Abstract
Background
Coronary artery obstruction is a rare, devastating complication of transcatheter aortic valve replacement (TAVR). Transcatheter electrosurgical aortic leaflet laceration (BASILICA) is a novel technique to prevent coronary artery obstruction. We report the 1-year outcomes of the BASILICA trial. Primary endpoints of 30-day success and safety have been reported previously.
Methods
The BASILICA trial was a prospective, multicenter, single-arm safety and feasibility study. Subjects with severe native or bioprosthetic aortic valve disease at high or extreme risk for surgery, and high risk of coronary artery obstruction, were included. Endpoints at 1 year included death, stroke, and myocardial infarction. Source-data was independently verified, and endpoints independently adjudicated.
Results
30 subjects were enrolled between February 2018 and July 2018. At 30 days, BASILICA was successful in 28 subjects (93.3%), there were 3 strokes (10%), including 1 disabling stroke (3.3%), 1 death (3.3%), and 1 periprocedural myocardial infarction (3.3%). Between 30-days and 1 year, there were no additional strokes, no myocardial infarction, and two deaths (10% 1-year mortality). No subject needed repeat intervention for aortic valve or coronary disease. Two subjects had infective endocarditis (6.7%) but neither was isolated to the aortic valve. There were no hospital admissions for heart failure. 14 (46.7%) subjects required repeat hospital admission for other causes. Aortic valve gradients on echocardiography, NYHA functional class and KCCQ scores improved from baseline to 30-days and were maintained at 1 year.
Conclusions
In these subjects with multiple comorbidities and restrictive anatomy that underwent TAVR, there was no late stroke, myocardial infarction, or death related to BASILICA. Mitigation of coronary obstruction remained intact at 1 year and was not related to recurrent readmission . These results are reassuring for patients and physicians who wish to avoid the long term complications related to snorkel stenting.
Clinical Trial Registration
Keywords: Bioprosthetic heart valve failure, Transcatheter aortic valve replacement, Coronary artery obstruction, Transcatheter electrosurgery, Structural heart disease
Introduction
Coronary artery obstruction occurs in 0.7% of all transcatheter aortic valve replacement (TAVR) procedures1. The incidence is higher in certain subgroups, especially for TAVR in bioprosthetic valves (valve-in-valve TAVR; 2.3% incidence)2. Coronary artery obstruction from TAVR is associated with up to 50% mortality despite attempted rescue percutaneous coronary intervention or coronary artery bypass surgery, even when successful1, 2. Therefore, it is important to predict and prevent this complication.
At present, two strategies are used for prevention of coronary obstruction. The first is “snorkel stenting”, where a coronary stent is pre-positioned in the coronary artery prior to TAVR and deployed protruding into the aorta after TAVR. The second strategy, called BASILICA, utilizes transcatheter electrosurgery to lacerate the aortic leaflets prior to TAVR allowing them to splay to the sides after TAVR and therefore maintaining coronary perfusion.
The BASILICA technique was investigated in a prospective early feasibility clinical trial and the 30-day results have been reported previously3. Here we report the 1-year outcomes.
Methods
Trial design and oversight
The BASILICA trial (NCT03381989) design has been described previously3. Briefly, this was an investigator initiated and sponsored prospective, multicenter, single arm study of BASILICA and TAVR. There was 100% independent on-site source-data verification and data monitoring. A Clinical Event Committee independently adjudicated the primary endpoints and death at 1 year. A central core laboratory analyzed baseline and post-procedure echocardiography and computed tomography (CT) images up to 30 days but not 1 year. The US Food and Drug Administration granted Investigational Device Exemption (IDE) for the study under the Early Feasibility pathway. The first and senior authors had full access to all the data in the study and take responsibility for its integrity and the data analysis. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects and Eligibility
30 subjects were enrolled between February and July 2018 at four centers in the United States. Subjects were eligible if they were at least high risk for surgical aortic valve replacement and high risk of coronary artery obstruction from TAVR as determined by a central eligibility committee based on previously published guidelines3. The institutional review board at each site and at NHLBI approved the study protocol and all subjects consented to participate in writing.
