Abstract
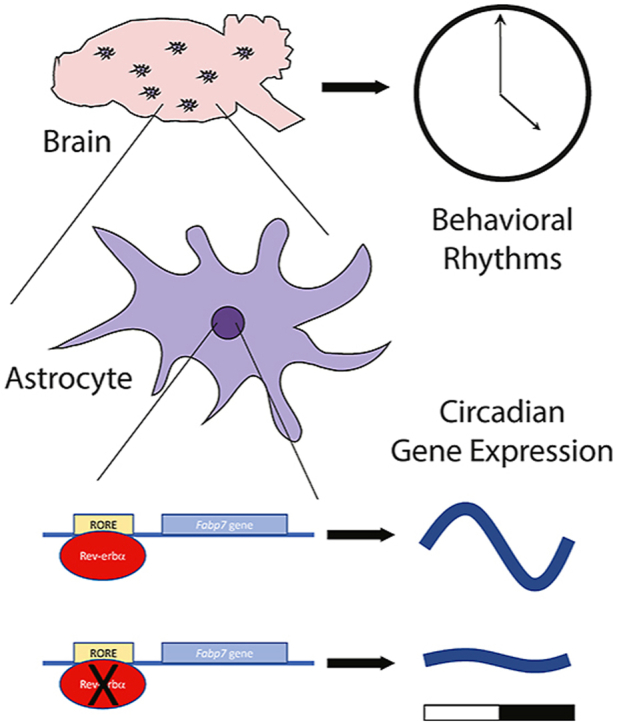
The astrocyte brain-type fatty-acid binding protein (Fabp7) circadian gene expression is synchronized in the same temporal phase throughout mammalian brain. Cellular and molecular mechanisms that contribute to this coordinated expression are not completely understood, but likely involve the nuclear receptor Rev-erbα (NR1D1), a transcriptional repressor. We performed ChIP-seq on ventral tegmental area (VTA) and identified gene targets of Rev-erbα, including Fabp7. We confirmed that Rev-erbα binds to the Fabp7 promoter in multiple brain areas, including hippocampus, hypothalamus, and VTA, and showed that Fabp7 gene expression is upregulated in Rev-erbα knock-out mice. Compared to Fabp7 mRNA levels, Fabp3 and Fabp5 mRNA were unaffected by Rev-erbα depletion in hippocampus, suggesting that these effects are specific to Fabp7. To determine whether these effects of Rev-erbα depletion occur broadly throughout the brain, we also evaluated Fabp mRNA expression levels in multiple brain areas, including cerebellum, cortex, hypothalamus, striatum, and VTA in Rev-erbα knock-out mice. While small but significant changes in Fabp5 mRNA expression exist in some of these areas, the magnitude of these effects are minimal to that of Fabp7 mRNA expression, which was over 6-fold across all brain regions. These studies suggest that Rev-erbα is a transcriptional repressor of Fabp7 gene expression throughout mammalian brain.
Keywords: Lipid, Metabolism, Glia, BLBP, B-FABP, Clock
Graphical abstract
Highlights
-
•
The transcriptional repressor Rev-erbα binds to the Fabp7 promoter across brain areas.
-
•
Multiple Rev-erbα response element binding sites exist on the Fabp7 promoter.
-
•
Rev-erbα is required for Fabp7 transcriptional repression and circadian expression.
-
•
Rev-erbα depletion does not affect other Fabp-type gene expression in brain.
1. Introduction
Fatty-acid binding proteins (Fabp) comprise a family of small (~15 kDa) hydrophobic ligand binding carriers with high affinity for long-chain fatty-acids for intracellular transport, and are associated with metabolic, inflammatory, and energy homeostasis pathways (Furuhashi and Hotamisligil, 2008; Storch and Corsico, 2008). These include three that are expressed in the adult mammalian central nervous system (CNS), and are Fabp3 (H-Fabp), Fabp5 (E-Fabp), and Fabp7 (B-Fabp). Fabp3 is primarily expressed in neurons, Fabp5 is expressed in various cell types, including both neurons and glia, and Fabp7 is most abundant in astrocytes and neural progenitors. While performing microarray analysis of transcripts in mouse brain to characterize novel diurnally regulated genes, Fabp7 was identified as a unique transcript elevated in multiple hypothalamic brain regions during the sleep phase (Gerstner et al., 2006). Unlike other circadian regulated gene products, Fabp7 has a synchronized pattern of global diurnal expression in adult murine brain (Gerstner et al., 2006, Gerstner et al., 2008; Gerstneret al., 2012), is regulated by the core clock gene BMAL1 (Gerstner and Paschos, 2020) and has a general role in governing aspects of sleep behavior in multiple species, including flies, mice, and humans (Gerstneret al., 2017). Fabp7 has been shown to regulate dendritic morphology and excitatory cortical neuron synaptic function (Ebrahimiet al., 2016), as well as locomotor responses to NMDA-receptor activity (Watanabeet al., 2007), and other behavioral conditions including fear memory and anxiety (Owadaet al., 2006). Therefore, Fabp7 may play an important role in regulating time-of-day dependent changes in astrocyte-derived and evolutionarily conserved plasticity-related processes (Lavialleet al., 2011; Nagaiet al., 2020; Gerstner, 2012).
Here we were interested in validating findings that Fabp7 is a target of Rev-erbα (Schnellet al., 2014) and determining whether Fabp7 mRNA is regulated by Rev-erbα across multiple brain areas. We also wanted to examine whether these effects are specific to Fabp7, or whether other Fabps expressed in the CNS are similarly affected.
