Abstract
Diffuse midline gliomas harboring histone H3 K27M mutations are most commonly found in the brainstem of children. This mutation confers a WHO grade IV designation and is associated with a particularly poor prognosis. Although traditionally considered to be a disease of children and young adults, a number of recent reports have described H3 K27M mutations in older adults with diffuse midline gliomas. Here, we present the unusual case of a diffuse midline glioma in the pons and cerebellum of an 83-year-old woman and review the evolving clinical literature on this entity in adults. This case underscores that it may occur even in older adults, in whom prognostic and treatment paradigms used in pediatrics may not be directly applicable.
Keywords: : diffuse intrinsic pontine glioma, diffuse midline glioma, elderly, H3 K27M
Summary
Diffuse midline gliomas with H3 K27M mutations are a particularly aggressive form of primary glial brain tumors. They are most commonly found in children and young adults. Here, we present the unusual case of a diffuse midline glioma with H3 K27M mutation in an 83-year-old woman. To our knowledge, this is the oldest reported patient with this disease in the medical literature. We review the evolving clinical literature on this rare entity in older adults.
Practice points.
Although typically a disease of children and young adults, diffuse midline glioma with H3 K27M mutation may occur in older adults.
Providers should consider routine H3 K27M testing for diffuse gliomas in midline locations, regardless of age group.
It is unclear whether diffuse midline glioma with H3 K27M mutation in older adults behaves similarly to their pediatric/young adult counterparts.
There is no consensus on the treatment of diffuse midline glioma in adults.
Providers should consider clinical trials, when available, in treating this disease.
Outside of clinical trials, we favor treating diffuse midline glioma with radiation therapy and temozolomide as for glioblastoma.
Diffuse midline glioma (DMG) is a particularly aggressive CNS tumor that primarily affects children and young adults. Previously termed diffuse intrinsic pontine glioma, they are most commonly found in the brainstem, although they may arise in other midline structures, including the thalamus, cerebellum and spinal cord. This diagnosis was formally defined by the 2016 WHO classification of CNS tumors to require the presence of the key histone H3 K27M mutation [1]. This mutation results in loss of K27 trimethylation that is important for transcriptional regulation, ultimately resulting in gliomagenesis [2]. Diffuse midline gliomas with this mutation carry a poor prognosis and are defined to be WHO Grade IV, regardless of histopathologic features. Although historically considered a disease of pediatrics and young adulthood, some recent reports have described DMG in adults. Here we describe the case of DMG in the pons and cerebellum of an 83-year-old woman, which is to our knowledge the oldest reported diagnosis of this disease.
Case description
An 83-year-old woman with a history of multiple myeloma and carfilzomib-induced heart failure with reduced ejection fraction presented to the emergency department after several weeks of gradually worsening left-sided fine motor dysfunction, gait instability and persistent low-grade headache. Neurologic exam demonstrated left-sided dysmetria and nystagmus. MRI revealed a diffuse mass with patchy T2 signal hyperintensity in the left cerebellum (Figure 1A, arrowheads) and pons (Figure 1A, arrows). Postcontrast T1 sequences showed left cerebellar nodular enhancement (Figure 1B, arrowheads) and leptomeningeal enhancement in the cerebellar folia (Figure 1B, arrows). Multiple calvarial enhancing foci were seen, consistent with the history of multiple myeloma. A biopsy of the left paramedian cerebellum was performed. Hematoxylin and eosin stained sections show an infiltrative neoplasm with high cellularity and brisk mitotic activity (Figure 2A & B). There is no microvascular proliferation or necrosis. Immunohistochemistry for IDH1 R132H mutation is negative (data not shown). The initial pathology impression was consistent with an anaplastic astrocytoma, isocitrate dehydrogenase wild-type, WHO grade III. She underwent 3 weeks of radiation therapy (total dose 40 Gy) with concurrent daily temozolomide (75 mg/m2). During her treatment, immunohistochemistry results were reported, which revealed an H3 K27M mutation (Figure 2C) and loss of H3 K27 trimethylation (Figure 2D). The diagnosis was therefore changed to diffuse midline glioma, H3 K27M-mutant, WHO grade IV. Clinically the patient deteriorated rapidly, with the development of significant fatigue, cognitive dysfunction, diplopia and motor weakness. She underwent no further therapy and transitioned to hospice 3 months after completion of radiation therapy. She survived for an additional 4.5 months.