BASILICA technique
The BASILICA technique has been described in detail in previous publications4, 5. Briefly, a guidewire is electrified to traverse the base of the target aortic leaflet. It is snare-externalized and the resulting guidewire loop, after minor benchtop modification to concentrate charge, is further electrified whilst being retracted together with sheathing guiding catheters. Blood is displaced during electrification with non-ionic dextrose solution to concentrate charge on the leaflet and reduce char and thrombus formation6. The result is to lacerate the leaflet down the centerline. This may be repeated if double leaflet BASILICA is performed. TAVR is then performed as usual.
Study endpoints
The primary endpoints of the study were procedure success on exit from the catheter laboratory and freedom from major adverse clinical events at 30-days, both as defined by Valve Academic Research Consortium-2 (VARC-2). Secondary endpoints included death, stroke and myocardial infarction at one year.
Statistical analysis
All analyses were based on the intention-to-treat principle with data from all enrolled patients. The sample size of 30 subjects was not derived statistically. Baseline subject and procedural characteristics were summarized as medians and interquartile ranges (IQR) for continuous variables and counts and percentages for categorical variables. McNemar’s test and paired t-test were used to assess the difference in the percentage of NYHA class and KCCQ quality of life measure between baseline, 30 day and 1-year visits, respectively. Statistical analyses were performed using R statistical software 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 30 subjects were enrolled in the BASILICA IDE trial [Figure 1]. One-year clinical follow-up was available for all the surviving study participants. Table 1 shows the baseline demographics and the coronary obstruction risk for enrolled subjects.
Figure 1.
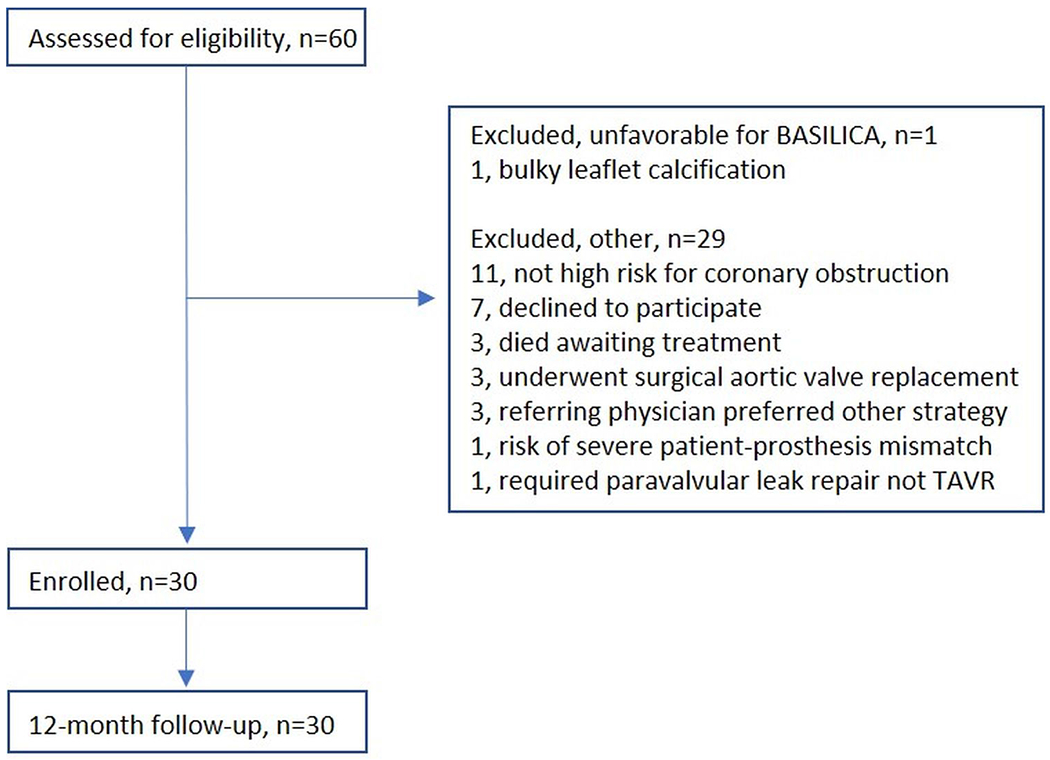
Consort flow diagram for the BASILICA trial.