2. Results
Since the time-of-day profile of Fabp7 mRNA expression is abolished in BMAL1 KO mice (Gerstner and Paschos, 2020), we performed bioinformatic analysis to locate core canonical E-box elements (CACGTG) within the Fabp7 promoter. We did not detect any canonical E-box elements, so we considered whether other cis-acting elements influenced by circadian output in the Fabp7 promoter exist. Analysis of the promoter for Fabp7 gene revealed several sites known to be involved in the metabolic arm of the clock (Choet al., 2012; Buggeet al., 2012; Zhanget al., 2015), including multiple sites for the transcriptional co-repressor nuclear receptor Rev-erbα (NR1D1), termed Rev-erbα response elements (RORE) (Table S1).
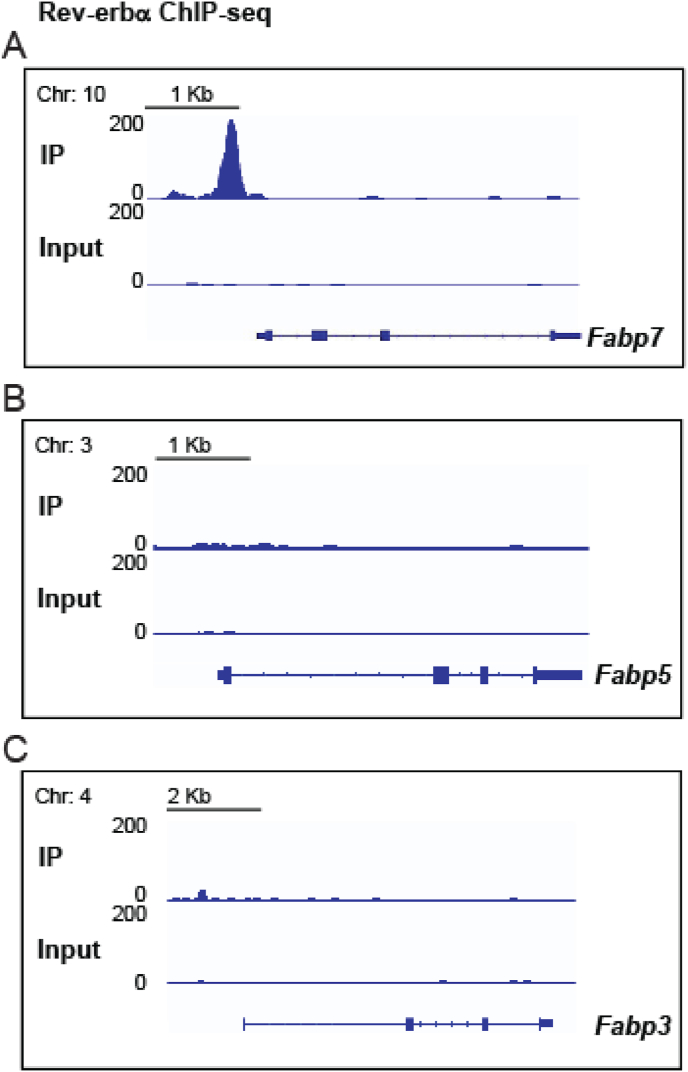
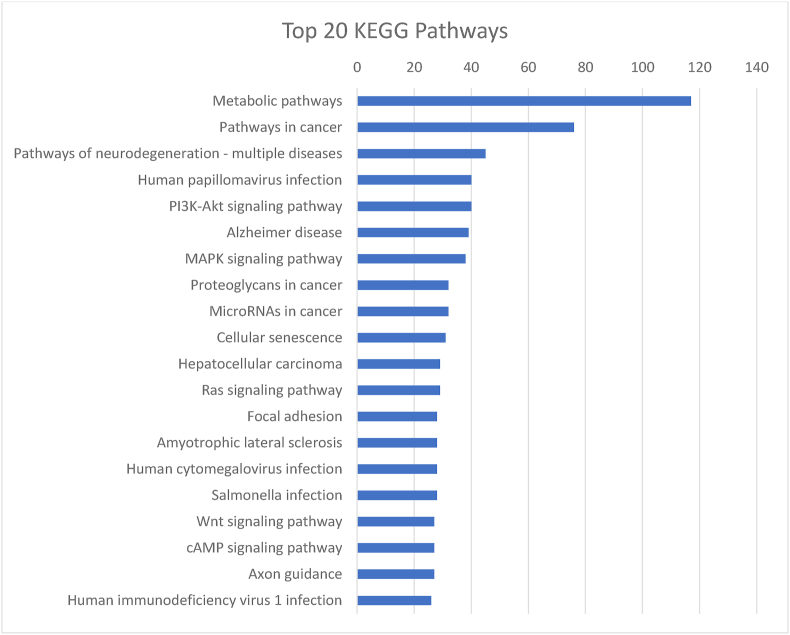
To determine whether these RORE sites were functional, we performed chromatin immunoprecipitation experiments followed by DNA-sequencing (ChIP-seq) on tissue from the ventral tegmental area (VTA), a brain region known to regulate motivational/reward behaviors (Morales and Margolis, 2017; Russo and Nestler, 2013), wakefulness, and sleep (Takataet al., 2018; Yuet al., 2019; Eban-Rothschild et al., 2016). Here we identified positive Rev-erbα interactions within the first kilobase upstream of the transcription start site of the Fabp7 promoter, but not in the Fabp3 or Fabp5 promoters (Fig. 1A–C). The top 20 Rev-erbα binding site loci, peak score, distance to the translational start site and gene names are listed in Table 1. Gene Ontology (GO) analysis revealed significant enrichment of several biological processes, molecular functions, and cellular components (Table 2) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis shows the top 20 pathways in Rev-erbα ChIP-seq genes (Fig. 2). The complete list of Rev-erbα ChIP-seq genes is provided in [SUPPLEMENTAL dataset 1].
Fig. 1.
