Abstract
PURPOSE
Time to antibiotic administration (TTA) is a commonly used standard of care in pediatric cancer settings in high-income countries. Effective interventions to improve outcomes in cancer patients with febrile neutropenia (FN) often address timely and appropriate antibiotic administration. We assessed the effectiveness of a locally adapted multimodal strategy in decreasing TTA in a resource-constrained pediatric cancer center in Mexico.
METHODS
We conducted a prospective observational study between January 2014 and April 2019. A three-phase (phase I: execution, phase II: consolidation, phase III: sustainability) multimodal improvement strategy that combined system change, FN guideline development, education, auditing and monitoring, mentoring, and dissemination was implemented to decrease TTA in inpatient and ambulatory areas. Sustainability factors were measured by using a validated tool during phases I and III.
RESULTS
Our population included 105 children with cancer with 204 FN events. The baseline assessment revealed that only 50% of patients received antibiotics within 60 minutes of prescription (median time: inpatient, 75 minutes; ambulatory, 65 minutes). After implementing our improvement strategy, the percentage of patients receiving antibiotics within 60 minutes of prescription increased to 88%. We significantly decreased median TTA in both clinical areas during the three phases of the study. In phase III (sustainability), the median TTA was 40 minutes (P = .023) in the inpatient area and 30 minutes (P = .012) in the ambulatory area. The proportion of patients with sepsis decreased from 30% (baseline) to 5% (phase III) (P = .001).
CONCLUSION
Our results demonstrate that locally adapted multimodal interventions can reduce TTA in resource-constrained settings. Mentoring and dissemination were novel components of the multimodal strategy to improve FN-associated clinical outcomes. Improving local infrastructure, ongoing monitoring systems, and leadership engagement have been key factors to achieving sustainability during the 5-year period.
INTRODUCTION
Febrile neutropenia (FN) is a significant cause of morbidity and mortality in patients with pediatric cancer receiving chemotherapy.1,2 Prompt empiric broad-spectrum antibiotic administration is recommended by professional medical societies and by FN guidelines targeting this population.3–7 Some studies have reported prolonged time to antibiotic administration (TTA) being associated with death and higher rates of sepsis and intensive care unit admissions in pediatrics settings.8,9 Consequently, TTA is commonly used as a measure of the quality of care provided in cancer facilities in high-income countries (HICs).10 Current guidelines recommend initial doses of empirical antibiotic therapy within 1 hour of triage.6,7 Because TTA is used as a standard of care in pediatric cancer settings, initiatives to decrease morbidity and mortality in patients with cancer and FN should address prompt and appropriate TTA.11
CONTEXT
Key Objective
To determine the effectiveness of a locally adapted multimodal strategy in decreasing time to antibiotic administration (TTA) in a resource-constrained pediatric cancer center in Mexico.
Knowledge Generated
Our results suggest that locally adapted multimodal interventions can reduce TTA in resource-constrained settings. Mentoring and dissemination were novel components of the multimodal strategy to improve febrile neutropenia–associated clinical outcomes, such as decrease in sepsis rates.
Relevance
Our findings highlight the feasibility of implementing a multimodal improvement strategy that targets TTA improvement and combines an assessment of the recommended core set of minimal clinical outcomes and associated sustainability factors. Improving local infrastructure, ongoing monitoring systems, and leadership engagement seem to be key factors to achieving sustainability.
Several single-site quality improvement projects have been implemented to overcome barriers associated with TTA delays in pediatric cancer facilities in HICs.11 Interventions targeting health care providers, including FN-Alert cards, skill training and education, FN guideline implementation, electronic health record best practice alerts, and feedback on performance to staff, have successfully decreased TTA in these settings.12–15 Although barriers associated with TTA delays and successful interventions to decrease TTA in HICs have been well-established,11 implementation of interventions to decrease TTA and assessment of their feasibility and associated clinical outcomes are lacking in low- and middle-income countries.
Barriers associated with TTA delays in pediatric cancer facilities in low- and middle-income countries include several factors that are usually absent in HICs.16 The use of traditional medicine, lack of transportation, low level of caregiver's literacy, low socioeconomic status, and shortage of health care providers and antibiotics have been identified as potential areas to target when developing a TTA improvement strategy.16,17 The most common barriers for prolonged TTA in HICs might not be common causes of delays in TTA in resource-constrained settings. For example, difficulties in obtaining central venous access because of inadequate topical analgesia are not issues for pediatric cancer facilities that do not have access to either central venous catheters or topical analgesia. Because the barriers involved may vary among centers, the improvement strategy should be tailored to the local resources and context.
Multimodal improvement strategies have been effectively implemented to improve behavior-driven performance among health care providers in resource-constrained settings.18,19 Implementation of more than three elements or components developed by local multidisciplinary teams is recommended to improve and sustain patient safety practices in health care facilities.20 Here, we aimed (1) to assess the effectiveness of implementing a multimodal strategy to decrease TTA in a pediatric cancer center in Mexico, (2) to determine the impact of a TTA < 60 minutes in patients' clinical outcomes, and (3) to define sustainability factors that increased our center's capacity to maintain a TTA < 60 minutes during the past 5 years.
METHODS
Settings and Context
The Hospital General Tijuana is a 191-bed general hospital with an average bed occupancy rate of 86%. It is the largest public hospital in Northwestern Mexico and the only one to provide cancer care to pediatric patients. The pediatric cancer center comprises two consulting areas: a separate 10-bed inpatient ward and an ambulatory area with 10 beds. The inpatient ward has an average daily bed occupancy of 90%, with a nurse-to-patient ratio of 1:3 during weekdays and weekends. The ambulatory area receives an average of 3,900 ambulatory visits per year, and the nurse-to-patient ratio is 1:5 during weekdays; no services are provided during the weekends. The pediatric hematology/oncology physician-to-patient ratio is 1:3 in the inpatient ward and 1:6 in the ambulatory area.
