ABSTRACT
As a chaperone protein of progesterone receptor (PR), FK-506 Binding Protein 52 (FKBP52) can enhance the activity of PR, but the mechanism of FKBP52 affecting PR expression levels is difficult to clarify. Here, we report a novel in vitro model of ectopic endometrial stromal cells (ESCM) established through the primary culture method of endometrial stromal cells, which is used to study the details of relationship between FKBP52 abnormality and PR expression level in endometriosis (Ems). At the same time, the clinical study of the relationship between FKBP52 and PR expression levels in endometriosis patients was used to verify our conclusions. The results showed that the expression levels of PR-A mRNA and protein in endometriosis are positively correlated with FKBP52 and the abnormality of FKBP52 leads to the decrease of PR-B mRNA and protein expression. When FKBP52 was deleted or reduced, the expression levels of m RNA and protein of PR-A and PR-B have decreased leading to the proliferation of ectopic endometrium cells (ESC) and the occurrence of endometriosis, which is consistent with the expression levels of clinical endometriosis patients and fully confirms our conclusions and reliability of the model, and has great guiding significance for the research of Ems disease occurrence mechanism and clinical treatment.
KEYWORDS: FKBP52, PR (PR-APR-B), ectopic endometrial stromal cells model, influence mechanism
Graphical abstract
Introduction
Endometriosis is a benign gynecological disease, but its biological behaviors of invasion and metastasis are similar to tumors, often causing inflammation, pain, and reducing fertility.1,2 So far, the pathogenesis of Ems has not been fully elucidated and it is ineffective to treat it through various treatment methods.3,4 Therefore, it is extremely urgent to explore the mechanism of unknown Ems and seek new clinical treatment plan. FK-506 Binding Protein 52 (FKBP52) is a chaperone protein of progesterone receptor (PR including PR-A and PR-B) and can enhance the activity of PR,5–7 which has unique biological functions in cancer,8–11 neurons12–15 and other aspects.16,17 Faber et al. discovered FKBP52 by constructing antibodies against the EC1 epitope of rabbit progesterone receptor complex.18 The early researches on the function of FKBP52 mainly focused on its influence on the steroid hormone receptor signaling pathway and the biological influence of related diseases.19–23 Pratt et al found that FKBP52 can form a stable biologically functional complex with steroid hormones under the participation of Hsp90.23 In addition, it has also been reported that FKBP52 was involved in the regulation of neuronal microtubule function and was closely related to the occurrence of various diseases. Similarly, progesterone can form the structure of FKBP52–Hsp90–receptor complex under the participation of FKBP52 and Hsp90, which makes the progesterone enter the cell to promote the transcription and translation process and complete the activation of progesterone.24–26 When FKBP52 was absent or reduced, the progesterone receptor’s binding and transport function of progesterone were directly affected, inhibiting blastocyst implantation and hindering the normal reproductive process.27,28 Therefore, the regulatory role of FKBP52 in female diseases has attracted wide attention, while the specific interactive regulation mechanism of progesterone and FKBP52 still needs further exploration.
It was reported that the expression level of FKBP52 was related to endometriosis.29 Hirota et al. utilized mice lacking FKBP52 expression to study the effect of FKBP52 in endometriosis.28 The results showed that the lack of FKBP52 expression promoted the inflammatory response, cell proliferation and angiogenesis at the transplantation site during the heterotopic transplantation. In addition, the team also reported through clinical cases that the serum FKBP52 level in patients with endometriosis was significantly lower than healthy people.29 Combined with experimental investigation and clinical investigation, the results of this study indicated that the level of FKBP52 was reduced, which promoted the formation of endometriosis. In addition, the study found that the expression of FKBP52 in ectopic tissue of endometriosis patients decreased significantly and it was related to the growth of endometriosis lesions, which inhibited the binding ability of PR-A and progesterone by affecting the binding ability of PR-A and HSP90, and made the function of PR-A in normal endometrium (including inhibiting abnormal uterine hyperplasia and limiting potential abnormal proliferation mediated by PR-B) inhibited.30,31 The low expression of FKBP52 causes dysfunction of the progesterone receptor complex and leads to embryo cessation, which indicates that FKBP52 affects steroid hormones by affecting the function of steroid hormone receptors.23,32 The researchers have proposed that epigenetic silencing of the PR gene and post-translational modification of PR can promote the degradation of proteins, while the loss of important activated T cell nuclear factor 3 can lead to suboptimization of PR activity.33–35 However, these studies only explained the effect of PR on progesterone resistance and could not explain the synergistic effect between PR (PR-A, PR-B) and other chaperone proteins (eg. FKBP52). These studies are mainly based on clinical and animal experiments to study the mechanism of progesterone resistance and it is difficult to study the specific relationship between the mechanisms due to the complex human or animal environment.
Therefore, we established a new in vitro model of ectopic endometrial stromal cells through the primary culture method of endometrial stromal cells and the vitro model is used to study the relationship between FKBP52 abnormalities and PR expression levels in endometriosis, which makes the research level from the clinical organization to the molecular level of cytology. Western Blotting (WB), Real-time Quantitative PCR Detecting System (qPCR), RNA interference (siRNA), synthetic clone primer overexpression, Cell Counting Kit (CCK-8), flow cytometry and other experimental techniques were used to explore the effects of abnormal immunophilin FKBP52 on the expression levels of PR (PR-A, PR-B) in endometriosis. Finally, the relationship between FKBP52 and PR (PR-A, PR-B) expression levels in clinical endometriosis patients was used to further confirm the reliability of the ESCM model and conclusions.
Materials and methods
Antibodies and reagents
The complete medium (serum concentration is 20%) is prepared by solution containing double antibodies (both penicillin and streptomycin are 100 IU/mL) 1640: fetal bovine serum at a ratio of 4:1. Mouse anti-human vimentin monoclonal antibody (vimentin) is usually prepared by diluting it with double-distilled water (ddH2O) at a ratio of 1:100 and storing it at 4°C. The mouse anti-human keratin monoclonal antibody is prepared by diluting it with ddH2O at a ratio of 1:200 and storing it at 200°C for later use. All details of other antibodies and reagents are included in the supporting information.
