Abstract
Scope:
Lipotoxicity-induced endothelial dysfunction is an important vascular complication associated with diabetes. Clinical studies support the vascular benefits of blueberry anthocyanins, but the underlying mechanism is unclear. The hypothesis that metabolites of blueberry anthocyanins attenuate lipotoxicity-induced endothelial dysfunction was tested.
Methods and results:
Human aortic endothelial cells (HAECs) were treated for 6 h with either: (i) the parent anthocyanins (malvidin-3-glucoside and cyanidin-3-glucoside); or (ii) the blueberry metabolites (hydroxyhippuric acid, hippuric acid, benzoic acid-4-sulfate, isovanillic acid-3-sulfate, and vanillic acid-4-sulfate), at concentrations known to circulate in humans following blueberry consumption. For the last 5 h HAECs were treated with palmitate or vehicle. HAECs treated with palmitate displayed elevated reactive oxygen species generation, increased mRNA expression of NOX4, chemokines, adhesion molecules, and IκBα, exaggerated monocyte binding, and suppressed nitric oxide production. Of note, the damaging effects of palmitate were ameliorated in HAECs treated with blueberry metabolites but not parent anthocyanins. Further, important translational relevance of these results was provided by our observation that palmitate-induced endothelial dysfunction was lessened in arterial segments that incubated concurrently with blueberry metabolites.
Conclusion:
The presented findings indicate that the vascular benefits of blueberry anthocyanins are mediated by their metabolites. Blueberries might complement existing therapies to lessen vascular complications.
Keywords: blueberry metabolites, endothelial dysfunction, inflammation, nitric oxide, vascular effects
1. Introduction
Endothelial dysfunction is a characteristic phenotype of cardiovascular complications associated with diabetes and related metabolic disorders.[1-3] Factors such as oxidative stress, hyperglycemia, lipotoxicity, and increased pro-inflammatory cytokines are characteristic systemic disturbances in patients with insulin-resistant conditions.[1-5] Each component has potential to contribute independently and synergistically to endothelial dysfunction by increasing endothelial inflammation and decreasing endothelial cell nitric oxide (NO) bioavailability.[3-5] This issue is clinically relevant because endothelial dysfunction contributes to pathological processes specific to large (atherosclerosis) and small (retinopathy, nephropathy, neuropathy) blood vessels.[3,6,7] Treating cardiovascular complications associated with insulin-resistant conditions contributes to increasing medical costs that burden the patient and society. Hence, there is a need to elucidate effective, low-cost, adjunct strategies to traditional therapies for treating vascular disease.
Evidence from clinical and pre-clinical models supports the vascular benefits of anthocyanins, one class of flavonoids widely available in berries.[8-12] Anthocyanins are glycosides which are comprised of the anthocyanidin aglycone and a sugar moiety. Blueberries are an excellent source of anthocyanins that include galactosides, glucosides, and arabinosides of cyanidin, delphinidin, malvidin, peonidin, and petunidin.[13] Recent studies showed that consumption of blueberries improves endothelial function in subjects with metabolic syndrome, reduces blood pressure in postmenopausal women with hypertension, and increases flow-mediated dilation in healthy individuals.[14-17] However, the mechanisms responsible for the vascular benefits of blueberry anthocyanins are unknown.
Anthocyanins are quickly metabolized by digestive enzymes and intestinal microbiota in humans suggesting vasculoprotection might be mediated by their circulating metabolites.[14] The role of blueberry metabolites in contributing to or being responsible for the vascular benefits of blueberry consumption has not been studied in a comprehensive manner because several of these compounds are not commercially available.[18] We addressed this issue by synthesizing the key blueberry metabolites and tested the hypothesis that lipotoxicity-induced endothelial dysfunction is lessened by blueberry metabolites.
2. Experimental Section
2.1. Materials
Human aortic endothelial cells (HAECs), endothelial basal medium-2 (EBM-2), and EGM-2 SingleQuot kit supplements and growth factors were purchased from Lonza (Allendale, NJ). Fetal bovine serum (FBS), cell culture supplements, calcein-AM, and RPMI 1640 were from Invitrogen (Carlsbad, CA); cell titer blue from Promega (Madison, WI); protein assay kits were from Bio-Rad (Hercules, CA); fatty acid-free BSA from Fisher Scientific (Hampton, NH); palmitate, protease and phosphatase inhibitor cocktails, malvidin-3-glucoside, cyanidin-3-glucoside, hippuric acid, hydroxyhippuric acid, 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF), 2′,7′-dichlorofluorescein diacetate, and other general chemicals were obtained from Sigma–Aldrich (St. Louis, MO). Human monocytic THP1 cells were from American Type Culture Collection (Manassas, VA). RNeasy plus mini kit, QuantiTech reverse transcription kit, SYBR qPCR (quantitative PCR) green master mix, and primers were purchased from Qiagen (Valencia, CA).