The center treats approximately 60 new cancer cases annually in patients younger than 18 years and follows up more than 360 children and adolescents with cancer in the region. In 2019, 264 inpatient admissions and 15 deaths were reported at the center. The most common cancer diagnosis was acute leukemia. Infection was the leading cause of treatment-related morbidity and mortality. The Hospital General Tijuana has provided pediatric cancer care since 2000. In 2007, the hospital started a partnership with stakeholders at St Jude Children's Research Hospital (St Jude, Memphis, TN) and Rady Children's Hospital (San Diego, CA) to establish a pediatric oncology program.21 The program was aimed at improving clinical outcomes and survival of children with cancer in the Mexican State of Baja California. The introduction of a multidisciplinary team contributed to decreasing treatment-related mortality in our center.21,22 Members of the local team obtained a certificate of training in the St Jude infection prevention and control course.23 Then, they were mentored on a biweekly basis by Rady Children's Hospital and St Jude mentors; an average of 24 meetings was conducted per year by using the St Jude24 virtual rooms to discuss quality improvement projects, infectious diseases, and mortality cases. Moreover, the Hospital General Tijuana joined the Mexico in Alliance with St Jude cooperative group in 2017 and participates in ongoing collaboration, evidence, and quality improvement initiatives.25
In 2013, as a part of the mentoring activities, FN standardized assessment and treatment guidelines were reviewed by the local team in collaboration with experts from St Jude and Rady Children's Hospital. In contrast to most common practice in general pediatric hospitals in Mexico, pediatric cancer patients with fever at our center were directly assessed by trained staff in oncology in either the inpatient ward or ambulatory area. Although most febrile patients were already avoiding delays in treatment by not consulting in the general hospital emergency room,26 baseline data from both consulting areas (inpatient ward and ambulatory area) showed that patients with FN were receiving antibiotics within 60 minutes only 50% of the time. The median TTA was 75 minutes in the inpatient ward and 65 minutes in the ambulatory area.
Improvement Strategy
Over a 5-year period, a three-phase (phase I: execution, phase II: consolidation, phase III: sustainability) multimodal improvement strategy, named the Golden Hour, was implemented to decrease TTA in children with cancer and FN presenting to either inpatient or ambulatory areas (Fig 1; Appendix).
FIG 1.
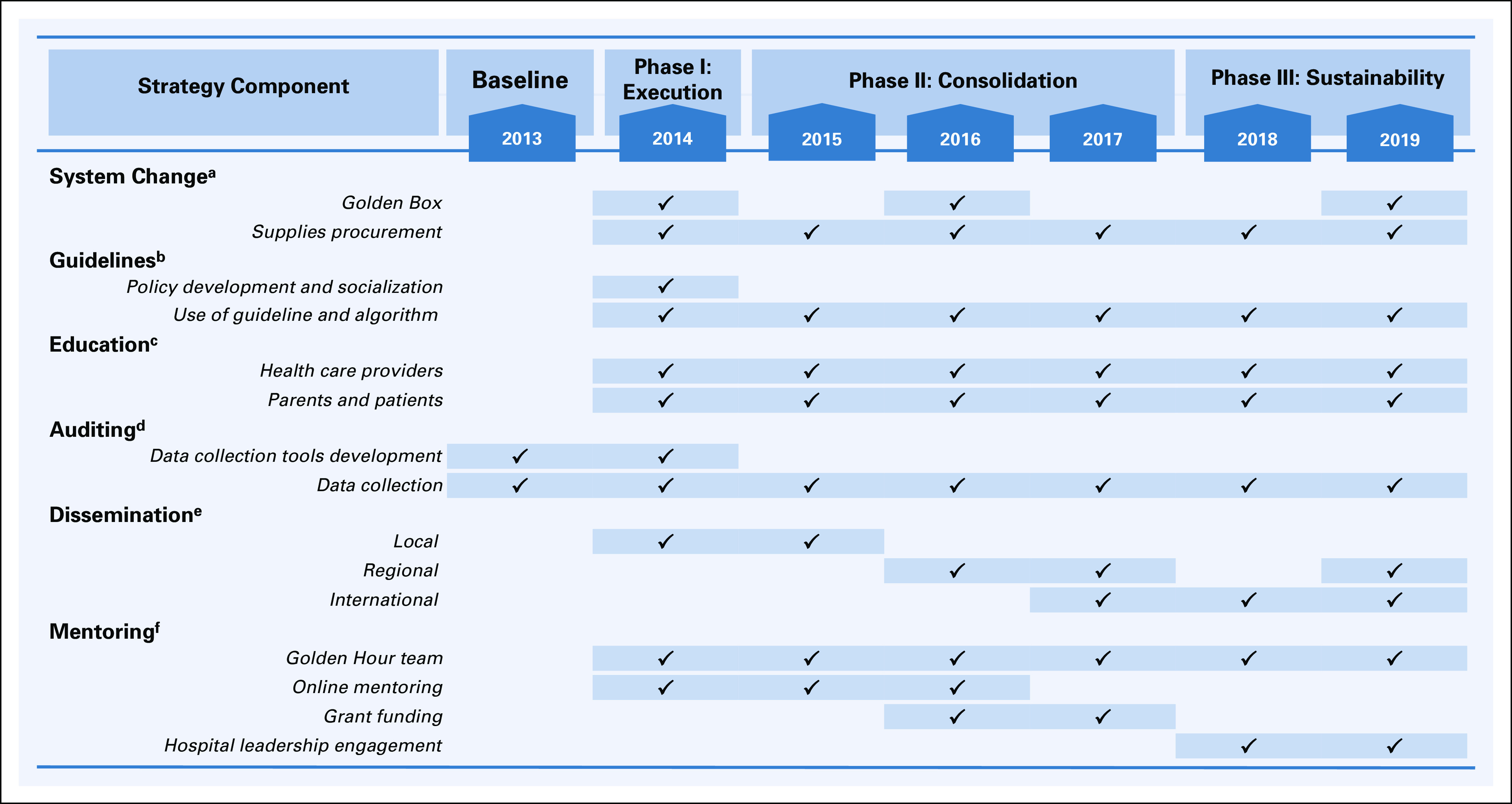
Multimodal strategy components and elements stratified by implementation phase. aSystem change assured availability of supplies at the point of care through the Golden Box to enable adequate practices. bA febrile neutropenia guideline and algorithm were developed by local providers. cFebrile neutropenia workshops were delivered at least twice a year for health care providers and once per year for parents. New employees and newly diagnosed patients and families received a one-to-one training during the onboarding process. dThree data collection tools were developed to facilitate systematic auditing of practices. eOral and poster presentations at the local, regional, and international levels were key to disseminating and providing feedback to all health care providers and leadership. fOnline mentoring was provided on a biweekly basis during the intervention phase and on a monthly basis thereafter.
Study Population and Design
In this prospective observational study, we included all FN events in children with cancer of age 1 month to 18 years who consulted the inpatient ward or the ambulatory area between January 1, 2014, and April 31, 2019.
