Abstract
Background
In-hospital hyperglycemia (HH) is frequent and related to higher morbidity and mortality. Despite the benefits of HH treatment, glycemic control is often poor and neglected. The use of health applications to support diagnosis and therapy is now incorporated into medical practice. Medical applications for inpatient glycemic management have potential to standardize this handling by the nonspecialist physician. However, related studies are scarce. We aim to evaluate the efficacy in inpatient glycemic control parameters of medical software applications in non-critical care settings.
Methods
This systematic review on in-hospital insulin applications was performed according to PRISMA guidelines. Data were extracted in triplicate and methodological quality was verified. Specific outcomes of interest were glycemic control efficacy, hypoglycemia risk, length of in-hospital stay, integration with the electronic medical record and healthcare staff acceptance.
Results
Among the 573 articles initially identified and subsequent revision of the references of each one, seven studies involving six applications were eligible for the review. A better glycemic control was reported with the use of most in-hospital insulin applications in the studies evaluated, but there was no mention of the time to reach the glycemic goal. The risk of hypoglycemia was low. Different reasons influenced the varied acceptance of the use of applications among health professionals.
Conclusion
The six applications of inpatient insulin therapy in a non-critical care environment proved to be useful and safe compared to the usual management. Medical apps are tools that can help improve the quality of patient care.
Keywords: Medical informatics applications, insulin therapy, hospital, blood glucose, diabetes mellitus, mobile applications
Introduction
In-hospital hyperglycemia (HH) is defined as a pre-meal blood glucose value greater than 140 mg/dL (7.8 mmol/L).1 The exact prevalence is not known, but observational studies report that HH affects 32% to 38% in community hospitals,2,3 70% in hospitalizations for acute coronary syndromes4 and about 80% after cardiac surgeries.5 HH increases the mortality and morbidity of the underlying cause of hospitalization, regardless of whether patients have diabetes mellitus (DM).2,6
In 2017, it was estimated that there were approximately 425 million adults with diabetes in the world and 14.25 million in Brazil, corresponding to about 8.9% of the Brazilian population.7 Patients with diabetes are hospitalized more frequently than general population,8 representing a high cost of health care.
In 2012, the Endocrine Society’s clinical guidelines on management of hyperglycemia in hospitalized patients in non-critical care setting recommended safe and practical glycemic goals, description of protocols and standardization of subcutaneous insulin prescription in the hospital setting.9 Despite the effort invested in the development and dissemination of medical guidelines, adherence is still limited in healthcare.10 Complexity of insulin protocols and fear of hypoglycemia are obstacles to achieve optimal treatment and also contribute to poor adherence.11
Many studies have provided evidence of benefits of HH treatment, such as reduction in hospital infections,12 better prognosis after acute myocardial infarction13,14 and after stroke, and adverse thrombotic events risk reduction.15 However, glycemic control remains deficient and neglected.16,17 Inpatient diabetes management is generally considered secondary in importance compared with the condition that led to admission, promoting a clinical inertia related to in-hospital glycemic management.18
The subcutaneous insulin basal-bolus regimen is the recommended therapy for non-critical patients because it is the safest in most patients.19–22 However, clinical variables of the patients make the in-hospital insulin protocol more dynamic and complex. There are specialized teams in hospital glycemic control in several hospitals, involving endocrinologists, diabetologists, hospitalists, diabetes nurse practitioners, among others,23–25 but they are not always available or sufficient to meet demand. In 2018, there were 5,210 endocrinologists in Brazil,26 and only the minority of them works in hospital setting. Considering the high prevalence of HH, the number of endocrinologists is not enough. Most cases of HH do not require the presence of the specialist for management; however, the non-specialist physicians barriers to insulin therapy protocol limit their performance and HH is often neglected.18 In Brazilian public hospitals, the scenario may be even worse, due to the lack of resources and professional staff.
Solutions are needed to standardize the inpatient dysglycemia (hypo and hyperglycemia) management, including insulin therapy, to reduce complexity, facilitate adherence to the recommendations in guidelines and reduce inappropriate variations in care.18
There are several medical software applications (medical apps) that facilitate the daily life of the physician and the patients with diabetes,27 including for the management of insulin therapy. Most of them are for outpatient insulin management, lack validation and often provide recommendations for inadequate doses of insulin28 or have a cost to use.
There is a need to improve the inpatient glycemic management due to the lack of standardization of treatment, barriers to insulin therapy and the limitation on skilled specialist availability make medical apps very attractive and useful tool for inpatient glucose management. The objective of this study is to compare the efficacy in glycemic control parameters of medical inpatient hospital applications in non-critical care settings.
Methods
Search strategy: Study was guided by the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) protocol.29 Articles were identified by searches in the following databases in January 2020: Pubmed, Cochrane library, Embase, Academic Google, Scopus, Virtual Health Library (VHL), UpToDate, Fiocruz Public Health Library (EBSCO Fiocruz), Gideon. We search all articles published retrospectively until 2020.