TABLE 1.
Baseline demographics and the coronary obstruction risk for enrolled subjects
| DEMOGRAPHICS | median (IQR) or n (%) (n=30) |
|---|---|
| Age, years | 76 (69 - 82) |
| Female | 24 (80%) |
| COMORBIDITIES | |
| Prior stroke | 7 (23%) |
| Coronary artery disease | 19 (63%) |
| Diabetes | 12 (40%) |
| End-stage kidney disease on dialysis | 3 (10%) |
| Severe pulmonary disease | 12 (40%) |
| Liver cirrhosis | 2 (7%) |
| Hypertension | 26 (87%) |
| Frail | 14 (47%) |
| STS predicted risk of mortality (%) | 6 (3 - 15) |
| ANTIPLATELET/ANTICOAGULATION | |
| Aspirin at baseline / discharge / 12 months | 73% / 87% / 77% |
| P2Y12 at baseline / discharge / 12 months | 40% / 37% / 23% |
| Anticoagulation at baseline / discharge / 12 months | 27% / 37% / 45% |
| TAVR SETTING | |
| Native | 13 (43%) |
| Bioprosthetic | 17 (57%) |
| CORONARY OBSTRUCTION RISK ON CT | |
| Coronary artery height, mm | 7.2 (5.2 – 9.7) |
| Sinus of Valsava width, mm | 25.9 (24.8 – 29.0) |
| VTC, mm | 3.3 (2.7 – 4.0) |
| VTSTJ, mm | 2.2 (0.5 – 3.1) |
| PROCEDURE | |
| Sapien 3 | 16 (53%) |
| Evolut R/Pro | 14 (47%) |
| Transfemoral access | 23 (77%) |
| Transcaval access | 6 (20%) |
| Percutaneous axillary access | 1 (3%) |
| Solo BASILICA | 23 (77%) |
| Doppio BASILICA | 7 (23%) |
IQR = Interquartile range; STS = Society of Thoracic Surgeons; CT = Computed Tomography; VTC = virtual transcatheter to coronary distance; VTSTJ = virtual transcatheter to sinotubular junction distance;
One-year clinical endpoints
Table 2 lists 30-day and 1-year outcomes for subjects after BASILICA TAVR. Between 30-days and 1 year, there were two deaths and no strokes. One subject died on day 110 of bacterial endocarditis with vegetations on all four valves, with multiple septic emboli including to the spine and brain, and acute respiratory failure on a background of liver cirrhosis and permanent indwelling central port. This was adjudicated as a cardiovascular death not related to BASILICA. One subject died on day 203 following a fall and head injury. This was adjudicated as a non-cardiovascular death not related to BASILICA. No subject suffered myocardial infarction requiring revascularization. There was one late troponin positive event in a subject with known atrial fibrillation and prior myocardial infarction treated with PCI who was admitted 5 months post BASILICA-TAVR procedure with palpitations and shortness of breath. She was found to be in atrial fibrillation with a rapid ventricular rate and was treated with rate control medications and continued on existing anticoagulation and aspirin therapy. No subject required repeat intervention for aortic valve or coronary artery disease.
TABLE 2.