Clinical tissue samples of the human body
The following tissues were obtained from laparoscopy performed by 30 women. 1) Endometrial tissues of women without Ems due to fallopian tube infertility (n = 15); 2) Ovary ectopic endometrial tissue of women with Ems (n = 15). Median age of infertile patient: 28 ± 0.7 y/26–35 y. Median age of EMS patient: 29 ± 0.2 y old/21–35 y. No significant difference was observed between the two groups. Both groups were from the proliferative endometrium, and Ems had been confirmed by laparoscopic surgery and histopathology. The severity of Ems was distinguished according to the report of the American Society of Reproductive Medicine Classification. The third and fourth stages had seven and eight cases, respectively. At least 6 months prior to surgery, all subjects did not receive any hormonal treatment during their normal menstrual cycle. Tissue samples were obtained under aseptic conditions and stored in the refrigerator at −80°C for qPCR and WB. The experimental procedure was approved by the Institutional Review Committee of Guangxi Medical University. Informed consent was obtained from the woman whose tissue was used.
Primary culture endometrial stromal cells
Normal endometrium (NSC) was obtained by vaginal curettage. The ectopic endometrium (ESC) was obtained from Ems cyst, which was adopted by an improved culture method. (1) According to the thickness of tissue fibers, the digestion time of normal endometrium is 30–40 min, and the digestion time of the inner wall of Ems cyst is 120–150 min. (2) The use of 0.25% EDTA-free trypsin digestion tissue can improve cell attachment rate. (3) The six-well plate is used to assist the initial colony growth of cultured cells. (4) Because the cells have tumor-like properties, 20% serum and 1640 medium are used. The stromal cells with cell immunohistochemistry were identified after the cultured cells grew on glass coverslips. Vimentin can be used as a primary antibody. During the staining process, the primary antibody was replaced with the mouse anti-human keratin monoclonal antibody as negative control.
The endometrial stromal cell model in vitro
The tissue specimens of endometriotic cysts will be cleaned several times with PBS solution containing double antibodies (both penicillin and streptomycin are 100 IU/mL) or sterile saline in a sterile biological safety cabinet to remove blood clots, and cut off the connective tissue as much as possible. In a sterile petri dish, the endometrial tissue was cut into fragments with a volume of about 1.0 mm3 by using tissue scissors and ophthalmic scissors, and the tissue pieces were washed with PBS solution until the cleaning solution was clear. Wait for the tissue mass to settle to the bottom of the tube naturally, and discard the supernatant. Then, the chopped intimal tissue was transferred to a centrifuge tube with sterile forceps, PBS was added to it, and the mixture was centrifuged at 1000 r/min for 5 min. The supernatant was removed, and blood clots and other impurities were removed again. And, 0.25% EDTA-free trypsin digestion tissue (approximately 3 times the volume of the tissue) was added, and digested in a 37°C water bath (digestion time: 30–40 min for normal endometrium; 120–150 min for inner wall of endometriotic cysts), and then the digestion was terminated with complete culture solution. After the digestion was terminated, the mixed solution was centrifuged at a speed of 1000 r/min for 5 min, and the supernatant is removed. The 1640 medium was added and the tissue mass was repeatedly pipetted with a pipette to separate the cells and stand still to sink the undispersed tissue mass. After grinding and filtering with a 200-mesh screen, the filtered tissue is gently moved with sterile tweezers to make it fully filtered, and the filtrate is collected in a sterile centrifuge tube. Serum (concentration 20%) containing double-antibody complete medium is added into the filtrate. After mixing, the cells were inoculated into a six-well plate, and 2 mL of cell growth medium was added to it, and cultured in a CO2 incubator at 37°C. After 24 h, the medium was removed, and new medium (2 mL) was added. Subsequently, the medium was changed every 2–3 d, and the morphology of interstitial cells was observed under an inverted microscope to obtain uniform cells. Subsequently, the medium was changed every 2–3 d, and the morphology of mesenchymal cells was observed under an inverted microscope to obtain uniform ectopic endometrium cell, which was used to construct an in vitro endometrial stromal cell model.
Identification of ESC
Interstitial cells were identified by cellular immunohistochemistry. During this staining process, a mouse anti-human keratin monoclonal antibody was used instead of the primary antibody as a negative control. The specific process is as follows: (1) sterile coverslips were placed in a six-well plate and the logarithmic growth phase cells were digested and inoculated with trypsin, which was placed on the sterile coverslips for cell slide culture. (2) When the cells were gone into a monolayer (the confluence is about 70%-80%), the cover glass was removed and the cells were washed three times with PBS to remove dead cells. At 40°C, the cells were fixed with 4% paraformaldehyde for 30 min and washed three times with PBS. (3) 0.5% Triton-X-100 liquid prepared with deionized water was added to the cells for 15 min that has been penetrated the cell membrane, which was washed three times with PBS. (4) The endogenous peroxidase was blocked with 0.3% hydrogen peroxide in methanol and placed at room temperature for 20 min, which was washed with PBS for three times. The secondary antibody was placed in a humid box and sealed with 5% normal animal serum of the host at room temperature for 20 min. (5) Mouse anti-human vimentin monoclonal antibody was added and incubated at 37°C for 2 h, which were washed three times with PBS and placed overnight at 40°C. The biotinylated secondary antibody was added dropwise and incubated at room temperature for 15 min, which were washed 3 times with PBS. (6) The peroxidase-labeled streptomycin working solution was added and incubated at room temperature for 15 min, which were washed three times with PBS. (7) DBA developer was added and developed at room temperature for 10 min, which were washed thoroughly with tap water, counterstained with hematoxylin, sealed with neutral gum and observed under a microscope.
siRNA screening of FKBP52
The siRNA interference sequence of FKBP52 was designed and synthesized by Guangzhou Ruibo Biological Company. Real-time fluorescence quantitative PCR was used to verify the silencing efficiency of the three siRNAs and siRNAs with silencing efficiency over 70% were selected for subsequent experiments. The information of the siRNA interference sequence of FKBP52 is shown in S Table 1 (Supporting information).