The fixed text descriptors used in searches were: medical informatics applications (decision making, computer-assisted, information systems and decision support systems), insulin therapy and hospital. Additional searches could be performed, according to the availability of each database. After the careful selection of articles, which was performed by three independent researchers and in three specific technical-evaluative processes (Exclusion of clearly irrelevant titles, exclusion of abstracts that did not address the main theme of the review, and, finally, exclusion of articles that did not presented data on inpatient subcutaneous insulin therapy), two authors, Feitosa ACR and Lavinas-Jones JM, analyzed the selected papers, respecting the pre-established inclusion and exclusion criteria, and defined which documents would proceed in the data extraction process.
Inclusion criteria: Studies on medical apps used for over 18 years inpatient insulin therapy were included. The following criteria were adopted for the inclusion of the articles: I) medical apps used for subcutaneous insulin dose calculation in non-critical care hospital setting; II) observational studies or randomized clinical trials with data on hospital glycemic control parameters; III) papers published in English, Spanish or Portuguese; There were no restrictions on date or place of study.
Data extraction and quality assessment: Methodological quality was not an exclusion criterion and was carried out using the Jadad scale (Figure 1)30 and the Delphi list.31 Three independent reviewers (Lavinas-Jones JM, Fonseca EM, Pato RB) used a standardized form for extracting data from the studies: general characteristics, information on participants, interventions, comparisons and results.
Figure 1.
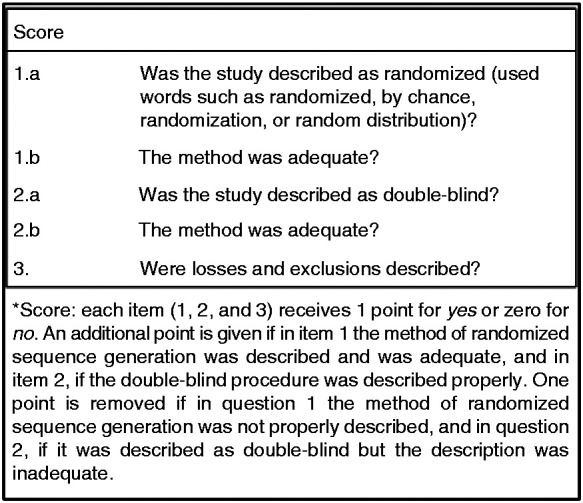
Jadad scale.
Data analysis and synthesis: Each included article was summarized in a structured narrative way. A narrative synthesis includes a complete description of the participants, type of diabetes, interventions received (type and dose of insulin), monitoring of efficacy and adherence, and outcome measures (mean blood glucose, percentage of blood glucose within the target, percentage of hypoglycemia and hyperglycemia episodes, amount of insulin used).
Results
A total of 573 records were identified in the electronic databases. Based on the review of titles, 83 articles were found to have met the initial selection criteria. Abstract analysis was performed and 25 articles remained. After examination of the full texts, five articles were eligible for the systematic review.32–36 And additional two studies37,38 that met the inclusion criteria were identified by checking the references of located papers. The seven selected studies involving six different applications were included in the review (Table 1).
Table 1.
Summary of included studies and patient demographics.
|
Study identification |
Study design | Application or electronic protocol name | Cause of hyperglycemia |
Total number of study participants |
Participant age, years | Insulin type | Total insulin dose (IU/kg/day) | Glycemic target (mg/dL) |
|---|---|---|---|---|---|---|---|---|
| Study #1: Schnipper32 |
Clinical Trial | Glycemic management protocol - Computer provider order entry (CPOE) | T2DM SH |
Pre-intervention: 63 Post-intervention: 106 |
Pre-intervention: 63 ± 15,7 Post-intervention: 64,7 ± 14,3 |
NPH, Glargine e Aspart |
0.5–0.7 | 60–180 |
| Study #2: Maynard37 |
Prospective Observational Study |
Structured subcutaneous insulin order sets and Insulin Management algorithm - Computer provider order entry (CPOE) | T2DM T1DM SH |
Pre-intervention: 2504 Post-intervention: 2295 |
Pre-intervention: 56 ± 17 Post-intervention: 56 ± 16 |
Glargine, Rapid-Acting and Short-Acting |
0.3–0.6 (according to BMI) | 60–180 |
| Study #3: Murphy38 |
Retrospective Observational Study |
Insulin protocol LUMC – Eletronic medical record (EMR) | T2DM T1DM SH |
No data | No available data | Glargine, NPH Lispro and Aspart |
0.2–0.8 (according to renal function, BMI and type of DM | 60–180 |
| Study #4: Wexler33 |
Clinical Trial | Computer order template for support basal-bolus insulin | Decompensated T2DM |
Intervention: 63 Control: 65 |
Intervention: 68 ± 14,3 Control: 70 ± 13,4 |
Glargine and Aspart | 0.5 | No data |
| Study #5: Schnipper34 |
Clinical Trial | Glycemic management protocol - Computer provider order entry (CPOE) | T2DM SH |
Intervention: 90 Control: 79 |
Intervention: 64,8 ± 15,5 Control: 65,4 ± 12,2 |
Glargine,NPH,Rapid-Acting and Short-Acting | 0.5–0.7 | 60–180 |
| Study #6: Neubauer35 |
Clinical Trial | GlucoTab | T2DM | 99 | 67 ± 11 | Glargine and Aspart | 0.5, but 0.3 if >70 years-old or Cr > 2 mg/dL | 70–140 |
| Study #7: Gregory36 |
Prospective Observational Study |
Comprehensive computadorized insulin order set and titration algorithm - Computer provider order entry (CPOE) | T2DM T2DM SH |
6526 | No available data | Glargine and Aspart | 0.1–0.3, according to age, type of DM, diet, BMI, renal function or history of pancreatectomy. | 70–180 |
T1DM1, Type 1 Diabetes mellitus; T2DM, Type 2 Diabetes mellitus; SH, stress hyperglycemia; BMI, body mass index; Cr, serum creatinine.