Detailed causes for re-admission
| CLINICAL OUTCOMES | At 30 days (n=30) | At 1 year (n=30) |
|---|---|---|
| All death Cardiovascular Non-Cardiovascular |
1 (3.3%) 1 (3.3%) 0 (0%) |
3 (10%) 2 (6.7%) 1 (3.3%) |
| All stroke Disabling Non-disabling |
3 (10%) 1 (3.3%) 2 (6.7%) |
3 (10%) 1 (3.3%) 2 (6.7%) |
| Repeat hospital admission | 5 (16.7%) Cardiovascular: 3 Stroke: 1 Rhythm of palpitations: 2 Non-cardiovascular: 2 GI bleed: 1 Pneumonia: 1 |
14 (46.7%) Cardiovascular: 8 Stroke: 1 Rhythm or palpitation: 5 Endocarditis: 2 Heart failure: 0 Non-cardiovascular: 6 Anemia or GI bleed: 3 Pneumonia: 1 Septic arthritis: 1 Fall and fracture: 1 |
| Coronary artery obstruction | 0 (0%) | 0 (0%) |
| Valve-related dysfunction requiring repeat procedure | 0 (0%) | 0 (0%) |
| Need for second valve | 0 (0%) | 0 (0%) |
| Spontaneous myocardial infarction | 0 (0%) | 0 (0%) |
| Peri-procedural myocardial infarction | 1 (3.3%) | NA |
| Any coronary intervention | 0 (0%) | 0 (0%) |
| Endocarditis | 0 (0%) | 2 (7%) |
| New pacemaker | 3 (10%) | 3 (10%) |
| Hemolytic anemia | 0 (0%) | 0 (0%) |
In the first 30 days, there was one major stroke and cardiovascular death (3%) in a patient who suffered distributive shock on induction of anesthesia resulting in systemic inflammatory response syndrome and widespread diffusion restriction and enhancement on brain MRI. This subject also suffered a periprocedural secondary myocardial infarction with troponin rise and EKG changes that was managed conservatively. There were two non-disabling strokes (7%) in subjects who fully recovered baseline neurological function. A Sentinel cerebral embolic protection device was used in 43% and embolic debris was recovered in 46% of those cases, though no systematic inspection technique was mandated. One patient with non-disabling stroke had a Sentinel in situ.
Including the two subjects that died after discharge, 14 subjects required repeat hospital admission in the first year, of which 10 subjects were readmitted between 30 days and 1 year. Four admissions were related to atrial flutter/fibrillation and one to complete AV block. Two subjects had endocarditis, one mentioned above and one with vegetations on the mitral valve. The causes for re-admission are detailed in Table 2. Three subjects had multiple admissions, not captured in the table, with GI bleed the cause of two re-admissions, palpitations in two, and fall in one.
Sub-group outcomes
Stroke was seen in 1/7 (14%) doppio BASILICA and 2/23 (8.7%) solo BASILICA during the peri-procedural period. Stroke was also observed in 1/13 (7.7%) native and 2/17 (11.8%) bioprosthetic valves.
Three subjects had hypoattenuated leaflet thickening (HALT) on 30-day CT and were alive at 1 year. Their peak aortic velocities post-TAVR and at 1 year were 2.7 m/s and 2.8 m/s; 3.1 m/s and 3.9 m/s; and 3.7 m/s and 4.2 m/s respectively. One of these, included above, had a non-disabling procedural stroke.
Three subjects had successful bioprosthetic valve fracture with no stroke or coronary obstruction with residual gradients at 1 year (peak aortic valve velocity 2.6 m/s, 3.2 m/s, 4.2 m/s; mean aortic valve gradient 14 mmHg, 23 mmHg, 34 mmHg; aortic valve area 2.0 cm2, 0.81 cm2, 0.98 cm2).
Functional and hemodynamic results
Functional assessment was complete in 26 subjects (87%) at 1 year. The remaining 4 subjects were alive per local medical notes but without a formal follow-up visit due to travel constraints. Figure 2 shows the change in NYHA class and KCCQ scores from baseline to 30-days to 1 year. Patients have significant improvement in NYHA class and KCCQ scores at 30 days and 1 year compared to baseline (both P<0.001), with no significant differences in NYHA and KCCQ summary score between 30-day and 1-year visits.
Figure 2.

NYHA class and KCCQ Summary scores improved from baseline to 30 days after BASILICA TAVR and the improvement was maintained at 1 year. NYHA CHF= New York Heart Association Congestive Heart Failure, KCCQ = Kansas City Cardiomyopathy Questionnaire
Echocardiography follow-up was complete in 22 of 27 subjects alive at 1 year (81%). The results are shown in Table 3. As expected, hemodynamics improved with TAVR and were maintained out to one year.