2.2. Synthesis of Blueberry Metabolites
The blueberry metabolites benzoic acid-4-sulfate (BAS; compound 3a), isovanillic acid-3-sulfate (IVAS; compound 3b), vanillic acid-4-sulfate (VAS; compound 3c) were generated using a three-step protocol (Figure 1A) that was modified from a previously published procedure.[18] Starting with 4-hydroxybenzoic acid (1a), isovanillic acid (IVA) (1b), or vanillic acid (1c), selective protection of carboxylic acid with cesium carbonate and benzyl bromide provided the benzyl esters. Installation of the protected sulfate was accomplished with the use of the 2,2,2-trichloroethyl sulfurochloridate Cl3CCH2OSO2Cl (TCE) group[19,20] to afford the sulfonylated intermediates 2a–c. Although a deprotection step is required, utilization of TCE is a reliable method for sulfonylation of phenolic groups.[21] Compounds (3a–c) were obtained as the ammonium salts by using atmospheric pressure H2(g) and stirring overnight in the presence of ammonium formate. The purity of each compound was verified by NMR (Supporting Information Figure S1A-C). The locally synthesized compounds VAS, IVAS, and BAS (Figure 1B) were combined with commercially available blueberry metabolites hydroxy hippuric acid (HHA) and hippuric acid (HA), and their combination is referred to as the blueberry metabolite mixture.
Figure 1.
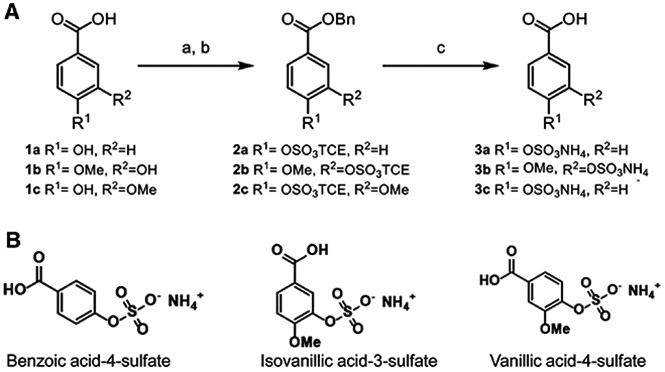
A) Three step protocol utilized to synthesize benzoic acid 4-sulfate (3a), isovanillic acid 3-sulfate (3b), and vanillic acid 4-sulfate (3c). Starting material: 4-hydroxy benzoic acid (1a), isovanillic acid (1b), and vanillic acid (1c). B) Structures of benzoic acid 4-sulfate, isovanillic acid 3-sulfate, and vanillic acid 4-sulfate.
2.3. In Vitro Analyses of Cellular Function
2.3.1. Cell Culture
HAECs were cultured in EBM-2 supplemented with EGM-2 SingleQuot endothelial growth supplements (Lonza, MD) and 2% heat inactivated FBS at 37 °C in a 5% CO2/95% oxygen environment. HAECs were passaged at 70–80% confluency and studied at passages 5–7.[22,23] Human monocytic THP1 cells (American Type Culture Collection, VA) were cultured in RPMI-1640 medium with 10% FBS.[23]
2.3.2. Palmitate, Blueberry Metabolites, and Parent Anthocyanins Treatment
HAECs were treated for 6 h with either: (i) parent anthocyanins (16 nm malvidin-3-glucoside and 12 nm cyanidin-3-glucoside); (ii) blueberry metabolites mixture (75 nm VAS, 75 nm IVAS, 700 nm BAS, 3 μm HHA, and 5 μM HA); (iii) individual metabolites; or (iv) the solubilizing vehicle DMSO. For the last 5 h HAECs in each condition were treated with 500 μm palmitate or the vehicle [bovine serum albumin (BSA)]. Palmitate (C16:0) (Sigma, MO) was coupled to fatty acid-free BSA (Fisher Scientific, NH) in the ratio of 2 m palmitate to 1 m BSA (palmitate-BSA).[24-26] The dose of palmitate was chosen based on earlier studies indicating its ability to evoke a lipotoxic stress in cells and arteries without causing cell death or apoptosis.[24-26] Further, studies indicate that palmitate is a predominant circulating saturated free fatty acid, and 500 μm palmitate mimics circulating pathophysiological conditions.[25] Upon completing the respective treatments, the following end-points were assessed in HAECs: monocyte binding; mRNA expression of NOX4, chemokines, and adhesion molecules; indices of nuclear factor κB (NFκB) signaling; and estimates of reactive oxygen species (ROS) and NO production.
2.3.3. Rationale for the Concentration of Parent Anthocyanins and Blueberry Metabolites
Evidence shows that less than 2% of parent anthocyanin reaches the circulation or target organs following the consumption of berries.[13,27] A recent study showed that only three anthocyanins (malvidin-3-glucoside, cyanidin-3-glucoside, and delphinidin-3-glucoside) appear in the plasma of humans following the consumption of blueberry puree which contains 12 anthocyanins.[28] Further, in this study delphinidin-3-glucoside was not detected in the plasma of all volunteers. Based on this study we used two parent anthocyanins (16 nm malvidin-3-glucoside and 12 nm cyanidin-3-glucoside) in our experiment. This dosage represents the concentration of these parent anthocyanins detected in the plasma following consumption of 300 g of blueberry puree.[28]
Anthocyanins are extensively metabolized by phase II enzymes and intestinal microbiota leading to the formation of diverse phenolic metabolites.[14] Indeed, 61 phenolic metabolites appear in the plasma following the consumption of blueberry.[13] However, only selected metabolites may be responsible for the specific biological effects of blueberry. A recent study measured the blueberry metabolites circulating in plasma at the time of the observed biological effect.[16] Intake of anthocyanins that is equivalent to ≈240 g ( ≈2 cups) of blueberries increased flow-mediated dilation in humans that was accompanied by increases in the plasma concentrations of phenolic metabolites such as VA, homovanillic acid, benzoic acid, HA, and HHA.[16] This suggests that these metabolites may be responsible for the improvements in endothelial function. In this study, samples were prepared by using enzymatic hydrolysis with sulfatase to produce non-sulfated metabolites for plasma metabolite analysis. Human studies reported that some of these metabolites exist as sulfated metabolites such as VAS, IVAS, and BAS in the plasma of humans.[29,30] Based on these human studies, a physiologically relevant mixture of blueberry metabolites was calculated i.e., 75 nm VAS, 75 nm IVAS, 700 nm BAS, 3 μm HHA, and 5 μm HA. This mixture/dosage closely represents the circulating levels of these metabolites in humans that consume ≈ 240 g of blueberries. Collectively, the choice and dosages of parent anthocyanins and metabolites used in our study are based on available published evidence, and physiologically relevant concentrations that have been reported.
