenic assemblies of amyloid-β (probably amyloid-β oligomers) that cannot be detected by PiB imaging.
Notably, amyloid-β oligomers cannot yet be accurately measured in the brain parenchyma of live patients, which has greatly hampered clinical testing of the amyloid-β hypothesis. For example, it is not clear whether amyloid-β oligomer levels were significantly lowered in any of the failed clinical trials of anti-amyloid-β compounds for the treatment of AD. Nor is it clear how much, or by which mechanism, they would have to be lowered to reduce amyloid-β-dependent synaptic and network dysfunction. Anti-amyloid-β treatments that failed to lower amyloid-β oligomer levels in brain also failed to reduce cognitive deficits in hAPP-J20 mice51,52.
In conclusion, testing the amyloid-β hypothesis in humans will probably require therapeutics that effectively lower or counteract the most pathogenic amyloid-β assemblies as well as better methods to confirm their target engagement in the brain. The above evidence strongly supports the hypothesis that amyloid-β causally contributes to cognitive decline in AD. However, the mechanisms remain to be fully elucidated, and alternative interpretations have been offered53–56.
Network dysfunction and preclinical AD
Brain activity can be monitored by functional MRI (fMRI), PET, single-photon emission computed tomography (SPECT), EEG or LFP recordings. The corresponding signals emerge from the complex relationships among electrical activity, cerebrovascular hemodynamics, oxygen consumption and the metabolism of neurons and glia57. Attention-demanding tasks, such as sensory processing and memory encoding, require the coordinated regulation of many neuronal ensembles. In healthy people, attention-demanding cognitive tasks increase fMRI signals in specific brain regions (for example, the hippocampus during learning) but also cause a profound large-scale deactivation in brain regions that are collectively referred to as the default mode network (DMN)58,59 (FIG. 2a). This network is most active during inwardly oriented mental activity, such as introspection, daydreaming, mind wandering, wakeful rest, imagination and recalling, and it becomes deactivated during outwardly directed mental tasks such as acquisition and encoding of new information.
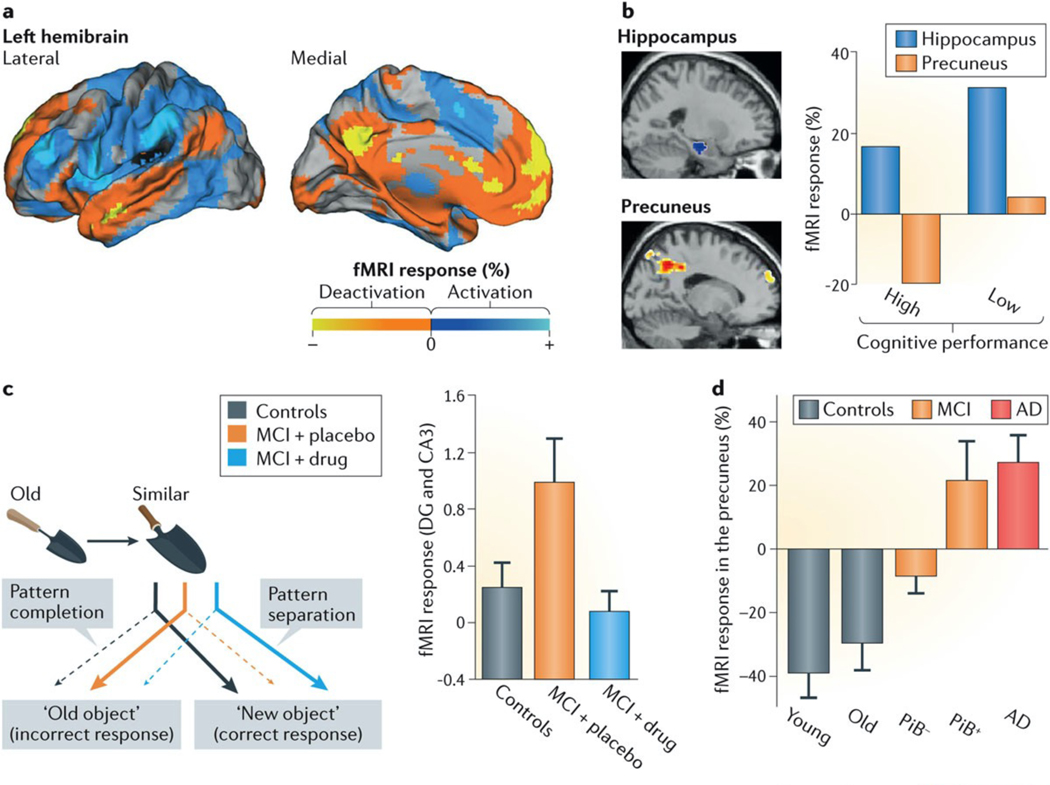
Figure 2 |. Encoding reveals network dysfunction in people with mild cognitive impairment.
Memory encoding causes specific changes in brain activity-related functional MRI (fMRI) signals across different networks. People at increased risk for Alzheimer disease (AD) show early changes in the activation and deactivation of specific networks. a | Lateral (left panel) and medial (right panel) views of a healthy left hemibrain. During encoding in healthy people, network activity increases in task-related networks (blue) and decreases in non-task-related networks (yellow/orange). Anti-correlated activity (blue versus yellow/orange) in these two widely distributed and non-overlapping networks is also evident when spontaneous fluctuations of fMRI signals during resting states are examined (not shown). The default mode network (yellow and orange regions) includes brain regions that show decreased fMRI activity during attention-demanding tasks but become active during inwardly oriented mental activity. b | In healthy individuals whose brain activity was monitored by fMRI during a cognitive task, a relatively smaller extent of hippocampal activation and a relatively greater extent of precuneal deactivation were associated with better cognitive performance. c | Pattern separation and completion are cognitive functions that heavily rely on the hippocampal formation and allow us to discriminate (pattern separation) or merge (pattern completion) similar representations or episodes. In a pattern-separation task, in which individuals were asked to discriminate between slightly different trowels (left panel), patients with amnestic mild cognitive impairment (MCI) made more pattern-completion errors (that is, they failed to discriminate slightly different trowels) and had aberrant hyperactivation of the dentate gyrus (DG) and CA3 regions of the hippocampus on fMRI (right panel). Treatment with the antiepileptic drug levetiracetam reversed the hippocampal hyperactivation and improved the patients’ ability to discriminate between images that were similar but not identical. d | During a face–name association task, patients with MCI and amyloid deposits in the brain (as revealed by a Pittsburg compound B-positive (PiB+) signal on positron emission tomography) and patients with AD showed deactivation deficits in the precuneus. Part a is adapted from REF. 58. Part b is adapted with permission from REF. 61, Springer. Part c is adapted with permission from REF. 67, Cell Press/Elsevier. Part d is adapted with permission from REF. 4, Cell Press/Elsevier.
The DMN includes the precuneus, posterior cingulate cortex, lateral and inferior parietal cortex, and regions of the temporal and medial prefrontal cortex59. A widely overlapping, but not identical, DMN that includes the hippocampus has been revealed by resting-state functional connectivity MRI60. Task-induced activation and deactivation of networks correlate well with cognitive performance in young healthy people61. Notably, task-induced deactivation of regions of the DMN (for example, the precuneus) can be a better predictor of good cognitive performance than the activation of regions that have been more extensively studied with regard to their involvement in cognitive functions (for example, the hippocampus)61. In fact, task-induced hippocampal activation without adequate DMN deactivation is associated with poor memory formation in healthy people (FIG. 2b), which highlights that proper execution of these complex functions requires well-coordinated regulation of widely distributed networks.
In AD, schizophrenia and other cognitive disorders, deactivation of DMN components is impaired during learning1–5. Because synchrony regulates the functional states of networks (FIG. 1), the abnormal activation or deactivation of brain regions during specific tasks raises the possibility that cognitive dysfunction in these disorders results from synchrony deficits. Although AD is increasingly viewed as a heterogeneous, multicausal syndrome, its fMRI signature seems to be remarkably consistent, particularly during the early stages of the disease. Hippocampal hyperactivation and reduced deactivation of DMN components during memory-encoding tasks have been observed in cognitively normal individuals with cerebral amyloid deposits4 (a potential harbinger of AD), cognitively normal carriers of the APOE ε4 allele6,62–64 (the major genetic risk factor for AD), presymptomatic carriers of FAD-causing mutations65,66 and patients with MCI67–69, which often develops into AD. In later stages of AD, the hippocampal formation is hypoactive during memory encoding, whereas the reduced deactivation of the DMN persists4,9,69,70.
Early hippocampal hyperactivation has been interpreted as a mechanism that may compensate for emerging cognitive decline in early AD71 and APOE ε4 carriers64. However, accumulating evidence suggests that this hyperactivation is primarily pathogenic and may impair learning and memory67,72,73. In cognitively normal APOE ε4 carriers, increased hippocampal activation was associated with reduced grid cell-like representations in the entorhinal cortex during a virtual spatial-memory task64, suggesting a potential link between entorhinal grid cell dysfunction and hippocampal hyperactivation.
In patients with MCI, hippocampal hyperactivation occurred during a pattern-separation task, which strongly depends on hippocampal functions; treatment with low doses of the antiepileptic drug levetiracetam for 2 weeks reversed the hyperactivation and improved performance on the task67,73 (FIG. 2c). In FAD mice (lines hAPP-J20 and 3xTg-AD (Supplementary information S1 (table)), levetiracetam suppressed network hyperexcitability, improved cognitive functions74,75 and reversed excessive neuronal DNA double-strand breaks76, which may contribute to neurodegeneration77,78. Thus, this drug may have both symptomatic and disease-modifying therapeutic potential. Collectively, these studies suggest that network hyperactivity is an early and detrimental alteration in the pathogenesis of AD-related cognitive dysfunction and that preventing or reversing this hyperactivity may be of therapeutic benefit.
Remarkably, there is strong evidence that functional brain alterations begin decades before the clinical onset of AD. The Dominantly Inherited Alzheimer Network (DIAN) consortium revealed that pathophysiological changes in the brain in people carrying autosomal dominant FAD mutations begin at least two decades before the predicted onset of clinical symptoms, including changes in cerebrospinal fluid (CSF) biomarkers, cerebral amyloid deposition, and brain metabolism8. Increased brain activity precedes the diagnosis of AD by 30 years in PSEN1 mutation carriers7,9. Finally, 20–35-year-old asymptomatic carriers of APOE ε4 show task-related hippocampal hyperactivation and altered DMN activity on fMRI6. Thus, FAD-causing mutations and APOE4 modulate brain function several decades before any clinical manifestations of the disease.
Network activity and amyloid deposits
Impaired deactivation of the DMN in older people with or without AD is closely linked to amyloid deposition in brain regions that make up this network4,9,70 (FIG. 2d, 3a). Whether the dysfunction of the DMN is a cause or a consequence of amyloid deposition is not known. When studying clinicopathological associations in humans, it is often impossible to conclusively discriminate between detrimental, beneficial and bystander alterations. Transgenic mouse or rat models expressing proteins that are suspected of contributing to the pathogenesis of AD can help to test related hypotheses in vivo31–33,79,80.
