Abstract
Background:
In real-world clinical practice, biologics in inflammatory bowel diseases (IBD) may be discontinued for a variety of reasons, including discontinuation initiated by gastroenterologists. The aims of the study are to report outcomes after discontinuation and predictors of prognosis after a minimum follow-up of 24 months; outcomes of gastroenterologist-initiated discontinuation with resulting direct cost implications on the health system were also studied.
Methods:
IBD patients who discontinued their first-use biologics between January 2013 and December 2016 were identified at our tertiary centre. Reasons for discontinuation and pre-defined adverse outcomes (AO) were recorded. Data were analysed using univariable and multivariable logistic regressions within a machine learning technique to predict AO. Gastroenterologist-initiated discontinuations were analysed separately, and Kaplan–Meier survival analysis performed; direct costs of AO due to discontinuation were assessed.
Results:
A total of 147 patients discontinued biologics (M = 74; median age 39 years; Crohn’s Disease = 110) with median follow-up of 40 months (range 24–60 months). In the total cohort, there were fewer AO among gastroenterologist-initiated discontinuations compared with patient-initiated; 54% (of the total group) had AO within 6 months. Among 59 gastroenterologist-initiated discontinuations, 23 (40%) had IBD-related AO within 6 months and 53 (90%) patients had AO by end of follow-up. Some 44 (75%) patients needed to restart biologics during follow-up, and direct costs due to AO and restart of biologics were high.
Conclusions:
The proportion of patients who have AO following discontinuation of biologics is high; clinicians need to carefully consider predictors of poor prognosis and high relapse rates when discussing discontinuation. The direct costs of managing AO probably offset theoretical economic gains, especially in the era where cost of biologics is reducing. Biologics should probably be continued without interruptions in most patients who have achieved remission for the duration these remain effective and safe.
Keywords: biologics discontinuation, biologics withdrawal, Crohn’s disease, direct costs, health economics, IBD, predictors of prognosis, ulcerative colitis
Background
Inflammatory bowel diseases (IBD) are increasing in incidence and prevalence, including in newly industrialised countries, and are now considered a global disease.1,2 The use of biological therapies for management of patients with IBD is part of guidelines from all societies, including the European Crohn’s and Colitis Organisation (ECCO) as well as the British Society of Gastroenterology, UK.3,4 The approval of drugs as well as guidance are based on pivotal trials,5–10 and have given clinicians multiple options to treat patients. However, real-world data suggest response rates as low as 30%, with a significant proportion needing to discontinue biologics due to a variety of adverse events;11,12 some patients choose to discontinue due to personal choice (e.g. pregnancy, patient-perceived risk of malignancy). Further, the 2019 updated guidance from the National Institute for Health and Care Excellence (NICE) in the UK says: ‘Biologics should only be started by clinicians with experience of their use in IBD and their clinical benefit should be reviewed regularly’.13 Review of biologic therapy for patients in remission is advised to be undertaken every 12 months,14 audited by local commissioning groups, and hence this practice is common in most hospitals managing IBD. NICE, however, does not give any recommendations about specific investigations prior to the decision.15 ECCO guidance on treatment withdrawal (exit strategies) has been published to help clinicians make rational cost-effective decisions while managing patients, but the recommendations are based on limited evidence.16 Most often in the UK, payers follow the NICE guidance.
It is known that biologics have been the main driver of expenditure of healthcare costs in IBD in the past decade,17 and such guidelines aim to rationalise therapy and ensure maintenance of economic feasibility, but also bring challenges to both clinicians and patients alike. In accordance with the NICE guidance clinicians often consider biological discontinuation based on absence of clinical symptoms, and we wished to understand the consequences of such practice in terms of costs and outcomes. There is a real and definite risk of relapse of disease, although current evidence shows considerable variation in the proportion of patients who relapse within a year of discontinuation. As per ECCO review on biologic therapy, the risk of relapse after anti-TNF withdrawal is between 30% and 40% at 1 year, and >50% beyond 2 years.16 This ranges from 30% to as high as 60% in some studies, depending on follow-up periods.18–20
Based on current evidence, patients who have ‘deep’ remission confirmed objectively prior to biologic withdrawal are more likely to remain in remission at 12 months of follow-up, and this is more often seen in Ulcerative Colitis (UC) than Crohn’s Disease (CD).21 To help aid the decision of withdrawal, some studies have attempted to identify risk factors for relapse after discontinuation of therapy18 but at present there are no controlled studies to confirm the best strategy and benefits of withdrawal.16
Aims
In clinical practice, patients with IBD have their biologic therapies withdrawn for a variety of reasons. The aims of the study were:
- to report adverse outcomes following discontinuation of biologics
- to identify predictors of prognosis
- to study direct cost implications on the health system following gastroenterologist-initiated discontinuation of first-use biologics.
Patients and methods
All IBD patients who discontinued their first biologic between January 2013 and December 2016 were identified from electronic medical records at University Hospitals Birmingham, UK, which is a tertiary referral centre. To capture adverse outcomes with sufficient follow-up period after discontinuation of therapy, we ensured at least 24 months follow-up for each patient, until end of December 2018, but most patients had a longer duration of follow-up.
Data on reasons for discontinuation of biologics were recorded; relevant clinical information was collected using the hospital biochemistry, radiology and endoscopy databases. A set of pre-defined adverse outcomes that included steroid and other rescue therapies, hospitalisations, and surgery including perianal were recorded. We also included patients who were classed as primary or secondary non-responders to their first biologic, if they had not been switched to another biologic for a period more than 12 weeks since discontinuation. For purposes of our study, primary non-response was defined as no improvement in clinical symptoms and/or biomarkers at 12 weeks since start of biologics, as recorded by the clinician; secondary loss of response was defined as relapse of clinical symptoms and/or worsening biomarkers after a period of remission on biologic therapy, as recorded by the clinician.
The outcomes of patients were recorded and analysed in two different contexts:
as an entire cohort (gastroenterologist-initiated + patient-initiated discontinuation) to investigate predictors of prognosis
as a sub-group who had gastroenterologist-initiated discontinuation only.
The gastroenterologist-initiated cohort was considered separately as this was the principal focus of the study. In this cohort, patients in clinical remission had the drug discontinued as per guidance by NICE, UK. We were interested in investigating the outcomes after discontinuation and also wanted to assess the direct costs after discontinuation of biologic; the burden of these outcomes on the healthcare system were recorded in the form of ‘episodes’, during the follow-up period. Each pre-defined adverse outcome (including any form of unscheduled patient contact with the hospital) was considered an episode which required some form of action to be taken by healthcare professionals to resolve it. Only those episodes which were related to IBD were considered after assessing the details available for each episode, and unrelated contacts with health care were not counted as an episode.