Data Collection
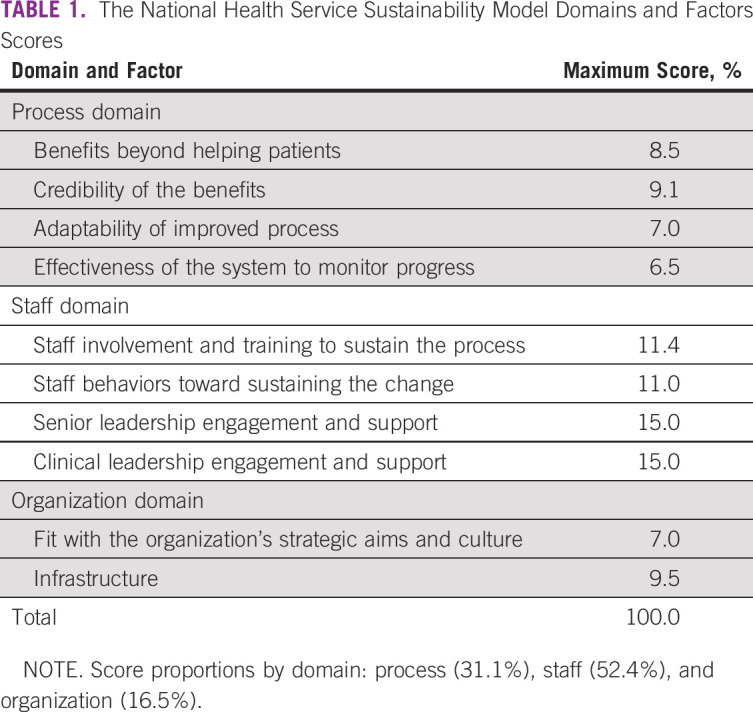
FN event characteristics and associated clinical outcome variables were prospectively documented in a paper-based form by a member of the Golden Hour team. The form included demographic data, date and time of antibiotic indication, date and time of antibiotic administration, identified causes of fever, length of stay (LOS), admission to the intensive care unit, sepsis, septic shock, and death. The information was collected from the paper medical records by a pediatrician and a pediatric nurse (trained in infection control and data collection) and then entered into an electronic database and reviewed by a member of the St Jude team (M.L.G.) to ensure that inclusion criteria were met. Sustainability factors (process, staff, and organization) (Table 1) were documented in phase I (2014) and at the end of phase III (2019) by using a validated score-based sustainability tool.27,28 The Sustainability Model tool was retrospectively completed, and scores represented mentors' and point-of-care staff's perceptions.27
TABLE 1.
The National Health Service Sustainability Model Domains and Factors Scores
Measurements and Definitions
The primary process measure to compare intervention phases was the TTA. It was defined as the elapsed time between the time of antibiotic prescription by the medical staff and the time of antibiotic administration by the nursing staff. The proportion of patients who received antibiotics in ≥ 60 minutes was also calculated for each phase of the intervention. Clinical outcome measures included intensive care unit admission, sepsis, septic shock, LOS, and death.
Fever, neutropenia, sepsis, and septic shock were defined by using recognized standard definitions29,30 (Appendix). We defined sustainability as the continuation or integration of all components of the Golden Hour multimodal strategy (Fig 1) within the organization whereby it has become a routine part of care delivery and continues to deliver desired outcomes. We measured 10 factors depicting before and after score-based results in three key domain areas: process, staff, and organization27 (Table 1).
Analysis and Ethical Considerations
Analysis was performed by using SPSS version 19.0 software. We used Pearson chi-square testing for categorical variables and independent t-testing for continuous variables to compare intervention phases. TTA comparison of baseline with each phase was done by using the nonparametric Mann-Whitney test. All comparisons were two-sided, and P values ≤ .05 were considered statistically significant. This study was approved by the Institutional Review Board of the Hospital General Tijuana. The requirement for informed consent was waived.
RESULTS
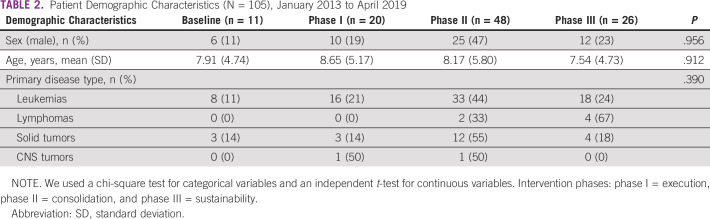
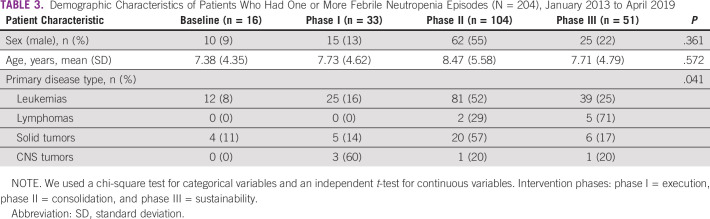
Our study population was composed of 105 patients with pediatric cancer who were admitted with 204 FN events during the study period. Patients' demographic characteristics over the study period were not statistically different from those at baseline (Table 2). Most of the FN events were documented in patients with acute leukemia, with an increased number of such patients during phase II of the improvement strategy (Table 3).
TABLE 2.
Patient Demographic Characteristics (N = 105), January 2013 to April 2019
TABLE 3.
Demographic Characteristics of Patients Who Had One or More Febrile Neutropenia Episodes (N = 204), January 2013 to April 2019
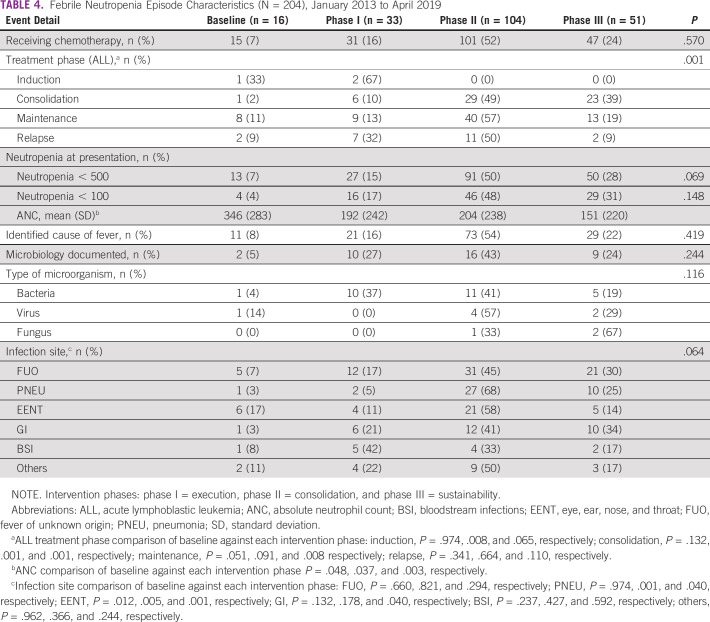
Table 4 shows the FN events' characteristics. Most patients had received chemotherapy during the 15 days before admission. Overall, 47% of the patients had profound neutropenia; a clinical infectious disease cause for the FN event was identified in 66% of the events (135 of 204), and a causative microorganism was identified in only 18% of those events (13 of 135). The most common microbiologically documented infections were bloodstream infections (30%), gastro-intestinal tract infections (22%), and skin and soft-tissue infections (19%). Most bloodstream infections were caused by Gram-negative bacteria (64%). Klebsiella pneumoniae was the predominant microorganism. Pneumonia was the most common infectious disease diagnosis (30%, 41 of 135) when combining clinically suspected and microbiologically documented infections. Only the acute lymphoblastic leukemia treatment phase in which the FN episode occurred was significantly different over the study period compared with baseline (P = .001). Seventy percent of the FN events were consulted in the inpatient area.
