Abstract
Background:
Noninvasive biomarkers predicting immune checkpoint inhibitor (ICI) response are urgently needed. We evaluated the predictive value of pretreatment neutrophil-to-lymphocyte ratio (NLR), smoking history, smoking intensity, BMI and programmed death ligand 1 (PD-L1) expression in non-small-cell lung cancer (NSCLC) patients treated with ICIs.
Materials & methods:
Single-center retrospective study included 137 patients from July 2015 to February 2018. Outcomes included 3-month disease control rate, progression-free survival, and overall survival. Predictive value of biomarkers was assessed independently and in a multivariable model.
Results:
NLR was associated with all outcomes. Smoking history was predictive of progression-free survival and smoking intensity was predictive of disease control rate. BMI and PD-L1 were not associated with any outcome. High BMI was associated with low NLR.
Conclusion:
Simple clinical biomarkers can predict response to ICIs. A score incorporating both clinical factors and established tissue/serum biomarkers may be useful in identifying NSCLC patients who would benefit from ICIs.
Keywords: : BMI, NLR, NLR and BMI, NSCLC, PD-L1, smoking
Immune checkpoint inhibitors (ICIs) have transformed the standard of care of non-small-cell lung cancer (NSCLC) over recent years. Based on large prospective clinical trials, pembrolizumab, a programmed death-1 (PD-1) inhibitor, received US FDA approval as first-line therapy as both a single agent in patients with PD-L1-expressing tumors and in combination with chemotherapy for all NSCLC patients regardless of programmed death ligand 1 (PD-L1) status [1–4]. Other ICIs, such as nivolumab and atezolizumab, have been approved for second-line and subsequent lines of stage IV NSCLC treatment [3]. However, despite impressive improvements in median outcomes with ICIs over chemotherapy in clinical trials, a majority of NSCLC patients do not respond to immunotherapy.
PD-L1 expression on tumor cells, a proof of principle biomarker, has been validated as a predictor for the potential benefit of pembrolizumab monotherapy in the first-line setting. Multiple studies have shown that outcomes are often directly proportional to the intensity of PD-L1 staining [2,4]. Nonetheless, many patients with high PD-L1 expression (≥50%) demonstrate poor responses to PD-L1 inhibitors [1]. Conversely, tumors with no PD-L1 expression can sometimes respond to ICI therapy alone or with additional agents [5,6]. The addition of chemotherapy to pembrolizumab improved survival relative to chemotherapy alone, as did the addition of ipilimumab to nivolumab [2,6]. Given the lack of the predictive accuracy of PD-L1 expression, additional or alternative biomarkers are greatly needed. Besides its suboptimal clinical utility, another limitation of PD-L1 as a biomarker is the lack of uniformity among available testing methods. Each approved PD-1/PD-L1 inhibitor has been studied with a companion PD-L1 immunohistochemical assay that utilizes different antibodies. As such, the definitions and cut-offs for PD-L1 positivity are not uniform among studies and they seem to be highly dependent on the reviewing pathologist. Moreover, there are known inter- and intra-tumor heterogeneity and variations of PD-L1 expression that occur over time [7,8]. Finally, PD-L1 testing has been validated only on surgical tissue specimens which are frequently hard to obtain. In clinical practice, cytologic aspirates are used for testing and serial monitoring of tumoral PD-L1 expression is impractical.
Multiple serum and tissue-based biomarkers have been studied for their value in predicting response to ICIs. Many of these markers focus on the tumor immune environment which has been found to play an intricate role in lung cancer progression. Some recent studies have suggested that tumor-associated inflammation may be reflected in the peripheral blood. For example, peripheral blood tumor mutational burden (TMB) has been shown to correlate with tissue TMB in clinical trials, and flow cytometry for peripheral T-cell subsets have been shown to predict ICI responses in preclinical models [9,10]. However, with conflicting results in prospective trials, both tissue-based and plasma TMB remain exploratory at this time [11–14]. Like PD-L1, these tests are also limited by differences in sequencing methodology, number of genes studied, turnaround time and cost [15].
Peripheral blood neutrophil-lymphocyte ratio (NLR) is a biomarker that has shown promise in forecasting ICI response. Although the mechanism is not completely understood, peritumoral neutrophils have been shown to promote tumor angiogenesis, cell invasion and metastasis as well as inhibit apoptosis. On the other hand, peritumoral lymphocytes are involved in enhancing tumor defense and inhibiting tumor growth. It has been postulated that peripheral NLR could provide insight into the intratumoral inflammatory microenvironment [16]. Indeed, elevated NLR has been associated with a worse prognosis and there are now many observations reported in literature suggesting that NLR may predict response to ICIs in both NSCLC and other cancer types [17–20].
Patient characteristics, such as age, performance status, smoking history (SH) and nutritional status have also been associated with disease control rate (DCR) of ICIs. History of smoking has been shown to increase the likelihood of response to ICIs in NSCLC [21]. The proposed mechanism for this observation is related to increased mutagenesis from carcinogens in tobacco smoke, leading to a greater TMB in smokers compared with nonsmokers [22]. Patients with a higher BMI are also more likely to have better outcomes with immunotherapy compared with patients with a lower BMI, the mechanism of which is poorly understood. A possible explanation is that obesity is a state of heightened inflammatory response that interferes with optimal immune function, inhibits antitumor immunity and results in immune exhaustion. ICI therapy might reverse this process and enhance antitumor immunity [23].
Given the predictive potential of these various factors, we assessed the value of pretreatment NLR, SH, smoking intensity (SI) and nutritional status (as determined by BMI) of NSCLC patients in predicting response to ICIs independently and compared them with that of PD-L1 expression.
Materials & methods
We conducted a single-center retrospective analysis of NSCLC patients treated with ICIs from July 2015 to February 2018. The study was granted an exempt status by our institutional review board. Patient’s age, gender, SH, SI in pack-years, NLR, BMI and tumor PD-L1 status were captured prior to starting an ICI as baseline covariates. PD-L1 expression was described as positive (≥1%) or negative (0%). SI was designated as either heavy (>20 pack-years) or nonheavy (≤20 pack-years). A previously established cutoff of five categorized NLR as high or low, and a cut-off of 25 kg/m2 classified BMI as high or low.