Articles were excluded because they did not address the subject being searched, such as applications for the calculation of intravenous insulin dose39–41 or for outpatient use.42,43 Other articles were excluded because they did not have glycemic control parameters necessary for inclusion in the study.44–46 One study was excluded because it was a calculator for the pediatric population.47 The complete search strategy and results are shown in Figure 2.
Figure 2.
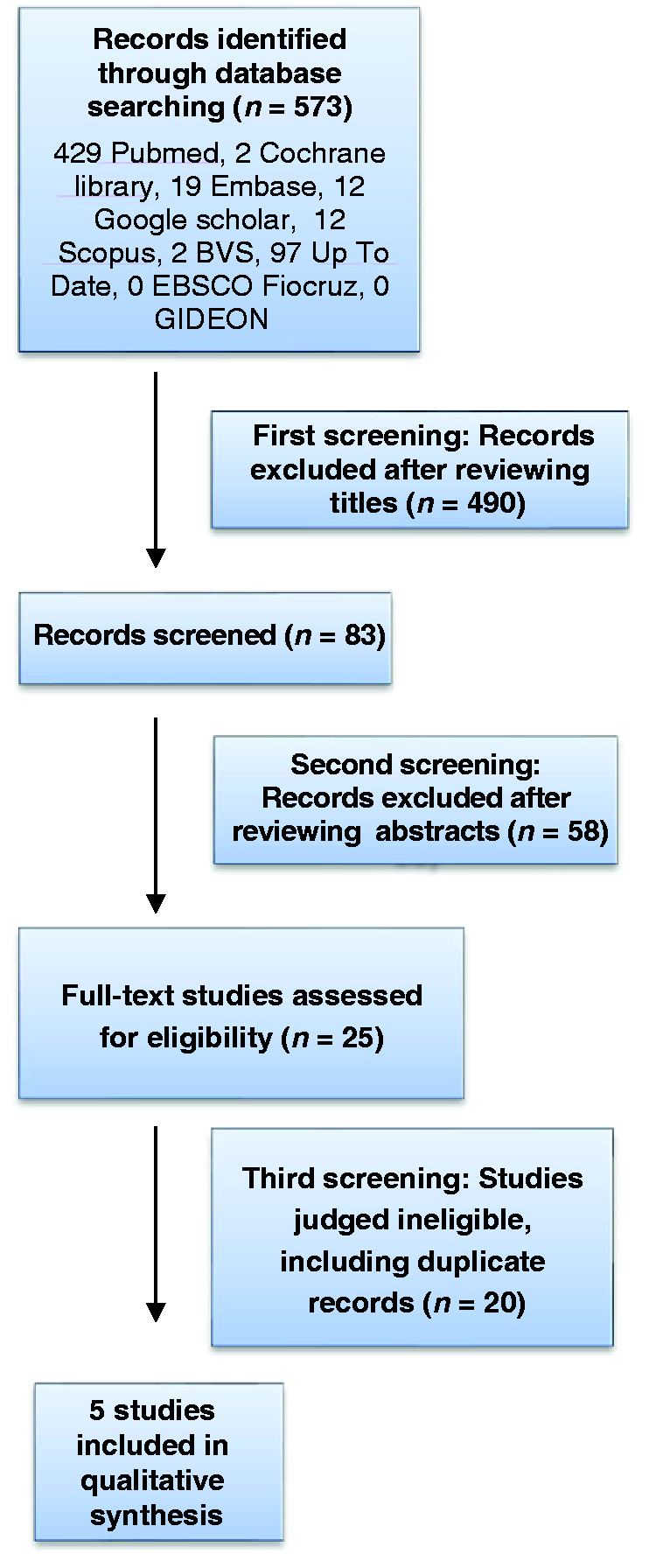
PRISMA (Preferred Reporting Items for Systematic Meta-analyses) flowchart.