TABLE 4.
Febrile Neutropenia Episode Characteristics (N = 204), January 2013 to April 2019
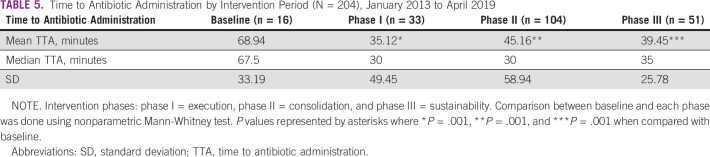
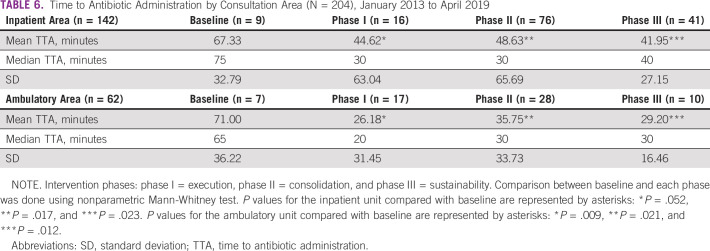
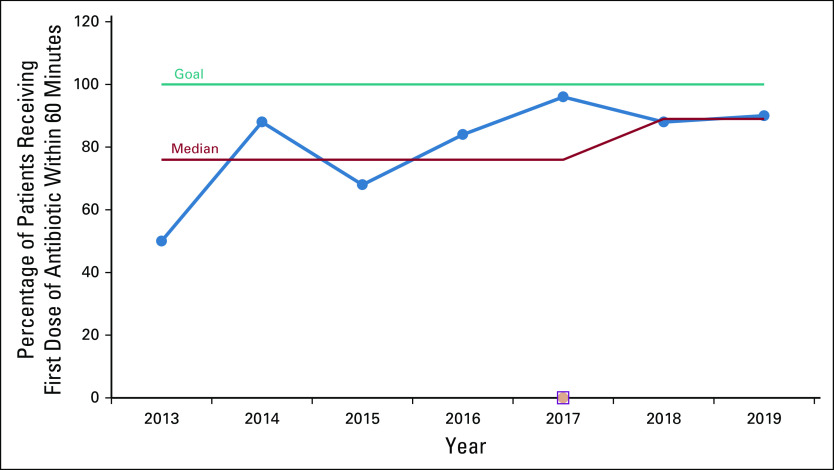
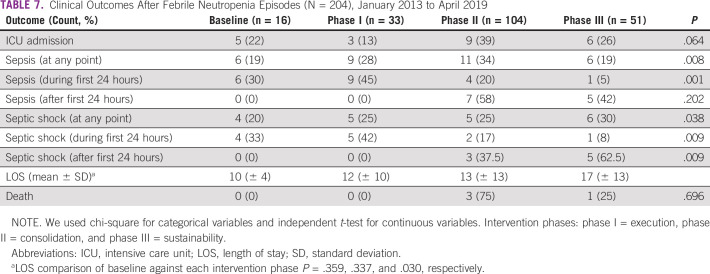
Table 5 displays the comparison between the TTA at baseline and at each of the three phases of the improvement strategy. A significant reduction in the overall median TTA from 67.5 minutes in the baseline group to 30 minutes in phase I and phase II was found (P = .001). The improvement was maintained through phase III (median, 35 minutes). When categorized by consultation area (inpatient v ambulatory), the TTA improvement was significant and sustained throughout the intervention phases (Table 6). The proportion of patients receiving antibiotics on time increased from 50% at baseline to 88% during phase III (Fig 2). Table 7 illustrates a statistically significant decrease in the proportion of patients in whom sepsis developed in the first 24 hours after admission during phase III (P = .001).
TABLE 5.
Time to Antibiotic Administration by Intervention Period (N = 204), January 2013 to April 2019
TABLE 6.
Time to Antibiotic Administration by Consultation Area (N = 204), January 2013 to April 2019
FIG 2.
Proportion of patients with antibiotics administered within 60 minutes of physicians' prescription stratified by year of the intervention. Intervention phases: baseline (2013); phase I, execution (2014); phase II, consolidation (2015-2017); and phase III, sustainability (2018-2019).
TABLE 7.
Clinical Outcomes After Febrile Neutropenia Episodes (N = 204), January 2013 to April 2019
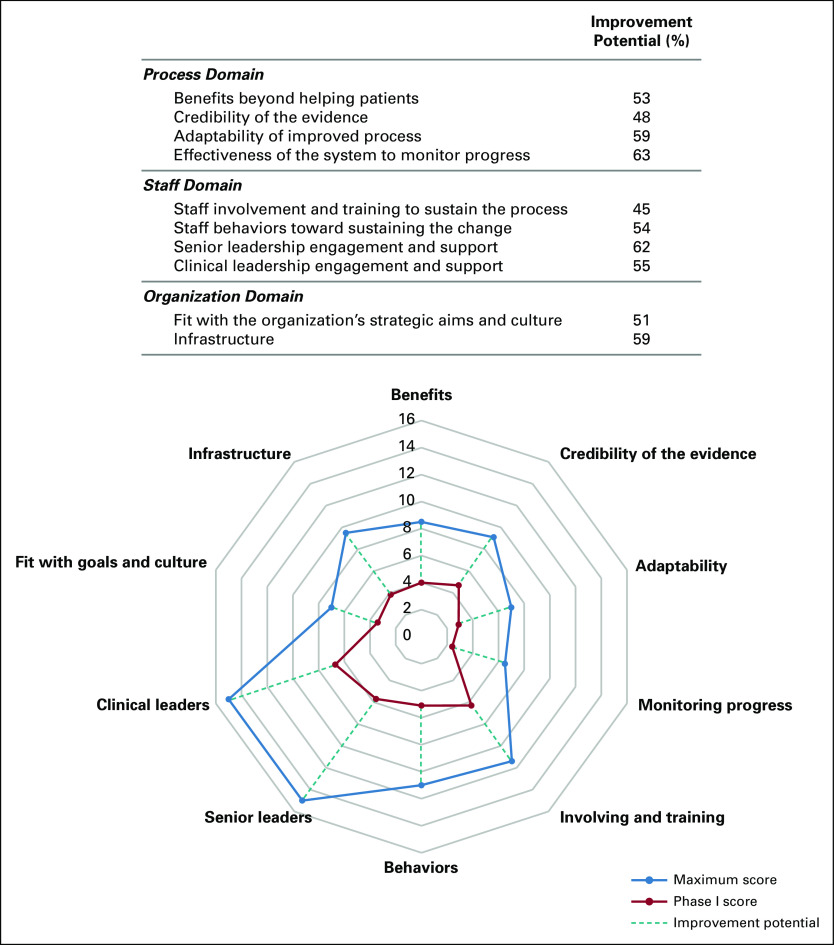
All the sustainability domains assessed had an improvement potential when documented for phase I of the improvement strategy (Fig 3). The two factors of the process domain with the highest improvement potential were the adaptability of improved processes and the system's effectiveness to monitor progress. Hospital leadership engagement was the most critical factor to address the staff domain. In the organization domain, the infrastructure factor was the most important (Fig 3). After completing phase III of the improvement strategy, 7 of 10 sustainability factors reached the maximum improvement potential. Two factors within the organization domain and one factor in the staff domain did not reach the maximum improvement potential (Fig 4).