Real-time quantitative PCR (qPCR) assay
Total RNA was isolated from human tissues and cultured cells using TRIzol RNA Isolation Reagents (Invitrogen, CA, USA). cDNAs were synthesized by using reverse transcriptase M-MLV (RNase H−) (Takara, Dalian, China). qRT-PCRs were performed on an Applied Biosystems® 7500 Real-Time PCR Systems (ABI, CA, USA) by using the 2× SYBR Green qPCR ProMix (EnzyValley, Guangzhou, China) following the manufacturer’s instructions. The following primers are shown in S Table 2 (Supporting information)
Western blot analysis (WB)
A proper amount of tissue was taken and cultured cells were harvested by scraping and centrifugation, which all were washed with PBS and re-suspended in RIPA buffer. Soluble proteins were collected by centrifugation at 12,000x g. Protein lysates were subjected to 10% and 12% of SDS-PAGE and transferred onto an NC membrane (Merck Millipore, Billerica, MA, USA). After blocking with 5% skim milk, the membranes were incubated with the respective primary antibodies in Tris-buffered saline (TBS) that contains 0.1% Tween-20 overnight at 4°C. The membranes were then incubated with the appropriate secondary horseradish peroxidase-conjugated IgG antibodies at a 1:5000 dilution (Proteintech Group Inc., Rosemont, IL, USA), followed by incubation with specific secondary antibodies. The reactive bands were detected by infrared fluorescence and exposed to the Odyssey Analysis System (LI-COR Biosciences, Lincoln, Nebraska, USA). The following protein antibodies were used: anti-FKBP52 antibody (abcam, ab97306), progesterone receptor A/B (C89F7) and Rabbit mAb antibody (CST,3151S).
Cell proliferation detection
Cell proliferation was determined by using the CCK8 assay. Briefly, the normal and ectopic endometrial stromal cells in logarithmic growth phase were taken. To cover the cell surface, 0.25% trypsin (1 mL) was added, until the cells were digested to become round. The digestion is terminated by adding the full culture liquid (1 mL). It was transferred to the 2-mL centrifuge tube at 1000 rpm centrifugation for 5 min and discarded the supernatant. At the same time, detection culture (1 mL) was added to suspend to count cells. Cell density was adjusted to 1.5 × 105/mL, and the suspension (100 μL) was added into a 96-hole plate. Cells were incubated for 0, 24, 48, 72, and 96 h or other different times. Then, the CCK-8 chromogenic solution was added into the holes and incubated at 37°C with 5% CO2 incubator. The 96-hole plate was removed, and the OD450 absorptivity of each hole was read by the enzyme-labeling instrument (ThermoFisher, CA, USA), which is used to draw cell proliferation curves.
Assessment of apoptosis by flow cytometry
Apoptosis and necrosis were identified by double fluorescence staining with Annexin V-propidium iodide (PI). The normal endometrial stromal cells and ectopic endometrium stromal cells (1 × 105 cells per sample) were loaded with 5 μL PI and 10 μL Annexin V-FITC (BD Pharmingen, San Diego, CA, USA) at room temperature for 15 min in the dark. Flow cytometric analysis was performed using a flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) after Annexin-PI labeling.
Gene silencing and overexpression of FKBP52
Three FKBP52 siRNAs were synthesized by Guangzhou Ruibo Biological Company and one of the most potent silencing cellular models was screened. The amplification primers were designed according to the sequence at the CDS region about the FKBP52 gene of NCBI species (human) by using PCR. Then, the total RNA reverse transcribed cDNA extracted from human endometrial tissue was used as a template. The gene sequence was amplified and built into the overexpression vector. FKBP52 over-expression plasmid, empty vector, FKBP52-siNRA, and NC-siRNA were transfected into human endometriotic cells by lipofectamine 2000 cationic liposome method. The information about the siRNA interference sequence of FKBP52 is shown in S Table 1(Supporting information).
Statistical analysis
Results are expressed as mean ± standard deviation (SD). All data analyses were performed using GraphPad Prism 6 software (GraphPad, Inc., La Jolla, CA, USA) and IBM SPSS 19.0 software (IBM SPSS, IBM Corporation, USA). Differences among the mean values of multiple groups were analyzed by one-way analysis of variance with Tukey’s test for post hoc comparisons. Statistical significance was considered at P < .05.
Results
Establishment of an endometrial stromal cell model in vitro
Endometriosis is a benign and common gynecological disease, which seriously affects the quality of life of patients. In order to strengthen the basic research on its etiology and pathogenesis and provide better methods for clinical treatment, researchers have been constantly exploring the establishment of experimental models in vivo or in vitro to study the disease. The vitro models of endometriosis mainly include tissue models and cell models. The cell model can well reflect the biological characteristics of the cells in the body and the interaction between the cells, which is closer to the real environment of the human body. Therefore, the cell model is still an important way to study the pathogenesis of Ems. In order to better study the biological function of FKBP52 in Ems and its specific regulatory mechanism, an endometrial stromal cell model was established and conducted a preliminary analysis of the characteristics about the cell model to provide reliable research models for studying the influence mechanism of abnormal immunophilin FKBP52 on the expression levels of PR-A and PR-B in endometriosis.
In order to establish the best vitro cell model, primary cultured endometrial stromal cells were used to isolate and culture normal endometrial specimens (15, success rate 93.33%) and EMS ectopic endometrium specimens (15, success rate 86.67%), respectively. ESC vimentin was positive by cell type identification (Figure 1(a)) and the cell purity was 85%. After a generation of training, the purity could reach more than 90%. The isolated NSC and ESC cells were observed with HE staining only to prove that they were successfully isolated. It is found that NSC cells are spun-cone-shaped and the nucleus is not easy to see, which is uniform in the morphology of cells (Figure 1(cand Figure 1d)). However, ESC cells have large nuclei and many cell divisions, which is uneven in the morphology of cells (Figure 1(eand Figure 1f)). Through the cultivation, it was found that the ESC cell population has grown slowly and it usually took 4 weeks to reach the logarithmic growth phase (Figure S1), while NSC only took 1 week (Figure S2). The growth of NSC cells weakened after five passages of culture and the growth of ESC cells began to weaken after seven passages of growth. Finally, Cell Counting Kit-8 (CCK-8) and flow cytometry were used to detect cell proliferation and apoptosis, respectively. The growth curve of cell (Figure 2(a)) shows the strong value-added of both NSC and ESC. The cell relative proliferation rate shows that ESC cells proliferate more vigorously than NSC cells (Figure 2(b)). Apoptosis and survival analysis of NSC and ESC confirmed that NSC had more apoptosis than ESC (Figure 3). The above test fully shows that an endometrial stromal cell model has been successfully established.