ChIP-seq binding profile of Rev-erbα around the Fabp7 locus (A), but not in the Fabp5 (B) or Fabp3 (C) loci in the VTA of WT mice.
Table 1.
The top 20 Rev-erbα binding site loci, peak score, distance to the translational start site (TSS), and gene names, as identified by Rev-erbα ChIP-seq.
| Chromosome | Peak Score | Distance to TSS | Gene Name |
|---|---|---|---|
| chr14 | 151.18628 | 506 | Nr1d2 |
| chr1 | 139.22659 | −2189 | Igsf8 |
| chr11 | 135.35667 | −27206 | Hlf |
| chr7 | 131.72737 | 2519 | Dbp |
| chr7 | 102.16678 | −206 | Dbp |
| chr2 | 102.00463 | 10184 | Cry2 |
| chr11 | 97.30782 | 1526 | Nr1d1 |
| chr6 | 95.75218 | 9516 | Bhlhe41 |
| chr3 | 94.62258 | −69 | Ciart |
| chr6 | 81.56895 | −1171 | Bhlhe40 |
| chr6 | 80.73732 | 83133 | Lsm3 |
| chr2 | 76.52149 | 401 | Aven |
| chr11 | 76.4099 | −4068 | Per1 |
| chr10 | 74.53577 | −153 | Fabp7 |
| chr15 | 71.89268 | −459 | Tef |
| chr9 | 67.80914 | −112 | Nptn |
| chr1 | 62.37303 | −197 | Coq10b |
| chr16 | 61.30798 | −793 | Ubald1 |
| chr7 | 60.32358 | 37 | Arntl |
| chr15 | 59.34713 | 45290 | Nfam1 |
Table 2.
Analysis of Gene Ontology in Rev-erbα ChIP-seq genes. Highest fold enriched Gene Ontology classes for Biological Process, Molecular Function and Cellular Component are listed with most highly enriched on top.
| PANTHER GO-Slim Biological Process | Number of Genes | Fold Enrichment | Raw P-value | FDR |
|---|---|---|---|---|
| circadian regulation of gene expression | 11 | 10.65 | 0.000000207 | 0.00000705 |
| neg. reg. of transforming growth factor beta receptor signaling pathway | 4 | 10.33 | 0.0021 | 0.0312 |
| regulation of circadian rhythm | 4 | 8.85 | 0.00314 | 0.0448 |
| chondroitin sulfate proteoglycan biosynthetic process | 5 | 6.46 | 0.00272 | 0.0393 |
| protein demethylation | 5 | 6.46 | 0.00272 | 0.0391 |
| protein autophosphorylation | 10 | 3.87 | 0.000719 | 0.0118 |
| regulation of actin filament organization | 15 | 2.8 | 0.000803 | 0.013 |
| response to abiotic stimulus | 16 | 2.75 | 0.000638 | 0.0106 |
| positive regulation of transcription by RNA polymerase II | 38 | 2.52 | 0.00000206 | 0.0000599 |
| regulation of cellular component size | 17 | 2.42 | 0.00196 | 0.0295 |
| positive reg. of nucleobase-containing compound metabolic process | 54 | 2.34 | 9.73E-08 | 0.00000342 |
| PANTHER GO-Slim Molecular Function | ||||
| demethylase activity | 9 | 5.17 | 0.000228 | 0.00452 |
| flavin adenine dinucleotide binding | 10 | 4.56 | 0.000242 | 0.00463 |
| transcription coregulator activity | 40 | 3.04 | 8.74E-09 | 0.000000606 |
| phosphoprotein phosphatase activity | 19 | 2.21 | 0.00295 | 0.0409 |
| small molecule binding | 44 | 1.76 | 0.000706 | 0.0112 |
| protein kinase activity | 51 | 1.62 | 0.00177 | 0.0266 |
| PANTHER GO-Slim Cellular Component | ||||
| vacuolar membrane | 13 | 2.76 | 0.00196 | 0.0344 |
| Golgi membrane | 14 | 2.55 | 0.00255 | 0.0418 |
| transcription regulator complex | 31 | 2.54 | 0.0000114 | 0.000386 |
| neuron projection | 42 | 1.72 | 0.00181 | 0.0328 |
| transferase complex | 50 | 1.69 | 0.000921 | 0.0187 |
| bounding membrane of organelle | 43 | 1.66 | 0.00248 | 0.0421 |
| nucleoplasm | 54 | 1.65 | 0.000836 | 0.0185 |
| chromatin | 67 | 1.56 | 0.000896 | 0.019 |
Fig. 2.
Analysis of the top 20 KEGG pathways enriched in Rev-erb ChIP-seq genes plotted with number of hits per pathway.