Subjects with complete response, partial response or stable disease per modified RECIST 1.1 criteria on a CT scan performed approximately 3 months after ICI therapy was initiated were considered to have disease control [24]. DCR at 3 months was used as an outcome measure. Subjects who had progression of disease on a 3-month scan or died prior to the scan were not considered to have disease control. For subjects who did not have a scan at 3 months or were lost to follow-up prior to 3 months, clinical benefit was considered unknown, and these patients were excluded from the analysis of DCR at 3 months. Associations between patient clinical factors at baseline and DCR at 3 months were examined using the Chi-square test or Fisher’s exact test, as appropriate. Multivariable logistic regression was used to examine the joint effects of NLR at baseline, BMI and SH on DCR.
Progression-free survival (PFS) was measured as the time from start of treatment to progression of disease or death, whichever came first. Subjects who had not progressed and were alive as of their last follow-up were considered censored, and time to last follow-up was used. Overall survival (OS) was measured as the time from start of treatment to death. Subjects who were alive as of their last follow-up were considered censored, and time to last follow-up was used. Separate analyses were carried out for each time-to-event outcome. PFS and OS were estimated using the Kaplan–Meier product-limit method and compared using the log-rank test. A multivariable Cox regression model was used to examine the joint effects of NLR at baseline, BMI and smoking on outcomes [25].
For all multivariable models, SH (current/prior or never) and SI in pack-years were combined into a single variable. This variable has three levels: heavy current/prior smokers (>20 pack-years), nonheavy current/prior smokers (1-20 pack-years), and never smokers (0 pack-years). It was not feasible to include the SH and intensity as separate variables because never smokers would essentially have 0 pack-years, and current/prior smokers could not have 0 pack-years. For all multivariable models, factors that have been shown to be associated with prognosis in published literature were chosen for inclusion. Although PD-L1 has also been shown to be associated with outcomes, it was not feasible to include it in any of the multivariable models due to the large number of subjects who did not have PD-L1 status available.
Results
Table 1 depicts the baseline characteristics of patients included in this study. A total of 137 patients were captured for analysis which included 80 male and 57 female patients. Within this population, 86 patients had adenocarcinoma and 39 patients had squamous cell carcinoma. The remaining 12 patients had a different histology which included poorly differentiated (5), neuroendocrine (5) and pleomorphic (2). Table 2 shows the incidence of histology among each clinical factor. An ICI was used in the first-line setting for 25 patients and it was given as a subsequent line of therapy for 112 patients. Within the first-line cohort, 17 patients received ICI monotherapy and 8 patients received an ICI in combination with chemotherapy. In regard to the clinical factors being investigated, there was a significant association between BMI (<25, ≥25) and NLR at baseline (p < 0.0381, Chi-square test). About 56.5% (39/69) of subjects with BMI <25 had an NLR <5 at baseline and 73.2% (52/71) of subjects with BMI ≥25 had an NLR <5. No other factors were associated with NLR at baseline.
Table 1. . Characteristics of patients at baseline.
| Age (n = 137) | Histology (n = 137) | Line of therapy ICI was initiated (n = 137) | Baseline PD-L1 status (n = 55) | Smoking status (n = 137) | Pack-years (n = 134) | Baseline NLR (n = 137) | BMI (n = 137) | ||
|---|---|---|---|---|---|---|---|---|---|
| n | 80 (male) | 86 (adenocarcinoma) | 1st (25) | Monotherapy (17) | 35 (positive) | 124 (current/prior) | 34 (≤20) | 90 (<5) | 67 (<25) |
| 39 (squamous) | Triple therapy (8) | ||||||||
| 57 (female) | 12 (other†) | 2nd or later (112) | 20 (negative) | 13 (never) | 100 (>20) | 47 (≥5) | 70 (≥25) | ||
| Mean | 68.4 | NA | NA | NA | NA | 39.1 | 5.3 | 25.6 | |
| Range | 28–92 | NA | NA | NA | NA | 0-110 | 0.9–35 | 15–41.4 | |
Includes poorly differentiated, neuroendocrine and pleomorphic.
ICI: Immune checkpoint inhibitor; NA: Not applicable; NLR: Neutrophil-to-lymphocyte ratio; PD-L1: Programmed death ligand 1.
Table 2. . Incidence of histology among clinical factors.
| PD-L1 (+) [n = 35] | PD-L1 (-) [n = 20] | Smoking status (current/prior) [n = 124] | Smoking status (never) [n = 13] | Pack years (≤20) [n = 34] | Pack years (>20) [n = 100] | Baseline NLR (<5) [n = 90] | Baseline NLR (≥5) [n = 47] | BMI (<25) [n = 67] | BMI (≥25) [n = 70] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Adenocarcinoma | 23 | 18 | 75 | 11 | 26 | 57 | 61 | 25 | 39 | 47 |
| Squamous | 8 | 1 | 39 | 0 | 4 | 35 | 19 | 20 | 24 | 15 |
| Other† | 4 | 1 | 10 | 2 | 4 | 8 | 10 | 2 | 4 | 8 |
Includes poorly differentiated, neuroendocrine and pleomorphic.
NLR: Neutrophil-to-lymphocyte ratio; PD-L1: Programmed death ligand 1.
NLR <5 was significantly associated with improvement in all measures of outcome (Table 3). Median survival for subjects with NLR <5 was 15 months (95% CI: 11.75–22.25) compared with 5.25 months for subjects with NLR ≥5 (95% CI: 2.75–9.75) (p < 0.0005, Figure 1). Median PFS was 8 months (95% CI: 6.0–11.25) and 3 months (95% CI: 2.0–4.00) for patients with low and high NLR respectively (p < 0.0001, Figure 2). DCR was 68.9% (62/90) in patients with an NLR <5 as opposed to 38.3% (18/47) of patients with an NLR ≥5 (p < 0.0006).