TABLE 3.
Results of echocardiography follow-up of subjects
| ECHOCARDIOGRAPHY HEMODYNAMICS | Baseline (n=30), median (IQR) | 30-days (n=28), median (IQR) | 1 year (n=22), median (IQR) |
|---|---|---|---|
| LVEF, % | 55 (44 – 60) | 60 (55 – 63) | 61 (55 – 69) |
| Aortic valve peak velocity, m/s | 4.3 (3.9 – 4.7) | 2.7 (2.4 – 3.3) | 2.6 (2.2 – 3.2) |
| Aortic valve mean gradient, mmHg | 43 (37 – 53) | 15 (11 – 23) | 14 (12 – 23) |
| Aortic valve area cm2 | 0.7 (0.6 – 0.8) | 1.1 (0.9 – 1.4) | 1.2 (1.0 – 1.8) |
| ≥ moderate aortic insufficiency | 7 (23%) | 0 (0%) | 1 (5%) |
IQR = interquartile range; LVEF = Left ventricular ejection fraction;
Discussion
The main findings at 30-days for the BASILICA trial were that the procedure was feasible in 93% of patients, the two failures relating to heavy target leaflet calcification at the traversal target. No patient had coronary obstruction despite the high predicted risk. There were one death and major stroke (3%) and two additional non-disabling strokes (7%).
In this paper, we reported the 1-year results of the BASILICA trial and we found there was no late stroke or myocardial infarction after BASILICA TAVR up to one year. There were no repeat interventions. Theoretically, the lacerated leaflet edge could be a nidus for thrombus, infection, and tissue proliferation. The absence of events is therefore reassuring and suggests the lacerated leaflets may be benign in the short and medium term. The one case of prosthetic valve endocarditis also involved the other three cardiac valves and was in the setting of an indwelling catheter.
Another theoretical concern was late mobilization of the leaflet, causing delayed coronary artery obstruction, new late paravalvular leak, or valve embolization. These events were not seen in the BASILICA trial.
Almost half of patients had been admitted to hospital within a year of discharge, although none for heart failure. This readmission rate probably reflects the elderly, frail, and high risk patient cohort treated in the BASILICA trial. This is consistent with data from the TVT Registry showing 50% readmission within one year for high risk patients undergoing TAVR7.
This study suggests the short to medium-term outcomes for these patients are similar to high and extreme surgical risk patients without risk of coronary artery obstruction undergoing contemporary TAVR. 1 year mortality was 12% and disabling stroke 2.9% at 1 year in the commercial arm (Sapien and Evolut) of the PORTICO IDE trial 8, and 9% mortality and 4% disabling stroke at 1 year in the Evolut arm of SCOPE 29, compared with 10% mortality and 3.3% disabling stroke at 1 year in the BASILICA trial.
Another option to prevent coronary artery obstruction is snorkel stenting. While the technique is simple and achieves flow in the coronary arteries on exit from the catheter laboratory10, stents may be under-expanded or crushed next to the transcatheter heart valve and the long term durability of this technique is questionable, especially with regards to late stent thrombosis and delayed coronary obstruction11. Moreover, re-engaging the coronary artery after snorkel stenting may be impossible, limiting downstream options for these patients.
BASILICA may be more technically demanding than snorkel stenting but our findings suggest there appear no downstream negative sequelae of BASILICA. Coronary access is maintained and may even be easier after BASILICA TAVR than after standalone TAVR as the outer leaflet has been parted. We speculate there may be some positive impact on rates of structural valve degeneration or late thromboembolism as BASILICA may increase flow in the neo-sinus12, but this study was not powered to assess this and would require lengthy (5-10 year) follow-up. Although antiplatelet and anticoagulation was not mandated for the BASILICA procedure, many patients received these therapies due to prior comorbidities or by operator discretion after TAVR.
Limitations
This trial was designed to show feasibility of the BASILICA procedure. The main limitations of the trial relate to demonstrating efficacy and safety. Demonstrating efficacy relied on accurate prediction of coronary artery obstruction, which is currently lacking. Despite this shortcoming, no patient developed coronary obstruction despite high predicted risk in all.
