In Canada, MMR (measles, mumps and rubella) vaccination of young children has been largely successful in preventing congenital rubella through vaccine-induced immunity and reduced circulation of rubella virus. However, outbreaks of rubella continue to occur,1 and cases of congenital rubella syndrome are reported every year.1 Many more cases go unreported.2 About half of the cases of congenital rubella syndrome in Canada and the United States result from missed opportunities for postpartum MMR vaccination.1,3,4 MMR vaccination before discharge from hospital of postpartum women without documented rubella immunity is recommended by the Canadian National Advisory Committee on Immunization5 and the US Advisory Committee on Immunization Practices.6 Relying on physician-initiated prescription of postpartum MMR vaccination has been repeatedly documented to result in a high proportion of women remaining unvaccinated.3,4,7 Recently, Gyorkos and colleagues showed that only 27% of seronegative women who delivered in 16 Canadian hospitals were vaccinated before hospital discharge, and even fewer (2%) were vaccinated during the following 3 months.7
Institution-based policies for postpartum MMR vaccination have been recommended.3,4,8,9 We were, however, unable to find documentation of the effectiveness of institutional approaches.
At the Ottawa Hospital, General Campus, comprehensive printed postpartum orders replaced handwritten orders in July 1997. The printed orders include the following: “MMR vaccine 0.5 mL SC if not rubella immune. If no result available, do titre, to be sent to attending staff.” If MMR vaccination is contraindicated, the physician is responsible for cancelling the vaccine order. (Rubella titre is supposed to be routinely entered on the Ontario antenatal record.) The postpartum nurse informs the patient that her doctor has recommended that she be immunized with MMR, because her antenatal test has shown that she is susceptible to rubella. The nurse then obtains written consent and administers the vaccine.
We carried out a study to determine whether these printed orders increased the proportion of rubella-susceptible women who received MMR vaccination before hospital discharge. This audit was approved by the quality assurance committee (the Ottawa General Hospital Research Ethics Board did not review quality assurance projects at that time.) We studied retrospectively a random sample of women who delivered a live infant or had a stillbirth during 1-year periods before (1996) and after (1998) the introduction of printed postpartum orders. The target sample size for each period was 60 rubella-susceptible women. If a patient was susceptible to rubella, we determined whether any health care provider was aware of this during admission for delivery and whether vaccination was given. If subjects did not receive indicated MMR vaccination, we tried to find out why. The complete medical record was searched for rubella serostatus, but we did not contact health care providers or laboratories outside the hospital regarding either antepartum serostatus or MMR vaccination after discharge.
For each study period, the number of deliveries, the number of medical records reviewed and the rubella serology results are shown in Fig. 1. Susceptibility to rubella was identified in 4.9% (58/1172) of women who delivered before and 6.7% (60/893) who delivered after the orders were introduced. MMR was given to 12.1% (7/58) of susceptible women before and 81.7% (49/60) after implementation of the preprinted orders, which is a 69.6% absolute increase (risk ratio 6.8, 95% confidence interval [CI] 3.3–13.7.) The intervention continues to be effective: a review of 10 seronegative women who delivered in March and April 2001 showed that all received MMR.
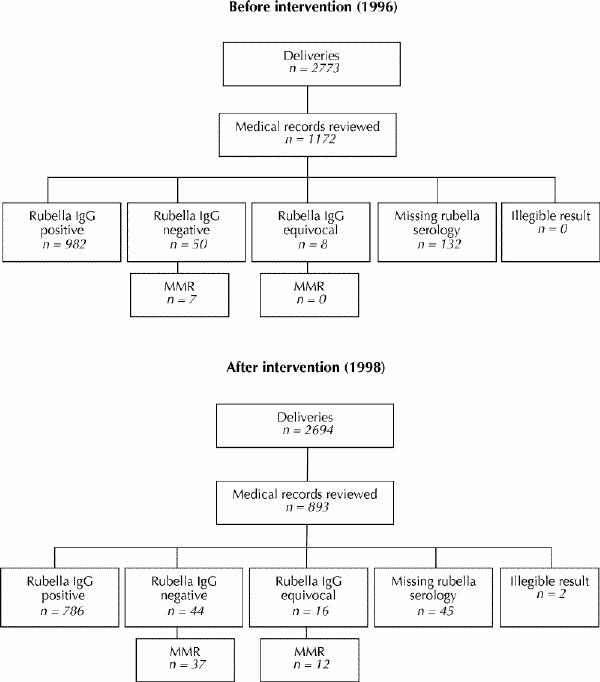
Fig. 1: Rubella serology and number of patients vaccinated. IgG = immunoglobulin G, MMR = measles, mumps and rubella vaccination.
Before printed orders were introduced, no health care provider noted rubella susceptibility in 48 of 51 women who had not been vaccinated. After the introduction of printed orders, susceptibility was not noted in 2 of 11 women who had not been vaccinated: in 7, susceptibility was noted but the vaccine was withheld and no reason documented; and 2 patients refused vaccination, one because she believed she was immune and one gave no reason.