Figure 1.
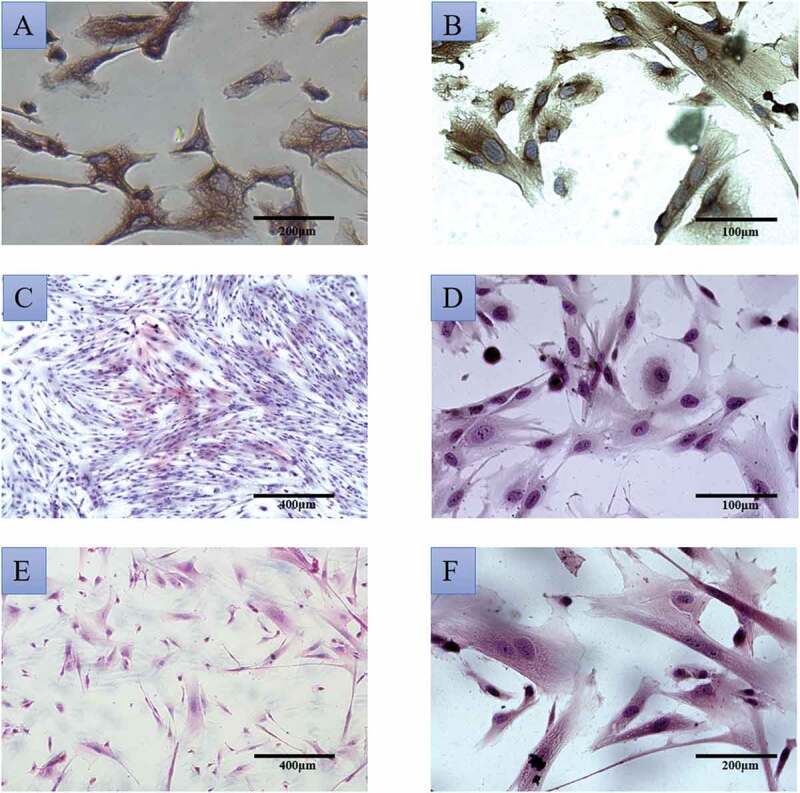
(a) immunohistochemical identification of normal endometrial stromal cells (20×); (b) Immunohistochemical identification of ectopic endometrial stromal cells (40×); HE staining of normal endometrial stromal cells (C:10×; D:40×); ectopic endometrial stromal cell morphology with HE staining (E:10×; F:20×)
Figure 2.
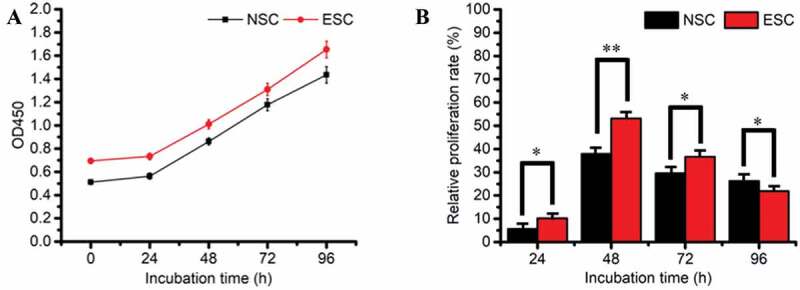
(a) The growth curve of cell; (b) cell relative proliferation rate, * refers to the significant statistical difference (P < .05). ** refers to the extremely significantly difference (P < .01)
Figure 3.
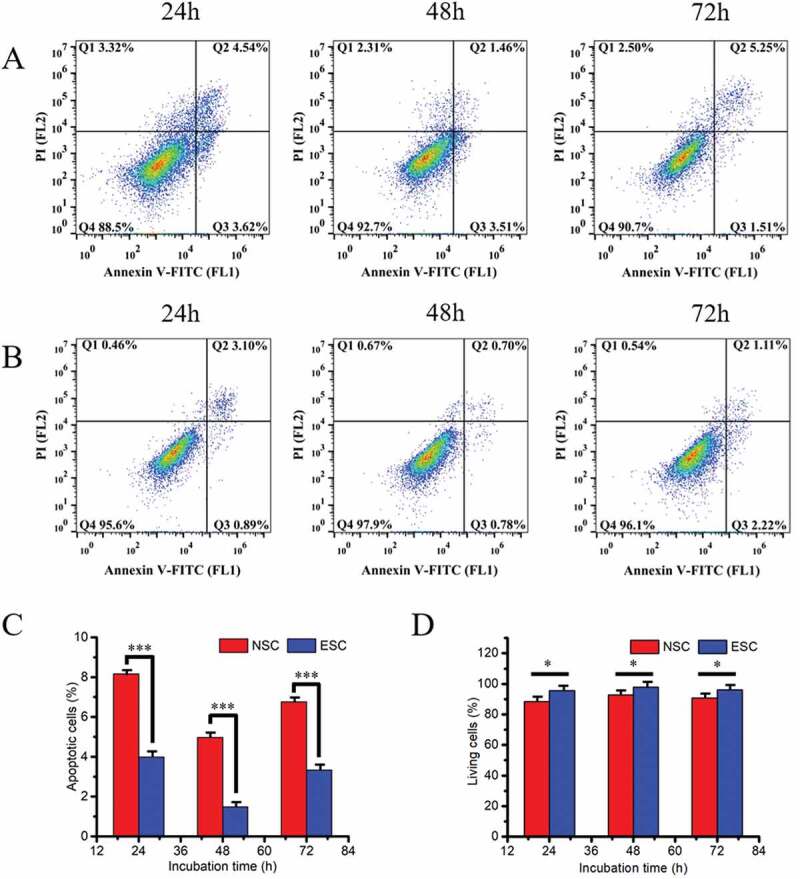
Apoptosis and living analysis of NSC (a) and ESC (b) cells at different point-in-time under normal culture conditions. (c) The total apoptosis rate statistics in NSC and ESC at different time points; (d) The total living rate statistics in NSC and ESC at different time points, * refers to the significant statistical difference (P < .05). *** refers to the extremely significantly difference (P < .001)
Relationship between abnormal FKBP52 and expression levels of PR-A and PR-B in endometriosis
In order to study the effect mechanism of FKBP52 on the expression levels of PR-A and PR-B in endometriosis, the gene silencing and overexpression of the FKBP52 model should be constructed, which used sequencing to determine whether the target sequence was built into the vector (the specific sequence showing in the supporting information). The lipofectamine 2000 cationic liposome method was used to transfer FKBP52 overexpression plasmid, empty, FKBP52-siRNA and NC-siRAN into human endometriosis cells. Figure 4(a and Figure 4c) shows cell transfection efficiency after transfection of FKBP52 overexpression plasmid and FKBP52-siNRA transfection, respectively. Figure 4(b and Figure 4b) is used to compare with fluorescence images to highlight cell transfection efficiency after transfection of FKBP52 overexpression plasmid and FKBP52-siNRA transfection. According to different transfection conditions, endometriotic stromal cells were divided into five groups: blank control group (Blank); overexpression empty vector group (pcDNA 3.1); FKBP52 overexpression group (pcDNA 3.1-FKBP52); FKBP52 silence group (siRNA-FKBP52) and siRNA blank control (siRNA-NC). The Cell proliferation curves (Figure 5(a)) of five groups showed that the cell proliferation decreased after FKBP52 overexpression and increased after silencing FKBP52. The relative proliferation rates of the five groups show the relative cell proliferation rate of siRNA-FKBP52 was significantly higher than that of pcDNA 3.1-FKBP52. However, Figure 5(a) also showed that the pcDNA3.1 was significantly decreased compared with the blank and the siRNA-NC. This may be because the pcDNA3.1 and pcDNA3.1-FKBP52 were transfected with large fragments of circular DNA, which had a greater impact on the growth of primary cells. Therefore, compared with other groups, cell growth dropped sharply or even negatively within 24 hours after transfection, resulting in