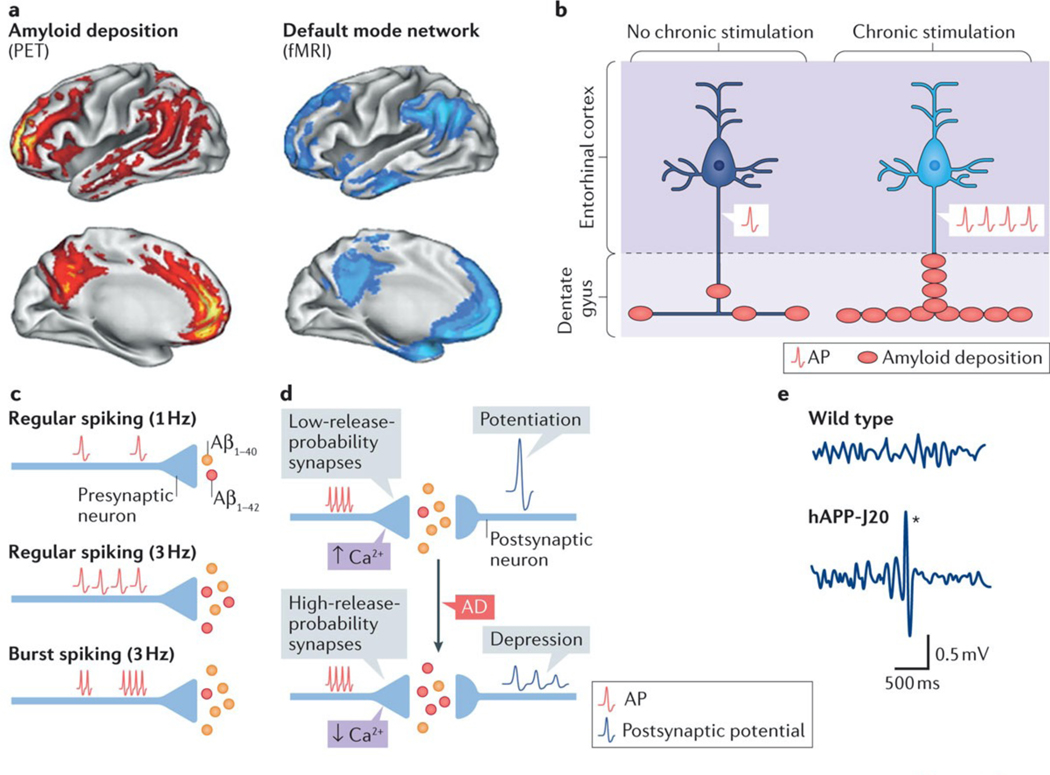
Figure 3 |. Neuronal activity regulates amyloid-β production and deposition.
a | Most patients with Alzheimer disease (AD) and some people without dementia have increased Pittsburg compound B-positive amyloid deposits in the brain243 (red). Amyloid deposits predominate in brain regions of the default mode network (blue), which shows deactivation deficits in AD (FIG. 2), suggesting a potential link between aberrant neuronal activity and amyloid deposition. b | In APP-A7 mice, chronic, optogenetic stimulation of pyramidal cells in the entorhinal cortex triggered epileptiform activity and increased amyloid deposition in the molecular layer of the dentate gyrus, directly supporting the notion that aberrant neuronal activity can promote amyloid deposition in vivo. c | At the synaptic level, an increase in the frequency of action potentials (APs) proportionally enhances amyloid-β1–42 (Aβ1–42) and amyloid-β1–40 (Aβ1–40) production. Compared with regular firing, burst firing reduces the Aβ1–42/Aβ1–40 ratio. d | Basal neurotransmitter release determines the ‘filter’ mode of synapses and regulates synaptic plasticity. The top panel indicates that excitatory synapses with lower release probability (high-pass-filter synapses) have greater presynaptic Ca2+ build-up, produce lower Aβ1–42/Aβ1–40 ratios, exhibit synaptic facilitation and primarily transfer potentiated responses. The bottom panel depicts synapses with higher release probability (low-pass-filter synapses), which have less presynaptic Ca2+ build-up, produce higher Aβ1–42/Aβ1–40 ratios and show synaptic depression as well as enhanced spike transfer. In AD, synapses may shift from high- to low-pass synaptic filtering. e | Electroencephalograhy recordings capture the combined electrical output of neuronal ensembles. In such recordings, most familial AD (FAD) mice, including hAPP-J20 (REF. 96), APP/PSEN1dE9 (REFS 102,103), Tg2576 (REF. 104), 5xFAD122, 3xTg-AD75, APP/TTA-EC105, APP/TTA-CaMKIIα106, and APP23 (REF. 107) mice (Supplementary information S1 (table)), show intermittent large-amplitude epileptiform discharges (denoted by the asterisk in hAPP-J20 mice; bottom panel), which provide evidence of network hypersynchrony. fMRI, functional MRI; PET, positron emission tomography. Part a is republished with permission of Society for Neuroscience, from Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory, Buckner, R. L. et al., 25, 34, 2005; permission conveyed through Copyright Clearance Center, Inc. Part e is adapted with permission from REF. 172, Cell Press/Elsevier.
In humans and mice, soluble amyloid-β levels in the interstitial fluid of different brain regions fluctuate with the brain state. Amyloid-β levels are higher during wakefulness than sleep81–83. In mice, sleep promotes amyloid-β clearance83, whereas sleep deprivation increases amyloid-β levels and amyloid deposition82, suggesting that brain states can modulate amyloid deposition. In young FAD mice (line Tg2576) (Supplementary information S1 (table)), interstitial levels of soluble amyloid-β vary markedly in different regions and relate closely to regional differences in metabolic activity84. Higher levels of metabolic activity are associated with higher levels of soluble amyloid-β and predict the regional amyloid burden in older mice. Thus, networks with higher metabolic rates may be more prone to amyloid deposition. In addition, experimental increases in neuronal activity by chronic optogenetic activation promote amyloid deposition and trigger epileptiform activity in FAD mice (line APP-A7)85 (FIG. 3b; see Supplementary information S1 (table)). Thus, the vulnerability of the DMN to amyloid deposition may be related to its relatively high baseline activity and to deactivation deficits that are associated with early stages of AD.
Synaptic functions and amyloid-β
Amyloid-β production and the amyloid-β1–42/amyloid-β1–40 ratio, which influence the formation of pathogenic oligomers as well as amyloid deposition, are regulated by neuronal action potential firing and calcium-dependent conformational changes in PSEN1 at presynaptic terminals86. Increasing the rates of regular or burst firing enhances amyloid-β1–40 and amyloid-β1–42 production in an activity-dependent manner (see also REFS 87,88)(FIG. 3c). Regular firing proportionally increases both amyloid-β1–40 and amyloid-β1–42 levels, whereas burst firing, which raises presynaptic calcium concentrations, selectively increases the level of amyloid-β1–40, reducing the amyloid-β1–42/amyloid-β1–40 ratio (FIG. 3c).
Synaptic plasticity is controlled by neurotransmitter release probability and calcium concentrations at presynaptic terminals. Excitatory synapses with a low initial probability of neurotransmitter release typically display greater facilitation during high-frequency stimulation (potentiation) and act as high-pass filters. Thus, they tend to be unresponsive to infrequent or disorganized action potentials but potentiate their responses to coordinated firing (FIG. 3d). By contrast, excitatory synapses with a high initial release probability respond preferentially to single-spike firing patterns and act as low-pass filters89,90. They tend to exhibit depression or reduced potentiation during sequential stimulation (FIG. 3d). In one study, the authors concluded that high-pass-filter synapses with higher calcium loading produce a lower amyloid-β1–42/amyloid-β1–40 ratio, whereas low-pass-filter synapses with lower calcium loading produce a higher amyloid-β1–42/amyloid-β1–40 ratio86.
Amyloid-β and other APP metabolites seem to participate in pre- and postsynaptic homeostatic mechanisms that regulate synaptic activity86,88,91–95. Amyloid-β may enhance presynaptic facilitation at low concentrations93 and promote postsynaptic depression at high concentrations88. Application of synthetic or recombinant amyloid-β1–42 oligomers or overexpression of FAD-mutant human APP reduces paired-pulse facilitation — a measure of increased release probability — and impairs long-term potentiation (LTP) at some, but not other, synapses in the mouse hippocampus91,93,96. These alterations may shift the filtering properties of the affected synapses from high-pass to low-pass. This shift could enhance the transfer of single action potentials, increase synaptic depression and elevate the amyloid-β1–42/amyloid-β1–40 ratio (FIG. 3d), promoting both hyperactivity in vulnerable networks and amyloid deposition. Reducing presynaptic release might counteract these processes. Indeed, levetiracetam, which has beneficial effects on network activity in FAD mice74,75 and humans with MCI67,73, reduces presynaptic neurotransmitter release in a use-dependent manner90,97,98. Thus, it may counteract amyloid-β-induced alterations in release probability.
In general, it is difficult to extrapolate from the effect that factors exert on specific synapses or neurons to the overall effect they have on microcircuits and complex networks. Indeed, this has been true for APP and amyloid-β. Although early studies suggested that increased neuronal amyloid-β production may be part of a homeostatic feedback loop that primarily reduces neuronal activity88, we now know that amyloid-β accumulation can also cause neuronal hyperexcitability in vitro and in vivo32,96,99–111. Acute amyloid-β application initially and transiently (10–20 minutes) increases the levels of surface AMPA-type glutamate receptors and GluN2B-containing NMDA-type glutamate receptors (NMDARs)100,103 and the frequency of spontaneous excitatory postsynaptic currents in primary neuronal cultures100,103. In brain slices, amyloid-β application also acutely increases the rate of action potential firing by hippocampal pyramidal cells99. FAD mice (lines hAPP-J20, hAPP-J9, APP23xPS45 and APP/PSEN1dE9) (Supplementary information S1 (table)) contain both hyperactive and hypoactive neurons — reflected in abnormally high or low rates of action potential-dependent calcium transients or levels of transcripts for the immediate early genes Arc and Fos — both before96,112,113 and after109,114,115 amyloid deposition becomes detectable.
Neurons exposed to pathologically elevated levels of amyloid-β in vitro or in vivo show signs of atrophy, including lower dendritic spine density and shorter dendrites116,117, alterations that can increase intrinsic cellular excitability118 and may explain the hyperactivity of some excitatory neurons. These morphological and functional effects of amyloid-β may be mediated, at least in part, by neuronal depletion of the DNA repair factor BRCA1 and the resulting accumulation of activity-induced DNA double-strand breaks77. Compensatory increases in inhibitory interneuron activity may account for the hypoactivity of other excitatory neurons101,109. Notably, hippocampal neuronal hyperactivity preceded amyloid plaque deposition and neuronal hypoactivity in APP23xPS45 mice and could be induced by local application of amyloid-β in wild-type mice101, suggesting that it is a very early step in the pathogenic cascade that is triggered by amyloid-β accumulation.
Hypersynchrony in AD and animal models
Besides network activation and deactivation deficits detectable by fMRI (FIG. 2), AD also results in network hypersynchrony (Table 1: see Supplementary information S3 (table)). Unlike normal fluctuations in network synchrony that are associated with physiological changes in behaviour and brain states (FIG. 1), network hypersynchrony is a pathological phenomenon in which aberrant synchronization of neuronal networks results in epileptiform discharges and seizures.
Table 1 |.
Network hypersynchrony in humans with FAD caused by mutations used in mouse models.
| Affected genes | FAD mutations | Number of patients with seizures (study population percentage) | Age at onset of cognitive deficits, years (mean) | Refs |
|---|---|---|---|---|
| APP | KM670/671NL | 10 (50%) | 44–61 (53) | 244 |
| APP | V717L, V717G or V717I | 11 (40%) | 40–67 (51) | 245–248 |
| APP | T714I or 714A | 6 (85%) | 33–55 (NA) | 249,250 |
| APP | Duplication | 27 (45%) | 39–62 (NA) | 144,145,251–253 |
| APP; PSEN1 | KM670/671NL (APP); H163Y (PSEN1) | 8 (89%) | 44–65 (54) | 254 |
| PSEN1 | M146L | 7 (70%) | 33–46 (39) | 255 |
| PSEN1 | M146V | 1 (100%) | 39 (NA) | 256 |
| PSEN1 | L286V | 7 (64%) | 39–56 (48) |
257
|
APP, amyloid precursor protein; FAD, familial Alzheimer’s disease; NA, not available; PSEN1, presenilin-1.
Neuronal expression or overexpression of proteins that accumulate in AD brains or are genetically linked to the disease, such as APP, amyloid-β, tau, APOE4 or α-synuclein, causes network hypersynchrony in transgenic mice96,119–121. Spontaneous epileptiform activity has been documented in many FAD models, including hAPP-J20 (REF. 96), APP/PSEN1dE9 (REFS 102,103), Tg2576 (REF. 104), 5xFAD122, 3xTg-AD75, APP/TTA-EC105, APP/TTA-CaMKIIα106, APP23 (REF. 107) and hAPPJ9/FYN108 transgenic mice (FIG. 3e; see Supplementary information S1 (table)). Behavioural seizures and lower thresholds for chemically induced seizures have also been described in some of these models96,103,108,111,123,124. Interestingly, network hypersynchrony in mouse models of AD and of epilepsy depends on the presence of endogenous, soluble wild-type tau, which seems to enable or promote aberrant synchronization32,35,110,111,125,126.