Stock solution of 20 000 × blueberry metabolites mixture or parent anthocyanins in DMSO was prepared and stored at −80 °C before use.
2.3.4. Cell Viability Assessment
We measured the effect of palmitate and/or blueberry metabolites on cell viability. Cell viability following palmitate or metabolites treatment was estimated as [(number of cells per field before treatment – number of cells per field after treatment) / (number of cells per field before treatment)] × 100.[24-26,31,32] Cell viability was also assessed using cell titer blue based on manufacturer’s instruction.
2.3.5. Monocyte Adhesion Assay
Monocyte adhesion was determined using fluorescent-labelled THP-1 human monocytic cells as we described.[23] Tumor necrosis factor-α (TNF-α) was used as a positive control and HAEC were incubated with 10 μg L−1 TNF-α for 6 h before adhesion assay.
2.3.6. Gene Expression Analysis by Quantitative Real-Time PCR
Gene expression analysis of IL-8, monocyte chemotactic protein-1 (MCP-1), vascular adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), NFκB - p65, inhibitor κB kinase (IκKβ), IκBα, and NADPH oxidase 4 (NOX4) was determined by qPCR as described.[33] Briefly, mRNA was isolated from HAECs using the RNeasy plus mini kit and cDNA was synthesized using the reverse transcription kit.[33] qPCR analysis was completed with cDNA template, SYBR Green Master Mix, and the respective Qiagen primer sets for IL-8, MCP-1, VCAM-1, ICAM-1, IκKβ, IκBα, and NOX4. The obtained signal was analyzed in Applied Biosystems QuantStudio 12K Flex Real-Time PCR System (Thermo Fisher Scientific). Copy numbers of cDNA targets were quantified using Ct values. Respective gene expression levels were calculated by normalizing to the level of the housekeeping gene GAPDH.[32]
2.3.7. ROS and NO Assay
Estimates of ROS and NO production were made using 2′,7′-dichlorofluorescein diacetate and 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (diaminofluorescein diacetate; DAF-FM diacetate) (Sigma), respectively, as we described.[31] To determine insulin-stimulated NO production HAECs were treated with 100 nm insulin for the last 30 min of each respective treatment as we described.[24-26,31,32]
2.4. Ex Vivo Analyses of Vascular Function
2.4.1. Animals
Seven-week old male C57Bl/6J mice were obtained from the Jackson Laboratories, USA. Animals were housed under controlled temperature and light conditions and were provided with food and water ad libitum. C57BL/6 male mice are a widely used animal model to study vascular function and we have previously used these mice for ex vivo vessel function experiments.[24-26] We used segments of aortae from male mice for ex vivo vessel experiments due to concerns that ovary-intact female animals are intrinsically more variable than males because of cyclical reproductive hormones.[34] Mice live to be ≈ 24 months and our model would be equivalent to a young-adult i.e., 20 years of age. We used eight segments of aortae per group for the vessel function experiment. The major difference in outcome was expected between BSA vs Pal and Pal vs Pal + blueberry metabolite treated vessels. The sample size was based on a previous study that determined vessel function in BSA vs palmitate treated vessel.[35] With a comparable effect size, we estimated that the sample size of eight will provide 80% power (estimated by f-test, analysis of variance (ANOVA), alpha = 0.05). All experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Utah (Protocol number: 15–12011).
2.4.2. Tissue Preparation and Measurement of Vascular Function
Mice were anesthetized using 2–5% isoflurane. When an adequate plane of anesthesia was achieved, the chest was opened, the heart excised, and the entire aorta was isolated while immersed in ice-cold normal physiological saline solution, and removed from the chest. The aortic segments isolated from male C57Bl/6J mice (7.1 ± 0.3 weeks of age; 25.8 ± 0.8 g body weight) were cut into equal segments that were incubated for 16 h with BSA, 500 μm palmitate + DMSO, or 500 μm palmitate + blueberry metabolites. After each treatment segments were used to assess vascular function (Supporting Information Table) using isometric tension techniques as we described.[24-26]
2.5. Data Analyses
Data are presented as mean ± SE, and p < 0.05 was considered significantly different. Comparison of one time point among groups was made using one-way ANOVA with SPSS/10. Comparison of multiple time points among groups was made using a one-way or two-way repeated-measure ANOVA using Prism. Tukey post hoc tests were performed when significant main effects were obtained.