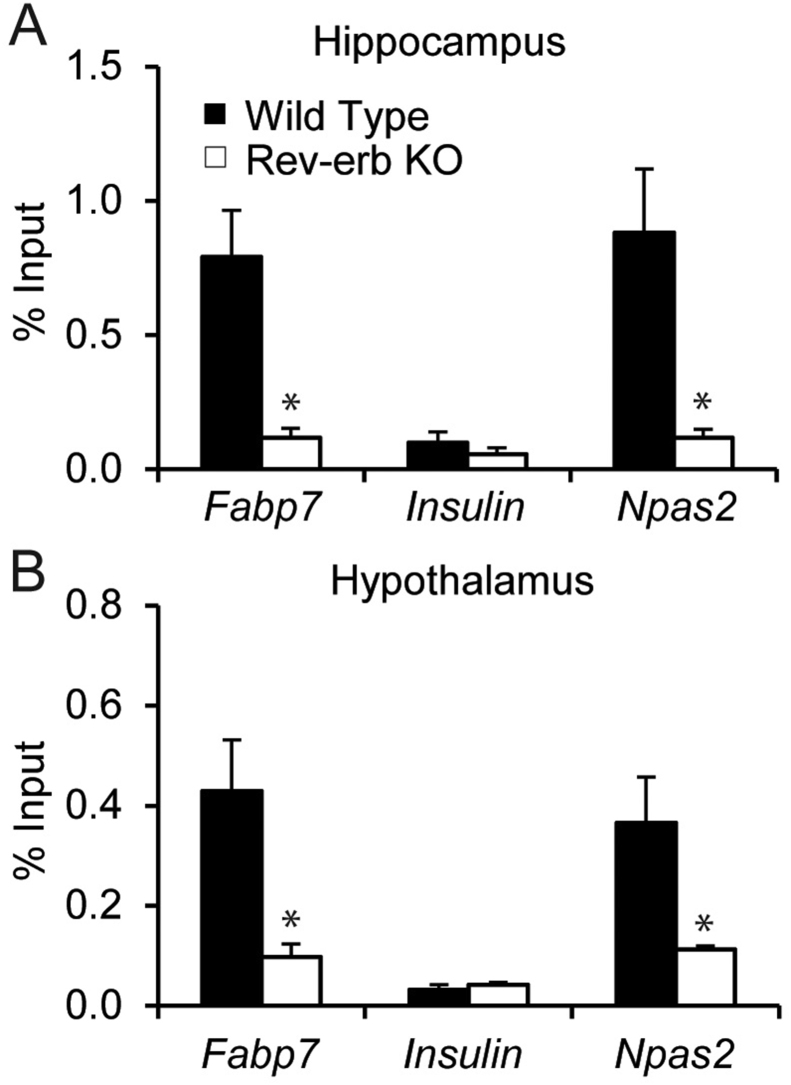
To confirm that Rev-erbα binds the Fabp7 promoter in multiple brain regions, we compared Rev-erbα binding in the Fabp7 promoter against the negative control insulin, and the positive control NPAS2 in WT and Rev-erbα KO mice. We observed Rev-erbα binding to the Fabp7 and NPAS2 promoters in WT, but not Rev-erbα KO mice, in both hippocampus (Fig. 3A) and hypothalamus (Fig. 3B). Binding of Rev-erbα was not observed for insulin, regardless of genotype (Fig. 3A and B). Since BMAL1 is known to transactivate Rev-erbα (Mohawk et al., 2012; Albrecht, 2012), a transcriptional repressor, BMAL1 could influence Fabp7 gene expression (Gerstner and Paschos, 2020) indirectly through Rev-erbα.
Fig. 3.
ChIP-qPCR measurements of Rev-erbα relative occupancy at Fabp7 locus, Insulin locus (negative control) and Npas2 locus (positive control) in hippocampus (A) and hypothalamus (B) of WT and Rev-erbα KO mice. Data are expressed as the percent of input and are the mean ± SEM. (Student’s t-test, ∗p < 0.05, n = 3 per group).
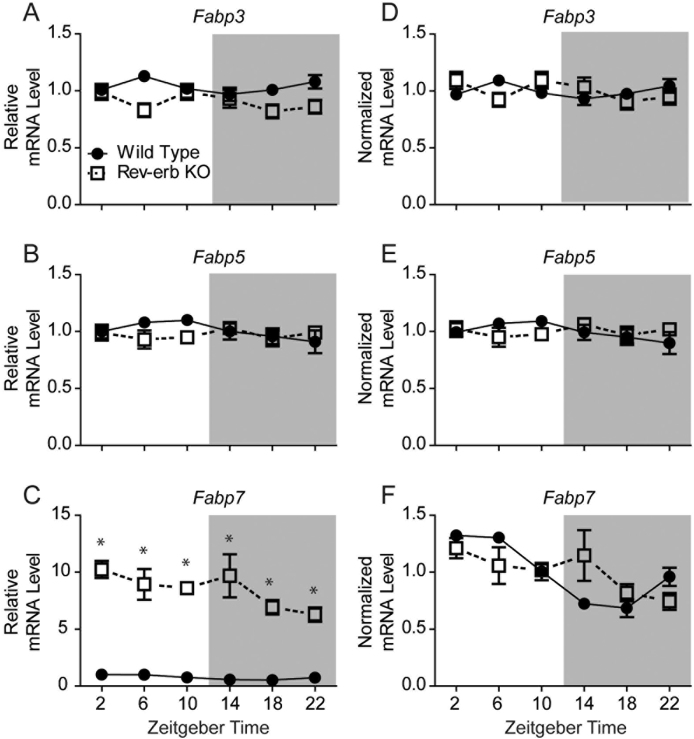
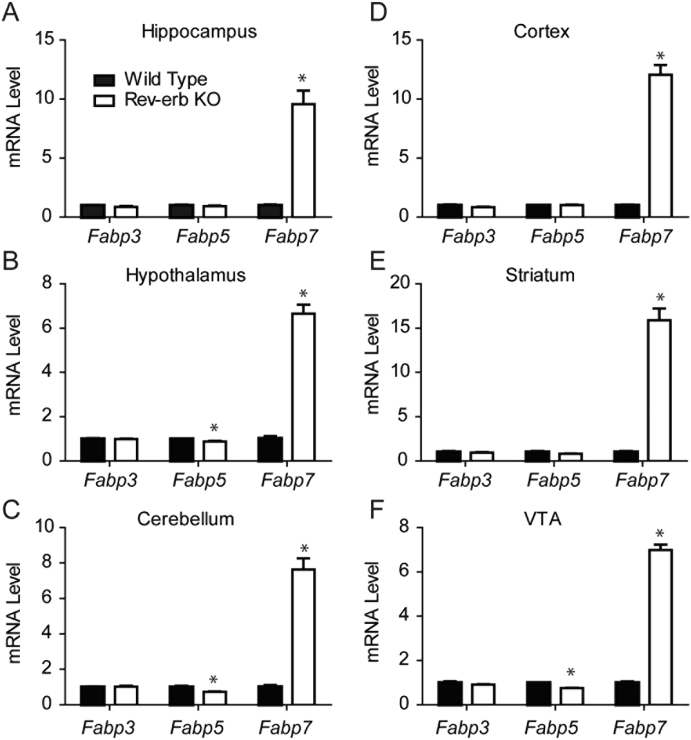
To test the hypothesis that Rev-erbα represses Fabp7 gene expression, we examined the diurnal profile of Fabp7 mRNA in Rev-erbα KO mice. If Fabp7 expression is repressed by Rev-erbα, this predicts that Fabp7 mRNA should be elevated in the Rev-erbα KO. We confirmed that Fabp7 mRNA is elevated in hippocampus of Rev-erbα KO mice, while Fabp3 and Fabp5 mRNA levels are not affected (Fig. 4A–C). To determine whether time-of-day mRNA levels are affected by Rev-erbα, we analyzed the normalized mRNA expression for Fabp3, Fabp5, and Fabp7 from six time-points over 24h of Rev-erbα KO and WT mice. While Fabp3 (Fig. 4D) and Fabp5 (Fig. 4E) mRNA do not oscillate in WT mice and remain unaffected in Rev-erbα KOs, the Fabp7 mRNA circadian oscillation is disrupted in the Rev-erbα KO compared to WT hippocampus (Fig. 4F). Since Fabp7 expression is diurnally regulated throughout murine brain (Gerstner et al., 2006; Gerstner, 2008; Gerstneret al., 2012), we wanted to determine if Fabp7 mRNA levels were regulated by Rev-erbα broadly in multiple brain regions. Analysis of multiple brain regions including striatum, VTA, cerebellum, hippocampus, hypothalamus, and cortex of Rev-erbα KO compared to WT mice revealed analogous increases in Fabp7 mRNA levels (~6–15 fold), but not Fabp3 or Fabp5 mRNA levels (Fig. 5). Together, these data suggest that the circadian clock control of Fabp7 mRNA expression requires Rev-erb⍺ broadly across many brain regions.
Fig. 4.
A–C: Relative hippocampal mRNA expression of various Fabps in Rev-erbα KO vs. WT mice under normal (LD) conditions. Levels of Fabp3 (A) or Fabp5. (B) are unaffected by Rev-erbα deficiency, however, Fabp7 shows a significant increase in expression based on genotype. ∗p < 0.001, N = 4–7 per group, Student’s t-test. ZT = zeitgeber time. Hippocampal mRNA expression normalized to genotype to visualize the circadian rhythmicity of Fabp3 (D), Fabp5 (E), and Fabp7 (F). Fabp7 circadian rhythmicity was significantly disrupted in Rev-erba KO mice (adj. p = 0.184; JTK_Cycle) compared to WT mice (adj. p < 0.001; JTK_Cycle).
Fig. 5.