Quality assessment: Using the Jadad scale,30 there were two studies with moderate methodological quality (Studies #433 and #534) and the other studies were considered to have low quality. According to the Delphi Scale,31 all studies were classified as being of good methodological quality, except one that was considered as of poor methodological quality (Study #237).
Demographic data and intervention: Table 1 describes demographic data of the studies: number of participants, mean age, type of hyperglycemia (T1DM, T2DM and stress hyperglycemia); and data related to the intervention, such as type and dose of insulin used. The results of each of the studies related to glycemic control parameters, hypoglycemia and length of in-hospital stay are described in Table 2.
Table 2.
Glycemic control parameters and length of in-hospital stay.
| Study identification | A1c (%) | BG within the target range (%) | Mean BG (mg/dL) | Reduçtion of mean BG | Frequency of hyperglicemia (%) (mg/dL) | Frequency of hypoglycemia (mg/dL) (%) | Lenght of in-hospital stay (days) |
|---|---|---|---|---|---|---|---|
| Study #1: Schnipper32 | Pre-intervention: 8.5Post-intervention: 8.3 | 65.0 (p = 0.04) | 165 | 5.78 | No data | <60 mg/dL: 6.1<40 mg/dL: 1.2 (patients-day) | Pre-intervention: 4.6Post-intervention: 3.5 |
| Study #2: Maynard37 | No data | 69.9 (p < 0.005) | 165 ± 58 | 7.82 | No data | <60 mg/dL: 9.8<40 mg/dL: 2.4 (patients-day) | Pre-intervention: 4.6Post-intervention: 4.8 |
| Study #3: Murphy38 | No data | Pre-intervention: 66Post-intervention: 53 | <135 | 15 | No data | <60 mg/dL: 6 | No data |
| Study #4:Wexler33 | No data | No data | Intervention: 194 ± 66Control: 224 ± 57 | No data | Intervention: 26Control: 38 BG > 240 mg/dL) | Intervention: <60 mg/dL: 12<40 mg/dL: 0Control: <60 mg/dL: 14<40 mg/dL: 1 | Intervention: 6Control: 5 |
| Study #5:Schnipper34 | Intervention: 7.6 ± 2.4Control: 7.4 ± 1.6 | Intervention: 74.6 (p = 0.05)Control: 71.3 | Intervention: 148 ± 42Control: 158 ± 54 | No data | Intervention: 7.3Control: 14.8(BG > 300 mg/dL) | Intervention: <60:mg/dL 6.8<40 mg/dL: 0.5Control: <60 mg/dL: 3.5<40 mg/dL: 0.3 (patients-day) | Intervention: 6.2Control: 5.7 |
| Study #6: Neubauer35 | 8.1 ± 4.1 | 50.2 (p = 0.001) | 154 ± 35 | No data | No data | 60–70 mg/dL:1.440–60 mg/dL: 0.5 <40 mg/dL: 0 | No data |
| Study #7: Gregory36 | T1DM: 9.3 ± 2.7T2DM2: 7.7 ± 2.0 | Pre-intervention: 65.7Post-intervention: 56.9 | No data | No data | Pre-intervention: 31.8Post-intervention: 41.3 | <70 mg/dL:1.8 | No data |
T1DM1, Type 1 Diabetes mellitus; T2DM, Type 2 Diabetes mellitus; BG, blood glucose; A1c, glycated hemoglobin.
Glycemic control: The studies #237 and #433 demonstrated a reduction in hyperglycemia in the intervention group. Mean blood glucose of studies #237 and #433 were 148 and 195 mg/dL in the intervention group and 158 and 224 mg/dL in the control group, respectively. Considering the glycemic target of 60 to 180 mg/dL, the study #534 found 74.6 and 71.3% of blood glucose measurements within the target in the intervention and control groups, respectively, with no statistically significant difference. There was a reduction in the proportion of patients-day with severe hyperglycemia (glucose >300 mg/dL), being 14.8% in the control group and 7.3% in the intervention group of study #5.34
Mean blood glucose of studies #132 and #635 were 165 and 154 mg/dL, respectively. The mean percent of glucose readings within the target range after the use of the applications was 65% in the study #132 and 50.2% in the study #6,35 but cut-off points were different: 60 to 180 mg/dL in the study #132 and 70 to 140 mg/dL in the study #635. Although these studies were uncontrolled clinical trials, the study #635 compared the group of patients using the application with the routine care group (paper-based algorithm), and it was shown that that the percentage of blood glucose between 70 and 180 mg/dL was significantly higher with application use (73% vs. 53%). In observational studies #237 and #3,38 mean blood glucose was 165 ± 58 and lower than 135 mg/dL, respectively. The use of the application resulted in 69.9% of the blood glucose within the target (60 to 180 mg/dL). Studies #338 and #736 compared the percentage of blood glucose within the target before and after the intervention. The study #338 considered a glycemic target of 60 to 180 mg/dL and the percentage before and after the intervention were 66% vs. 53%. Study #736 showed a reduction in the percentage of blood glucose values within the target (70 to 180 mg/dL) after the intervention, with results of 65.67% vs. 56.85%, respectively before and after the intervention. Studies #237 and #338 reduced the mean blood glucose by 7.82% and 15%, respectively, with the use of the applications.