FIG 3.
Improvement potential for the 10 sustainability factors (phase I).
FIG 4.
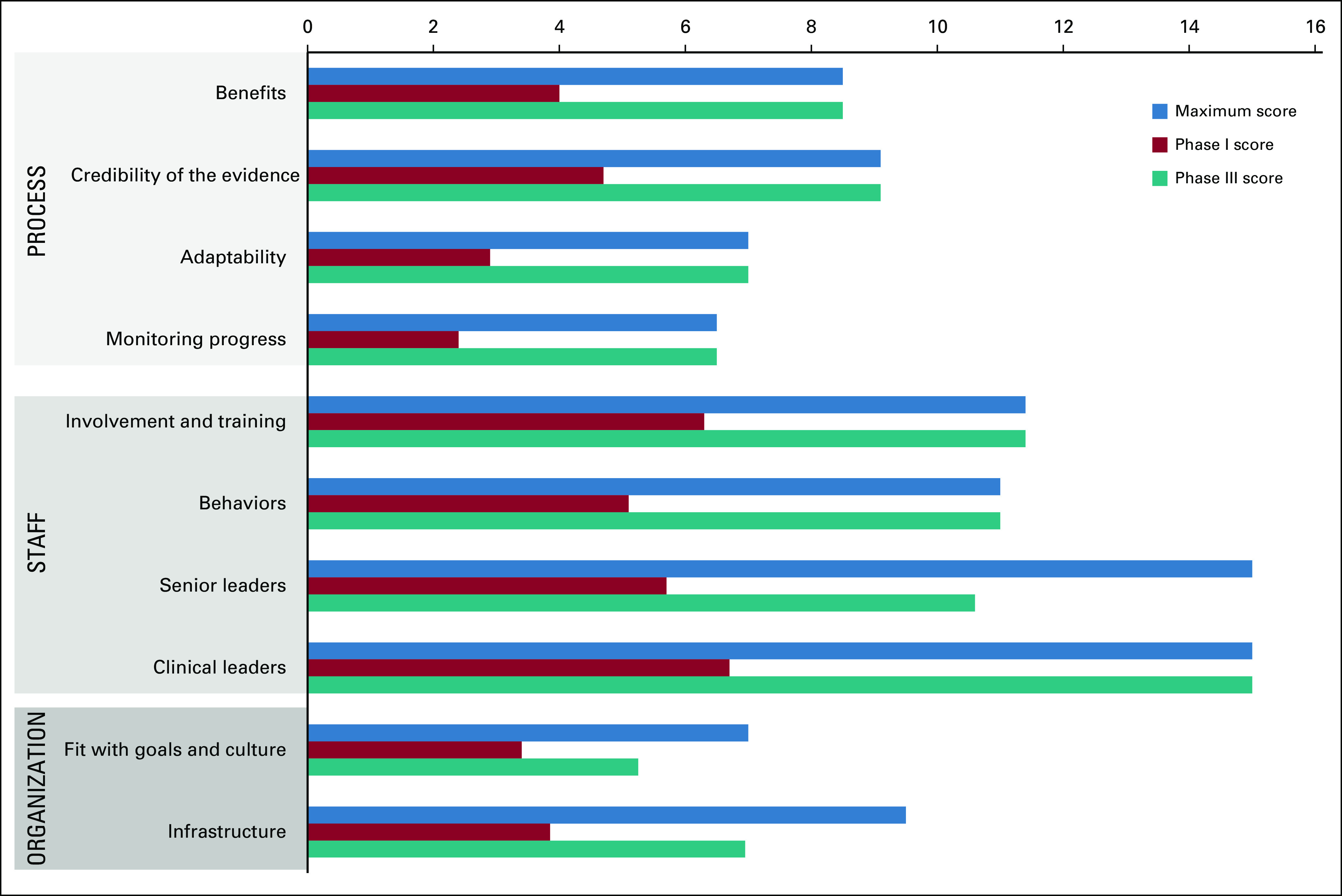
Sustainability factors comparison (phase I v phase III).
DISCUSSION
Our findings show that the use and implementation of a multimodal improvement strategy were associated with a significant decrease in TTA in patients with pediatric cancer and FN consulting in the inpatient and ambulatory areas in our center. Using a locally adapted multimodal strategy combining system change, the use of standardized FN guidelines, education, auditing and monitoring, mentoring, and dissemination increased the proportion of patients with pediatric cancer receiving antibiotics in a timely manner. A TTA < 60 minutes decreased the proportion of patients in whom sepsis developed over time. We did not find a decrease in intensive care admission and death rates. The LOS during phase III was higher than that at baseline. Our data demonstrate that the TTA in patients with FN in both assessed consultation areas has been maintained throughout a 5-year period. Factors such as improving local infrastructure, ongoing monitoring systems, and leadership engagement have been vital in ensuring the Golden Hour improvement strategy's sustainability.
Most interventions to decrease TTA in pediatric cancer facilities in HICs have been implemented in the Emergency Department (ED).11 Our intervention was implemented in the inpatient and ambulatory areas. Although the implementation areas differed, our improvement strategy components included the most commonly used interventions in HICs, with a mindful and collaborative local adaptation. For example, some pediatric cancer facilities in HICs have expedited the availability of antibiotics by implementing standing orders or incorporating an automatic antibiotic dispenser system such as the Pyxis in the ED9,31; the Golden Box was our adapted system to make sure that antibiotics were available at the point of care. This element not only required a physical space to place the Golden Box but also continued efforts to ensure funding for supplies and medications.