Statistical analysis
The data were analysed using multivariable and univariable logistic regressions within a machine learning technique in order to predict adverse outcomes, within the stated timeframe by means of R [R Core Team (2019)] package CARRoT Alina Bazarova and Marko Raseta, 2019.22 The latter combines principles of good practice from machine learning, such as cross-validation23 and those in medical statistics, such as best subset regression24 restricted by the rule of ten events per variable (‘one in ten rule’).25 CARRoT has been previously successfully used for outcome prediction in the clinical outcomes.26,27 Results of this analysis were used as a guide to stratify patients in the survival analysis. The outcomes stricturing disease, surgery and hospitalisation were merged together and analysed separately by means of univariable regression. We tested the significance of the identified predictors and performed analysis for confounders using bivariate regression for those predictors that were significant. We performed Kaplan–Meier28 and Cox proportional hazards29 survival analysis to compare patients with gastroenterologist-initiated versus patient-initiated discontinuation of biologics. The patients who had their biologics electively discontinued were analysed again as a separate group to detect any differences in their outcomes. Survival analysis and likelihood-ratio test to assess the separation between survival curves were performed by R package survival and visualised via the package R survminer.30
Ethical considerations
This study was registered with the hospital research governance and ethics committee and was given ethical clearance in April 2019 (Reference number CARMS-15164).
Results
Overall discontinuations (Gastroenterologist-initiated + patient-initiated discontinuation of biologics)
A total of 147 patients who discontinued biologics (M = 74, median age 39 years; CD = 110) were identified. Follow-up ranged from 24 to 60 months (median 40 months). The details of demographics and patient characteristics are given in Table 1.
Table 1.
Patient demographics characteristics n = 147.
| Age and gender | Total group n = 147 | |
|---|---|---|
| Median age | 39 years (range 21–82 years) | |
| Gender | Male = 74; Female = 73 | |
| Follow-up duration | ||
| 24 months | 147 (100%) | |
| 24 months | 80 (54.5%) | |
| Disease type | Ulcerative colitis | Crohn’s disease |
| Number of patients | 37 (25.2%) | 110 (74.8%) |
| Classification | NA | |
| Ulcerative colitis extent | E1 = 1 | |
| E2 = 9 | ||
| E3 = 18 | ||
| Unknown = 9 | ||
| Montreal classification for CD | NA | |
| Age | 37 | |
| A1 | 70 | |
| A2 | 0 | |
| A3 | 3 | |
| Unknown | ||
| Location | NA | |
| L1 | 24 | |
| L2 | 34 | |
| L3 | 39 | |
| L4 | 12 | |
| Unknown | 5 | |
| Behaviour | NA | |
| B1 | 3 | |
| B2 | 26 | |
| B3 | 17 | |
| Perianal | 23 | |
| Unknown | 41 | |
| Race | ||
| Caucasian | 20 (54%) | 83 (74.5%) |
| Asian | 11 (29.7%) | 12 (10.9%) |
| Afro-Caribbean | 2 (5.3%) | 2 (1.8%13/110) |
| Unknown/Unreported | 4 (11%) | 13 (11.8%) |
Fifty-nine (40%) patients had their therapy discontinued by their gastroenterologist, recorded as a joint decision by the treating clinician and patient. This decision was based on assessment of clinical remission using global assessment and blood markers of activity of disease [C-reactive protein (CRP), full blood counts]. In our cohort we noted that endoscopy, cross-sectional imaging or histology were used at the discretion of the clinician but not routinely used before discontinuation of the drug. Details of definitions used for each reason behind discontinuation as well as to definitions used for adverse outcomes are as given in Table 2.
Table 2.
Criteria/study definitions used for reasons for discontinuation and adverse outcomes in the study.
| Reason for discontinuation | Criteria used to define reason in this study | Proportion of patients |
|---|---|---|
| Primary non-response | • No improvement in disease status despite at least 12 weeks of therapy + • Delay of >12 weeks to start next biologic |
10% |
| Secondary non-response | • Worsening disease status while on biologic therapy + • Delay of >12 weeks to start next biologic |
12% |
| Immunogenicity | • Documented proven antibodies to biologic with loss of response | 2% |
| Adverse effects to biologics | • No other specific reason mentioned by clinician | 14% |
| Elective discontinuation | • Biologic discontinuation as planned by clinician if patient had been in clinical remission for >12 months | 40% |
| Pregnancy | • Patient choice to discontinue biologic when pregnancy confirmed (at any trimester) | 2.5% |
| Patient choice | • Patient choice to discontinue biologic (personal choice) | 6.8% |
| Patient non-compliance/Non-attendance | • Patient non-attendance for infusions on >3 consecutive sessions | 2.0% |
| Other | Reasons not falling into any of the above | 10.7% |
| Adverse outcomes | Criteria used to define adverse outcomes in this study | |
| Flare-up of disease | • Flare-up as recorded by clinician | |
| Steroid therapy | • Patient started on either topical +/– oral OR intravenous steroids | |
| Hospitalisation | • Treated as inpatient for IBD-related complications | |
| Rescue therapy with biologics | • Reintroduction of biologic to manage worsening disease activity | |
| Stricturing disease | • Disease progression to new or recurring stricture | |
| Fistulising disease | • Disease progression with new or worsening fistulating disease | |
| Perianal disease | • Disease progression with new or worsening perianal disease | |
| Surgery | • Surgical procedure indicated for IBD-related complications (included EUA + seton placements, perianal abscess drainage, defunctioning loop ileostomy formation, ileo-caecal resection, sub-total colectomy with ileostomy in our cohort) |
EUA, examination under anaesthesia; IBD, inflammatory bowel disease
Gastroenterologist-initiated versus patient-initiated discontinuation of biologics
Among patients who had their biologics discontinued, 60% of discontinuations were patient-initiated. The most common reason in this group of patients was side effects (not otherwise specified). Details of other reasons definitions used and proportion of patients are given in Table 2. Using data from all 147 patients, multivariable logistic regression analysis was performed to identify significant predictors of poor and favourable outcomes. These are presented in Table 3. A separate analysis done for patients with CD only has been included in Supplemental Material Table 8.
Table 3.
Predictors of outcomes in entire cohort.