Figure 1. . Initial MRI images.
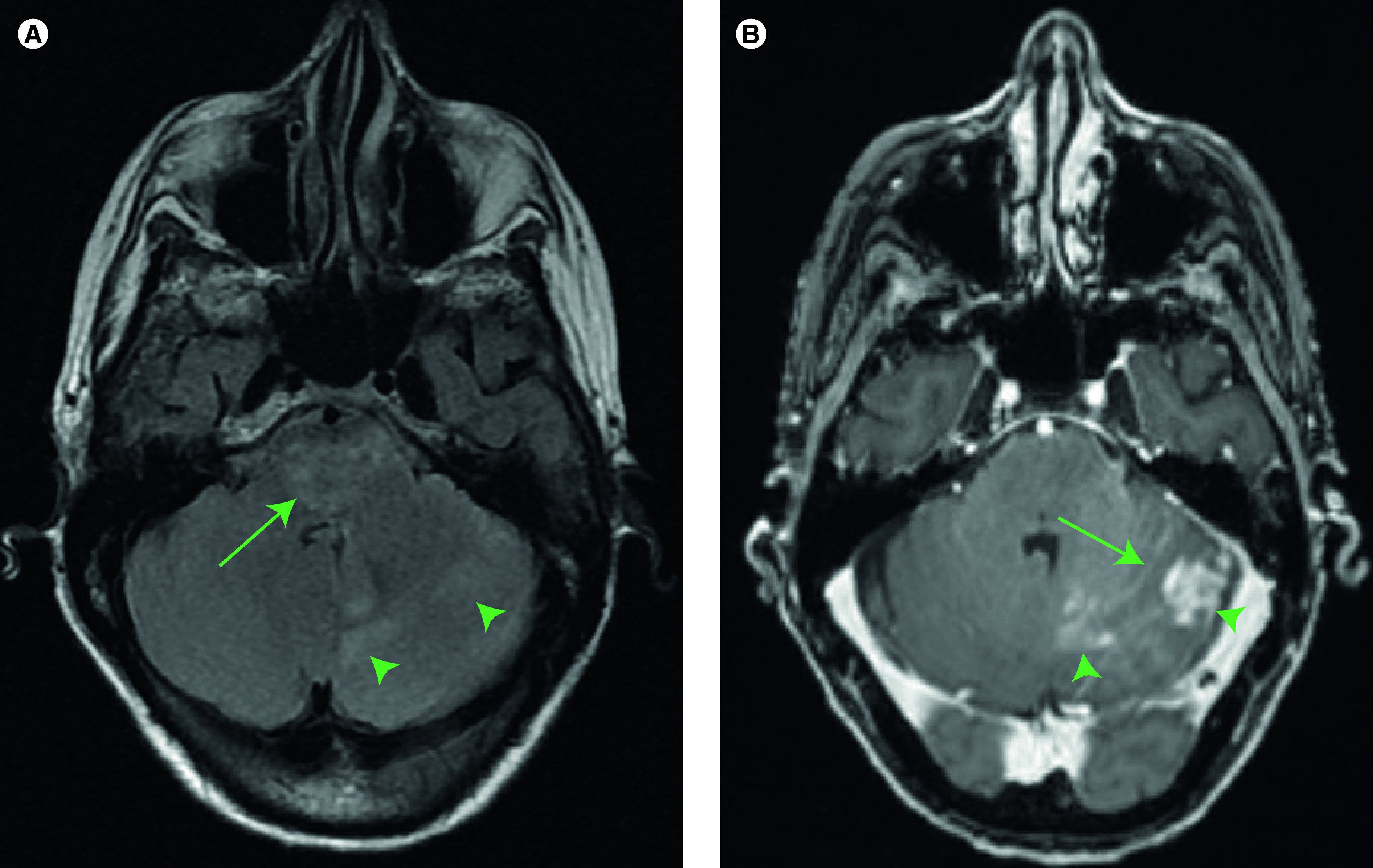
(A) T2 fluid-attenuated inversion recovery images showing patchy hyperintensity in the pons (arrows) and cerebellum (arrowheads). (B) T1 postcontrast images showing leptomeningeal enhancement in the cerebellar folia (arrows) and areas of nodular enhancement (arrowheads).