A–F: Relative mRNA expression from various brain regions of Fabps in Rev-erbα KO vs. WT mice. Levels of Fabp3 mRNA are not affected by loss of Rev-erbα, while lower levels of Fabp5 mRNA are observed in Hypothalamus (B), Cerebellum (C), and VTA (F) based on Rev-erbα deficiency compared to WT. Fabp7 mRNA, however, shows significant increases in expression in Rev-erbα KO compared to WT in all brain regions studied. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, N = 3–5 per group, Student’s t-test.
3. Discussion
The astrocyte Fabp7 gene expression is known to cycle in a synchronized fashion throughout the mammalian CNS (Gerstner et al., 2006; Gerstner, 2008; Gerstneret al., 2012; Schnellet al., 2014). Previous studies have shown Fabp7 circadian gene expression is under control of the core clock transcription factor BMAL1 (Gerstner and Paschos, 2020), however the Fabp7 promoter lacks a canonical E-box element, suggesting that BMAL1 may indirectly exert its effects on Fabp7 circadian expression via Rev-erbα, a transcriptional repressor, and known BMAL1 target (Guillaumond et al., 2005). Here we provide evidence that Fabp7 contains canonical ROREs and that Rev-erbα binds to the RORE regions in the Fabp7 gene locus in the VTA (Fig. 1A). The current study validates a previous report that also showed Rev-erbα binding to Fabp7 in the hippocampus (Schnellet al., 2014) (Fig. 3A), and extends these findings to show this also occurs in the hypothalamus (Fig. 3B). Taken together, these results suggest that the coordinated and synchronized expression of Fabp7 transcription is controlled by Rev-erbα direct binding in multiple brain regions throughout mammalian brain.
Rev-erbα KO mice showed a greater than 6-fold increase in Fabp7 mRNA expression across multiple brain areas, including cerebellum, cortex, hippocampus, hypothalamus, striatum, and VTA, compared to WT mice. We also observed minimal, but significant, reduction in Fabp5 mRNA in a few brain areas (cerebellum, hypothalamus, and VTA; Fig. 5) and no differences in Fabp3 mRNA in any brain region, in Rev-erbα KO compared to WT mice. These reductions in Fabp5 mRNA may represent compensatory mechanisms that are in response to the large increases in Fabp7 mRNA expression in glial cells, however, to rule out a direct role of Rev-erbα in transcriptional regulation of these other Fabp types throughout brain, binding assays for Rev-erbα at their respective genetic loci across multiple brain regions would be required. Recently, local oscillators have been discovered in multiple brain regions throughout the mammalian brain (Paulet al., 2019), therefore it will be important to determine the extent to which Fabp7 oscillations require ‘global’ vs. ‘local’ coordinated control. Stability of Rev-erbα and the role of degradation processes that control the protein half-life in downstream signaling may also contribute to alterations in periodicity of gene expression (DeBruyne et al., 2015). Future studies determining the cell-type specificity of these observations are also needed to better understand lipid-mediated signaling cascades (Gooley and Chua, 2014; Gooley, 2016) downstream of circadian- and metabolically (Buggeet al., 2012; Kumar Jha et al., 2015; Bass and Takahashi, 2010; Eckel-Mahan and Sassone-Corsi, 2013) driven changes in Rev-erbα expression both within and between neurons and glia.