Hypoglycemia: Risk of hypoglycemia was low in all seven studies included in this systematic review, and with frequency comparable to data found in other studies.22,48–50 The GlucoTab application study35 showed the occurrence of blood glucose ranges of 60 to 70, 40 to 60 and <40 mg/dL was, respectively, 1.4; 0.5 and 0% of measurements. In studies #132 and #2,37 episodes of hypoglycemia with blood glucose less than 60 mg/dL occurred in 6.1% and 9.8% patients-day, respectively, and blood glucose values lower than 40 mg/dL occurred in 1.2% and 2.4% patients-day. The percentage of patients who had hypoglycemia episodes was 6% (blood glucose <60mg/dL) and 1.82% (blood glucose <70 mg/dL), respectively, in the study #534 and #7.36 The studies #433 and #534 compared hypoglycemia episodes between intervention and control groups. In the study #5,34 blood glucose values less than 60 mg/dL and less than 40 mg/dL were 6.8% and 0.5% patient-days in the intervention group and 3.5% and 0.3% patient-days in the control group.34 The study #433 reported hypoglycemia <60 and <40 mg/dL in 12 and 0% in the intervention group and 14 and 1% in the control group, respectively.33
Time to obtain the target for glycemic control: All seven studies did not report the time to reach the target of the glycemic mean after use of the applications.
Length of in-hospital stay: Studies #1,32 #5,34 #433 and #237 compared the length of hospital stay before and after intervention. In study #1,32 the length of hospital stay was 25% shorter in the post-intervention (112 vs. 86 hours, pre and post-intervention respectively) after adjusting for patient insurance, race, gender, and Charlson comorbidity score. The studies #2,37 #534 and #433 showed length of hospital stay similar before and after intervention.
Integration with the electronic medical record (EMR): All study tools presented integration with EMR.
Health professional who is app users: Studies #1,32 #2,37 #4,33 #534 and #736 only physicians operate de tool. Studies #338 and #6,35 Physicians, nurses and pharmacists and Physicians and nurses, respectively.
Healthcare staff acceptance: A total of 65 healthcare professionals answered a questionnaire about the usability of the GlucoTab application.35 The results were that 91% of health care professionals referred confidence to use the application for in-hospital glycemic management, 89% reported being a practical tool for routine use, 80% believed that its use could prevent medical errors, and 85% thought glycemic control was better when using GlucoTab app. Regarding the increase in work after the application of the application, different perceptions were reported: 13 healthcare professionals reported that the work increased, 33 had a decrease in work, 12 there was no change in workload and seven did not answer the question. Study #736 reported that adherence to the digital protocol was low, but no objective data were shown.
The study #534 reported that physicians who used the tool had less acceptance of the new resource because they were professionals still in training whose preference was to perform glycemic control in the conventional manner. The study #433 identified that one of the reasons for the lower acceptance of the health team to the application was the fact that its use was optional by health professionals. The authors suggest the use of the application in a mandatory way, associated with an intensive implementation support with professionals who are in contact with the target population.
The studies #132, #237 and #338 did not report the team's acceptance of the new tool used, but in the discussion as one of the future improvements they cited the need to pay attention to usability.
Discussion
Although there is strong evidence of treatment benefits,35–38 glycemic control remains poor and neglected.16 Management of in-hospital diabetes is generally considered less important than the condition that led to admission, resulting in a clinical inertia to the diabetes care in the hospital.18 The use of electronic insulin dose calculation tools may standardize the in-hospital insulin therapy protocol, reduce the complexity and facilitate adherence to the recommendations of the guidelines, allowing a better control of HH.
Most of the applications aimed at glycemic management in critically ill patients39–41,51 or in an outpatient setting.42,43 Few medical applications have been identified for calculation of subcutaneous insulin doses for non-critical patients in an in-hospital setting. About the Glucostabilizer, it was not initially identified in our searches as a tool for subcutaneous insulinization. Possibly the article had not been identified due to the use of non-specific keywords. On the official website of the application, there is information that draws attention of the same in the performance in insulin IV and absence of information about subcutaneous insulinization.52
Six applications32–38 were identified. They have been shown to be effective and safe for HH management. The mean blood glucose after tool use was within the glycemic targets set by the guidelines for non-critical patients.9
On the other hand, the risk of hypoglycemia was low in all seven studies included in this systematic review. When compared with other studies,20,53 the frequency was similar. It is known that the physicians fear of hypoglycemia is one of the reasons for clinical inertia in the treatment of HH. Therefore, the use of strategies such as insulin dose calculators - which does not imply an increase or reduction in the risk of hypoglycemia - can generate knowledge and safety in insulinization, reducing episodes of hyperglycemia and contribute directly to the patient's better glycemic control.