Figure 2. . Histopathology of patient's tumor specimen with H3 K27M mutation.
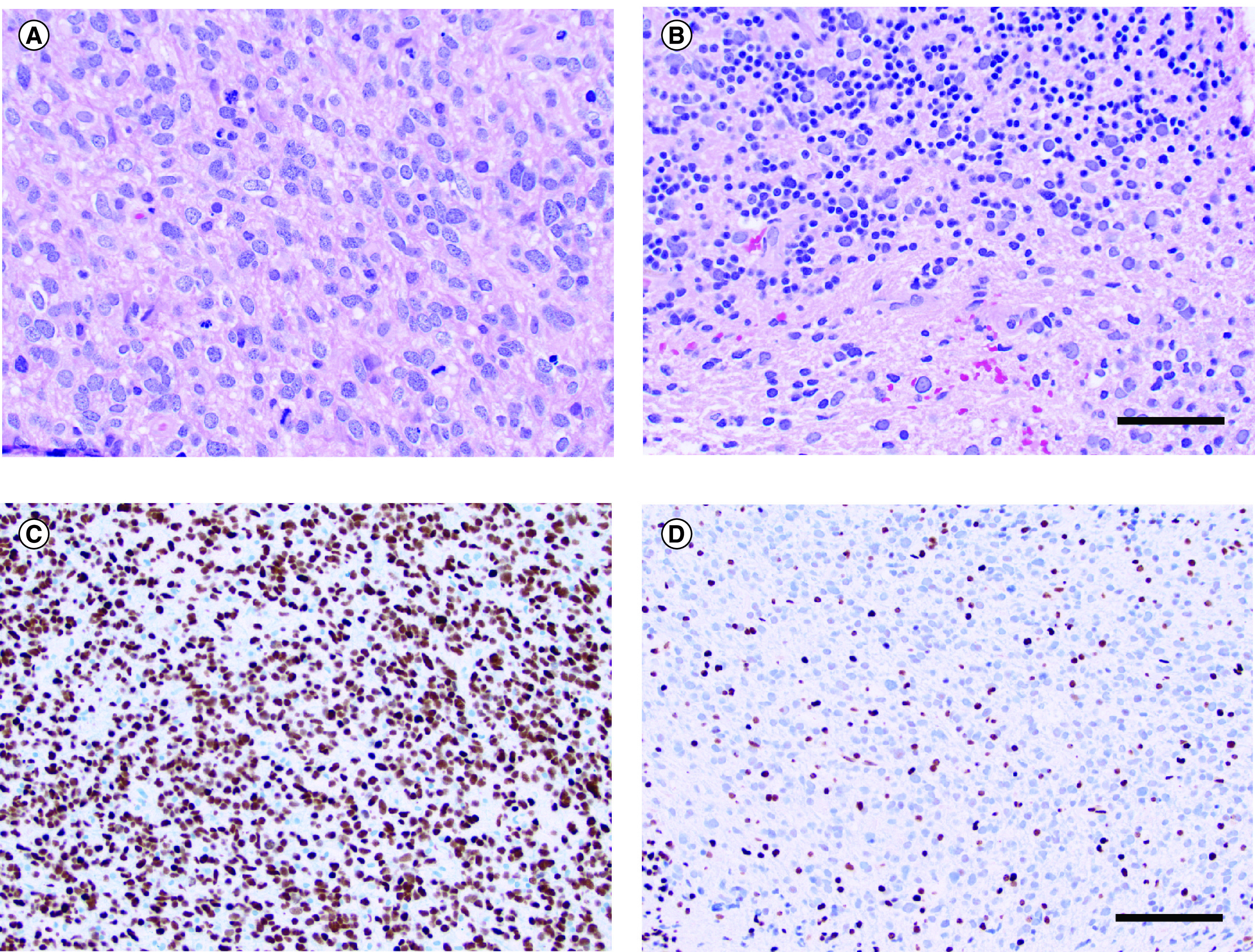
(A) Hematoxylin and eosin (H&E) stained sections show a highly cellular glial neoplasm with brisk mitotic activity. Microvascular proliferation and necrosis are not present. (B) H&E stained section highlights infiltration of neoplastic cells into the granular cell layer. (C) H3 K27M mutant protein is detected by immunohistochemistry. (D) H3 K27me3 tri-methylation is lost in neoplastic cells. Scale bar in B = 50 μm for (A & B). Scale bar in (D) = 100 μm for (C & D).
Discussion
The histone H3 K27M mutation was discovered in 2012 and found to distinguish the particularly aggressive pediatric diffuse gliomas that primarily affect the pons [3,4]. At the time of the 2016 WHO classification, the H3 K27M mutation was thought to occur exclusively in the brainstem and other midline structures. Since then, however, a number of reports describing its presence in nonmidline structures have been published [5]. Although an H3 K27M mutation is associated with a dismal prognosis in DMG [6], its prognostic significance for nonmidline and circumscribed gliomas is less clear [7]. The Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW) recently emphasized the necessary inclusion of all three key features (diffuse rather than circumscribed, midline location and H3 K27M mutation) for the diagnosis of DMG [8].