Understanding the molecular and cellular components that regulate Fabp7 expression will have important implications for public health. For example, pathological states associated with Fabp7 overexpression exist for a variety of diseases, including multiple types of cancer (Zhouet al., 2015; Liuet al., 2012; Mita et al., 2010; Corderoet al., 2019; Kagawaet al., 2019; Maet al., 2018), and neurodegenerative disease, including Alzheimer’s disease (Teunissenet al., 2011; Johnsonet al., 2018). Given the role of the circadian clock in cancer (Masri and Sassone-Corsi, 2018; Sulli et al., 2018, 2019) and neurodegeneration (Musiek and Holtzman, 2016; Hood and Amir, 2017; Lanannaet al., 2020), future studies determining the role in how circadian Fabp7 and Fabp7 lipid-signaling may feedback onto metabolic (Choet al., 2012; Bass and Takahashi, 2010; Panda, 2016) and inflammatory pathways (Carteret al., 2016; Scheiermann et al., 2013; Castanon-Cervanteset al., 2010) may provide novel links between clock-regulated mechanisms, fatty-acid pathways, and disease.
4. Materials and methods
Animals. The Rev-erbα knock out (KO) mice were obtained from B. Vennström and were backcrossed for >7 generations with C57/Bl6 mice. Mice (N = 3–7 per group) were housed under standard 12h-light/12h-dark (LD) cycles and were sacrificed at specific times (zeitgeber time (ZT) 2, 6, 10, 14, 18, 22 with ZT0 corresponding to 7 a.m.). Animal care and use procedures followed the guidelines of the Institutional Animal Care and Use Committee of the University of Pennsylvania in accordance with the guidelines of the US National Institutes of Health.
Chromatin immunoprecipitation (ChIP). ChIP experiments were performed as previously described (Fenget al., 2011) with minor changes. Mouse brain tissue was harvested at ZT10, minced and cross-linked in 1% formaldehyde for 20min, followed by quenching with 1/20 volume of 2.5M glycine solution for 5 min, and then two washes with PBS. Cell lysates with fragmented chromatin were prepared by probe sonication in ChIP dilution buffer (50 mM HEPES, 155 mM NaCl, 1.1% Triton X-100, 0.11% sodium deoxycholate, 0.1% SDS, 1 mM phenylmethylsulfonyl fluoride [PMSF], and a complete protease inhibitor tablet [pH 7.5]). Proteins were immunoprecipitated in ChIP dilution buffer, using 1 μg of Rev-erbα antibody (Cell signaling). Cross-linking was reversed overnight at 65 °C in elution buffer (50 mM Tris-HCL, 10 mM EDTA, 1% SDS, pH8), and DNA isolated using phenol/chloroform/isoamyl alcohol. Precipitated DNA was analyzed by quantitative PCR or high-throughput sequencing.
ChIP-qPCR. Precipitated DNA was analyzed by quantitative PCR, using the following primers: Fabp7, forward: 5′-GGG GAT CAG GAT TGT GAT GT-3’; Fabp7, reverse: 5′-AGA TGG CTC CAA TCC TCC TT-3’; Arbp, forward: 5′- CTG GGA CGA TGA ATG AGG AT-3’; Arbp, reverse: 5′- AGC AGC TGG CAC CTA AAC AG-3’; Npas2, forward: 5′-TTG CAG AAG CTT GGG AAA AG-3’; Npas2, reverse: 5′-TTT CCT GTG GGA GGA GAC AG-3’.
ChIP-seq and cistromic analysis. For ChIP-seq, material from three mice was pooled prior to library generation. ChIP DNA was prepared for sequencing according to the amplification protocol provided by Illumina, using adaptor oligo and primers from Illumina, enzymes from New England Biolabs and PCR Purification Kit and MinElute Kit from Qiagen. Deep sequencing was performed by the Functional Genomics Core (J. Schug and K. Kaestner) of the Penn Diabetes Endocrinology Research Center using the Illumina HiSeq2000, and sequences were obtained using the Solexa Analysis Pipeline. Sequenced reads were aligned to the mouse reference genome (mm9) and peak calling was performed with HOMER (Heinzet al., 2010). ChIP-seq data are deposited in NCBI GEO GSE67973 (Zhang et al., 2015), for GSM1659684 and GSM1659685 datasets.
4.1. MEME package
Analysis of the Fabp7 promoter was done using the MEME package (http://meme.nbcr.net/meme/). 2000 base pairs upstream and 2000 base pairs downstream of the murine Fabp7 transcription start site (TSS) was used for promoter analysis. Reference to site location of cis-elements were expressed 0–4000, with 2000 being at the TSS.
4.2. GO and KEGG analysis
Gene ontology analysis was performed on the ranked list of Rev-erbα ChIP-seq genes with peak score >2 [SUPPLEMENTAL dataset 1], using Panther GO-Slim against the mouse gene list (http://geneontology.org release 2021-01-01: 44,091; (Ashburneret al., 2000; The Gene Ontology resourc, 2021). Top non-redundant categories are presented.
KEGG pathway analysis was performed on the same gene list using KEGG Mapper https://www.genome.jp/kegg/tool/map_pathway1.html (Kanehisa and Sato, 2020) against mouse pathways.