3. Results and Discussion
We tested the overall hypothesis that vascular benefits of blueberry anthocyanins reported to exist in clinical and preclinical models are mediated by their circulating metabolites. To this point, a comprehensive evaluation of this hypothesis has been difficult to explore because many of the metabolites are not available commercially. We addressed this issue by synthesizing three important blueberry metabolites (VAS, IVAS, and BAS) and combining them with two metabolites that do exist commercially (HHA and HA) to comprise a physiologically relevant blueberry metabolites mixture. The ability of blueberry metabolites to lessen lipotoxicity-induced endothelial dysfunction was assessed and compared to that exerted by the parent anthocyanins.
3.1. Characterization of Blueberry Metabolites and Cell Viability
The purity and identity of VAS, IVAS, and BAS were confirmed by NMR (Supplementary Figure 1A-C). Blueberry metabolites or palmitate treatment at the dosage and duration used in this study did not reduce cell viability (Supporting Information Figure S2A and B).
3.2. Palmitate-Induced Endothelial Inflammation is Prevented by Blueberry Metabolites but not by Parent Anthocyanins
We tested whether palmitate-induced adhesion and inflammation are attenuated by blueberry metabolites. As expected, palmitate increased the binding of monocytic THP1 cells to HAECs as compared to control (Figure 2A). In support of our hypothesis, palmitate-induced monocyte binding to HAECs was prevented in HAECs treated with blueberry metabolites (Figure 2A). This is clinically relevant because exaggerated monocyte binding to the endothelium is a key initial event in the pathogenesis of endothelial dysfunction.[14,22,36] In contrast, the parent anthocyanins failed to exert such a protective effect (Figure 2A). We further investigated the influence of individual metabolites at physiologically-relevant concentrations on monocyte binding to ECs. BAS (p = 0.077) and HA (p = 0.054) modestly reduced palmitate-induced monocyte binding to HAECs (Figure 2B). These data suggest beneficial vascular effects of blueberries could be secondary to the synergistic effect(s) of circulating metabolites.
Figure 2.
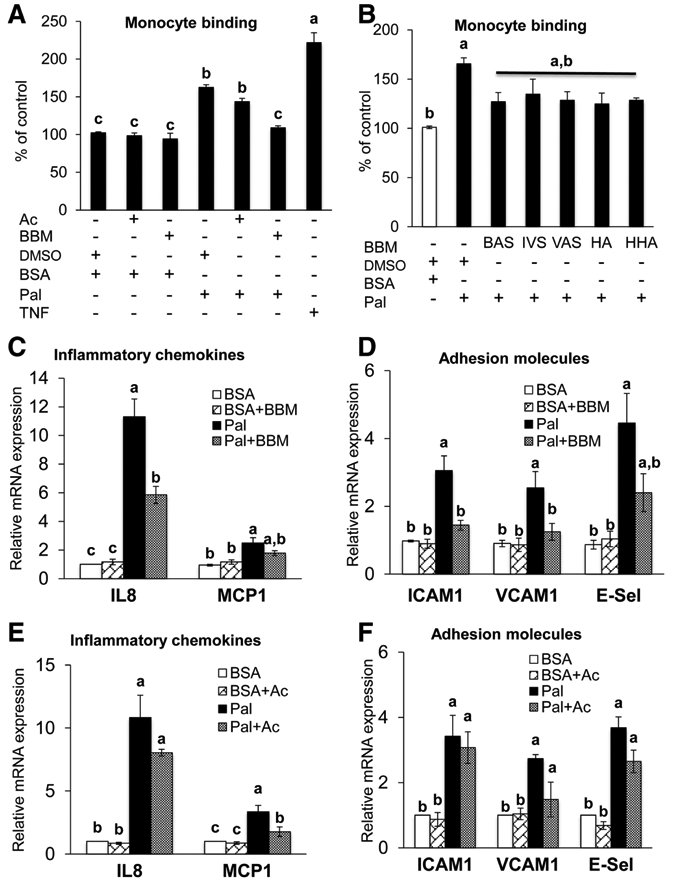
HAECs treated ± parent anthocyanins (Ac) ± blueberry metabolites ± vehicle for 6 h and with or without palmitate-BSA (Pal) or vehicle (BSA) for the last 5 h. A) The binding of monocyte to HAECs was determined by using fluorescent-labelled THP-1 human monocytic cells. TNF-α (6 h × 10 μg L−1) served as a positive control. Values are mean ± SEM, n = 8 (means of triplicate). B) The binding of monocyte to HAECs was determined by using fluorescent labelled THP-1 human monocytic cells. Values are mean ± SEM, n = 4 (means of triplicate). mRNA expression of IL-8 and MCP1 C), and ICAM1, VCAM1 and E-selectin D) were analyzed by qPCR using SYBR green. Values are mean ± SEM, n = 6 (means of duplicate). mRNA expression of IL-8 and MCP1 E), and ICAM1, VCAM1 and E-selectin F) and were analyzed by qPCR using SYBR green. Values are mean ± SEM, n = 4 (means of duplicate). Means without a common letter differ, p < 0.05.
Next we examined whether the beneficial effect of blueberry metabolites on monocyte binding might result from their ability to limit palmitate-induced activation of chemokines and adhesion molecules. Activation of chemokines and adhesion molecules is required for monocyte rolling, enhanced endothelium-monocyte interaction, and subsequent monocyte recruitment into the subendothelial space.[22,23,37] Palmitate increased the mRNA expression of IL8, MCP1, ICAM1, VCAM1, and E-Selectin (Figure 2C and D), major inflammatory factors involved in endothelial dysfunction.[22,23] Blueberry metabolites completely prevented palmitate-induced expression of IL-8, ICAM1, and VCAM1 in HAECs (Figure 2C and D). Although treatment with the parent anthocyanins did not negate palmitate-induced inflammation and adhesion, a modest attenuation of MCP1 activation was observed (Figure 2E and F). Thus, palmitate-induced endothelial inflammation and adhesion is sensitive to treatment with blueberry metabolites but not their parent anthocyanins.