Only one study35 evaluated the acceptance of the tool by health professionals. The users reported confidence, practicality and better glycemic control with the use of the application.35 The good acceptance of healthcare professionals may result in better adherence and quality to HH treatment. Most HH cases do not require the presence of the specialist for its management; however, the barriers to the use of in-hospital insulin therapy may limit the performance of the non-specialist physician.16 Public health service hospitals may have an even worse scenario, due to the lack of resources and professionals. The use of a reliable and practical tool could aid in the in-hospital glycemic management of these hospitals.
It is of great importance that each hospital defines in its institutional protocol the role of health professionals in conducting hospital glycemic control. In most studies only the physician used the tool.32–34,36,37 Only two studies, nurses35,38 and pharmaceuticals38 could also use. All study tools were integrated with EMR. This is of unique importance as the tool transcends its use in mobile-only applications and integrates the entire patient-linked hospital system. This directly implies as another factor that helps control the glycemic average of the patients studied, as it facilitates communication between health professionals and gives joint access to each patient's chart and their glycemic calculator.
The length of in-hospital stay was evaluated in four studies32–34,36 and was smaller in the intervention group in three of these studies.32–34 Such studies may represent a reduction in hospital costs and complications caused by long-term hospitalization.
Limitations were identified in the included studies, some being classified in the Jadad scale as having low methodological quality.32,35–38 Data that are considered important to evaluate the applicability of the tool have not been reported, such as the time to reach the target for glycemic control and the user acceptance report. We also meet the need for individualized algorithms for specific populations such as pregnancy, children and elderly.
Most in-hospital insulin dose calculation applications have helped to promote good glycemic control with low risk for hypoglycemia. In addition, they have the potential to standardize protocols, ensure applicability of the guidelines and assist the non-specialist physician in HH management. In Brazil, InsulinAPP application for in-hospital glycemic control is available54 and we are conducting a prospective randomized study to validate this application.
Conclusion
This review is unprecedented and useful to expose the applications available for inpatient insulin therapy, describing important parameters related to adequate control.
The information from the present study allowed identifying and comparing medical apps for hospital insulinization of non-critical patients. The tools identified for in-hospital glycemic management promoted better glycemic mean and lower risk of hypoglycemia than usual management. The results are of interest to the scientific community and clinical practice, since they demonstrate the impact of the applications, electronic tools, in the control of HH and allow the diffusion of knowledge about the importance of hospital insulinization and alternative forms of HH management. In Brazil, InsulinAPP application for in-hospital glycemic control is available54 and we are conducting a prospective randomized study to validate this application.
Acknowledgements
The authors express their gratitude to the Study Group GEPEN (Grupo de Estudo e Pesquisa em Endocrinologia e Metabologia), belonging to Escola Bahiana de Medicina e Saúde Pública. The funders of the six apps had no influence on the design of the review and were not involved in data collection or analysis, the writing of the article, or the decision to submit it for publication.
Footnotes
Contributorship: A.C.R.F. and J. M. L. J. wrote the initial research proposal and manuscript. A.C.R.F., J.M.L.J., M.T.K.T. and M.C.H. reviewed/edited the research proposal and manuscript and contributed to the discussion. J.M.L.J., M.C.H., E.M.F. and R.B.P. collected researched the data.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Marcos Tadashi K. Toyoshima is one of the developers of the InsulinAPP application - an app for glycemic control of non-critical hospitalized patients. The others authors declare that there are no conflicts of interests.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor: A.C.R.F.
Peer review: This manuscript was reviewed by reviewers who have chosen to remain anonymous.
ORCID iD: Julia Mandaro Lavinas Jones https://orcid.org/0000-0003-0609-4135
References
- 1.American Diabetes Association. Diabetes care in the hospital, nursing home, and skilled nursing facility. Diabetes Care 2015; 38: S80–S85. [DOI] [PubMed] [Google Scholar]
- 2.Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002; 87: 978–982. [DOI] [PubMed] [Google Scholar]
- 3.Cook CB, Kongable GL, Potter DJ, et al. Inpatient glucose control: a glycemic survey of 126 U.S. hospitals. J Hosp Med 2009; 4: E7–E14. [DOI] [PubMed] [Google Scholar]
- 4.Kosiborod M, Inzucchi SE, Spertus JA, et al. Elevated admission glucose and mortality in elderly patients hospitalized with heart failure. Circulation 2009; 119: 1899–1907. [DOI] [PubMed] [Google Scholar]
- 5.Schmeltz LR, DeSantis AJ, Thiyagarajan V, et al. Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy. Diabetes Care 2007; 30: 823–828. [DOI] [PubMed] [Google Scholar]
- 6.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglicemia and increased risk of death after myocardical infarction in patients with and without diabetes: a systematic overview. Lancet 2000; 355: 773–778. [DOI] [PubMed] [Google Scholar]
- 7.IDF diabetes atlas. 8th ed. Internacional Federation of Diabetes, 2015, Belgium.
