Abstract
Background
Inhibition of activated factor XI (FXIa) is a promising antithrombotic drug target. BMS‐724296 is a selective, reversible, small‐molecule inhibitor of human FXIa (Ki 0.3 nM).
Objectives
This study assessed effects of BMS‐724296 versus standard‐of‐care oral anticoagulants apixaban (activated factor X inhibitor) and dabigatran (thrombin inhibitor) on arterial thrombosis, kidney bleeding time (KBT), and clotting time (CT) in nonhuman primate (NHP) cynomolgus monkey models.
Methods
Carotid artery thrombosis was produced by electrical stimulation in anesthetized NHPs. Hemostasis was assessed with a provoked KBT model. Thrombosis, KBT, and CT were monitored. Vehicle and various doses of BMS‐724296, apixaban, and dabigatran were administered as bolus (intravenous [i.v.]) followed by infusion starting 30 minutes before initiation of thrombosis and continued until the experiment’s end (n = 3‐8/group). Primary end points included thrombus weight reduction (TWR), KBT, and CT (activated partial thromboplastin time [aPTT], prothrombin time [PT], and thrombin time [TT]).
Results
BMS‐724296 at 0.025 + 0.05, 0.05 + 0.1, 0.102 + 0.2, and 0.4 + 0.8 mg/kg+mg/kg/h i.v. (bolus + infusion) reduced thrombus weight by 0 ± 0, 35 ± 7*, 72 ± 4*, and 86 ± 4%*, respectively (*P < .05 vs vehicle; n = 5‐6/group). BMS‐724296 at the highest dose (0.4 + 0.8 mg/kg+mg/kg/h) did not increase KBT compared to vehicle (109 ± 6 vs 113 ± 20 seconds, respectively) and increased ex vivo aPTT by 2.9 ± 0.1‐fold without changing PT and TT. In companion NHP studies, high doses of apixaban and dabigatran produced similar TWR as BMS‐724296, but increased KBT 4.3 ± 0.5‐fold and 5.8 ± 0.5‐fold, respectively (n = 3‐4/group).
Conclusions
BMS‐724296 produced similar antithrombotic efficacy as apixaban and dabigatran but with no increase in KBT in NHPs. These findings suggest that FXIa inhibitors may provide safe and effective antithrombotic therapy.
Keywords: anticoagulant, antithrombotic agent, apixaban, blood coagulation, dabigatran, factor XIa inhibitor, hemostasis, thrombosis
Essentials.
BMS‐724296 is an inhibitor of factor XIa, a novel antithrombotic drug target.
Effects of BMS‐724296 versus apixaban and dabigatran were assessed in cynomolgus monkey models.
Antithrombotic efficacy was similar with BMS‐724296, apixaban, and dabigatran.
Kidney bleeding time was increased with apixaban and dabigatran but not BMS‐724296.
1. INTRODUCTION
Direct oral anticoagulants (DOACs), including four activated factor X (FXa) inhibitors (rivaroxaban, apixaban, edoxaban, and betrixaban) and one thrombin inhibitor (dabigatran), have been approved for the prevention and treatment of cardiovascular events. 1 Although these new agents are effective anticoagulants and are associated with a lower risk of bleeding compared with warfarin, bleeding still remains a concern. 2 , 3 , 4 This residual bleeding risk has prompted a search for new effective anticoagulants that are associated with even less bleeding liability than DOACs. Activated factor XI (FXIa) inhibition has been proposed as a promising, safe antithrombotic target. 2 , 3 , 4 , 5
FXIa is a key component of the intrinsic coagulation pathway and is activated by factor XIIa and thrombin feedback. 6 The formation of FXIa leads to the sequential activation of factor IX and factor X. FXIa plays a major role in amplifying thrombin production, maintaining and propagating a formed thrombus, and downregulating fibrinolysis. 3 , 4 Human genetic, experimental, and epidemiologic data suggest that FXIa is important in thrombosis but has little role in hemostasis. 2 , 3 , 4 A phase 2, proof‐of‐concept study showed that decreasing circulating FXI via an antisense oligonucleotide reduced the incidence of venous thrombosis and appeared safe with respect to bleeding. 7 A monoclonal antibody against FXIa also showed efficacy in preventing venous thromboembolism among patients undergoing knee arthroplasty. 8
BMS‐262084 was first reported by Sutton et al as a small‐molecule tryptase inhibitor. 9 It was subsequently demonstrated that BMS‐262084 is an irreversible inhibitor of FXIa, with good antithrombotic efficacy and low bleeding liability in rats and rabbits. 10 , 11 This prompted the initiation of a drug discovery program targeting FXIa, which led to the discovery of reversible FXIa inhibitors represented by BMS‐654457 and BMS‐724296. 12 , 13 , 14 BMS‐724296 (Figure 1) is a reversible direct inhibitor of human FXIa (FXIa Ki, 0.3 nM) with >5000‐fold selectivity over most of the human coagulation proteases 14 and is a strong antithrombotic agent in rabbits. 15
FIGURE 1.
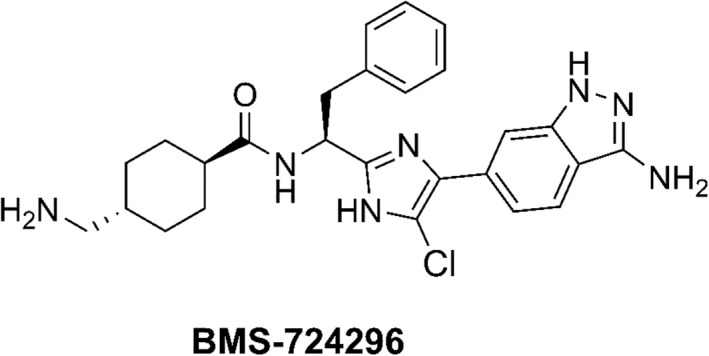
Chemical structure of the FXIa inhibitor BMS‐724296. FXIa, activated factor XI
There are currently no approved small‐molecule FXIa inhibitors, and head‐to‐head clinical studies evaluating inhibitors of FXIa, FXa, and thrombin are lacking. Similarly, comparative preclinical studies of DOACs are limited. 2 , 3 , 4 , 5 Previous in vivo studies with small‐molecule FXIa inhibitors have mostly been conducted in rodents and rabbits 2 , 5 , 10 , 11 instead of larger animals such as monkeys, which are physiologically more similar to humans. 16 To help fill the gaps in preclinical knowledge, this study evaluated the efficacy/safety profiles of the FXIa inhibitor BMS‐724296 versus standard‐of‐care anticoagulants apixaban (FXa inhibitor) and dabigatran (thrombin inhibitor) in cynomolgus monkeys. Efficacy was determined by prevention of arterial thrombosis in the carotid artery, and hemostasis was assessed with a provoked kidney bleeding time (KBT) model in anesthetized animals, followed by measurements of ex vivo clotting times. 17
2. METHODS
2.1. Reagents
The drugs and chemicals used in this study included activated partial thromboplastin time (aPTT) reagent (Dade Actin FS Activated Reagent; Dade Behring, Deerfield, IL, USA), prothrombin time (PT) reagent (Dade Thromboplastin C Plus, Dade Behring), and thrombin time (TT) reagent (Test Thrombin Reagent; Dade Behring). BMS‐724296, 14 apixaban, and dabigatran were synthesized at Bristol Myers Squibb.