3.3. Palmitate-Induced Suppression of Insulin-Stimulated NO Production is Prevented by Blueberry Metabolites
Studies in cultured cells,[26] isolated arteries,[24-26,38] animal models,[24-26,39] and humans[40,41] demonstrate that lipotoxicity has strong potential to impair NO production. Endothelial cell derived NO has vasodilatory, anti-inflammatory, and antiproliferative properties.[3,7,14,42] As such, any mismatch between production and destruction of this molecule has potential to precipitate cardiovascular complications. Therefore, we sought to determine whether blueberry metabolites might protect against the suppressed NO production that occurs in the context of lipotoxicity.[24-26] Proof of concept for this hypothesis exists. For example, increased blood NO was reported in postmenopausal women with hypertension following blueberry consumption.[15] As predicted, palmitate reduced insulin-stimulated NO production in HAECs, and this response was restored by concurrent treatment with blueberry metabolites (Figure 3A).
Figure 3.
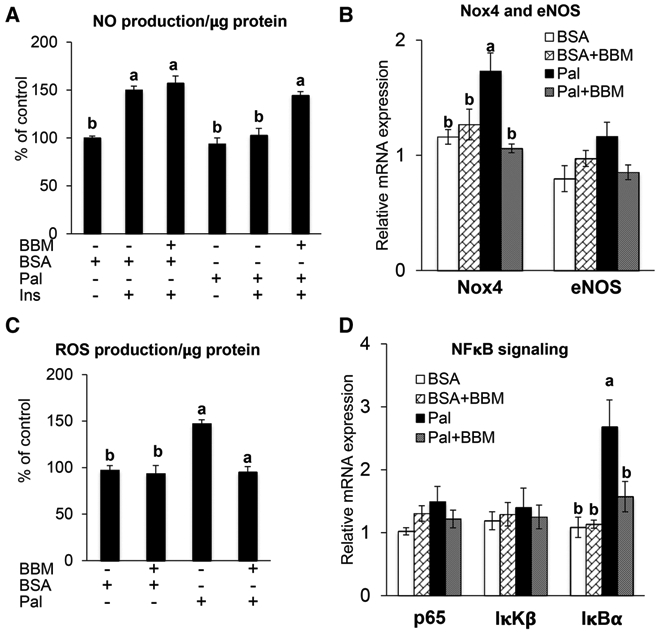
HAECs were treated for 6 h with either: (i) Parent anthocyanins (Ac) [16 nM MG and 12 nM CG]; (ii) Blueberry metabolites [75 nm VAS, 75 nm IVAS, 700 nm BAS, 3 μm HHA, and 5 μm HA]; or (iii) the solubilizing vehicle DMSO. For the last 5 h HAECs in each condition were treated with 500 μM palmitate or the vehicle BSA. A) To determine insulin-stimulated NO production HAEC were treated with 100 nm insulin (Ins) for the final 30 min of each treatment. Values are mean ± SEM, n = 6 (means of duplicate). B) mRNA expression of endothelial nitric oxide synthase and NOX4 were analyzed by qPCR using SYBR green. Values are mean ± SEM, n = 4 (means of duplicate). C) ROS was determined by DCF assay. Values are mean ± SEM, n = 6 (means of duplicate). D) mRNA expression of NFκB-p65, IκKβ and IκBα were analyzed by qPCR using SYBR green. Values are mean ± SEM, n = 4 (means of duplicate). Means without a common letter differ, p < 0.05.
3.4. Palmitate-Induced ROS Generation, NOX4 Expression, and IκBα Expression is Prevented by Blueberry Metabolites
ROS play a major role in the development of endothelial dysfunction and NOX is an important source of ROS in the vasculature.[7,14] Palmitate can increase NOX levels in ECs that are associated with ROS production.[43,44] Congruent with this, we observed that palmitate increased NOX4 expression (Figure 3B) and ROS production (Figure 3C). As hypothesized, both effects were prevented by concurrent treatment with blueberry metabolites (Figure 3B and C). This finding suggests strongly that reduced NOX activity observed in healthy humans following blueberry intake[16] might be mediated by their metabolites rather than by the parent anthocyanins.
NFκB plays a major role in endothelial dysfunction by up-regulating chemokines and adhesion molecules.[2] Inhibitor κB kinase activates the nuclear translocation of NFκB-p50/p65 by degrading the inhibitor IκBα.[45] In the nucleus, p50/p65 binds to the promoters of NFκB-dependent inflammatory genes to thereby mediate endothelial dysfunction.[46,47] In the present study, the expression of NFκB-p65 and IκKβ were refractory to palmitate-treatment (Figure 3D). However, palmitate robustly increased the expression of IκBα and this effect was prevented by treatment with blueberry metabolites (Figure 3D). Although speculative, increased IκBα expression in palmitate-treated HAECs might be an adaptive response to inhibit the nuclear translocation of p50/p65. Further, diminished IκBα expression after blueberry metabolite treatment could be secondary to a reduction of palmitate induced metabolic stress.