4.3. qPCR
Total RNA was extracted from tissue using the RNeasy Mini Kit (QIAGEN) and treated with DNase (QIAGEN). The RNA was reversed transcribed using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) and analyzed by quantitative PCR. Quantitative PCR was performed with Power SYBR Green PCR Mastermix on the PRISM 7500 (Applied Biosystems). Gene expression was normalized to mRNA levels of housekeeping gene 36B4 and the level of the gene of interest in the control samples. Circadian oscillations in gene expression were calculated using JTK_cyclev3.1 scripts (Hughes et al., 2010) run on R. Amplitude confidence intervals were calculated according to Miyazaki et al. (2011).
4.4. Primers
36B4 Forward TCC-AGG-CTT-TGG-GCA-TCA.
36B4 Reverse CTT-TAT-CAG-CTG-CAC-ATC-ACT-CAG-A
Fabp3 Forward CTG-ACT-CTC-ACT-CAT-GGC-AGT-GT
Fabp3 Reverse GCC-AGG-TCA-CGC-CTC-CTT
Fabp5 Forward CGA-CAG-CTG-ATG-GCA-GAA-AAA
Fabp5 Reverse GAC-CAG-GGC-ACC-GTC-TTG
Fabp7 Forward CTC-TGG-GCG-TGG-GCT-TT
Fabp7 Reverse TTC-CTG-ACT-GAT-AAT-CAC-AGT-TGG-TT
Funding
This work was supported by National Institute of Heath grant R35GM133440 to J.R.G.
CRediT author statement
William M. Vanderheyden: Formal analysis, Writing – Review & Editing, Visualization. Bin Fang: Formal analysis, Investigation, Data Curation, Resources, Writing – Review & Editing, Visualization. Carlos C. Flores: Formal analysis, Investigation, Data Curation, Writing – Review & Editing, Visualization. Jennifer Jager: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing – Review & Editing, Visualization. Jason R. Gerstner: Conceptualization, Methodology, Resources, Writing – Original Draft, Writing – Review & Editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Dr. A. Pack and the UPENN Center for Sleep and Circadian Neurobiology and Dr. M. Lazar and the UPENN Institute for Diabetes, Obesity, and Metabolism for advice and support.
Footnotes
A Peer Review Overview and (sometimes) Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.crneur.2021.100009.
Appendix A. Peer Review Overview and Supplementary data
A Peer Review Overview and (sometimes) Supplementary data associated with this article:
References
- Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74:246–260. doi: 10.1016/j.neuron.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Ashburner M., et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J., Takahashi J.S. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugge A., et al. Rev-erbalpha and Rev-erbbeta coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657–667. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S.J., et al. A matter of time: study of circadian clocks and their role in inflammation. J. Leukoc. Biol. 2016;99:549–560. doi: 10.1189/jlb.3RU1015-451R. [DOI] [PubMed] [Google Scholar]
- Castanon-Cervantes O., et al. Dysregulation of inflammatory responses by chronic circadian disruption. J. Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H., et al. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero A., et al. FABP7 is a key metabolic regulator in HER2+ breast cancer brain metastasis. Oncogene. 2019;38:6445–6460. doi: 10.1038/s41388-019-0893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruyne J.P., Baggs J.E., Sato T.K., Hogenesch J.B. Ubiquitin ligase Siah2 regulates RevErbα degradation and the mammalian circadian clock. Proc. Natl. Acad. Sci. U. S. A. 2015;112:12420–12425. doi: 10.1073/pnas.1501204112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eban-Rothschild A., Rothschild G., Giardino W.J., Jones J.R., de Lecea L. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat. Neurosci. 2016;19:1356–1366. doi: 10.1038/nn.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi M., et al. Astrocyte-expressed FABP7 regulates dendritic morphology and excitatory synaptic function of cortical neurons. Glia. 2016;64:48–62. doi: 10.1002/glia.22902. [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan K., Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol. Rev. 2013;93:107–135. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D., et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M., Hotamisligil G.S. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner J.R., et al. Brain fatty acid binding protein (Fabp7) is diurnally regulated in astrocytes and hippocampal granule cell precursors in adult rodent brain. PloS One. 2008;3 doi: 10.1371/journal.pone.0001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner J.R. On the evolution of memory: a time for clocks. Front. Mol. Neurosci. 2012;5:23. doi: 10.3389/fnmol.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner J.R., Paschos G.K. Circadian expression of Fabp7 mRNA is disrupted in Bmal1 KO mice. Mol. Brain. 2020;13:26. doi: 10.1186/s13041-020-00568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner J.R., Vander Heyden W.M., Lavaute T.M., Landry C.F. Profiles of novel diurnally regulated genes in mouse hypothalamus: expression analysis of the cysteine and histidine-rich domain-containing, zinc-binding protein 1, the fatty acid-binding protein 7 and the GTPase, ras-like family member 11b. Neuroscience. 2006;139:1435–1448. doi: 10.1016/j.neuroscience.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner J.R., et al. Time of day regulates subcellular trafficking, tripartite synaptic localization, and polyadenylation of the astrocytic Fabp7 mRNA. J. Neurosci. 2012;32:1383–1394. doi: 10.1523/JNEUROSCI.3228-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner J.R., et al. Normal sleep requires the astrocyte brain-type fatty acid binding protein FABP7. Sci Adv. 2017;3 doi: 10.1126/sciadv.1602663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley J.J. Circadian regulation of lipid metabolism. Proc. Nutr. Soc. 2016;75:440–450. doi: 10.1017/S0029665116000288. [DOI] [PubMed] [Google Scholar]