2.2. Animals
Experiments were conducted with a nonhuman primate (NHP) cynomolgus monkey (Macaca fascicularis) animal model. Healthy NHPs included in the study were retired from other pharmacokinetic and pharmacodynamic studies. The animals often had implanted vascular access ports in one or both femoral arteries and/or veins. They were previously treated one or more times with experimental compounds in previous studies but had a washout period of at least 4 weeks before being included in the current study; thus, treatments administered in previous studies were unlikely to have influenced results of the present study.
Animals were treated with vehicle (10% N,N‐dimethylacetamide:95% dextrose, n = 8) or various drugs that were administered as a bolus (intravenous [i.v.]) followed by an i.v. infusion; BMS‐724296 0.025 + 0.05 (n = 5), 0.05 + 0.1 (n = 6), 0.102 + 0.2 (n = 6), and 0.4 + 0.8 (n = 6) mg/kg+mg/kg/h i.v.; apixaban 0.024 + 0.036 (n = 4), 0.12 + 0.18 (n = 4), and 0.6 + 0.9 (n = 4) mg/kg+mg/kg/h i.v.; and dabigatran 0.024 + 0.036 (n = 3), 0.12 + 0.18 (n = 3), and 0.6 + 0.9 (n = 3) mg/kg+mg/kg/h i.v. The number of NHPs in each group was chosen on the basis of past experience with this model.
Experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the regulations of the Animal Care and Use Committee of Bristol Myers Squibb. The Animal Study Protocol Number internally assigned to this protocol is 2009H016.
2.3. Thrombosis and bleeding time studies
Thrombosis, KBT, and ex vivo clotting time studies were conducted in the same animals. Arterial thrombosis was studied with the NHP model of electrolytic‐mediated arterial thrombosis (ECAT). 17 Briefly, NHPs were anesthetized as described previously, both common carotid arteries were carefully isolated, and carotid blood flow (CBF) was measured using an appropriately sized Transonic flow probe and a Transonic perivascular flowmeter (TS420 model; Transonic Systems Inc, Ithaca, NY, USA). Thrombosis was induced by electrical stimulation of the control left carotid artery for 5 minutes at 10 mA, using an external stainless steel bipolar electrode (Figure 2). CBF was continuously recorded for 90 minutes to monitor thrombosis‐induced occlusion. Integrated blood flow (IBF) through the injured left carotid artery was measured using area under the flow‐time curve. IBF was expressed as the percentage of the total control CBF, if the control CBF had been maintained continuously for 90 minutes. In addition, the thrombus from the injured left artery was removed, blotted twice on a weighing paper to remove residual fluid, and weighed.
FIGURE 2.
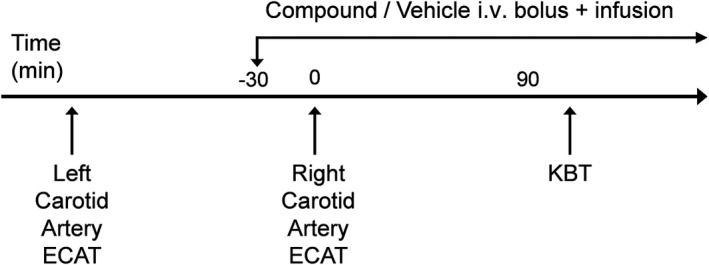
Schematic representation of the NHP study protocol. ECAT, electrolytic‐mediated arterial thrombosis; KBT, kidney bleeding time; i.v., intravenous; NHP, nonhuman primate
After determination of the IBF and thrombus weight in the control left carotid artery, all animals were allowed 30 minutes to recover from the ligation of the left carotid artery for stabilization of the right carotid blood flow (Figure 2). The 30‐minute recovery period was also the pretreatment period. Vehicle (10% dimethylacetamide:90% of 5% dextrose), BMS‐724296, apixaban, and dabigatran were given intravenously as a bolus injection supplemented with a continuous i.v. infusion 30 minutes before the electrical stimulation until the end of the experiment (Figure 2). The 30‐minute pretreatment period allowed the infusion of the compounds to achieve their steady‐state plasma levels. Thrombosis was induced in the right common carotid artery using the method described previously for the left carotid artery (Figure 2). CBF was continuously recorded for 90 minutes to monitor thrombosis‐induced occlusion. IBF and thrombus weight were determined as described previously. IBF and thrombus weight reduction (TWR) were used as indicators of antithrombotic effects of the compounds.
Analysis of KBT was started in the same animal after the antithrombotic study was completed (Figure 2). KBT is a provoked bleeding time model that has been described previously. 17 Briefly, a midline abdominal incision was made to expose the kidney and kidney bleeding was induced by renal cortex incision. KBT was defined as the time from injury until bleeding stopped without rebleeding for 30 seconds. Animals were monitored for up to 20 minutes, and KBT was determined in triplicate. After the KBT test was completed, animals were euthanized with an overdose of sodium pentobarbital (100 mg/kg i.v.).
2.4. Ex vivo coagulation assays
Arterial blood samples for the determination of ex vivo aPTT, PT, and TT were collected in tubes containing one‐tenth the volume of 0.129 M sodium citrate before the start of the infusion of vehicle or test compound and at the end of the study. Clotting times were measured with an automated coagulation analyzer (Sysmex, Dade Behring) as described previously. 18 aPTT, PT, and TT reagents were reconstituted, and assays performed according to the manufacturer’s instructions.
2.5. Statistical analysis
Statistical analyses included analysis of variance and Dunnett’s test or Tukey’s test for multiple comparison using Prism version 8.4 for Windows (GraphPad Software, San Diego, CA, USA). A value of P < .05 was considered statistically significant. All data are presented as mean ± standard error of the mean.
3. RESULTS
Table 1 shows the baseline characteristics of the NHPs included in the study. The age of the NHPs included in this study ranged from 3.2 to 8.2 years (Table 1), which was estimated to correspond to humans aged 10 to 24 years, based on data from Kohama et al. 19
TABLE 1.