- 8.Donnan PT, Leese GP, Morris AD. Hospitalizations for people with type 1 and type 2 diabetes compared with the nondiabetic population of Tayside, Scotland: a retrospective cohort study of resource use. Diabetes Care 2000; 23: 1774–1779. [DOI] [PubMed] [Google Scholar]
- 9.Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012; 97: 16–38. [DOI] [PubMed] [Google Scholar]
- 10.Woolf S, Grol R, Hutchinson A, et al. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. Br Med J 1999; 318: 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turchin A, Matheny ME, Shubina M, et al. Hypoglicemia and clinical outcomes in patients with diabetes with diabetes hospitalized in the general ward. Diabetes Care 2009; 32: 1153–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pomposelli JJ, Baxter JK, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr 1998; 22: 77–81. [DOI] [PubMed] [Google Scholar]
- 13.Malmberg K, Rydén L, Efendic S, et al. Randomized trial of insulin glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardical infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995; 26: 57–65. [DOI] [PubMed] [Google Scholar]
- 14.Malmberg K. Prospective randomized study of intensive insulin treatment on long term survival after acute myocardical infarction in patients with diabetes mellitus. DIGAMI study group. BMJ 1997; 314: 1512–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 2001; 32: 2426–2432. [DOI] [PubMed] [Google Scholar]
- 16.McDonnell ME, Umpierrez GE, Insulin therapy for the management of hyperglycemia in hospitalized patients. Endocrinol Metab Clin North Am 2012; 41 175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyoshima MT, Admoni SN, Lottenberg AS, et al. Controle glicêmico no HCFMUSP: importância de um protocolo institucional unificado. Arq Bras Endocrinol Metab 2014; 58: S204. [Google Scholar]
- 18.Cook CB, Castro JC, Schmidt RE, et al. Diabetes care in hospitalized non-critically ill patients: more evidence for clinical inertia and negative therapeutic momentum. J Hosp Med 2007; 2: 203–211. [DOI] [PubMed] [Google Scholar]
- 19.Krikorian A, Ismail-Beigi F, Moghissi ES. Comparisons of different insulin infusion protocols: a review of recent literature. Curr Opin Clin Nutr Metab Care 2010; 13: 198–204. [DOI] [PubMed] [Google Scholar]
- 20.Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007; 30: 2181–2186. [DOI] [PubMed] [Google Scholar]
- 21.Qureshi A, Deakins DA, Reynolds LR. Obstacles to optimal management of inpatient hyperglycemia in noncritically ill patients. Hosp Pract (1995) 2012; 40: 36–43. [DOI] [PubMed] [Google Scholar]
- 22.Mendez CE, Umpierrez GE. Pharmacotherapy for hyperglycemia in noncritically ill hospitalized patients. Diabetes Spectr 2014; 27: 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caimari F, González C, Ramos A, et al. Efficacy of a hyperglycemia treatment program at a vascular surgery department supervised by endocrinology. Cir Esp 2015; 94: 392–98. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Roschkov S, Alkhodair A, et al. The effect of nurse practitioner-led intervention in diabetes care for patients admitted to cardiology services. Can J Diabetes 2017; 41: 10–16. [DOI] [PubMed] [Google Scholar]
- 25.Draznin B, Gilden J, Golden SH, for the PRIDE investigators et al. Pathways to quality inpatient management of hyperglycemia and diabetes: a call to action. Diabetes Care 2013; 36: 1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheffer M, Cassenote A, Guilloux AGA, et al. Demografia Médica no Brasil 2018. São Paulo, SP: FMUSP, CFM, Cremesp, 2018, 286p. [Google Scholar]
- 27.Seabrook H, Stromer J, Shevkenek C, et al. Medical applications: a database and characterization of apps in apple iOS and android platforms. BMC Res Notes 2014; 7: 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huckvale K, Adomaviciute S, Prieto J T, et al. Smartphone apps for calculating insulin dose: a systematic assessment. BMC Med 2015; 13: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff, J, et al. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA Statement. PLoS Medicine 2009; 6: 1–7. [DOI] [PMC free article] [PubMed]
- 30.Jadad AR, Moore RA, Carrol D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 31.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998; 51: 1235–1241. [DOI] [PubMed] [Google Scholar]
- 32.Schnipper JL, Ndumele CD, Liang CL, et al. Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: results of a clinical trial. Hoboken: Wiley InterScience, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Wexler DJ, Shrader P, Burns SM, et al. Effectiveness of a computerized insulin order template in general medical inpatients with type 2 diabetes: a cluster randomized trial. Diabetes Care 2010; 33: 2181–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnipper JL, Liang CL, Ndumele CD, et al. Effects of a computerized order set on the inpatient management of hyperglycemia: a cluster-randomized controlled trial. Endocr Pract 2010; 16: 209–218. [DOI] [PubMed] [Google Scholar]