The vaccination rate for both periods combined was similar whether the rubella serostatus was negative (46.8%, 44/94) or equivocal (50%, 12/24) (risk ratio 1.1, 95% CI 0.7–1.7).
Rubella serology results were missing from the hospital record in 11.3% (132/1172) of women before and 5.3% (47/893) after the introduction of printed orders. This decrease was probably due to the initiative taken spontaneously by postpartum nurses, who called physicians' offices when results were missing. Of the women with missing results for whom a rubella titre was performed post partum (this only occurred after preprinted orders were introduced), 32.3% (10/31) were susceptible, which is significantly more than the 6.3% (118/1886) who were susceptible among all subjects with recorded antenatal results (risk ratio 5.0, 95% CI 2.9–8.6). Women whose serology results were missing delivered earlier (mean gestational age 35.2 weeks) than women with known results (mean gestational age 38.5 weeks) (p < 0.001). Serology may have been missing for women who delivered prematurely because antenatal records were not routinely sent to the hospital until a later gestational age, or were not forwarded with high-risk patients transferred to this tertiary institution.
Of all the rubella-susceptible women studied, 56.8% (67/118) had at least one previous missed postpartum opportunity to receive vaccination: 37 women already had one child, 15 had 2, and 15 had 3 or more. Rubella immunity was not predicted by parity (6.5% of nulliparous, 5.3% of primiparous and 7.6% of multiparous women were susceptible, p = 0.29), maternal age (mean 29.6 years if susceptible, mean 30.3 years if immune, p = 0.07) or gestational age at delivery (38.8 weeks and 38.5 weeks respectively.)
A key element in the success of the preprinted MMR order may be drawing susceptibility to rubella to the attention of health care personnel. Physicians do not have to remember to address the issue and do not have to search through the chart for serostatus. Research on clinical guidelines has demonstrated that “implementation strategies which are nearer the end user and integrated into the process of healthcare delivery are more likely to be effective.”10 Including MMR vaccination and other preventive measures in standard postpartum orders effectively integrates them directly into the routine process of nursing care.
Our strategy is a pragmatic one that avoids challenging institutional practices regarding the roles of health care personnel and maintains the usual professional medicolegal responsibilities. Nurses' adherence to the written order was excellent, and patient acceptance was high. If the efficacy of this simple approach is confirmed in other centres, its widespread adoption could prevent about half the cases of congenital rubella syndrome in Canada.
Footnotes
This article has been peer reviewed.
Competing interests: None declared.
Correspondence to: Dr. Erica Eason, Box 803, 501 Smyth Rd., Ottawa ON K1H 8L6; fax 613 739-6266; eeason@ohri.ca (reprints will not be available)
References
- 1.Infectious Diseases and Immunization Committee, Canadian Paediatric Society (CPS). Prevention of congenital rubella syndrome. J Paediatr Child Health 1999;4:155-7. [DOI] [PMC free article] [PubMed]
- 2.Survey of congenital rubella syndrome, Montreal, Laval, and Monteregie, Quebec, 1985–1991. Can Commun Dis Rep 1996;22(5):33-5. [PubMed]
- 3.Schluter WW, Reef SE, Redd SC, Dykewicz CA. Changing epidemiology of congenital rubella syndrome in the United States. J Infect Dis 1998;178:641. [DOI] [PubMed]
- 4.Lee SH, Ewert DP, Frederick PD, Mascola L. Resurgence of congenital rubella syndrome in the 1990s. JAMA 1992;267:2616-20. [PubMed]
- 5.National Advisory Committee on Immunization. Canadian immunization guide. 5th ed. Ottawa: Health Canada; 1998.
- 6.Watson JC, Hadler SC, Dykewicz CA, Reef S, Phillips L. Measles, mumps, and rubella-vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 1998;47(RR-8):1-57. [PubMed]
- 7.Gyorkos TW, Tannenbaum TN, Abrahamowicz M, Delage G, Carsley J, Marchand S. Evaluation of rubella screening in pregnant women. CMAJ 1998;159(9):1091-7. Abstract available: www.cma.ca/cmaj/vol-159/issue-9/1091.htm [PMC free article] [PubMed]
- 8.Tam TWS. Following up on unfinished business — prenatal rubella screening and postpartum vaccination. CMAJ 1998;159(9):1117-8. Available: www .cma .ca /cmaj/vol-159/issue-9/1117.htm [PMC free article] [PubMed]
- 9.Berkeley MIK, Moffat MAJ, Russell D. Surveillance of antibody to rubella virus in Grampian: closing the immunity gap. BMJ 1991;303:1174-6. [DOI] [PMC free article] [PubMed]
- 10.Grimshaw J, Freemantle N, Wallace S, Russell I, Hurwitz B, Watt I, et al. Developing and implementing clinical practice guidelines. Qual Health Care 1995;4(1):55-64. [DOI] [PMC free article] [PubMed]