Table 3. . NLR at baseline and outcomes.
| NLR <5 | NLR ≥5 | p-value | |
|---|---|---|---|
| Disease control rate | 0.0006 | ||
| – Yes | 62 (68.9%) | 18 (38.3%) | |
| – No | 28 (31.1%) | 29 (61.7%) | |
| Median overall survival (months) | 15.00 (95% CI: 11.75–22.25) | 5.25 (95% CI: 2.75, 9.75) | 0.0005 |
| Median progression-free survival (months) | 8.00 (95% CI: 6.00–11.25) | 3.00 (95% CI: 2.00, 4.00) | <0.0001 |
NLR: Neutrophil-to-lymphocyte ratio.
Figure 1. . Overall survival (in months) by neutrophil-to-lymphocyte ratio (high vs low) at baseline.
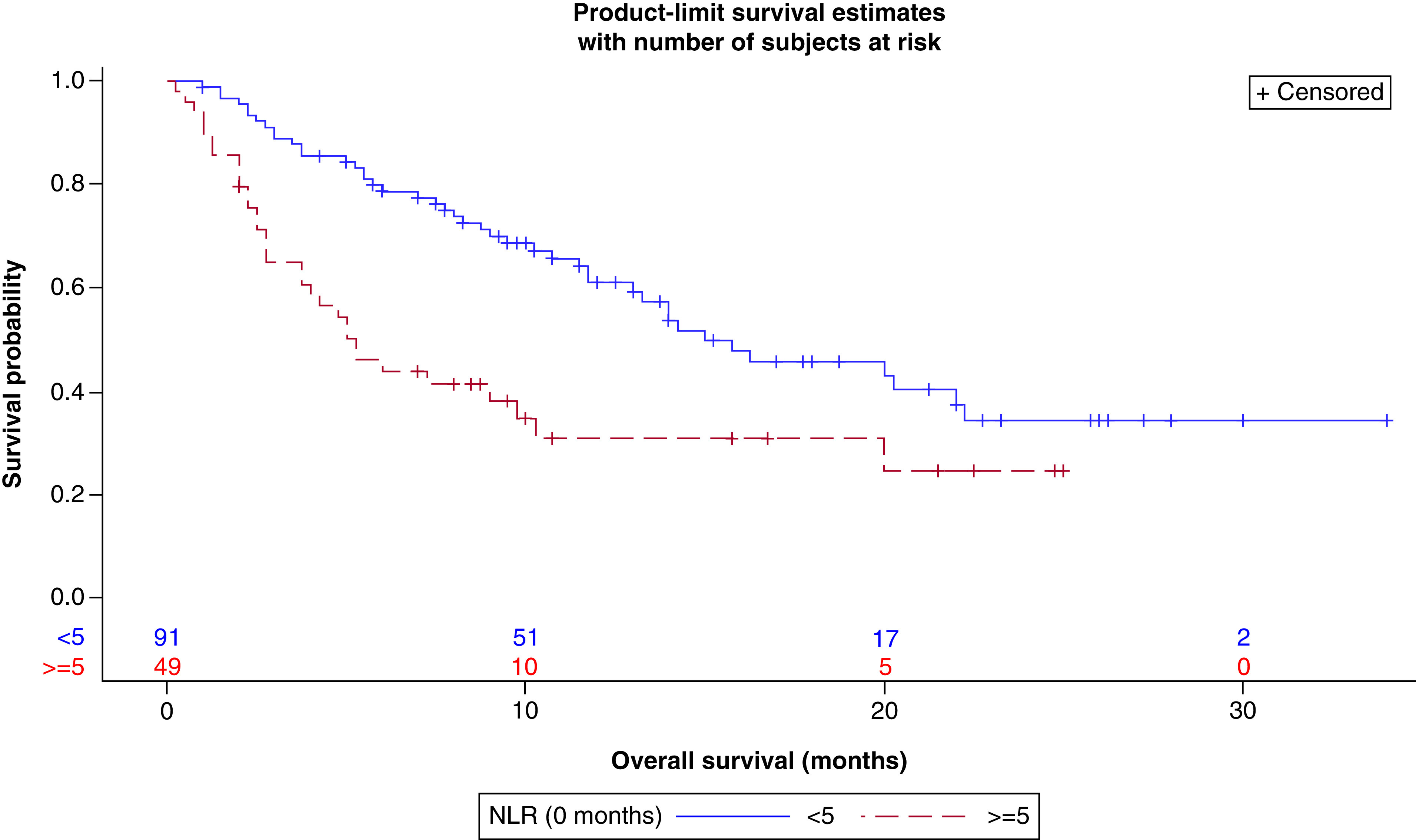
NLR: Neutrophil-to-lymphocyte ratio.
Figure 2. . Progression-free survival (in months) by neutrophil-to-lymphocyte ratio (high vs low) at baseline.