3.5. Palmitate-Induced Endothelium-Dependent Dysfunction is Attenuated by Blueberry Metabolites
We determined the extent to which our findings from HAECs might be translated to intact arteries. As hypothesized, the severity of palmitate-induced endothelium-dependent dysfunction was less in arteries treated with blueberry metabolites (Figure 4A), whereas no differences existed among groups concerning responses to the endothelium-independent vasodilator sodium nitroprusside (Figure 4B). Collectively, these data suggest that blueberry metabolites normalize a palmitate-induced defect that is specific to the endothelium. These data are congruent with reports indicating that blueberry consumption is beneficial concerning blood pressure and endothelial function in subjects with metabolic syndrome.[11,17] Taken together results from our study suggest that the benefits of blueberry consumption might be mediated through their circulating metabolites.
Figure 4.
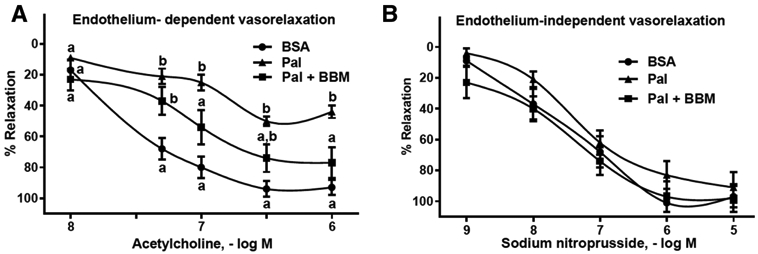
Aorta segments were incubated for 16 h with BSA, 500 μM palmitate, or 500 μm palmitate + blueberry metabolites. A) Endothelium-dependent vasorelaxation was determined by evaluating responses to acetylcholine. B) Endothelium-independent vasorelaxation was determined by evaluating responses to sodium nitroprusside. Values are mean ± SEM, n = 8 (means of duplicate). Means without a common letter differ, p < 0.05.
4. Concluding Remarks
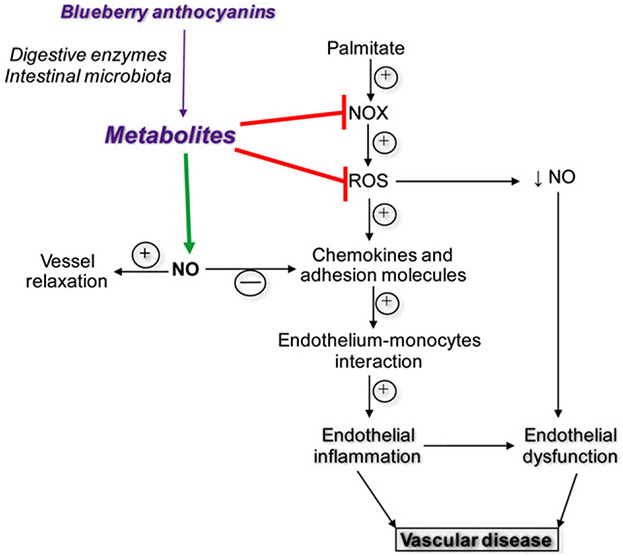
Blueberry metabolites lessen indices of lipotoxicity-induced oxidant stress, inflammation, and monocyte adhesion in HAECs, and attenuate lipotoxicity-evoked endothelial dysfunction in arteries. A possible explanation for these findings is that blueberry metabolites suppress NOX-mediated ROS production, which increases bioavailable NO to an extent that improves endothelial cell function (Figure 5). These results indicate that the benefits of blueberry consumption in the context of lipotoxicity-induced vascular dysfunction, at least in part, are secondary to their metabolites. Herein we provide solid proof of concept that blueberries might prevent, delay the onset, or lessen the severity of lipotoxicity-induced endothelial dysfunction.
Figure 5.
Working hypothesis for the protective effect of blueberry metabolites on endothelial cell function.
Supplementary Material
Acknowledgments
D.B., R.R.M.C., C.P., N.B., and B.R.C. contributed equally to this work. This work was supported by research grants from the University of Utah research start-up fund, University of Utah Seed Grant, and College of Health Pilot grant (to P.V.A.B.); the University of Utah Undergraduate Research Opportunities Program award (to B.C.); Native American Research Internship (to N.B.); and Brazil Scientific Mobility Program (to R.R.M.C., M.M.A.C., R.K.L.G.R., M.R.F.). J.D.S was supported by the American Heart Association (AHA:16GRNT31050004) and National Institutes of Health (NIH:RO3AGO52848). N.S.R. was supported by the National Institutes of Health (HL118067 and AG042860).
Abbreviations
- ANOVA
analysis of variance
- BAS
benzoic acid-4-sulfate
- DAF
4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate
- HA
hippuric acid
- HAEC
human aortic endothelial cells
- HHA
hydroxy hippuric acid
- ICAM-1
intercellular adhesion molecule-1
- IVAS
isovanillic acid-3-sulfate
- IVS
isovanillic acid; IκKβ, inhibitor κB kinase
- MCP
monocyte chemoattractant protein-1
- NFκB
nuclear factor κB
- NO
nitric oxide
- NOX
NADPH oxidases
- ROS
reactive oxygen species
- TCE
2,2,2-trichloroethyl sulfurochloridate Cl3CCH2OSO2Cl
- TNF-α
Tumor necrosis factor-α
- VAS
vanillic acid-4-sulfate
- VCAM-1
vascular adhesion molecule-1
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest
The authors have declared no conflict of interest.