- 35.Neubauer KM, Mader JK, Holl B, et al. Standardized glycemic management with a computerized workflow and decision support system for hospitalized patients with type 2 diabetes on different wards. Diab Technol Ther 2015; 17: 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregory SN, Seley JJ, Gerber LM, et al. Decreased rates of hypoglycemia following implementation of a comprehensive computerized insulin order set and titration algorithm in the inpatient setting. Hosp Pract (1995) 2016; 44: 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maynard G, Lee J, Phillips G, et al. Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm. J Hosp Med 2009; 4: 3–15. [DOI] [PubMed] [Google Scholar]
- 38.Murphy DM, Vercruysse RA, Bertucci TM, et al. Reducing hyperglycemia hospitalwide: the basal-bolus concept. Jt Comm J Qual Patient Saf 2009; 35: 216–223. [DOI] [PubMed] [Google Scholar]
- 39.Blagev DP, Hirshberg EL, Sward K, et al. The evolution of eProtocols that enable reproducible clinical research and care methods. J Clin Monit Comput 2012; 26: 305–317. [DOI] [PubMed] [Google Scholar]
- 40.Fort A, Narsinghani U, Bowyer F. Evaluating the safety and efficacy of glucommander, a computer-based insulin infusion method, in management of diabetic ketoacidosis in children, and comparing its clinical performance with manually titrated insulin infusion. J Pediatr Metal 2009; 22: 119–125. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Xie H, Jiang X, et al. Intelligent closed-loop insulin delivery systems for ICU patients Youqing. IEEE J Biomed Health Inform 2014; 18: 290–299. [DOI] [PubMed] [Google Scholar]
- 42.Cook CB, Mann LJ, King EC, et al. Management of insulin therapy in urban diabetes patients is facilitated by use of an intelligent dosing system. Diab Technol Ther 2004; 6: 326–335. [DOI] [PubMed] [Google Scholar]
- 43.Mougiakakou SG, Bartsocas CS, Bozas E, et al. SMARTDIAB: a communication and information technology approach for the intelligent monitoring, management and follow-up of type 1 diabetes patients. IEEE Trans Inf Technol Biomed 2010; 14: 622–633. [DOI] [PubMed] [Google Scholar]
- 44.Holl B, Spat S, Plank J, et al. Design of a mobile, safety critical in-patient glucose management system. In: Moen A, et al. (eds) User centred networked health care. Amsterdam: IOS Press, 2011; 169: 950–954. [PubMed] [Google Scholar]
- 45.Roemer LK, Borsato EP, Hulse NC, et al. Glycemic control through computerized subcutaneous insulin calculators. In: Saranto K, et al. (eds) Connecting health and humans. Vol 146. Amsterdam: IOS Press, 2009, pp. 473–477. [PubMed] [Google Scholar]
- 46.Spat S, Holl B, Petritsch G, et al. Automatic system testing of a decision support system for insulin dosing using google android. In: Blobel B, et al. (eds) Data and knowledge for medical decision support. Vol 186. Amsterdam: IOS Press, 2013, pp. 187–191. [PubMed] [Google Scholar]
- 47.Ateya MB, Aiyagari R, Moran C, et al. Insulin bolus calculator in a pediatric hospital. Appl Clin Inform 2017; 8: 529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011; 34: 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diabetes Care 2013; 36: 3430–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nirantharakumar K, Chen YF, Marshall T, et al. Clinical decision support systems in the care of in patients with diabetes in non-critical care setting: systematic review. Diabetes Med 2012; 29: 698–708. [DOI] [PubMed] [Google Scholar]
- 51.John SM, Waters KL, Jivani K. Evaluating the implementation of the EndoTool glycemic control software system. Diabetes Spectr 2018; 31: 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juneja R, Golas AA, Varroll J, et al. Safety and effectiveness of a computerized subcutaneous insulin program to treat inpatient hyperglycemia. J Diab Sci Technol 2008; 2: 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mader JK, Neubauer KM, Schaupp L, et al. Efficacy, usability and sequence of operations of a workflow-integrated algorithm for basal–bolus insulin therapy in hospitalized type 2 diabetes patients. Diabetes Obes Metab 2014; 16: 137–146. [DOI] [PubMed] [Google Scholar]
- 54.Toyoshima MT, De Souza ABC, Admoni SN, et al. New digital tool to facilitate subcutaneous insulin therapy orders: an inpatient insulin dose calculator. Diabetol Metab Syndr 2015; 21: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]