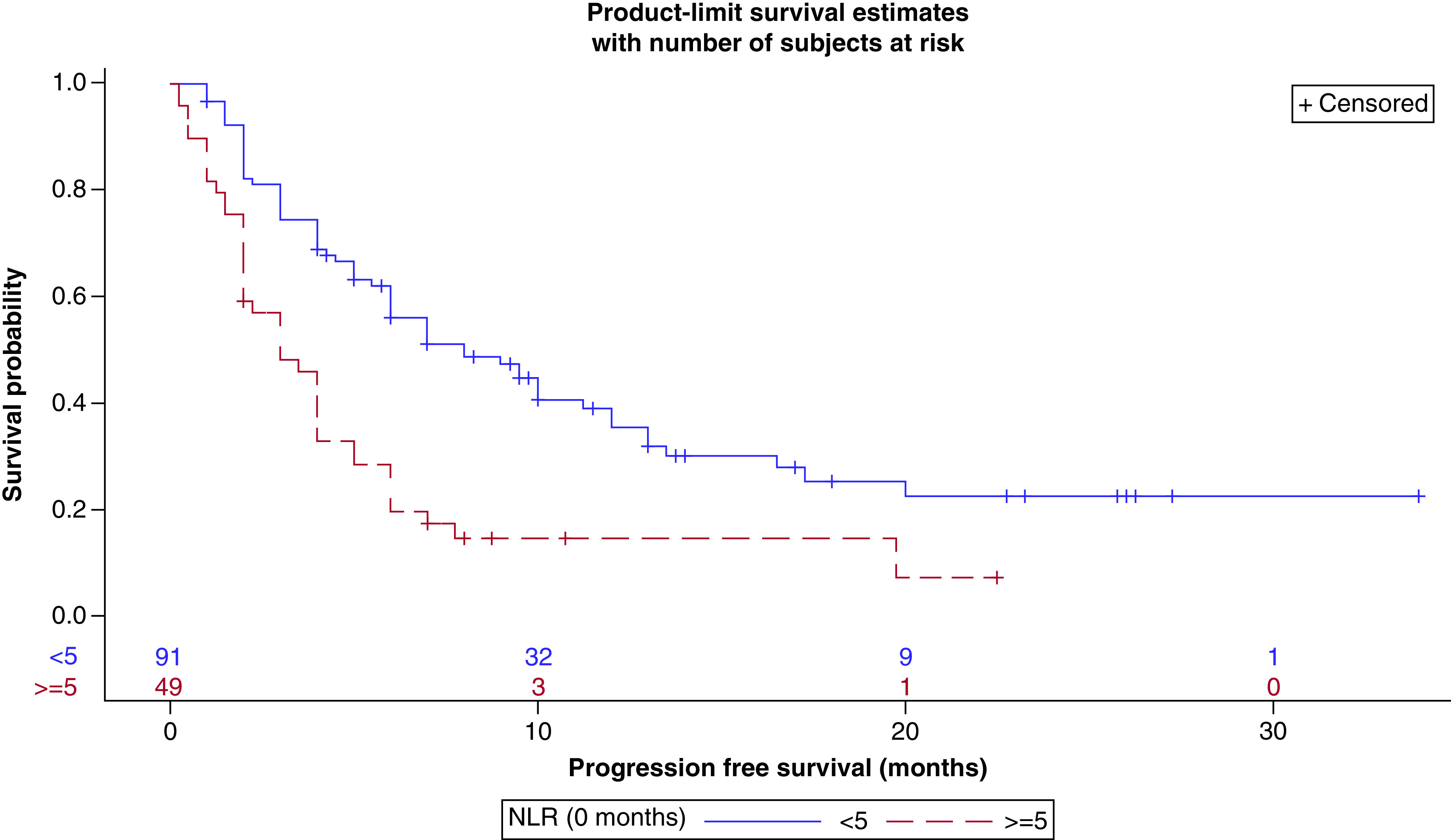
NLR: Neutrophil-to-lymphocyte ratio
In regard to smoking, there was a positive association between pack-years (0-20, >20) and DCR at 3 months (p < 0.0320). 44.1% (15/34) of subjects with ≤20 pack-years had disease control at 3 months compared with 65.0% (65/100) of heavy smokers (Table 4). There was also a statistically significant difference in PFS in regard to SH (Table 5). Patients with history of tobacco smoking (current or prior) had a median PFS of 6 months (95% CI: 4.00–7.00) compared with 4 months (95% CI: 2.00–6.00) for patients who have never smoked (Figure 3) (p < 0.0267). Neither SH nor SI had an impact on OS in our analysis. In addition, patients with a high BMI were observed to have improvement in all measures of outcome in our analysis; however, none of these associations were found to be statistically significant (Table 6).
Table 4. . Smoking intensity and outcomes.
| 0-20 pack-years | >20 pack-years | p-value | |
|---|---|---|---|
| Disease control rate | 0.0320 | ||
| – Yes | 15 (44.1%) | 65 (65.0%) | |
| – No | 19 (55.9%) | 35 (35.0%) | |
| Median overall survival (months) | 10.25 (95% CI: 5.25–20.25) | 14.00 (95% CI: 10.25, 22.25) | 0.2864 |
| Median progression-free survival (months) | 4.00 (95% CI: 3.00–7.00) | 6.00 (95% CI: 4.50, 9.50) | 0.0624 |
Table 5. . Smoking history and outcomes.
| Current/prior | Never | p-value | |
|---|---|---|---|
| Disease control rate | 0.1254 | ||
| – Yes | 75 (60.5%) | 5 (38.5%) | |
| – No | 49 (39.5%) | 8 (61.5%) | |
| Median overall survival (months) | 11.75 (95% CI: 8.75–16.25) | 15.75 (95% CI: 2.75, NE) | 0.7711 |
| Median progression-free survival (months) | 6.00 (95% CI: 4.00–7.00) | 4.00 (95% CI: 2.00, 6.00) | 0.0267 |
NE: Not estimable based on the pattern of the data.
Figure 3. . Progression-free survival (in months) by smoking history (current/prior vs never).