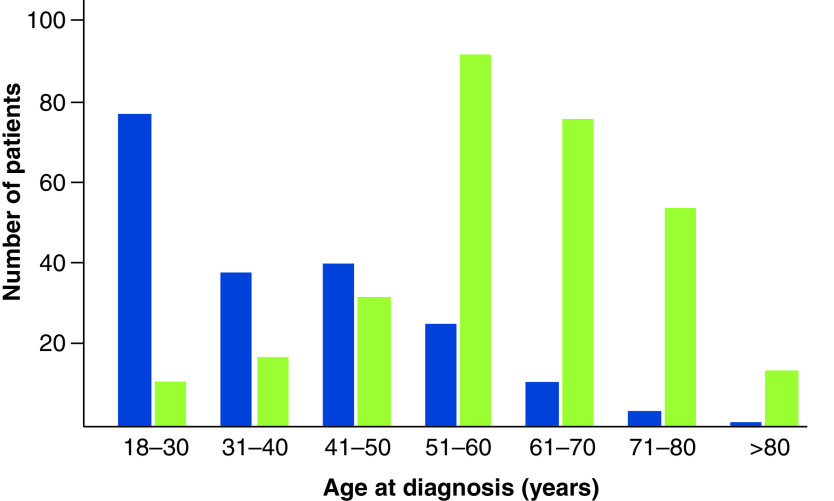
Classically described in children and young adults, H3 K27M mutations have recently been described in adult diffuse midline gliomas [9–14]. These mutations are less common with advancing age and are extremely rare in older adults (Figure 3, blue). This contrasts with the situation in H3 wild-type grade IV glioma (i.e., glioblastoma), which is much more common with increasing age (Figure 3, green). Our current report represents, to our knowledge, the oldest reported patient with an H3 K27M DMG. Her atypical age and somewhat unusual imaging appearance (heterogeneous rather than homogeneous T2 signal and enhancement) raise the possibility of a different pathogenesis to typical DMG. Unfortunately, we did not have next-generation sequencing data available for this patient. We were therefore unable to evaluate whether the molecular landscape of her tumor was similar to DMGs reported in the existing literature. Additionally, despite the lack of a known mechanistic link between her prior history of multiple myeloma and her DMG diagnosis, we cannot exclude the possibility of a relationship between the two diagnoses.