Contributor Information
Divya Bharat, Department of Nutrition and Integrative Physiology, College of Health, University of Utah, Salt Lake City, USA.
Rafaela Ramos Mororo Cavalcanti, Department of Nutrition and Integrative Physiology, College of Health, University of Utah, Salt Lake City, USA.
Chrissa Petersen, Department of Nutrition and Integrative Physiology, College of Health, University of Utah, Salt Lake City, USA.
Nathan Begaye, Department of Nutrition and Integrative Physiology, College of Health, University of Utah, Salt Lake City, USA.
Brett Ronald Cutler, Department of Nutrition and Integrative Physiology, College of Health, University of Utah, Salt Lake City, USA.
Marcella Melo Assis Costa, Department of Nutrition and Integrative Physiology, College of Health, University of Utah, Salt Lake City, USA.
Renata Kelly Luna Gomes Ramos, Department of Nutrition and Integrative Physiology, College of Health, University of Utah, Salt Lake City, USA.
Marina Ramos Ferreira, Department of Nutrition and Integrative Physiology, College of Health, University of Utah, Salt Lake City, USA.
Youyou Li, Department of Nutrition and Integrative Physiology, College of Health, University of Utah, Salt Lake City, USA.
Leena P. Bharath, Department of Nutrition and Integrative Physiology, College of Health, University of Utah, Salt Lake City, USA
Emma Toolson, Department of Nutrition and Integrative Physiology, College of Health, University of Utah, Salt Lake City, USA.
Paul Sebahar, Synthetic and Medicinal Chemistry Core, University of Utah, Salt Lake City, USA.
Ryan E. Looper, Synthetic and Medicinal Chemistry Core, University of Utah, Salt Lake City, USA
Thunder Jalili, Department of Nutrition and Integrative Physiology, College of Health, University of Utah, Salt Lake City, USA.
Namakkal S. Rajasekaran, Cardiac Aging and Redox Signaling Laboratory, Division of Molecular and Cellular Pathology, Department of Pathology, University of Alabama at Birmingham, Birmingham, USA
Zhenquan Jia, Department of Biology, University of North Carolina at Greensboro, Greensboro, USA.
J. David Symons, Department of Nutrition and Integrative Physiology, College of Health, University of Utah, Salt Lake City, USA; Division of Endocrinology, Metabolism, Diabetes, and Molecular, Medicine Program, University of Utah, Salt Lake City, USA.
Pon Velayutham Anandh Babu, Department of Nutrition and Integrative Physiology, College of Health, University of Utah, Salt Lake City, USA.
References
- [1].Park KH, Park WJ, J. Korean Med. Sci 2015, 30, 1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Savoia C, Schiffrin EL, Clin. Sci 2007, 112, 375. [DOI] [PubMed] [Google Scholar]
- [3].Symons JD, Abel ED, Rev. Endocr. Metab. Disord 2013, 14, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tabit CE, Chung WB, Hamburg NM, Vita JA, Rev. Endocr. Metab. Disord 2010, 11, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chistiakov DA, Orekhov AN, Bobryshev YV, Front. Physiol 2015, 6, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Triggle CR, Ding H, J. Am. Soc. Hypertens 2010, 4, 102. [DOI] [PubMed] [Google Scholar]
- [7].Symons JD, Diabetes 2013, 62, 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhu Y, Xia M, Yang Y, Liu F, Li Z, Hao Y, Mi M, Jin T, Ling W, Clin. Chem 2011, 57, 1524. [DOI] [PubMed] [Google Scholar]
- [9].Cassidy A, Mukamal KJ, Liu L, Franz M, Eliassen AH, Rimm EB, Circulation 2013, 127, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jennings A, Welch AA, Fairweather-Tait SJ, Kay C, Minihane AM, Chowienczyk P, Jiang B, Cecelija M, Spector T, Macgregor A, Cassidy A, Am. J. Clin. Nutr 2012, 96, 781. [DOI] [PubMed] [Google Scholar]
- [11].Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, Aston CE, Lyons TJ, J. Nutr 2010, 140, 1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Basu A, Lyons TJ, J. Agric. Food Chem 2012, 60, 5687. [DOI] [PubMed] [Google Scholar]
- [13].Feliciano RP, Istas G, Heiss C, Rodriguez-Mateos A, Molecules 2016, 21, E1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cutler BR, Petersen C, Anandh Babu PV, Mol. Nutr. Food Res 2017, 61, 1600271. [DOI] [PubMed] [Google Scholar]
- [15].Johnson SA, Figueroa A, Navaei N, Wong A, Kalfon R, Ormsbee LT, Feresin RG, Elam ML, Hooshmand S, Payton ME, Arjmandi BH, J. Acad. Nutr. Diet 2015, 115, 369. [DOI] [PubMed] [Google Scholar]
- [16].Rodriguez-Mateos A, Rendeiro C, Bergillos-Meca T, Tabatabaee S, George TW, Heiss C, Spencer JP, Am. J. Clin. Nutr 2013, 98, 1179. [DOI] [PubMed] [Google Scholar]