In humans, epidemiological studies have consistently shown that AD is a risk factor for epileptic seizures127,128. However, the reported incidence of seizures in patients with AD that were detected by observation has ranged from 0.5% to 64%129,130. A recent review of 17 clinical studies that assessed epileptic activity in AD reported an average incidence of 15.1% (median: 9.0%)131. The incidence of seizures is roughly 7–8-fold higher in individuals with AD than in people without dementia132,133. In a recent nationwide study of data from over 140 million hospitalizations, patients with AD were fourfold more likely to be hospitalized for a seizure than for non-seizure-related condition134. Moreover, people with early-onset AD (50–65 years old) were more likely to be hospitalized for seizures or epilepsy than those with a later onset of AD (>81 years old)134. These new findings are consistent with earlier reports indicating that early-onset AD (<65 years old) is a major risk factor for seizures129,133,135. Conversely, patients with amnestic MCI or AD who had seizures or subclinical epileptic activity typically had an earlier onset and faster progression of cognitive decline than those without detectable epilepsy129,136,258.
Although seizures in AD are widely thought to result from end-stage neurodegeneration, several reports indicate that they can occur early in the disease course and before a neurodegenerative disease diagnosis is made136,137. Indeed, in patients who had MCI or early AD with epilepsy, seizure onset clustered near the onset of cognitive decline136 or preceded the MCI diagnosis by 4 to 7 years138. At the time MCI was diagnosed, the latter group of patients had mild hippocampal atrophy138. Seizures in MCI or early AD with epilepsy responded best to the antiepileptic drugs levetiracetam or lamotrigine and least well to phenytoin136. The lower relative risk of seizures in patients with late-onset AD may reflect the fact that strokes and other ageing-related comorbidities increase seizure risk in people without dementia. It is also possible that old brains are less susceptible to amyloid-β-induced network hypersynchrony than younger brains and that amyloid-β has a less important role in the pathogenesis of late-onset AD, which may be more multifactorial than early-onset AD.
Epileptic activity is even more prominent in pedigrees of early-onset autosomal dominant FAD91,139,140. Seizures have been observed in patients with FAD who carry any of 66 different PSEN1 mutations141,142 (Supplementary information S3 (table)), in 31% of patients with AD carrying PSEN2 mutations143, and in 58% of patients with APP duplications144,145. More recently, a multicenter study including 132 patients with FAD showed that 48% of patients harboring an FAD mutation had seizures, including 43% of PSEN1 mutation carriers (n = 94), 43% of PSEN2 mutation carriers (n = 7), 47% of APP mutation carriers (n = 15), and 81% of APP duplication carriers (n = 16)146. Seizure frequency in patients with FAD seems to be even higher than that observed in most FAD mice (Supplementary information S1 (table)).
In addition, 84% of patients with Down’s syndrome who progress to dementia also develop seizures147. Interestingly, very early onset of AD (<40 years old) further increases the proportion of FAD pedigrees with epileptic phenotypes (>80%)148. Some FAD mutations (for example, PSEN1 L166P) and APP duplications even cause epileptic activity in childhood or adolescence, preceding cognitive decline by many years145,149. Thus, network hypersynchrony can be a very early and prominent clinical feature of FAD. Indeed, many FAD-PSEN1 pedigrees qualify as epileptic syndromes or disorders140,141. This intriguing association between AD and epilepsy probably holds important clues about the mechanisms by which amyloid-β, tau, and APOE4 promote the development of AD and about potential entry points for therapeutic intervention.
The power of gamma oscillations
Whereas abnormal neuronal synchrony can result in epileptic activity, normal neuronal synchrony underlies the generation of oscillatory rhythmic activities, or brain rhythms, that promote cognitive functions. Neuronal ensembles coordinate their firing within a range of oscillatory frequencies (0–300 Hz). Within each frequency band, neuronal ensembles can increase or decrease their firing rate and synchrony and thus generate higher or lower oscillatory amplitudes (FIG. 4a), although it should be noted that only a small fraction of the cells contributes to each oscillatory cycle. The amplitude, phase and frequency in each oscillatory band modulate each other and neuronal firing rates in precise ways150. For example, the firing of hippocampal pyramidal cells shows phase-coupling with low-frequency oscillations, such as theta (4–8 Hz). Thus, during periods of high theta, the action potential firing rate of pyramidal cells is increased and its timing is synchronized with this rhythm151. By contrast, the firing of several types of interneurons shows phase-coupling with high-frequency oscillations, such as gamma (30–150 Hz)150–152. Thus, during periods of high gamma power, action potential firing of interneurons is more prominent and synchronized with the phase of gamma151 (FIG. 4b,c).
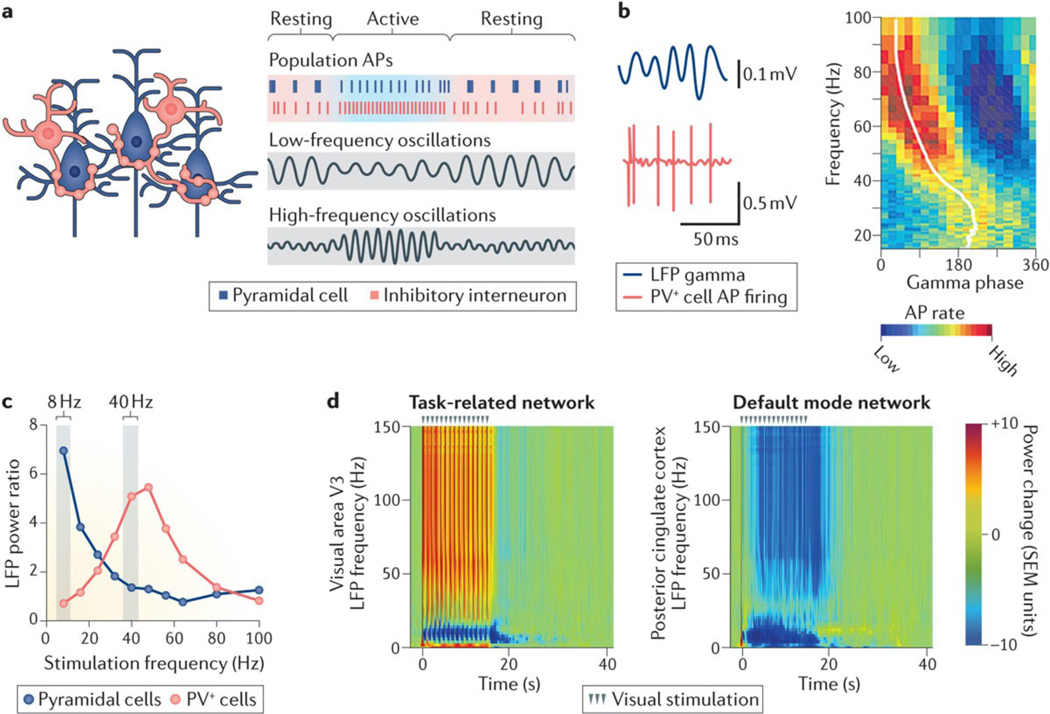
Figure 4 |. Neuronal ensembles generate oscillatory activity patterns.
a | Oscillatory rhythms (grey boxes) emerge from, and control, the activity of neuronal ensembles that are formed by excitatory neurons (blue) and inhibitory interneurons (red). The amplitude, or power, of low-frequency oscillations increases during rest, whereas the amplitude of high-frequency oscillations increases during activity. Action potentials (APs) of excitatory neurons are phase-locked to high-power, lower-frequency oscillations (top grey box), whereas APs of inhibitory cells are phase-locked to high-power, high-frequency oscillations (bottom grey box). b | The left panel shows local field potential (LFP) gamma oscillations (blue) and APs (red) for a parvalbumin-positive (PV+) cell in area CA3, revealing a close association between APs and the phase of gamma oscillations. In the right panel, the AP firing rate (colour coded) is shown as a function of gamma phase for the same PV+ cell, which prominently fires during the ascending phase of gamma oscillations. c | Optogenetic stimulation of PV+ and pyramidal cells at different frequencies resulted in a cell type- and frequency-specific generation of oscillatory activity. 40-Hz stimulation of PV+, but not pyramidal, cells increased gamma power, whereas 8-Hz stimulation of pyramidal, but not PV+, cells increased theta power. d | In macaques, visual stimulation (15s of dim 1 Hz illumination) increased the power of LFP gamma (30–150 Hz) oscillations in a task-related network (visual area V3; left panel) and decreased LFP power across multiple oscillatory frequencies in a default mode network region (posterior cingulate cortex; right panel). SEM, standard error of the mean. Part b is from REF.151, Nature Publishing Group. Part c is from REF. 190, Nature Publishing Group. Part d is adapted from Bentley, W. J., Li, J.M., Snyder, A. Z., Raichle, M.E. & Snyder, L.H., Oxygen level and LFP in task-positive and task-negative areas: bridging BOLD fMRI and electrophysiology, Cerebral Cortex, 2014, 26, 1, 346–357, by permission of Oxford Journals.
The amplitude (or power) of gamma oscillatory activity increases during sensory processing or memory encoding, and such increases in gamma power predict successful memory formation in humans and mice153–158. Encoding during sensory, motor or memory tasks is associated not only with increased power of high-frequency oscillations (gamma) but also with reduced power of lower-frequency oscillations (alpha, beta and theta) in task-related brain regions57,154,159,160 (FIG. 4d). However, the relationship between gamma power and attention can differ across cortical areas and tasks161. Increases in gamma power tend to be localized to networks that are directly involved in the task (task-related networks), whereas reductions in the power of lower-frequency oscillations typically involve multiple cortical regions162,163. During sensory encoding, individuals with schizophrenia have reduced increases in gamma power in task-related networks and reduced decreases in the power of lower-frequency oscillations across multiple components of the DMN164. It is tempting to speculate that these large-scale deficits of oscillatory activity may be related to the activation and deactivation deficits of fMRI signals in patients with schizophrenia or AD5 (FIG. 2). Patients with AD also have reductions in gamma power165; however, the relationship between task-related deficits in fMRI signals, oscillatory frequencies and the observed prominent memory-encoding problems does not seem to have been directly studied in this disease.
In healthy individuals, the strength of fMRI blood-oxygen-level–dependent (BOLD) signals correlates positively with the power of gamma oscillations166,167 and negatively with the power of alpha oscillations168–170. By contrast, decreases in DMN fMRI BOLD signals are associated with reductions in gamma power171. During visual stimulation in monkeys, an increase in oxygen level, which was associated with a rise in fMRI signals, coincided with increases in LFP gamma power in task-related networks (for example, visual area V3), whereas a decrease in oxygen level coincided with prominent decreases in LFP power in all oscillatory frequencies in DMN regions (for example, the posterior cingulate cortex)57 (FIG. 4d). These findings suggest that fMRI signals may directly reflect changes in oscillatory activity. It should be informative to further explore the relationship between fMRI signals and oscillatory alterations in AD and other cognitive disorders.
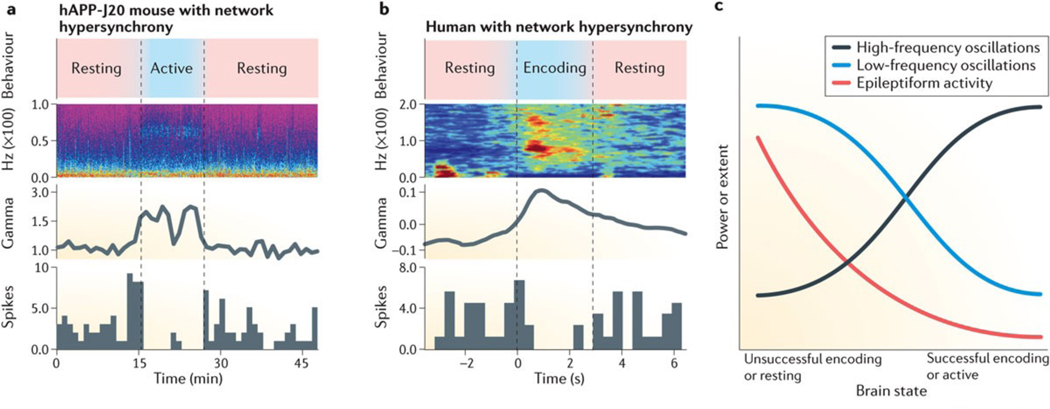
Longitudinal video-EEG recordings revealed abnormal behaviour-dependent fluctuations in the power of gamma oscillations in hAPP-J20 mice (FIG. 5a). Notably, spontaneous epileptiform discharges emerged primarily during resting periods in these animals, when the intensity of gamma oscillations was low172. In two mouse models of absence epilepsy, pharmacologically induced increases or decreases of gamma power were associated with decreases or increases in epileptic activity, respectively173. Thus, network hypersynchrony and reduced gamma power may be mechanistically linked across different conditions, and behaviour-induced increases in gamma oscillatory power may counteract AD-related epileptiform activity by activating task-related brain regions and reducing network synchrony.