Figure 3. . Distribution of age at diagnosis for adult patients with diffuse midline glioma.
H3 K27M-mutant (blue) reported in published series (total number of patients: 196) [9–15]. The age distribution at diagnosis for glioblastoma is shown for comparison (green, total number of patients: 296). Glioblastoma data are from The Cancer Genome Atlas Pan Cancer Atlas and downloaded from cBioPortal [21,22].
The prognostic implications of the H3 K27M mutation in adult DMG are poorly understood. Retrospective series have identified this mutation in a significant proportion of adult brainstem and midline diffuse gliomas [15]. Compared with H3 wild-type diffuse midline gliomas, some studies have found H3 K27M mutations to be associated with shorter survival, similar to the pediatric situation [15]. In contrast, others have reported no significant survival difference in series of 21 [10] and 29 [9] adult DMGs. Additionally, Schreck et al. [12] identified H3 K27M mutations in 18/123 (15%) adult midline gliomas and found that the presence of this mutation was actually associated with improved survival compared with H3 wild-type midline gliomas.
The anatomic distribution of H3 K27M mutant gliomas in adults also appears to differ from those in children, with less pronounced preference for the brainstem. Small studies of cerebellar adult gliomas have noted a prevalence of H3 K27M mutations of up to 21% [16,17]. This mutation is even more commonly found in adult thalamic gliomas and may not be associated with a survival difference from H3 wild-type tumors [18]. Data summarizing age distribution of previously published adult diffuse midline glioma series are available in Supplementary Table 1.
Histopathologic features are diverse in DMG. The most common morphology is that of a high-grade astrocytic tumor as seen in the present case, although oligodendroglial morphologies have also been documented. DMGs may lack mitotic figures, microvascular proliferation and necrosis, thus conferring the histologic appearance of a low-grade glioma [1]. However, in the presence of an H3 K27M mutation, these features have no impact on the uniformly poor prognosis, and consequently these tumors are defined to be WHO grade IV.
Radiation therapy is the only modality that prolongs survival for children with H3 K27 DMG. Multiple trials of systemic agents have failed to improve survival, including temozolomide, tyrosine kinase inhibitors and radiosensitizers [19,20]. Owing to ongoing uncertainty as to whether H3 K27 DMG represents a distinct disease in older adults, many providers choose to include temozolomide in analogy to the treatment of their H3 wild-type counterparts, given the dearth of literature guidance.
Conclusion & future perspective
This case represents a rare diagnosis of an H3 K27M diffuse midline glioma in the elderly. The diagnostic and treatment implications of this entity in older adults remain unclear. Additional cases will need to be collected to understand better the clinical behavior of this disease. Cases that incorporate sequencing information will be indispensable for exploring the molecular landscape of DMG in older adults compared with their younger counterparts.
Acknowledgments
The authors thank K Seagroves for administrative support in the preparation of this manuscript.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/cns-2020-0030
Author contributions
KB Peters treated the patient and identified the uniqueness of this case. SH Wang rendered the diagnosis and created the histopathologic figures. JT Low and KB Peters wrote the manuscript. All authors read and approved the final manuscript.