Baseline characteristics of NHPs included in the study
| VEH | BMS LD | BMS MD1 | BMS MD2 | BMS HD | API LD | API MD | API HD | DAB LD | DAB MD | DAB HD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group size | 8 | 5 | 6 | 6 | 6 | 4 | 4 | 4 | 3 | 3 | 3 |
| Sex | 5 M, 3 F | 1 M, 4 F | 3 M, 3 F | 2 M, 4 F | 2 M, 4 F | 4 F | 2 M, 2 F | 3 M, 1 F | 3 M | 3 M | 3 M |
| Age, y, mean (range) | 6.4 (5‐7.1) | 5 (4.4‐5.9) | 5 (4.1‐6.2) | 4.2 (3.8‐4.7) | 4.5 (3.4‐6.9) | 4.1 (3.8‐4.8) | 3.8 (3.2‐4.2) | 4.7 (4.4‐5.0) | 4.6 (4.0‐5.8) | 7.0 (5.9‐8.2) | 6.0 a |
| Body weight, kg, mean (range) | 5.9 (3.1‐8.7) | 3.9 (4.4‐5.9) | 4.9 (3.1‐8.5) | 4.1 (3.1‐5.5) | 4 (3.2‐4.7) | 3.3 (3.0‐3.9) | 3.9 (3.1‐4.9) | 5.9 (4.7‐6.7) | 7.4 (5.6‐9.8) | 7.8 (7.5‐8.4) | 8.0 (7.6‐8.4) |
VEH; BMS at 0.025 + 0.05 (LD), 0.05 + 0.1 (MD1), 0.102 + 0.2 (MD2), and 0.4 + 0.8 (HD) mg/kg+mg/kg/h i.v.; API at 0.024 + 0.036 (LD), 0.12 + 0.18 (MD), and 0.6 + 0.9 (HD) mg/kg+mg/kg/h i.v.; and DAB at 0.024 + 0.036 (LD), 0.12 + 0.18 (MD), and 0.6 + 0.9 (HD) mg/kg+mg/kg/h i.v.
Abbreviations: API, apixaban; BMS, BMS‐724296; DAB, dabigatran; HD, high dose; i.v., intravenous; LD, low dose; MD, medium dose; VEH, vehicle.
Age data were missing for two nonhuman primates.
3.1. Arterial thrombosis
The right CBF initially increased after ligation of the left carotid artery, then gradually stabilized at a lower level. In vehicle‐treated NHPs, the right CBF was slightly, but not significantly, greater than the control left CBF in the same animal 30 minutes after the ligation of the left carotid artery with an average CBF of 55 ± 13 and 61 ± 11 mL/min, respectively (n = 8).
After stimulation with an electric current, thrombosis formation occurred rapidly, resulting in the reduction of CBF to 0 within 30 minutes in all vehicle‐treated animals (Figure 3). BMS‐724296, apixaban, and dabigatran were effective in preserving vascular patency in a dose‐dependent manner during the induction of occlusive thrombosis in NHPs (Figure 3).
FIGURE 3.
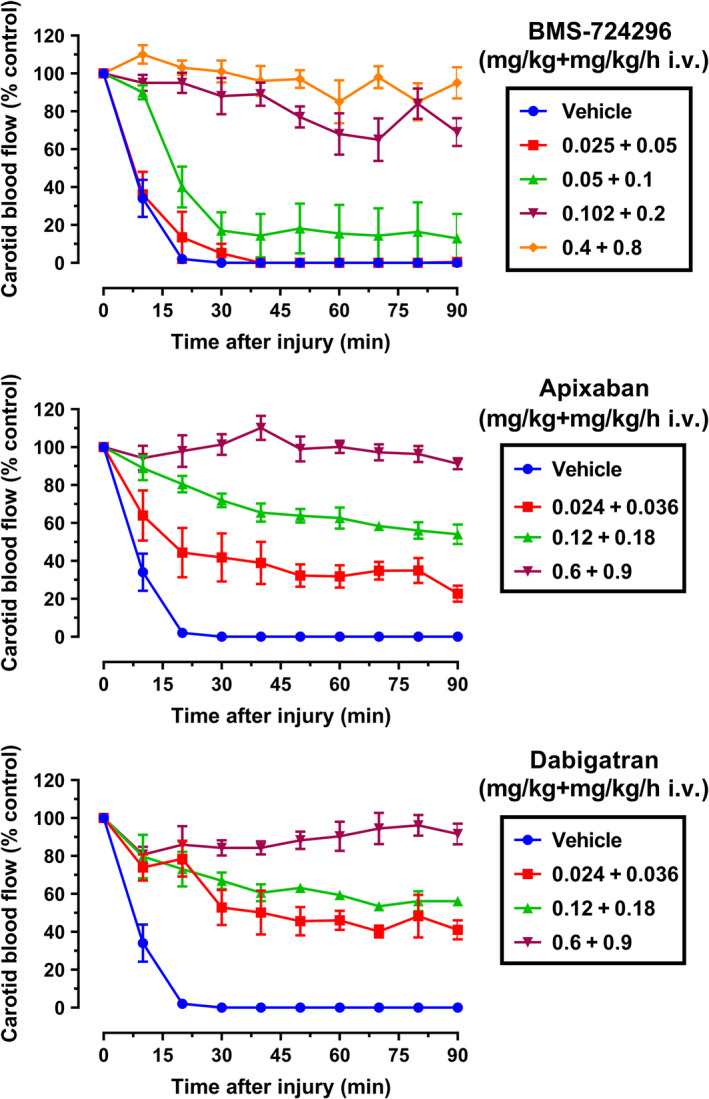
Antithrombotic effects in the NHP model of electrolytically mediated carotid arterial thrombosis. Effects of vehicle, BMS‐724296, apixaban, and dabigatran on carotid blood flow after thrombus induction in the injured carotid artery. Carotid blood flow was expressed as a percentage of control carotid blood flow. Vehicle (n = 8); BMS‐724296 at 0.025 + 0.05 (LD; n = 5), 0.05 + 0.1 (MD1; n = 6), 0.102 + 0.2 (MD2; n = 6), and 0.4 + 0.8 (HD; n = 6) mg/kg+mg/kg/h i.v.; apixaban at 0.024 + 0.036 (LD; n = 4), 0.12 + 0.18 (MD; n = 4), and 0.6 + 0.9 (HD; n = 4) mg/kg+mg/kg/h i.v.; and dabigatran at 0.024 + 0.036 (LD; n = 3), 0.12 + 0.18 (MD; n = 3), and 0.6 + 0.9 (HD; n = 3) mg/kg+mg/kg/h i.v. Data are mean ± SEM. HD, high dose; i.v., intravenous; LD, low dose; MD, medium dose; NHP, nonhuman primate; SEM, standard error of the mean
Figure 4 shows effects of vehicle, BMS‐724296, apixaban, and dabigatran on IBF and thrombus weight in the ECAT NHP model. IBF in the vehicle‐treated group averaged 9% ± 1% of the control level. The high doses of BMS‐724296 (n = 6), apixaban (n = 4), and dabigatran (n = 3) increased IBF to a similar extent (98% ± 3%, 98% ± 4%, and 89% ± 4% of the control level, respectively; Figure 4). Thrombus weight in the vehicle‐treated group averaged 7.8 ± 0.3 mg. Thrombus weight averaged 1.1 ± 0.3, 0.8 ± 0.3, and 0 ± 0 mg in NHPs treated with the high doses of BMS‐724296, apixaban, and dabigatran, respectively (Figure 4).