| Predictors of poor outcomes | Time interval when significant | AUROC | Odds ratio (OR) and confidence interval (CI) | Statistical significance |
|---|---|---|---|---|
| Asian race | • <6 months | • 0.6283 | • OR 0.68 95% CI (0.49, 0.95) | • p = 0.02 |
| Secondary non-response | • <6 months • 6–12 months • >24 months |
• 0.6110 • 0.6715 • 0.6412 |
• OR 4.46 95% CI (1.37, 13.7) • OR 7.09 95% CI (1.60, 29.97) • OR 1.82 95% CI (1.98, 19.03) |
• p = 0.01 • p = 0.01 • p = 0.001 |
| Steroid therapy | • 12–24 months | • 0.6219 | • OR 0.24 95% CI (0.04, 0.88) | • p = 0.06 |
| Predictors of good outcomes | ||||
| Male sex | • >24 months | • 0.638 | • OR 0.31 95% CI (0.09, 0.85) | • p = 0.03 |
| Elective stop | • <6 months | • 0.6398 | • OR 0.24 95% CI (0.05, 0.77) | • p = 0.03 |
| Other predictors | ||||
| Sex | • <6 months • 6–12months • 12–24 months |
• 0.5131 • 0.5314 • 0.5147 |
• OR 1.11 95% CI (0.42, 2.97) • OR 0.78 95% CI (0.19, 3.06) • None of the women experienced adverse outcomes |
• p = 0.83 • p = 0.72 |
| Race | • 6–12 months • 12–24 months • >24 months |
• 0.5861 • 0.5052 • 0.5201 |
• OR 1.51 95% CI (0.87, 2.84) • OR 1.05 95% CI (0.73, 1.58) • OR 0.97 95% CI (0.69, 1.4) |
• p = 0.18 • p = 0.79 • p = 0.84 |
| Rectal 5-ASA at baseline | • <6 months • 6–12 months • 12–24 months • >24 months |
• 0.5664 • 0.5616 • 0.5321 • 0.5664 |
• None of the patients who were on this treatment • experienced adverse outcomes • OR 0.45 95% CI (0.02, 2.42) • None of the patients who were on this treatment experienced adverse outcomes |
• NA • NA • p = 0.45 • NA |
| Thiopurine at baseline | • <6 months • 6–12 months • 12–24 months • >24 months |
• 0.5611 • 0.6365 • 0.5081 • 0.5296 |
• OR 1.66 95% CI (0.62, 4.41) • OR 0.2 95% CI (0.01, 1.14) • OR 0.93 95% CI (0.3, 2.61) • OR 0.77 95% CI (0.26, 2.09) |
• p = 0.31 • p = 0.13 • p = 0.9 • p = 0.62 |
| Steroid therapy | • < 6months • 6–12months • >24 months |
• 0.5101 • 0.5000 • 0.5403 |
• OR 0.91 95% CI (0.30, 2.48) • OR 1 95% CI (0.2, 3.97) • OR 0.68 95% CI (0.21, 1.91) |
• p = 0.86 • p = 1.0 • p = 0.49 |
| Endoscopic activity | • <6 months • 6–12 months • 12–24 months • >24 months |
• 0.5047 • 0.5134 • 0.5344 • 0.5260 |
• OR 0.99 95% CI (0.73, 1.41) • OR 0.94 95% CI (0.62, 1.55) • OR 1.06 95% CI (0.76, 1.56) • OR 1.05 95% CI (0.76, 1.54) |
• p = 0.96 • p = 0.79 • p = 0.76 • p = 0.77 |
| First biologic | • <6 months • 6–12 months • 12–24 months • >24 months |
• 0.5192 • 0.6297 • 0.6265 • 0.5055 |
• OR 1.56 95% CI (0.85, 2.42) • OR 1.5 95% CI (0.68, 2.88) • OR 1.57 95% CI (0.89, 2.66) • OR 0.85 95% CI (0.4, 1.56) |
• p = 0.15 • p = 0.25 • p = 0.1 • p = 0.64 |
| Primary non-response | • <6 months • 6–12 months • 12–24 months • >24 months |
• 0.5623 • 0.5048 • 0.5244 • 0.5284 |
• OR 2.84 95% CI (0.72, 9.54) • OR 1.11 95% CI (0.06, 6.71) • OR 0.52 95% CI (0.03, 2.86) • OR 0.45 95% CI (0.02, 2.47) |
• p = 0.11 • p = 0.93 • p = 0.54 • p = 0.46 |
| Secondary non-response | • 12–24 months | • 0.5638 | • OR 2.55 95% CI (0.65, 8.42) | • p = 0.14 |
| Side effects | • <6 months • 6–12 months • 12–24 months • >24 months |
• 0.5216 • 0.5761 • 0.5475 • 0.5518 |
• OR 0.67 95% CI (0.10, 2.62) None of the patients who had side effects experienced adverse outcomes • OR 0.34 95% CI (0.02, 1.84) • OR 0.3 95% CI (0.02, 1.59) |
• p = 0.62 • NA • p = 0.31 • p = 0.25 |
| Elective stop | • 12–24 months • >24 months |
• 0.5606 • 0.5794 |
• OR 0.59 95% CI (0.18, 1.68) • OR 0.49 95% CI (0.15, 1.36) |
• p = 0.34 • p = 0.19 |
| Rectal 5-ASA following discontinuation | • <6 months • 6–12 months • 12–24 months • >24 months |
• 0.5127 • 0.5399 • 0.5090 • 0.5430 |
• OR 0.66 95% CI (0.03, 3.74) None of the patients who were on this treatment experienced adverse outcomes • OR 0.75 95% CI (0.04, 4.32) None of the patients who were on this treatment experienced adverse outcomes |
• p = 0.70 • NA • p = 0.79 • NA |
| Thiopurine following discontinuation | • <6 months • 6–12 months • 12–24 months • >24 months |
• 0.5621 • 0.6667 • 0.5439 • 0.5286 |
• OR 1.72 95% CI (0.62, 4.60) None of the patients who were on this treatment experienced adverse outcomes in this time frame • OR 0.64 95% CI (0.17, 1.95) • OR 0.76 95% CI (0.23, 2.13) |
• p = 0.28 • NA • p = 0.47 • p = 0.62 |
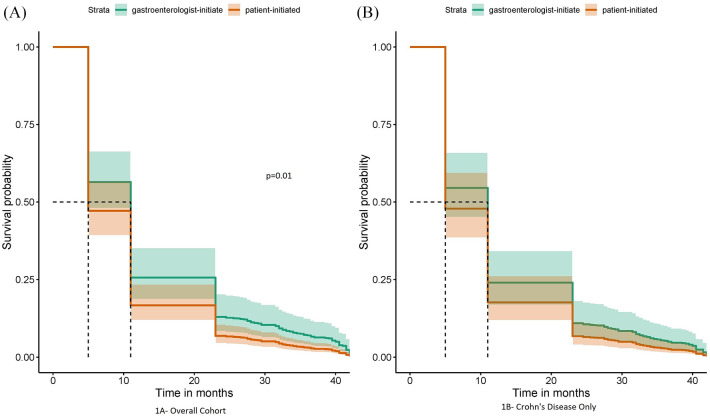
In this overall cohort, gastroenterologist-initiated discontinuation resulted in fewer IBD-related pre-defined adverse outcomes compared with patient-initiated (p = 0.01). Figure 1 shows Cox Proportional Hazards curves comparing the two cohorts.
Figure 1.
Survival without pre-defined adverse outcomes (gastroenterologist-initiated versus patient-initiated discontinuation); (A) overall cohort and (B) for Crohn’s disease only.
There is good separation between the two groups initially; it was noted that a significant proportion of patients had adverse outcomes within 6 months of biologic discontinuation in both groups and the curves merge at about 40 months, which was the median duration of follow-up in this study.
In the total cohort, significant number of patients (n = 96, 65%) needed biologics to be restarted by the end of the study follow-up period.
Gastroenterologist-initiated discontinuation of first-use biologic
Of the total cohort, 59 patients had their therapy discontinued electively; median duration of therapy prior to discontinuation was 24 months (range 1 month–96 months); median CRP at point of discontinuation was 1 mg/L (IQR 5 mg/L) and median haemoglobin at discontinuation was 138 g/L (range 111–169 g/L) This group was analysed separately to better understand outcomes. The decision to discontinue biologics was made by the treating clinician after discussion with the patient, keeping in line with guidance provided by NICE, UK. Further clinical details were collected for these 59 patients, which are as given in Table 4.