little difference in absolute cell proliferation rates between the two groups. However, the proliferation curve and trend are different, which indicates that the difference in FKBP52 expression will affect cell proliferation. However, only small fragments were transfected into siRNA-NC and siRNA-FKBP52, which had little effect on the state of cell growth. The difference between the two groups also indicates that the difference in FKBP52 expression has a great influence on cell proliferation.
Figure 4.
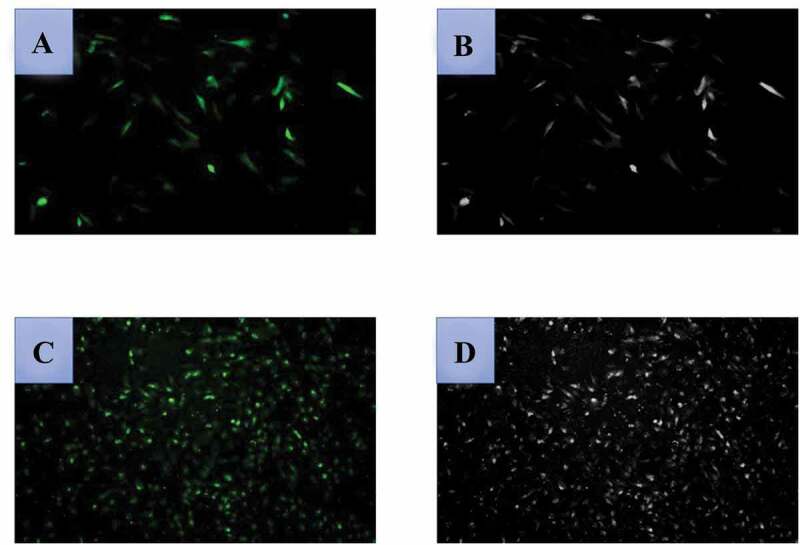
Detection of cell transfection efficiency after transfection of FKBP52 overexpression plasmid ((a): fluoroscopy×100; (b): optical microscope×100); cell transfection efficiency after FKBP52-siNRA transfection ((c): fluoroscopy×100; (d): optical microscope×100). The optical microscope refers to a picture of cells without fluorescence excitation
Figure 5.
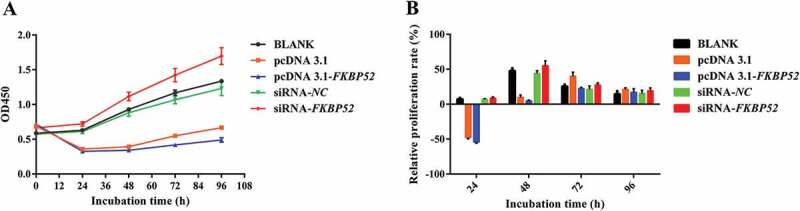
Cell proliferation curves (a) and relative proliferation rates (b) of five groups. The relative proliferation rates at 24 and 48 h have a significant level (P-value = .0001 at 24 h, P-value = 0.0001 at 48 h, statistically significant (P < .05); P-value = 0.6862 at 72 h, P-value = 0.8726 at 96 h, no statistically significant (P > .05)). The difference between the last two time points may cause by the factors such as the elimination of the transfection effect during the proliferation and growth of cells after transfection
Real-time quantitative PCR assay (qPCR) and Western blot analysis (WB) were used to analyze the mRNA expression level of each gene and the protein expression of five groups of cells, respectively. Figure 6(a) shows that the mRNA of FKBP52 increased significantly after FKBP52 gene overexpression and the mRNA of FKBP52 decreased significantly after FKBP52 gene silencing, compared with the blank group. Figure 6(b) shows that the mRNA of PR-A increased significantly after FKBP52 gene overexpression and the mRNA of PR-A decreased significantly after FKBP52 gene silencing, compared with the blank group. Figure 6(c) shows that the mRNA of PR-A decreased significantly after FKBP52 gene overexpression and the mRNA of PR-A decreased significantly after FKBP52 gene silencing, compared with the blank group. Expression of FKB52 in five groups showed that FKBP52 protein expression (Figure 7) increased significantly after FKBP52 gene overexpression and FKBP52 protein expression decreased significantly after FKBP52 gene silencing, which is consistent with the gene expression change of FKBP52. Expression of PR-A in five groups showed that the protein expression of PR-A was up-regulated after FKBP52 gene overexpression and the protein expression of PR-A was down-regulated after FKBP52 gene silencing, which is consistent of gene mRNA expression levels. Expression of PR-B in five groups showed that PR-B protein expression was down-regulated after FKBP52 gene overexpression and PR-B protein expression decreased after FKBP52 gene silencing, which is consistent with gene mRNA expression levels.