TAVR is associated with risk of stroke and vascular complications. It is unknown whether BASILICA increases the risk of stroke. A larger sample size is needed to evaluate whether BASILICA is associated with excess risk of stroke.
Long-term patency of the channel created by BASILICA was assumed by the absence of cardiovascular death, coronary obstruction, myocardial infarction or repeat intervention, but no dedicated imaging was mandated to confirm this. Indeed, non-invasive imaging is unlikely to make this determination.
An intention to treat analysis was used throughout for this study as there was no control group and we felt this reflected the procedure risk better than an as-treated analysis in these subjects. In the two subjects where BASILICA failed, multiple traversal attempts were made but without success, involving extra manipulation of leaflets, radiofrequency energy delivered, and time taken. Furthermore, in one subject, double BASILICA was planned, and BASILICA was successful in the left cusp but failed in the right cusp. We classified this as a failure of BASILICA in the patient. These would create ambiguity in an as-treated analysis. The one patient who did not have BASILICA had multiple hospital admissions with gastrointestinal bleeding and palpitations as reported in this manuscript.
Finally, the sample size and event rate were too small for meaningful comparison in outcomes between native and bioprosthetic valves, solo and doppio BASILICA, or between subjects with and without HALT at 30 days.
Future directions
A randomized controlled trial may help clinicians decide between BASILICA, snorkel stenting, or surgery when faced with intermediate or high surgical risk patients with high risk of TAVR related coronary artery obstruction. However, such a trial is unlikely to be conducted due to cost and perceived lack of equipoise. Operators who are facile with the BASILICA procedure are reluctant to revert to deploying snorkel stents. Data from BASILICA registries will add to our understanding of the safety of the procedure, particularly whether BASILICA is associated with an excess risk of stroke, and whether cerebral embolic devices may be useful in this setting.
The BASILICA technique has undergone modest refinement since this trial. Dedicated pachyderm-shaped guiding catheters assist in the technique and reduce procedure time13. Further dedicated device development for leaflet laceration is likely to make the procedure easier and more reproducible. In performing these procedures and assessing these registries it is important to assure appropriate technique including use of dextrose-water flush to minimize char and thromboembolism during transcatheter electrosurgery. Dextrose or iodine contrast injection during attempted traversal may enhance traversal success beyond what was observed in this protocol. Favorable and unfavorable patient anatomical features, in the opinion of the authors, are tabulated in Table 4.
TABLE 4.
Favorable and unfavorable anatomical features of patients.
| Anatomy | Favorable for BASILICA | Unfavorable for BASILICA |
|---|---|---|
| Calcium | • Typical calcium pattern which spares the nadir of the leaflet | • Confluent calcium at the leaflet nadir • Bulky calcium mass on the leaflet |
| Bioprosthesis | • Commissures aligned with native commissures | • Bioprosthetic valve post in-front of coronary artery ostium |
| Access | • Femoral access for BASILICA catheters preferred for ergonomics | • Non-femoral access (femoral artery or transcaval) for double leaflet BASILICA |
| Coronary obstruction risk | • Single leaflet BASILICA • Risk from sinus sequestration |
• Double BASILICA not recommended for new operators in their first 2-3 cases |
As TAVR is offered to progressively lower-risk patients, the risk of TAV-in-TAV induced coronary obstruction is expected to increase over time. The applicability of BASILICA for failed transcatheter heart valves is potentially large. Early benchtop studies suggest that BASILICA may not be suitable in all patients undergoing TAV-in-TAV because of transcatheter heart valve design, and the randomness of commissural alignment in the common transcatheter heart valves14. In appropriately selected cases, particularly where the risk is from sinus sequestration, BASILICA may be useful in preventing coronary obstruction in TAV-in-TAV.