Table 4.
Details of patients with gastroenterologist-initiated discontinuation of biologics (n = 59).
| Type of first-use biologic | 59/59 |
|---|---|
| Adalimumab | 32 (54%) |
| Infliximab | 27 (46%) |
| Disease classification | |
| Ulcerative Colitis | 9 (15%) |
| Crohn’s Disease | 50 (85%) |
| Montreal classification for Crohn’s Disease | |
| Age | |
| A1 | 18 |
| A2 | 27 |
| A3 | 5 |
| Unknown | 0 |
| Location | |
| L1 | 10 |
| L2 | 17 |
| L3 | 22 |
| L4 | 7 |
| Unknown | 0 |
| Behaviour | |
| B1 | 1 |
| B2 | 14 |
| B3 | 8 |
| Perianal | 8 |
| Unknown | 29 |
| Ulcerative colitis extent | |
| E1 | 0 |
| E2 | 2 |
| E3 | 7 |
| Duration of biologic therapy prior to stop | 59/59 |
| <6 months | 9 (15%) |
| 6–12 months | 6 (10%) |
| 13–24 months | 16 (27%) |
| 25–36 months | 11 (19%) |
| >36 months | 17 (29%) |
| Median C-reactive protein at discontinuation | 1 mg/L (IQR 5 mg/L) |
| Median haemoglobin at discontinuation | 138 g/L (IQR 111–169) |
| Thiopurine continued after biologic stop | 59/59 |
| Yes | 26 (44%) |
| No | 33 (56%) |
| Patients needing restart of biologic therapy (44/59) | 44/59 |
| <6 months | 9 (20%) |
| 6–12 months | 13 (30%) |
| 13–24 months | 10 (23%) |
| >24 months | 12 (27%) |
Among these patients, all had been on anti-TNFs; 44 (75%) had received therapy for more than 12 months with no differences noted between infliximab and adalimumab and are therefore considered together.
Some 26 (44%) patients continued thiopurines after biologic stoppage. Patients not on thiopurines had a trend towards lower probability of adverse outcomes during the follow-up period; however, the difference between two groups of patients was not statistically significant (p = 0.32, likelihood ratio = 0.98) (Figure 2).
Figure 2.
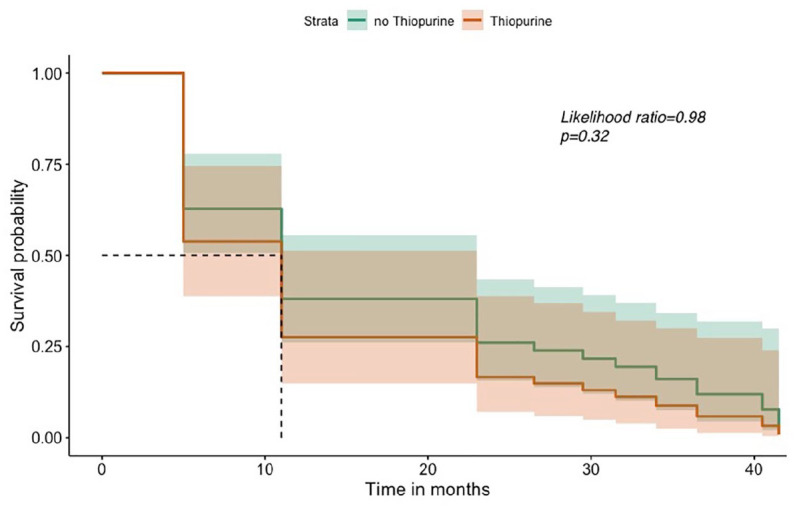
Survival without any pre-defined adverse outcomes in thiopurine versus no thiopurine.
In this sub-group, 23 (40%) patients had IBD-related adverse outcomes within 6 months after stoppage and 53 (90%) patients had at least one adverse outcome recorded by the end of follow-up. It was also noted that 44 (75%) patients needed to restart biologics at some point during the follow-up period of this study. The proportion of patients without any adverse outcomes after gastroenterologist-initiated discontinuation is demonstrated in using a Kaplan–Meier survival curve (Figure 3). More than 50% of patients had to restart biological drugs within 2 years of discontinuation.
Figure 3.
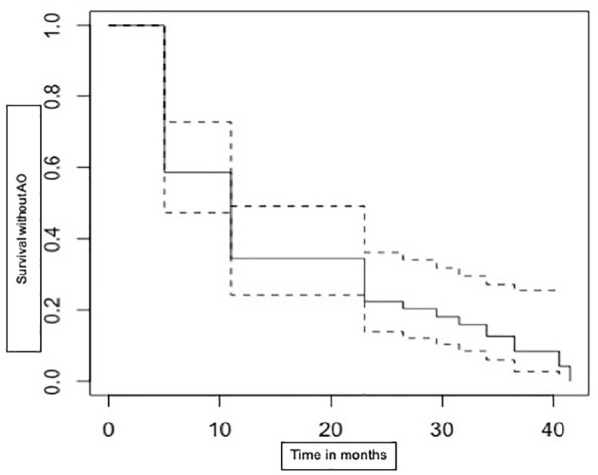
Survival without any pre-defined adverse outcomes among gastroenterologist-initiated discontinuations.
AO, adverse outcomes.
Episodes of contact with healthcare team
In this cohort of gastroenterologist-initiated discontinuations, we also studied the number of IBD-related unscheduled contacts (triggered by adverse outcomes) these patients had with the healthcare team following gastroenterologist-initiated discontinuation of biologics. Each contact was termed as an ‘episode’ (included telephone consults + face-to-face reviews + hospitalisations) and data were collected for pre-specified time frames. Each patient could have had more than one episode during the follow-up period, and this was collected as cumulative data. The pre-defined adverse outcomes were flare-up, corticosteroid prescription, hospitalisation, rescue therapy (using urgent biologic therapy as inpatient or urgent ambulatory care) and surgery (including procedures for perianal disease). Only those episodes which were related to IBD were considered after assessing the details available for each episode, and unrelated contacts with health care were not counted as an episode.
Consequently, each patient could have had multiple episodes over the follow-up period. These data were collected in a cumulative fashion to assess the economic burden on the healthcare system as a result of these multiple episodes. The costing was calculated using data from ‘unit pricing’ at a national level, and service and drug costs at our centre.
For purposes of the study and to calculate financial burden, we made the following assumptions:
- all episodes which followed discontinuation of biologics were a consequence of the discontinuation itself
- all of the episodes could have been avoided had the biologic been continued.
While these are assumptions, these allow calculations that are likely to be a close reflection of the real-world experience of most teams caring for patients with IBD.
The breakdown of the number of episodes at pre-specified time points and the cumulative total is given in Table 5. In our cohort, it was noted that an episode of contact recorded due to a flare-up was the commonest, followed by rescue therapy with biologics. The number of episodes varied with time points, but was noted to increase beyond 24 months of follow-up. Similar detailed follow-up data for episodes of contact with the healthcare team for the physician-initiated discontinuations were not collected, as this was not the focus of our study.