Figure 6.
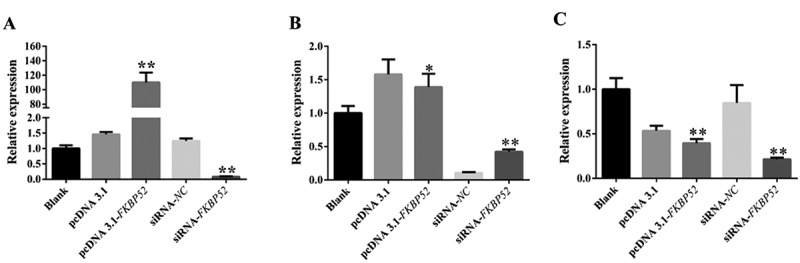
qPCR was used to detect the level of mRNA of each gene after FKBP52 overexpression and silencing. (a) Relative expression of FKBP52; (b) relative expression of PR-A; (c) relative expression of PR-B. * refers to the significant statistical difference (P < .05). ** refers to the extremely significantly difference (P < .01)
Figure 7.
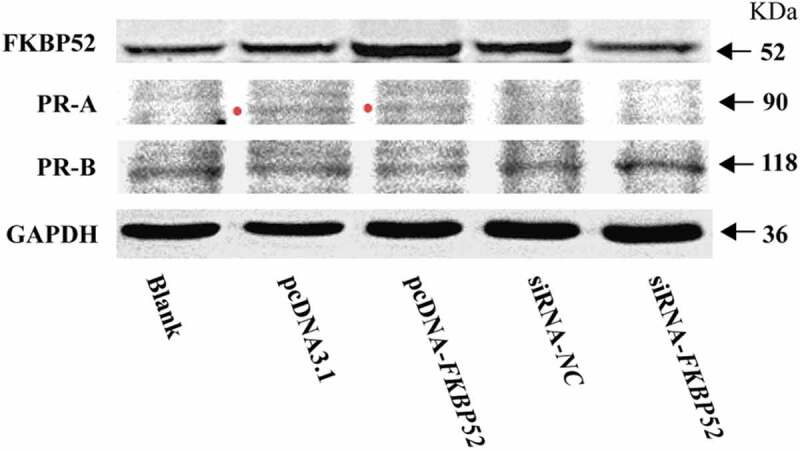
Expression of FKB52, PR-A and PR-B in five groups at vitro endometrial stromal cell model
Verifying the reliability of the mechanism and model through clinical testing
In order to further verify the correctness of the conclusions obtained from endometrial stromal cell model in vitro, we conducted a clinical study on the relationship between FKBP52 and PR expression levels in patients with endometriosis. Figure 8 shows that compared with normal endometrial tissue, the mRNA expression of FKBP52 in the ectopic endometrial tissue for the case group was significantly decreased and the mRNA expression of PR (PR-A, PR-B) in the ectopic endometrial tissue was decreased, which is consistent with the conclusion on the mRNA expression of the endometrial stromal cell model in vitro. Figure 9 shows that compared with normal endometrial tissue, the protein expression of FKBP52, PR-A, PR-B in ectopic endometrial tissue decreased, which is consistent with the conclusion on the protein expression of the endometrial stromal cell model in vitro.
Figure 8.
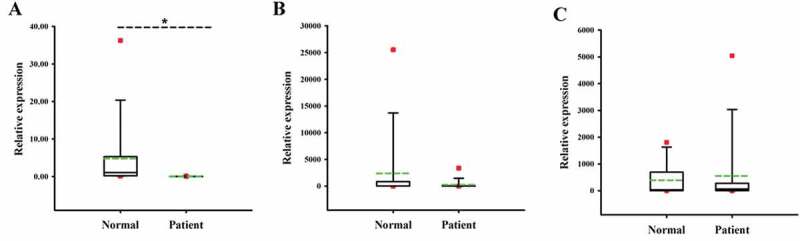
Relative expression of FKBP52, PR-A, and PR-B in normal and Ems tissues. (a) FKBP52 relative expression; (b) PR-A relative expression; (c) PR-B relative expression. The red dot is the abnormal value in the box graph analysis; the green dotted line is the mean line; *P < .05 means that the difference is of statistical significance
Figure 9.