Figure 5 |. Close association between behavioural state, gamma oscillations and epileptiform activity in mice and humans.
a,b | Behavioural activity, full-frequency range spectrograms, gamma oscillatory power, and distribution of epileptic discharges in cortical networks of an hAPP-J20 mouse (part a) and a human with epilepsy (part b). In mice, exploration (active) of a novel environment robustly increased gamma oscillatory power and reduced epileptiform discharges, suggesting that the brain state modulates brain rhythms and network hypersynchrony. In humans, successful, but not unsuccessful, memory encoding also increased gamma oscillatory power and reduced epileptiform discharges. c | In our hypothetical model, memory encoding requires frequency-specific modulation of oscillatory frequencies, which reduces network hypersynchrony. Part a is adapted with permission from REF. 172, Cell Press/Elsevier. Part b is adapted from Matsumoto, J.Y. et al., Network oscillations modulate interictal epileptiform spike rate during human memory, Brain, 2013, 136, 8, 2444–2456, by permission of Oxford Journals.
These ideas are supported by findings in humans with epilepsy154 (FIG. 5b). During EEG recordings, individuals were shown a set of images (encoding trials), and their memory of the images was probed 24 hours later (recognition trials). Encoding trials were deemed ‘correct’ or ‘incorrect’, depending on whether or not the old images were recognized during the recognition trial. Remarkably, gamma power increased and epileptiform activity reduced only during correct memory encoding.
Overall, these LFP, EEG and fMRI findings suggest that effective memory formation requires, or induces, a pattern of brain activity that increases the power of gamma oscillations and reduces the power of low-frequency oscillations, and that this ‘brain state’ reduces aberrant network hypersynchrony in humans and mice (FIG. 5c). Social interactions and mental activity may benefit some patients with AD174, perhaps, at least in part, by promoting this state. Pharmacological interventions that more persistently improve brain rhythms could be of greater therapeutic benefit.
Interneuron impairments in AD
Network synchrony and oscillatory brain rhythms are promoted and controlled by the activity of inhibitory GABAergic interneurons175. These cells are highly diverse, and different subtypes form electrically coupled functional networks176. Their combined inhibitory synaptic input onto excitatory principal neurons and other interneurons generates precise oscillatory rhythms that in turn coordinate the timing of pyramidal cell firing (see above). Some types of inhibitory interneurons, such as somatostatin-positive (SOM+) or neuropeptide Y-positive (NPY+) cells, tend to fire tonically and independently of brain and behavioural states177,178. Others, such as parvalbumin-positive (PV+) or vasoactive intestinal polypeptide-positive (VIP+ ) cells, fire predominantly during brain states that promote encoding177,178. Interneurons and the oscillatory network activities that they regulate are altered in AD, epilepsy, schizophrenia and autism91,165,172,179–182. Indeed, interneuron impairment is emerging as a potential common mechanism of brain dysrhythmias and cognitive dysfunction in many neurological and psychiatric disorders172,179,180,183,184. What is more, recent findings support the hypothesis that modulating interneuron function may improve brain rhythms and cognitive functions in AD and related disorders172,182,185,186.
Proper functioning of inhibitory interneurons is required for the generation of high-frequency gamma oscillatory activity, coordination among different oscillatory frequencies (cross-frequency interactions) and regulation of neuronal firing by brain rhythms (frequency-action potential interactions). Several lines of evidence suggest that fast-spiking PV+ cells are impaired in disorders associated with hypersynchronous network activity such as schizophrenia and epilepsy187, and that other types of interneurons — for example, those containing cholecystokinin or NPY — are impaired in mood disorders and anxiety187–189.
PV+ cells constitute ~40% of inhibitory interneurons and are the major source of perisomatic inhibition onto excitatory pyramidal cells. Because PV+ cells are electrically coupled by dendritic gap junctions and form an electrically coordinated interneuron network, they are particularly well suited to synchronously modulate the activity of many pyramidal cells176. In optogenetic stimulation studies in wild-type mice, increasing the firing rate of PV+ cells increased the power of gamma oscillations but not of other oscillations, whereas increasing the firing rate of pyramidal cells specifically increased the power of low-frequency oscillations179,190 (FIG. 4c), indicating a causal link between gamma and PV+ cell function. Increasing gamma power improved the signal-to-noise ratio of pyramidal cell firing, an effect that is likely to enhance neuronal processing179,190.
In FAD mice (lines hAPP-J20, Tg2576, APP23, APOE4-KI, and APP/PSEN1dE9) (Supplementary information S1 (table)), gamma oscillatory activity is altered107,172,191–193, suggesting that these animals have deficits in interneuron function. hAPP-J20 mice have brief peaks of increased gamma power and long periods of decreased gamma power172,192. Similar abnormal fluctuations in gamma power occur in Tg2576 mice191. Increased gamma power is associated with behaviour-dependent brain activation, increased firing rate of PV+ cells177,194 and the suppression of epileptiform discharges in hAPP-J20 mice172 and humans with epilepsy154. By contrast, gamma power is decreased during resting activity and is associated with reduced firing rate of PV+ cells177,194 and increased epileptiform discharges in hAPP-J20 mice172. These behaviour-related modulations of gamma oscillations probably differ mechanistically from aberrant increases in gamma power during seizures or hyper-synchronized network activity and from the overall increases in oscillatory power across multiple frequency bands in APP23 and APP/PSEN1dE9 mice107,193. In APP23xPS45 mice, hyperactivity of cortical neurons was associated with decreased GABAergic inhibition rather than increased glutamatergic transmission, suggesting impaired inhibitory function109. More recently, reduced inhibitory function has been linked to amyloid-β-induced deficits in slow-wave propagation195,196. Aberrant increases in gamma power in the auditory cortex during evoked auditory stimulation in patients with AD have also been related to decreased inhibition197,198. Overall, however, humans with AD typically show decreases in the power of higher-frequency oscillations and increases in the power of lower-frequency oscillations199.
APOE4-KI mice (Supplementary information S1 (table)), in which the Apoe gene is replaced by knocking in the human ε4 allele, also have prominent GABAergic dysfunction and show reduced hippocampal power of slow gamma oscillations during sharp-wave ripple activity200. Amyloid-β application99,201 and APP overexpression202–204 markedly suppress the power of kainate-induced gamma oscillations in neuronal cultures. The mechanisms of gamma degradation by amyloid-β in brain slices are unclear but may involve changes in inhibition–excitation balance and cellular excitability99. Amyloid-β treatment increased excitatory and decreased inhibitory postsynaptic currents in pyramidal cells and increased the firing rate of these excitatory neurons99. Interestingly, although the mean phase of action potential firing did not change, the temporal firing window of excitatory neurons became wider, suggesting desynchronization of action potential firing99. Perhaps, spike-theta phase dysregulation204 also contributes to the reduced gamma power and the epileptiform activity that are observed in FAD mice. Hippocampal injections of amyloid-β reduced firing rates in rhythmically bursting interneurons (probably PV+ cells) but not in tonically firing interneurons (probably cholinergic interneurons) in the septum203.
Across brain regions and behavioural tasks, effective encoding also seems to depend on cross-frequency interactions, particularly the coupling between the phase of theta oscillations and the power of gamma oscillations (phase–amplitude coupling)205–209, which is regulated by the synaptic activity of PV+ cells210,211. Recordings from acute brain slices of young FAD mice and in vivo recordings from adult FAD mice (lines TgCRND8 and APP/PSEN1dE9, respectively) (Supplementary information S1 (table)) revealed deficits in theta-gamma coupling193,212, which were associated with cognitive deficits212 and could result from the kind of PV+ cell dysfunction that we identified in hAPP-J20 mice. Indeed, by impairing PV+ cell functions, APP and amyloid-β could disrupt multiple aspects of neuronal coordination.
Single-unit recordings of inhibitory cells in behaving rodents have revealed that behavioural and brain states affect interneuron subtypes differentially177,178,189,213. For example, PV+ and VIP+ cells drastically increase their action potential firing rate during specific behavioural states (for example, locomotor exploration), whereas NPY+ and SOM+ cells show persistent or tonic firing across multiple behavioural states (for example, locomotion, sleep and quiet wakefulness). These findings imply that certain behavioural states may be able to overcome, at least partly and for short periods, PV+ cell impairments that cause low gamma power, hypersynchronous network activity and cognitive deficits in FAD mice and humans with AD (FIG. 5c). Optogenetically suppressing the activity of interneurons that express the homeobox protein DLX1 in the dentate gyrus impaired learning and acutely suppressed memory retrieval in mice, further highlighting the crucial role of interneurons in cognitive tasks214. It is tempting to speculate that fluctuations in interneuron activity may help to explain the fluctuations in cognitive performance of patients with AD that are frequently reported by care takers but have been difficult to document by clinical measurements.
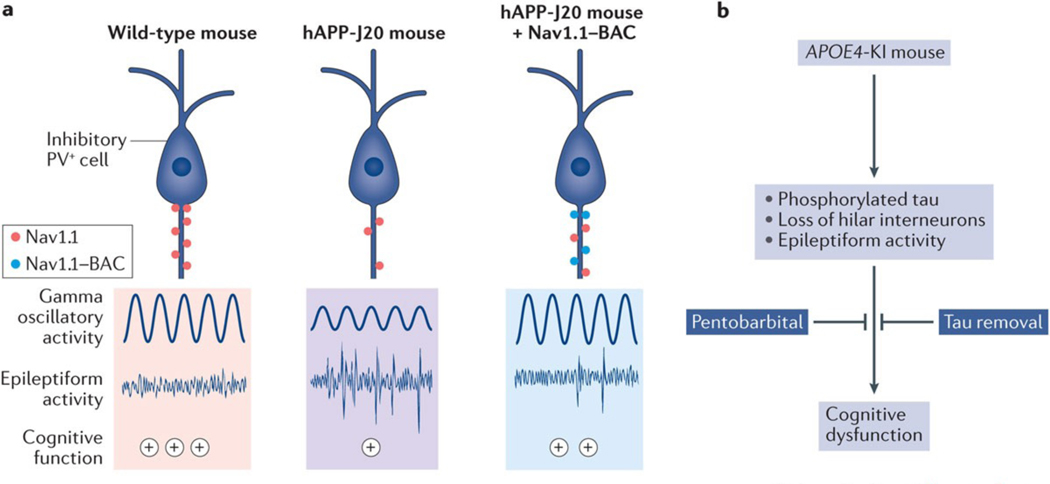
The mechanisms by which interneuron deficits contribute to network and cognitive dysfunction in AD and related conditions remain to be fully elucidated, although much progress has recently been made on this front. Decreased synaptic inhibition and excitation–inhibition imbalances might explain deactivation deficits in the DMN of individuals with MCI or AD4,67,72, but these hypotheses have not yet been tested experimentally. Impaired inhibition is another potential mechanism for plaque-related neuronal hyperactivity in APP23xPS45 mice109. In hAPP-J20 mice, we found that PV+ cell impairments are caused by the depletion of the voltage-gated sodium channel subunit Nav1.1 and crucially contribute to network and cognitive dysfunctions172 (FIG. 6a). Voltage-gated sodium channels control intrinsic cellular excitability by modulating action potential firing in specific neuronal subtypes. Nav1.1 is expressed predominantly in interneurons, including PV+ cells, in both mice172 and humans215, and its levels in the parietal cortex are reduced in hAPP-J20 mice and patients with AD172. Hypofunction of Nav1.1 has been also described in Tg2576 mice216 and in transgenic mice overexpressing BACE1 (REF. 217). As indicated earlier, the activity of BACE1, which is expressed at high levels in the brains of patients with AD218, is required for the release of amyloid-β from APP. Because this enzyme also cleaves the β-subunit of voltage-gated sodium channels, increased BACE1 levels lead to aberrant β-subunit cleavage and reduced transport of Nav1.1 to the membrane, resulting in Nav1.1 hypofunction216,217. Neuroblastoma cells expressing the FAD-linked PSEN1 E280A mutation also had reduced levels of Nav1.1 mRNA and protein levels219.