FIGURE 4.
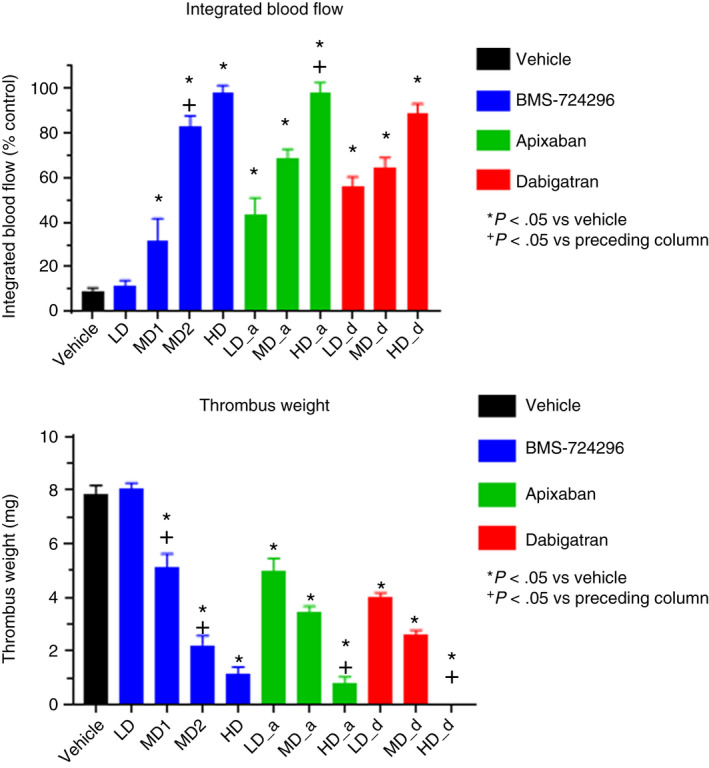
Top panel: Effects of vehicle, BMS‐724296, apixaban, and dabigatran on integrated blood flow expressed as a percentage of control carotid blood flow (ie, preinjury blood flow) in NHPs. Bottom panel: Effects of vehicle, BMS‐724296, apixaban, and dabigatran on thrombus weight reduction (percent inhibition) in ECAT NHPs. Vehicle (n = 8); BMS‐724296 at 0.025 + 0.05 (LD; n = 5), 0.05 + 0.1 (MD1; n = 6), 0.102 + 0.2 (MD2; n = 6), and 0.4 + 0.8 (HD; n = 6) mg/kg+mg/kg/h i.v.; apixaban at 0.024 + 0.036 (LD; n = 4), 0.12 + 0.18 (MD; n = 4), and 0.6 + 0.9 (HD; n = 4) mg/kg+mg/kg/h i.v.; and dabigatran at 0.024 + 0.036 (LD; n = 3), 0.12 + 0.18 (MD; n = 3), and 0.6 + 0.9 (HD; n = 3) mg/kg+mg/kg/h i.v. Data are mean ± SEM. *P < .05 compared to vehicle. + P < .05 compared to preceding column. ECAT, electrolytic‐mediated arterial thrombosis; HD, high dose; i.v., intravenous; LD, low dose; MD, medium dose; NHP, nonhuman primate; SEM, standard error of the mean
By blocking the formation of thrombus, BMS‐724296, apixaban, and dabigatran appear to cause dose‐dependent increases in IBF and reductions in thrombus weight. As shown in Figure 4, there was at least one dose showing statistical significance versus the preceding dose, suggesting a dose‐dependent effect, except that no significant dose‐dependent effect of dabigatran on IBF was observed. However, the high dose of dabigatran appears to show a greater effect on IBF versus the low dose with a P value of .053, which hints at a dose‐dependent effect.
3.2. Kidney bleeding time
Figure 5 shows the effects of vehicle, BMS‐724296, apixaban, and dabigatran on KBT in NHPs. KBT in the vehicle group averaged 113 ± 20 seconds. BMS‐724296 did not increase KBT at any dose. In contrast, apixaban and dabigatran increased KBT dose dependently. As shown in Figure 5, there was at least one dose of both apixaban and dabigatran showing a significant effect versus the preceding dose, suggesting a dose‐dependent effect. The high doses of apixaban and dabigatran increased KBT to 489 ± 59 seconds and 653 ± 122 seconds, respectively, versus 113 ± 20 seconds in the vehicle‐treated group (Figure 5).
FIGURE 5.
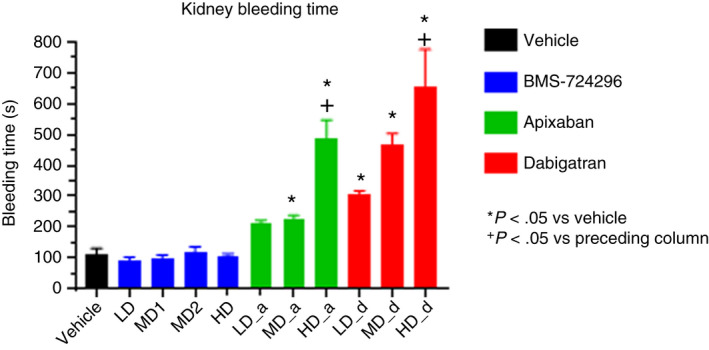
Effects of vehicle, BMS‐724296, apixaban, and dabigatran on kidney bleeding time model in NHPs. Vehicle (n = 8); BMS‐724296 at 0.025 + 0.05 (LD; n = 5), 0.05 + 0.1 (MD1; n = 6), 0.102 + 0.2 (MD2; n = 6), and 0.4 + 0.8 (HD; n = 6) mg/kg+mg/kg/h i.v.; apixaban at 0.024 + 0.036 (LD; n = 4), 0.12 + 0.18 (MD; n = 4), and 0.6 + 0.9 (HD; n = 4) mg/kg+mg/kg/h i.v.; and dabigatran at 0.024 + 0.036 (LD; n = 3), 0.12 + 0.18 (MD; n = 3), and 0.6 + 0.9 (HD; n = 3) mg/kg+mg/kg/h i.v. Data are mean ± SEM. *P < .05 compared to vehicle. + P < .05 compared to preceding column. HD, high dose; i.v., intravenous; LD, low dose; MD, medium dose; NHP, nonhuman primate; SEM, standard error of the mean
3.3. Ex vivo clotting times
Figure 6 shows the effects of BMS‐724296, apixaban, and dabigatran on ex vivo aPTT, TT, and PT. aPTT, PT, and TT in the vehicle‐treated animals averaged 27 ± 1 seconds, 16 ± 2 seconds, and 40 ± 3 seconds, respectively. At all doses, BMS‐724296 increased aPTT without affecting PT and TT. The high dose of BMS‐724296 increased aPTT by 2.9 ± 0.1‐fold. The high dose of apixaban increased aPTT and PT by 1.75 ± 0.05‐fold and 1.93 ± 0.09‐fold, respectively, without altering TT. Dabigatran increased aPTT and TT at all doses, and increased PT at the two highest doses. The high dose of dabigatran increased aPTT by 4.67 ± 0.18‐fold, PT by 8.4 ± 0.4‐fold, and TT by 4.3 ± 0.7‐fold.