Table 5.
Details of episodes at pre-specified time points and cumulative total.
| Adverse outcomes (‘Episodes’) |
<6 months | 6–12 months | 12–24 months | >24 months | Total for each adverse outcome |
|---|---|---|---|---|---|
| Flare-up | 20 | 18 | 20 | 24 | 82 |
| Corticosteroid therapy | 6 | 5 | 5 | 5 | 21 |
| Rescue therapy (using biologics) |
10 | 14 | 14 | 20 | 58 |
| Hospitalisation | 1 | 0 | 1 | 5 | 7 |
| Progress of disease (Stricturing, penetrating or perianal complication) | 3 | 3 | 5 | 5 | 16 |
| Surgery | 2 | 0 | 3 | 3 | 8 |
| TOTAL EPISODES as per time frame | 42 | 40 | 48 | 62 |
The estimated economic burden of episodes following discontinuation of biologics was calculated using the National Health Service’s UK unit-price costs for health and social care list for 2017.31 A similar approach has been reported by previous studies that have investigated the economic aspects.32
A pragmatic approach was used to calculate the direct costs. In our centre, flare-up of disease generally triggers a telephone call by the patient to the dedicated IBD helpline for advice. The unit cost is calculated using the costing for a telephone clinic. The total cumulative cost for all gastroenterologist-initiated discontinuations by type of episode is given in Table 6.
Table 6.
Details of estimated immediate direct costs following gastroenterologist-initiated discontinuation.
| Episode | Services utilised | Unit price in GBP (£) | Total number of episodes | Total cumulative cost |
|---|---|---|---|---|
| Flare-up | Telephone call/clinic | 25 | 82 | 2050 |
| Corticosteroid therapy | Prescription | 9 | 21 | 189 |
| Outpatient attendance | 137 | 11 | 1507 | |
| Rescue therapy using biologics | Outpatient attendance | 137 | 58 | 7946 |
| Multi-disciplinary team meeting | 243 | 58 | 14,094 | |
| Cost of drug/s | ||||
| Infliximab* | 1200 | 66 infusions (11 pts) | 79,200 | |
| Adalimumab** | 140 | 754 injections (29 pts) | 105,560 | |
| Ustekinumab*** | 2150 | 24 injections (4 pts) | 51,600 | |
| Day-case unit for infusion (only for IV drugs) | 300 | 66 | 19,800 | |
| Hospitalisation | Inpatient stay | 727 | 7 | 5089 |
| Surgery including hospital stay | 3900 | 8 | 31,200 | |
| GRAND TOTAL | 266,635 |
Calculated for 12 months at 8 weekly infusions (presumed avg dose of 300 mg/dose).
Calculated for 12 months at fortnightly injections (presumed avg of 40 mg/dose).
Calculated for 12 months at 8 weekly injections (avg dose of 90 mg; induction dose excluded).
Discussion
Biologics are routinely used in IBD. With a large number of IBD patients now on biologics, they account for a major proportion of costs incurred. Hence the expenditure in health systems, for management of IBD is now driven by medical therapies which are expensive.33 As a result, healthcare agencies such as NICE in the UK provide guidance to clinicians to rationalise therapies.15 One of the ways this is done is by reviewing need for biologic therapy every 12 months and encouraging gastroenterologist-initiated discontinuation in patients who have achieved disease remission. No specific standards to define remission are recommended. In practice, this enables clinicians to suggest discontinuation of biologic based on clinical symptoms. In this study, we included only the first biologic used in a patient and therefore there was no experience of previous discontinuation. While this practice is encouraged keeping economic feasibility in mind, it is debatable whether the strategy does in fact pay dividends. Although expensive, it has been shown that among patients who respond to biologics, a significant proportion remain in sustained remission for up to 5 years,34,35 with a reduction in need for surgery and hospitalisation,36 and over years the direct expenditure on the health system reduces.37 Despite the high perceived costs it could be argued that these drugs bring net benefits and should be continued without forced elective interruptions. The rates of relapse and resulting need for high-intensity care in patients who have had their drugs discontinued probably offset the costs saved by gastroenterologist-initiated discontinuation. This is especially true given the changing landscape, with the introduction of biosimilar drugs driving down the cost of medications.
Apart from gastroenterologist-initiated discontinuation, there is a proportion of patients in whom treatment has to be discontinued for reasons that have been discussed before. In patients with primary non-response, secondary loss of response or drug intolerance, the treatment is usually switched to another approved agent. However, despite the best efforts of most teams managing patients with IBD, it is seldom possible to ensure that the switch is done in a timely manner. There could be multiple reasons that lead to a delay, for example, delays due to patient-related factors, delays in the system, lack of therapeutic options on offer and many more. Regardless of reasons for the delay, it could be argued that delays have the same detrimental effect as discontinuations, invariably leading to a higher rate of complications. Also, discontinuations for other reasons are generally unplanned events, as a result of which a switch to another agent can be delayed due to the healthcare set-up. This is far from ideal, but unfortunately a reality in practice.
Predictors of poor and favourable outcomes after discontinuation
The outcomes after discontinuation have been reported by other studies previously, most of which have been assessed in patients who have objective confirmation of remission prior to discontinuation. As per a systematic review by Torres et al., the rates of relapse after discontinuation range from 20% to as high as 80%, particularly in some high-risk patients.38
In our total cohort, a larger proportion of patients had patient-initiated discontinuation of therapy. The predictors of poor outcomes were Asian race, secondary loss of response (at any time point), and need for steroid therapy at 12 months or more after discontinuation, all of which reached statistical significance (Table 3). The category of poor outcomes due to secondary loss of response is perhaps not unexpected, and could represent refractory or aggressive disease. This highlights the group of patients who need closer monitoring and a more proactive management plan. This is also true of patients requiring steroid therapy, and such patients should be considered for a swift change to a different biologic or other interventions as appropriate.
The analysis also showed male sex and gastroenterologist-initiated discontinuation as predictors of good outcomes, reaching statistical significance. Although patients who have gastroenterologist-initiated discontinuation have been shown to have slightly better outcomes,39 male patients doing better after discontinuation has so far not been reported commonly. Male sex was strongly associated with absence of relapse (good prognostic factor) in our cohort both when analysed together for disease type (UC + CD) and separately for CD (as they constituted the majority). Though the numbers in our study are limited, this is an interesting finding and underlying reasons need to be explored further.
It is possible that gastroenterologist-initiated discontinuations do better mainly because the patients are more likely to have achieved clinical remission and are also more likely to have biomarker assessments undertaken prior to withdrawal of therapy.