Figure 6 |. Targeting interneurons to improve Alzheimer disease-related network dysfunction.
a | In hAPP-J20 mice (middle panel), reduced levels of the voltage-gated sodium chanel subunit Nav1.1 in parvalbumin-positive (PV+) cells were associated with reduced gamma power, epileptiform activity and cognitive impairment (the level of cognitive performance is denoted by the plus symbols). Restoring Nav1.1 levels in PV+ cells with an Nav1.1-BAC (bacterial artificial chromosome) transgene (right panel) reduced all of these deficits. b | APOE4-KI mice have increased neuronal levels of phosphorylated tau, age-dependent loss of somatostatin-positive interneurons in the hilus of the dentate gyrus, and seizures. Memory deficits in these mice were reduced by pentobarbital treatment221, tau removal221 and transplantation of interneuron precursor cells160. Part a is adapted with permission from REF. 172, Cell Press/Elsevier.
APOE4-KI mice also develop spontaneous epileptic activity and seizures120. In humans without dementia, hyperventilation induces network hypersynchronization (for example, sharp waves) more frequently in APOE ε4 carriers than non-carriers220. Although network hypersynchrony has not been directly linked to interneuron dysfunction in APOE4-KI mice, these mice lose around 30% of SOM+ cells in the hilus of the dentate gyrus, and this reduction is associated with learning and memory deficits221. Interestingly, chronic treatment with pentobarbital reversed the latter deficits but not the loss of hilar interneurons221, suggesting that this positive allosteric modulator of GABA type A receptors can compensate for the partial loss of inhibitory input (FIG. 6b).
Synaptic depression and hypersynchrony
Synaptic loss is a pathological hallmark of AD and correlates well with cognitive decline 222,223. In experimental models, high levels of amyloid-β cause synaptic loss, reduce glutamatergic synaptic transmission and LTP, and increase long-term depression25,88,94,224–228. Intriguingly, several proposed mechanisms of amyloid-β-induced synaptic depression might also contribute to network hypersynchrony229. For example, amyloid-β blocks neuronal glutamate uptake at synapses, which could result in glutamate spillover around the synaptic cleft25. The rise in glutamate may desensitize synaptic NMDARs and aberrantly activate extra- or perisynaptic GluN2B-containing NMDARs and metabotropic glutamate receptors (mGluRs), and both GluN2B-containing NMDARs and mGluRs can promote long-term synaptic depression and spine retraction25,228,230. Amyloid-β-induced NMDAR- and mGluR-dependent long-term depression can be prevented by lowering extracellular glutamate25 and can be mimicked by application of the glutamate reuptake inhibitor thero-β-benzyloxyaspartate (TBOA), which can also trigger epileptiform discharges in wild-type brain slices 231. Thus, amyloid-β-induced network hypersynchrony may be directly linked to synaptic depression.
Future therapies
The effective therapy of AD will probably require combining treatments targeting the root causes of the disease with therapeutic strategies that can block or counteract the pathogenic processes that these causes trigger33. From the evidence reviewed above, we think that brain dysrhythmias caused by aberrant interneuron activity and other mechanisms probably contribute to cognitive deficits and behavioural alterations in AD and related conditions. We further hypothesize that enhancing the function of interneurons in these conditions, particularly of PV+ and SOM+ cells, will improve network activities and cognitive performance.
In hAPP-J20 mice, restoring Nav1.1 levels enhanced PV+ cell-dependent gamma oscillatory power, reduced network hypersynchrony and improved cognitive performance (FIG. 6a), pinpointing Nav1.1- and PV+ cell-dependent oscillatory rhythms as potential therapeutic targets. Although it is never certain that therapeutic findings obtained in experimental models (or in early clinical trials in humans, for that matter) will hold up in large heterogeneous human populations, Nav1.1 depletion and various other molecular alterations identified in hAPP-J20 mice have been found in people with AD (Supplementary information S2 (table)). These include depletions of calbindin in the dentate gyrus and of reelin in the entorhinal cortex, increased neuronal production of collagen VI, higher hippocampal levels of activated group IVA phospholipase A2 and increased expression of the adenosine A2A receptor by hippocampal astrocytes100,172,232–236. In addition, the anti-epileptic drug levetiracetam, which reduces synaptic, network and cognitive deficits in hAPP-J20 mice74, also exerts beneficial effects in patients with amnestic MCI67,73, which is widely viewed as an early stage of AD237–239.
Manipulating interneuron function to improve brain rhythms is a promising therapeutic strategy for disorders involving interneuron and network dysfunctions, including AD, epilepsy, schizophrenia and autism. But how might these dysfunctions be approached therapeutically? Interneurons have highly specialized functions, electrophysiological properties and molecular profiles. Targeting molecules such as ion channels or receptors that are predominantly expressed in specific types of interneurons might make it possible to improve and harness the function of these cells without impairing other cell types. Indeed, Nav1.1 enhancers that preferentially activate Nav1.1-containing interneurons have been proposed for the treatment of epilepsy, schizophrenia and AD240. Because the firing rate of PV+ cells and gamma power strongly depend on behavioural activity154,177, behavioural or sensorial interventions that promote brain states associated with increased power ratios in high- to low-frequency oscillations might also be of benefit.
Finally, embryonic interneuron precursors transplanted into adult brains can migrate and integrate into appropriate circuits and mature into fully functional interneurons182. Although cell therapy for cognitive disorders may seem to be rather daring and must be approached with appropriate caution, it has already been explored in a small clinical trial in patients with AD241. In AD-related mouse models with prominent loss of hilar interneurons, including pilocarpine-treated wild-type mice185 and untreated APOE4-KI mice186, hippocampal transplants of wild-type interneuron precursors improved behavioural functions. However, it is not known how well such transplants would survive and function in the proteopathic, toxic microenvironment in AD brains172,203. Genetic modifications may be required to render interneuron precursors resistant to such conditions. Potentially beneficial modifications include overexpression of proteins that protect against the disease, such as Nav1.1 (REF. 172) in PV+ cells, or reduction of proteins that promote or enable AD-related pathogenesis, such as tau110 and APOE4 fragments242. These therapeutic opportunities also call for the development of reprogramming strategies to convert skin or blood cells into precursors of specific interneuron subtypes.
Conclusions
Network activities that support cognition are altered decades before the expected onset of clinical signs and symptoms in AD, and the affected networks predict future pathology and brain atrophy. Because neuronal synchrony regulates the functional state of brain circuits and networks, deficits in synchrony, including network hypersynchrony and altered oscillatory rhythmic activity, could contribute to AD-related cognitive dysfunction. Although the precise causes of these synchrony deficits remain to be defined, diverse lines of evidence suggest that interneuron dysfunction and network imbalance may be crucially involved. Therapeutic strategies that improve the function of interneurons and counteract such abnormalities might improve cognitive functions in AD and related disorders. By preventing excitotoxic overstimulation of neurons and maladaptive compensatory processes, they may also help to prevent or stall disease progression.
Supplementary Material
Acknowledgements
The authors thank P. Sanchez, Y. Huang, A. Kreitzer and members of their laboratories for helpful discussions; S. Ordway for editorial review; and C. Dickerson and A. Cheung for administrative assistance. This work was supported by US National Institutes of Health grants AG011385 (L.M.), AG053981 (L.M.), and AG047313 (J.J.P.); an Alzheimer’s Association grant IIRG-13–284779 (J.J.P.); an S.D. Bechtel, Jr. Young Investigator Award (J.J.P.); and a gift from the Dolby Family.
L. M. has received research funding from Bristol-Myers Squibb and consulting fees from AbbVie; he serves on the scientific advisory boards of Acumen Pharmaceuticals, Alkahest, Dolby Family Ventures, E-scape Bio, and Neuropore Therapies. J. P. declares no competing interests.
Footnotes
Competing interests statement
The authors declare completing interests. See Web version for details.
References
- 1.Seidman LJ et al. Medial temporal lobe default mode functioning and hippocampal structure as vulnerability indicators for schizophrenia: A MRI study of non-psychotic adolescent first-degree relatives. Schizophr. Res 159, 426–434 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Fahoum F, Zelmann R, Tyvaert L, Dubeau F. & Gotman J. Epileptic discharges affect the default mode network--fMRI and intracerebral EEG evidence. PLoS One 8, e68038 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oser N. et al. Default mode network alterations during language task performance in children with benign epilepsy with centrotemporal spikes (BECTS). Epilepsy Behav. 33, 12–17 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Sperling RA et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 63, 178–188 (2009).This study shows that PiB+ amyloid deposits are associated with deactivation deficits in a default network component in non-demented individuals and patients with MCI, linking aberrant neuronal activity and pathological changes.
- 5.Anticevic A. et al. The role of default network deactivation in cognition and disease. Trends Cogn. Sci 16, 584–592 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filippini N. et al. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc. Natl. Acad. Sci. USA 106, 7209–7214 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mondadori CR et al. Enhanced brain activity may precede the diagnosis of Alzheimer’s disease by 30 years. Brain 129, 2908–2922 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Bateman RJ et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med 367, 795–804 (2012).This study demonstrates changes in CSF biomarkers, cerebral amyloid deposition, and brain metabolism in carriers of autosomal dominant FAD mutations at least two decades before the estimated onset of clinical symptoms.
- 9.Reiman EM et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: A case-control study. Lancet. Neurol 11, 1048–1056 (2012).This study shows hippocampal hyperactivity and deactivation deficits in the DMN in 18–26-year-old asymptomatic PSEN1 mutation carriers.
- 10.Jasper HH Cortical excitatory state and variability in human brain rhythms. Science 83, 259–260 (1936). [DOI] [PubMed] [Google Scholar]
- 11.Destexhe A, Contreras D. & Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J. Neurosci 19, 4595–4608 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poulet JF & Petersen CC Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454, 881–885 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Poulet JF, Fernandez LM, Crochet S. & Petersen CC Thalamic control of cortical states. Nat. Neurosci 15, 370–372 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Karran E, Mercken M. & De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov 10, 698–712 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Benilova I, Karran E. & De Strooper B. The toxic Aβ oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci 15, 349–357 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Karch CM, Cruchaga C. & Goate AM Alzheimer’s disease genetics: from the bench to the clinic. Neuron 83, 11–26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez-Isla T. et al. The impact of different presenilin 1 andpresenilin 2 mutations on amyloid deposition, neurofibrillary changes and neuronal loss in the familial Alzheimer’s disease brain: Evidence for other phenotype-modifying factors. Brain. 122, 1709–1719 (1999). [DOI] [PubMed] [Google Scholar]
- 18.Jankowsky JL et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ _secretase. Hum. Mol. Genet 13, 159–170 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Steiner H. & Haass C. Intramembrane proteolysis by presenilins. Nat. Rev. Mol. Cell Biol 1, 217–224 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Tsubuki S, Takaki Y. & Saido TC Dutch, Flemish, Italian, and Arctic mutations of APP and resistance of Aβ to physiologically relevant proteolytic degradation. Lancet 361, 1957–1958 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Nilsberth C. et al. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Aβ protofibril formation. Nat. Neurosci 4, 887–893 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Shimada H. et al. Clinical course of patients with familial early-onset Alzheimer’s disease potentially lacking senile plaques bearing the E693 Δ mutation in amyloid precursor protein. Dement. Geriatr. Cogn. Disord 32, 45–54 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Jonsson T. et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488, 96–99 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Maloney JA et al. Molecular mechanisms of Alzheimer disease protection by the A673T allele of amyloid precursor protein. J. Biol. Chem 289 30990–31000, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S. et al. Soluble oligomers of amyloid β-protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 62, 788–801 (2009).This study shows that amyloid-β blocks neuronal glutamate uptake at synapses, which results in glutamate spillover, aberrant activation of extra- or perisynaptic GluN2B-containing NMDARs, and enhanced long-term depression.