FIGURE 6.
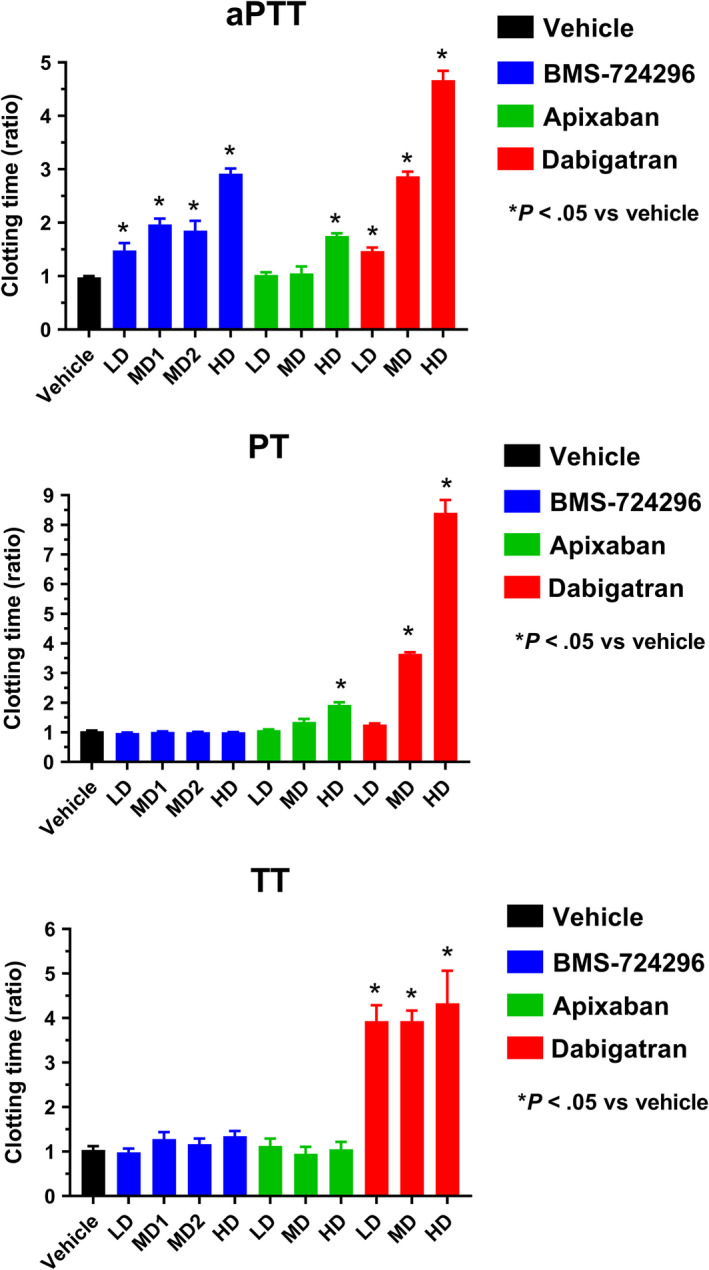
Effects of vehicle, BMS‐724296, apixaban, and dabigatran on clotting times (top panel: aPTT; middle panel: PT; bottom panel: TT) in NHPs. Vehicle (n = 8); BMS‐724296 at 0.025 + 0.05 (LD; n = 5), 0.05 + 0.1 (MD1; n = 6), 0.102 + 0.2 (MD2; n = 6), and 0.4 + 0.8 (HD; n = 6) mg/kg+mg/kg/h i.v.; apixaban at 0.024 + 0.036 (LD; n = 4), 0.12 + 0.18 (MD; n = 4), and 0.6 + 0.9 (HD; n = 4) mg/kg+mg/kg/h i.v.; and dabigatran at 0.024 + 0.036 (LD; n = 3), 0.12 + 0.18 (MD; n = 3), and 0.6 + 0.9 (HD; n = 3) mg/kg+mg/kg/h i.v. Data are mean ± SEM. *P < .05 compared to vehicle. aPTT, activated partial thromboplastin time; HD, high dose; i.v., intravenous; LD, low dose; MD, medium dose; NHP, nonhuman primate; PT, prothrombin time; TT, thrombin time; SEM, standard error of the mean
3.4. Therapeutic index
The relative antithrombotic effects of BMS‐724296, apixaban, and dabigatran, expressed as TWR and KBT, were plotted as a function of dose (Figure 7). BMS‐724296 produced a greater separation between the TWR and KBT dose‐response relationships than was observed with apixaban and dabigatran. At doses resulting in >90% antithrombotic activity, apixaban and dabigatran produced 4.3 ± 0.5‐fold and 5.8 ± 1.1‐fold increases in KBT, whereas BMS‐724296 did not increase KBT.
FIGURE 7.