There have been reports on different aspects of discontinuation (including patients at risk of discontinuation itself), and several investigators have attempted to recognise predictors of outcomes. The STORI study stratified patients and identified those at low risk of relapse using predictive factors.40 A recent study published in 2019 reported that female sex was associated with a higher risk of discontinuation of anti-TNF therapy in IBD due to higher rates of drug intolerance.41 In the same study, other factors such as greater age at start of anti-TNF therapy and dose escalation were found to be associated with discontinuation.41 Similar findings were reported by another study where a greater age of patient was associated with higher risk of discontinuation and treatment failure.42 Our cohort identified some new risk factors which predict poor outcomes after discontinuation of biologics which could guide decision making.
Gastroenterologist-initiated discontinuation of anti-TNF therapy
This group of gastroenterologist-initiated discontinuations was the focus of further analysis in our study to explore the true benefits of discontinuation to patients as well as the health economic consequences.
In our centre, patients were assessed for clinical remission based on symptoms and serum biomarkers prior to discontinuation of therapy. Mucosal assessment by way of endoscopy was not always undertaken and faecal calprotectin was available only after 2017, hence not used in this cohort. Among the gastroenterologist-initiated discontinuations in our cohort we made some interesting observations. Patients who continued to remain on thiopurine therapy after withdrawal of biologic had more adverse outcomes recorded during follow-up period (Figure 2). This is contrary to most reports which show thiopurines to have a protective effect, particularly in CD.39
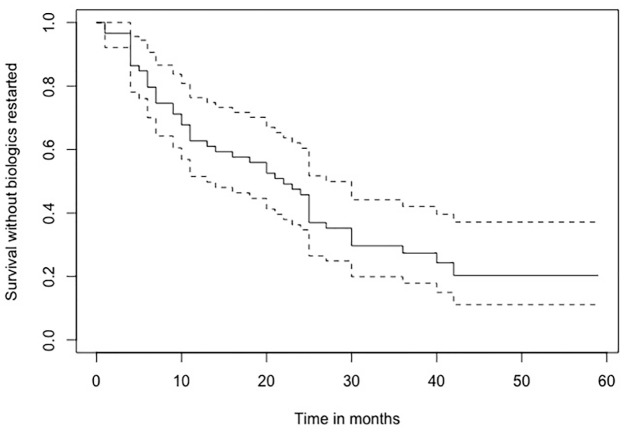
The cause for concern is the large number of patients who have adverse outcomes within a very short duration after discontinuation of therapy. About 40% of patients had at least one or more adverse outcome within 6 months of discontinuation and this number steadily increased with time, with nearly 90% of patients having at least one adverse outcome at the end of follow-up period (Figure 3). Also, a very high proportion of patients (71%) required restart of biologics during the follow-up period to manage adverse outcomes (Figure 4). The relapse rate in our cohort was much higher than reports in most studies, including a systematic review by Gisbert et al.43
Figure 4.
Survival without biologic restart among gastroenterologist-initiated discontinuations.
It is unclear whether these patients had relapsed due to underlying disease activity which was asymptomatic and not detected on serum biomarkers. It is now well established that patients who achieve mucosal healing are more likely to remain in remission for longer durations as well as have lower rates of hospitalisation and surgery;44–46 it is also recommended that treatment should be withdrawn only after objective confirmation of mucosal healing.16 The significantly high number of patients with adverse outcomes in this cohort emphasises the importance of objective confirmation of mucosal healing in order to reduce rates of relapse.
Cost of treatment withdrawal/discontinuation
The number of episodes where patients came in contact with the healthcare team following gastroenterologist-initiated discontinuation brings cost implications. A calculation of cumulative direct costs for all patients showed a high expenditure in managing these episodes. We used the Unit costs of health and social care document for our cost calculations.31 These episodes also increase time pressures on teams as they consume valuable resources (Table 6). The indirect costs due to patient morbidity are probably higher, but this was not explored as it was outside the scope of this study. A very high proportion of patients also needed to restart biologics to manage relapses. Any theoretical economic benefits gained by discontinuation of therapy are offset by costs incurred in managing the adverse outcomes. These episodes and resulting costs probably outweigh the theoretical benefits of discontinuation. This is especially more relevant considering the introduction of biosimilars which are available at much lower prices, as well as the significant reduction in the cost of the originator drugs due to discounted pricing. The annual cost of maintaining patients on these drugs is now significantly less. The lower cost has to be capitalised on in the form of maintaining therapies to maintain remission for as long as the drug remains effective. The guidelines by health agencies to review use of biologics annually were partially driven by the high cost. However, since the approval of biosimilar agents for both infliximab and adalimumab, the health services are projected to make large savings.47 The end of patent and the introduction of biosimilars have resulted in pharmaceutical companies offering heavy discounts of up to 70% on originator drugs, resulting in further reduction of costs.48 Biosimilars have been reported to be as safe and effective as originators.49 Hence, there has been a drastic overall reduction in the annual cost per patient in managing diseases without any compromise in efficacy. This brings into question whether treatment withdrawal in patients who have achieved remission is a sound strategy. ECCO guidelines do not recommend treatment withdrawal in high-risk patients (perianal disease, penetrating disease), but given the lowering cost and comparable safety profile of anti-TNF monotherapy to thiopurines, this should probably be extended to most patients who have been successfully and safely maintained in remission.
Our study has several limitations. As this was a retrospective study, there were some missing data, particularly data such as smoking status and faecal calprotectin levels. Another limitation of our cohort, which probably reflects real-world practice at the time, is that even when biologics were discontinued electively, the decision was not always after confirming endoscopic or histological remission. This was more often based on clinical remission as identified by the clinician together with blood and serum biomarkers. This approach is unlikely to have been a reliable representation of true disease activity, but pressure on endoscopy services made ready access often challenging. For the patient-initiated discontinuations, lab results were not consistently recorded at time of discontinuation. There are various reasons for this including that some were unplanned events and some had tests in primary care but results inaccessible to us. About 22% of patients faced significant delays in switching to alternate biologic therapies after primary non-response or secondary loss of response was identified, due to various reasons, and this delay probably had an effect on frequency of adverse outcomes. Therapeutic drug-level monitoring was not available for routine care at the time of the study.
To assess the economic aspects the calculations of cost were based on a pragmatic approach where we assumed that a particular episode of adverse outcome would generally trigger certain services in the health system. It is quite possible that the estimates could be an underestimate, as it was difficult to track how each episode was resolved. Another limitation is the challenge in assessing direct costs of progression of disease or its behaviour. When patients have penetrating or perianal complications multiple services are inevitably utilised, but it may not always be possible to capture this in retrospective studies.
Conclusion
The discontinuation of biologics for a variety of reasons is a common clinical scenario in real-world practice. In our cohort, among patients who discontinued biologics, nearly 54% had at least one adverse outcome within 6 months of discontinuation. Our results show that there were fewer adverse outcomes when discontinuation was elective, even when disease assessment prior to discontinuation was based on clinical symptoms and serum biomarkers alone. However, despite gastroenterologist-initiated (planned) discontinuation, a large proportion of patients had adverse outcomes rapidly and about 75% required restart of biologics. These adverse outcomes resulted in an increased number of episodes of contact with the healthcare team, thereby resulting in added expenditure in managing them.