- 26.Shankar GM et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med 14, 837–842 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S. et al. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J. Neurosci 31, 6627–6638 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cleary JP et al. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat. Neurosci 8, 79–84 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Musiek ES & Holtzman DM Three dimensions of the amyloid hypothesis: time, space and ‘wingmen’. Nat. Neurosci 18, 800–806 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu P. et al. Quaternary structure defines a large class of amyloid-β oligomers neutralized by sequestration. Cell Rep. 11, 1760–1771 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashe KH & Zahs KR Probing the biology of Alzheimer’s disease in mice. Neuron 66, 631–645 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris M, Maeda S, Vossel K. & Mucke L. The many faces of tau. Neuron 70, 410–426 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y. & Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell 148, 1204–1222 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heppner FL, Ransohoff RM & Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci 16, 358–372 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Maeda S. et al. Expression of A152T human tau causes age-dependent neuronal dysfunction and loss in transgenic mice. EMBO Rep. 17, 530–551 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SantaCruz K. et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandelkow EM & Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect. Med 2, a006247 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Min SW et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med 21, 1154–1162 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahley RW & Huang Y. Apolipoprotein E sets the stage: response to injury triggers neuropathology. Neuron 76, 871–885 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pike KE et al. β-Amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 130, 2837–2844 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Savva GM et al. Age, neuropathology, and dementia. N. Engl. J. Med 360, 2302–2309 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Ingelsson M. et al. Early Aβ accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 62, 925–931 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Lesne SE et al. Brain amyloid-β oligomers in ageing and Alzheimer’s disease. Brain 136, 1383–1398 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selkoe DJ Soluble oligomers of the amyloid β-protein impair synaptic plasticity and behavior. Behav. Brain Res 192, 106–113 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klein WL, Krafft GA & Finch CE Targeting small Aβ oligomers: The solution to an Alzheimer’s disease conundrum. Trends Neurosci. 24, 219–224 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Walsh DM & Selkoe DJ Aβ oligomers - a decade of discovery. J. Neurochem 101, 1172–1184 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Shankar GM et al. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci 27, 2866–2875 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng IH et al. Accelerating amyloid- β fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J. Biol. Chem 282, 23818–23828 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Tomiyama T. et al. A mouse model of amyloid β oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation, and neuronal loss in vivo. J. Neurosci 30, 4845–4856 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lesné S. et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Meilandt WJ et al. Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Aβ oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice. J. Neurosci 29, 1977–1986 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mably AJ et al. Anti- Aβ antibodies incapable of reducing cerebral Aβ oligomers fail to attenuate spatial reference memory deficits in J20 mice. Neurobiol. Dis 82, 372–384 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Strooper B. Loss-of-function presenilin mutations in Alzheimer disease. EMBO Rep. 8, 141–146 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pimplikar SW Neuroinflammation in Alzheimer’s disease: from pathogenesis to a therapeutic target. J. Clin. Immunol 34, S64–S69 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Xia D. et al. Presenilin-1 knockin mice reveal loss-of-function mechanism for familial Alzheimer’s disease. Neuron 85, 967–981 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bredesen DE Neurodegeneration in Alzheimer’s disease: caspases and synaptic element interdependence. Mol. Neurodegener 4, 27 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bentley WJ, Li JM, Snyder AZ, Raichle ME & Snyder LH Oxygen level and LFP in task-positive and task-negative areas: bridging BOLD fMRI and electrophysiology. Cereb. Cortex, 26, 346–357 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boyatzis RE, Rochford K. & Jack AI Antagonistic neural networks underlying differentiated leadership roles. Front. Hum. Neurosci 8, 114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raichle ME et al. A default mode of brain function. Proc. Natl. Acad. Sci. USA 98, 676–682 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greicius MD, Krasnow B, Reiss AL & Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 100, 253–258 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sperling RA et al. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med. 12, 27–43 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bookheimer SY et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med 343, 450–456 (2000).This study shows hippocampal hyperactivity during memory tasks in asymptomatic APOE ε4 carriers.
- 63.Trivedi MA et al. fMRI activation during episodic encoding and metacognitive appraisal across the lifespan: risk factors for Alzheimer’s disease. Neuropsychologia 46, 1667–1678 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kunz L. et al. Reduced grid-cell-like representations in adults at genetic risk for Alzheimer’s disease. Science 350, 430–433 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Quiroz YT et al. Hippocampal hyperactivation in presymptomatic familial Alzheimer’s disease. Ann. Neurol 68, 865–875 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sepulveda-Falla D, Glatzel M. & Lopera F. Phenotypic profile of early-onset familial Alzheimer’s disease caused by presenilin-1 E280A mutation. J. Alzheimers Dis 32, 1–12 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Bakker A. et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74, 467–474 (2012).This study shows that treatment with low doses of the antiepileptic drug levetiracetam reduces hippocampal hyperactivity and improves cognition in patients with MCI.
- 68.Dickerson BC et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65, 404–411 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Celone KA et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J. Neurosci 26, 10222–10231 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Persson J. et al. Altered deactivation in individuals with genetic risk for Alzheimer’s disease. Neuropsychologia 46, 1679–1687 (2008). [DOI] [PubMed] [Google Scholar]
- 71.Dickerson BC et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann. Neurol 56, 27–35 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Putcha D. et al. Hippocampal hyperactivation associated with cortical thinning in Alzheimer’s disease signature regions in non-demented elderly adults. J. Neurosci 31, 17680–17688 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bakker A, Albert MS, Krauss G, Speck CL & Gallagher M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention asessed by fMRI and memory task performance. NeuroImage Clin. 7, 688–698 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanchez PE et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc. Natl. Acad. Sci. USA 109, E2895–E2903 (2012).This study shows that levetiracetam reduces network hypersynchrony, synaptic deficits and behavioral abnormalities in hAPP-J20 mice.
- 75.Nygaard HB et al. Brivaracetam, but not ethosuximide, reverses memory impairments in an Alzheimer’s disease mouse model. Alzheimer Res. Ther 7, 25 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suberbielle E. et al. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat. Neurosci 16, 613–621 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suberbielle E. et al. DNA repair factor BRCA1 depletion occurs in Alzheimer brains and impairs cognitive function in mice. Nat. Commun 6, 8897 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Madabhushi R, Pan L. & Tsai LH DNA damage and its links to neurodegeneration. Neuron 83, 266–282 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim J, Basak JM & Holtzman DM The role of apolipoprotein E in Alzheimer’s disease. Neuron 63, 287–303 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saito T. et al. Single App knock-in mouse models of Alzheimer’s disease. Nat. Neurosci 17, 661–663 (2014). [DOI] [PubMed] [Google Scholar]
- 81.Roh JH et al. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer’s disease pathology. Sci. Transl. Med 4, 150ra122 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang JE et al. Amyloid- β dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xie L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bero AW et al. Neuronal activity regulates the regional vulnerability to amyloid- β deposition. Nat. Neurosci 14, 750–756 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamamoto K. et al. Chronic optogenetic activation augments Aβ pathology in a mouse model of Alzheimer disease. Cell Rep. 11, 859–865 (2015). [DOI] [PubMed] [Google Scholar]
- 86.Dolev I. et al. Spike bursts increase amyloid- β 40/42 ratio by inducing a presenilin-1 conformational change. Nat. Neurosci 16, 587–595 (2013).This study shows that amyloid-β production and the amyloid-β1–42/amyloid-β1–40 ratio are regulated by neuronal action potential firing through calcium-dependent conformational changes in PSEN1 at presynaptic terminals.
- 87.Nitsch RM, Farber SA, Growdon HJ & Wurtman RJ Release of amyloid β-protein precursor derivatives by electrical depolarization of rat hippocampal slices. Proc. Natl. Acad. Sci. USA 90, 5191–5193 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kamenetz F. et al. APP processing and synaptic function. Neuron 37, 925–937 (2003).This study shows that neuronal activity regulates amyloid-β production.
- 89.Abbott LF & Regehr WG Synaptic computation. Nature 431, 796–803 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Laviv T. et al. Basal GABA regulates GABABR conformation and release probability at single hippocampal synapses. Neuron 67, 253–267 (2010). [DOI] [PubMed] [Google Scholar]
- 91.Palop JJ & Mucke L. Amyloid-β-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat. Neurosci 13, 812–818 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cirrito JR et al. Synaptic activity regulates interstitial fluid amyloid- β levels in vivo. Neuron 48, 913–922 (2005). [DOI] [PubMed] [Google Scholar]
- 93.Abramov E. et al. Amyloid-β as a positive endogenous regulator of release probability at hippocampal synapses. Nat. Neurosci 12, 1567–1576 (2009). [DOI] [PubMed] [Google Scholar]
- 94.Puzzo D. et al. Picomolar amyloid- β positively modulates synaptic plasticity and memory in hippocampus. J. Neurosci 28, 14537–14545 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Willem M. et al. η-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature, 526, 443–447 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palop JJ et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55, 697–711 (2007).This study shows that hAPP-J20 mice have network hyperexcitability that is associated with extensive anatomical and physiological alterations in learning and memory centers.
- 97.Yang XF, Weisenfeld A. & Rothman SM Prolonged exposure to levetiracetam reveals a presynaptic effect on neurotransmission. Epilepsia 48, 1861–1869 (2007). [DOI] [PubMed] [Google Scholar]
- 98.Meehan AL, Yang X, McAdams BD, Yuan L. & Rothman SM A new mechanism for antiepileptic drug action: vesicular entry may mediate the effects of levetiracetam. J. Neurophysiol 106, 1227–1239 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kurudenkandy FR et al. Amyloid-β-induced action potential desynchronization and degradation of hippocampal gamma oscillations is prevented by interference with peptide conformation change and aggregation. J. Neurosci 34, 11416–11425 (2014).This study shows that amyloid-β reduces gamma oscillations by desynchronizing the action potentials of hippocampal pyramidal cells.
- 100.Sanchez-Mejia RO et al. Phospholipase A2 reduction ameliorates cognitve deficits in mouse model of Alzheimer’s disease. Nat. Neurosci 11, 1311–1318 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Busche MA et al. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 109, 8740–8745 (2012).This study shows that amyloid-β increases hippocampal neuronal activity in vivo by calcium imaging.
- 102.Minkeviciene R. et al. Amyloid β-induced neuronal hyperexcitability triggers progressive epilepsy. J. Neurosci 29, 3453–3462 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Um JW et al. Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat. Neurosci 15, 1227–1235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bezzina C. et al. Early onset of hypersynchronous network activity and expression of a marker of chronic seizures in the Tg2576 mouse model of Alzheimer’s disease. PLoS ONE 10, e0119910 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harris JA et al. Transsynaptic progression of amyloid-β-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron 68, 428–441 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Born HA et al. Genetic suppression of transgenic APP rescues hypersynchronous network activity in a mouse model of Alzeimer’s disease. J. Neurosci 34, 3826–3840 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ittner AA, Gladbach A, Bertz J, Suh LS & Ittner LM p38 MAP kinase-mediated NMDA receptor-dependent suppression of hippocampal hypersynchronicity in a mouse model of Alzheimer inverted question marks disease. Acta Neuropathol. Commun 2, 149 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roberson ED et al. Amyloid-β/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J. Neurosci 31, 700–711 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Busche MA et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 321, 1686–1689 (2008).This study shows that APP23xPS45 mice have hyperactive neurons and synchronized neuronal activity by calcium imaging.
- 110.Roberson ED et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science 316, 750–754 (2007).This study shows that endogenous tau promotes APP-dependent network hypersynchrony and cognitive decline.