Financial & competing interests disclosure
This case report was funded with internal departmental funds. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
The authors state that they have obtained verbal and written informed consent from the patient for the inclusion of their medical and treatment history within this case report.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Louis DN, Ohgaki H, Wiestler OD et al. World Health Organization Histological Classification of Tumours of the Central Nervous System. International Agency for Research on Cancer, Lyon, France: (2016). [Google Scholar]
- 2.Fontebasso AM, Liu XY, Sturm D, Jabado N. Chromatin remodeling defects in pediatric and young adult glioblastoma: a tale of a variant histone 3 tail. Brain Pathol. 23(2), 210–216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartzentruber J, Korshunov A, Liu XY et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482(7384), 226–231 (2012). [DOI] [PubMed] [Google Scholar]; •• One of two seminal papers describing histone H3 mutations in DMG.
- 4.Wu G, Broniscer A, Mceachron TA et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 44(3), 251–253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• One of two seminal papers describing histone H3 mutations in DMG.
- 5.Lopez G, Oberheim Bush NA, Berger MS et al. Diffuse non-midline glioma with H3F3A K27M mutation: a prognostic and treatment dilemma. Acta Neuropathol. Commun. 5(1), 38 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karremann M, Gielen GH, Hoffmann M et al. Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol 20(1), 123–131 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pratt D, Natarajan SK, Banda A et al. Circumscribed/non-diffuse histology confers a better prognosis in H3K27M-mutant gliomas. Acta Neuropathol. 135(2), 299–301 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis DN, Ellison DW, Brat DJ et al. cIMPACT-NOW: a practical summary of diagnostic points from Round 1 updates. Brain Pathol. 29(4), 469–472 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebrahimi A, Skardelly M, Schuhmann MU et al. High frequency of H3 K27M mutations in adult midline gliomas. J. Cancer Res. Clin. Oncol. 145(4), 839–850 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyronet D, Esteban-Mader M, Bonnet C et al. Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol. 19(8), 1127–1134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu T, Chanchotisatien A, Qin Z et al. Imaging characteristics of adult H3 K27M-mutant gliomas. J. Neurosurg. (2019). [DOI] [PubMed] [Google Scholar]
- 12.Schreck KC, Ranjan S, Skorupan N et al. Incidence and clinicopathologic features of H3 K27M mutations in adults with radiographically-determined midline gliomas. J. Neurooncol. 143(1), 87–93 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon DA, Wood MD, Tihan T et al. Diffuse midline gliomas with histone H3-K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol. 26(5), 569–580 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Li Z, Zhang M et al. H3 K27M-mutant diffuse midline gliomas in different anatomical locations. Hum. Pathol. 78, 89–96 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Daoud EV, Rajaram V, Cai C et al. Adult brainstem gliomas with H3K27M mutation: radiology, pathology, and prognosis. J. Neuropathol. Exp. Neurol. 77(4), 302–311 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Picart T, Barritault M, Berthillier J et al. Characteristics of cerebellar glioblastomas in adults. J. Neurooncol. 136(3), 555–563 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Tauziede-Espariat A, Saffroy R, Pages M et al. Cerebellar high-grade gliomas do not present the same molecular alterations as supratentorial high-grade gliomas and may show histone H3 gene mutations. Clin. Neuropathol. 37(5), 209–216 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Feng J, Hao S, Pan C et al. The H3.3 K27M mutation results in a poorer prognosis in brainstem gliomas than thalamic gliomas in adults. Hum. Pathol. 46(11), 1626–1632 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Cohen KJ, Pollack IF, Zhou T et al. Temozolomide in the treatment of high-grade gliomas in children: a report from the Children's Oncology Group. Neuro. Oncol. 13(3), 317–323 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vitanza NA, Monje M. Diffuse intrinsic pontine glioma: from diagnosis to next-generation clinical trials. Curr. Treat. Options Neurol. 21(8), 37 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerami E, Gao J, Dogrusoz U et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2(5), 401–404 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao J, Aksoy BA, Dogrusoz U et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6(269), pl1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]