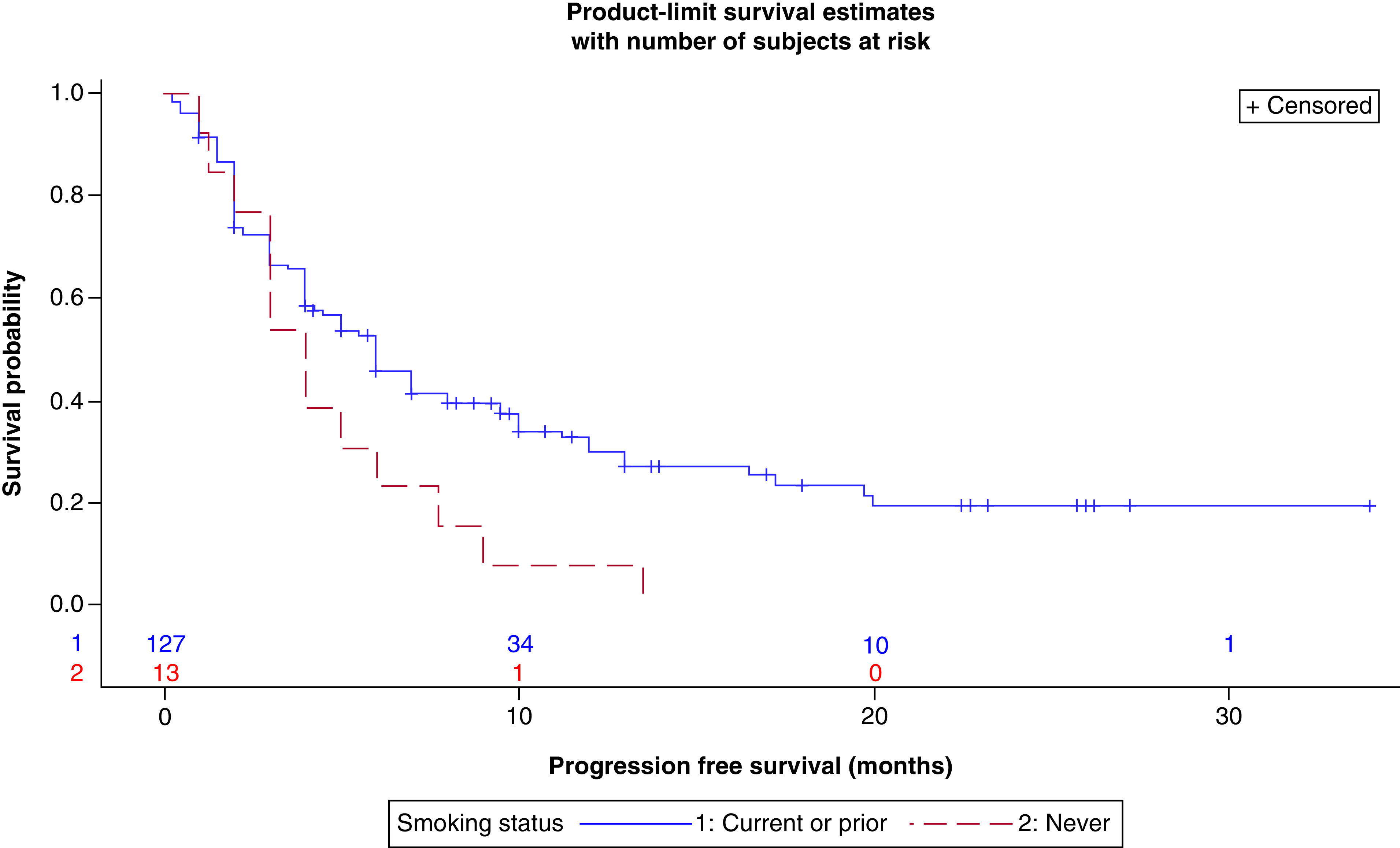
Table 6. . BMI at baseline and outcomes.
| <25 kg/m2 | ≥25 kg/m2 | p-value | |
|---|---|---|---|
| Disease control rate | 0.6967 | ||
| – Yes | 38 (56.7%) | 42 (60.0%) | |
| – No | 29 (43.3%) | 28 (40.0%) | |
| Median overall survival (months) | 10.25 (95% CI: 6.00–16.25) | 14.00 (95% CI: 10.25, NE) | 0.1166 |
| Median progression-free survival (months) | 5.00 (95% CI: 3.50–6.00) | 7.00 (95% CI: 4.00, 10.00) | 0.0743 |
NE: Not estimable based on the pattern of the data.
The total number of patients with PD-L1 expression available was limited (N = 55) which restricted our analysis. DCR in this small patient cohort was similar in the positive and negative groups (Table 7). There was no difference noted in either OS or PFS. The median OS was not reached for both positive and negative cohorts in this subgroup.
Table 7. . PD-L1 expression at baseline.
| Positive | Negative | p-value | |
|---|---|---|---|
| Disease control rate | 0.7445 | ||
| – Yes | 23 (65.7%) | 14 (70.0%) | |
| – No | 12 (34.3%) | 6 (30.0%) | |
| Median overall survival (months) | NE (7.75-NE) | NE (95% CI: 10.25, NE) | 0.9364 |
| Median progression-free survival (months) | 5.00 (95% CI: 4.00-NE) | 8.00 (95% CI: 4.00, 12.00) | 0.8585 |
NE: Not estimable; PD-L1: Programmed death ligand 1.
Tables 8 and 9 summarize results of the multivariable model for DCR and PFS. Low NLR at baseline was associated with significant improvement in both of these outcomes. Also, the heavy current/prior smokers cohort was significantly associated with a better PFS when directly compared with the never smokers cohort. In the multivariable model for OS, the assumption of proportional hazards was not met for NLR ratio. Log of negative log of the estimated survivor functions versus the log of time was plotted in order to estimate that the hazard changed at about 6 months. Therefore, an extended Cox model using a step function for NLR at baseline (≤6 months, >6 months) was used. The results of the multivariable model for OS are summarized in Table 10. NLR at baseline was significantly associated with survival during the first 6 months after the start of treatment; subjects with NLR ≥5 had a higher hazard of dying. After 6 months, there was no significant difference in the hazard.
Table 8. . Multivariable analysis (disease control rate)†.
| Factor | Level | Odds ratio (95% CI) | p-value |
|---|---|---|---|
| NLR at baseline | <5 | Ref | 0.0014 |
| ≥5 | 3.59 (1.64, 7.87) | ||
| BMI | ≥25 | Ref | 0.7968 |
| <25 | 1.11 (0.52, 2.36) | ||
| Smoking history | Never | Ref | 0.0725 |
| Current/prior smokers (1–20 pack-years) | 0.76 (0.18, 3.31) | ||
| Current/prior smokers (>20 pack-years) | 0.32 (0.09, 1.11) |
The outcome was ‘No DCR at 3 months.’ An odds ratio (OR) >1 indicates higher odds of no DCR.
NLR: Neutrophil-to-lymphocyte ratio.