- 111.Ittner LM et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell 142, 387–397 (2010). [DOI] [PubMed] [Google Scholar]
- 112.Palop JJ et al. Vulnerability of dentate granule cells to disruption of Arc expression in human amyloid precursor protein transgenic mice. J. Neurosci 25, 9686–9693 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chin J. et al. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer’s disease. J. Neurosci 25, 9694–9703 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grienberger C. et al. Staged decline of neuronal function in vivo in an animal model of Alzheimer’s disease. Nat. Commun 3, 774 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rudinskiy N. et al. Orchestrated experience-driven Arc responses are disrupted in a mouse model of Alzheimer’s disease. Nat. Neurosci 15, 1422–1429 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spires TL & Hyman BT Neuronal structure is altered by amyloid plaques. Rev. Neurosci 15, 267–278 (2004). [DOI] [PubMed] [Google Scholar]
- 117.Koffie RM et al. Oligomeric amyloid β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl. Acad. Sci. USA 106, 4012–4017 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Siskova Z. et al. Dendritic structural degeneration is functionally linked to cellular hyperexcitability in a mouse model of Alzheimer’s disease. Neuron 84, 1023–1033 (2014). [DOI] [PubMed] [Google Scholar]
- 119.Garcia-Cabrero AM et al. Hyperexcitability and epileptic seizures in a model of frontotemporal dementia. Neurobiol. Dis 58, 200–208 (2013). [DOI] [PubMed] [Google Scholar]
- 120.Hunter JM et al. Emergence of a seizure phenotype in aged apolipoprotein epsilon 4 targeted replacement mice. Brain Res. 1467, 120–132 (2012). [DOI] [PubMed] [Google Scholar]
- 121.Morris M. et al. Network dysfunction in α-synuclein transgenic mice and human Lewy body dementia. Ann. Clin. Transl. Neurol 2, 1012–1028 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Siwek ME et al. Altered theta oscillations and aberrant cortical excitatory activity in the 5XFAD model of Alzheimer’s disease. Neural Plast. 2015, 781731 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lalonde R, Dumont M, Staufenbiel M. & Strazielle C. Neurobehavioral characterization of APP23 transgenic mice with the SHIRPA primary screen. Behav. Brain Res 157, 91–98 (2005). [DOI] [PubMed] [Google Scholar]
- 124.Hsiao KK et al. Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron 15, 1203–1218 (1995). [DOI] [PubMed] [Google Scholar]
- 125.Vossel KA et al. Tau reduction prevents Aβ-induced impairments in axonal transport. Science 330, 198 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Devos SL et al. Antisense reduction of tau in adult mice protects against seizures. J. Neurosci 33, 12887–12897 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Romanelli MF, Morris JC, Ashkin K. & Coben LA Advanced Alzheimer’s disease is a risk factor for late-onset seizures. Arch. Neurol 47, 847–850 (1990). [DOI] [PubMed] [Google Scholar]
- 128.Hauser WA, Morris ML, Heston LL & Anderson VE Seizures and myoclonus in patients with Alzheimer’s disease. Neurology 36, 1226–1230 (1986). [DOI] [PubMed] [Google Scholar]
- 129.Irizarry MC et al. Incidence of new-onset seizures in mild to moderate Alzheimer disease. Arch. Neurol 69, 368–372 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Risse SC et al. Myoclonus, seizures, and paratonia in Alzheimer disease. Alzheimer Dis. Assoc. Disord 4, 217–225 (1990). [DOI] [PubMed] [Google Scholar]
- 131.Horvath A, Szucs A, Barcs G, Noebels JL & Kamondi A. Epileptic seizures in Alzheimer disease: a review. Alzheimer Dis. Assoc. Disord 30, 186–192 (2016). [DOI] [PubMed] [Google Scholar]
- 132.Imfeld P, Bodmer M, Schuerch M, Jick SS & Meier CR Seizures in patients with Alzheimer’s disease or vascular dementia: a population-based nested case-control analysis. Epilepsia 54, 700–707 (2013). [DOI] [PubMed] [Google Scholar]
- 133.Amatniek JC et al. Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia 47, 867–872 (2006).This study shows that early onset of AD is a risk factor for seizures.
- 134.Sherzai D, Losey T, Vega S. & Sherzai A. Seizures and dementia in the elderly: Nationwide Inpatient Sample 1999–2008. Epilepsy Behav. 36, 53–56 (2014). [DOI] [PubMed] [Google Scholar]
- 135.Scarmeas N. et al. Seizures in Alzheimer disease: who, when, and how common? Arch. Neurol 66, 992–997 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vossel KA et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 70, 1158–1166 (2013).This study shows that epileptic activity in patients with MCI or early AD can occur early in the disease course and is frequently non-convulsive in nature.
- 137.Hesdorffer DC, Hauser WA, Annegers JF, Kokmen E. & Rocca WA Dementia and adult-onset unprovoked seizures. Neurology 46, 727–730 (1996). [DOI] [PubMed] [Google Scholar]
- 138.Cretin B. et al. Epileptic prodromal Alzheimer’s disease, a retrospective study of 13 new cases: expanding the spectrum of Alzheimer’s disease to an epileptic variant? J. Alzheimers Dis 52, 1125–1133 (2016). [DOI] [PubMed] [Google Scholar]
- 139.Palop JJ & Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch. Neurol 66, 435–440 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Noebels J. A perfect storm: converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia 52 (Suppl. 1), 39–46 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Larner AJ Presenilin-1 mutation Alzheimer’s disease: a genetic epilepsy syndrome? Epilepsy Behav. 21, 20–22 (2011). [DOI] [PubMed] [Google Scholar]
- 142.Larner AJ & Doran M. Clinical phenotypic heterogeneity of Alzheimer’s disease associated with mutations of the presenilin-1 gene. J. Neurol 253, 139–158 (2006).This study shows that seizures are a clinical feature of many pedigrees of FAD patients carrying PSEN1 mutations.
- 143.Jayadev S. et al. Alzheimer’s disease phenotypes and genotypes associated with mutations in presenilin 2. Brain 133, 1143–1154 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cabrejo L. et al. Phenotype associated with APP duplication in five families. Brain 129, 2966–2976 (2006).This study shows that seizures are a clinical feature of many FAD patients carrying wild-type APP duplications.
- 145.McNaughton D. et al. Duplication of amyloid precursor protein (APP), but not prion protein (PRNP) gene is a significant cause of early onset dementia in a large UK series. Neurobiol. Aging 33, e13–21 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zarea A. et al. Seizures in dominantly inherited Alzheimer disease. Neurology 87, 912–919 (2016). [DOI] [PubMed] [Google Scholar]
- 147.Lai F. & Williams RS A prospective study of Alzheimer disease in Down syndrome. Arch. Neurol 46, 849–853 (1989). [DOI] [PubMed] [Google Scholar]
- 148.Snider BJ et al. Novel presenilin 1 mutation (S170F) causing Alzheimer disease with Lewy bodies in the third decade of life. Arch. Neurol 62, 1821–1830 (2005). [DOI] [PubMed] [Google Scholar]
- 149.Moehlmann T. et al. Presenilin-1 mutations of leucine 166 equally affect the generation of the Notch and APP intracellular domains independent of their effect on Aβ42 production. Proc. Natl Acad. Sci. USA 99, 8025–8030 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jensen O. & Colgin LL Cross-frequency coupling between neuronal oscillations. Trends Cognit. Sci 11, 267–269 (2007). [DOI] [PubMed] [Google Scholar]
- 151.Viney TJ et al. Network state–dependent inhibition of identified hippocampal CA3 axo–axonic cells in vivo. Nat. Neurosci 16, 1802–1811 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tukker JJ, Fuentealba P, Hartwich K, Somogyi P. & Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J. Neurosci 27, 8184–8189 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Uhlhaas PJ et al. Neural synchrony in cortical networks: history, concept and current status. Front. Integr. Neurosci 3, 17 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Matsumoto JY et al. Network oscillations modulate interictal epileptiform spike rate during human memory. Brain 136, 2444–2456 (2013).This study shows that memory-induced oscillatory changes, including increaed gamma power, reduce epileptiform discharges in humans with epilepsy.
- 155.Sederberg PB et al. Gamma oscillations distinguish true from false memories. Psychol. Sci 18, 927–932 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sederberg PB et al. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb. Cortex 17, 1190–1196 (2007).This study shows that increases in cortical and hippocampal gamma oscillations during memory tasks predict successful encoding of new memories in humans.
- 157.Jensen O, Kaiser J. & Lachaux JP Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 30, 317–324 (2007). [DOI] [PubMed] [Google Scholar]
- 158.Yamamoto J, Suh J, Takeuchi D. & Tonegawa S. Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell 157, 845–857 (2014). [DOI] [PubMed] [Google Scholar]
- 159.Tallon-Baudry C, Bertrand O, Delpuech C. & Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J. Neurosci 16, 4240–4249 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lachaux JP et al. The many faces of the gamma band response to complex visual stimuli. Neuroimage 25, 491–501 (2005). [DOI] [PubMed] [Google Scholar]
- 161.Harris KD & Thiele A. Cortical state and attention. Nat. Rev. Neurosci 12, 509–523 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Crone NE, Miglioretti DL, Gordon B. & Lesser RP Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 121, 2301–2315 (1998). [DOI] [PubMed] [Google Scholar]
- 163.Burke JF et al. Synchronous and asynchronous theta and gamma activity during episodic memory formation. J. Neurosci 33, 292–304 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sun L. et al. Evidence for dysregulated high-frequency oscillations during sensory processing in medication-naive, first episode schizophrenia. Schizophr. Res 150, 519–525 (2013). [DOI] [PubMed] [Google Scholar]
- 165.Herrmann CS & Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin. Neurophysiol 116, 2719–2733 (2005). [DOI] [PubMed] [Google Scholar]
- 166.Niessing J. et al. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science 309, 948–951 (2005). [DOI] [PubMed] [Google Scholar]
- 167.Logothetis NK, Pauls J, Augath M, Trinath T. & Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157 (2001). [DOI] [PubMed] [Google Scholar]
- 168.Goldman RI, Stern JM, Engel J Jr. & Cohen MS Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport 13, 2487–2492 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Laufs H. et al. EEG-correlated fMRI of human alpha activity. Neuroimage 19, 1463–1476 (2003). [DOI] [PubMed] [Google Scholar]
- 170.Scheeringa R. et al. Trial-by-trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. Neuroimage 44, 1224–1238 (2009). [DOI] [PubMed] [Google Scholar]
- 171.Ossandon T. et al. Transient suppression of broadband gamma power in the default-mode network is correlated with task complexity and subject performance. J. Neurosci 31, 14521–14530 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Verret L. et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708–721 (2012).This study shows that impairments of inhibitory interneurons contribute to network hypersynchrony, altered oscillatory rhythms, and behavioural deficits in hAPP-J20 mice.
- 173.Maheshwari A, Marks RL, Yu KM & Noebels JL Shift in interictal relative gamma power as a novel biomarker for drug response in two mouse models of absence epilepsy. Epilepsia 57, 79–88 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Crooks VC, Lubben J, Petitti DB, Little D. & Chiu V. Social network, cognitive function, and dementia incidence among elderly women. Am. J. Public Health 98, 1221–1227 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Buzsaki G. & Draguhn A. Neuronal oscillations in cortical networks. Science 304, 1926–1929 (2004). [DOI] [PubMed] [Google Scholar]
- 176.Hestrin S. & Galarreta M. Electrical synapses define networks of neocortical GABAergic neurons. Trends Neurosci. 28, 304–309 (2005). [DOI] [PubMed] [Google Scholar]
- 177.Lapray D. et al. Behavior-dependent specialization of identified hippocampal interneurons. Nat. Neurosci 15, 1265–1271 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fu Y. et al. A cortical circuit for gain control by behavioral state. Cell 156, 1139–1152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sohal VS, Zhang F, Yizhar O. & Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (2009).This study shows that parvalbumin cell activity is mechanistically linked to gamma oscillations.
- 180.Fazzari P. et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature 464, 1376–1380 (2010). [DOI] [PubMed] [Google Scholar]
- 181.Kepecs A. & Fishell G. Interneuron cell types are fit to function. Nature 505, 318–326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Southwell DG et al. Interneurons from embryonic development to cell-based therapy. Science 344, 1240622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Han S. et al. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature 489, 385–390 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chao HT et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 468, 263–269 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hunt RF, Girskis KM, Rubenstein JL, Alvarez-Buylla A. & Baraban SC GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat. Neurosci 16, 692–697 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tong LM et al. Inhibitory interneuron progenitor transplantation restores normal learning and memory in ApoE4 knock-in mice without or with Aβ accumulation. J. Neurosci 34, 9506–9515 (2014).This study shows that transplants of inhibitory interneurons restore learning and memory in APOE4-KI mice.