A recent meta-analysis found that the number of interventions needed to decrease TTA in pediatric cancer facilities varied from one single intervention up to 3-year interventions.11 Our 5-year improvement strategy not only included interventions that assured readily available antibiotics, standardized FN guidelines, education, and auditing/monitoring tools but also incorporated strong mentoring and dissemination components. Ongoing mentoring has contributed to promoting collaboration, team accountability, and local health care providers' empowerment and has facilitated extramural grant funding. Disseminating results in national and international forums has increased the visibility and empowerment of the hospital's Golden Hour team.
Extensive data related to the average TTA in different pediatric cancer facilities in HICs have been published.11 Although the definition of TTA has varied from one institution to another, TTA before an improvement intervention in 16 pediatric cancer facilities in the United States, Australia, Brazil, and Thailand averaged 137 minutes (range, 79-221 minutes); the reported reduction of the average TTA after implementing an intervention was 18-156 minutes (22%-73% reduction).11 However, data about the average TTA in resource-constrained settings are limited. One study in a pediatric cancer center in El Salvador reported that the time elapsed from hospital arrival and intravenous antibiotic administration was 3.5 hours.17 Similarly, a study in the Philippines reported that when assessing from fever onset to antibiotic administration, as much as 3.62 days elapsed, and the TTA was 15 hours.16 Our baseline TTA was lower (75 minutes and 65 minutes, inpatient and ambulatory area, respectively) than the TTA reported in other studies.12,13,16,17 This difference could be explained by the consultation area where care is provided and the TTA definition used. Our patients are assessed directly in the inpatient and ambulatory areas and do not face delays in care in the ED, such as overcrowding or triage failure. Longer TTA has been described when patients are seen in the ED upon presentation of FN.26 Our TTA definition assessed time from the initial provider evaluation to intravenous antibiotic administration; the studies in El Salvador and the Philippines used the time to hospital arrival to calculate their TTA.16,17 However, when compared with a study carried out in an outpatient unit that used the same TTA definition,32 the median TTA before intervention in our inpatient area (75 minutes) was similar to their findings (79.6 minutes); the reported TTA reduction after the intervention was 48%. We reduced the median TTA by 47% in the inpatient area and 53% in the ambulatory area.
Clinical outcomes reported by single-center TTA quality improvement projects in cancer settings have included decreases in mortality, intensive care admission, and sepsis rates.8,9,33 However, a recent meta-analysis found it difficult to generate solid conclusions about an unequivocal direct association between TTA and death, admission to intensive care, or sepsis and/or septic shock. The heterogeneity of the published studies, triage biases, and the lack of control of other confounding factors made it difficult to provide a clear conclusion.34 Similarly, we found no association between TTA < 60 minutes and a decrease in either mortality or intensive care admission rates in our center. The mortality prevalence reported in TTA improvement in pediatric studies has been between 0.7% and 3.4%. During our study period, the prevalence was 3.8%. The proportion of intensive care admissions in the sustainability phase did not significantly differ from that at baseline (22% v 26%). Unlike other studies, we found a significant increase in LOS and the proportion of patients with septic shock during the sustainability phase. The proportion of sepsis and septic shock decreased after phase I of the intervention.
Our study has several limitations that should be considered, including those inherent to a single-center design. First, our sample before the intervention was small, which may affect the impact of comparisons pre- and postintervention, and we did not control for confounding factors such as the initial illness severity, sepsis at initial clinical presentation, patients' FN risk status, prior use of prophylactic antibiotics, worsening of sepsis because of immediate administration of antibiotics, and levels of staffing (time and day of the week). Simultaneous to our TTA improvement strategy, in 2018-2019 (during phase III), another quality improvement initiative that included the implementation of a pediatric early warning system in children with cancer might have also influenced improvement in our target outcomes. As previously mentioned, the proportion of sepsis significantly decreased in phase III, possibly as a result of the combination of the pediatric early warning system and Golden Hour interventions. Our TTA definition did not include either the time from fever onset to hospital arrival or that from hospital arrival to the first health care provider assessment. We did not assess adherence to the FN guideline or adequacy of supportive management. The number of patients and patient flow might be similar to that in other medium-sized public pediatric centers in other states in Mexico but do not represent the number or flow of patients in larger cancer institutions in our country.
Our results suggest that locally adapted multimodal interventions can reduce TTA in resource-constrained settings. Our study demonstrates the feasibility of implementing a multimodal improvement strategy that targets TTA improvement and combines an assessment of the recommended core set of minimal clinical outcomes35 and associated sustainability factors.27 The appropriate validation of these findings would require a multisite study in our region to address the limitations reported in our study. Mentoring and dissemination are novel components to include in future TTA improvement strategies. Assessing adherence to FN guidelines and improving treatment adequacy by assuring optimal infectious disease diagnostic capabilities are areas to be addressed in future research investigating the role of TTA in clinical outcomes in resource-constrained settings.
ACKNOWLEDGMENT
We thank all patients, families, and oncology nurses and physicians who participated in or supported the Golden Hour initiative at Hospital General Tijuana, Patronato Foundation, and Casa de La Amistad, especially Alfonso Valenzuela, MD; Gloria Monforte, MD; Salma Amaya, MA; Pedro Perez, PhD; Leonardo Arana, ENGR; Silvia Ornelas, MS; Alberto Torres, ENGR; Baltasar Madrid, MS; Clemente Zuniga, MD; Alfredo Ornelas, MD; Alberto Reyes, MD; Angelica Martinez, MD; Martha Magdaleno, MD; Gabriela Tamayo, MD; Magdalena Perez, MD; Gabriela Aguiar, MD; Raquel Ruiz, BA; Marcela Baltazar, MA; Dora Bastidas, LSW; and Martha Garcia, LSW. We thank Francois Desbrandes, Valentine Leuenberger, and Valerie Faillat at the Sanofi Espoir Foundation, My Child Matters Pediatric Oncology Program. We also thank the members in the St Jude Children's Research Hospital Global Pediatric Medicine Department and Rady Children's Hospital San Diego who mentored the Golden Hour team. The authors thank Cherise Guess, PhD, ELS, for editing the manuscript.
Appendix
Improvement Strategy Description
Phase I (12 months, 2014)
Phase I is referred to as the execution phase of several components of the improvement strategy (Fig 1). In January 2014, a local improvement team was formed consisting of a pediatrician leader (A.L.-R.), three nurses (M.A.C., A.S., and M.R.), an oncologist (R.R.-G.), and an infectious disease physician (D.T.). The team named the improvement initiative The Golden Hour. The first component of the strategy to implement was system change, which refers to the act of ensuring the availability of supplies needed at the point of care to enable adequate practices.36 To ensure a timely time to antibiotic administration (TTA), the team provided access to venipuncture or line care supplies, blood culture bottles, and first-line broad-spectrum antibiotics. A portable toolbox was adapted to serve as centralized storage of all needed supplies and medications at the point of care. The box was named The Golden Box and was placed in the inpatient pediatric oncology ward.