Table 9. . Multivariable analysis (progression-free survival).
| Factor | Level | Hazard ratio (95% CI) | p-value |
|---|---|---|---|
| NLR at baseline | <5 | Ref | 0.0005 |
| ≥5 | 2.18 (1.41, 3.38) | ||
| BMI | ≥25 | Ref | 0.1743 |
| <25 | 1.34 (0.88, 2.04) | ||
| Smoking history | Never | Ref | 0.0375† |
| Current/prior smokers (1–20 pack-years) | 0.71 (0.34, 1.47) | ||
| Current/prior smokers (>20 pack-years) | 0.48 (0.26, 0.88) |
Current/prior smokers (1-20 pack-years) vs never: p < 0.3498.
Current/prior smokers (>20 pack-years) vs never: p < 0.0175.
NLR: Neutrophil-to-lymphocyte ratio.
Table 10. . Multivariable analysis (overall survival).
| Factor | Level | Hazard ratio (95% CI) | p-value | |
|---|---|---|---|---|
| NLR at baseline | <5 | ≤6 months | Ref | 0.0001 |
| ≥5 | 3.37 (1.81, 6.26) | |||
| NLR at baseline | <5 | >6 months | Ref | 0.7681 |
| ≥5 | 0.86 (0.32, 2.30) | |||
| BMI | ≥25 | Ref | 0.1491 | |
| <25 | 1.43 (0.88, 2.32) | |||
| Smoking history | Never | Ref | 0.1694 | |
| Current/prior smokers (1–20 pack-years) | 1.75 (0.71, 4.33) | |||
| Current/prior smokers (>20 pack-years) | 1.00 (0.45, 2.22) |
NLR: Neutrophil-to-lymphocyte ratio.
Discussion & conclusion
Neutrophils are associated with conditions that cause systemic inflammation such as rheumatological disorders, infections and tobacco smoking. Cancer is also considered a state of chronic inflammation and indeed neutrophilia frequently occurs in patients with advanced cancer. In fact, neutrophilia is promoted by cancer through release of various cytokines. These, in turn, support cancer growth as well as T-cell suppression through various mechanisms, thus triggering a cyclical process that progressively supports survival and progression of cancer [26]. In contrast, lymphocytes, particularly cytotoxic CD8+ effector T cells, play a major role in mounting an immune response to cancer [27].
Checkpoint inhibitors work by blocking inhibitor engagement between cancer cells and immune cells in the tumor microenvironment. In order for this strategy to work successfully, an adequate number of effector T lymphocytes needs to be deployed into the tumor microenvironment. High-density infiltration of these cells into the tumor has been associated with improved responses to ICIs and a favorable prognosis. As a proof of this principle, increased NLR in the tumor microenvironment has been associated with cancer progression and decreased survival in different cancer subtypes [28–31].
Peripheral blood NLR, which reflects NLR in the tumor microenvironment, renders itself as an easily measurable biomarker that could help predict responses to ICI treatment. In our retrospective analysis, we found that pretreatment NLR was strongly prognostic of outcome and predictive of response to ICIs. An NLR <5 correlated with improvement in all measures of outcome (DCR, PFS and OS) and each association was statistically significant (Table 3). As depicted in Figures 1 & 2, the Kaplan–Meier curves demonstrated both an OS and PFS advantage for those with a baseline NLR <5. In both figures, there is a clear separation of the curves at 8 months. These associations were also demonstrated in our multivariable analysis, though in our model, there was no significant difference between NLR score and OS after 6 months of starting ICI therapy (Tables 8–10). As observed with our data, a few other recent studies have shown a similar predictive value of NLR as well (Table 11).
Table 11. . Neutrophil-to-lymphocyte ratio studies.
| Study | Year | NLR cut-off | Findings | Patient population | Ref. |
|---|---|---|---|---|---|
| Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis Gu, Xiao-Bin, et al. | 2015 | 5 | Elevated pretreatment NLR was associated with poor OS and PFS | NSCLC patients who received surgery vs nonsurgical treatments (Chemo/RT/Targeted therapy) | [32] |
| A reliable and feasible way to predict the benefits of nivolumab in patients with non-small cell lung cancer: a pooled analysis of 14 retrospective studies Cao, Dedong, et al. | 2018 | 5 | Elevated pretreatment NLR was associated with poor OS and PFS | NSCLC patients treated with nivolumab | [17] |
| Peripheral blood biomarkers correlate with outcomes in advanced non-small cell lung cancer patients treated with anti-PD-1 antibodies Soyano, Aixa E., et al. | 2018 | 5.9 | Elevated pretreatment NLR was associated with poor OS and PFS | NSCLC patients treated with ICIs (nivolumab or pembrolizumab) | [18] |
| Neutrophil–lymphocyte ratio (NLR) predicted prognosis for advanced non-small-cell lung cancer (NSCLC) patients who received immune checkpoint blockade (ICB) Ren, Fangping, et al. | 2019 | 2.5 | Elevated pretreatment NLR was associated with poor OS and PFS | NSCLC patients treated with ICIs (nivolumab or pembrolizumab) | [19] |
ICI: Immune checkpoint inhibitor; ICB: Immune checkpoint blockade; NLR: Neutrophil–lymphocyte ratio; NSCLC: Non-small-cell lung cancer; OS: Overall survival; PFS: Progression-free survival.
In our study, there was an association between SI and DCR. As noted above, 65.0% patients who were heavy smokers had a DCR compared with 44.1% of mild/never-smokers. Though no significant difference was found for median OS or median PFS with respect to SI, both were notably higher in the heavy-smokers subset (Table 4). A history of smoking was also seen to correlate with median PFS. Current or prior smokers had a median PFS of 6 months compared with 4 months for patients who had never smoked (Table 5). Of note, in our multivariable analysis, the heavy current/prior smokers cohort (>20 pack-years) was significantly associated with a better PFS when directly compared with the never smokers cohort (Table 9). Interestingly, smoking status has been shown to predict outcomes with ICI treatment [21]. There are some hypotheses explaining this paradoxical impact of SH on outcomes of NSCLC patients treated with ICIs. One possible explanation is a higher TMB in these patients resulting from chronic exposure to mutagens in cigarette smoke [21]. As noted in CheckMate 227, patients with a high TMB (defined as ≥10 mutations per megabase) were found to have a greater chance of benefit from ICIs than patients with a low TMB [11].