- [17].Stull AJ, Cash KC, Champagne CM, Gupta AK, Boston R, Beyl RA, Johnson WD, Cefalu WT, Nutrients 2015, 7, 4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang Q, Raheem KS, Botting NP, Slawin AMZ, Kay CD, O’Hagan D, Tetrahedron 2012, 68, 4194. [Google Scholar]
- [19].Liu Y, Lien IF, Ruttgaizer S, Dove P, Taylor SD, Org. Lett 2004, 6, 209. [DOI] [PubMed] [Google Scholar]
- [20].Ingram LJ, Desoky A, Ali AM, Taylor SD, J. Org. Chem 2009, 74, 6479. [DOI] [PubMed] [Google Scholar]
- [21].Pitts AK, O’Hara F, Snell RH, Gaunt MJ, Angew. Chem. Int. Ed. Engl 2015, 54, 5451. [DOI] [PubMed] [Google Scholar]
- [22].Babu PV, Si H, Fu Z, Zhen W, Liu D, J. Nutr 2012, 142, 724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Babu PV, Si H, Liu D, Mol. Nutr. Food Res 2012, 56, 1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bharath LP, Ruan T, Li Y, Ravindran A, Wan X, Nhan JK, Walker ML, Deeter L, Goodrich R, Johnson E, Munday D, Mueller R, Kunz D, Jones D, Reese V, Summers SA, Babu PV, Holland WL, Zhang QJ, Abel ED, Symons JD, Diabetes 2015, 64, 3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang QJ, Holland WL, Wilson L, Tanner JM, Kearns D, Cahoon JM, Pettey D, Losee J, Duncan B, Gale D, Kowalski CA, Deeter N, Nichols A, Deesing M, Arrant C, Ruan T, Boehme C, McCamey DR, Rou J, Ambal K, Narra KK, Summers SA, Abel ED, Symons JD, Diabetes 2012, 61, 1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED, Circ. Res 2009, 104, 1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lila MA, Burton-Freeman B, Grace M, Kalt W, Annu. Rev. Food Sci. Technol 2016, 7, 375. [DOI] [PubMed] [Google Scholar]
- [28].Del Bo C, Riso P, Brambilla A, Gardana C, Rizzolo A, Simonetti P, Bertolo G, Klimis-Zacas D, Porrini M, J. Agric. Food Chem 2012, 60, 9298. [DOI] [PubMed] [Google Scholar]
- [29].de Ferrars RM, Cassidy A, Curtis P, Kay CD, Mol. Nutr. Food Res 2014, 58, 490. [DOI] [PubMed] [Google Scholar]
- [30].de Ferrars RM, Czank C, Zhang Q, Botting NP, Kroon PA, Cassidy A, Kay CD, Br. J. Pharmacol 2014, 171, 3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bharath LP, Mueller R, Li Y, Ruan T, Kunz D, Goodrich R, Mills T, Deeter L, Sargsyan A, Anandh Babu PV, Graham TE, Symons JD, Can. J. Physiol. Pharmacol 2014, 92, 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bharath LP, Cho JM, Park SK, Ruan T, Li Y, Mueller R, Bean T, Reese V, Richardson RS, Cai J, Sargsyan A, Pires K, Anandh Babu PV, Boudina S, Graham TE, Symons JD, Arterioscler. Thromb. Vasc. Biol 2017, 37, 1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Muthusamy VR, Kannan S, Sadhaasivam K, Gounder SS, Davidson CJ, Boeheme C, Hoidal JR, Wang L, Rajasekaran NS, Free Rad. Biol. Med 2012, 52, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zucker I, Beery AK, Nature 2010, 465, 690. [DOI] [PubMed] [Google Scholar]
- [35].Toral M, Romero M, Jimenez R, Mahmoud AM, Barroso E, Gómez-Guzmán M, Sánchez M, Cogolludo Á, García-Redondo AB, Briones AM, Vázquez-Carrera M, Pérez-Vizcaíno F, Duarte J, Clin. Sci 2015, 129, 823. [DOI] [PubMed] [Google Scholar]
- [36].Pittet MJ, Swirski FK, Eur. J. Immunol 2011, 41, 2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Durpes MC, Morin C, Paquin-Veillet J, Beland R, Paré M, Guimond MO, Rekhter M, King GL, Geraldes P, Cardiovasc. Res 2015, 106, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW, Circ. Res 2007, 100, 1589. [DOI] [PubMed] [Google Scholar]
- [39].Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M, J. Clin. Invest 2006, 116, 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD, Diabetes 2000, 49, 1231. [DOI] [PubMed] [Google Scholar]
- [41].Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD, J. Clin. Invest 1997, 100, 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wende AR, Symons JD, Abel ED, Curr. Hypertens. Rep 2012, 14, 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H, Diabetes 2000, 49, 1939. [DOI] [PubMed] [Google Scholar]
- [44].Kroller-Schon S, Jansen T, Schuler A, Oelze M, Wenzel P, Hausding M, Kerahrodi JG, Beisele M, Lackner KJ, Daiber A, Münzel T, Schulz E, Arterioscler. Thromb. Vasc. Biol 2013, 33, 1928. [DOI] [PubMed] [Google Scholar]
- [45].Kim F, Tysseling KA, Rice J, Gallis B, Haji L, Giachelli CM, Raines EW, Corson MA, Schwartz MW, J. Mol. Cell Cardiol 2005, 39, 327. [DOI] [PubMed] [Google Scholar]
- [46].Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R, Diabetes 1999, 48, 855. [DOI] [PubMed] [Google Scholar]
- [47].Bartchewsky W Jr. Martini MR, Masiero M, Squassoni AC, Alvarez MC, Ladeira MS, Salvatore D, Trevisan M, Pedrazzoli J Jr. Ribeiro ML, Scand. J. Gastroenterol 2009, 44, 153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.