The second component included the development, socialization, and implementation of a local febrile neutropenia (FN) guideline and algorithm. The FN guideline and algorithm were developed based on local antimicrobial susceptibilities and adapted from international pediatric guidelines.4,29 Cefepime was the initial broad-spectrum antibiotic administered to patients with low and high-risk FN without septic shock. Patients with septic shock were treated with meropenem and vancomycin. Aminoglycoside was added when antimicrobial resistance was suspected. The local guideline recommendation was to prescribe empirical antibiotics as soon as the laboratory test and blood cultures were drawn without waiting for the complete blood cell count results to confirm neutropenia.
The educational component targeted health care providers and caregivers. Training materials for health care providers addressed (1) recognition of FN as an oncology emergency, (2) the Golden Hour strategy, (3) early recognition of sepsis, and (4) blood culture technique. Pre- and post-tests were conducted to assess knowledge gain. Caregivers were instructed on basic concepts of FN and procedures to measure body temperature at home. This training was delivered at least twice a year for health care providers and once per year for caregivers. New employees and caregivers of patients with newly-diagnosed cancer received one-to-one training during their onboarding process. As part of the auditing and monitoring component, the team developed two data collection tools: (1) a checklist to monitor supplies availability and (2) a database to document every FN event. Each FN event's characteristics were prospectively documented as part of the infection prevention and control surveillance.22 The mentoring component allowed The Golden Hour team to receive online mentoring by mentors at Rady Children's Hospital (P.A.) and St Jude (M.L.G. and M.A.C.) on a biweekly basis during this phase.
Finally, the dissemination component encouraged The Golden Hour team to provide feedback locally to staff and to share results in local, regional, and international forums.
Phase II (36 months, 2015-2017)
Phase II or consolidation phase was centered on adding another Golden Box in the ambulatory infusion area, ongoing education, auditing and monitoring, mentoring, and dissemination (Fig 1). Nurse educators reinforced caregivers' knowledge at each hospital admission. The Golden Hour team partnered with a local philanthropic foundation and obtained extramural funding to ensure continuous availability of supplies and antibiotics. The results were shared with local health care providers and hospital leadership at least monthly.
Phase III (16 months, 2018-2019)
Phase III or sustainability phase focused on institutionalization of the improvement strategy components. In addition to the education, auditing and monitoring, and dissemination activities that had been adopted and incorporated as a part of the daily workflow at Hospital General Tijuana, hospital leadership engagement was needed to achieve long-term sustainability. Moreover, the Golden Hour Team joined the Mexico in Alliance with St Jude Quality Improvement Collaborative and served as one of the pioneering institutions in Mexico to model the success in decreasing TTA in similar settings.
Key Definitions
Fever was defined as a single oral temperature of ≥ 38.3°C (101°F) or a temperature of ≥ 38.0°C (100.4°F) sustained over a 1-hour period. Neutropenia was defined as an absolute neutrophil count (ANC) < 500 cells/μL or an ANC that was expected to decrease to < 500 cells/μL over the next 48 hours, and profound neutropenia, as an ANC < 100 cells/μL.29 Sepsis was defined as the presence of systemic inflammatory response syndrome in the presence of or as a result of suspected or proven infection.30 Sepsis was compared at two points in time: before and 24 hours after admission. Septic shock was defined as a patient having cardiovascular organ dysfunction in addition to meeting the sepsis criteria.30
Patients were recorded as having received chemotherapy if they received any type of chemotherapy medications during the 15 days before the current admission. We used standardized Centers for Disease Control and Prevention surveillance infection definitions; each infection event was categorized by the major and specific site of infection.37
Dara Torres
Honoraria: Merck
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or National Cancer Institute.
PRIOR PRESENTATION
Presented as a poster presentation at St Jude/Pediatric Infectious Diseases Society Pediatric Infectious Diseases Research Conference, Memphis, TN, March 10-11, 2017; as an oral and poster presentation in The American Society of Hematology Annual Meeting, San Diego, CA, December 1-4, 2018; and as a poster presentation at the International Society of Pediatric Oncology, Lyon, France, October 23-26, 2019.
SUPPORT
Supported by the Sanofi Espoir Foundation, My Child Matters Pediatric Oncology Program, the American Lebanese Syrian Associated Charities (ALSAC) and partially supported by the National Cancer Institute K08 CA230306 (P.A.).
AUTHOR CONTRIBUTIONS
Conception and design: Paula Aristizabal, Rebeca Rivera-Gómez, Miguela A. Caniza
Financial support: Miguela A. Caniza
Administrative support: Paola Friedrich, Miguela A. Caniza
Provision of study material or patients: Mario Ornelas-Sánchez, Marco Aguilera, Mitzy Romano
Collection and assembly of data: Miriam L. Gonzalez, Paula Aristizabal, Adriana Loera-Reyna, Dara Torres, Laura Nuño-Vázquez, Marco Aguilera, Alicia Sánchez, Mitzy Romano
Data analysis and interpretation: Miriam L. Gonzalez, Paula Aristizabal, Mario Ornelas-Sánchez, Laura Nuño-Vázquez, Alicia Sánchez, George Relyea, Paola Friedrich
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Dara Torres
Honoraria: Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.Pizzo PA.Management of fever in patients with cancer and treatment-induced neutropenia N Engl J Med 3281323–13321993 [DOI] [PubMed] [Google Scholar]
- 2.Pizzo PA.Management of patients with fever and neutropenia through the arc of time: A narrative review Ann Intern Med 170389–3972019 [DOI] [PubMed] [Google Scholar]
- 3.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol 31431–4552010 [DOI] [PubMed] [Google Scholar]
- 4.Lehrnbecher T, Phillips R, Alexander S, et al. Guideline for the management of fever and neutropenia in children with cancer and/or undergoing hematopoietic stem-cell transplantation J Clin Oncol 304427–44382012 [DOI] [PubMed] [Google Scholar]
- 5.Lehrnbecher T, Robinson P, Fisher B, et al. Guideline for the management of fever and neutropenia in children with cancer and hematopoietic stem-cell transplantation recipients: 2017 update J Clin Oncol 352082–20942017 [DOI] [PubMed] [Google Scholar]
- 6.Klastersky J, de Naurois J, Rolston K, et al. Management of febrile neutropaenia: ESMO clinical practice guidelines Ann Oncol 27v111–v1182016suppl 5 [DOI] [PubMed] [Google Scholar]