Conclusions
There were no late complications of BASILICA observed in the BASILICA IDE trial, including no stroke, myocardial infarction, or repeat intervention. There were no BASILICA related deaths. The lacerated leaflets did not appear to be a persistent nidus for thrombosis or infection. Mitigation of coronary obstruction remained intact at 1 year and was not related to recurrent readmission in this sick and frail patient group. Coronary artery obstruction remains uncommon and this small early feasibility trial cannot exclude rare complications. Unlike the lifetime management burden of a snorkeled coronary stent with antiplatelet therapy, difficult coronary access and risks of restenosis and stent thrombosis, there does not appear to be any specific management required after a split leaflet.
What is Known
BASILICA is a novel technique to prevent coronary artery obstruction from TAVR
Prior to this study, only 30-day outcomes have been reported.
What the Study Adds
One-year clinical data from the BASILICA trial show no late complications of the BASILICA procedure, particularly no late stroke or coronary artery obstruction.
These results are reassuring for patients and physicians who wish to avoid the long term complications related to snorkel stenting.
Acknowledgements
We thank clinical and research staff at University of Washington (Christopher Rumer, Data Manager; Samantha Solis, Research Coordinator; Kate Jordan, Senior Research Coordinator; Ikki Komatsu, MD; Gabriel Aldea, MD; G. Burkhard Mackensen, MD, PhD); Emory University (Brad Leshnower, MD; Lauren Wheeler, DNP; James Lee, RN; Patricia Keegan, DNP; Elizabeth Charles, Research Coordinator; Kristy Pitts, Research Coordinator; Kimberly McWhorter, Research Coordinator; Hima Patel, Research Coordinator; Jennifer James, Research Coordinator); Medstar Washington Hospital Center (Petros Okubagzi, Clinical Research Director; Erin Collins, Research Coordinator); Henry Ford Hospital (Ashish Solanki, Research Coordinator); and independent Data Monitors (Olha Katynska; Valeriy Matveev; Artur Karapetyan). We thank the Medstar Clinical Events Adjudication Committee (Hector Garcia-Garcia, Eugene McFadden, Alexandre Kajita, and Kayode Kuku).
Funding
Supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, USA (Z01-HL006040-7), and by the intramural programs of the participating centers.
Disclosures
JMK, TR, and RJL are co-inventors on patents, assigned to NIH, on catheter devices to lacerate valve leaflets.
JMK has proctored for Edwards Lifesciences and Medtronic
ABG is a proctor for Edwards Lifesciences, Medtronic, and Abbott Vascular. He has equity in Transmural Systems.
VCB is a consultant for Edwards Lifesciences, Abbott Vascular and Transmural Systems, and his employer has research contracts for clinical investigation of transcatheter aortic and mitral devices from Edwards Lifesciences, Abbott Vascular, Medtronic, St Jude Medical, and Boston Scientific.
TR is a consultant/proctor for Edwards Lifesciences and Medtronic. He has equity in Transmural Systems.
MHE is a proctor for Edwards Lifesciences.
RW is a consultant for Medtronic and is a consultant and receives grant support from Abbott Vascular.
DD is a consultant for Edwards Lifesciences, Medtronic and Abbott Vascular.
RJL is the principal investigator on a Cooperative Research and Development Agreement between NIH and Edwards Lifesciences on transcatheter modification of the mitral valve.
No other author has a financial conflict of interest related to this research.