Clinicians need to be cautious when considering biologic discontinuation (including gastroenterologist-initiated) given the high proportion of patients who relapse and need re-escalation to biologics. This should be discussed with patients when considering discontinuation of biologics. It can be argued that the direct costs of increased frequency of contacts with the healthcare system offset the economic gains made by drug withdrawal. This is especially true considering the reducing costs of drugs and increasing use of biosimilars. Clinicians and health agencies should take cognisance of the changing economic equations in making decisions and policies.
Supplemental Material
Supplemental material, sj-pdf-1-tag-10.1177_1756284820981216 for Clinical outcomes, predictors of prognosis and health economics consequences in IBD patients after discontinuation of the first biological therapy by Uday N. Shivaji, Alina Bazarova, Tamsin Critchlow, Samuel C. L. Smith, Olga Maria Nardone, Melanie Love, Joanne Davis, Subrata Ghosh and Marietta Iacucci in Therapeutic Advances in Gastroenterology
Supplemental material, sj-pdf-2-tag-10.1177_1756284820981216 for Clinical outcomes, predictors of prognosis and health economics consequences in IBD patients after discontinuation of the first biological therapy by Uday N. Shivaji, Alina Bazarova, Tamsin Critchlow, Samuel C. L. Smith, Olga Maria Nardone, Melanie Love, Joanne Davis, Subrata Ghosh and Marietta Iacucci in Therapeutic Advances in Gastroenterology
Footnotes
Authors’ note: Alina Bazarova is now affiliated with Centre for Computational Biology, University of Birmingham Institute for Biological Physics, University of Cologne, Cologne, Germany.
Author contribution: Shivaji, UN – literature search, data collection, data analysis, concept development, writing and editing manuscript, revision and approval
Bazarova, A – statistical analysis, writing sections of manuscript, revision and approval
Critchlow, T – data collection, data analysis, writing and editing manuscript, revision and approval
Smith, SCL – writing sections of the manuscript, revision and final approval
Nardone, OM – data input, analysis, revision and approval of manuscript
Love, M – management of patient database, patient data selection, revision and approval
Davis, J – management of patient database, revision and approval
Ghosh, S – plan of study, critical review of manuscript, editing, revision, overall supervision and final approval
Iacucci, M – revision, critical review of manuscript, final approval
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This paper presents independent research funded by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
ORCID iDs: Uday N. Shivaji  https://orcid.org/0000-0002-6800-584X
https://orcid.org/0000-0002-6800-584X
Samuel C. L. Smith  https://orcid.org/0000-0001-8351-1081
https://orcid.org/0000-0001-8351-1081
Olga Maria Nardone  https://orcid.org/0000-0002-9554-4785
https://orcid.org/0000-0002-9554-4785
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Uday N. Shivaji, National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, Birmingham, UK Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK; University Hospitals Birmingham, UK.
Alina Bazarova, Institute of Translational Medicine, Birmingham, UK.
Tamsin Critchlow, University Hospitals Birmingham, Birmingham, UK.
Samuel C. L. Smith, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK University Hospitals Birmingham, Birmingham, UK; Institute of Translational Medicine, Birmingham, UK.
Olga Maria Nardone, Institute of Immunology and Immunotherapy, University of Birmingham, UK; University Hospitals Birmingham, Birmingham, UK.
Melanie Love, University Hospitals Birmingham, Birmingham, UK.
Joanne Davis, University Hospitals Birmingham, Birmingham, UK.
Subrata Ghosh, Professor of Medicine and Gastroenterology, Director, Institute of Translational Medicine, University of Birmingham, Edgbaston, Birmingham, B15 2TH, UK; National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, Birmingham, UK; Institute of Immunology and Immunotherapy, University of Birmingham (UK) University Hospitals Birmingham, UK.
Marietta Iacucci, National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, Birmingham, UK; Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, UK; University Hospitals Birmingham, Birmingham, UK; Institute of Translational Medicine, Birmingham, UK.
References
- 1. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018; 390: 2769–2778. [DOI] [PubMed] [Google Scholar]
- 2. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015; 12: 720–727. [DOI] [PubMed] [Google Scholar]
- 3. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis 2020; 14: 4–22. [DOI] [PubMed] [Google Scholar]
- 4. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019; 68(Suppl. 3): s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462–2476. [DOI] [PubMed] [Google Scholar]
- 6. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012; 142: 257–265.e1–e3. [DOI] [PubMed] [Google Scholar]
- 7. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- 8. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014; 146: 85–95; quiz e14–e15. [DOI] [PubMed] [Google Scholar]
- 9. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019; 381: 1201–1214. [DOI] [PubMed] [Google Scholar]
- 10. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376: 1723–1736. [DOI] [PubMed] [Google Scholar]
- 11. Lehtola E, Haapamaki J, Farkkila MA. Outcome of inflammatory bowel disease patients treated with TNF-alpha inhibitors: two-year follow-up. Scand J Gastroenterol 2016; 51: 1476–1481. [DOI] [PubMed] [Google Scholar]
- 12. Juillerat P, Sokol H, Froehlich F, et al. Factors associated with durable response to infliximab in Crohn’s disease 5 years and beyond: a multicenter international cohort. Inflamm Bowel Dis 2015; 21: 60–70. [DOI] [PubMed] [Google Scholar]
- 13. National Institute for Health and Care Excellence. Inflammatory bowel disease: NICE updates advice on remission, https://www.guidelinesinpractice.co.uk/gastrointestinal/inflammatory-bowel-disease-nice-updates-advice-on-remission/454783.article (2019; accessed 16 September 2020).
- 14. National Institute for Health and Care Excellence. Crohn’s disease: management 2019, https://www.nice.org.uk/guidance/ng129/chapter/Update-information (accessed 16 September 2020).
- 15. National Institute for Health and Care Excellence. Infliximab and adalimumab for the treatment of Crohn’s disease: national institute for health and care excellence, https://www.nice.org.uk/guidance/ta187/chapter/4-Evidence-and-interpretation#cost-effectiveness (2010; accessed 29 March 2020).
- 16. Doherty G, Katsanos KH, Burisch J, et al. European Crohn’s and Colitis Organisation topical review on treatment withdrawal [‘exit strategies’] in inflammatory bowel disease. J Crohns Colitis 2018; 12: 17–31. [DOI] [PubMed] [Google Scholar]
- 17. Targownik LE, Kaplan GG, Witt J, et al. Longitudinal trends in the direct costs and health care utilization ascribable to inflammatory bowel disease in the biologic era: results from a Canadian population-based analysis. Am J Gastroenterol 2020; 115: 128–137. [DOI] [PubMed] [Google Scholar]
- 18. Lee JM, Kim YJ, Lee KM, et al. Long-term clinical outcome after infliximab discontinuation in patients with inflammatory bowel disease. Scand J Gastroenterol 2018; 53: 1280–1285. [DOI] [PubMed] [Google Scholar]
- 19. Molander P, Farkkila M, Kemppainen H, et al. Long-term outcome of inflammatory bowel disease patients with deep remission after discontinuation of TNFalpha-blocking agents. Scand J Gastroenterol 2017; 52: 284–290. [DOI] [PubMed] [Google Scholar]
- 20. Waugh AW, Garg S, Matic K, et al. Maintenance of clinical benefit in Crohn’s disease patients after discontinuation of infliximab: long-term follow-up of a single centre cohort. Aliment Pharmacol Ther 2010; 32: 1129–1134. [DOI] [PubMed] [Google Scholar]
- 21. Molander P, Farkkila M, Salminen K, et al. Outcome after discontinuation of TNFalpha-blocking therapy in patients with inflammatory bowel disease in deep remission. Inflamm Bowel Dis 2014; 20: 1021–1028. [DOI] [PubMed] [Google Scholar]
- 22. Bazarova A, Raseta M. CARRoT: predicting categorical and continuous outcomes using one in ten rule, https://cran.r-project.org/web/packages/CARRoT/index.html (2019; accessed 9 March 2020).