No statistically significant associations were discovered in the univariate analyses of BMI; however, patients with a high BMI were observed to have improvement in all measures of outcome (Table 6). Of note, there was a significant association between BMI and NLR at baseline. 73.2% of patients with a BMI ≥25 had an NLR <5 compared with 56.5% of patients with a BMI <25 which could possibly influence the results in a favorable way for the high BMI cohort. Additionally, in animal models, obesity has been found to correlate with T-cell dysfunction, enhanced PD-1 protein expression on T cells, and more aggressive tumor growth [23]. This immune disturbance is suspected to be influenced by leptin signaling to an uncertain degree. Leptin, a hormone that inhibits hunger and is produced in excess in obesity, has been shown to enhance the expression of PD-1 by T cells. Recent studies have shown better outcomes in obese patients with cancer treated with ICIs [33]. These positive results are possibly related to the increased expression of checkpoint proteins such as PD-1 which make the tumors more responsive to PD-1/PD-L1 blockade [23]. Thus, obesity is a potential moderator of immune dysfunction that can be effectively targeted by ICIs, which demonstrates that host factors are important aspects to consider when choosing ICIs.
Unfortunately, the total number of patients with PD-L1 expression available in our analysis was limited. It is important to mention that PD-L1 testing had not been mandated in our institution until 2018 once pembrolizumab was approved in the front-line setting for PD-L1 positive NSCLC. Prior to this, ICIs such as nivolumab and atezolizumab were approved as second-line treatment for NSCLC regardless of PD-L1 status, and so there was no clear benefit to reflex PD-L1 testing. It was only completed upon request from the oncologist. Additionally, this test was only performed at outside laboratories initially.
Though the total number of patients with known PD-L1 expression was limited, it is important to note that the DCR did not differ between the positive and negative groups (Table 7). As mentioned earlier, pembrolizumab is FDA approved as first-line monotherapy for patients with PD-L1 positive NSCLC [3]. However, several studies have shown that PD-L1 is an imperfect predictive biomarker. Response rates are variable even among patients with high PD-L1 expression (≥50%) and tumors without PD-L1 expression can sometimes respond to ICI therapy [1,5,6]. Moreover, PD-L1 testing is tissue-based and it cannot be tested sequentially for dynamic changes that are known to occur over time and with exposure to chemotherapy and radiation [8]. Overall, PD-L1 expression should be considered just one piece of a complex puzzle and other predictive biomarkers are urgently needed to help not only identify patients who would benefit from ICIs, but also not exclude those who could potentially benefit. Given the limitations of PD-L1, creating a score or calculator that incorporates multiple factors to help select these patients could be helpful moving forward. The LIPI score (lung immune prognostic index), which incorporates elements such as derived NLR (ANC/[WBC-ANC]) and LDH, is an example of a risk calculator that has been shown to predict response to ICI therapy for patients with NSCLC [34]. In light of our data, a score that includes clinical factors as well may improve the prognostic value of currently available tissue and liquid-based biomarkers.
Limitations
While our data is impactful and adds to recently published studies, it is important to mention that the main limitation of our study is that it is single-centered and retrospective in nature which creates the possibility of confounders and selection bias. In addition, as mentioned earlier, PD-L1 status was unavailable for a large number of patients included in the study and so it was not feasible to include PD-L1 status in any of the multivariable analyses. It is also essential to recognize certain factors which could potentially influence the baseline NLR calculation. For instance, if a patient had an active/recent infection or was recently started on steroids, a transient left-shift could possibly be present on the differential which can falsely affect the results. Furthermore, the sample size was small for a few subgroups which can explain the wider confidence intervals present in our data. However, despite these limitations, our study demonstrates that the predictive potential of NLR, smoking status and BMI is promising and a score incorporating these factors may be beneficial in choosing patients for ICIs. Nevertheless, the clinical value of these elements and others should be substantiated in future large, multi-center, randomized, prospective studies.
Summary points.
A majority of non-small-cell lung cancer (NSCLC) patients do not respond to immune checkpoint inhibitors (ICIs), highlighting the need for predictive biomarkers to help identify patients who would benefit.
PD-L1 expression on tumor cells, a validated biomarker, has many limitations.
The tumor immune environment has been found to play an intricate role in lung cancer progression.
Peripheral blood neutrophil-to-lymphocyte ratio is a biomarker that can reflect the intratumoral inflammatory microenvironment.
In our retrospective analysis, a pretreatment neutrophil-to-lymphocyte ratio <5 correlated with improvement in all measures of outcome (disease control rate, progression-free survival, and overall survival) and each association was statistically significant.
A positive smoking history has been shown to increase the likelihood of response to ICIs in NSCLC which is possibly due to a higher tumor mutational burden in these patients resulting from chronic exposure to mutagens in cigarette smoke.
Obesity, a potential moderator of immune dysfunction, may be associated with a higher probability of response to ICIs.
Given the limitations of PD-L1 expression, a score or calculator that incorporates multiple factors is greatly needed to help select NSCLC patients who would benefit from ICIs.
Footnotes
Financial & competing interests disclosure
I Preeshagul served on advisory boards for Pfizer, AstraZeneca, Blueprint Medicines, and Eli Lilly this past year. C Devoe has been a consultant for Pfizer this past year. N Seetharamu served on advisory boards for Pfizer, Takeda, Amgen, AstraZeneca, and Genentech this past year. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Reck M, Rodríguez-Abreu D, Robinson A et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375(19), 1823–1833 (2016). [DOI] [PubMed] [Google Scholar]; • KEYNOTE-024 helped validate PD-L1 expression as a useful biomarker in predicting response to immune checkpoint inhibitor (ICIs) in NSCLC patients.
- 2.Gandhi L, Rodríguez-Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378(22), 2078–2092 (2018). [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute. non-small-cell lung cancer treatment (PDQ®)–health professional version. http://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq#link/_485177
- 4.Mok T, Wu Y, Kudaba I et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomized, open-label, controlled, Phase III trial. Lancet 393(10183), 1819–1830 (2019). [DOI] [PubMed] [Google Scholar]; • KEYNOTE-042 demonstrates a benefit of ICIs even for patients with low PD-L1 expression (≥1%) which helped expand pembrolizumab’s indication for first-line treatment of NSCLC.