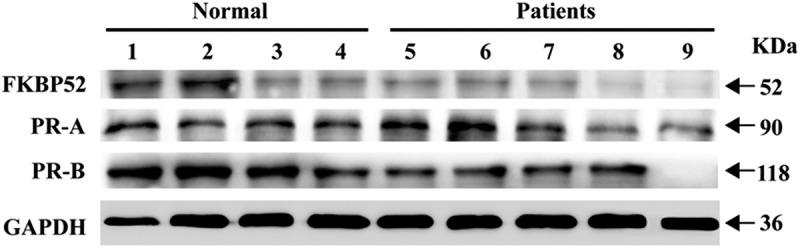
Expression of FKB52, PR, PR-A and PR-B in endometriotic tissue
Discussion
Endometriosis is a benign and common gynecological disease, which seriously affects the quality of life of patients. In order to better study the biological function of FKBP52 in Ems and its specific regulatory mechanism, an endometrial stromal cell model in vitro was established and was conducted a preliminary analysis of the characteristics for the cell model to provide reliable research models for studying the influence mechanism of abnormal immunophilin FKBP52 on the expression levels of PR-A and PR-B in endometriosis, which makes the research level from the clinical organization to the molecular level of cytology. Western Blotting, Real-time Quantitative PCR Detecting System, RNA interference, synthetic clone primer overexpression, CCK-8, flow cytometry and other experimental techniques were used to explore the effects of abnormal immunophilin FKBP52 on the expression levels of PR (PR-A, PR-B) in endometriosis. Finally, the relationship between FKBP52 and PR (PR-A, PR-B) expression levels in clinical endometriosis patients was used to further confirm the reliability of the endometrial stromal cell model in vitro and conclusions. The results show that the expression levels of PR-A mRNA and protein in endometriosis are positively correlated with FKBP52 and the abnormality of FKBP52 leads to the decrease of PR-B mRNA and protein expression. When FKBP52 is deleted or reduced, the expression levels of m RNA and protein of PR-A and PR-B have decreased leading to the proliferation of ESC cells and the occurrence of endometriosis, which is consistent with the expression levels of clinical endometriosis patients and fully confirms our conclusions and reliability of the model. The ESCM is built successfully and the influence mechanism that the decrease of FKBP52 will cause the reduction of PR-A and PR-B expression levels (ie, the expression level of PR is reduced) is confirmed by ESCM, which has great guiding significance for the research of Ems disease occurrence mechanism, clinical treatment and organ chips.
Supplementary Material
Acknowledgments
We are grateful to all family members who contributed to the study.
Funding Statement
This study was supported by the Guangxi Natural Science Foundation Youth Fund [2018GXNSFBA138047] and by the grant from the Guangxi Key Research Plan [GUIKEAB18050024].
Declaration of Potential Conflict of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article. All the relevant data in the manuscript are available.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Giudice LC, Kao LC.. Endometriosis. Lancet. 2004;364(9447):1789–99. PMID:15541453. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 2.Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. J Urology. 2006;175(2):573–573. doi: 10.1016/S0022-5347(05)00355-1. [DOI] [PubMed] [Google Scholar]
- 3.Kyama CM, Overbergh L, Mihalyi A, Meuleman C, Mwenda JM, Mathieu C, D’Hooghe TM. Endometrial and peritoneal expression of aromatase, cytokines, and adhesion factors in women with endometriosis. Fertil Steril. 2008;89(2):301–10. PMID: 17678915. doi: 10.1016/j.fertnstert.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 4.Storer CL, Dickey CA, Galigniana MD, Rein T, Cox MB. FKBP51 and FKBP52 in signaling and disease. Trends Endocrin Met. 2011;22(12):481–90. PMID: 21889356. doi: 10.1016/j.tem.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zgajnar NR, De Leo SA, Lotufo CM, Erlejman AG, Piwien-Pilipuk G, Galigniana MD. Biological actions of the Hsp90-binding immunophilins FKBP51 and FKBP52. Biomolecules. 2019;9(2):52. PMID: 30717249. doi: 10.3390/biom9020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song S, Tan Y. Expression of FKBP52 in the ovaries of PCOS rats. Int J Mol Med. 2019;43(2):868–78. PMID: 30483787. doi: 10.3892/ijmm.2018.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sivils JC, Storer CL, Galigniana MD, Cox MB. Regulation of steroid hormone receptor function by the 52-kDa FK506-binding protein (FKBP52). Curr Opin Pharmacol. 2011;11(4):314–19. PMID: 21511531. doi: 10.1016/j.coph.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romano S, D’Angelillo A, Romano MF. Pleiotropic roles in cancer biology for multifaceted proteins FKBPs. Biochim Biophys Acta. 2015;1850(10):2061–68. PMID: 25592270. doi: 10.1016/j.bbagen.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 9.De Leon JT, Iwai A, Feau C, Garcia Y, Balsiger HA, Storer CL, Suro RM, Garza KM, Lee S, Kim YS, et al. Targeting the regulation of androgen receptor signaling by the heat shock protein 90 cochaperone FKBP52 in prostate cancer cells. Proc Natl Acad Sci USA. 2011;108(29):11878–83. PMID: 21730179. doi: 10.1073/pnas.1105160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solassol J, Mange A, Maudelonde T. FKBP family proteins as promising new biomarkers for cancer. Curr Opin Pharmacol. 2011;11(4):320–25. PMID: 21514221. doi: 10.1016/j.coph.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Joshi JB, Patel D, Morton DJ, Sharma P, Zou J, Hewa Bostanthirige D, Gorantla Y, Nagappan P, Komaragiri SK, Sivils JC, et al. Inactivation of ID4 promotes a CRPC phenotype with constitutive AR activation through FKBP52. Mol Oncol. 2017;11(4):337–57. PMID: 28252832. doi: 10.1002/1878-0261.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meduri G, Guillemeau K, Dounane O, Sazdovitch V, Duyckaerts C, Chambraud B, Baulieu EE, Giustiniani J. Caspase-cleaved Tau-D (421) is colocalized with the immunophilin FKBP52 in the autophagy-endolysosomal system of Alzheimer’s disease neurons. Neurobiol Aging. 2016;46:124–37. PMID: 27479154. doi: 10.1016/j.neurobiolaging.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Giustiniani J, Sineus M, Sardin E, Dounane O, Panchal M, Sazdovitch V, Duyckaerts C, Chambraud B, Baulieu EE. Decrease of the immunophilin FKBP52 accumulation in human brains of Alzheimer’s disease and FTDP-17. J Alzheimers Dis. 2012;29(2):471–83. PMID: 22233767. doi: 10.3233/JAD-2011-111895. [DOI] [PubMed] [Google Scholar]