- Gooley J.J., Chua E.C. Diurnal regulation of lipid metabolism and applications of circadian lipidomics. J Genet Genomics. 2014;41:231–250. doi: 10.1016/j.jgg.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Guillaumond F., Dardente H., Giguère V., Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J. Biol. Rhythm. 2005;20:391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- Heinz S., et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood S., Amir S. Neurodegeneration and the circadian clock. Front. Aging Neurosci. 2017;9:170. doi: 10.3389/fnagi.2017.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M.E., Hogenesch J.B., Kornacker K. JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J. Biol. Rhythm. 2010;25:372–380. doi: 10.1177/0748730410379711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E.C.B., et al. Deep proteomic network analysis of Alzheimer’s disease brain reveals alterations in RNA binding proteins and RNA splicing associated with disease. Mol. Neurodegener. 2018;13:52. doi: 10.1186/s13024-018-0282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa Y., et al. Role of FABP7 in tumor cell signaling. Adv Biol Regul. 2019;71:206–218. doi: 10.1016/j.jbior.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Kanehisa M., Sato Y. KEGG Mapper for inferring cellular functions from protein sequences. Protein Sci. 2020;29:28–35. doi: 10.1002/pro.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Jha P., Challet E., Kalsbeek A. Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol. Cell. Endocrinol. 2015;418(Pt 1):74–88. doi: 10.1016/j.mce.2015.01.024. [DOI] [PubMed] [Google Scholar]
- Lananna B.V., et al. Chi3l1/YKL-40 is controlled by the astrocyte circadian clock and regulates neuroinflammation and Alzheimer’s disease pathogenesis. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aax3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavialle M., et al. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc. Natl. Acad. Sci. U. S. A. 2011;108:12915–12919. doi: 10.1073/pnas.1100957108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.Z., et al. A fatty acid-binding protein 7/RXRβ pathway enhances survival and proliferation in triple-negative breast cancer. J. Pathol. 2012;228:310–321. doi: 10.1002/path.4001. [DOI] [PubMed] [Google Scholar]
- Ma R., et al. FABP7 promotes cell proliferation and survival in colon cancer through MEK/ERK signaling pathway. Biomed. Pharmacother. 2018;108:119–129. doi: 10.1016/j.biopha.2018.08.038. [DOI] [PubMed] [Google Scholar]
- Masri S., Sassone-Corsi P. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 2018;24:1795–1803. doi: 10.1038/s41591-018-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita R., Beaulieu M.J., Field C., Godbout R. Brain fatty acid-binding protein and omega-3/omega-6 fatty acids: mechanistic insight into malignant glioma cell migration. J. Biol. Chem. 2010;285:37005–37015. doi: 10.1074/jbc.M110.170076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M., et al. Age-associated disruption of molecular clock expression in skeletal muscle of the spontaneously hypertensive rat. PloS One. 2011;6 doi: 10.1371/journal.pone.0027168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk J.A., Green C.B., Takahashi J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M., Margolis E.B. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 2017;18:73–85. doi: 10.1038/nrn.2016.165. [DOI] [PubMed] [Google Scholar]
- Musiek E.S., Holtzman D.M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354:1004–1008. doi: 10.1126/science.aah4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai J., et al. Behaviorally consequential astrocytic regulation of neural circuits. Neuron. 2020 doi: 10.1016/j.neuron.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owada Y., et al. Altered emotional behavioral responses in mice lacking brain-type fatty acid-binding protein gene. Eur. J. Neurosci. 2006;24:175–187. doi: 10.1111/j.1460-9568.2006.04855.x. [DOI] [PubMed] [Google Scholar]
- Panda S. Circadian physiology of metabolism. Science. 2016;354:1008–1015. doi: 10.1126/science.aah4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J.R., et al. Circadian regulation of membrane physiology in neural oscillators throughout the brain. Eur. J. Neurosci. 2019 doi: 10.1111/ejn.14343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S.J., Nestler E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C., Kunisaki Y., Frenette P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013;13:190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell A., et al. The nuclear receptor REV-ERBalpha regulates Fabp7 and modulates adult hippocampal neurogenesis. PloS One. 2014;9 doi: 10.1371/journal.pone.0099883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch J., Corsico B. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu. Rev. Nutr. 2008;28:73–95. doi: 10.1146/annurev.nutr.27.061406.093710. [DOI] [PubMed] [Google Scholar]
- Sulli G., Manoogian E.N.C., Taub P.R., Panda S. Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol. Sci. 2018;39:812–827. doi: 10.1016/j.tips.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulli G., Lam M.T.Y., Panda S. Interplay between circadian clock and cancer: New frontiers for cancer treatment. Trends Cancer. 2019;5:475–494. doi: 10.1016/j.trecan.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata Y., et al. Sleep and wakefulness are controlled by ventral medial midbrain/pons GABAergic neurons in mice. J. Neurosci. 2018;38:10080–10092. doi: 10.1523/JNEUROSCI.0598-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen C.E., et al. Brain-specific fatty acid-binding protein is elevated in serum of patients with dementia-related diseases. Eur. J. Neurol. 2011;18:865–871. doi: 10.1111/j.1468-1331.2010.03273.x. [DOI] [PubMed] [Google Scholar]
- The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021;49:D325–d334. doi: 10.1093/nar/gkaa1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A., et al. Fabp7 maps to a quantitative trait locus for a schizophrenia endophenotype. PLoS Biol. 2007;5:e297. doi: 10.1371/journal.pbio.0050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., et al. GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat. Neurosci. 2019;22:106–119. doi: 10.1038/s41593-018-0288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., et al. GENE REGULATION. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science. 2015;348:1488–1492. doi: 10.1126/science.aab3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., et al. Overexpression of FABP7 promotes cell growth and predicts poor prognosis of clear cell renal cell carcinoma. Urol. Oncol. 2015;33:113. doi: 10.1016/j.urolonc.2014.08.001. e119-117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.