- 23. Geisser S. Predictive inference. New York: Chapman and Hall, 1993. [Google Scholar]
- 24. Zhang Z. Variable selection with stepwise and best subset approaches. Ann Transl Med 2016; 4: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49: 1373–1379. [DOI] [PubMed] [Google Scholar]
- 26. Smith SCL, Bazarova A, Ejenavi E, et al. A multicentre development and validation study of a novel lower gastrointestinal bleeding score-the Birmingham Score. Int J Colorectal Dis 2020; 35: 285–293. [DOI] [PubMed] [Google Scholar]
- 27. Rutter AV, Crees J, Wright H, et al. Identification of a glass substrate to study cells using Fourier transform infrared spectroscopy: are we closer to spectral pathology? Appl Spectrosc 2020; 74: 178–186. [DOI] [PubMed] [Google Scholar]
- 28. Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 29. Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol 1972; 34: 187–202. [Google Scholar]
- 30. Kassambara A, Kosinski M, Biecek P. Survminer: drawing survival curves using ‘ggplot2’, https://cran.r-project.org/package=survminer (2019; accessed 9 March 2020).
- 31. Curtis L, Burns A. Unit costs of health and social care 2017: personal social services research unit, University of Kent, Canterbury, 10.22024/UniKent/01.02/65559 (2017; accessed 25 March 2020). [DOI]
- 32. Travis S, Feagan BG, Peyrin-Biroulet L, et al. Effect of adalimumab on clinical outcomes and health-related quality of life among patients with ulcerative colitis in a clinical practice setting: results from InspirADA. J Crohns Colitis 2017; 11: 1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Targownik LE, Benchimol EI, Witt J, et al. The effect of initiation of anti-TNF therapy on the subsequent direct health care costs of inflammatory bowel disease. Inflamm Bowel Dis 2019; 25: 1718–1728. [DOI] [PubMed] [Google Scholar]
- 34. Peters CP, Eshuis EJ, Toxopeus FM, et al. Adalimumab for Crohn’s disease: long-term sustained benefit in a population-based cohort of 438 patients. J Crohns Colitis 2014; 8: 866–875. [DOI] [PubMed] [Google Scholar]
- 35. Eshuis EJ, Peters CP, van Bodegraven AA, et al. Ten years of infliximab for Crohn’s disease: outcome in 469 patients from 2 tertiary referral centers. Inflamm Bowel Dis 2013; 19: 1622–1630. [DOI] [PubMed] [Google Scholar]
- 36. van der Valk ME, Mangen MJ, Leenders M, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFalpha therapy: results from the COIN study. Gut 2014; 63: 72–79. [DOI] [PubMed] [Google Scholar]
- 37. Burisch J, Vardi H, Schwartz D, et al. Health-care costs of inflammatory bowel disease in a pan-European, community-based, inception cohort during 5 years of follow-up: a population-based study. Lancet Gastroenterol Hepatol 2020; 5: 454–464. [DOI] [PubMed] [Google Scholar]
- 38. Torres J, Boyapati RK, Kennedy NA, et al. Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology 2015; 149: 1716–1730. [DOI] [PubMed] [Google Scholar]
- 39. Casanova MJ, Chaparro M, Garcia-Sanchez V, et al. Evolution after anti-TNF discontinuation in patients with inflammatory bowel disease: a multicenter long-term follow-up study. Am J Gastroenterol 2017; 112: 120–131. [DOI] [PubMed] [Google Scholar]
- 40. Louis E, Mary JY, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology 2012; 142: 63–70.e5; quiz e31. [DOI] [PubMed] [Google Scholar]
- 41. Schultheiss JPD, Brand EC, Lamers E, et al. Earlier discontinuation of TNF-alpha inhibitor therapy in female patients with inflammatory bowel disease is related to a greater risk of side effects. Aliment Pharmacol Ther 2019; 50: 386–396. [DOI] [PubMed] [Google Scholar]
- 42. de Jong ME, Smits LJT, van Ruijven B, et al. Increased discontinuation rates of anti-TNF therapy in elderly inflammatory bowel disease patients. J Crohns Colitis 2020; 14: 888–895. [DOI] [PubMed] [Google Scholar]
- 43. Gisbert JP, Marin AC, Chaparro M. The risk of relapse after anti-TNF discontinuation in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2016; 111: 632–647. [DOI] [PubMed] [Google Scholar]
- 44. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis 2009; 15: 1295–1301. [DOI] [PubMed] [Google Scholar]
- 45. Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology 2010; 138: 463–468; quiz e10–e11. [DOI] [PubMed] [Google Scholar]
- 46. Pineton de, Chambrun G, Peyrin-Biroulet L, Lemann M, et al. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol 2010; 7: 15–29. [DOI] [PubMed] [Google Scholar]
- 47. National Health Service England. NHS set to save £150 million by switching to new versions of most costly drug: NHS England, https://www.england.nhs.uk/2018/10/nhs-set-to-save-150-million-by-switching-to-new-versions-of-most-costly-drug/ (2018; accessed 20 March 2020).
- 48. Biopharmadive. Merck lowers cost of Remicade in UK as biosimilars enter market, https://www.biopharmadive.com/news/merck-lowers-cost-of-remicade-in-uk-as-biosimilars-enter-market/408249/ (2015, accessed 20 April 2020).
- 49. White C. Infliximab biosimilars are safe, effective, and cheap, UK audit shows. BMJ 2016; 354: i5084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tag-10.1177_1756284820981216 for Clinical outcomes, predictors of prognosis and health economics consequences in IBD patients after discontinuation of the first biological therapy by Uday N. Shivaji, Alina Bazarova, Tamsin Critchlow, Samuel C. L. Smith, Olga Maria Nardone, Melanie Love, Joanne Davis, Subrata Ghosh and Marietta Iacucci in Therapeutic Advances in Gastroenterology
Supplemental material, sj-pdf-2-tag-10.1177_1756284820981216 for Clinical outcomes, predictors of prognosis and health economics consequences in IBD patients after discontinuation of the first biological therapy by Uday N. Shivaji, Alina Bazarova, Tamsin Critchlow, Samuel C. L. Smith, Olga Maria Nardone, Melanie Love, Joanne Davis, Subrata Ghosh and Marietta Iacucci in Therapeutic Advances in Gastroenterology