- 5.Borghaei H, Paz-Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373(17), 1627–1639 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellmann M, Paz-Ares L, Caro R et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 381(21), 2020–2031 (2019). [DOI] [PubMed] [Google Scholar]; •• CheckMate 227 highlights the inaccuracies of PD-L1 expression since first-line treatment with nivolumab plus ipilimumab in NSCLC patients resulted in a survival benefit over chemotherapy independent of the PD-L1 expression level.
- 7.Teixidó C, Vilariño N, Reyes R, Reguart N. PD-L1 expression testing in non-small-cell lung cancer. Ther. Adv. Med. Oncol. 10, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shukuya T, Carbone D. Predictive markers for the efficacy of anti–PD-1/PD-L1 antibodies in lung cancer. J. Thorac. Oncol. 11(7), 976–988 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inomata M, Kado T, Okazawa S et al. Peripheral PD1-positive CD4 T-lymphocyte count can predict progression-free survival in patients with non-small-cell lung cancer receiving immune checkpoint inhibitor. Anticancer Res. 39(12), 6887–6893 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Duan J, Cai S et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small-cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. 5(5), 696 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellmann M, Ciuleanu T, Pluzanski A et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 378(22), 2093–2104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates the utility of other biomarkers in predicting response to ICIs such as tumor mutational burden (TMB).
- 12.Rizk C. Lung cancer studies provide contradictory results on utility of TMB as biomarker for treatment. Precision Oncol. News (2019). http://www.precisiononcologynews.com/genetic-research/lung-cancer-studies-provide-contradictory-results-utility-tmb-biomarker-treatment [Google Scholar]
- 13.Greillier L, Tomasini P, Barlesi F. The clinical utility of tumor mutational burden in non-small-cell lung cancer. Transl. Lung Cancer Res. 7(5), 639–646 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedlaender A, Nouspikel T, Christinat Y, Ho L, Mckee T, Addeo A. Tissue-plasma TMB comparison and plasma TMB monitoring in patients with metastatic non-small-cell lung cancer receiving immune checkpoint inhibitors. Front. Oncol. 10, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendriks L, Rouleau E, Besse B. Clinical utility of tumor mutational burden in patients with non-small-cell lung cancer treated with immunotherapy. Transl. Lung Cancer Res. 7(5), 647–660 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin Y, Wang J, Wang X et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: a meta-analysis. Clinics (Sao Paulo). 70(7), 524–530 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao D, Xu H, Xu X, Guo T, Ge W. A reliable and feasible way to predict the benefits of nivolumab in patients with non-small-cell lung cancer: a pooled analysis of 14 retrospective studies. OncoImmunology 7(11), e1507262 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soyano A, Dholaria B, Marin-Acevedo J et al. Peripheral blood biomarkers correlate with outcomes in advanced non-small -cell lung cancer patients treated with anti-PD-1 antibodies. J. Immunother. Cancer 6(1), (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren F, Zhao T, Liu B, Pan L. Neutrophil–lymphocyte ratio (NLR) predicted prognosis for advanced non-small-cell lung cancer (NSCLC) patients who received immune checkpoint blockade (ICB). Onco. Targets Ther. 12, 4235–4244 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bilen M, Dutcher G, Liu Y et al. Association between pretreatment neutrophil-to-lymphocyte ratio and outcome of patients with metastatic renal-cell carcinoma treated with nivolumab. Clin. Genitourin Cancer 16(3), e563–e575 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Kim H, Kim B. Prognostic value of smoking status in non-small-cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Oncotarget 8(54), 93149–93155 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Illustrates the potential benefit of ICIs over chemotherapy in NSCLC patients with a history of smoking.
- 22.Davis A, Chae Y, Agte S et al. Association of tumor mutational burden with smoking and mutation status in non-small-cell lung cancer (NSCLC). J. Clin. Oncol. 35(Suppl. 7), 24–24 (2017).28034071 [Google Scholar]
- 23.Lowry F. Paradox: obesity promotes tumors but boosts immunotherapy. Medscape (2019). http://www.medscape.com/viewarticle/905660. [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumors: revised RECIST guideline (Version 1.1). Eur. J. Cancer 45(2), 228–247 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Lin D, Wei L, Ying Z et al. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika 80(3), 557–572 (1993). [Google Scholar]
- 26.Singel K, Segal B. Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal. Immunol. Rev. 273(1), 329–343 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu S, Liao R, Tu H et al. Stromal PD-L1–positive regulatory T cells and PD-1–positive CD8-positive T cells define the response of different subsets of non-small-cell lung cancer to PD-1/PD-L1 blockade immunotherapy. J. Thorac Oncol. 13(4), 521–532 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Yu Y, Qian L, Cui J. Value of neutrophil-to-lymphocyte ratio for predicting lung cancer prognosis: a meta-analysis of 7219 patients. Mol. Clin. Oncol. 7(3), 498–506 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilie M, Hofman V, Ortholan C et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable non-small-cell lung cancer. Cancer 118(6), 1726–1737 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Jia Y, Wang N et al. The clinical significance of tumor-infiltrating neutrophils and neutrophil-to-CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J. Transl. Med. 12(1), 7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Qiu S, Fan J et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J. Hepatol. 54(3), 497–505 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Gu X, Tian T, Tian X, Zhang X. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small-cell lung cancer: a meta-analysis. Sci. Rep. 5(1), (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Aguilar E, Luna J et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 25(1), 141–151 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Provides a helpful insight into the effect obesity has on immune dysfunction and how this process can make tumors more responsive to ICIs.
- 34.Mezquita L, Auclin E, Ferrara R et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small-cell lung cancer. JAMA Oncol. 4(3), 351–357 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Helps demonstrate the utility of a risk calculator that incorporates multiple factors in predicting response to ICI therapy in NSCLC patients.