Abbreviations
- BASILICA
Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction
- TAVR
Transcatheter Aortic Valve Replacement
- VTC
Virtual Transcatheter Valve to Coronary distance
- IDE
Investigational device exemption
- NHLBI
National Heart Lung and Blood Institute
- VARC
Valve Academic Research Consortium
- NYHA
New York Heart Association
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- HALT
Hypoattenuated Leaflet Thickening
References
- 1.Ribeiro HB, Webb JG, Makkar RR, Cohen MG, Kapadia SR, Kodali S, Tamburino C, Barbanti M, Chakravarty T, Jilaihawi H, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol. 2013;62:1552–62. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro HB, Rodes-Cabau J, Blanke P, Leipsic J, Kwan Park J, Bapat V, Makkar R, Simonato M, Barbanti M, Schofer J, et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J. 2018;39:687–695. [DOI] [PubMed] [Google Scholar]
- 3.Khan JM, Greenbaum AB, Babaliaros VC, Rogers T, Eng MH, Paone G, Leshnower BG, Reisman M, Satler L, Waksman R, et al. The BASILICA Trial: Prospective Multicenter Investigation of Intentional Leaflet Laceration to Prevent TAVR Coronary Obstruction. JACC Cardiovasc Interv. 2019;12:1240–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lederman RJ, Babaliaros VC, Rogers T, Khan JM, Kamioka N, Dvir D and Greenbaum AB. Preventing Coronary Obstruction During Transcatheter Aortic Valve Replacement: From Computed Tomography to BASILICA. JACC Cardiovasc Interv. 2019;12:1197–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan JM, Dvir D, Greenbaum AB, Babaliaros VC, Rogers T, Aldea G, Reisman M, Mackensen GB, Eng MHK, Paone G, et al. Transcatheter Laceration of Aortic Leaflets to Prevent Coronary Obstruction During Transcatheter Aortic Valve Replacement: Concept to First-in-Human. JACC Cardiovasc Interv. 2018;11:677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan JM, Rogers T, Greenbaum AB, Babaliaros VC, Yildirim DK, Bruce CG, Herzka DA, Schenke WH, Ratnayaka K and Lederman RJ. Transcatheter Electrosurgery: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:1455–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes DR Jr., Brennan JM, Rumsfeld JS, Dai D, O’Brien SM, Vemulapalli S, Edwards FH, Carroll J, Shahian D, Grover F, et al. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313:1019–28. [DOI] [PubMed] [Google Scholar]
- 8.Makkar RR, Cheng W, Waksman R, Satler LF, Chakravarty T, Groh M, Abernethy W, Russo MJ, Heimansohn D, Hermiller J, et al. Self-expanding intra-annular versus commercially available transcatheter heart valves in high and extreme risk patients with severe aortic stenosis (PORTICO IDE): a randomised, controlled, non-inferiority trial. Lancet. 2020;396:669–683. [DOI] [PubMed] [Google Scholar]
- 9.Tamburino C, Bleiziffer S, Thiele H, Scholtz S, Hildick-Smith D, Cunnington M, Wolf A, Barbanti M, Tchetche D, Garot P, et al. Comparison of Self-Expanding Bioprostheses for Transcatheter Aortic Valve Replacement in Patients with Symptomatic Severe Aortic Stenosis: The SCOPE 2 Randomized Clinical Trial. Circulation. 2020;142(25):2431–2442. [DOI] [PubMed] [Google Scholar]
- 10.Mercanti F, Rosseel L, Neylon A, Bagur R, Sinning JM, Nickenig G, Grube E, Hildick-Smith D, Tavano D, Wolf A, et al. Chimney Stenting for Coronary Occlusion During TAVR: Insights From the Chimney Registry. JACC Cardiovasc Interv. 2020;13:751–761. [DOI] [PubMed] [Google Scholar]
- 11.Jabbour RJ, Tanaka A, Finkelstein A, Mack M, Tamburino C, Van Mieghem N, de Backer O, Testa L, Gatto P, Purita P, et al. Delayed Coronary Obstruction After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2018;71:1513–1524. [DOI] [PubMed] [Google Scholar]
- 12.Hatoum H, Maureira P, Lilly S and Dasi LP. Impact of Leaflet Laceration on Transcatheter Aortic Valve-in-Valve Washout: BASILICA to Solve Neosinus and Sinus Stasis. JACC Cardiovasc Interv. 2019;12:1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lisko JC, Babaliaros VC, Lederman RJ, Khan JM, Rogers T and Greenbaum AB. Pachyderm-Shape Guiding Catheters to Simplify BASILICA Leaflet Traversal. Cardiovasc Revasc Med. 2019;20:782–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan JM, Bruce CG, Babaliaros VC, Greenbaum AB, Rogers T and Lederman RJ. TAVR Roulette: Caution Regarding BASILICA Laceration for TAVR-in-TAVR. JACC Cardiovasc Interv. 2020;13:787–789. [DOI] [PMC free article] [PubMed] [Google Scholar]