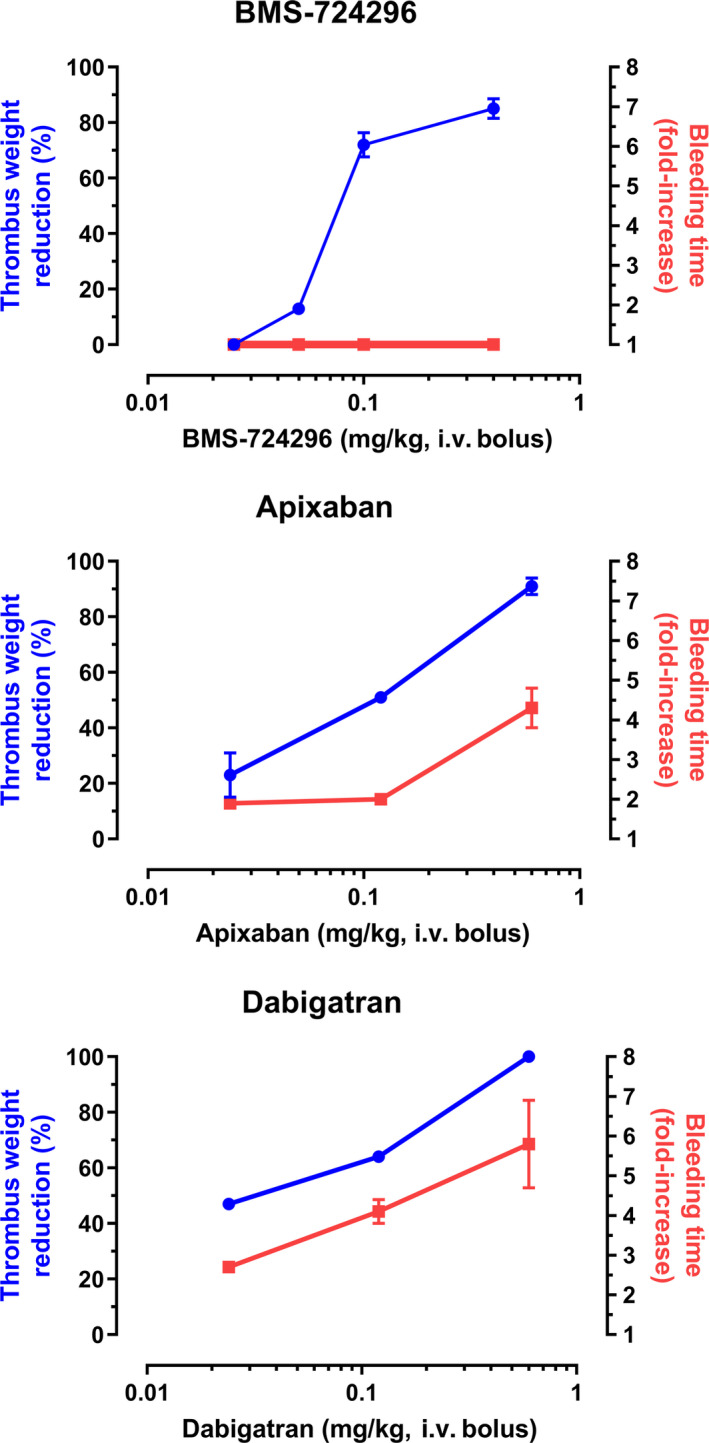
Dose‐dependent effect of BMS‐724296 (top panel), apixaban (middle panel), and dabigatran (bottom panel) on thrombus reduction and BT in NHPs. Thrombus reduction was expressed as a percentage reduction of drug‐treated thrombus weight relative to thrombus weight in the control artery. BT effect was expressed as a ratio of drug‐treated value versus the mean vehicle‐treated value. Data are mean ± SEM. Group sizes were reported in the Figure 2 legend. BT, bleeding time; i.v., intravenous; NHP, nonhuman primate; SEM, standard error of the mean
4. DISCUSSION
This was a comparative dose‐response study of the FXIa inhibitor BMS‐724296 versus the FXa inhibitor apixaban and the thrombin inhibitor dabigatran in NHP models of thrombosis and hemostasis. Robust and antithrombotic efficacy was observed among BMS‐724296, apixaban, and dabigatran in the prevention of arterial thrombosis, and efficacy was comparable between all drugs. BMS‐724296 did not increase KBT; in contrast, apixaban and dabigatran increased KBT at equivalent antithrombotic doses.
NHP models of thrombosis and hemostasis were selected to study FXIa, FXa, and thrombin blockade in this study because the blood coagulation system, platelet function, and cardiovascular physiology of NHPs are similar to that of humans. 20 , 21 In recent experimental models of thrombosis and hemostasis in NHPs, arterial thrombosis was induced by electrical stimulation of the carotid artery, which triggered the formation of platelet‐ and fibrin‐rich thrombi attaching to the damaged endothelium 17 —a thrombus phenotype mimicking that seen in humans. NHP thrombosis models have been benchmarked previously with the standard‐of‐care antithrombotic agents, including the parenteral antiplatelet agents, the glycoprotein (GP) IIb/IIIa receptor antagonist abciximab, and the P2Y12 antagonist cangrelor, 17 and the oral antiplatelet agent clopidogrel, a P2Y12 antagonist. 22 Abciximab, cangrelor, and clopidogrel inhibited thrombus formation and increased KBT dose dependently in NHPs, 17 , 22 and their antithrombotic efficacy and bleeding profiles were similar to those reported in humans. 23 , 24 These studies of standard‐of‐care antiplatelet agents in NHPs validated NHPs as a viable translational model to evaluate efficacy and safety profiles of new antiplatelet agents in support of studies of arterial thrombosis prevention in humans.
This study extended the findings of previous benchmarking studies of standard antiplatelet agents in ECAT and KBT NHP models and validated the use of these models for assessing antithrombotic efficacy and bleeding profiles of DOACs. 17 , 22 Both apixaban and dabigatran were strong antithrombotic agents in NHPs, which effectively prevented the formation of occlusive thrombus and completely preserved the vascular patency of the injured carotid artery. Like other standard antithrombotic agents, the antithrombotic effects of apixaban and dabigatran in NHPs come with an increased bleeding risk. This study used the bleeding time as an index of the bleeding risk. As clinically relevant bleeds often occur in internal organs, the KBT model was used to quantitate the bleeding liability of antithrombotic agents. 25
This study showed that apixaban at a high antithrombotic dose produced a 4.3‐fold increase in KBT in NHPs, which was less than the KBT increase of 5.8‐fold observed with dabigatran at a similar dose. This result is consistent with a previous report showing a greater bleeding time increase with dabigatran than apixaban at maximal antithrombotic doses in rabbits. 26 Interestingly, these results appear to translate into human patients, as evidenced by the demonstration of lower risks of major bleeding and gastrointestinal bleeding with apixaban compared with warfarin, dabigatran, and rivaroxaban in patients on warfarin and DOACs for stroke prevention in atrial fibrillation in real‐world studies. 27 It should be noted that the increases in KBT with apixaban and dabigatran were still significantly less than the >10‐fold increases in KBT observed with the standard antiplatelet agents abciximab, cangrelor, and clopidogrel. 17 , 22
This study demonstrated that BMS‐724296 produces robust dose‐dependent antithrombotic activity that prevents the formation of occlusive thrombus in injured carotid arteries and causes similar full antithrombotic efficacy as apixaban and dabigatran in the ECAT model in NHPs. However, therapeutic doses of BMS‐724296 produced no increase in KBT in NHPs, suggesting that it has a wide therapeutic window. The potential for a wide therapeutic window was also suggested when comparing BMS‐724296 with apixaban and dabigatran in this study and with the GPIIb/IIIa antagonist abciximab 17 and the P2Y12 antagonists cangrelor 17 and clopidogrel 22 in previous studies of the same model. This study also confirms and extends previous reports that showed potent antithrombotic effects of FXIa inhibitors and antisense factor XI oligonucleotide, with a lower bleeding tendency in animals and humans. 3 , 4 , 5 , 7 , 10 , 11 , 13 It is tempting to speculate that a possible explanation for why BMS‐724296 does not appear to affect KBT in NHPs is that BMS‐724296 does not inhibit thrombin generation via the extrinsic coagulation pathway (ie, the tissue factor/factor VIIa pathway), thus allowing enough thrombin production for hemostasis.
The KBT procedure involved a midline abdominal incision to expose the kidney. During the laparotomy, there was usually some minor bleeding from the incision site in animals treated with direct anticoagulants. The bleeding was stopped by putting pressure on the area without the need to cauterize the incision. However, the quantity of bleeding from the incision sites was not determined and which treatment group had the most bleeding cannot be determined. This will be an interesting topic for future studies. One hypothesis is that the minor bleeding at the incision site may be related to the fact that these direct anticoagulants do not have a direct inhibitory effect on platelet aggregation and function, which allows the formation of a platelet plug to minimize bleeding.
The translation of preclinical findings, such as effects on bleeding time, to humans should be viewed with caution, especially in healthy subjects. Bleeding risk may vary due to differences between species and sites of bleeding. Further, the critical effect of any antithrombotic agent on bleeding liability cannot be projected solely on the basis of a provoked bleeding time model since the provoked bleeding time in an animal model may not be directly comparable with spontaneous bleeding in humans. Finally, bleeding may be influenced by factors specific to individual patients like complications of cardiovascular metabolic disease and polypharmacy, which are not replicated in animal models. However, by comparing the efficacy and bleeding profile of BMS‐724296 to standard‐of‐care antithrombotic agents such as apixaban, dabigatran, and clopidogrel, these preclinical findings may generate hypotheses that can be tested in clinical studies.
The laboratory tests traditionally used for adjusting anticoagulant doses of heparin (aPTT) and warfarin (PT) lack sensitivity for more selective anticoagulants. 28 This study used several ex vivo coagulation biomarker assays (aPTT, PT, and TT), which may have different sensitivities to anticoagulant activity, and showed that apixaban prolonged both aPTT and PT only at the high antithrombotic dose but not at the lower antithrombotic doses in NHPs. Similar findings of mild PT and aPTT effects of apixaban have been observed in rabbits. 18 This mild effect may be related to the binding kinetics of apixaban for FXa. 29 Apixaban exhibited a slower association rate to bind FXa either in free form or associated with the prothrombin complex. 29 As expected, apixaban did not prolong TT at all doses since it did not inhibit thrombin activity. 18 Dabigatran is a direct thrombin inhibitor 30 and thus, as expected, prolonged aPTT, PT, and TT profoundly in NHPs. 31 Consistent with the mechanism of FXIa inhibition, BMS‐724296 prolonged aPTT but not PT or TT. The ex vivo aPTT appeared to track the antithrombotic activity of BMS‐724296 closely in NHPs. In addition to the aPTT test, it appears the whole blood clotting time test is also a sensitive indicator of the anticoagulant activity of FXIa inhibitors 32 and may warrant inclusion in future studies of small‐molecule inhibitors.
In summary, this study demonstrated that BMS‐724296, apixaban, and dabigatran have similar efficacy in preventing the formation of occlusive thrombus in the injured carotid artery in the NHP ECAT model. At doses with equivalent antithrombotic efficacy, BMS‐724296 was not associated with an increase in KBT, while apixaban was associated with a moderate increase in KBT, and dabigatran was associated with the greatest increase in KBT. These findings suggest that FXIa inhibitors may be a safe and effective antithrombotic therapy.
RELATIONSHIP DISCLOSURE
PCW and MLQ are employees and stockholders of Bristol Myers Squibb.
AUTHOR CONTRIBUTIONS
PCW contributed to the establishment of thrombosis and hemostasis models in NHPs; designed the study; supervised C. Watson, who performed the study; analyzed and interpreted the data; and wrote the manuscript. MLQ contributed to the discovery chemistry of BMS‐724296 and supervised C. Wang, who synthesized BMS‐724296. All authors approved the final version of the manuscript to be published.
ACKNOWLEDGMENTS
We thank Carol Watson for experimental assistance in the animal studies, Cailan Wang for the synthesis of BMS‐724296, and the BMS Veterinary Sciences for technical assistance in the animal studies. Editorial support was provided by Alanna Kahhan, ELS, of MedErgy and was funded by Bristol Myers Squibb and Janssen Global Services, LLC.
Handling Editor: Pantep Angchaisuksiri.
Work carried out at: Bristol Myers Squibb, Princeton, NJ, USA.
Presented in part at the International Society on Thrombosis and Haemostasis Congress; July 8–13, 2017; Berlin, Germany. Abstract OC 40.4.
Funding information
This study was supported by Bristol Myers Squibb.
REFERENCES
- 1. Chen A, Stecker E, A. Warden B. Direct oral anticoagulant use: a practical guide to common clinical challenges. J Am Heart Assoc. 2020;9(13):e017559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schumacher WA, Luettgen JM, Quan ML, Seiffert DA. Inhibition of factor XIa as a new approach to anticoagulation. Arterioscler Thromb Vasc Biol. 2010;30(3):388‐392. [DOI] [PubMed] [Google Scholar]
- 3. Gailani D, Gruber A. Factor XI as a therapeutic target. Arterioscler Thromb Vasc Biol. 2016;36(7):1316‐1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weitz JI, Fredenburgh JC. 2017 Scientific Sessions Sol Sherry Distinguished Lecture in Thrombosis: factor XI as a target for new anticoagulants. Arterioscler Thromb Vasc Biol. 2018;38(2):304‐310. [DOI] [PubMed] [Google Scholar]
- 5. Quan ML, Pinto DJP, Smallheer JM et al. Factor XIa inhibitors as new anticoagulants. J Med Chem. 2018;61(17):7425‐7447. [DOI] [PubMed] [Google Scholar]
- 6. Emsley J, McEwan PA, Gailani D. Structure and function of factor XI. Blood. 2010;115(13):2569‐2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Büller HR, Bethune C, Bhanot S et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372(3):232‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weitz JI, Bauersachs R, Becker B et al. Effect of osocimab in preventing venous thromboembolism among patients undergoing knee arthroplasty: the FOXTROT randomized clinical trial. JAMA. 2020;323(2):130‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sutton JC, Bolton SA, Hartl KS et al. Synthesis and SAR of 4‐carboxy‐2‐azetidinone mechanism‐based tryptase inhibitors. Bioorg Med Chem Lett. 2002;12(21):3229‐3233. [DOI] [PubMed] [Google Scholar]
- 10. Schumacher WA, Seiler SE, Steinbacher TE et al. Antithrombotic and hemostatic effects of a small molecule factor XIa inhibitor in rats. Eur J Pharmacol. 2007;570(1–3):167‐174. [DOI] [PubMed] [Google Scholar]
- 11. Wong PC, Crain EJ, Watson CA, Schumacher WA. A small‐molecule factor XIa inhibitor produces antithrombotic efficacy with minimal bleeding time prolongation in rabbits. J Thromb Thrombolysis. 2011;32(2):129‐137. [DOI] [PubMed] [Google Scholar]
- 12. Quan ML, Wong PC, Wang C et al. Tetrahydroquinoline derivatives as potent and selective factor XIa inhibitors. J Med Chem. 2014;57(3):955‐969. [DOI] [PubMed] [Google Scholar]
- 13. Wong PC, Quan ML, Watson CA et al. In vitro, antithrombotic and bleeding time studies of BMS‐654457, a small‐molecule, reversible and direct inhibitor of factor XIa. J Thromb Thrombolysis. 2015;40(4):416‐423. [DOI] [PubMed] [Google Scholar]
- 14. Hangeland JJ, Friends TJ, Rossi KA et al. Phenylimidazoles as potent and selective inhibitors of coagulation factor XIa with in vivo antithrombotic activity. J Med Chem. 2014;57(23):9915‐9932. [DOI] [PubMed] [Google Scholar]
- 15. Wong PC, Quan M, Watson CA et al. Potent antithrombotic effect of a novel, small‐molecule and direct inhibitor of factor XIa in prevention and treatment of thrombosis in rabbit model of arterial thrombosis at doses that preserve haemostasis. J Thromb Haemost. 2015;3(suppl 1):P0162. [Google Scholar]
- 16. Jagadeeswaran P, Cooley BC, Gross PL, Mackman N. Animal models of thrombosis from zebrafish to nonhuman primates: use in the elucidation of new pathologic pathways and the development of antithrombotic drugs. Circ Res. 2016;118(9):1363‐1379. [DOI] [PubMed] [Google Scholar]
- 17. Wong PC, Watson C, Crain EJ. The P2Y1 receptor antagonist MRS2500 prevents carotid artery thrombosis in cynomolgus monkeys. J Thromb Thrombolysis. 2016;41(3):514‐521. [DOI] [PubMed] [Google Scholar]
- 18. Wong PC, Crain EJ, Xin B et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost. 2008;6(5):820‐829. [DOI] [PubMed] [Google Scholar]
- 19. Kohama SG, Rosene DL, Sherman LS. Age‐related changes in human and non‐human primate white matter: from myelination disturbances to cognitive decline. Age (Dordr). 2012;34(5):1093‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Birndorf NI, Pearson JD, Wredman A. The clotting system of monkeys: a comparison of coagulation factors and tests between cynomolgus monkeys (Macaca irus) and humans. Comp Biochem Physiol. 1971;38(1):157‐161. [DOI] [PubMed] [Google Scholar]
- 21. Iwase H, Ekser B, Zhou H, Dons EM, Cooper DK, Ezzelarab MB. Platelet aggregation in humans and nonhuman primates: relevance to xenotransplantation. Xenotransplantation. 2012;19(4):233‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong PC, Seiffert D, Bird JE et al. Blockade of protease‐activated receptor‐4 (PAR4) provides robust antithrombotic activity with low bleeding. Sci Transl Med. 2017;9(371):eeaf5294. [DOI] [PubMed] [Google Scholar]
- 23. Eikelboom JW, Hirsh J, Spencer FA, Baglin TP, Weitz JI. Antiplatelet drugs: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):e89S‐e119S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. von Beckerath N, Taubert D, Pogatsa‐Murray G, Schömig E, Kastrati A, Schömig A. Absorption, metabolization, and antiplatelet effects of 300‐, 600‐, and 900‐mg loading doses of clopidogrel: results of the ISAR‐CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) trial. Circulation. 2005;112(19):2946‐2950. [DOI] [PubMed] [Google Scholar]
- 25. Schumacher WA, Bostwick JS, Stewart AB, Steinbacher TE, Xin B, Wong PC. Effect of the direct factor Xa inhibitor apixaban in rat models of thrombosis and hemostasis. J Cardiovasc Pharmacol. 2010;55(6):609‐616. [DOI] [PubMed] [Google Scholar]
- 26. Wong PC, Crain EJ, Watson CA, Xin B. Favorable therapeutic index of the direct factor Xa inhibitors, apixaban and rivaroxaban, compared with the thrombin inhibitor dabigatran in rabbits. J Thromb Haemost. 2009;7(8):1313‐1320. [DOI] [PubMed] [Google Scholar]
- 27. Proietti M, Romanazzi I, Romiti GF, Farcomeni A, Lip GYH. Real‐world use of apixaban for stroke prevention in atrial fibrillation: a systematic review and meta‐analysis. Stroke. 2018;49(1):98‐106. [DOI] [PubMed] [Google Scholar]
- 28. Walenga JM, Hoppensteadt DA. Monitoring the new antithrombotic drugs. Semin Thromb Hemost. 2004;30(6):683‐695. [DOI] [PubMed] [Google Scholar]
- 29. Kim PY, Yeh CH, Dale BJ et al. Mechanistic basis for the differential effects of rivaroxaban and apixaban on global tests of coagulation. TH Open. 2018;2(2):e190‐e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Ryn J, Goss A, Hauel N et al. The discovery of dabigatran etexilate. Front Pharmacol. 2013;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wienen W, Stassen JM, Priepke H, Ries UJ, Hauel N. In‐vitro profile and ex‐vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate. Thromb Haemost. 2007;98(1):155‐162. [PubMed] [Google Scholar]
- 32. Thomas D, Thelen K, Kraff S et al. BAY 1213790, a fully human IgG1 antibody targeting coagulation factor XIa: first evaluation of safety, pharmacodynamics, and pharmacokinetics. Res Pract Thromb Haemost. 2019;3(2):242‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]