- 14.Rohrer JD, Ridgway GR, Modat M, Ourselin S, Mead S, Fox NC, Rossor MN, Warren JD. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. Neuroimage. 2010;53(3):1070–76. PMID: 20045477. doi: 10.1016/j.neuroimage.2009.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giustiniani J, Chambraud B, Sardin E, Dounane O, Guillemeau K, Nakatani H, Paquet D, Kamah A, Landrieu I, Lippens G, et al. Immunophilin FKBP52 induces Tau-P301L filamentous assembly in vitro and modulates its activity in a model of tauopathy. Proc Natl Acad Sci USA. 2014;111(12):4584–89. PMID: 24623856. doi: 10.1073/pnas.1402645111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandleon S, Strunz PP, Pickel S, Tiapko O, Cellini A, Miranda-Laferte E, Eder-Negrin P. FKBP52 regulates TRPC3-dependent Ca2+ signals and the hypertrophic growth of cardiomyocyte cultures. J Cell Sci. 2019;132(20). PMID: 31540954. doi: 10.1242/jcs.231506. [DOI] [PubMed] [Google Scholar]
- 17.Liang S, Bian X, Liang D, Sivils JC, Neckers LM, Cox MB, Xie H. Solution formulation development and efficacy of MJC13 in a preclinical model of castration-resistant prostate cancer. Pharm Dev Technol. 2016;21(1):121–26. PMID: 25380396. doi: 10.3109/10837450.2014.979946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies TH, Sanchez ER. Fkbp52. Int J Biochem Cell B. 2005;37(1):42–47. PMID: 15381148. doi: 10.1016/j.biocel.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Bracher A, Kozany C, Hahle A, Wild P, Zacharias M, Hausch F. Crystal structures of the free and ligand-bound FK1-FK2 domain segment of FKBP52 reveal a flexible inter-domain hinge. J Mol Biol. 2013;425(22):4134–44. PMID: 23933011. doi: 10.1016/j.jmb.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 20.Stechschulte LA, Sanchez ER. FKBP51-a selective modulator of glucocorticoid and androgen sensitivity. Curr Opin Pharmacol. 2011;11(4):332–37. PMID: 21565552. doi: 10.1016/j.coph.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daneri-Becerra C, Zgajnar NR, Lotufo CM, Ramos Hryb AB, Piwien-Pilipuk G, Galigniana MD. Regulation of FKBP51 and FKBP52 functions by post-translational modifications. Biochem Soc Trans. 2019;47(6):1815–31. PMID: 31754722. doi: 10.1042/BST20190334. [DOI] [PubMed] [Google Scholar]
- 22.Makkonen H, Kauhanen M, Paakinaho V, Jaaskelainen T, Palvimo JJ. Long-range activation of FKBP51 transcription by the androgen receptor via distal intronic enhancers. Nucleic Acids Res. 2009;37(12):4135–48. PMID: 19433513. doi: 10.1093/nar/gkp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen HY, Li OY, Pang LH, Xu H, Fan XJ, Liang HF, Chen XF, Qing JZ, Huang RD, Deng BY. Expression of FK506-binding protein 52 (FKBP52) in chorionic villi with early recurrent spontaneous abortion. J Matern Fetal Neonatal M. 2015;28(10):1165–69. PMID: 25053194. doi: 10.3109/14767058.2014.947572. [DOI] [PubMed] [Google Scholar]
- 24.Erlejman AG, De Leo SA, Mazaira GI, Molinari AM, Camisay MF, Fontana V, Cox MB, Piwien-Pilipuk G, Galigniana MD. NF-kappaB transcriptional activity is modulated by FK506-binding proteins FKBP51 and FKBP52: a role for peptidyl-prolyl isomerase activity. J Biol Chem. 2014;289(38):26263–76. PMID: 25104352. doi: 10.1074/jbc.M114.582882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratajczak T. Steroid receptor-associated immunophilins: candidates for diverse drug-targeting approaches in disease. Curr Mol Pharmacol. 2015;9(1):66–95. PMID: 25986567. doi: 10.2174/1874467208666150519113639. [DOI] [PubMed] [Google Scholar]
- 26.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18(3):306–60. PMID: 9183567. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 27.Harris DC, Garcia YA, Samaniego CS, Rowlett VW, Ortiz NR, Payan AN, Maehigashi T, Cox MB. Functional comparison of human and zebra fish FKBP52 confirms the importance of the proline-rich loop for regulation of steroid hormone receptor activity. Int J Mol Sci. 2019;20(21). PMID: 31661769. doi: 10.3390/ijms20215346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirota Y, Tranguch S, Daikoku T, Hasegawa A, Osuga Y, Taketani Y, Dey SK. Deficiency of immunophilin FKBP52 promotes endometriosis. Am J Pathol. 2008;173(6):1747–57. PMID: 18988805. doi: 10.2353/ajpath.2008.080527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tranguch S, Wang H, Daikoku T, Xie H, Smith DF, Dey SK. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest. 2007;117(7):1824–34. PMID: 17571166. doi: 10.1172/JCI31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hildenbrand ZL, Bernal RA. Interplay between Hsp90 and TPR domain-containing proteins in steroidogenic signaling. Cell Cycle. 2012;11(7):1263–64. PMID: 22421152. doi: 10.4161/cc.19889. [DOI] [PubMed] [Google Scholar]
- 31.Riggs LD, Roberts JP, Chirillo CS, Cheung-Flynn J, Prapapanich V, Ratajczak T, Gaber R, Picard D, Smith FD. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. Embo J. 2003;22(5):1158–67. PMID: 12606580. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong YY, Her J, Oh SY, Chung IK. Hsp90-binding immunophilin FKBP52 modulates telomerase activity by promoting the cytoplasmic retrotransport of hTERT. Biochem J. 2016;473(20):3517–32. PMID: 27503910. doi: 10.1042/BCJ20160344. [DOI] [PubMed] [Google Scholar]
- 33.Dyson MT, Roqueiro D, Monsivais D, Ercan CM, Pavone ME, Brooks DC, Kakinuma T, Ono M, Jafari N, Dai Y, et al. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 2014;10(3):e1004158. PMID: 24603652. doi: 10.1371/journal.pgen.1004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer JL, Zimbardi D, Podgaec S, Amorim RL, Abrao MS, Rainho CA. DNA methylation patterns of steroid receptor genes ESR1, ESR2 and PGR in deep endometriosis compromising the rectum. Int J Mol Med. 2014;33(4):897–904. PMID: 24481237. doi: 10.3892/ijmm.2014.1637. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1(2):106–11. PMID: 17965625. doi: 10.4161/epi.1.2.2766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