- 187.Freund TF & Katona I. Perisomatic inhibition. Neuron 56, 33–42 (2007). [DOI] [PubMed] [Google Scholar]
- 188.Schmidt MJ et al. Modulation of behavioral networks by selective interneuronal inactivation. Mol. Psychiatry 19, 580–587 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kvitsiani D. et al. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature 498, 363–366 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cardin JA et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cramer PE et al. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 335, 1503–1506 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rubio SE et al. Accelerated aging of the GABAergic septohippocampal pathway and decreased hippocampal rhythms in a mouse model of Alzheimer’s disease. FASEB J. 26, 4458–4467 (2012). [DOI] [PubMed] [Google Scholar]
- 193.Gurevicius K, Lipponen A. & Tanila H. Increased cortical and thalamic excitability in freely moving APPswe/PS1dE9 mice modeling epileptic activity associated with Alzheimer’s disease. Cereb. Cortex 23, 1148–1158 (2013). [DOI] [PubMed] [Google Scholar]
- 194.Kemere C, Carr MF, Karlsson MP & Frank LM Rapid and continuous modulation of hippocampal network state during exploration of new places. PLoS ONE 8, e73114 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Busche MA et al. Rescue of long-range circuit dysfunction in Alzheimer’s disease models. Nat. Neurosci 18, 1623–1630 (2015). [DOI] [PubMed] [Google Scholar]
- 196.Mander BA et al. β-Amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat. Neurosci 18, 1051–1057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.van Deursen JA, Vuurman EF, van Kranen-Mastenbroek VH, Verhey FR & Riedel WJ 40-Hz steady state response in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 32, 24–30 (2011). [DOI] [PubMed] [Google Scholar]
- 198.van Deursen JA, Vuurman EF, Verhey FR, van Kranen-Mastenbroek VH & Riedel WJ Increased EEG gamma band activity in Alzheimer’s disease and mild cognitive impairment. J. Neural Transm. (Vienna) 115, 1301–1311 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nimmrich V, Draguhn A. & Axmacher N. Neuronal network oscillations in neurodegenerative diseases. Neuromolecular Med. 17, 270–284 (2015). [DOI] [PubMed] [Google Scholar]
- 200.Gillespie AK et al. Apolipoprotein E4 causes age-dependent disruption of slow gamma oscillations during hippocampal sharp-wave ripples. Neuron 90, 740–751 (2016).This study shows reduced gamma power during hippocampal sharp-wave ripples in APOE4-KI mice.
- 201.Pena-Ortega F, Solis-Cisneros A, Ordaz B, Balleza-Tapia H. & Javier Lopez-Guerrero J. Amyloid beta 1–42 inhibits entorhinal cortex activity in the beta-gamma range: role of GSK-3. Curr. Alzheimer Res 9, 857–863 (2012). [DOI] [PubMed] [Google Scholar]
- 202.Driver JE et al. Impairment of hippocampal gamma-frequency oscillations in vitro in mice overexpressing human amyloid precursor protein (APP). Eur. J. Neurosci 26, 1280–1288 (2007). [DOI] [PubMed] [Google Scholar]
- 203.Villette V. et al. Decreased rhythmic GABAergic septal activity and memory–associated theta oscillations after hippocampal amyloid-β pathology in the rat. J. Neurosci 30, 10991–11003 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Royer S. et al. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat. Neurosci 15, 769–775 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tort AB, Komorowski RW, Manns JR, Kopell NJ & Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc. Natl Acad. Sci. USA 106, 20942–20947 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lakatos P, Karmos G, Mehta AD, Ulbert I. & Schroeder CE Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 320, 110–113 (2008). [DOI] [PubMed] [Google Scholar]
- 207.Canolty RT et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science 313, 1626–1628 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bragin A. et al. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J. Neurosci 15, 47–60 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sirota A. et al. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60, 683–697 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Freund TF Interneuron diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 26, 489–495 (2003). [DOI] [PubMed] [Google Scholar]
- 211.Belluscio MA, Mizuseki K, Schmidt R, Kempter R. & Buzsaki G. Cross-frequency phase-phase coupling between theta and gamma oscillations in the hippocampus. J. Neurosci 32, 423–435 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Goutagny R. et al. Alterations in hippocampal network oscillations and theta-gamma coupling arise before Aβ overproduction in a mouse model of Alzheimer’s disease. Eur. J. Neurosci 37, 1896–1902 (2013). [DOI] [PubMed] [Google Scholar]
- 213.Klausberger T. et al. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421, 844–848 (2003).This study shows that different types of interneurons contribute to specific oscillatory frequencies.
- 214.Andrews-Zwilling Y. et al. Hilar GABAergic interneuron activity controls spatial learning and memory retrieval. PLoS ONE 7, e40555 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang WZ et al. The developmental changes of Nav1.1 and Nav1.2 expression in the human hippocampus and temporal lobe. Brain Res. 1389, 61–70 (2011). [DOI] [PubMed] [Google Scholar]
- 216.Corbett BF et al. Sodium channel cleavage is associated with aberrant neuronal activity and cognitive deficits in a mouse model of Alzheimer’s disease. J. Neurosci 33, 7020–7026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kim DY et al. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat. Cell Biol 9, 755–764 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yang LB et al. Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat. Med 9, 3–4 (2003). [DOI] [PubMed] [Google Scholar]
- 219.Kim DY et al. The E280A presenilin mutation reduces voltage-gated sodium channel levels in neuronal cells. Neurodegener. Dis 13, 64–68 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ponomareva NV, Korovaitseva GI & Rogaev EI EEG alterations in non-demented individuals related to apolipoprotein E genotype and to risk of Alzheimer disease. Neurobiol. Aging 29, 819–827 (2008). [DOI] [PubMed] [Google Scholar]
- 221.Andrews-Zwilling Y. et al. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J. Neurosci 30, 13707–13717 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Terry RD et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol 30, 572–580 (1991). [DOI] [PubMed] [Google Scholar]
- 223.DeKosky ST & Scheff SW Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol 27, 457–464 (1990). [DOI] [PubMed] [Google Scholar]
- 224.Walsh DM et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 (2002). [DOI] [PubMed] [Google Scholar]
- 225.Hsia AY et al. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc. Natl. Acad. Sci. USA 96, 3228–3233 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mucke L. et al. High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci 20, 4050–4058 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kim JH, Anwyl R, Suh YH, Djamgoz MB & Rowan MJ Use-dependent effects of amyloidogenic fragments of β-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. J. Neurosci 21, 1327–1333 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hsieh H. et al. AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron 52, 831–843 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Palop JJ & Mucke L. Synaptic depression and aberrant excitatory network activity in Alzheimer’s disease: two faces of the same coin? Neuromolecular Med. 12, 48–55 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Liu L. et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304, 1021–1024 (2004). [DOI] [PubMed] [Google Scholar]
- 231.Campbell SL, Hablitz JJ & Olsen ML Functional changes in glutamate transporters and astrocyte biophysical properties in a rodent model of focal cortical dysplasia. Front. Cell. Neurosci 8, 425 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Palop JJ et al. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc. Natl Acad. Sci. USA 100, 9572–9577 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cheng JS et al. Collagen VI protects neurons against Aβ toxicity. Nat. Neurosci 12, 119–121 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chin J. et al. Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer’s disease. J. Neurosci 27, 2727–2733 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Meilandt WJ et al. Enkephalin elevations contribute to neuronal and behavioral impairments in a transgenic mouse model of Alzheimer’s disease. J. Neurosci 28, 5007–5017 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Orr AG et al. Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat. Neurosci 18, 423–434 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Albert MS et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gainotti G, Quaranta D, Vita MG & Marra C. Neuropsychological predictors of conversion from mild cognitive impairment to Alzheimer’s disease. J. Alzheimers Dis 38, 481–495 (2014). [DOI] [PubMed] [Google Scholar]
- 239.Budson AE & Solomon PR New criteria for Alzheimer disease and mild cognitive impairment: implications for the practicing clinician. Neurologist 18, 356–363 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jensen HS, Grunnet M. & Bastlund JF Therapeutic potential of Nav1.1 activators. Trends. Pharmacol. Sci 35, 113–118 (2014). [DOI] [PubMed] [Google Scholar]
- 241.Tuszynski MH et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat. Med 11, 551–555 (2005). [DOI] [PubMed] [Google Scholar]
- 242.Knoferle J. et al. Apolipoprotein E4 produced in GABAergic interneurons causes learning and memory deficits in mice. J. Neurosci 34, 14069–14078 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Buckner RL et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci 25, 7709–7717 (2005).This study shows that PiB+ amyloid preferentially deposits in brain regions of the DMN.
- 244.Axelman K, Basun H, Winblad B. & Lannfelt L. A large Swedish family with Alzheimer’s disease with a codon 670/671 amyloid precursor protein mutation: a clinical and genealogical investigation. Arch. Neurol 51, 1193–1197 (1994). [DOI] [PubMed] [Google Scholar]
- 245.Godbolt AK et al. A second family with familial AD and the V717L APP mutation has a later age at onset. Neurology 66, 611–612 (2006). [DOI] [PubMed] [Google Scholar]
- 246.Abe M. et al. Phenotypical difference of amyloid precursor protein (APP) V717L mutation in Japanese family. BMC Neurol. 12, 38 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kennedy AM et al. Familial Alzheimer’s disease. A pedigree with a mis-sense mutation in the amyloid precursor protein gene (amyloid precursor protein 717 valine→glycine). Brain 116, 309–324 (1993). [DOI] [PubMed] [Google Scholar]
- 248.Mullan M. et al. Clinical comparison of Alzheimer’s disease in pedigrees with the codon 717 Val→Ile mutation in the amyloid precursor protein gene. Neurobiol. Aging 14, 407–419 (1993). [DOI] [PubMed] [Google Scholar]
- 249.Lindquist SG et al. Atypical early-onset Alzheimer’s disease caused by the Iranian APP mutation. J. Neurol. Sci 268, 124–130 (2008). [DOI] [PubMed] [Google Scholar]
- 250.Edwards-Lee T. et al. An African American family with early-onset Alzheimer disease and an APP (T714I) mutation. Neurology 64, 377–379 (2005). [DOI] [PubMed] [Google Scholar]
- 251.Sleegers K. et al. APP duplication is sufficient to cause early onset Alzheimer’s dementia with cerebral amyloid angiopathy. Brain 129, 2977–2983 (2006). [DOI] [PubMed] [Google Scholar]
- 252.Remes AM et al. Hereditary dementia with intracerebral hemorrhages and cerebral amyloid angiopathy. Neurology 63, 234–240 (2004). [DOI] [PubMed] [Google Scholar]
- 253.Rovelet-Lecrux A. et al. APP locus duplication in a Finnish family with dementia and intracerebral haemorrhage. J. Neurol. Neurosurg. Psychiatry 78, 1158–1159 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Axelman K, Basun H. & Lannfelt L. Wide range of disease onset in a family with Alzheimer disease and a His163Tyr mutation in the presenilin-1 gene. Arch. Neurol 55, 698–702 (1998). [DOI] [PubMed] [Google Scholar]
- 255.Mangone CA et al. Early onset Alzheimer’s disease in a South American pedigree from Argentina. Acta Neurol. Scand 91, 6–13 (1995). [DOI] [PubMed] [Google Scholar]
- 256.Mann DMA, et al. Amyloid angiopathy and variability in amyloid β deposition is determined by mutation position in presenilin-1-linked Alzheimer’s disease. Am. J. Pathol 158, 2165–2175 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Frommelt P, Schnabel R, Kuhne W, Nee LE & Polinsky RJ Familial Alzheimer disease: a large, multigeneration German kindred. Alzheimer Dis. Assoc. Disord 5, 36–43 (1991). [DOI] [PubMed] [Google Scholar]
- 258.Vossel KA et al. Incidence and impact of subclinical epileptiform activity in alzheimer’s disease. Ann. Neurol 10.1002/ana.24794 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.