- 7.Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical Practice Guideline update J Clin Oncol 361443–14532018 [DOI] [PubMed] [Google Scholar]
- 8.Fletcher M, Hodgkiss H, Zhang S, et al. Prompt administration of antibiotics is associated with improved outcomes in febrile neutropenia in children with cancer Pediatr Blood Cancer 601299–13062013 [DOI] [PubMed] [Google Scholar]
- 9.Salstrom JL, Coughlin RL, Pool K, et al. Pediatric patients who receive antibiotics for fever and neutropenia in less than 60 min have decreased intensive care needs Pediatr Blood Cancer 62807–8152015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCavit TL, Winick N.Time-to-antibiotic administration as a quality of care measure in children with febrile neutropenia: A survey of pediatric oncology centers Pediatr Blood Cancer 58303–3052012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koenig C, Schneider C, Morgan JE, et al. Interventions aiming to reduce time to antibiotics (TTA) in patients with fever and neutropenia during chemotherapy for cancer (FN), a systematic review Support Care Cancer 282369–23802020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cash T, Deloach T, Graham J, et al. Standardized process used in the emergency department for pediatric oncology patients with fever and neutropenia improves time to the first dose of antibiotics Pediatr Emerg Care 3091–932014 [DOI] [PubMed] [Google Scholar]
- 13.Cohen C, King A, Lin CP, et al. Protocol for reducing time to antibiotics in pediatric patients presenting to an emergency department with fever and neutropenia: Efficacy and barriers Pediatr Emerg Care 32739–7452016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emerson BL, Prozora S, Jacob A, et al. An initiative to decrease time to antibiotics for patients with fever and neutropenia Am J Med Qual 34158–1642019 [DOI] [PubMed] [Google Scholar]
- 15.Volpe D, Harrison S, Damian F, et al. Improving timeliness of antibiotic delivery for patients with fever and suspected neutropenia in a pediatric emergency department Pediatrics 130e201–e2102012 [DOI] [PubMed] [Google Scholar]
- 16.Kirby J, Dolendo M, Guimera D, et al. Predictors of wait-time for antibiotic initiation and association of wait-time with hospital length of stay and ICU admission among children with cancer at the Southern Philippines Medical Center Pediatr Blood Cancer 61680–6862014 [DOI] [PubMed] [Google Scholar]
- 17.Gavidia R, Fuentes SL, Vasquez R, et al. Low socioeconomic status is associated with prolonged times to assessment and treatment, sepsis and infectious death in pediatric fever in El Salvador. PLoS One. 2012;7:e43639. doi: 10.1371/journal.pone.0043639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfäfflin F, Tufa TB, Getachew M, et al. Implementation of the WHO multimodal hand hygiene improvement strategy in a university hospital in Central Ethiopia. Antimicrob Resist Infect Control. 2017;6:3. doi: 10.1186/s13756-016-0165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allegranzi B, Gayet-Ageron A, Damani N, et al. Global implementation of WHO's multimodal strategy for improvement of hand hygiene: A quasi-experimental study Lancet Infect Dis 13843–8512013 [DOI] [PubMed] [Google Scholar]
- 20.Storr J, Twyman A, Zingg W, et al. Core components for effective infection prevention and control programmes: New WHO evidence-based recommendations. Antimicrob Resist Infect Control. 2017;6:6. doi: 10.1186/s13756-016-0149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aristizabal P, Fuller S, Rivera R, et al. Improving pediatric cancer care disparities across the United States-Mexico border: Lessons learned from a transcultural partnership between San Diego and Tijuana. Front Public Health. 2015;3:159. doi: 10.3389/fpubh.2015.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres D, González ML, Loera A, et al. The Centers for Disease Control and Prevention definition of mucosal barrier injury-associated bloodstream infection improves accurate detection of preventable bacteremia rates at a pediatric cancer center in a low- to middle-income country Am J Infect Control 44432–4372016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caniza MA, Maron G, McCullers J, et al. Planning and implementation of an infection control training program for health care providers in Latin America Infect Control Hosp Epidemiol 281328–13332007 [DOI] [PubMed] [Google Scholar]
- 24. www.cure4kids.org
- 25.Friedrich P, Echeandia N, Romo-Rubio H, et al. Scaling-up effective interventions in global pediatric oncology: Mexico in alliance with St. Jude, a breakthrough model Pediatr Blood Cancer 67366–3672020suppl 4 [Google Scholar]
- 26.De la Maza V, Simian D, Castro M, et al. Administration time for the first dose of antimicrobials in episodes of fever and neutropenia in children with cancer Pediatr Infect Dis J 341069–10732015 [DOI] [PubMed] [Google Scholar]
- 27.Maher L, Gustafson D, Evans A. NHS sustainability model. https://improvement.nhs.uk/resources/Sustainability-model-and-guide/
- 28.Doyle C, Howe C, Woodcock T, et al. Making change last: Applying the NHS institute for innovation and improvement sustainability model to health care improvement. Implement Sci. 2013;8:127. doi: 10.1186/1748-5908-8-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America Clin Infect Dis 52e56–e932011 [DOI] [PubMed] [Google Scholar]
- 30.Goldstein B, Giroir B, Randolph A.International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics Pediatr Crit Care Med 62–82005 [DOI] [PubMed] [Google Scholar]
- 31.Jobson M, Sandrof M, Valeriote T, et al. Decreasing time to antibiotics in febrile patients with central lines in the emergency department Pediatrics 135e187–e1952015 [DOI] [PubMed] [Google Scholar]
- 32.Vanderway J, Vincent C, Walsh SM, et al. Implementation of a pathway for the treatment of fever and neutropenia in pediatric patients with cancer J Pediatr Oncol Nurs 34315–3212017 [DOI] [PubMed] [Google Scholar]
- 33.Pakakasama S, Surayuthpreecha K, Pandee U, et al. Clinical practice guidelines for children with cancer presenting with fever to the emergency room Pediatr Int 53902–9052011 [DOI] [PubMed] [Google Scholar]
- 34.Koenig C, Schneider C, Morgan JE, et al. Association of time to antibiotics and clinical outcomes in patients with fever and neutropenia during chemotherapy for cancer: A systematic review Support Care Cancer 281369–13832020 [DOI] [PubMed] [Google Scholar]
- 35.Haeusler GM, Phillips RS, Lehrnbecher T, et al. Core outcomes and definitions for pediatric fever and neutropenia research: A consensus statement from an international panel Pediatr Blood Cancer 62483–4892015 [DOI] [PubMed] [Google Scholar]
- 36.Allegranzi B, Sax H, Pittet D.Hand hygiene and health care system change within multi-modal promotion: A narrative review J Hosp Infect 83S3–S102013suppl 1 [DOI] [PubMed] [Google Scholar]
- 37.CDC NHSN patient safety component manual. http://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf